Abstract
The severe acute respiratory syndrome coronavirus 2, which causes coronavirus disease 2019 (COVID-19), has infected more than 200 million and led to the deaths of more than 4.3 million people. Although there are known risk factors for severe disease, asthma was initially hypothesized to be a risk factor for severe disease given the association between asthma exacerbations and respiratory viral illnesses in general. Fortunately, clinical outcomes for patients with asthma overall are similar to those for patients without asthma, without convincing evidence that asthma is a risk factor for severe disease. This may be explained in part by the decreasing gradient of angiotensin-converting enzyme-2 receptor from the upper to lower respiratory epithelium and that aeroallergen-sensitized patients with asthma can have up to 50% reduction in angiotensin-converting enzyme-2 receptor expression. Vaccination for patients with asthma is recommended for all without clear contraindications. COVID-19–specific treatment options are available depending on the severity of disease. We caution the use of systemic corticosteroids in patients with asthma not requiring supplemental oxygen given an association with worse outcomes. Postacute COVID-19 syndrome or long-haul COVID does not appear to be more prevalent in the population with asthma, and a multidisciplinary approach to care is a reasonable option.
Key words: Asthma, COVID-19, Corticosteroids, Vaccine
Abbreviations used: ACE2, angiotensin-converting enzyme-2; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has infected more than 200 million and led to the deaths of more than 4.3 million people.1 There are well-established risk factors associated with severe disease and increased mortality such as older age, race, sex, obesity, hypertension, cardiovascular disease, and chronic obstructive pulmonary disease.2, 3, 4 Given the propensity for respiratory viral illnesses to cause asthma exacerbations, it was initially postulated that patients with asthma would be at a higher risk for both infection and severe COVID-19.5 Despite this abundance of data for non-COVID respiratory viruses, several groups have noted a reduction in overall asthma exacerbation rates during periods of social lockdown.6, 7, 8, 9 In this article, we will discuss the clinical outcomes of patients with asthma who acquire infection, the influence of asthma and its comorbidities on SARS-CoV-2 receptor expression and risk of COVID-19 infection, the safety and efficacy of the COVID-19 vaccinations, and how to differentiate between asthma-related symptoms and residual COVID-19 symptoms in patients with asthma who have recovered from COVID-19.
Clinical Outcomes of Patients with Asthma who Acquired Sars-Cov-2 Infection
Respiratory viral illnesses are well known to be important causes of asthma exacerbations, in particular human rhinoviruses; these illnesses are a major contributor to asthma morbidity, need for acute care, and mortality.5 Given this association, there was reasonable concern regarding patients with asthma and their susceptibility for COVID-19 and their subsequent outcomes. The Centers for Disease Control and Prevention initially declared asthma to be a risk factor for severe disease and currently still warns that patients with moderate to severe or uncontrolled asthma are more likely to be hospitalized from COVID-19 as of September 2021.10 The prevalence of asthma in the initially described Wuhan cohort was relatively low, suggestive that asthma may not be a risk factor for severe disease; however, there was subsequent data suggesting a higher prevalence of patients with asthma with COVID-19 compared with the general population.3 , 11 , 12 Subsequent data from the first few months of the pandemic had conflicting results regarding asthma as a risk factor for severe disease, with some reports supporting this theory and others supportive of asthma not being a risk factor for severe disease.12, 13, 14, 15, 16, 17 In the subsequent time since the beginning of the pandemic, more robust data regarding both pediatric and adult outcomes have been published.
