Abstract
The promyelocytic leukemia zinc finger (PLZF) protein is a transcription factor disrupted in patients with t(11;17)(q23;q21)-associated acute promyelocytic leukemia. PLZF contains an N-terminal BTB/POZ domain which is required for dimerization, transcriptional repression, formation of high-molecular-weight DNA-protein complexes, nuclear sublocalization, and growth suppression. X-ray crystallographic data show that the PLZF BTB/POZ domain forms an obligate homodimer via an extensive interface. In addition, the dimer possesses several highly conserved features, including a charged pocket, a hydrophobic monomer core, an exposed hydrophobic surface on the floor of the dimer, and two negatively charged surface patches. To determine the role of these structures, mutational analysis of the BTB/POZ domain was performed. We found that point mutations in conserved residues that disrupt the dimer interface or the monomer core result in a misfolded nonfunctional protein. Mutation of key residues from the exposed hydrophobic surface suggests that these are also important for the stability of PLZF complexes. The integrity of the charged-pocket region was crucial for proper folding of the BTB/POZ domain. In addition, the pocket was critical for the ability of the BTB/POZ domain to repress transcription. Alteration of charged-pocket residue arginine 49 to a glutamine (mutant R49Q) yields a domain that can still dimerize but activates rather than represses transcription. In the context of full-length PLZF, a properly folded BTB/POZ domain was required for all PLZF functions. However, PLZF with the single pocket mutation R49Q repressed transcription, while the double mutant D35N/R49Q could not, despite its ability to dimerize. These results indicate that PLZF requires the BTB/POZ domain for dimerization and the charged pocket for transcriptional repression.
The promyelocytic leukemia zinc finger (PLZF) protein is a DNA binding transcriptional repressor disrupted in patients with t(11;17)(q23;q21)-associated acute promyelocytic leukemia (APL) (33). In this setting, the N-terminal 455 amino acids of PLZF are fused to retinoic acid receptor alpha (RARα) to form the PLZF-RARα fusion product. PLZF belongs to a large family of proteins that contain an N-terminal, evolutionarily conserved motif known as the BTB (bric-a-brac, tram track, broad complex) or POZ (poxvirus, zinc finger) domain (2, 5, 50). In humans, about half of BTB/POZ domain proteins also contain C-terminal zinc fingers, and several of these, including PLZF, B-cell lymphoma 6 (BCL-6), and hypermethylated in cancer 1 (HIC-1), are implicated in human malignancy (1, 33, 45, 47). The BTB/POZ domain of PLZF allows PLZF to self-associate (12) and to form heteromeric complexes with other BTB/POZ proteins, such as Fanconi anemia zinc finger, a protein closely related to PLZF (17).
PLZF functions as a transcriptional repressor, binding to promoters of target genes, such as those for cyclin A and the interleukin 3 (IL-3) receptor α chain, via its C-terminal zinc fingers (4, 48). PLZF has a second repression domain (RD2) between amino acids 200 and 300 which mediates powerful repression when fused to a heterologous DNA binding domain (DBD) (27) and which interacts with the ETO corepressor (34). The biological consequences of PLZF expression in hematopoietic cell lines include growth suppression, cell cycle arrest in the G1/S phase, and differentiation blockade (43, 48).
PLZF is believed to repress transcription by recruitment through the BTB/POZ domain of corepressor molecules, such as N-CoR, SMRT, and Sin3A, which in turn draw histone deacetylases (HDACs) to the promoter (9, 15, 16, 18, 32). BTB/POZ-dependent formation of such a complex may result in nucleosomal remodeling and local changes in chromatin structure which modulate transcriptional regulation (26, 33, 38, 39). This results in PLZF-induced repression of genes which govern mammal embryonal development and myeloid differentiation (7, 40). Similarly, BCL-6 is associated with a related corepressor-HDAC complex and is critical for normal differentiation of follicular center lymphocytes (11, 19).
In the usual form of APL, the t(15:17) fusion product PML-RARα responds to pharmacological concentrations of the RARα agonist all-trans retinoic acid by releasing corepressors from the RARα moiety, thus abrogating the dominant negative inhibition of RARα target gene expression. In PLZF-RARα-associated APL, the BTB/POZ domain recruits corepressors and HDACs to RARα target genes, inhibiting the expression of key genes required for normal myeloid differentiation. In these patients, high-dose all-trans retinoic acid is ineffective, since this ligand cannot mediate the release of corepressors from the BTB/POZ domain within the fusion protein (15, 16, 32).
The BTB/POZ domain may also affect chromatin structure by multimerizing (1, 29) and cooperatively binding multiple DNA target sequences, leading to DNA bending (13, 24). Consistent with this idea, the PLZF BTB/POZ domain allows PLZF to bind to DNA as a high-molecular-mass complex of over 600 kDa (4). In addition, the BTB/POZ domain is required for PLZF to localize to nuclear speckles, which likely represent sites of concentration of the protein on chromatin (12).
When produced in Escherichia coli and purified, the PLZF BTB/POZ domain formed a stable dimer highly resistant to trypsin digestion (1, 29). Crystallographic analysis revealed that the POZ monomers interact via an extensive hydrophobic interface with interlocked α helices and β sheets, consistent with the observation that the domain is an obligate dimer (1, 28). In addition, there is crystallographic evidence for higher-order associations between the BTB dimers through β-sheet interactions on the hydrophobic floor of the dimer (1, 29). The structure of the dimer has several notable features (Fig. 1), including a highly conserved charged pocket, an exposed hydrophobic surface, buried hydrophobic monomer cores, and two negatively charged surface patches (1, 29). We undertook a structure-function analysis to determine the role of these features in the ability of the BTB/POZ domain to mediate transcriptional and other biological effects of PLZF in order to further understand the mechanism of action of this transcription factor. For this purpose, we created a panel of mutant BTB/POZ domains and studied the ability of the mutant proteins to dimerize in vitro and in vivo and to repress gene transcription. The mutant BTB/POZ domains were reinserted into PLZF and tested for their ability to repress transcription, to bind DNA as a multimeric complex, to localize to nuclear speckles, and to inhibit cell growth. We found that conserved residues in the core of the PLZF BTB/POZ domain as well as along the interface of each monomer were required for the proper folding and dimerization of the structure. The charged pocket of the BTB/POZ domain was essential for transcriptional repression. Mutation of residue arginine 49 of the pocket to a glutamine abrogated the ability of the domain to repress transcription and converted the PLZF BTB/POZ domain into an activator. However, when this mutation was inserted into full-length PLZF, repression could still occur. Adding a second mutation to the pocket abrogated repression but allowed for some dimerization. These results indicate that the BTB/POZ domain may be critical for multimerization of PLZF, cooperation between the two repression domains of PLZF, and perhaps interaction with cofactors.
FIG. 1.
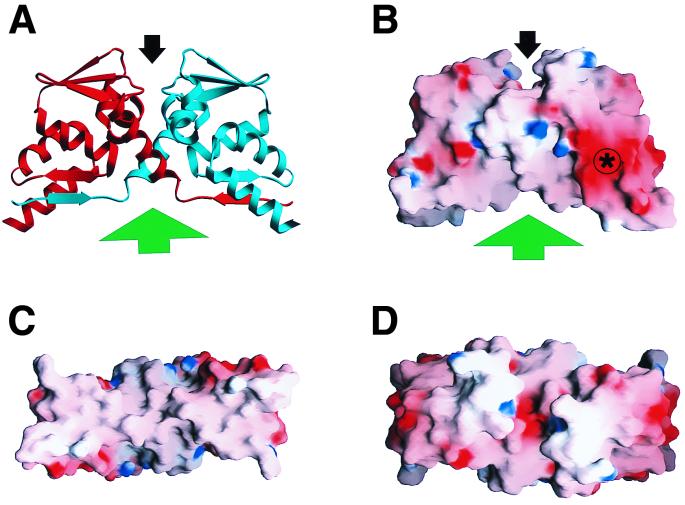
Structural features of the PLZF BTB/POZ dimer. (A) Ribbon view of the dimer, with one monomer in red and the other in blue. The location of the charged-pocket motif is identified by the black arrow, and the exposed hydrophobic surface is indicated by the green arrow. (B to D) Three views of the BTB/POZ surface colored by the electrostatic potential (−18 kT to +18 kT, where k is the Boltzman constant and T is the temperature in kelvins. Electropositive features are indicated in blue, electronegative features are indicated in red, and neutral surfaces are indicated in white. The asterisk denotes the negatively charged surface feature. (B) Side view, same orientation as in panel A. (C) View from below (as seen from the green arrow in panels A and B) showing the extended hydrophobic surface on the bottom of the dimer. (D) Top view (as seen from the black arrow in panels A and B), directly into the charged pocket. Both the charged pocket and the bottom hydrophobic surface are formed from residues contributed by both halves of the dimer. The figure was prepared using the graphics programs SETOR (14) and GRASP (36).
MATERIALS AND METHODS
Plasmids and mutant BTB/POZ domains.
The DNA segment encoding residues 1 to 137 of PLZF, comprising the BTB/POZ region, was amplified by PCR from human PLZF cDNA using a 5′ primer creating a BamHI site (5′-CGCGGATCCGTATGGATCTGACAAAAATG-3′) and a 3′ primer containing XbaI and SfiI sites (5′-TCACTCTAGAGCGGCCATGGTGGCCTCCGTGTCATT-3′). The following BTB/POZ domain mutations were created by PCR-mediated mutagenesis using the indicated specific oligonucleotides (in parentheses): L11A (5′-ATGATCCAGGCGCAGAACCCT-3′ and 5′-AGGGTTCTGCGCCTGGATCAT-3′), L20A (5′-CCCACGGGGGCACTGTGCAAG-3′ and 5′-CTTGCACAGTGCCCCCGTGGG-3′), M27A (5′-GCCAACCAGGCGCGGCTGGCC-3′ and 5′-GGCCAGCCGCGCCTGGTTGGC-3′), D35N (5′-ACTTTGTGCAATGTGGTCATC-3′ and 5′-GATGACCACATTGCACAAAGT-3′), D41R (5′-ATCATGGTGCGCAGCCAGGAG-3′ and 5′-CTCCTGGCTGCGCACCATGAT-3′), R49D (5′-CACGCCCACGACACGGTGCTG-3′ and 5′-CAGCACCGTGTCGTGGGCGTG-3′), R49Q (5′-CACGCCCACCAGACGGTGCTG-3′ and 5′-CAGCACCGTCTGGTGGGCGTG-3′), S56A (5′-GCCTGCACCGCCAAGATGTTT-3′ and 5′-AAACATCTTGGCGGTGCAGGC-3′), Y88A (5′-CTGGAGTATGCAGCTACAGCCACG-3′ and 5′-CGTGGCTGTAGCTGCATACTCCAG-3′), A90A (5′-GCATATACATCCACGCTGCAA-3′ and 5′-TTGCAGCGTGGATGTATATGC-3′), L103E (5′-GATGACCTGGAGTATGCGGCC-3′ and 5′-GGCCGCATACTCCAGGTCATC-3′), and C118A (5′-CTGGAGGAACAGGCCCTGAAGATG-3′ and 5′-CATCTTCAGGGCCTGTTCCTCCAG-3′). The ALA48–52 mutation (an alanine replacement spanning residues 48 to 52) was generated by PCR using the internal oligonucleotide primers 5′-GCAGCTGCGGCCGCTGCCTGCACCAGCAAGATGTTTGAG-3′ and 5′-AGCGGCCGCAGCTGCGGCGTGGAACTCCTGGCTGTCCAC-3′. BTB/POZΔ1–56 was generated using an N-terminal BamHI-containing primer (5′-CGCGGATCCGTATGAAGATGTTTGAGATC-3′) and the SfiI-XbaI primer mentioned above. Finally, BTB/POZΔ83–114 was generated using the N-terminal primer 5′-AAGACCTTCCAGCAGGAGGAACAGTGCCTGAAGATG-3′ and the C-terminal primer 5′-CATCTTCAGGCACTGTTCCTCCTGCTGGAAGGTCTT-3′.
