Abstract
Purpose of Review
Brain and other central nervous system (CNS) tumors, while rare, cause significant morbidity and mortality across all ages. This article summarizes the current state of the knowledge on the epidemiology of brain and other CNS tumors.
Recent Findings
For childhood and adolescent brain and other CNS tumors, high birth weight, non-chromosomal structural birth defects and higher socioeconomic position were shown to be risk factors. For adults, increased leukocyte telomere length, proportion of European ancestry, higher socioeconomic position, and HLA haplotypes increase risk of malignant brain tumors, while immune factors decrease risk.
Summary
Although no risk factor accounting for a large proportion of brain and other CNS tumors has been discovered, the use of high throughput “omics” approaches and improved detection/measurement of environmental exposures will help us refine our current understanding of these factors and discover novel risk factors for this disease.
Keywords: Brain and other CNS tumors, Epidemiology, Risk factor, Incidence, Survival
Introduction
Brain and other CNS tumors, while rare, cause significant mortality and morbidity across all ages. Despite decades of research on the etiology of brain and other CNS tumors, no risk factor accounting for a large proportion of cases has been identified. Brain and other CNS tumors are unique in that they are histologically complex, with over 100 types as listed by the World Health Organization International Classification of Diseases Oncology [1] and they display many of the well know Hallmarks of Cancer [2, 3] with dysregulated cell growth, metabolism, etc. However, with the use of novel high throughput “omics” approaches our understanding of causes and risk factors for brain and other CNS tumor continues to be refined and grow. In this review, we describe current and up to date knowledge about causes and risk factors for brain and other CNS tumors in children/adolescents and adults.
Updates on Causes and Risk Factors for Brain and Other CNS Tumors in Children and Adolescents
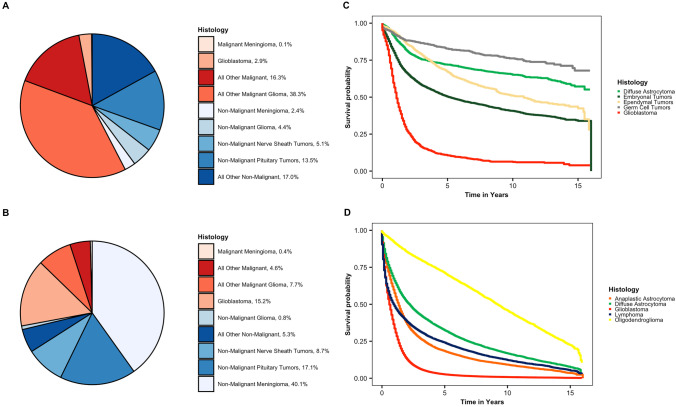
Brain and other CNS tumors the most common cancer in children diagnosed at 0–14 years old and the second most common cancer in adolescents diagnosed at 15–19 years old [4••]. In particular, the incidence of brain and other CNS tumors is highest for those 5 and younger at diagnosis. In children and adolescents, the majority of brain and other CNS tumors are malignant tumors (age-adjusted incidence of 3.55 per 100,000) while non-malignant brain and other CNS tumors are less common in this age group (age-adjusted incidence 2.60 per 100,000) [4••]. The most common malignant histologies in this age group are glioma, embryonal tumors and germ cell tumors while the most common specific non-malignant histology is tumors of the pituitary (Fig. 1a). There have been no significant changes in incidence of these tumors in this age group over the last few decades [4••, 5]. In addition, brain and other CNS tumors are the number one cause of cancer related mortality in children diagnosed at 0–14 years old and overall survival for childhood and adolescent brain and other CNS tumors varies greatly by brain and other CNS tumor histology (Fig. 1c).
Fig. 1.
Incidence and survival for primary brain and other CNS tumors by age group, behavior and histology (CBTRUS incidence: data provided by CDC’s National Program of Cancer Registries (NPCR) and NCI’s Surveillance, Epidemiology and End Results (SEER) Program, 2013–2017; NPCR Survival Analytic file (2001–2016)), distribution of primary brain and other CNS tumors by behavior for a children (0–19 years), and b adults (20 years and older); CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2013–2017; Kaplan–Meier survival curves for the five most common histologies within c children (0–19 years), and d adults (20 years and older); National Program of Cancer Registries SEER*Stat Database: NPCR Survival Analytic file (2001–2016).*Percentages may not add up to 100% due to rounding. “All Other Malignant” includes histologies with ICD − O − 3 behavior code of /3 from choroid plexus tumors, neuronal and mixed neuronal − glial tumors, tumors of the pineal region, embryonal tumors, nerve sheath tumors, mesenchymal tumors, primary melanocytic lesions, other neoplasms related to the meninges, lymphoma, other hematopoietic neoplasms, germ cell tumors, cysts and heterotopias, tumors of the pituitary, craniopharyngioma, hemangioma, neoplasm unspecified, and all other. “All Other Non-Malignant” includes histologies with ICD − O − 3 behavior code of /0 or /1 from neuronal and mixed neuronal − glial tumors, tumors of the pineal region, embryonal tumors, other tumors of cranial and spinal nerves, mesenchymal tumors, primary melanocytic lesions, other neoplasms related to the meninges, other hematopoietic neoplasms, germ cell tumors, cysts and heterotopias, craniopharyngioma, hemangioma, neoplasm unspecified, and all other
Many factors, both environmental and genetic, have been studied in order to identify a factor that accounted for a large proportion of childhood and adolescent brain and other CNS tumors (as reviewed in [6•]). Unfortunately, no such factor has been identified. There are two primary risk factors for brain and other CNS tumors in children, adolescents and adults that have been well validated: single gene inherited disorders (~ 4% of childhood cases) and ionizing radiation (as reviewed in [6•, 7]). In fact, carcinogenic effects of radiation seem to be stronger in children, particularly in younger children, and show a clear dose response relationship [8, 9]. Few genetic association studies have been performed in childhood brain and other CNS tumors and therefore our knowledge about genetic risk factors for these tumors in this age group is very limited. Some candidate gene studies have been performed and provide some evidence for shared genetic risk factors for brain and other CNS tumors between age groups (as reviewed in [6•]). Some recent work in childhood ependymoma suggests that European ancestry is associated with higher risk of a childhood ependymoma [10] and that genetic risk for longer telomere length was associated with a higher risk of ependymoma in children and adolescents aged 12–19 but not for those younger than 12 years old at diagnosis [11•].