Asthma currently affects approximately 7% of the pediatric population in the United States, making it one of the most common pediatric chronic illnesses, and it has been similarly represented in US pediatric cases of COVID-19, though admittedly the data are limited worldwide.18 Recent data support that there is no increased risk in children with asthma infected with COVID-19 with regard to severity of disease. Ruano et al19 reported that there were no differences in lung function, need for oral corticosteroids, emergency care, or hospitalizations for pediatric patients with asthma with and without COVID-19, though it was noted that in patients with probable COVID-19 there was increased use of controller and symptom reliever treatment. This was independent of asthma severity or control in the last year, though the increased use of controller and symptom reliever therapy suggests possible COVID-19–induced exacerbations.19 Beken et al20 reported that in children with asthma, with or without concurrent allergic diseases, there was no association with an increased risk of hospitalization for COVID-19. In addition, there is the impact of COVID-19 on availability of medical services for asthmatic children, with there being a clear reduction in in-person visits and new consultations.21 Despite this, there is clear evidence that during the pandemic there have been decreases in pediatric asthma-related hospitalizations, emergency department visits, exacerbations, and albuterol and oral corticosteroid use without increased adherence to controller therapy suggestive of improved asthma outcomes during this time period.20 , 21 There is a similar increase in telemedicine visits as well as decrease in influenza and particulate matter air population over this time period.20
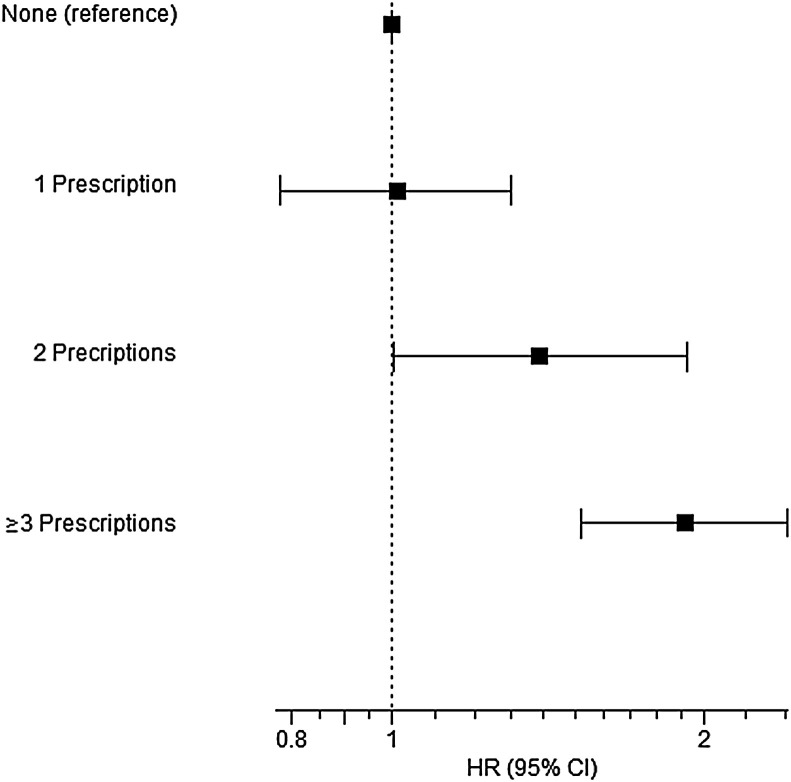
There have been multiple studies demonstrating that there is no increased risk in general between adult patients with asthma and severe COVID-19 infection.22, 23, 24, 25, 26, 27 However, there may exist a subset of patients in the population with asthma, those with less-than-optimal control, who are at more risk for severe disease. Huang et al28 demonstrated that adult patients with asthma who required clinical care in the previous 12 months before COVID-19 diagnosis were at increased risk of severe disease; conversely, those who did not receive clinical care were not at increased risk for severe disease. In addition, there are data to support that COVID-19 infection may not induce a severe exacerbation requiring oral corticosteroids or inhaled bronchodilators in patients with asthma.24 This is further supported because the prevalence of asthma in patients with COVID-19 is similar to that reported in the general population worldwide, and in some areas lower. The data suggest that asthma may in fact be a protective factor in COVID-19 infection for various proposed mechanisms including the differences in TH2 inflammation, decreased expression of angiotensin-converting enzyme, and use of inhaled corticosteroids in patients with asthma.22 , 27 , 29 , 30 Adult asthma patients with COVID-19 infection have been shown to have a decreased mortality, rate of hospitalization, and intensive care unit admission.26 Similarly, the presence of absolute eosinophilia patients with COVID-19, independent of