Amplified fragments were ligated into vector PCRII and then transferred to vectors pAS2.1 and pACTII (Clontech, Palo Alto, Calif.) for yeast-two-hybrid analysis, the GAL DBD mammalian expression vector pBXG1 (30), and the shuttle vector pSP73 (Promega, Madison, Wis.). Sequences encoding wild-type and mutant BTB/POZ domains were cloned into pET-32(a) (Novagen, Madison, Wis.) to yield an E. coli thioredoxin protein followed by a 56-amino-acid linker containing a six-histidine affinity tag and ending with amino acids 1 to 132 of PLZF. PLZFΔBTB/POZ, lacking the first 120 N-terminal amino acids of PLZF, was described previously (12). Full-length PLZF was cloned into the EcoRI site of pCDNA3.1myc/his+A (Invitrogen, Carlsbad, Calif.). This plasmid was digested with BamHI and SfiI to remove sequences encoding the wild-type BTB/POZ domain, which were replaced with BamHI/SfiI fragments derived from pSP73 vectors harboring the mutant BTB/POZ domains. All plasmids were confirmed by automated DNA sequencing (Utah State University Biotechnology Center, Logan, and ACGT Corp., Toronto, Ontario, Canada).
Expression and purification of PLZF.
The pET-32(a)-based constructs were used to transform E. coli BL21(DE3) cells. Transformants were grown at 37°C in 2 liters of Luria-Bertani medium containing 100 μM ampicillin to an A600 of 0.6. Isopropyl β-d-thiogalactopyranoside (IPTG) was then added to the culture to a final concentration of 0.2 mM. Growth was continued for an additional 4 h, and cells were harvested by centrifugation, resuspended in nickel column binding buffer (500 mM NaCl, 20 mM Tris-HCl [pH 8.0], 10 mM imidazole), and passaged three times at 20,000 lb/in2 through an Aminco French pressure cell (Heinemann, Schwäbisch Gmünd, Germany). The resulting lysate was subsequently centrifuged for 15 min at 29,000 × g to remove insoluble material, and the soluble supernatant was purified by metal chelation chromatography on a nickel-nitrilotriacetic acid column (Qiagen, Valencia, Calif.). The peak fractions containing the fusion protein were pooled, concentrated, and further purified by size exclusion chromatography on a Superdex-75 column (Pharmacia Biotech; 16 by 600 mm) equilibrated with buffer A (100 mM NaCl, 50 mM Tris-HCl [pH 7.5], 2.5 mM CaCl2, 1 mM Tris-[2-carboxyethyl]phosphine hydrochloride] [TCEP]) at a flow rate of 1 ml/min.
Trypsin sensitivity.
Trypsin was added to the wild-type and mutant BTB/POZ fusion proteins at a molar ratio of 1:1,000 in buffer A. Such fusions have several trypsin-sensitive sites within their linker regions. In the wild-type PLZF BTB/POZ fusion protein, both the N-terminal thioredoxin domain and the C-terminal BTB/POZ domain were resistant to digestion under these conditions for more than 24 h at room temperature. At specific times during digestion, approximately 5 μg of protein was removed for analysis. Pefabloc (Boehringer, Mannheim, Germany) was added to a final concentration of 1 mg/ml to inactivate the trypsin, and the samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Purification of the PLZF BTB/POZ domain.
The wild-type BTB/POZ domain and the mutants that were resistant to trypsin digestion were purified to homogeneity. The fusion proteins were digested with trypsin for 24 h, and the protease was inactivated with Pefablock as described above. The PLZF BTB/POZ domain was purified from the digest mixture by ion-exchange chromatography on a Q-Sepharose column preequilibrated with buffer A; elution was done with a 0.1 to 1.0 M linear NaCl gradient. Fractions containing the PLZF BTB/POZ domain were pooled, concentrated, and purified by size exclusion chromatography as described above.
CD spectroscopy.
Circular dichroism (CD) measurements were obtained using an AVIV 62A-DS CD spectrometer (Aviv Instruments, Lakewood, N.J.). Thermal denaturation analyses were carried out using the temperature scan mode and measuring the ellipticity at 222 nm of PLZF BTB/POZ solutions (55 μM monomer in 100 mM NaCl–50 mM boric acid [pH 8.5]–1 mM TCEP) in a 1-mm cell. Protein concentrations were determined by quantitative amino acid analysis (University of Toronto Biotechnology Service Center). All scans were done from 10 to 90°C in one-degree steps. The averaging time for each data point was 20 s, while the temperature equilibration time was 12 s. A bandwidth of 1 nm was used. Similar results were obtained at protein concentrations of 1 to 110 μM.
The fraction of unfolded protein as a function of temperature was calculated as ([θ222]obs − [θ222]f)/([θ222]u − [θ222]f), where [θ222]obs is the molar ellipticity at a particular temperature and [θ222]f and [θ222]u are the molar ellipticities of the fully folded protein (at a low temperature) and of the fully unfolded protein (at a high temperature), respectively.
Yeast two-hybrid assays.
Saccharomyces cerevisiae strain PJ69-4A (21) was used for transformations with plasmids containing GAL4(DBD)-BTB/POZ, GAL4(DBD)-BTB/POZ mutants, GAL4(AD)-BTB/POZ, GAL4(AD)-BTB/POZ mutants, GAL4(DBD)-PLZF, GAL4(AD)-PLZF, and control constructs (AD, activation domain). The interactions were tested as follows: mutant DBD-mutant AD, mutant DBD–wild-type AD, and wild-type DBD–mutant AD. Only the results from mutant-mutant and mutant–wild-type interactions are reported (see Fig. 4). The yeasts were then grown on media lacking leucine, tryptophan, and adenine. To control for transformation efficiency, the same yeasts were also grown on media lacking leucine and tryptophan. Yeast colonies were counted and then selected in duplicate for liquid β-galactosidase assays as directed elsewhere (Clontech). The results were normalized to the level of β-galactosidase generated by the dimerization of wild-type BTB/POZ. As positive controls, a full-length GAL4 plasmid or plasmids containing p53-GAL4(DBD) transformed together with simian virus 40 large T antigen fused to GAL4(AD) were used (Clontech). These same p53 and T antigen plasmids were used as negative controls for binding to PLZF, BTB/POZ, and mutant BTB/POZ. Western blotting was performed to confirm the expression of all proteins.
FIG. 4.
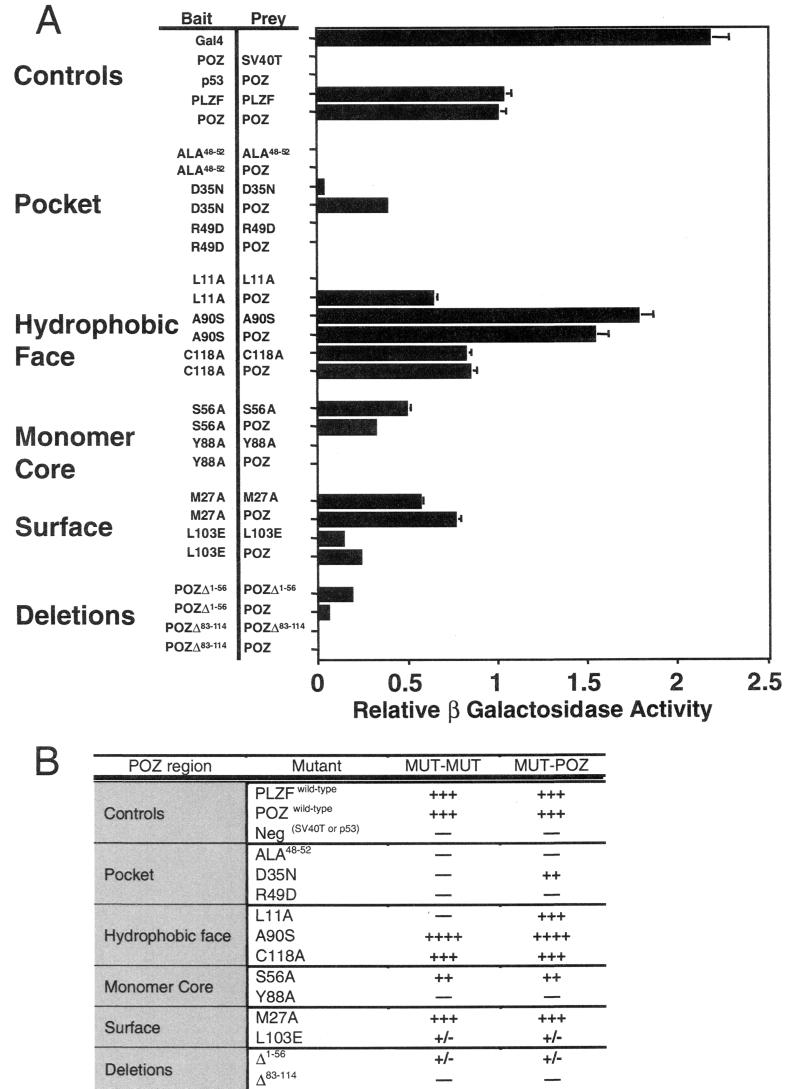
Yeast two-hybrid analysis of BTB/POZ mutant associations. (A) β-Galactosidase activity (± standard error of the mean [SEM]) of transformed yeast colonies depicted relative to the activity generated by wild-type BTB/POZ dimerization. Rows showing control experiments and pocket, hydrophobic-face, monomer core, surface, and deletion mutants are grouped separately. As positive controls, full-length GAL4 and wild-type PLZF and BTB/POZ (POZ) dimerization are shown. The wild-type BTB/POZ domain did not interact with simian virus 40 T antigen (SV40T) or p53, nor did it allow autoactivation of the yeast reporter genes. BTB/POZ mutant homodimerization and heterodimerization to wild-type BTB/POZ are shown. (B) Yeast two-hybrid analysis results tabulated according to the locations of the BTB/POZ mutations. Symbols refer to the relative strength of two-hybrid interaction.
Reporter assays.
Reporter constructs used in this study included (GAL4)5-tk-Luc (34) and (IL3R)4-tk-Luc, the latter containing PLZF binding sites (4). A thymidine kinase-Renilla luciferase construct lacking specific binding sites was used as a negative control for the above reporters, and a thymidine kinase-Renilla luciferase plasmid was included as an internal control. 293T cells were plated in 12-well tissue culture dishes at a density of 2 × 105 per well or in 6-well dishes at a density of 4 × 105 per well and transfected with Lipofectamine (Gibco BRL, Rockville, Md.) or Superfect (Qiagen). Dual luciferase assays were performed (Promega), and luciferase activity was measured using an MLX microtiter plate luminometer (Dynex Technologies, Chantilly, Va.). Immunoblotting confirmed the expression of the mutant GAL4-BTB/POZ and PLZF proteins, all transfection experiments were performed in duplicate 3 to 10 times, and results were normalized to those for the internal control. Percent transcriptional activity was calculated by comparison to the effect of the control empty vector (see Fig. 5) after normalization to the internal control. The fold repression of transcription was calculated relative to the transcription of the reporters in the presence of the relevant empty expression vector.
