Abstract
Recent studies have shown that chronic use of prescription or illicit opioids leads to an increased risk of cardiovascular events and pulmonary arterial hypertension. Indices of vascular age and arterial stiffness are also shown to be increased in opioid dependent patients, with the effects being more marked in women. There are currently no studies investigating sex-specific vascular dysfunction in opioid use, and the mechanisms leading to opioid-induced vascular damage remain unknown. We hypothesized that exposure to exogenous opioids causes sex-specific vascular remodeling that will be more pronounced in female. Acknowledging the emerging roles of cofilins and extracellular signal-regulated kinases (ERK) in mediating actin dynamics, we investigated the effects of morphine on these molecules. Twenty-four hour exposure to morphine increased inactivated cofilin and activated ERK in resistance arteries from female mice, which may promote stress fiber over-assembly. We also performed continuous intraluminal infusion of morphine in pressurized resistance arteries from male and female mice using culture pressure myographs. We observed that morphine reduced the vascular diameter in resistance arteries from female, but not male mice. These results have significant implications for the previously unexplored role of exogenous opioids as a modifiable cardiovascular risk factor, especially in women.
Keywords: Opioids, sex differences, vascular physiology
INTRODUCTION
According to the Center for Disease Control’s Annual Surveillance Report of Drug Related Risks and Outcomes, more than 47,600 people died from an opioid overdose in the United States in 2019 [1]. This means that on average, there were over 130 opioid overdose deaths on any given day. In response to the magnitude of the ongoing crisis that raises questions on the associations between opioid use and chronic diseases, recent studies have shown that exogenous opioids act extensively on the cardiovascular system [2-7]. Opioid use is shown to be a significant risk factor for coronary artery disease, and follows a dose dependent relationship with the severity of atherosclerosis as measured by clinical vessel score [8]. It was also observed that coronary disease occurred two years earlier in opioid dependent patients than in opioid naïve controls [9].
There are three major types of opioid receptors: μ-opioid receptor, δ-opioid receptor, and κ-opioid receptor, and it has been shown that they are all expressed in the cardiovascular system [10,11]. The μ-opioid receptor serves as the primary physiological target for most clinically important opioids including morphine, and is known to be expressed on the surface of endothelial cells [12,13]. Studies have shown that opioids can promote angiogenesis and endothelial cell proliferation, indicating that stimulation of opioid receptors can have adverse effects on the cardiovascular system [14,15]. From an endocrinological standpoint, studies suggest that long-term exposure to opioids decreases estrogen levels, an important cardioprotective hormone, and this can be reversed by the discontinuation of opioid treatment [16,17]. Furthermore, women were significantly more likely to report lifetime opioid use than men, and higher prescription rates were seen in female patients, leading to increased overdoses in women [18]. As estrogen was shown to exert a direct protective effect against ischemia/reperfusion injury on the myocardium, opioid suppression of the hypothalamic-pituitary-ovarian axis in females can certainly be a leading culprit in the increased number of opioid overdose deaths seen in women [19,20]. Additionally, indices of vascular age and arterial stiffness are worse in opioid dependent patients compared with opioid naïve controls, with mean calculated ages elevated by 1.97% in men and 13.43% in women [21]. Although the relationship between opioids and vascular disease, including sex differences are emerging, there remains a gap in the literature regarding the precise mechanisms associated with opioids and vascular changes. We hypothesized that opioids affect vascular remodeling in a sex-dependent manner. Although we observed that opioids induce vascular smooth muscle cell proliferation in cells from both males and females, culture pressure myographs revealed that female mice present higher risk of developing morphine-induced vascular dysfunction than male mice.
METHODS
1. Animals
Male and female C57BL/6NTac mice (12 weeks old) were purchased from Taconic (USA) and maintained at the animal facility, University of Toledo College of Medicine and Life Sciences. All animal procedures and protocols used were approved by the University of Toledo Institutional Animal Care and Use Committee (IACUC protocol approval numbers 108854, 108855, 104573, 108390). Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were maintained on a 12:12 hour light-dark cycle with both standard chow and water ad libitum. For vascular function and molecular biology experiments, mice used were euthanized via thoracotomy and exsanguination via cardiac puncture while anesthetized under isoflurane anesthesia (5% in 100% O2 administered via nose cone). Please note that the sample size indicated per experiment is the number of independent mice used.