Some of the newest environmental risk factors to be studied in relation to risk for childhood and adolescent brain and other CNS tumors are birth weight and non-chromosomal structural birth defects. There is reasonably consistent evidence that higher birth weight is associated with a higher risk of childhood brain and other CNS tumors as provided by 3 large meta-analyses [12–14]. Georgakis et al. performed a systematic review and meta-analysis and showed that birth weight > 4000 g was associated with in increased risk of a childhood brain and other CNS tumor (Odds Ratio 1.14, 95% confidence interval (1.08–1.20); higher risk for astrocytoma and embryonal tumors and non-significant for ependymoma [12]. Dahlhaus et al. performed a meta-analysis and showed that high birth weight (> 4000 g) increased the risk of astrocytoma and medulloblastoma and not for ependymoma [13]. However, Bailey et al. pooled data from multiple population-based case–control studies in France and found no association between birth weight and childhood brain and other CNS tumor risk [14].
Non-chromosomal structural birth defects are a strong and consistent risk factor for childhood cancers in general [15–17]; these findings were most pronounced in young children, aged 5 years or younger with cancer [18, 19]. For brain and other CNS tumors, ~ 7% of childhood brain and other CNS tumors are attributable to these defects [15–17]. Previous studies had suggested ~ twofold increased risk of childhood brain and other CNS tumor associated with a birth defect [18–21]. However, a very recent study using records from 10 million live births showed that particularly for children with a defect of the central nervous system or other neurological anomaly they are at a higher risk of development of a brain and other CNS tumor, with hazard ratios as high as 10 [17].
Updates on Causes and Risk Factors for Brain and Other CNS Tumors in Adults
Brain and other CNS tumors are the 8th most common cancer in adults 40 + [4••]. The majority of brain and other CNS tumors diagnosed in adults 20 + years old are non-malignant tumors (age-adjusted incidence of 22.38 per 100,000) while malignant brain and other CNS tumors are less common in this age group (age-adjusted incidence 8.5 per 100,000) [4••]. The most common malignant histology in adult is glioma, while the most common specific non-malignant histologies are meningioma and tumors of the pituitary (Fig. 1b). There have been no significant changes in incidence of glioma in this age group over the last few decades [4••, 5]. Malignant brain and other CNS tumors are the 6th most common cause of cancer death in adults 40 + years old in the USA [4••]. Overall, survival for adult brain and other CNS tumors varies greatly by brain and other CNS tumor histology (Fig. 1d).
Numerous environmental exposures have been evaluated as potential risk factors for brain and other CNS tumors in adults, but the only consistent risk factor that has been identified is exposure to high-dose ionizing radiation [22]. For meningioma, the excess relative risk (ERR) associated with one Gy of exposure to ionizing radiation was 4.63, while the ERR associated with glioma was 1.98. History of respiratory allergies has been consistently associated with decreased risk of glioma [23]. Due to the rarity of this level of radiation exposure, this does not account for the vast majority of brain tumor incidence.
Many environmental risk factors are still under investigation, though these have mixed or null results of association with brain and other CNS tumors. One of the most thoroughly investigated is cellular phones due to their frequent use globally. Cellular phones emit radiofrequency fields (RF), which were classified as a possible carcinogen by the International Agency for Research on Cancer (IARC) in 2011 [24]. The majority of epidemiological studies since the publication of the IARC report have found no significant associations between cellular phone use and risk of any type of brain and other CNS tumor. Extremely low frequency magnetic fields (ELFs) have also been studies extensively in relation to brain and other CNS tumor risk. The INTEROCC consortium was formed to evaluate the association between ELF and brain and other CNS tumors, and did not find an association with lifetime cumulative occupational exposure to ELF [25]. Power lines are another source of EMF exposure that have been investigated in relation to brain and other CNS tumor risk. A recent case–control study found a significant association between the highest level of estimated ELF from power lines and increased risk of brain and other CNS tumors, and glioma in particular [26]. More investigation is necessary to confirm this association. Other non-radiation occupational exposures have also been studied extensively in relation to risk for brain and other CNS tumors, and to date none have been consistently associated with risk of brain and other CNS tumors [6•].