the presence of comorbid asthma, may also be a protective factor because it is associated with decreased mortality.26 , 30 Adir et al30 showed that the use of biologics in patients with severe asthma has no increased risk of COVID-19 infection nor increased risk for moderate to severe COVID-19 infection or all-cause 90-day mortality. Interestingly, recent systemic corticosteroid use, within 1 year, was associated with both moderate to severe COVID-19 infection and a composite end point of moderate to severe COVID-19 infection and all-cause mortality.30 This association had a positive correlation with the number of prescriptions in the previous year, with greater than 2 prescriptions having the highest hazard ratio30 (Figure 1 ). There may exist a subset of patients in the population with asthma who are at more risk for severe disease. Huang et al28 demonstrated that adult patients with asthma who required clinical care in the previous 12 months before COVID-19 diagnosis were at increased risk of severe disease; however, those who did not receive clinical care were not at increased risk for severe disease. From a mechanistic perspective, common cold viruses are well known to induce postviral airway hyperresponsiveness lasting weeks to months.31 , 32 In this regard, the asthma exacerbations occurring in the year before COVID exposure might lead to extended airway hyperresponsiveness that subsequently contributes to a greater risk of COVID hospitalization or death. It is also reasonable to hypothesize that a similar COVID-19–induced postviral airway hyperresponsiveness could lead to worse asthma control.
Figure 1.
Association between systemic corticosteroid prescriptions and advanced COVID-19 illnesses in a population study with complete pharmacy records. Patients with asthma and a nasopharyngeal PCR diagnosis of COVID-19 (N = 8242) were identified from health records with complete pharmacy data. Systemic corticosteroids had been used in the past year by 1358 patients, and biologics had been used by 50 in the last 120 days. Adjusted HRs and 95% CI are shown for the association between the number of filled corticosteroid prescriptions in previous year and composite of moderate to severe COVID-19 or all-cause mortality within 90 days following PCR date among adult asthmatic patients with positive PCR result for SARS-CoV-2. For comparison, the association with biologic use was not significant (HR = 1.42; 95% CI, 0.70-2.88; P = .332). HR, Hazard ratio. This figure has been reproduced with permission.30
Impact of Asthma and Comorbid Conditions on Sars-Cov-2 Receptor Expression and Risk of COVID-19 Infection
Asthma is a well-recognized risk factor for more severe viral respiratory illnesses.5 This has been consistently observed for both seasonal influenza and H1N1.33 Furthermore, type 2 inflammation has been shown to impair antiviral responses and increase susceptibility to respiratory infections, particularly rhinoviruses.34 , 35 Thus, there was significant concern at the onset of the COVID-19 pandemic that patients with asthma could be particularly at risk for severe outcomes with SARS-CoV-2 infections. However, as noted above, asthma has not been consistently linked to enhanced COVID-19 risk. Mechanisms underlying this observation have been of interest since the beginning of the pandemic.