FIG. 5.
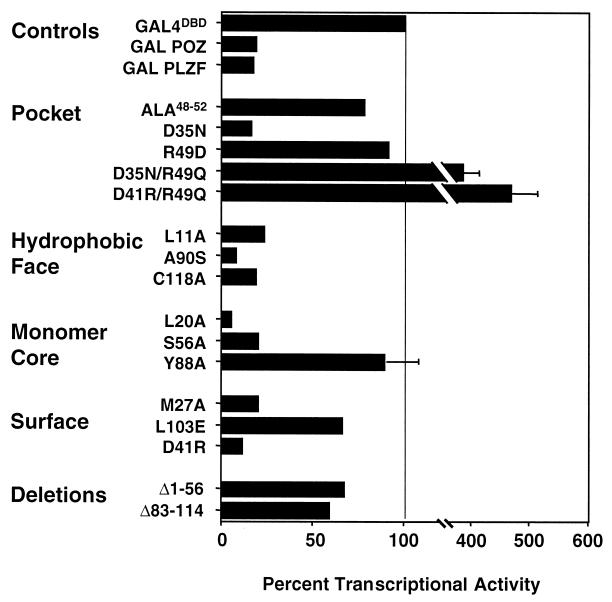
Transcriptional activities of BTB/POZ mutants. Mutant BTB/POZ domains were fused to the GAL4 DBD. These plasmids (400 ng) were transfected into 293T cells along with a reporter plasmid containing five GAL4 binding sites. A thymidine kinase-Renilla luciferase construct was also transfected as an internal control. The transcriptional effects are expressed as percent transcriptional activity (± SEM) compared to the activity of the GAL4 DBD alone (depicted as 100%).
EMSAs.
For electrophoretic mobility shift assays (EMSAs), 106 293T cells were transfected by the calcium phosphate method with 10 μg of expression vectors for wild-type PLZF, PLZFΔBTB/POZ, and the various PLZF constructs containing mutations within the BTB/POZ domain. At 48 h after transfection, the cells were harvested, nuclear extracts were prepared, and EMSAs were performed with an [α-32P]dCTP-labeled oligonucleotide containing a high-affinity binding site for PLZF as described previously (4).
Immunofluorescence.
293T cells were transfected using Superfect reagents and protocols. Wild-type or mutant PLZF plasmids (50 or 100 ng) and 200 ng of a green fluorescent protein (GFP)-spectrin plasmid (22) were transfected into 3 × 105 cells growing on sterile glass coverslips in six-well dishes. At 48 h after transfection, the cells were fixed in ice-cold methanol and immunostained. After being blocked in 10% donkey serum for 30 min, the cells were exposed to a 1:100 dilution of mouse PLZF monoclonal antibody for 1 h. Samples were then treated for 30 min with donkey anti-mouse antibody conjugated to Texas red (Jackson Immuno-Research, West Grove, Pa.). Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlington, Calif.) was then applied, and the slides were examined with a Leica-TCS-SP (UV) confocal microscope (Leica, Heidelberg, Germany). To eliminate the possibility of cross-channel bleed-through, the samples were scanned separately in the nonoverlapping portion of the spectrum of each fluorescent marker. These experiments were repeated two to four times, and multiple fields were imaged.
Colony suppression assays.
SaOS-2 cells (106) were plated in 10-cm dishes and transfected 24 h later with the pCDNA3.1+ expression vector (Invitrogen) containing the neomycin resistance gene and wild-type or mutant PLZF BTB/POZ domain constructs. The cells were subsequently split 1:5 and selected for 2 to 3 weeks in media containing G418. The dishes were stained with crystal violet, and the numbers of colonies were counted and averaged. Colony suppression by PLZF was measured relative to the number of colonies formed in the presence of the empty expression vector.
RESULTS AND DISCUSSION
Creation of BTB/POZ mutants.
The PLZF BTB/POZ domain is required for the dimerization, transcriptional repression, nuclear speckle localization, and corepressor binding functions of the PLZF protein (9, 12, 16, 27). Crystallographic analysis of the BTB/POZ dimer defined several major structural features (1) (Fig. 1), including an extensive hydrophobic dimer interface involving residues over the entire length of the protein. Given the extent of the interacting surfaces, we predicted that mutations that disrupt dimerization would result in misfolded nonfunctional protein aggregates. Another prominent structure within the BTB/POZ domain is a charged-pocket region composed of some of the most conserved residues of the BTB/POZ sequence (Fig. 1). The pocket is formed by symmetry-related residues from each of the monomers, including pairs of aspartates at position 35 and arginines at position 49 (1). The pocket has a high charge density with a central patch of negative potential flanked by two regions of positive potential but is electroneutral overall as a result of charge balancing. The physical structure and conserved nature of the pocket suggested that it might be a site of protein interaction, possibly involved in transcriptional regulation by the BTB/POZ domain. A second possible protein interaction motif is the extensive hydrophobic surface found on the side of the molecule opposite the pocket (Fig. 1) and formed by α helices and β sheets from both monomers. Furthermore, it was recently suggested that this hydrophobic surface could be a site for higher-order complex formation between BTB/POZ dimers (29). Other conserved structural features of note include a negatively charged surface patch and a hydrophobic core in each monomer. Although it is convenient to consider the dimer interface and each of the monomer cores as separate sites in the protein, an equally accurate description would be a single, extended hydrophobic core in the dimer consisting of both the core and the interface.
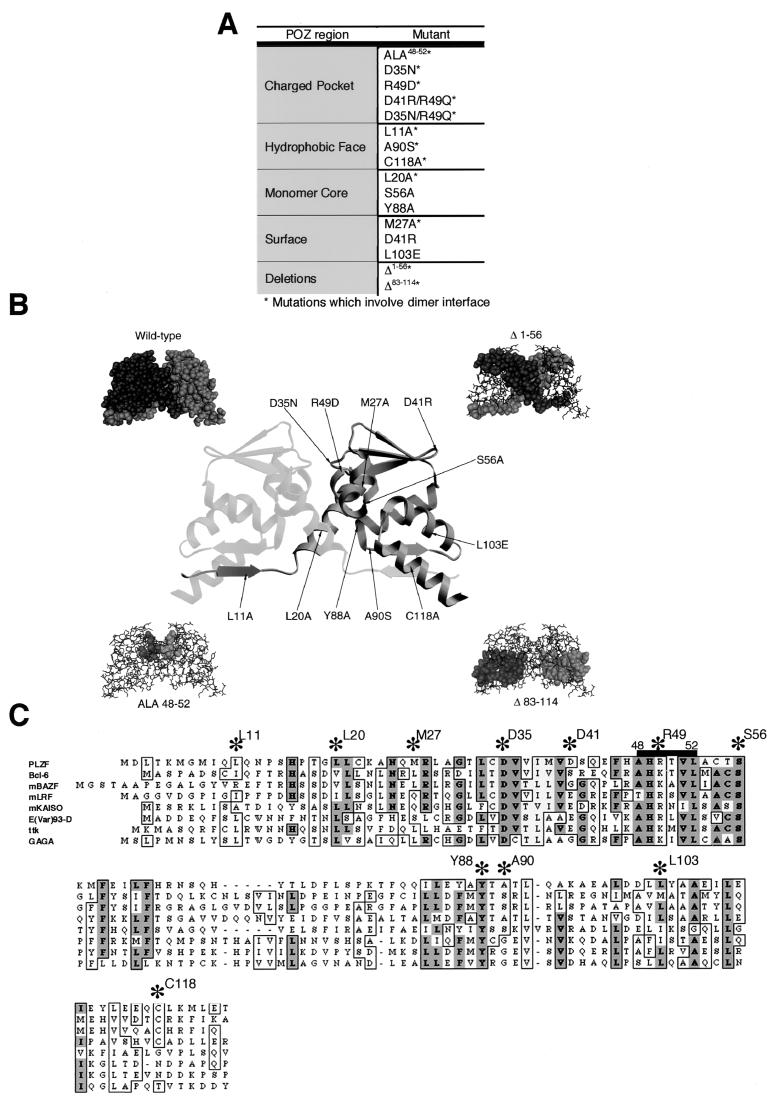
We hypothesized that the functions of the PLZF BTB/POZ domain in transcription and growth control require dimerization of the BTB/POZ domain. Thus, any PLZF molecule containing a BTB/POZ moiety unable to self-associate would be equivalent to PLZF lacking a BTB/POZ domain. We further postulated that conserved structures within the BTB/POZ domain mediate dimerization and transcriptional functions. To test this idea, we systematically mutated residues of the BTB/POZ domain within these conserved regions and assayed their effects on the biochemical and biological functions of PLZF (Fig. 2). The resulting mutants included (i) 13 mutants with point mutations of residues selected on the basis of their conservation throughout evolution, the existence of lethal missense mutations in the Drosophila E(var)93-D gene product BTB/POZ domain (Thomas Kornberg, personal communication), and predictions of the X-ray crystallographic model; (ii) two deletion mutants lacking N-terminal and C-terminal α-helical sequences which form the major portions of the dimer interface; and (iii) an alanine replacement mutant spanning residues 48 to 52, corresponding to the charged-pocket structure. Figure 2A lists all of the mutations and their localization within the BTB/POZ structures mentioned above.
FIG. 2.
Description and localization of BTB/POZ mutations. (A) List of the BTB/POZ mutations generated by PCR and their locations in the BTB/POZ dimer. Mutations which are located at the dimer interface are marked with an asterisk. (B) Ribbon view of the BTB/POZ dimer indicating the locations of residues selected for point mutations. The four surrounding diagrams denote, clockwise from top left, space-filling model of BTB/POZ dimer; BTB/POZΔ1–56 with deleted sequences shown in the space-filling mode; BTB/POZΔ83–114 with deleted sequences shown in the space-filling mode, and ALA48–52 with mutated sequences shown in the space-filling mode. (C) Sequence alignment of the PLZF BTB/POZ domain with other BTB/POZ domains showing conserved residues (asterisks) selected for mutational analysis. Darker shading indicates more highly conserved residues. m, mouse; ttk, tramtrack.
A number of the mutations affect the charged-pocket region. The alanine replacement mutation ALA48–52 would result in gross disruptions of the dimer interface as well as in a complete loss of the charged pocket. These effects would completely nullify participation of this structure in overall BTB/POZ function. The D35N mutation [lethal in Drosophila E(var)93-D] and the R49D mutation result in a change in the net charge of the pocket from 0 (−2 from the two D35 residues and +2 from the two R49 residues) to −2 and −4, respectively. Both of these mutations affect electrostatic interactions between the two pairs of residues. These simple electrostatic considerations are consistent with the results of more rigorous electrostatic potential calculations (36) (data not shown). The negative charge of the R49D mutation would probably repulse the D35 residue nearby and destabilize the pocket structure. We predicted that if mutations were created that could neutralize these charged residues, charge repulsion would be avoided. The resulting BTB/POZ domain might still dimerize but would have altered transcriptional function (Fig. 2). This result would indicate that dimerization is necessary although not sufficient for transcriptional repression. Therefore, we created the double mutant D35N/R49Q, where the charged-pocket residues are replaced by neutral, polar residues. The nominal charge in the pocket remains 0 in this mutant. We compared this mutant with a second mutant, (D41R)/R49Q, where the D41 amino acid is located at a remote surface location and is not predicted to alter BTB/POZ structure (Fig. 2). This mutation is thus considered equivalent to a single R49Q mutation in the rest of the study. These mutations in the charged pocket were predicted to have an effect on dimerization and possibly also on BTB/POZ function by affecting binding interactions in this region (1).