While the vast majority of brain and other CNS tumors occur in individuals without a known cancer syndrome, ~ 5–10% have a family history of brain and CNS tumor [27]. There are numerous mendelian cancer syndromes that affect risk of brain and other CNS tumors, including neurofibromatosis types I and II, tuberous sclerosis, and Li Fraumeni syndrome (as reviewed [6•]; Table 1). Due to the lack of known environmental risk factors, investigations into common inherited genetic polymorphisms have been conducted to identify genetic risk factors in individuals with no family history. The majority of these studies have focused on glioma, which is responsible for the vast majority of deaths due to malignant brain and other CNS tumors. In total, these have identified 25 single nucleotide polymorphisms (SNPs) associated with risk for glioma. The risk conferred by these variants is histology specific. There are 11 risk SNPs for glioblastoma and 19 risk SNPs for non-glioblastoma, where 5 SNPs are shared between these two broad glioma types [28••] (Table 1). The function of many gliomas associated SNPs are currently unknown, though some are part of known oncogenic pathways. The most common pathway identified as conferring risk in glioma are those associated with telomere maintenance, including risk variants near TERT and RTEL1. Many of these SNPs have further molecular subtype associations ([29•]; Table 1). Several candidate SNP studies have been conducted in East Asian populations, which have found novel association loci for glioma as well as validated those discovered in European-ancestry populations, including loci in TERC, TERT, EGFR, and PHLDB1 [30, 31] (Table 1). The only GWAS of glioma in an East Asian population confirmed associations near TERT, PHLDB1 and RTEL1, and identified two new variants [32•] (Table 1).
Table 1.
Genes implicated in inherited and sporadic brain tumor risk by chromosomal position (as reviewed in in [6•])
| Chromosomal location | Gene | Associated tumor type | Mendelian associations disorder/syndrome (OMIM ID) | Single SNP associations from genome-wide association studies |
|---|---|---|---|---|
| 2p16.3 | MSH6 | Medulloblastoma, glioma, glioblastoma, |
Lynch syndrome (120435), Biallelic mismatch repair deficiency, constitutional MMR deficiency Mismatch repair deficiency syndrome (276300) |
None |
| 2p21-p16.3 | MSH2 | Medulloblastoma, glioma, glioblastoma, |
Lynch syndrome (120435), Biallelic mismatch repair deficiency, constitutional MMR deficiency Mismatch repair deficiency syndrome (276300) |
None |
| 2q33.3 | C2orf80 | Lower grade glioma | None | rs7572263 |
| 2q33.3 | IDH1 | Glioma | Ollier disease | None |
| 3p14.1 | LRIG1 | Lower grade glioma | None | rs11706832 |
| 3p21.1 | BAP1 | Meningioma | BAP1 tumor predisposition syndrome (614327) | None |
| 3p22.2 | MLH1 | Medulloblastoma, glioma, glioblastoma, |
Turcot’s syndrome type 1 Lynch syndrome (120435), Biallelic mismatch repair deficiency, constitutional MMR deficiency Mismatch repair deficiency syndrome (276300) |
None |
| 3p25 | VHL | Hemangioblastoma | Von Hippel-Lindau syndrome (193300) | None |
| 3q26.2 | TERC | All glioma | None | rs1920116 |
| 5p13.3 | DROSHA | Pineoblastoma, pituitary blastoma | DICER1 syndrome | None |
| 5p15.33 | TERT | All glioma | None | rs10069690 |
| Astrocytoma | None | rs2853676 | ||
| 5q21 | APC | Medulloblastoma, glioma | Familial adenomatous polyposis (FAP, 175100), Turcot’s syndrome type 2 | None |
| 7p11.2 | EGFR | All glioma | None | rs2252586 |
| Glioblastoma | None | rs11979158; rs730437; rs1468727 | ||
| 7p22.1 | PMS2 | Medulloblastoma, glioma, glioblastoma, |
Turcot’s syndrome type 1 Lynch syndrome (120435), Biallelic mismatch repair deficiency, constitutional MMR deficiency Mismatch repair deficiency syndrome (276300) |
None |
| 8p12 | RECQL2 | Meningioma | Werner syndrome (277700) | None |
| 8q24.21 | CCDC26 | Lower grade glioma, in particular IDH-mutant tumors | None | rs55705857 |
| 9p21.3 | CDKN2A | Glioma | Melanoma-neural system tumor syndrome (155755) | None |
| CDKN2B-AS1 | Lower grade glioma, in particular WHO grade II-IV astrocytic tumors | None | rs4977756 | |
| 9q22.3 | PTCH1 | Medulloblastoma, meningioma | Gorlin’s syndrome (nevoid basal cell carcinoma) | None |
| 9q34.14 | TSC1 | Giant cell astrocytoma | Tuberous sclerosis (TSC) (191100, 613254) | None |
| 10p12.31 | MIR4675, NEBL | Pituitary adenoma | None | rs2359536 |
| MLLT10 | Meningioma | None | rs11012732 | |
| 10q21.1 | PCDH15 | Pituitary adenoma | None | rs10763170 |
| 10q23.31 | PTEN | Cerebellar gangliocytoma, meningioma | Cowden syndrome 1 (158350) | None |
| 10q24.32 | SUFU | Meningioma | Familial meningiomatoses (607174) | None |
| 10q24.33 | OBFC1 | Lower grade glioma | None | rs11598018 |
| 10q25.2 | VTI1A | Lower grade glioma | None | rs11599775 |
| 11p15.5 | RIC8A | Meningioma | None | rs2686876 |
| 11q13.1 | MEN1 | Pituitary prolactinoma, meningioma | Multiple endocrine neoplasia, type 1 (131100) | None |
| 11q13.2 | AIP | Pituitary adenomas | Pituitary adenoma predisposition (102200) | None |
| 11q14.1 | Intergenic | Glioblastoma | None | rs11233250 |
| 11q21 | MAML2 | Lower grade glioma | None | rs7107785 |