SARS-CoV-2 infects the respiratory epithelium through a process that involves binding the angiotensin-converting enzyme-2 (ACE2) receptor and cleavage of the viral spike protein by the transmembrane serine protease 2.36 Given the central role of the ACE2 receptor in SARS-CoV-2 infection, it has been hypothesized that it could relate to COVID-19 severity. There is a gradient of ACE2 expression from the upper to lower airway, with higher levels expressed in the upper airway epithelium, which corresponds to a greater susceptibility of upper airway cells to SARS-CoV-2 infection.37
Analyses of nasal epithelium in the Urban Environment and Childhood Asthma study identified significant impacts of asthma and respiratory allergy on epithelial ACE2 expression.38 Within the asthma population, there was an inverse association between the levels of aeroallergen sensitization and ACE2 expression. In fact, those with the greatest levels of allergen sensitization had nearly a 50% lower ACE2 expression compared with those with little or no allergen sensitization. Interestingly, allergen exposure is associated with further reductions in ACE2 expression in both the upper and lower airways.38 Multiple type 2 biomarkers associate with lower ACE2 expression, and this appears to be driven by IL-13 inflammatory pathways.39 , 40
Increased ACE2 expression has been associated with a number of risk factors for COVID-19 severity, notably increasing age.41 Peters et al42 examined factors that impact both ACE2 and transmembrane serine protease 2 expression in sputum cells in the Severe Asthma Research Program. Although they did not identify significant differences in expression in asthma versus control, other risk factors for COVID-19 morbidity and mortality associated significantly with receptor expression, including Black race, male sex, diabetes, hypertension, obesity, and cardiovascular disease. Furthermore, Kasela et al43 identified active smoking, hypertension, and obesity to be associated with greater ACE2 expression in bronchial epithelium. Collectively, these associations highlight the potential relevance of ACE2 expression to COVID-19 expression, and motivate further studies to directly evaluate the impact of ACE2 expression on susceptibility to SARS-CoV-2 infection and disease severity.
Covid Vaccine Safety and Effectiveness in Patients with Asthma
The Centers for Disease Control and Prevention recommends vaccination against SARS-CoV-2 for all patients older than 12 years, with the following 2 contraindications: (1) severe allergic reaction after a previous dose or to a component of the COVID-19 vaccine and (2) immediate allergic reaction of any severity to a previous dose of known (diagnosed) allergy to a component of the COVID-19 vaccine.44 Anaphylaxis typically occurs at a rate of less than 1 per million doses for most vaccines; however, the Pfizer-BioNTech COVID-19 vaccine showed initial data of 11.1 cases per million doses.45 , 46 Although no specific vaccine component is known to be responsible, polyethylene glycol is one ingredient known to previously cause anaphylaxis.46 Per the European Academy of Allergy and Clinical Immunology consensus statement, there are no current data showing higher risk for severe allergic reactions to the COVID-19 vaccines in patients with allergic rhinitis or asthma.47 There were no data in our literature review that patients with asthma in general will have less efficacy with the vaccine, though it would be reasonable to assume that those with severe asthma on high levels of corticosteroids chronically may have a decreased response to the vaccine given their immunocompromised state. It is our general recommendation given the reduction in COVID-19 illness, need for hospitalization, and mortality in patients who have received vaccination that all patients with asthma, in the absence of contraindications, should receive vaccination against SARS-CoV-2.29 , 48