Mutations were introduced into the exposed hydrophobic surface. A missense mutation at L11A was expected to result in a loss of hydrophobic packing in the lower β-sheet region with nonpolar residues from the opposite monomer. This leucine contributes to the exposed hydrophobic surface and also makes up 8% of the dimer interface, more than any other residue in the structure (1). Residue 11 is normally packed against L92′ from the paired β strand as well as against Y113′ and L114′ from the terminal α helix (numbers with primes indicate residues from the opposite monomer). Leucines 11, 92, and 114 are all highly conserved in the BTB/POZ domain, and the integrity of this area is important in maintaining the dimer interface (Fig. 2). The A90S mutation was designed to parallel the G96S lethal mutation in Drosophila E(var)93-D. However, the PLZF BTB/POZ domain has an alanine residue instead of a glycine residue in the equivalent position. This residue makes critical contacts in the loop region between β1 and α1 of the other monomer (1). The C118A mutation replaces a conserved Cys residue which is important to the C-terminal α5-α6 helical hairpin as well as to interactions with sheet β1′ (Fig. 2).
The hydrophobic monomer core was targeted by mutations L20A, S56A, and Y88A. S56 is highly conserved and is localized to a buried alpha-helical region in the BTB/POZ monomer. The switch to an alanine residue would not disrupt the helix, although it might result in a loss of hydrogen bonding. Tyrosine 88 is a large, strongly conserved residue that is fully buried and forms the “anchor” for the BTB/POZ monomer fold. Mutations at this position are expected to result in misfolding.
Finally, surface mutations M27A and D41R were not expected to cause major disruptions. However, L103E is a lethal E(var)93-D mutation and is distinguished from the previous two mutations by its location in the negatively charged patch on the lateral surface of the monomer (Fig. 2). This mutation could disrupt the BTB/POZ structure by charge repulsion due to the proximity of aspartate and glutamic acid residues in the same monomer. In addition, L103 could hydrogen bond with nearby L119 and L122, and a loss of these interactions could also destabilize the BTB/POZ dimer. The M27 side chain reaches toward the interface, raising the possibility that its mutation could be detrimental to dimer stability.
Dimerization.
Each of the PLZF BTB/POZ mutant domains was tested for its ability to self-associate. The mutant proteins were expressed in bacteria as thioredoxin fusion proteins, purified, and tested for their solubility and sensitivity to cleavage by trypsin. The proteins were analyzed by gel filtration chromatography to estimate the molecular weights of the BTB/POZ complexes formed (Fig. 3). In addition, the ability of the PLZF protein to dimerize was assessed in vivo by the yeast two-hybrid method, in which both homodimerization to the same mutant and heterodimerization to wild-type BTB/POZ were assessed (Fig. 4). Equivalent expression of GAL4-BTB/POZ mutant proteins in yeast was confirmed by immunoblotting with GAL4 DBD or AD monoclonal antibodies (data not shown). The yeast two-hybrid results are represented as β-galactosidase activity relative to that generated by wild-type BTB/POZ homodimerization. Overall, there was a very good correlation between the in vitro and the in vivo techniques; thus, these results are presented together as a measure of the ability of the mutant BTB/POZ domains to fold correctly and dimerize.
FIG. 3.
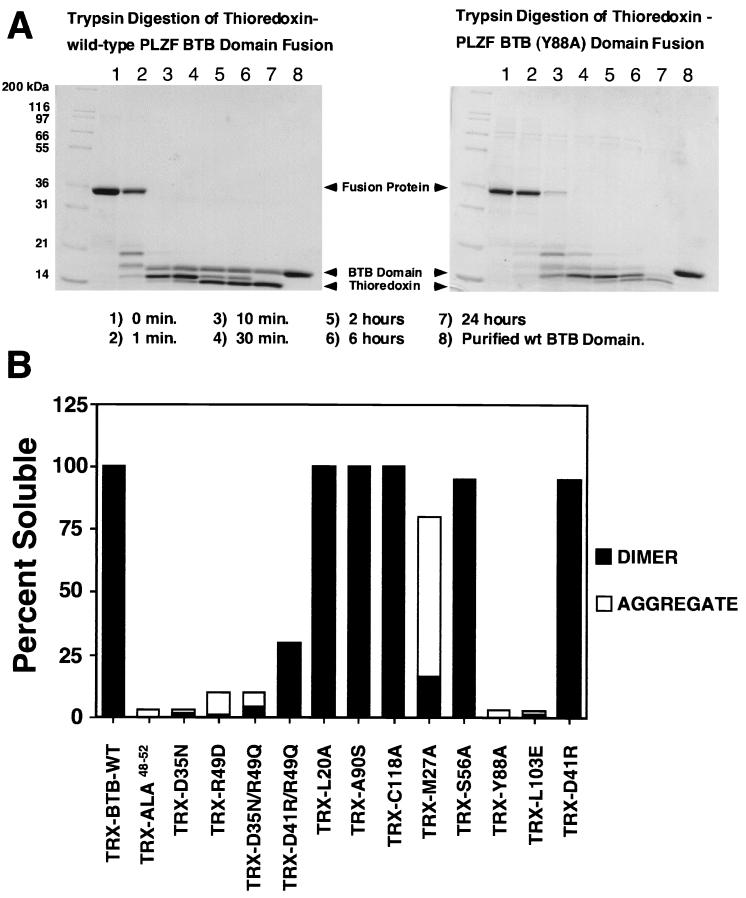
Biochemical analysis of BTB/POZ mutants. (A) Trypsin sensitivities of the wild-type (wt) PLZF BTB/POZ domain and mutant Y88A. Trypsin was added to thioredoxin fusion proteins of wild-type and mutant PLZF BTB/POZ domains at a ratio of 1:1,000 (wt/wt), and the digests were resolved by sodium dodecyl sulfate–14% polyacrylamide gel electrophoresis and staining with Coomassie blue. PLZF BTB/POZ domain mutant Y88A was degraded by trypsin, while the wild-type PLZF BTB/POZ domain remained intact for 24 h. Thioredoxin is resistant to trypsin under these conditions and serves as an internal control. (B and D) Solubility of thioredoxin (TRX)-BTB/POZ domain fusion proteins expressed in E. coli. Wild-type (WT) and mutant BTB/POZ fusion proteins were expressed to high levels in E. coli in all cases, but severe folding defects were identified in many of the mutants. Insoluble protein in the form of inclusion bodies was removed by centrifugation, and the soluble fractions of the fusion proteins in the supernatants were tested for their oligomeric state by gel filtration chromatography. Aggregates eluted in the voided volume of the column. As judged by molecular weight standards, the column could resolve monomers, dimers, and tetramers, although only dimers and aggregates larger than tetramers were observed. Note the strong correlation between aggregated protein and trypsin sensitivity, indicating that the soluble aggregates consisted of a misfolded BTB/POZ domain. (C) Thermal denaturation profiles of purified BTB/POZ domains, as measured by CD spectroscopy at 222 nm.
The wild-type PLZF BTB/POZ domain formed a highly soluble protein in bacteria, which was resistant to 24 h of trypsin digestion (Fig. 3), and yielded strong signals in the yeast two-hybrid system (Fig. 4). Deletion of the N-terminal or C-terminal portion of the BTB/POZ domain yielded peptide fragments with little or no interaction in the two-hybrid assay (Fig. 4).
When the charged pocket was completely disrupted, as in the ALA48–52 mutant, the BTB/POZ domain was expressed almost entirely as inclusion bodies in E. coli. A small amount of soluble protein could be isolated but was found to be in the form of large, nonspecific aggregates by gel filtration chromatography. Consistent with this finding, this material was very sensitive to tryptic digestion, indicating a severe folding defect in this mutant. Similarly, the charge swap R49D mutation resulted in a BTB/POZ species that was incapable of dimerizing and that was almost completely misfolded. The D35N mutant showed a slight improvement in the relative proportions of dimer versus aggregated protein in the soluble fraction, and the small amount of dimeric species that could be recovered was resistant to trypsin digestion. Even though the D35N mutant was impaired for homodimerization in vivo (Fig. 4), heterodimerization to wild-type BTB/POZ was still possible, consistent with the less severe biochemical phenotype. When the charge distribution of the D35N pocket mutant was further altered by the R49Q mutation, there was no additional disruption of BTB/POZ dimerization or folding. Thus, both the D35N and the D35N/R49Q pocket mutations resulted in a partial loss of folding and stability, but a significant proportion of the protein could still self-associate. Interestingly, the (D41R)/R49Q mutation resulted in a relatively functional BTB/POZ dimer that was resistant to trypsin, although some of the protein localized as insoluble inclusion bodies. Given that the behavior of the D41R mutant protein is indistinguishable from that of the wild-type protein, we attribute the partial folding defect in (D41R)/R49Q to the charged-pocket mutation. Except for the ALA48–52 mutation, which results in a misfolded protein, these pocket mutations offered an opportunity to determine the importance of the pocket structure of BTB/POZ in gene regulation.
In contrast to the above results, mutations in the exposed hydrophobic surface at residues A90 and C118 did not affect BTB/POZ dimerization, even though both residues also participate in the dimer interface. The L11A mutation resulted in a BTB/POZ domain that was defective for in vivo homodimerization, although heterodimerization between the mutant and the wild type was intact. Based on the predicted packing of the BTB/POZ dimer, it is not surprising that this mutant was less able to dimerize than the A90S or C118A mutant, since the latter two mutations are not anticipated to disrupt the dimer interface (see above). Interestingly, the β-galactosidase activity generated by GAL4-A90S was consistently higher than that of the wild type. Since the domain is an obligate dimer, a plausible explanation is that the increased signal is due to stronger dimer-dimer interactions in the hydrophobic face (Fig. 3 and 4).
The monomer core mutants L20A and S56A had a wild-type biochemical profile, although S56A yielded a weaker signal in the two-hybrid assay. In contrast, the Y88A mutation altered a deep monomer core residue, resulting in a completely misfolded BTB/POZ species unable to self-associate in vivo or in vitro (Fig. 3 and 4).
The external-face mutations resulted in several different phenotypes, according to their location. The negatively charged patch mutation L103E is lethal in Drosophila E(var)93-D. This result was reflected by the weak two-hybrid interaction and underlines the importance of the negatively charged patch for BTB/POZ integrity (Fig. 4). The M27A mutation resulted in a unique phenotype in that the molecule was mostly localized in a soluble, aggregated fraction that was sensitive to trypsin. However, this mutant was sufficiently functional to register an approximately 50% wild-type yeast two-hybrid signal. As expected (as mentioned above), the D41R mutant had properties similar to those of the wild type (Fig. 3 and 4).
In light of the biochemical data presented above, these two-hybrid experiments are not simply measuring monomer-dimer equilibria but instead reflect the functional state of the protein. In addition, even when a strong two-hybrid signal is obtained, we cannot distinguish between simple bait-prey dimer formation or higher-order complexes involving multiple bait and/or prey molecules.