2. Vascular Function and Mechanics
The mesenteric arcade was carefully removed from male and female mice. Fifth order mesenteric resistance arteries (MRA) were used to evaluate vascular function and mechanics [internal diameter μm: Female: 134±14 (n=4), Male: 112±11 μm (n=4)]. For this, arteries were mounted on DMT culture myographs (Danish MyoTech, Aarhus, Denmark). The 2-3 mm segments were bathed in filtered Krebs physiologic buffer (in mM: 115 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4.7H2O, 2.5 CaCl2, 1.2 KH2PO4, 11.1 glucose and 0.01 Na2EDTA) and cannulated at both ends in an arteriography using a glass microcannula (120-125 μm diameter) and then secured with surgical nylon suture. Subsequently, vessel length was adjusted to maintain the vessel walls parallel at increasing pressures. The arteries were then checked for the presence of leakage. HEPES physiologic buffer (in mM: 134 NaCl, 6 KCl, 1 MgCl, 2 CaCl2, 10 glucose, 0.03 Na2EDTA, 10 HEPES) was used to avoid salt crystal formation after 24 hours of intraluminal infusion, and Krebs physiologic buffer (in mM: 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4, 14.9 NaHCO3, 1.56 CaCl2, 5.5 glucose, 0.026 EDTA) was used for extraluminal. Flow in the vessel was generated through the distal pipette with a peristaltic pump. Intraluminal pressure was then slowly raised to 160 mmHg at 20 mmHg intervals, and again the vessel length was adjusted at each interval. The segment was set to a pressure of 60 mmHg and allowed to equilibrate for 30 min at 37°C, gassed with a mixture of 95% O2 and 5% CO2. After testing vascular smooth muscle cell integrity with KCl (120 mmol/L), arteries were equilibrated at 60 mmHg for 30 min in HEPES (intraluminal) and Krebs (extraluminal) solution, the intraluminal pressure was dropped to 3 mmHg and a pressure curve was obtained by increasing the intraluminal pressure in 20 mmHg steps to 160 mmHg every 2 min. Subsequently, the arteries were equilibrated at 60 mmHg for 30 min, and the arteries were continuously intraluminally infused with morphine (10 μM) or vehicle (HEPES physiologic buffer) for 24 hours. After the treatment, pressure-curve was repeated. After this curve the intraluminal and extraluminal buffers were exchanged with 0 mM Ca2+ Krebs (plus EGTA, 1 mM) and equilibrated at 60 mmHg for 10 min. After this period, another pressure curve was obtained. Videos and/or still images were captured using the Myoview data capture software (DMT, Aarhus, Denmark) and subsequently analyzed with the VasoTracker Offline Diameter Analyzer for accurate inner and outer diameter measurements [22]. From the internal and external diameter measurements in the passive conditions, structural parameters were calculated as previously described [23-25].
Changes in lumen diameter (Δ) was calculated by subtracting the lumen diameter measured at 0 hours from the lumen diameter measured at 24 hours, after the infusion of morphine or vehicle. The lumen diameter measurements that were used to calculate Δ lumen diameter were taken from curves that were performed in the presence of Ca2+.
2.1. Calculation of Mechanical Parameters
Diameter = (Di/Di0Ca2+60mmHg) x 100
Circumferential wall strain (ε) = (Di0Ca2+ - D00Ca2+)/D00Ca2+, where D00Ca2+ is the internal diameter at 3 mmHg.
Wall thickness (WT) = (De0Ca2+ – Di0Ca2+)/2
Wall:lumen = (De0Ca2+ – Di0Ca2+)/2Di0Ca2+
Cross-sectional area = (π/4) X (DeOCa2+2 – Di0Ca2+2)
Circumferential wall stress (σ) = (P × Di0Ca2+)/(2WT), where P is the intraluminal pressure (1 mmHg = 1.334 × 103 dynes·cm-2) and WT is wall thickness at each intraluminal pressure in 0Ca2+-KHS.
Arterial stiffness is determined by the Young’s elastic modulus (E=stress/strain). The stress-strain relationship is non-linear; therefore, it is important to obtain a tangential or incremental elastic modulus (Einc) by determining the slope of the stress-strain curve (Einc =δσ/δε) Einc was obtained by fitting the stress-strain data from each animal to an exponential curve using the equation: σ =σorigeβε, where σorig is the stress at the original diameter. Taking derivatives on the equation presented earlier, we see that Einc=βσ. For a given σ-value, Einc is directly proportional to β. An increase in β implies an increase in Einc, which means an increase in stiffness [23].
3. Western Blotting
Mesenteric resistance arteries were collected from male and female mice and treated with vehicle or morphine (10 μM, 24 hours). Subsequently, the non-pressurized arteries were incubated in a humidified chamber at 37°C, with 5% CO2, and low glucose Dulbecco's Modified Eagle's Medium (GE Healthcare, Logan, UT, USA) containing 10% fetal bovine serum and 1% penicillin/streptomycin solution (Corning, Manassas, VA, USA). After treatment, arteries were washed with an ice-cold phosphate-buffered saline (PBS) solution. Arteries were then homogenized using a mortar and pestle and lysed using tissue protein extraction reagent (Thermo Fisher Scientific), with protease inhibitors (sodium orthovanadate, phenylmethylsulfonyl fluoride, protease inhibitor cocktail) and phosphatase inhibitors (sodium fluoride and sodium pyrophosphate) (all Millipore Sigma). Protein concentration of lysates were subsequently determined, and then equal quantities of protein (15 μg) were loaded into polyacrylamide gels (12%). Gels were then transferred to nitrocellulose membranes (GE Healthcare, USA). The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4°C with primary antibodies. β-actin was used as the loading control. Further details on antibody concentrations, secondary antibody isotype, company from which the antibody was purchased, and the Research Resource Identifiers (RRID) antibody identification number are presented in Table 1. Phosphorylated proteins were normalized to β-actin given that opioids interfered in their total form. Densitometric analysis was performed by ImageJ (National Institutes of Health).