Summary of Evidence-Based Treatment Options for Patients with Acute Sars-Cov-2 Infection
What should I recommend if my patient with asthma has symptoms of COVID-19? Regardless of vaccination status, the increased prevalence and contagiousness of the delta variant warrants continued recommendation for COVID testing in all patients with acute symptoms. Asthma maintenance therapies including biologics should not be discontinued. The literature and misinformation regarding COVID-specific treatment options have been overwhelming at times. To address this need for an evidence-based approach to care that is more timely than traditional guideline statements, Cohen et al49 describe a Collaborative Writing Application involving ongoing review of articles by more than 200 experts in 30 disciplines to provide an open-access clinical guideline that is available online.50 In brief, for ambulatory patients with mild to moderate COVID symptoms not requiring hospitalization, the treatment is largely supportive of symptoms unless the patient also has risk factors including age more than 60 years, cardiovascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, cancer, immunosuppressive medications, detectable HIV viral load or CD4 less than 200, tuberculosis, pregnancy, and malnutrition. The presence of 1 or more of these risks of severe COVID illness should prompt closer monitoring of ambulatory patients and consideration of mAb treatments (eg, bamlanivimab-etesevimab, casirivimab-imdevimab, or sotrovimab) directed at the spike protein if available. Although the Randomized Evaluation of Covid-19 Therapy (RECOVERY)59 trial and several others have shown that dexamethasone use in hospitalized patients with COVID-related respiratory failure reduces mortality, this benefit was not seen in admitted patients not requiring supplemental oxygen. This information, combined with the findings of Adir et al30 discussed above, provides a cautionary note discouraging the empiric use of systemic corticosteroids for ambulatory asthma patients with an acute COVID illness. The Platform Randomized trial of INterventions against COVID-19 In older peoPLE (PRINCIPLE)60 trial and several others also show lack of efficacy for azithromycin in preventing hospitalization or other COVID-related end points, leading to a recommendation against use by several guideline statements. All other COVID-directed treatment options supported by clinical trials (remdesivir, therapeutic anticoagulation, tocilizumab, and baricitinib) are indicated only for subsets of hospitalized patients and independent of their comorbid asthma status.51
Evaluation of Patients with Asthma who have Previously Had Covid
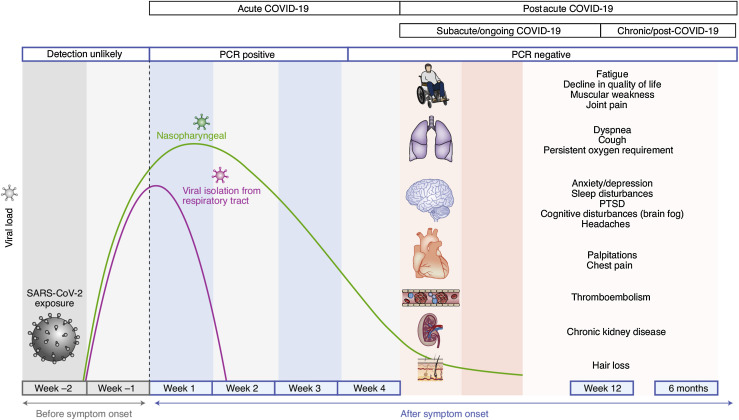
Perhaps more than any other respiratory virus, infection with SARS-CoV-2 has a high rate of prolonged symptoms beyond 4 weeks after the initial infection, in what has been termed postacute COVID-19 syndrome or long-haul COVID.52 Fatigue, cough, dyspnea, and loss of taste or smell are the most common findings in many studies, although the systemic inflammatory response to this infection can also lead to chronic symptoms including weakness, joint pain, sleep disruption, cognitive disturbances, chest pain, palpitation, thromboembolism, chronic kidney disease, and hair loss (Figure 2 ). Most studies to date have follow-up periods less than 3 months, with a few in the 6- to 12-month range.53 , 54 Severity of the acute illness is a risk for lingering symptoms in most studies. Although premorbid asthma has not been associated with an increased risk of postacute COVID-19 syndrome, this patient subset is relatively small in most of the published literature, suggesting a role for additional research using large prepandemic cohorts.
Figure 2.