| 11q22.3 | ATM | Astrocytoma and medulloblastoma | Ataxia-telangiectasia (208900) | None |
| 11q23.2 | PHLDB1 | All glioma | None | rs648044; rs17748; rs2236661; rs494560 |
| All glioma | None | rs494560 | ||
| Lower grade glioma, in particular IDH-mutant gliomas | None | rs498872 | ||
| 12p11.23 | STK38L | All glioma | None | rs10842893 |
| 12q21.2 | Intergenic | Lower grade glioma | None | rs1275600 |
| 13q12.13 | CDK8 | Pituitary adenoma | None | rs17083838 |
| 13q14 | RB1 | Retinoblastoma, pineoblastoma, Malignant glioma | Retinoblastoma | None |
| 14q12 | AKAP6 | Lower grade glioma | None | rs10131032 |
| 14q32.13 | DICER1 | Pineoblastoma, pituitary blastoma | DICER1 syndrome | None |
| 15q21.3 | RAB27A | All glioma | None | rs4774756 |
| 15q24.2 | ETFA | Lower grade glioma | None | rs1801591 |
| 15q26.1 | IDH2 | Glioma | Ollier disease | None |
| 16p13.3 | CREBBP | Medulloblastoma, oligodendroglioma, and meningioma | Rubinstein-Taybi syndrome (180849) | None |
| 16p13.3 | RHBDF1 | Glioblastoma | None | rs2562152 |
| Lower grade glioma | None | rs3751667 | ||
| TSC2 | Giant cell astrocytoma | Tuberous sclerosis (TSC) (191100, 613254) | None | |
| 16q12.1 | HEATR3 | Glioblastoma | None | rs10852606 |
| 16q24.3 | FANCA | Medulloblastoma | Fanconi anemia (227650) | None |
| 17p13.1 | TP53 | All glioma | Li-Fraumeni syndrome (151623) | rs78378222 |
| 17q11.2 | NF1 | Astrocytoma, schwannomas, optic nerve glioma | Neurofibromatosis 1 (NF1) (162200) | None |
| 17q21.2 | SMARCE1 | Meningioma | Familial meningiomatoses (607174) | None |
| 17q24.2 | PRKAR1A | Pituitary adenomas | Carney complex (160980) | None |
| 1p31.3 | RAVER2 | Glioblastoma | None | rs12752552 |
| 1q32.1 | MDM4 | Lower grade glioma | None | rs4252707 |
| 1q44 | AKT3 | Lower grade glioma | None | rs12076373 |
| 20q13.33 | RTEL1 | All glioma | None | rs6010620 |
| 22q11.23 | SMARCB1 | Meningioma | Familial meningiomatoses (607174) | None |
| 22q12.1 | MN1 | Meningioma | Familial meningiomatoses (607174) | None |
| 22q12.2 | NF2 | Acoustic neuromas, meningiomas, Ependymoma | Neurofibromatosis 2 (NF2) (101000) | None |
| 22q13.1 | PDGFB | Meningioma | Familial meningiomatoses (607174) | None |
| SLC16A8 | Glioblastoma | None | rs2235573 | |
| 22q13.2 | EP300 | Medulloblastoma, oligodendroglioma, and meningioma | Rubinstein-Taybi syndrome (180849) | None |
Ancestry and Brain Tumor Risk
Genetic studies have also been conducted in other brain and other CNS tumor types. In European ancestry populations, two SNPs have been identified as affecting risk for meningioma [33•] (Table 1), while two SNPs have been identified for primary CNS lymphomas [34•] (Table 1). In individuals of East Asian ancestry, three SNPs have been identified as increasing risk in pituitary adenoma [35]. Genetic factors other than specific SNPs have also been associated with risk of developing a brain tumor. Increased leukocyte telomere length (LTL) has been associated with increased risk of both glioma and meningioma [36, 37]. In addition to individual level variation in LTL, analysis of glioma samples has demonstrated that these tumors have significantly longer telomere length as compared to other cancers [38]. Malignant brain tumor incidence is highest in countries with primarily European-ancestry populations (such as Europe, the USA and Canada), and in white non-Hispanics in the USA [6•, 39]. Similar to associations identified with pediatric tumors, increased overall European-ancestry has been detected in African American and Hispanic glioma cases as compared to controls [40•].
Immune Related Factors: Viruses, Allergy, and HLA
Several infections have been epidemiologically evaluated in glioma. Members of the polyomavirus family including BK, JC, and SV40 have been inconsistently associated with glioma risk [41, 42]. Members of the family herpesviridae have been evaluated in multiple studies with inconsistent results. The herpesvirus’s Epstein-Barr virus, herpes-simplex 1/2, has been extensively evaluated in human cancers; yet, the evidence in central nervous system tumors is contradictory [43, 44]. Cytomegalovirus (CMV) was associated with glioma where serologic investigations into risk/survival and the presence of CMV within tumors have again provided inconsistent evidence of a causal link between CMV and glioma development [45–48]. However, recently two anti-CMV therapeutics have provided evidence of increased patient survival after receiving valganciclovir or a pp65 based treatment [49, 50]. Those observations and mechanistic studies have bolstered a theory of CMV as an ‘oncomodulator’ in glioma, where CMV may not necessarily be involved in the initiation of glioma but may play a role in tumor growth and immune evasion [51•]. The most recently associated infection with glioma risk is not a virus but a protozoan, toxoplasma gondii (T. gondii). In a relatively small study of serum samples from two separate cohorts antibodies to T. gondii were significantly associated (OR: 2.70; 95% CI: 0.96–7.62; OR: 1.32, 95% CI: 0.85–2.07) with glioma risk before diagnosis, eliminating reverse causation biasing the association [52]. Further serologic studies examining T. gondii are needed.