TABLE 1:
| Antibodies | Primary Antibody concentration |
Secondary Antibody concentration |
Company And Cat # |
RRID |
|---|---|---|---|---|
| Phospho-cofilin (pSer3) | 1:1,000 | 1:1,000 | Cell Signaling #3313 | RRID:AB_2080597 |
| Total Cofilin | 1:1,000 | 1:1,000 | Cell Signaling #5175 | RRID:AB_10622000 |
| Phospho-p44/42 (ERK 1/2) | 1:1,000 | 1:1,000 | Cell Signaling #4377 | RRID:AB_331775 |
| Total p44/42 (ERK 1/2) | 1:1,000 | 1:1,000 | Cell Signaling #9102 | RRID:AB_330744 |
| β-actin (HRP conjugated) | 1:8,000 | N/A | Sigma-Aldrich #A3854 | RRID:AB_262011 |
4. Vascular smooth muscle cells proliferation
Aortic vascular smooth muscle cells were isolated from male and female rats with a Sprague Dawley background (11-13-week-old). Primary cells were then cultured in DMEM (Dulbecco's Modified Eagle Medium) with 10% FBS (Fetal Bovine Serum) and 1% P/S (penicillin-streptomycin). The medium was changed every 48 hours until the cells were ready to be passaged. Cells were cultured up to passage 2, and subsequently they were plated with 2500 cells/well. Treatment consisted of fresh medium containing either morphine (10 nM or 30 μM), hydrocodone (10 nM or 30 μM), or 0.1% DMSO control. The medium was not replaced until the end of the treatment. The plates were allocated to the IncuCyte S3 from Essenbioscience and real time images from cell were captured in a time-dependent manner (every 16 hours and up to 120 hours or 5 days). Images were analyzed for percent confluence over time with the IncuCyte S3 from Essenbioscience.
5. Drugs
Our department is registered with the U.S. Drug Enforcement Agency (DEA) to purchase, possess, or use controlled substances (opioids). Morphine sulfate salt pentahydrate (# M8777) and hydrocodone (+)-bitartrate salt (# H4516) were purchased from Millipore-Sigma (Saint Louis, USA) and stored in a safety cabinet that was bolted to the wall. Access was limited to the laboratory PI, and no more than two laboratory members used the substance. Detailed inventory records were kept up to date, including amounts purchased, used, left on hand, and disposed of. Morphine and hydrocodone solutions were prepared in distilled water and DMSO, respectively.
Based on the U.S. Food and Drug administration (FDA), morphine dosing is 0.08-0.1 mg/kg for pediatrics, and the usual starting dose in adults is 0.1 mg - 0.2 mg/ kg (i.v.) every 4 hours as needed to manage pain. The morphine concentrations used in our experiments are based on reported therapeutic doses, but were adjusted for mice and rat models [26-30]. Additionally, the amount of hydrocodone used was strictly based on the morphine concentrations for comparison purposes, therefore no dosing calculation was done for hydrocodone specifically.
6. Statistical analysis
All statistical analyses were performed using GraphPad Prism 9.0.2 (La Jolla, CA, USA). Data are presented as mean ± S.E.M. and statistical significance was set at p < 0.05. Specific procedures used include: Student’s unpaired t-test to compare the means between 2 samples; one-way or two-way analysis of variance (ANOVA) to compare more than 2 conditions and time dependent curves, respectively. Tukey’s post-hoc test and Bonferroni post-hoc test were used in one-way ANOVA and two-way ANOVA, respectively. To test data for normal distribution, we used a normality test (D’ Agostino & Person and/or Shapiro-Wilk test). The sample size indicated per experiment is the number of independent animals used.
RESULTS
Vascular changes in female arteries upon acute exposure to morphine.