Graphic summary of the usual course for acute and postacute COVID-19 symptoms. The definition of postacute COVID-19 relates to symptoms lasting longer than 4 weeks, a time point beyond which it is very unlikely to find live virus in clinical samples. PTSD, Posttraumatic stress disorder. This figure has been reproduced with permission.52
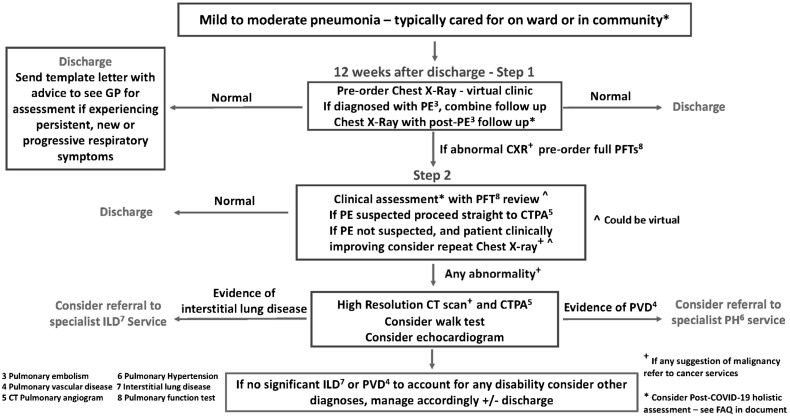
The British Thoracic Society has published guidance for managing patients during postacute recovery period with the full document available online and reviewed by George et al.55 , 56 Figure 3 shows the recommended follow-up algorithm for patients recovering from mild to moderate COVID pneumonia not requiring acute intensive care unit level care. Briefly, a plain chest radiograph with a clinical assessment 3 months after the acute infection is the first level of screening, with complete pulmonary function studies including lung volumes and diffusion capacity reserved for patients with abnormal radiographic findings. A 6-minute walk study, echocardiogram, and/or a chest computed tomography scan is recommended for patients with abnormal chest X-rays and pulmonary function tests. This pragmatic approach is also parsimonious for patients with lingering respiratory symptoms, but should be expanded often with multidisciplinary care for patients with extrapulmonary long-COVID symptoms.52 , 57
Figure 3.
Follow-up care and testing algorithm for patients with mild to moderate COVID-19 pneumonia not requiring acute care in the intensive care unit. The full guideline is available online.55,56
Although new onset of incident asthma may be rare, Maestre-Muñiz et al53 reported that approximately 20% of patients with asthma before their COVID-related hospitalization required more intensive asthma treatment in the following year. The workup for patients with difficult to control asthma has been recently reviewed by Couillard et al.58 Confirming the diagnosis with pulmonary function studies, attending to adherence, and addressing comorbidities are cornerstones for all patients before considering advanced options such as biologic treatments. Additional research will be needed to evaluate whether resolution of infection from SARS-CoV-2 modifies the underlying inflammatory phenotype of patients with established asthma.
Summary
The relationship between asthma and SARS-CoV-2 has fortunately not been one of worsened patient outcomes, but rather one demonstrating that overall patients with asthma have similar outcomes as their nonasthmatic counterparts. There does appear to be an inverse relationship between asthma control, demonstrated either by clinical contact or by corticosteroids use, and severity of COVID-19 disease, suggesting a subset of patients with asthma may do worse than those without asthma. This finding may be driven by SARS-CoV-2 use of the ACE2 receptor for infection, the decreasing gradient of ACE2 expression from upper to lower airway epithelium, and the overall decrease in ACE2 expression in aeroallergen-sensitized patients with asthma.
We recommend vaccination for COVID-19 for all eligible patients without contraindications, and COVID-19– specific therapies are discussed above. Although postacute COVID-19 syndrome or long-haul COVID does not appear to be more prevalent in the population with asthma, careful evaluation including chest imaging, pulmonary function tests, 6-minute walk test, and echocardiogram, in addition to confirmation of a diagnosis of asthma, may help guide care in patients with asthma who have postacute COVID-19 syndrome or long-haul COVID. It is uncertain at this time whether COVID-19 changes the underlying inflammatory phenotype in patients with established asthma.
Footnotes
A portion of this work was supported by The William W. and Judith H. Busse Professorship of Allergy & Asthma Research (L.C.D.).