The only consistently associated infection tied to glioma risk is the herpesvirus varicella zoster virus (VZV), the nearly ubiquitous virus that causes chickenpox and shingles [53]. Serologic studies of VZV antigens have also shown a similar reduction in glioma risk [54, 55]. In a large international meta-analysis of self-report VZV infection reported from 8704 cases included in the Glioma International Case Control Study, infection with VZV conferred a 20% reduced risk of glioma [56]. Although the mechanism remains a mystery, it has been hypothesized that interactions between the VZV and host immune response may be mediating glioma development. Parallel to the inverse association with VZV is the observation that allergic and ectopic conditions reduce glioma risk [23]. Allergies and other atopic conditions have consistently been shown to reduce risk of brain tumors, particularly glioma (as reviewed in [6•]).
Two large international meta-analyses have also concluded that allergy and ectopic conditions reduce the risk of glioma ~ 20% [23, 57]. Measurements of serum IgE in glioma cases and controls have mirrored the questionnaire based studies showing that increased serum IgE is associated with reduced glioma risk [58, 59]. To further investigate the underlying genetic architecture of allergy and its relation to glioma risk Mendelian randomization studies have been utilized to assess the genetic basis for this association [60–62]. The results from these studies have been suggestive showing small effects of reduced risk when comparing genetically programmed allergy/atopy with glioma risk, but not conclusive and may be due to the difficulty of constructing a genetic instrument for allergy and ectopic conditions.
Studies have demonstrated a significant heritable component (32–48%) of antibody responses to many viruses and have identified multiple host genetic loci relating to immune response for a variety of viruses [63]. The hereditable component for allergic response is estimated at ~ 65% and genetic loci relating to T-cell and signal transduction [64–66]. Genetic studies of both allergy and response to infections have highlighted the human leucocyte antigen (HLA) as a powerful genetic regulator. Specific HLA alleles have been associated with glioma, though the complexity of the HLA complicates studies based on SNP array data. One of the earliest studies to investigate this was the UCSF Adult Glioma Study, with risk-increasing effects observed for B*13 and B*07 ~ C*07 haplotype, and protective effects for C*01 allele [67]. In this same study, two class I HLA alleles, A*32 and B*55, were associated with longer survival in GBM AGS patients. A*32 was also inversely associated with GBM risk in a separate population [68]. The largest recent study of using SNPs1856 glioma cases and 4955 controls, observed a 50% greater risk of glioma in heterozygous compared to homozygous carriers of the DRB1*15:01 ~ DQA1*01:02 ~ DQB1*06:02 haplotype (p < 0.002), with significant non-additive/epistatic effects [69]. Intriguingly, this haplotype is associated with susceptibility to multiple autoimmune conditions, and antibody response to EBV and VZV antigens [70, 71], and a new analysis suggested that history of auto-immune disease may also decrease risk of developing a glioma [72•]. Recent analyses of expression of immune cell populations using LD score regression showed that the genomic architecture of T cells, NK cells, and myeloid cells is inversely correlated with glioma and may be mediating glioma predisposition [72•]. New approaches to categorizing immune cells in tumors include traditional immunohistochemistry-based approaches [73] and novel methylation based analyses to de-convolute cell types [74•]; both of these approaches seek to stratify tumor types based on tumor infiltrating immune cells. Recent studies show that methylation derived neutrophile to lymphocyte ratios less than 4.0 were associated with significantly decrease survival times (HR 2.02, 95% CI, 1.11–3.69) [75]. Further research examining the interaction between genetic loci, blood cell proportions and their relationship to allergy/infections are required to understand the complex involvement to glioma risk.
Socioeconomic Position
Mounting evidence from diverse studies suggests that higher socioeconomic position (SEP) is associated with an increased risk of adult CNS tumors when compared to individuals with a lower SEP [76–79, 80••]. An analysis of SEER data showed a significant relationship between the first quartile versus the second third, and fourth quartiles of county level income revealing a 10%, 11%, and 14% higher risk of glioma respectively [77]. A recent analysis of SEER data showed that the increased risk associated with higher SEP is primarily in non-Hispanic whites [80••]. Additionally, two recent registry-based studies of childhood CNS malignancies suggest that this relationship appears to not only exist in adult CNS tumors but also in childhood CNS tumors, where studies in both California and Denmark show similar effects in various measures of SEP [81•, 82•]. Possible explanations include a diagnostic bias where tumors in patients with lower SEP may go unreported; yet, the accuracy of surveillance and the magnitude of the effect suggest that this bias alone does not alone account for the association. Another explanation is an unidentified risk factor that is associated with higher SEP, possibly related to the ‘hygiene hypothesis’ [83] where immune exposures relating to allergy and infection maybe altered according to SEP.
Conclusions
Although no risk factor accounting for a large proportion of brain and other CNS tumors has been discovered, there are multiple directions that can be taken to add to our understanding of risk for brain and other CNS tumors. Specifically, the use of high throughput “omics” approaches, improved detection/measurement of environmental exposures, expansion to more diverse populations, synergy between germline and somatic variants, and incorporation of all types of clinical data to comprehensively study this disease (such as imaging). These novel directions will help us refine our current understanding of these factors and discover novel risk factors for this disease.