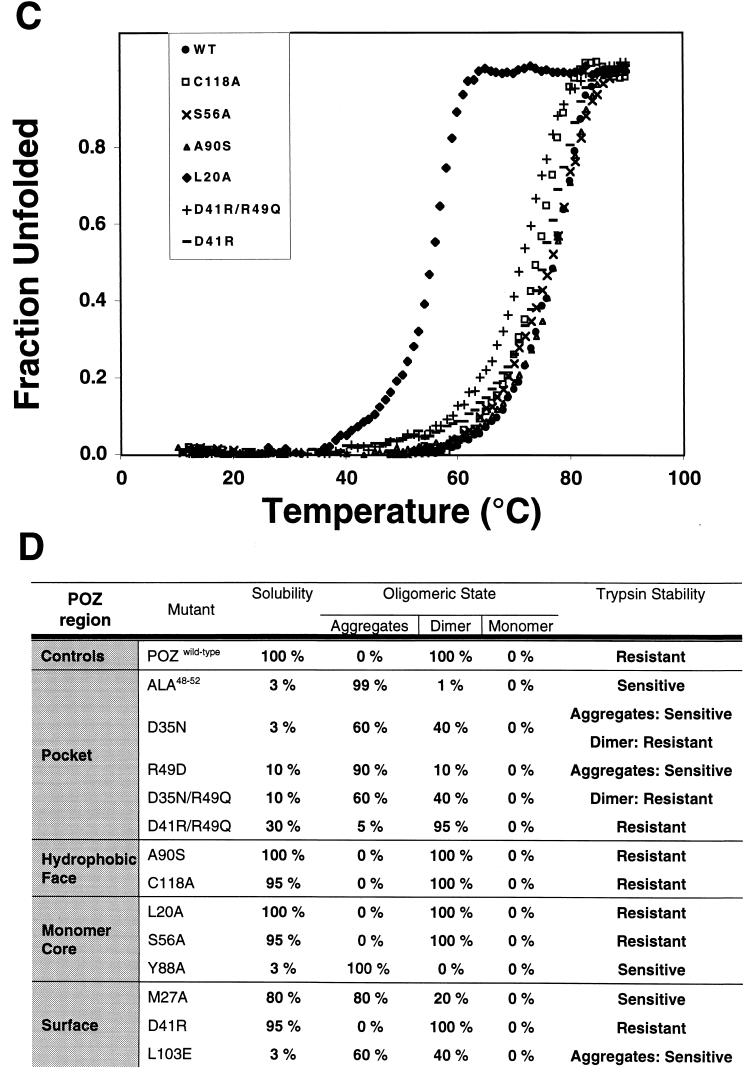
Finally, we studied the thermodynamic stability of the BTB/POZ mutants that produced sufficient amounts of folded, dimeric protein following trypsin treatment. The temperature dependence of unfolding was measured by CD spectroscopy. The wild-type protein had a midpoint-transition temperature (Tm) of 77.5°C, reflecting the stable nature of the domain, consistent with the findings of Li et al. (28). Most of the well-expressed, soluble mutants had similar melting profiles, with only slightly reduced melting temperatures, including the (D41R)/R49Q mutant (Tm = 71.5°C). The L20A mutant was the most affected and had a Tm of 55.5°C. In all cases, the domains melted in a single step, consistent with the transition from a folded dimer to two unfolded monomers. Thus, we have no evidence of an intermediate state consisting of dissociated, folded monomers.
Together, these results suggest that (i) as predicted by crystallographic analysis, BTB/POZ monomers are inherently unstable and mutations that destabilize the interface result in misfolding, (ii) the integrity of the charged-pocket domain is important for the structural stability of the BTB/POZ dimer, (iii) it is possible to modify electrostatic interactions within the charged pocket without losing the dimeric structure of the BTB/POZ domain, and (iv) the monomer core and negatively charged patches are important for proper folding and dimerization.
Transcriptional repression.
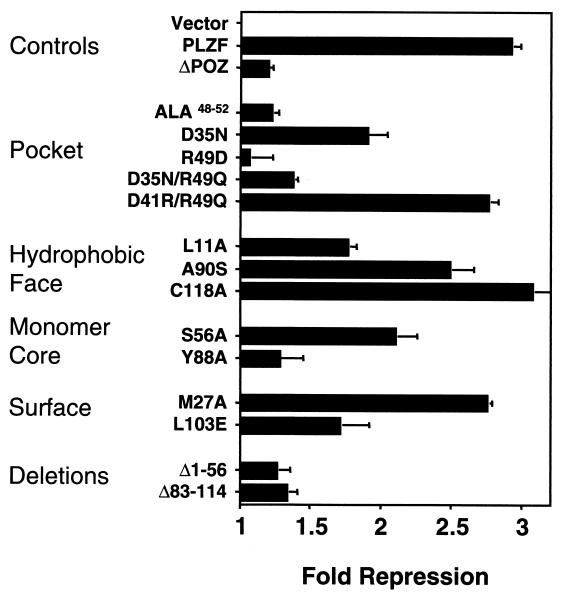
The BTB/POZ domains of PLZF and other BTB/POZ proteins were previously shown to mediate transcriptional repression (6, 19, 27); therefore, we tested the BTB/POZ mutants for transcriptional activity. We transiently expressed the BTB/POZ mutants as fusions with the GAL4 DBD (GAL4 residues 1 to 147 [GAL41–147]) along with a reporter construct containing GAL4 binding sites. The wild-type BTB/POZ fusion protein consistently repressed transcription by approximately 80%, similar to the repression effect of a PLZF1–400 construct, which contains residues 1 to 400 including the PLZF second repression domain (Fig. 5) (27). A reporter construct lacking GAL4 binding sites was unaffected (data not shown). Equivalent expression of all GAL4-BTB/POZ fusion proteins was confirmed by immunoblotting of a transfected cell extract with GAL4 antibodies (data not shown).
We next analyzed the BTB/POZ charged-pocket mutants. As anticipated, the insoluble ALA48–52 mutant was transcriptionally inactive, as was R49D (Fig. 5). Interestingly, the GAL4-D35N protein was able to repress almost to the same degree as the wild-type BTB/POZ protein. This result suggests that the protein fraction present in a dimer configuration is still functionally competent. Thus, an electrostatic interaction through D35 is not important for mediating transcriptional repression. In addition, the fact that the GAL4 DBD itself dimerizes may favor the dimeric state of the BTB/POZ moiety and contribute to the functional competence of the D35N mutant. In marked contrast, the double mutant D35N/R49Q showed no repression, even though the biochemical profiles of these mutants were similar. In fact, this double mutant activated the (GAL4)5-tk-Luc reporter (Fig. 5). This same phenomenon was observed with the (D41R)/R49Q mutant, which also activated transcription. Therefore, the R49 residue in the pocket domain is required for transcriptional repression although not for dimerization, since this mutant had an almost wild-type biochemical phenotype. Taken together, these results suggest a fundamental role for the PLZF-BTB/POZ charged-pocket domain in mediating BTB/POZ-dependent transcriptional repression.
The exposed hydrophobic-surface mutations did not affect the ability of PLZF to repress transcription (Fig. 5). This finding is consonant with the wild-type dimerization exhibited by C118A and A90S. Consistent with the slight increase in yeast two-hybrid β-galactosidase activity, the A90S mutant was a more powerful transcriptional repressor than wild-type BTB/POZ, yielding approximately 12-fold repression. Finally, like D35N, the partially dimerization-impaired mutant L11A could repress transcription (Fig. 5). Again, this action is probably mediated by the dimerized fraction of protein and could also be due to structural changes caused by the GAL4 DBD.
The effect of the L20A, S56A, and Y88A core mutations on repression was also analyzed (Fig. 5). Consistent with their biochemical and two-hybrid profiles, the L20A and S56A mutants were transcriptionally competent. Interestingly, L20A was enhanced in its ability to mediate repression compared to wild-type BTB/POZ. Like the ALA48–52 and R49D mutants, the Y88A mutant was a null mutant for both dimerization and transcriptional function.
Among the BTB/POZ surface residues, wild-type-like D41R was fully competent for repression (Fig. 5). The M27A protein was also competent for repression, indicating a relatively stable dimer in spite of the unusual biochemical profile noted above (Fig. 3B and C). The L103E negatively charged patch mutant was severely impaired in its ability to repress transcription (Fig. 5). This result was fully consistent with its impaired biochemical profile and therefore may not necessarily be attributed to a specific function of the negatively charged patch in interacting with negative cofactors. Finally, the two gross deletion mutants of the BTB/POZ domain were also impaired for repression.
Several conclusions can be drawn by comparing the structural and functional data. First, dimerization and transcriptional function are linked and inseparable features of the BTB/POZ domain. Thus, mutants with the most severe folding defects (Ala48–52, R49D, Y88A, BTB/POZΔ1–56, and BTB/POZΔ83–114) were also transcriptionally impaired, while mutants that formed dimers retained biological activity. Second, dimerization of the BTB/POZ domain is not sufficient for repression. Our data indicate that the charged pocket of the PLZF BTB/POZ domain is a key structure for transcriptional repression. When the R49 pocket residue was replaced by a polar residue, the domain retained its ability to dimerize, yet the BTB/POZ domain was transformed from a transcriptional repressor to a transcriptional activator. Third, although mutations in the exposed hydrophobic surface targeted highly conserved residues, there was little impact on BTB/POZ-mediated repression in protein species competent for dimerization. Hence, changing a single amino acid on this surface appeared to yield little effect. Fourth, both the A90S and the L20A surface residue mutations did repress to a greater extent than the wild type, suggesting that these residues might directly stabilize interactions with transcriptional corepressors, act indirectly by altering dimer conformation, or affect higher-order BTB/POZ domain structures. Alternatively, the change in conformation might favor improved DNA binding by the GAL4 portion of the fusion proteins.
Effects of BTB/POZ mutations on full-length PLZF.
The BTB/POZ domain was reported to be critical for transcriptional repression and dimerization of PLZF (12, 16, 27). By examining the effect of our panel of mutants in the context of full-length PLZF, we determined the contributions of BTB/POZ substructures to these processes by studying their impact on PLZF in the following assays (Table 1).
TABLE 1.
Analysis of full-length PLZF proteins with POZ mutationsa
| POZ region | Mutant | Rep | EMSA | Locat | GS |
|---|---|---|---|---|---|
| Control | PLZF | +++ | +++ | Speck | +++ |
| ΔBTB/POZ | + | − | Pu/Dff | + | |
| ALA48–52 | + | Dff | + | ||
| D35N | ++ | + | Sp/Dff | + | |
| R49D | + | − | Dff | + | |
| D35N/R49Q | + | ++ | Sp/Dff | ++ | |
| (D41R)/R49Q | +++ | +++ | Speck | +++ | |
| Hydrophobic face | L11A | ++ | + | Pu/Dff | ++ |
| A90S | ++ | ++ | Speck | +++ | |
| C118A | +++ | +++ | Sp/Dff | +++ | |
| Monomer core | S56A | ++ | ++ | Speck | ++ |
| Y88A | + | − | Dff | − | |
| Surface | M27A | +++ | + | Speck | ++ |
| L103E | + | + | Pu/Dff | − | |
| Deletion | Δ1–56 | + | − | Dff | − |
| Δ83–114 | + | − | Dff | − |
Rep, transcriptional repression by full-length PLZF of a PLZF-binding-site-containing reporter; EMSA, HMW complex formation; Locat, immunofluorescence localization (Speck, speckle localization; Pu, punctate localization; Dff, diffuse localization); GS, suppression of colony formation by wild-type PLZF and PLZF containing BTB/POZ mutations compared to results obtained with the vector alone after transfection into SaOS-2 cells.
(i) Transcriptional repression.
To assess transcriptional repression, PLZF mutations were transiently transfected in 293T cells along with a reporter gene containing cognate PLZF binding sites from the IL-3 receptor α-chain promoter (4). Protein expression was confirmed by immunoblotting of transfected cell lysates with anti-PLZF monoclonal antibody. As we previously reported, PLZF consistently represses luciferase production approximately threefold (4). In contrast, PLZFΔBTB/POZ was unable to repress transcription (Fig. 6).