Conflicts of interest: P. A. Palmon has no relevant conflicts of interest. D. J. Jackson reports receiving grant funding from the National Institute of Allergy and Infectious Diseases, the National Heart, Lung, and Blood Institute (NHLBI), and GlaxoSmithKline, and personal fees from Sanofi, Regeneron, Novartis, Astra Zeneca, Vifor Pharma, and Pfizer. L. C. Denlinger has consulted with AstraZeneca and Sanofi-Regeneron, and has grant funding from NHLBI outside the submitted work, including participation in the Severe Asthma Research Program (SARP) and PrecISE Trial networks. Within the last 3 years, the following companies provided financial support for study activities at the coordinating and clinical centers beyond the third year of patient follow-up for the SARP network: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative. Within the last 3 years, the following companies have provided study drugs for the PrecISE Trial Network: GlaxoSmithKline, Laurel, Sun Pharma, Vifor, Vitaeris/CSL Behring, and Vitaflo.
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 4.Chotirmall S.H., Leither L.M., Coruh B., Chan L.L.Y., Joudi A.M., Brown S.M., et al. Update in COVID-19 2020. Am J Respir Crit Care Med. 2021;203:1462–1471. doi: 10.1164/rccm.202102-0415UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K.-P.F., Kwok W.C., Ma T.F., Hui C.H., Tam T.C., Wang J.K., et al. Territory-wide study on hospital admissions for asthma exacerbation in COVID-19 pandemic. Ann Am Thorac Soc. 2021;18:1624–1633. doi: 10.1513/AnnalsATS.202010-1247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer G., Braunstahl G.J., Hendriks R., Tramper-Stranders G. Asthma exacerbation prevalence during the COVID-19 lockdown in a moderate-severe asthma cohort. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2020-000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst J.H., Zhao C., Fitzpatrick N.S., Goldstein B.A., Lang J.E. Reduced pediatric urgent asthma utilization and exacerbations during the COVID-19 pandemic. Pediatr Pulmonol. 2021;56:3166–3173. doi: 10.1002/ppul.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee L.E., Conceicao E.P., Tan J.Y., Sim J.X.Y., Venkatachalam I. Reduction in asthma admissions during the COVID-19 pandemic: consequence of public health measures in Singapore. Eur Respir J. 2021;57:2004493. doi: 10.1183/13993003.04493-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention People with moderate to severe asthma. 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html
- 11.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latz C.A., DeCarlo C., Boitano L., Png C.Y.M., Patell R., Conrad M.F., et al. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99:2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J.M., Koh H.Y., Moon S.Y., Yoo I.K., Ha E.K., You S., et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest. 2020;158:2290–2291. doi: 10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beurnier A., Jutant E.M., Jevnikar M., Boucly A., Pichon J., Preda M., et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56:2001875. doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Most recent national asthma data. 2021. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
- 19.Ruano F.J., Somoza Álvarez M.L., Haroun-Díaz E., Vázquez de la Torre M., López González P., Prieto-Moreno A., et al. Impact of the COVID-19 pandemic in children with allergic asthma. J Allergy Clin Immunol Pract. 2020;8:3172–3174.e1. doi: 10.1016/j.jaip.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beken B., Ozturk G.K., Aygun F.D., Aydogmus C., Akar H.H. Asthma and allergic diseases are not risk factors for hospitalization in children with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:569–575. doi: 10.1016/j.anai.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulos N.G., Custovic A., Deschildre A., Mathioudakis A.G., Phipatanakul W., Wong G., et al. Impact of COVID-19 on pediatric asthma: practice adjustments and disease burden. J Allergy Clin Immunol Pract. 2020;8:2592–2599.e3. doi: 10.1016/j.jaip.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terry P.D., Heidel R.E., Dhand R. Asthma in adult patients with COVID-19. Prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021;203:893–905. doi: 10.1164/rccm.202008-3266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao L., Lee S., Krings J.G., Rauseo A.M., Reynolds D., Presti R., et al. Asthma in patients with suspected and diagnosed coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:535–541.e2. doi: 10.1016/j.anai.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandbastien M., Piotin A., Godet J., Abessolo-Amougou I., Ederle C., Enache I., et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. 