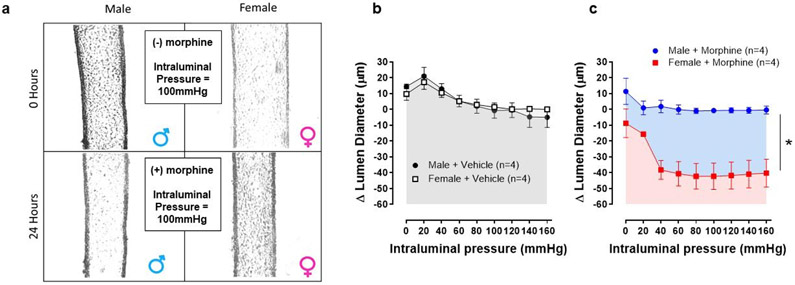
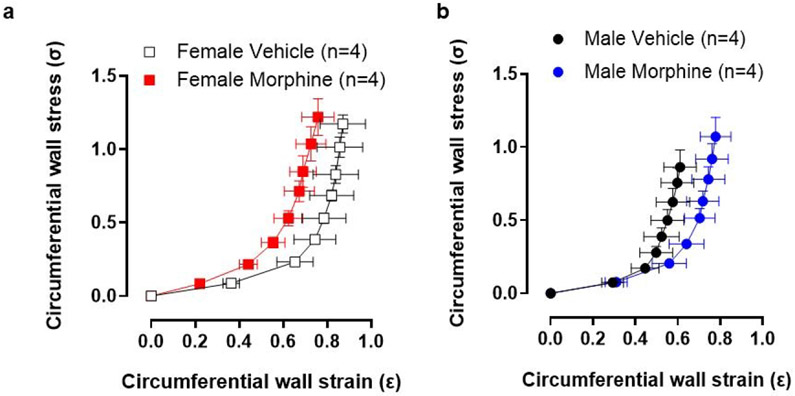
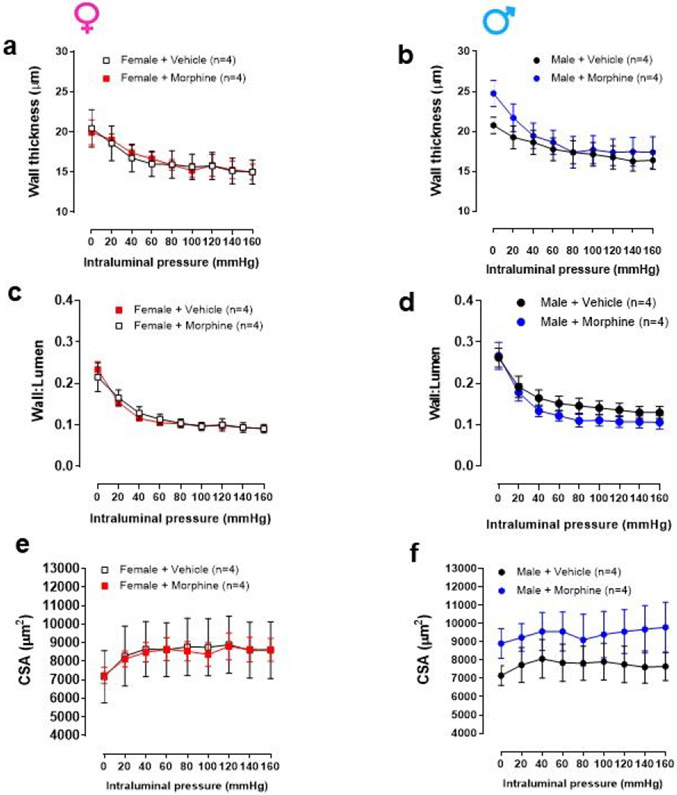
Intraluminal pressure curve showed that 24 hours of morphine intraluminal infusion reduced lumen diameter by 23% in mesenteric resistance arteries from female mice when compared to vehicle (Figure 1a-c). There was an average change of ~40 μm from baseline values (Figure 1b and c). In contrast, there was little to no change in the luminal diameter of male arteries (Figure 1b and c). Regarding strain-stress curves, a tendency of increasing vascular stiffness in arteries was observed from female mice treated with morphine, as shown by the leftward shift in the stress-strain curve (Figure 2a). However, no changes were observed in the elastic modulus β in arteries from female and male mice treated with morphine (Female: vehicle: 8.5 ±0.58 vs. Morphine: 5.9 ± 0.6; Male: vehicle: 9.3 ±1.1 vs. Morphine: 8.4 ± 0.9) (Figure 2a). Further, no changes were observed in wall thickness (WT), wall:lumen ratio, and cross-sectional area (CSA) in arteries from male or female treated with vehicle or morphine (Figure 3a-f)
Figure 1.
Morphine decreases lumen diameter in arteries from female mice. (a) Representative images of pressurized mesenteric resistance arteries from male and female mice prior to (0 hours), and after, continuous intraluminal infusion with morphine (10 μM, 24 hours). (b) and (c) Changes in internal diameter measured during incremental in intraluminal pressure (mmHg) in mesenteric resistance arteries from male and female mice after continuous intraluminal infusion with vehicle or morphine (10μM, 24 hours). Number of animals used per group are described within the graphs. Values are mean ± SEM. Two-ANOVA, *p<0.0001 vs. morphine.