Acknowledgements
The CBTRUS data were provided through an agreement with the Centers for Disease Control’s National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. The only human subject’s data included in this article was use of de-identified data from the Central Brain Tumor Registry of the United States (CBTRUS Incidence: Data provided by CDC’s National Program of Cancer Registries (NPCR) and NCI’s Surveillance, Epidemiology and End Results (SEER) Program, 2013–2017; NPCR Survival Analytic file (2001–2016), approved as exempt from the Duke University Institutional Review Board.
Disclaimer
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Footnotes
This article is part of the Topical Collection on Neuro-Oncology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of Importance in the last 3 years •• Of major importance in the last 3 years
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta neuropathologica. 2016;131(6):803–20. Epub 2016/05/10. 10.1007/s00401-016-1545-1. [DOI] [PubMed]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. Epub 2011/03/08. 10.1016/j.cell.2011.02.013. [DOI] [PubMed]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. Epub 2000/01/27. 10.1016/s0092-8674(00)81683-9. PubMed PMID: 10647931. [DOI] [PubMed]
- 4.•• Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-oncology. 2020;22(12 Suppl 2):iv1-iv96. Epub 2020/10/31. 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed]
- 5.Gittleman HR, Ostrom QT, Rouse CD, Dowling JA, de Blank PM, Kruchko CA, Elder JB, Rosenfeld SS, Selman WR, Sloan AE, Barnholtz-Sloan JS. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–12. Epub 2014/08/27. 10.1002/cncr.29015. [DOI] [PMC free article] [PubMed]
- 6.• Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME, Bondy ML. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019;21(11):1357–75. Epub 2019/07/14. 10.1093/neuonc/noz123. [DOI] [PMC free article] [PubMed]
- 7.Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, McKean-Cowdin R, Fisher JL, Lupo PJ, Partap S, Schwartzbaum JA, Scheurer ME. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(12):2716–36. Epub 2014/09/07. 10.1158/1055-9965.epi-14-0207. [DOI] [PMC free article] [PubMed]
- 8.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta neuropathologica. 2005;109(1):93–108. Epub 2005/02/03. 10.1007/s00401-005-0991-y. [DOI] [PubMed]
- 9.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatric radiology. 2006;36 Suppl 2(Suppl 2):121–5. Epub 2006/07/25. 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed]
- 10.Zhang C, Ostrom QT, Hansen HM, Gonzalez-Maya J, Hu D, Ziv E, Morimoto L, de Smith AJ, Muskens IS, Kline CN, Vaksman Z, Hakonarson H, Diskin SJ, Kruchko C, Barnholtz-Sloan JS, Ramaswamy V, Ali-Osman F, Bondy ML, Taylor MD, Metayer C, Wiemels JL, Walsh KM. European genetic ancestry associated with risk of childhood ependymoma. Neuro-oncology. 2020;22(11):1637–46. Epub 2020/07/02. 10.1093/neuonc/noaa130. [DOI] [PMC free article] [PubMed]
- 11.• Zhang C, Ostrom QT, Semmes EC, Ramaswamy V, Hansen HM, Morimoto L, de Smith AJ, Pekmezci M, Vaksman Z, Hakonarson H, Diskin SJ, Metayer C, Taylor MD, Wiemels JL, Bondy ML, Walsh KM. Genetic predisposition to longer telomere length and risk of childhood, adolescent and adult-onset ependymoma. Acta neuropathologica communications. 2020;8(1):173. Epub 2020/10/30. 10.1186/s40478-020-01038-w. [DOI] [PMC free article] [PubMed]
- 12.Georgakis MK, Kalogirou EI, Liaskas A, Karalexi MA, Papathoma P, Ladopoulou K, Kantzanou M, Tsivgoulis G, Petridou ET. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. European journal of cancer (Oxford, England : 1990). 2017;75:117–31. Epub 2017/02/22. 10.1016/j.ejca.2016.12.033. [DOI] [PubMed]
- 13.Dahlhaus A, Prengel P, Spector L, Pieper D. Birth weight and subsequent risk of childhood primary brain tumors: an updated meta-analysis. Pediatric blood & cancer. 2017;64(5). Epub 2016/11/03. 10.1002/pbc.26299. [DOI] [PubMed]
- 14.Bailey HD, Rios P, Lacour B, Guerrini-Rousseau L, Bertozzi AI, Leblond P, Faure-Conter C, Pellier I, Freycon C, Michon J, Puget S, Ducassou S, Orsi L, Clavel J. Factors related to pregnancy and birth and the risk of childhood brain tumours: The ESTELLE and ESCALE studies (SFCE, France). International journal of cancer. 2017;140(8):1757–69. Epub 2017/01/06. 10.1002/ijc.30597. [DOI] [PubMed]
- 15.Johnson KJ, Lee JM, Ahsan K, Padda H, Feng Q, Partap S, Fowler SA, Druley TE. Pediatric cancer risk in association with birth defects: a systematic review. PloS one. 2017;12(7):e0181246. Epub 2017/07/28. 10.1371/journal.pone.0181246. [DOI] [PMC free article] [PubMed]