FIG. 6.
Transcriptional repression by full-length PLZF with BTB/POZ mutations. The mutant BTB/POZ domains were engineered into full-length PLZF, and 400 ng was transfected into 293T cells along with 100 ng of a reporter construct containing four PLZF binding sites. A thymidine kinase-Renilla luciferase construct (5 ng) was also transfected as an internal control. Results are expressed as fold repression compared to the repression obtained with the plasmid vector alone.
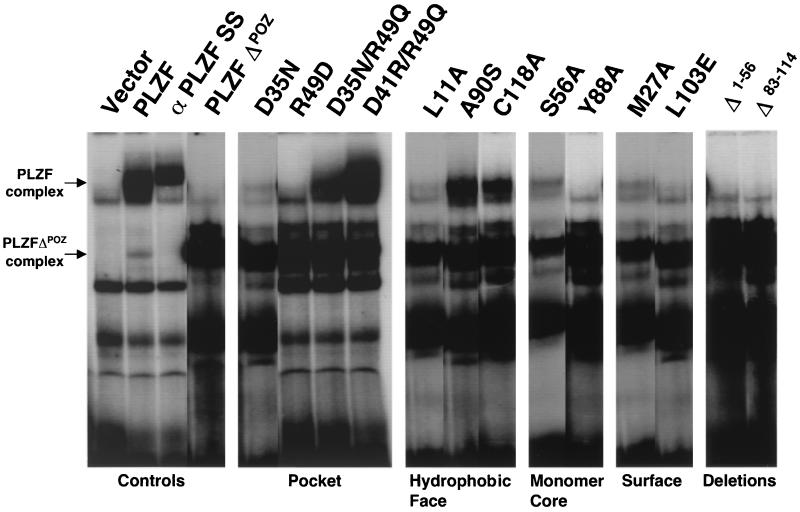
(ii) HMW complex formation.
Another measure of PLZF function is its ability to form high-molecular-weight (HMW) DNA-protein complexes when bound to an oligonucleotide containing the IL-3 receptor α-chain promoter binding site (4). To determine the impact of the BTB/POZ mutants on this process, lysates from transfected 293T cells were similarly analyzed by EMSAs (Fig. 7). These cell lysates were also immunoblotted to confirm the equivalence of expression (data not shown). PLZF formed a slowly migrating complex which was absent in the vector-transfected cells (Fig. 7). This complex failed to form in the PLZFΔBTB/POZ lysates, although a faster-migrating shift was noted. This smaller complex also occurred in the wild-type PLZF lysate and is a consequence of the expression of a truncated form of PLZF lacking the BTB/POZ domain, which is translated from an internal start site (4). The fact that both shifts are abolished by incubation with a PLZF monoclonal antibody indicates that these are both PLZF-dependent complexes (Fig. 7).
FIG. 7.
HMW complex formation. PLZF proteins harboring wild-type and mutant BTB/POZ domains were expressed in 293T cells. Cell lysates were allowed to bind to an oligonucleotide containing PLZF binding sites from the IL-3 receptor α-chain promoter. The top arrow marks the shift in mobility caused by PLZF. The bottom arrow marks the mobility shift caused by PLZFΔBTB/POZ. The higher-mobility shift is seen in all PLZF-containing lanes, since there is low-level expression of a truncated protein when PLZF is transfected into cell cultures. Lane α PLZF SS, supershifting of PLZF with a monoclonal antibody.
(iii) Immunolocalization.
Endogenous PLZF was previously described as localizing to nuclear speckles (31). We reproduced this expression pattern in 293T cells by titrating the amount of transfected PLZF plasmid (Fig. 8). A spectrin-GFP fusion product was coexpressed to mark transfected cells (22). The speckled staining pattern was not seen in cells expressing vector-transfected cells (Fig. 8) or when preimmune primary antibody was used (data not shown). In comparison, PLZFΔBTB/POZ was diffusely distributed throughout the nucleus and was also present in the cell cytoplasm (Fig. 8).
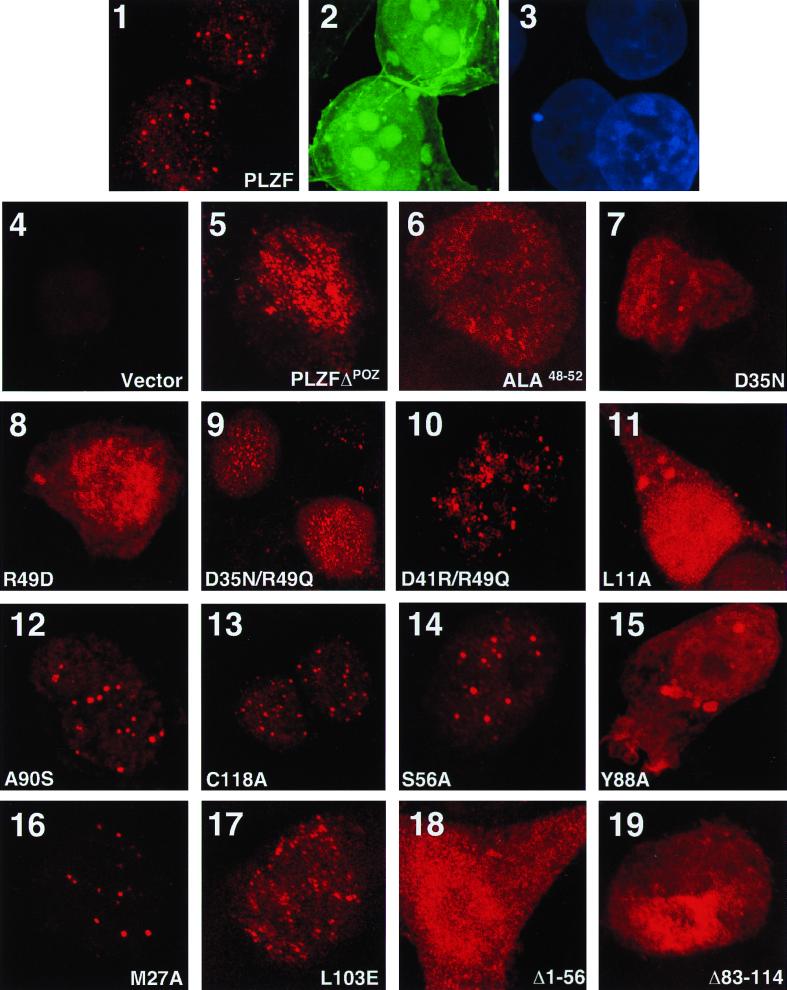
FIG. 8.
Immunolocalization of PLZF BTB/POZ mutants. Expression vectors for wild-type and mutant forms of PLZF were cotransfected along with GFP-spectrin in 293T cells. After incubation with PLZF antibody, a secondary Texas red-conjugated antibody was used to visualize PLZF. Cells were also stained with DAPI to precisely determine if proteins were confined to the nucleus. The slides were scanned at a magnification of ×1,000. Panels 1, 2, and 3, wild-type PLZF-transfected cells (1, PLZF-Texas red; 2, GFP; 3, DAPI); panels 4 to 19, mutant protein transfections (only Texas red panels are shown).
(iv) Growth suppression.
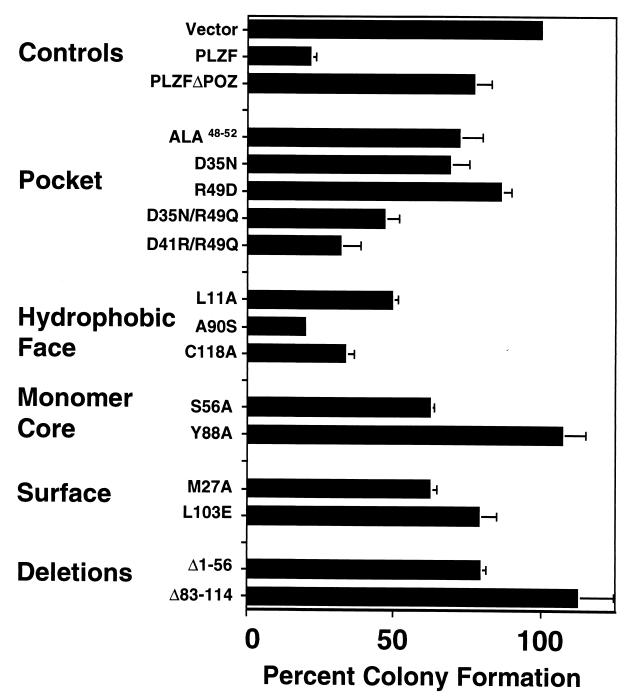
We previously showed that the stable expression of PLZF suppresses cell growth (43, 48). We established a more rapid colony suppression assay with transfected SaOS-2 cells to determine the effect of the mutants on cell growth (Fig. 9). PLZF repressed the formation of colonies by 80% compared to the number of colonies formed after transfection of the empty vector harboring the neo gene. In contrast, PLZFΔBTB/POZ yielded only approximately 20% inhibition of colony formation (Fig. 9). Overall, the correlation between these assays was excellent, with only a few inconsistencies.
FIG. 9.
Suppression of colony formation. SaOS-2 cells were transfected with PLZF and the PLZF BTB/POZ mutants. Cells were then diluted and allowed to grow for 2 weeks in the presence of neomycin. The number of colonies (± SEM) was determined and compared to the number of colonies which formed from vector-transfected cells. This value was set at 100%, and the percent reduction of colony formation by each protein was plotted.
The consequences of the BTB/POZ mutations for the function of full-length PLZF are discussed below and are summarized in Table 1.
Consequences of BTB/POZ mutations for full-length PLZF function. (i) Charged-pocket mutations.
Consistent with their misfolded and nonfunctional status as a BTB/POZ domain, the PLZFALA48–52 and PLZFR49D mutants manifested a PLZFΔBTB/POZ phenotype. Specifically, both mutants were deficient for transcription and did not form an HMW complex (Fig. 6 and 7). In confocal microscopy analysis, these mutant proteins were diffusely localized to both the nucleus and the cytoplasm and were unable to suppress cell growth in colony formation assays (Fig. 8 and 9). The PLZFD35N mutant manifested a more complex phenotype consistent with the effects observed in isolated BTB/POZ domain analysis. This mutant exhibited a weak transcriptional repression effect, was severely deficient in HMW complex formation, and formed very few nuclear speckles (Fig. 6 to 8). These weak effects were further reflected in the colony suppression assay, since this mutant was defective for growth suppression (Fig. 9). The “double-neutral” PLZFD35N/R49Q mutant was also deficient for transcriptional repression. However, this mutant did not activate transcription, although the isolated mutant BTB/POZ domain did (Fig. 6). Furthermore, the PLZF(D41R)/R49Q mutant was fully competent for repression (Fig. 6). This seemingly paradoxical result can be explained by the fact that PLZF contains RD2 between amino acids 200 and 300 (which is not within the BTB/POZ domain) (27, 34). This sequence is a powerful repressor when fused to GAL41–147; in the context of a PLZF species able to dimerize, such as PLZF(D41R)/R49Q, it likely is able to dominantly repress transcription, essentially overpowering the mutant BTB/POZ domain. The additional impairment of dimerization potential conferred by D35N in the PLZFD35N/R49Q mutant may have resulted in a protein unable to provide an effective repression platform for RD2. However, the double-pocket mutant might also disrupt an essential corepressor interaction. These results were consistent with observations from the other functional assays. Thus, the PLZF(D41R)/R49Q mutant was able to form an HMW complex, localize to nuclear speckles, and suppress colony formation (Fig. 7 to 9). The PLZFD35N/R49Q mutant manifested an intermediate phenotype, with weak complex formation, a combined speckle and diffuse nuclear localization pattern, and weak growth suppression (Fig. 7 to 9). These results are consistent with the central role of the charged-pocket domain in BTB/POZ repression. These results also suggest that it is necessary for the PLZF BTB/POZ domain to mediate dimerization for the protein to wield its transcriptional and cell biological functions. Furthermore, the ability to perform these functions correlates completely with formation of the HMW DNA-protein complex and localization to discrete nuclear speckles.