Figure 2.
Twenty-four hour treatment with morphine did not change stress-strain curve. (a) and (b) Stress-strain curves calculated during incremental increases in intraluminal pressure (mmHg) in mesenteric resistance arteries from male and female mice. Number of animals used per group are described within the graphs. Values are mean ± SEM, p>0.05.
Figure 3.
Twenty-four hour treatment with morphine did not change wall thickness (a and b), walllumen (c and d) and cross-sectional area (CSA) (e and f) calculated during incremental increases in intraluminal pressure (mmHg) in mesenteric resistance arteries from male and female mice. Number of animals used per group are described within the graphs. Values are mean ± SEM, p>0.05.
Female cells are more sensitive to morphine and hydrocodone-induced vascular smooth muscle cell proliferation.
Time-dependent proliferation in vascular smooth muscle cells was measured at 0 to 120 hours or up to five days. Our results showed that treatment with morphine, regardless of concentration (10 nM or 10 μM) increased proliferation in both female and male cells (Figure 4a and b), but the increase in proliferation induced by morphine started earlier in female cells when compared to male cells (Fig. 3a and b). Treatment with hydrocodone also induced vascular smooth muscle cell proliferation in both male and female cells. However, proliferation in female cells increased to both concentrations (10 nM or 10 μM), but they were more sensitive to the lower concentration (Figure 4c). On the other hand, male cells only responded to hydrocodone-induced proliferation in high concentration of hydrocodone (10 μM) (Figure 4d).
Figure 4.
Morphine increases proliferation in vascular smooth muscle cells. Percent confluence of vascular smooth muscle cells (VSMC) over 5 days of treatment with vehicle or two different concentration of (a) and (b) morphine (10 nM and 10 μM) and (c) and (d) hydrocodone (10 nM and 10 μM). Number of VSMC used per group are n=4. The plate was mapped to have quadruplicates per group. Two-ANOVA, *Both concentrations vs. Vehicle. #Hydrocodone (10 nM) vs. vehicle.
Morphine induces phosphorylation of ERK and cofilin.
We observed that morphine increased phosphorylated (inactivated) cofilin 1.6-fold [arbitrary units (AU), control: 0.59 ± 0.14 vs morphine: 0.95 ± 0.10*, *p<0.05] and increased phosphorylated (activated) ERK 1.8-fold (AU, control: 0.51 ± 0.11 vs morphine: 0.90 ± 0.13*, *p<0.05) in mesenteric resistance arteries from female mice (Figure 5a and b). On the other hand, morphine decreased phosphorylated ERK 1.5-fold (AU, control: 0.91 ± 0.08 vs MOR: 0.59 ± 0.11*, *p<0.05) but had no significant effect on the levels of phosphorylated cofilin (p>0.05) in mesenteric resistance arteries from male mice (Fig. 5a and b).
Figure 5.
Morphine alters actin dynamic protein expression in a sex-dependent manner. Panels show typical Western Blot images from phospho- and total cofilin (a) and phospho- and total ERK (b) in mesenteric resistance arteries from male and female mice after 24-hour treatment with vehicle or morphine (10 μM). Number of animals used per group are described within the graphs. t-test, p<0.05.
DISCUSSION
With substantial growth of prescription opioid use in the United States over the last decade, studies have examined associations between opioid therapy and cardiovascular disease. Several recent studies have implicated long-term prescription opioid use as a non-traditional risk factor of cardiovascular disease [2-7]. As analgesia elicited by opioids acts predominately on the μ-opioid receptor, we focused mostly on its exogenous ligands and their adverse effects [31]. Morphine, the prototypical μ-opioid receptor agonist, can stimulate vascular endothelial cell proliferation at pre-clinically relevant concentrations in animal models by enhancing the mitogenic and pro-survival activities of vascular endothelial growth factor [32]. It has also been shown that morphine promotes tumor neovascularization and cancer progression via stimulation of the ERK signaling pathway in a breast tumor model [33]. Morphine also increased migration and proliferation of endothelial cells from pulmonary vessels in humans, which was inhibited by the μ-opioid receptor antagonist methylnaltrexone, indicating that opioids promote angiogenesis by directly acting on the μ-opioid receptor [34,35]. Furthermore, it was demonstrated that methylnaltrexone markedly attenuated tumor growth in experimental mouse models by blocking opioid-induced angiogenesis [36]. Corroborating these prior studies, we observed that morphine and hydrocodone induced proliferation of vascular smooth muscle cells from male and female rats, but supporting the clinical data, this phenomenon was more sensitive in cells from females. Additionally, our results showed that male cells only proliferated in the higher concentration of hydrocodone (10 μM), whereas morphine-induced proliferation was seen in both concentrations (10 nM or 10 μM). The different effects of morphine and hydrocodone observed in cells from male rats in our proliferation assay can be explained by the binding capacities of each drug to opioid receptors. In prior studies, morphine was shown to have a high affinity for the μ-opioid receptor, and low affinities for the δ- and κ-opioid receptors [31]. Similarly, hydrocodone also produces its analgesic effects by activating the μ-opioid receptor but can also stimulate the