2020;8:2600–2607. doi: 10.1016/j.jaip.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L., Xu J., Xiao W., Wang Y., Jin Y., Chen S., et al. Asthma in patients with coronavirus disease 2019: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2021;126:524–534. doi: 10.1016/j.anai.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho K.S., Howell D., Rogers L., Narasimhan B., Verma H., Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021;127:42–48. doi: 10.1016/j.anai.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunjaya A.P., Allida S.M., Di Tanna G.L., Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021:1–14. doi: 10.1080/02770903.2021.1888116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang B.Z., Chen Z., Sidell M.A., Eckel S.P., Martinez M.P., Lurmann F., et al. Asthma disease status, COPD, and COVID-19 severity in a large multiethnic population. J Allergy Clin Immunol Pract. 2021;9:3621–3628.e2. doi: 10.1016/j.jaip.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adir Y., Humbert M., Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361–367.e13. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemanske R.F., Jr., Dick E.C., Swenson C.A., Vrtis R.F., Busse W.W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xepapadaki P., Papadopoulos N.G., Bossios A., Manoussakis E., Manousakas T., Saxoni-Papageorgiou P. Duration of postviral airway hyperresponsiveness in children with asthma: effect of atopy. J Allergy Clin Immunol. 2005;116:299–304. doi: 10.1016/j.jaci.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S., Kamimoto L., Bramley A.M., Schmitz A.M., Benoit S.R., Louie J., et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 34.Soto-Quiros M., Avila L., Platts-Mills T.A., Hunt J.F., Erdman D.D., Carper H., et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–1505.e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D.J., Evans M.D., Gangnon R.E., Tisler C.J., Pappas T.E., Lee W.M., et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajuthi S.P., DeFord P., Li Y., Jackson N.D., Montgomery M.T., Everman J.L., et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11:5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.J., Montgomery M.T., et al. COVID-19-related Genes in Sputum Cells in asthma. Relationship to demographic features and corticosteroids, Am J Respir Crit Care Med, 202. 2020:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasela S., Ortega V.E., Martorella M., Garudadri S., Nguyen J., Ampleford E., et al. Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium. Genome Med. 2021;13:66. doi: 10.1186/s13073-021-00866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Contraindications
- 45.Turner P.J., Ansotegui I.J., Campbell D.E., Cardona V., Ebisawa M., El-Gamal Y., et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy K.R., Patel N.C., Ein D., Hudelson M., Kodoth S., Marshall G.D., Jr., et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 Vaccines. Ann Allergy Asthma Immunol. 2021;126:319–320. doi: 10.1016/j.anai.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klimek L., Jutel M., Akdis C.A., Bousquet J., Akdis M., Torres M.J., et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—an EAACI-ARIA position paper. Allergy. 2021;76:1624–1628. doi: 10.1111/all.14726. [DOI] [PubMed] [Google Scholar]
- 48.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen C.L., Walker K.H., Hsiang M., Sonenthal P.D., Riviello E.D., Rouhani S.A., et al. Combating information chaos: a case for collaborative clinical guidelines in a pandemic. Cell Rep Med. 2021;2:100375. doi: 10.1016/j.xcrm.2021.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigham and Women's Hospital, Inc. Homepage - COVID-19 protocols. https://covidprotocols.org/en/ Accessed September 20, 2021.
- 51.PRINCIPLE Trial Collaborative Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maestre-Muñiz M.M., Arias Á., Mata-Vázquez E., Martín-Toledano M., López-Larramona G., Ruiz-Chicote A.M., et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.British Thoracic Society COVID-19: information for the respiratory community. 2021. https://britthoracic.org.uk/about-us/covid-19-information-forthe-respiratory- community/
- 56.George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 57.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 58.Couillard S., Jackson D.J., Wechsler M.E., Pavord I.D. How I do it. Work-up of severe asthma. Chest. 2021;160:2019–2029. doi: 10.1016/j.chest.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 59.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.PRINCIPLE Trial Collaborative Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]