(ii) Hydrophobic surface mutations.
The L11A mutant manifested an intermediate phenotype in functional assays, with moderate impairment of transcriptional repression and growth suppression (Fig. 6 and 9). HMW complex formation and speckle localization were more severely impaired (Fig. 7 and 8). This result is consonant with the weaker self-association properties of this mutant (Fig. 4). The PLZFA90S and PLZFC118A mutants were mildly impaired for transcriptional repression and growth suppression and yet had a wild-type pattern of HMW complex formation and nuclear localization (Fig. 6 to 9). Therefore, there was some difference between the ability to form HMW complexes and biological function. Although these results may represent artifacts of the experimental systems, subtle defects in BTB/POZ function cannot be ruled out. The fact that the L11A mutant was deficient in complex formation is interesting in light of the possible contribution of this surface to higher-order interactions among PLZF molecules (29). Interactions with other proteins remain possible as well.
(iii) Monomer core mutations.
The S56A and Y88A mutants were studied in the context of full-length PLZF. L20A was not studied, since it was believed to be equivalent to wild-type BTB/POZ and thus not informative. In the setting of full-length PLZF, the PLZFY88A mutant was similar to PLZFΔBTB/POZ (Fig. 6 to 9). This result was consistent with the loss of function of PLZFY88A observed when its BTB/POZ domain alone was analyzed. Interestingly, this mutant was diffusely localized throughout the cytoplasm as well as the nucleus, suggesting that the misfolded BTB/POZ domain moiety at the N terminus had an adverse impact on the folding or nuclear import of the remaining PLZF sequence (Fig. 8). It is also possible that misfolded proteins undergo increased proteolysis and that fragments containing the epitope recognized by the monoclonal antibody (between residues 120 and 200) are aberrantly localized. The PLZFS56A mutant was partially impaired in the functional assays. This result reflects the mild phenotype observed when the mutant was expressed as a GAL4-BTB/POZ fusion protein (Fig. 6 to 9).
(iv) Surface residue mutations.
Among the surface residue mutants, only PLZFL103E and PLZFM27A were selected for further analysis, since the D41R mutant was similar to the wild type. The PLZFL103E mutant displayed a severely impaired functional profile concordant with the dysfunctional status of its mutant BTB/POZ domain (Fig. 6 to 9). In contrast, the PLZFM27A mutant could both repress cell growth and localize to speckles, although EMSA complex formation and growth suppression of this mutant protein were less efficient than those of the wild-type protein (Fig. 6 to 9). These results could reflect the altered biochemical properties of the PLZFM27A BTB/POZ domain (Fig. 3).
(v) Deletion mutations.
Both BTB/POZ deletion mutants were analyzed and resulted in PLZF proteins indistinguishable from the PLZFΔBTB/POZ protein (Fig. 6 to 9).
Conclusions.
We analyzed structural features of the PLZF BTB/POZ domain dimer identified by X-ray crystallography (1, 29). Our results show that a number of evolutionarily conserved residues within the BTB/POZ domain are critical for proper folding, dimerization, and transcriptional repression. Mutations that disrupt the interface and abrogate dimerization of the BTB/POZ domain result in completely nonfunctional and misfolded proteins. This finding supports the concept that, for PLZF and probably for most BTB/POZ zinc finger-containing proteins, BTB/POZ dimerization is essential for the proper folding of the entire protein. In concordance with this notion, PLZFΔBTB/POZ was unable to dimerize, repress transcription, form an HMW DNA-protein complex, localize to nuclear speckles, or mediate growth suppression (reference 12 and data above). In agreement with these results, both N- and C-terminal deletions of BTB/POZ result in a completely nonfunctional protein, as predicted by the crystallographic structure. The finding that the domain cannot be subdivided is consistent with the observation that residues from the entire length of the domain participate in the formation of the dimer (1, 29). Several of the most highly conserved residues in the BTB/POZ domain are located in the hydrophobic monomer core region (1, 29). A significant amino acid substitution, Y88A, completely disrupted the ability of the monomer core to fold correctly and resulted in an insoluble, nonfunctional protein.
We determined the functional significance of dimer substructures formed by the interaction between two BTB/POZ monomers. The most salient of these features is the charged-pocket motif, which is one of the most highly conserved sequences in the BTB/POZ domain (Fig. 1 and 2). This region is required for the structural integrity of BTB/POZ, as demonstrated by the fact that replacement of key amino acids with alanine residues results in insoluble, nonfunctional protein aggregates. The charge balance in the pocket is also crucial. Indeed, the loss of intramonomeric electrostatic interactions between R49 and D35 resulted in a partially defective protein, and exchanging an arginine at position 49 for an aspartate resulted in a charge repulsion mutant with a severe folding defect.
The pocket region has been previously proposed to be a potential corepressor interaction motif involved in BTB/POZ transcriptional repression (1). The identification of such a structure is of importance not only for understanding the mechanism of action of BTB/POZ transcription factors but also as a potential therapeutic target in the setting of human malignancies related to aberrant expression of PLZF and BCL-6. The PLZF BTB/POZ domain is thought to play a major role in PLZF repression and was shown by several methods to interact with Sin3A, SMRT, N-CoR, and HDAC1 (9, 15, 16, 18, 19, 32, 46). A structure-function analysis performed by Li et al. (29) examined whether mutating residues from the pocket wall could affect repression. They created three mutations in nonconserved pocket residues (H64A, N66A, and Q68A); these mutants failed to disrupt dimerization or transcriptional repression as GAL4-BTB/POZ fusions. The authors concluded that the pocket was not important for the repression function of the PLZF BTB/POZ domain (29). In contrast, by mutating a highly conserved pocket residue, we found that the pocket is critical for transcriptional repression. The exchange of arginine 49 to a glutamine residue permits dimerization of the isolated BTB/POZ domain but results in a protein that not only is unable to mediate transcriptional repression but also is actually a transcriptional activator. Thus, BTB/POZ dimerization is necessary but not sufficient for transcriptional repression. In concordance with this conclusion, we found that the D35N/R49Q and (D41R)/R49Q mutant BTB/POZ domains are unable to cooperate with corepressors in transcriptional repression assays. In fact, the CREB binding protein coactivator protein enhanced transcriptional activation by the R49Q-containing BTB/POZ mutants (data not shown). In contrast, repression by wild-type BTB/POZ is enhanced by corepressors but is unaffected by coactivators, as is repression by the other functionally competent mutant BTB/POZ domains mentioned in this study (data not shown).
The fact that the R49Q dimers activated transcription could be explained in several ways. It is possible that the mutant configuration of the BTB/POZ domain is unable to bind corepressors and either reproduced a natural or resulted in a novel coactivator binding structure. The lack of corepressor binding may also allow a secondary coactivator binding site to become functionally dominant. In concordance with this notion, PLZF has been shown to associate with the p300 coactivator, although the interaction has not been further mapped (A. Zelent, personal communication). Furthermore, while most zinc finger family BTB/POZ domain proteins were shown to be transcriptional repressors (3, 6, 17, 23, 27, 37, 42), BTB/POZ domain proteins such as GAGA may function as antirepressors (8, 44), ZF5 can activate transcription from SP-1 sites in the human immunodeficiency virus long terminal repeat (23), and MAZR is a transcriptional activator of c-myc (25). The transcriptional effect of BTB/POZ proteins may be promoter dependent; for example, the ZF5 protein also represses transcription from specific binding sites in the β-actin promoter (23). Finally, the complex recruited by BTB/POZ may have the potential to act in both repression and activation, and the mutant BTB/POZ domains may physically alter this conglomerate, thus allosterically triggering the activation function. A mechanism which could explain functions in both activation and repression involves BTB/POZ-dependent formation of HMW oligomers, as in the case of Drosophila GAGA, which bend DNA and result in nucleosome remodeling (13, 24). It is possible that this remodeling favors either activation of transcription or silencing of transcription, depending on the positioning of nucleosomes and/or additional factors recruited to the oligomer complex by the BTB/POZ domain protein.
Interestingly, our results indicate that the PLZF BTB/POZ dimerization function is essential for PLZF function but that the repression function of the BTB/POZ domain is not always required. Thus, the (D41R)/R49Q mutant is functionally identical to the wild-type PLZF transcriptional repressor, even though the isolated BTB/POZ domain is unable to repress transcription. This finding suggests a mechanism of action for PLZF where the BTB/POZ domain mediates oligomerization, while RD2 is central to the transcriptional repression effect. This model is supported by several additional lines of evidence. First, the coordinated actions of both domains are required for PLZF function, since the absence of either one results in a protein incapable of repressing transcription (16, 34). Second, PLZF RD2 is a more powerful transcriptional repressor than the BTB/POZ domain when fused to GAL4 (27, 34). Third, RD2 also binds to corepressors, such as ETO, N-CoR, SMRT, and Sin3A (15, 18, 34; A. Melnick and J. D. Licht, unpublished data). Finally, other members of the PLZF family of zinc finger transcription factors that repress transcription, such as HIC-1 and γF1-binding protein isoform B, do not bind to N-CoR, SMRT, Sin3A, or HDACs (10).
The oligomerization property of PLZF BTB/POZ was proposed to be mediated by the exposed hydrophobic surface (29). Among the mutations introduced into this region, the L11A mutant manifested a phenotype that could indicate a partial oligomerization defect. Indications of this notion include the inability of this mutant to homodimerize as well as marked defects in HMW complex formation and nuclear sublocalization as a full-length PLZF protein. Oligomerization is a feature of BTB/POZ proteins across evolution, including BTB/POZ zinc finger proteins such as GAGA and BTB/POZ nonzinc finger proteins such as Drosophila kelch, Mac-2 binding protein, and Bach1 (13, 20, 24, 35, 41, 49). However, the role of oligomerization in PLZF function is only beginning to be understood, and further structure-function analysis will be useful in these studies. Mutation of the exposed hydrophobic surface formed by the dimer might affect the ability of the BTB/POZ domain to multimerize while leaving dimerization intact.
In summary, our structure-function analysis of the PLZF BTB/POZ domain has shed light on the molecular mechanism of action of the PLZF transcriptional factor. We have identified critical residues involved in transcription, dimerization, and formation of HMW complexes. These properties have been correlated with biological parameters of PLZF function, such as growth suppression and nuclear substructure localization. Finally, an understanding of the actions of BTB/POZ proteins involved in human malignancy at the molecular level may lead to novel small-molecule therapeutic agents which can attach to the key binding sites.
ACKNOWLEDGMENTS
This work was supported by NIH grant CA59936 (to J.D.L.) and ACS grant DHP160 (to J.D.L.). J.D.L. is a scholar of the Leukemia and Lymphoma Society. H.B. was supported by the Leukemia Research Foundation. A.M. is supported by NIH grant KO8 CA73762. G.G.P. is supported by the National Cancer Institute of Canada, and K.F.A. is supported by a Medical Research Council of Canada doctoral research award. Confocal laser scanning microscopy was performed at the Mount Sinai School of Medicine Confocal Laser Scanning Microscopy core facility, supported with funding from an NIH shared instrumentation grant (1S10 RR0 9145-01) and an NSF major research instrumentation grant (DBI-9724504).