δ and κ-opioid receptors at high doses [37]. However, hydrocodone is a prodrug that displays only weak binding capacity for the μ-opioid receptor in in vitro experiments [37,38]. Therefore, when the male cells were exposed to a low concentration of hydrocodone (10 nM), we observed a markedly lower percent confluence over time compared to the cells that were exposed to a higher concentration of the drug (10 μM). In fact, the male cells that were treated with 10 nM hydrocodone had a proliferation trend that was almost identical to that of the male control cells, which indicates little to no opioid receptor activation. On the other hand, cells from female rats showed similar proliferation trends at both doses of morphine and hydrocodone, which indicates that both drugs were able to activate opioid receptors, even at lower concentrations. In response to these findings, we questioned whether continuous intraluminal infusion of opioids for 24 hours would affect vascular mechanics in a sex-dependent manner. To answer this question, we used culture pressure myographs to digitally track changes in isolated sections of mesenteric resistance arteries from male and female mice. After continuous morphine infusion for 24 hours under near physiological conditions, we observed that arteries from female mice displayed decreased lumen diameter. These results were supported by expression analysis that showed increased levels of phospho-cofilin and phospho-ERK in arteries from female mice upon exposure to morphine. Although our results do not provide a full understanding of the mechanisms that are responsible for these observations, this study is the first to demonstrate that continuous exposure to morphine induces sex-specific vascular dysfunction.
It has been shown that the mechanical and morphological properties of arterial walls change over time, which lead to decreased tensile strength and increased vessel stiffness with age [39]. As described above, opioid use has been associated with increased arterial stiffness, particularly in women [21]. It has also been shown that the elevated vascular age and arterial stiffness associated with opioid dependence can be reduced by terminating opioid use in both males and females [40]. We observed that continuous treatment with morphine for 24 hours may cause a left shift in the stress-strain curve, especially in the elastin component or first half of the curve, in arteries from female mice. However, no significant differences were seen. Further, no changes were observed in wall thickness (WT), wall:lumen ratio, and cross-sectional area (CSA) in arteries treated with morphine for 24 hours. Given that we observed morphine and hydrocodone-induced proliferation in a time-dependent manner, we infer that long-term treatment (>24 hours) with morphine may lead to a significant change in stress-strain curve and/or vascular remodeling in arteries from female mice. Future studies are necessary to confirm these mechanical and structural results.
In physiological conditions, vasculature oscillates between contraction and relaxation to maintain vascular tone and homeostasis [41]. The vascular wall and its compounds, including endothelial cells and vascular smooth muscle cells, are not static. The cytoskeleton also displays a highly dynamic process characterized by polymerization and depolymerization based on cellular demand [42]. For example, pressure-induced actin polymerization in vascular smooth muscle cells is a mechanism underlying myogenic contraction [43]. One of the most important roles of cofilin in cells is to function as an actin depolymerizing factor, which is crucial for tissue homeostasis and the prevention of disease. As actomyosin bundles are the major contractile units in vascular smooth muscle cells, defects such as excessive cortical tension may arise when there are depleted levels of active cofilin [44]. ERK disturbs the role of cofilin in mediating actin treadmilling by activating Rho kinase, which in turn activates LIM kinase, leading to increased phosphorylation and deactivation of cofilin. Contraction of actin stress fibers is also enhanced via phosphorylation of myosin light chains by myosin light chain kinase, which is activated by ERK in a pathway that does not involve cofilin [45]. The signaling cascades involving ERK and cofilin in the regulation of actin dynamics are well understood, but the effects of opioids on these mechanisms remain largely unknown. We observed that morphine increased inactivated cofilin and activated ERK in arteries from female mice, suggesting that acute exposure to exogenous opioids increases actin polymerization in female but not male arteries (Figure 6). Therefore, our results indicate that the peripheral vasculature of female mice is more susceptible to opioid-induced vascular remodeling than that of male mice. Furthermore, it has been shown that agonists of μ-opioid receptors can activate ERK via the G protein-dependent pathway or the β-arrestin-dependent pathway, but not all agonists prefer both pathways [46,47]. A prior study found that morphine activates ERK via the G protein-dependent pathway only (in mouse embryonic fibroblast cells) and suggested that morphine-mediated ERK phosphorylation was β-arrestin-independent [47]. The results from this study demonstrated that morphine-induced ERK phosphorylation requires the activation of protein kinase C, and therefore is G protein-dependent [47].