- 16.Mili F, Khoury MJ, Flanders WD, Greenberg RS. Risk of childhood cancer for infants with birth defects. I. A record-linkage study, Atlanta, Georgia, 1968–1988. American journal of epidemiology. 1993;137(6):629–38. Epub 1993/03/15. 10.1093/oxfordjournals.aje.a116720. [DOI] [PubMed]
- 17.Lupo PJ, Schraw JM, Desrosiers TA, Nembhard WN, Langlois PH, Canfield MA, Copeland G, Meyer RE, Brown AL, Chambers TM, Sok P, Danysh HE, Carozza SE, Sisoudiya SD, Hilsenbeck SG, Janitz AE, Oster ME, Scheuerle AE, Schiffman JD, Luo C, Mian A, Mueller BA, Huff CD, Rasmussen SA, Scheurer ME, Plon SE. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA oncology. 2019;5(8):1150–8. Epub 2019/06/21. 10.1001/jamaoncol.2019.1215. [DOI] [PMC free article] [PubMed]
- 18.Botto LD, Flood T, Little J, Fluchel MN, Krikov S, Feldkamp ML, Wu Y, Goedken R, Puzhankara S, Romitti PA. Cancer risk in children and adolescents with birth defects: a population-based cohort study. PloS one. 2013;8(7):e69077. Epub 2013/07/23. 10.1371/journal.pone.0069077. [DOI] [PMC free article] [PubMed]
- 19.Janitz AE, Neas BR, Campbell JE, Pate AE, Stoner JA, Magzamen SL, Peck JD. Childhood cancer in children with congenital anomalies in Oklahoma, 1997 to 2009. Birth defects research Part A, Clinical and molecular teratology. 2016;106(7):633–42. Epub 2016/03/08. 10.1002/bdra.23494. [DOI] [PMC free article] [PubMed]
- 20.Fisher PG, Reynolds P, Von Behren J, Carmichael SL, Rasmussen SA, Shaw GM. Cancer in children with nonchromosomal birth defects. The Journal of pediatrics. 2012;160(6):978–83. Epub 2012/01/17. 10.1016/j.jpeds.2011.12.006. [DOI] [PMC free article] [PubMed]
- 21.Bjørge T, Cnattingius S, Lie RT, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(3):500–6. Epub 2008/02/26. 10.1158/1055-9965.epi-07-2630. [DOI] [PubMed]
- 22.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. doi: 10.1667/RR3329. [DOI] [PubMed] [Google Scholar]
- 23.Amirian ES, Zhou R, Wrensch MR, Olson SH, Scheurer ME, Il'yasova D, Lachance D, Armstrong GN, McCoy LS, Lau CC, Claus EB, Barnholtz-Sloan JS, Schildkraut J, Ali-Osman F, Sadetzki S, Johansen C, Houlston RS, Jenkins RB, Bernstein JL, Merrell RT, Davis FG, Lai R, Shete S, Amos CI, Melin BS, Bondy ML. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the Glioma International Case-Control Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(2):282–90. Epub 2016/02/26. 10.1158/1055-9965.epi-15-0847. [DOI] [PMC free article] [PubMed]
- 24.Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Islami F, Galichet L, Straif K. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–6. Epub 2011/08/17. 10.1016/s1470-2045(11)70147-4. [DOI] [PubMed]
- 25.Turner MC, Benke G, Bowman JD, Figuerola J, Fleming S, Hours M, Kincl L, Krewski D, McLean D, Parent ME, Richardson L, Sadetzki S, Schlaefer K, Schlehofer B, Schüz J, Siemiatycki J, van Tongeren M, Cardis E. Occupational exposure to extremely low-frequency magnetic fields and brain tumor risks in the INTEROCC study. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1863–72. Epub 2014/06/18. 10.1158/1055-9965.Epi-14-0102. [DOI] [PMC free article] [PubMed]
- 26.Carles C, Esquirol Y, Turuban M, Piel C, Migault L, Pouchieu C, Bouvier G, Fabbro-Peray P, Lebailly P, Baldi I. Residential proximity to power lines and risk of brain tumor in the general population. Environ Res. 2020;185:109473. Epub 2020/04/12. 10.1016/j.envres.2020.109473. [DOI] [PubMed]
- 27.Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R, Wiencke J, Neuhaus J. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. American journal of epidemiology. 1997;145(7):581–93. Epub 1997/04/01. 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed]
- 28.•• Melin BS, Barnholtz-Sloan JS, Wrensch MR, Johansen C, Il'yasova D, Kinnersley B, Ostrom QT, Labreche K, Chen Y, Armstrong G, Liu Y, Eckel-Passow JE, Decker PA, Labussiere M, Idbaih A, Hoang-Xuan K, Di Stefano AL, Mokhtari K, Delattre JY, Broderick P, Galan P, Gousias K, Schramm J, Schoemaker MJ, Fleming SJ, Herms S, Heilmann S, Nothen MM, Wichmann HE, Schreiber S, Swerdlow A, Lathrop M, Simon M, Sanson M, Andersson U, Rajaraman P, Chanock S, Linet M, Wang Z, Yeager M, Wiencke JK, Hansen H, McCoy L, Rice T, Kosel ML, Sicotte H, Amos CI, Bernstein JL, Davis F, Lachance D, Lau C, Merrell RT, Shildkraut J, Ali-Osman F, Sadetzki S, Scheurer M, Shete S, Lai RK, Claus EB, Olson SH, Jenkins RB, Houlston RS, Bondy ML. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nature genetics. 2017;49(5):789–94. Epub 2017/03/28. 10.1038/ng.3823. [DOI] [PMC free article] [PubMed]