We thank Avijit Chakrabartty for the use of the CD spectrometer. We thank Samuel Waxman for continued support. We thank Thomas Kornberg for information on and discussion of Drosophila E(var)93-D BTB/POZ mutants.
REFERENCES
- 1.Ahmad K F, Engel C K, Prive G G. Crystal structure of the BTB domain from PLZF. Proc Natl Acad Sci USA. 1998;95:12123–12128. doi: 10.1073/pnas.95.21.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–1198. [PubMed] [Google Scholar]
- 3.Aoki K, Meng G, Suzuki K, Takashi T, Kameoka Y, Nakahara K, Ishida R, Kasai M. RP58 associates with condensed chromatin and mediates a sequence-specific transcriptional repression. J Biol Chem. 1998;273:26698–26704. doi: 10.1074/jbc.273.41.26698. [DOI] [PubMed] [Google Scholar]
- 4.Ball H J, Melnick A, Shaknovich R, Kohanski R A, Licht J D. The promyelocytic leukemia zinc finger (PLZF) protein binds DNA in a high molecular weight complex associated with cdc2 kinase. Nucleic Acids Res. 1999;27:4106–4113. doi: 10.1093/nar/27.20.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardwell V J, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 6.Chang C C, Ye B H, Chaganti R S, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci USA. 1996;93:6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook M, Gould A, Brand N, Davies J, Strutt P, Shaknovich R, Licht J, Waxman S, Chen Z, Gluecksohn-Waelsch S, et al. Expression of the zinc-finger gene PLZF at rhombomere boundaries in the vertebrate hindbrain. Proc Natl Acad Sci USA. 1995;92:2249–2253. doi: 10.1073/pnas.92.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croston G E, Kerrigan L A, Lira L M, Marshak D R, Kadonaga J T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991;251:643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- 9.David G, Alland L, Hong S H, Wong C W, DePinho R A, Dejean A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene. 1998;16:2549–2556. doi: 10.1038/sj.onc.1202043. [DOI] [PubMed] [Google Scholar]
- 10.Deltour S, Guerardel C, Leprince D. Recruitment of SMRT/N-CoR-mSin3A-HDAC-repressing complexes is not a general mechanism for BTB/POZ transcriptional repressors: the Case of HIC-1 and γFBP-B. Proc Natl Acad Sci USA. 1999;96:14831–14836. doi: 10.1073/pnas.96.26.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhordain P, Albagli O, Lin R J, Ansieau S, Quief S, Leutz A, Kerckaert J P, Evans R M, Leprince D. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc Natl Acad Sci USA. 1997;94:10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong S, Zhu J, Reid A, Strutt P, Guidez F, Zhong H J, Wang Z Y, Licht J, Waxman S, Chomienne C, et al. Amino-terminal protein-protein interaction motif (POZ-domain) is responsible for activities of the promyelocytic leukemia zinc finger-retinoic acid receptor-alpha fusion protein. Proc Natl Acad Sci USA. 1996;93:3624–3629. doi: 10.1073/pnas.93.8.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinas M L, Jimenez-Garcia E, Vaquero A, Canudas S, Bernues J, Azorin F. The N-terminal POZ domain of GAGA mediates the formation of oligomers that bind DNA with high affinity and specificity. J Biol Chem. 1999;274:16461–16469. doi: 10.1074/jbc.274.23.16461. [DOI] [PubMed] [Google Scholar]
- 14.Evans S V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993;11:127–128. doi: 10.1016/0263-7855(93)87009-t. , 134–138. [DOI] [PubMed] [Google Scholar]
- 15.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara F F, Zamir I, Seiser C, Lazar M A, Minucci S, Pelicci P G. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 16.Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]
- 17.Hoatlin M E, Zhi Y, Ball H, Silvey K, Melnick A, Stone S, Arai S, Hawe N, Owen G, Zelent A, Licht J D. A Novel BTB/POZ transcriptional repressor protein interacts with the Fanconi anemia group C protein and PLZF. Blood. 1999;94:3737–3747. [PubMed] [Google Scholar]
- 18.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARalpha) and PLZF-RARalpha oncoproteins associated with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh K D, Bardwell V J. The BCL-6 POZ domain and other POZ domains interact with the co-repressors N-CoR and SMRT. Oncogene. 1998;17:2473–2484. doi: 10.1038/sj.onc.1202197. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi K, Hoshino H, Muto A, Suwabe N, Nishikawa S, Nakauchi H, Yamamoto M. Multivalent DNA binding complex generated by small Maf and Bach1 as a possible biochemical basis for a beta-globin locus control region complex. J Biol Chem. 1998;273:11783–11790. doi: 10.1074/jbc.273.19.11783. [DOI] [PubMed] [Google Scholar]
- 21.James P, Halladay J, Craig E A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalejta R F, Shenk T, Beavis A J. Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry. 1997;29:286–291. doi: 10.1002/(sici)1097-0320(19971201)29:4<286::aid-cyto4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan J, Calame K. The ZiN/POZ domain of ZF5 is required for both transcriptional activation and repression. Nucleic Acids Res. 1997;25:1108–1116. doi: 10.1093/nar/25.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsani K R, Hajibagheri M A, Verrijzer C P. Co-operative DNA binding by GAGA transcription factor requires the conserved BTB/POZ domain and reorganizes promoter topology. EMBO J. 1999;18:698–708. doi: 10.1093/emboj/18.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi A, Yamagiwa H, Hoshino H, Muto A, Sato K, Morita M, Hayashi N, Yamamoto M, Igarashi K. A combinatorial code for gene expression generated by transcription factor bach2 and MAZR (MAZ-related factor) through the BTB/POZ domain. Mol Cell Biol. 2000;20:1733–1746. doi: 10.1128/mcb.20.5.1733-1746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornberg R D, Lorch Y. Chromatin-modifying and -remodeling complexes. Curr Opin Genet Dev. 1999;9:148–151. doi: 10.1016/S0959-437X(99)80022-7. [DOI] [PubMed] [Google Scholar]
- 27.Li J Y, English M A, Ball H J, Yeyati P L, Waxman S, Licht J D. Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J Biol Chem. 1997;272:22447–22455. doi: 10.1074/jbc.272.36.22447. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Lopez-Guisa J M, Ninan N, Weiner E J, Rauscher III F J, Marmorstein R. Overexpression, purification, characterization, and crystallization of the BTB/POZ domain from the PLZF oncoprotein. J Biol Chem. 1997;272:27324–27329. doi: 10.1074/jbc.272.43.27324. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Peng H, Schultz D C, Lopez-Guisa J M, Rauscher III F J, Marmorstein R. Structure-function studies of the BTB/POZ transcriptional repression domain from the promyelocytic leukemia zinc finger oncoprotein. Cancer Res. 1999;59:5275–5282. [PubMed] [Google Scholar]
- 30.Licht J D, Ro M, English M A, Grossel M, Hansen U. Selective repression of transcriptional activators at a distance by the Drosophila Krüppel protein. Proc Natl Acad Sci USA. 1993;90:11361–11365. doi: 10.1073/pnas.90.23.11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Licht J D, Shaknovich R, English M A, Melnick A, Li J Y, Reddy J C, Dong S, Chen S J, Zelent A, Waxman S. Reduced and altered DNA-binding and transcriptional properties of the PLZF-retinoic acid receptor-alpha chimera generated in t(11;17)-associated acute promyelocytic leukemia. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- 32.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 33.Melnick A, Licht J D. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 34.Melnick A M, Westendorf J J, Polinger A, Carlile G W, Arai S, Lutterbach B, Hiebert S W, Licht J D. The ETO protein disrupted in t(8;21)-associated acute myeloid leukemia is a corepressor for the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2000;20:2075–2086. doi: 10.1128/mcb.20.6.2075-2086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller S A, Sasaki T, Bork P, Wolpensinger B, Schulthess T, Timpl R, Engel A, Engel J. Domain organization of Mac-2 binding protein and its oligomerization to linear and ring-like structures. J Mol Biol. 1999;291:801–813. doi: 10.1006/jmbi.1999.2996. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls A, Sharp K A, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 37.Okabe S, Fukuda T, Ishibashi K, Kojima S, Okada S, Hatano M, Ebara M, Saisho H, Tokuhisa T. BAZF, a novel Bcl6 homolog, functions as a transcriptional repressor. Mol Cell Biol. 1998;18:4235–4244. doi: 10.1128/mcb.18.7.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pazin M, Kadonaga J. What's up and down with histone deacetylation and transcription. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 39.Redner R L, Wang J, Liu J M. Chromatin remodeling and leukemia: new therapeutic paradigms. Blood. 1999;94:417–428. [PubMed] [Google Scholar]
- 40.Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, Licht J, Waxman S, Krumlauf R, Zelent A. Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood. 1995;86:4544–4552. [PubMed] [Google Scholar]
- 41.Robinson D N, Cooley L. Drosophila kelch is an oligomeric ring canal actin organizer. J Cell Biol. 1997;138:799–810. doi: 10.1083/jcb.138.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seyfert V L, Allman D, He Y, Staudt L M. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- 43.Shaknovich R, Yeyati P L, Ivins S, Melnick A, Lempert C, Waxman S, Zelent A, Licht J D. The promyelocytic leukemia zinc finger protein affects myeloid cell growth, differentiation, and apoptosis. Mol Cell Biol. 1998;18:5533–5545. doi: 10.1128/mcb.18.9.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukiyama T, Becker P B, Wu C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature. 1994;367:525–532. doi: 10.1038/367525a0. [DOI] [PubMed] [Google Scholar]
- 45.Wales M M, Biel M A, el Deiry W, Nelkin B D, Issa J P, Cavenee W K, Kuerbitz S J, Baylin S B. p53 activates expression of HIC-1, a new candidate tumour suppressor gene on 17p13.3. Nat Med. 1995;1:570–577. doi: 10.1038/nm0695-570. [DOI] [PubMed] [Google Scholar]
- 46.Wong C W, Privalsky M L. Components of the SMRT corepressor complex exhibit distinctive interactions with the POZ domain oncoproteins PLZF, PLZF-RARalpha, and BCL-6. J Biol Chem. 1998;273:27695–27702. doi: 10.1074/jbc.273.42.27695. [DOI] [PubMed] [Google Scholar]
- 47.Ye B H, Lista F, Lo Coco F, Knowles D M, Offit K, Chaganti R S, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 48.Yeyati P L, Shaknovich R, Boterashvili S, Li J, Ball H J, Waxman S, Nason-Burchenal K, Dmitrovsky E, Zelent A, Licht J D. Leukemia translocation protein PLZF inhibits cell growth and expression of cyclin A. Oncogene. 1999;18:925–934. doi: 10.1038/sj.onc.1202375. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida C, Tokumasu F, Hohmura K I, Bungert J, Hayashi N, Nagasawa T, Engel J D, Yamamoto M, Takeyasu K, Igarashi K. Long range interaction of cis-DNA elements mediated by architectural transcription factor bach1. Genes Cells. 1999;4:643–655. doi: 10.1046/j.1365-2443.1999.00291.x. [DOI] [PubMed] [Google Scholar]
- 50.Zollman S, Godt D, Prive G G, Couderc J L, Laski F A. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc Natl Acad Sci USA. 1994;91:10717–10721. doi: 10.1073/pnas.91.22.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]