Figure 6.
Graphical abstract shows a possible mechanism of how continuous intraluminal infusion with morphine leads to changes in vascular remodeling in arteries from female mice.
In conclusion, opioid-induced cardiovascular disorders and the related sex differences have only recently been studied. Although the effects of exogenous opioids on the nervous system are well known, various adverse effects on other body systems have not yet been extensively studied. As a result, there is currently not enough research to have a clear understanding of how opioids affect the cardiovascular system, especially the mechanisms that are directly and indirectly involved. Conflicting evidence has surfaced over the years, and while some studies suggest that opioids are cardioprotective, several published works (as well as our own data) support the concept that chronic exposure to exogenous opioids can be cardiotoxic, especially for women. Based on our observations, we question whether treating chronic pain with opioids is clinically beneficial, given its many disadvantages.
FUNDING
This work was supported by National Institutes of Health (R01HL149762 and R00GM118885 to C.F.W., K99HL151889 to C.G.M., R01HL1430820 to B.J.,), American Heart Association (18POST34060003 to C.G.M.) and NSF (AGEP1432878 to J.M.E.).
Footnotes
STATEMENT OF ETHICS
The University of Toledo College of Medicine and Life Sciences Institutional Animal Care and Use Committee (IACUC) approved all protocols #108854, 108855, 104573, 108390. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
CONFLICT OF INTEREST
None
DATA AVAILABILITY STATMENT
The data that support the findings of this study are available from the corresponding author, C.F.W., upon reasonable request.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. 2019. [Google Scholar]
- 2.Khodneva Y, Muntner P, Kertesz S, Safford MM. Prescription Opioid Use and Risk of Cardiovascular Disease among Older Adults from a Community Sample. Drug Alcohol. Depend 2014;140:e103. [Google Scholar]
- 3.Khodneva Y, Muntner P, Kertesz S, Kissela B, Safford MM. Prescription Opioid Use and Risk of Coronary Heart Disease, Stroke, and Cardiovascular Death among Adults from a Prospective Cohort (REGARDS Study) Pain Med. 2016;17:444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen A, Ashburn MA. Cardiac effects of opioid therapy. Pain Med. 2015;16(Suppl. 1):S27–S31. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med 2015;162:276–286. [DOI] [PubMed] [Google Scholar]
- 6.Rawal H, Patel BM. Opioids in Cardiovascular Disease: Therapeutic Options. J. Cardiovasc. Pharmacol. Ther 2018;23:279–291. [DOI] [PubMed] [Google Scholar]
- 7.Ogungbe O, Akil L, Ahmad HA. Exploring Unconventional Risk-Factors for Cardiovascular Diseases: Has Opioid Therapy Been Overlooked? Int J Environ Res Public Health. 2019; 16(14):2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeghian S, Darvish S, Davoodi G, Salarifar M, Mahmoodian M, Fallah N, Karimi AA. The association of opium with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2007;14(5):715–7. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghian S, Dowlatshahi S, Karimi A, Tazik M. Epidemiology of opium use in 4398 patients admitted for coronary artery bypass graft in Tehran Heart Center. J Cardiovasc Surg (Torino). 2011;52(1):140–1. [PubMed] [Google Scholar]
- 10.Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13(2):230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed RW, Stefano GB, Murga JD, Short TW, Qi F, Bilfinger TV, Magazine HI. Expression of functional delta opioid receptors in vascular smooth muscle. Int J Mol Med. 2000;6(6):673–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;9;95(12):7157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Liu Y, Casares F, Goligorsky MS. Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem. 1995;22;270(51):30290–3. [DOI] [PubMed] [Google Scholar]
- 14.Blebea J, Mazo JE, Kihara TK, Vu JH, McLaughlin PJ, Atnip RG, Zagon IS. Opioid growth factor modulates angiogenesis. J Vasc Surg. 2000;32(2):364–73. [DOI] [PubMed] [Google Scholar]
- 15.Lennon Frances E., Moss Jonathan, Singleton Patrick A., Riou Bruno; The μ-Opioid Receptor in Cancer Progression: Is There a Direct Effect? Anesthesiology 2012;116:940–945. [DOI] [PubMed] [Google Scholar]
- 16.Fountas A, Chai ST, Kourkouti C, Karavitaki N. Mechanisms of Endocrinology: Endocrinology of opioids. Eur J Endocrinol. 20181;179(4):R183–R196. [DOI] [PubMed] [Google Scholar]
- 17.Seyfried O, Hester J. Opioids and endocrine dysfunction. Br J Pain. 2012;6(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin Psychiatry. 2017. l;30(4):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moolman Johannes A., Unravelling the cardioprotective mechanism of action of estrogens, Cardiovasc Res. 2006; 69(4): 777–780. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y Arenas IA Armstrong SJ Plahta WC Xu H Davidge ST Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-α Cardiovasc Res. 2006;69:836–844. [DOI] [PubMed] [Google Scholar]
- 21.Reece AS, Hulse GK. Impact of lifetime opioid exposure on arterial stiffness and vascular age: cross-sectional and longitudinal studies in men and women. BMJ Open. 2014;4:e004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton PF, Lee MD, Saunter CD, Girkin JM, McCarron JG, Wilson C. VasoTracker, a Low-Cost and Open Source Pressure Myograph System for Vascular Physiology. Front Physiol. 2019;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol A Biol Sci Med Sci. 2007;62 696–706. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, Edwards JM, Tomcho JC, Schreckenberger Z, Bearss NR, Zhang Y, Morgan EE, Cheng Xi, Spegele AC, Vijay-Kumar M, McCarthy CG, Koch LG, Joe B, Wenceslau CF. Intrinsic Exercise Capacity and Mitochondrial DNA Lead to Opposing Vascular-Associated Risks. Function (Oxf). 2021;2(1):zqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenceslau CF, McCarthy CG, Earley S, England SK, Filosa JA, Goulopoulou S, Gutterman DD, Isakson BE, Kanagy NL, Martinez-Lemus LA, Sonkusare SK, Thakore P, Trask AJ, Watts SW, Webb RC. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am J Physiol Heart Circ Physiol, 202; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347(14):1094–1103. [DOI] [PubMed] [Google Scholar]
- 27.American Pain Society (APS). Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th ed, Glenview, IL: American Pain Society; 2008. [Google Scholar]
- 28.Coté CJ, Lerman J, Anderson B, eds. A Practice of Anesthesia for Infants and Children. 6th ed. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 29.Kliegman RM and St. Geme J, eds. Nelson Textbook of Pediatrics. 21st ed. Philadelphia, PA: Saunders Elsevier; 2020. [Google Scholar]
- 30.Thigpen JC, Odle BL, Harirforoosh S. Opioids: A review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. Eur J Drug Metab Pharmacokinet. 2019;44(5):591–609. [DOI] [PubMed] [Google Scholar]
- 31.McDonald J, Lambert DG. Opioid receptors, Continuing Education in Anaesthesia Critical Care & Pain. 2005;5(1)22–25. [Google Scholar]
- 32.Leo S, Nuydens R, Meert TF. Opioid-induced proliferation of vascular endothelial cells. J Pain Res. 2009;2:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP: Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 34.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J: Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvasc Res. 2006;72:3–11. [DOI] [PubMed] [Google Scholar]
- 35.Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc. 2008;83(10):1116–30. [DOI] [PubMed] [Google Scholar]
- 36.Singleton PA, Moss J. Effect of perioperative opioids on cancer recurrence: a hypothesis. Future Oncol. 2010;6:1237–1242. [DOI] [PubMed] [Google Scholar]
- 37.Cardia L, Calapai G, Quattrone D, Mondello C, Arcoraci V, Calapai F, Mannucci C and Mondello E. Preclinical and Clinical Pharmacology of Hydrocodone for Chronic Pain: A Mini Review. Front. Pharmacol 2018;9:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–53. [PubMed] [Google Scholar]
- 39.Nogata F, Yokota Y, Kawamura Y, Walsh WR, Morita H, Uno Y, Kawamura T, Nagashima M, and Hotta N. A Technique for Estimating Sclerosis of Carotid Artery with Ultrasonic Echo. Dössel O and Schlegel WC. (Eds.): WC 2009, IFMBE Proceedings 25/IV, pp. 655–658, 2009. 28. [Google Scholar]
- 40.Reece AS, Hulse GK. Reduction in arterial stiffness and vascular age by naltrexone-induced interruption of opiate agonism: a cohort study. BMJ Open 2013;3:e002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards JM, McCarthy CG, Wenceslau CF. The Obligatory Role of the Acetylcholine-Induced Endothelium-Dependent Contraction in Hypertension: Can Arachidonic Acid Resolve this Inflammation? Curr Pharm Des. 2020;26(30):3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh MP & Cole WC. The Role of Actin Filament Dynamics in the Myogenic Response of Cerebral Resistance Arteries. J Cereb Blood Flow Metab. 2013;33:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. [DOI] [PubMed] [Google Scholar]
- 44.Kanellos G, Frame MC. Cellular functions of the ADF/cofilin family at a glance. J Cell Sci. 2016;129(17):3211–8. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard CA, Hayes L, Wojnowski L, Zimmer A, Marais RM, Norman JC. B-Raf Acts via the ROCKII/LIMK/Cofilin Pathway to Maintain Actin Stress Fibers in Fibroblasts. Molecular and Cellular Biology. 2004;(13):5937–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. [DOI] [PubMed] [Google Scholar]
- 47.Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73(1):178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, C.F.W., upon reasonable request.