- 29.• Labreche K, Kinnersley B, Berzero G, Di Stefano AL, Rahimian A, Detrait I, Marie Y, Grenier-Boley B, Hoang-Xuan K, Delattre JY, Idbaih A, Houlston RS, Sanson M. Diffuse gliomas classified by 1p/19q co-deletion, TERT promoter and IDH mutation status are associated with specific genetic risk loci. Acta neuropathologica. 2018;135(5):743–55. Epub 2018/02/21. 10.1007/s00401-018-1825-z. PubMed PMID: 29460007; PMCID: PMC5904227 NF S96 900), and received the authorization for genetic analysis from ethical committee (CPP Ile de France VI, ref A39II and 2013–1962), and French Ministry for research (AC 2013–1962). Conflict of interest: The authors declare no conflict of interest. [DOI] [PMC free article] [PubMed]
- 30.Li G, Jin T, Liang H, Zhang Z, He S, Tu Y, Yang H, Geng T, Cui G, Chen C, Gao G. RTEL1 tagging SNPs and haplotypes were associated with glioma development. Diagnostic pathology. 2013;8:83. Epub 2013/05/21. 10.1186/1746-1596-8-83. [DOI] [PMC free article] [PubMed]
- 31.Liu HB, Peng YP, Dou CW, Su XL, Gao NK, Tian FM, Bai J. Comprehensive study on associations between nine SNPs and glioma risk. Asian Pacific journal of cancer prevention : APJCP. 2012;13(10):4905–8. Epub 2012/12/19. doi: 10.7314/apjcp.2012.13.10.4905. [DOI] [PubMed]
- 32.• Chen H, Chen G, Li G, Zhang S, Chen H, Chen Y, Duggan D, Hu Z, Chen J, Zhao Y, Zhao Y, Huang H, Zheng SL, Trent JM, Yu L, Jiang D, Mo Z, Wang H, Mou Y, Jiang T, Mao Y, Xu J, Lu D. Two novel genetic variants in the STK38L and RAB27A genes are associated with glioma susceptibility. International journal of cancer. 2019;145(9):2372–82. Epub 2019/02/05. 10.1002/ijc.32179. [DOI] [PubMed]
- 33.• Claus EB, Cornish AJ, Broderick P, Schildkraut JM, Dobbins SE, Holroyd A, Calvocoressi L, Lu L, Hansen HM, Smirnov I, Walsh KM, Schramm J, Hoffmann P, Nöthen MM, Jöckel KH, Swerdlow A, Larsen SB, Johansen C, Simon M, Bondy M, Wrensch M, Houlston RS, Wiemels JL. Genome-wide association analysis identifies a meningioma risk locus at 11p15.5. Neuro-oncology. 2018;20(11):1485–93. Epub 2018/05/16. 10.1093/neuonc/noy077. [DOI] [PMC free article] [PubMed]
- 34.• Labreche K, Daniau M, Sud A, Law PJ, Royer-Perron L, Holroyd A, Broderick P, Went M, Benazra M, Ahle G, Soubeyran P, Taillandier L, Chinot OL, Casasnovas O, Bay JO, Jardin F, Oberic L, Fabbro M, Damaj G, Brion A, Mokhtari K, Philippe C, Sanson M, Houillier C, Soussain C, Hoang-Xuan K, Houlston RS, Alentorn A. A genome-wide association study identifies susceptibility loci for primary central nervous system lymphoma at 6p25.3 and 3p22.1: a LOC Network study. Neuro-oncology. 2019;21(8):1039–48. Epub 2019/05/19. 10.1093/neuonc/noz088. [DOI] [PMC free article] [PubMed]
- 35.Ye Z, Li Z, Wang Y, Mao Y, Shen M, Zhang Q, Li S, Zhou L, Shou X, Chen J, Song Z, Ma Z, Zhang Z, Li Y, Ye H, Huang C, Wang T, He W, Zhang Y, Xie R, Qiao N, Qiu H, Huang S, Wang M, Shen J, Wen Z, Li W, Liu K, Zhou J, Wang L, Ji J, Wang Y, Chen H, Cheng H, Shi Z, Zhu Y, Geng D, Yao Z, Tang W, Lu B, Pan L, Zhang Y, Bao W, Wu J, Zheng K, Shi Y, Zhao Y. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nature genetics. 2015;47(7):793–7. Epub 2015/06/02. 10.1038/ng.3322. [DOI] [PubMed]
- 36.Walsh KM, Codd V, Rice T, Nelson CP, Smirnov IV, McCoy LS, Hansen HM, Elhauge E, Ojha J, Francis SS, Madsen NR, Bracci PM, Pico AR, Molinaro AM, Tihan T, Berger MS, Chang SM, Prados MD, Jenkins RB, Wiemels JL, Samani NJ, Wiencke JK, Wrensch MR. Longer genotypically-estimated leukocyte telomere length is associated with increased adult glioma risk. Oncotarget. 2015;6(40):42468–77. Epub 2015/12/10. 10.18632/oncotarget.6468. PubMed PMID: 26646793; PMCID: PMC4767445. [DOI] [PMC free article] [PubMed]
- 37.Muskens IS, Hansen HM, Smirnov IV, Molinaro AM, Bondy ML, Schildkraut JM, Wrensch M, Wiemels JL, Claus EB. Longer genotypically-estimated leukocyte telomere length is associated with increased meningioma risk. Journal of neuro-oncology. 2019;142(3):479–87. Epub 2019/02/24. 10.1007/s11060-019-03119-w. [DOI] [PMC free article] [PubMed]
- 38.Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang Q, Lichtenberg T, Hu J, Zhang J, Zheng S, Verhaak RG. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nature genetics. 2017;49(3):349–57. Epub 2017/01/31. 10.1038/ng.3781. [DOI] [PMC free article] [PubMed]
- 39.Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro-oncology. 2017;19(11):1553–64. Epub 2017/05/10. 10.1093/neuonc/nox091. [DOI] [PMC free article] [PubMed]
- 40.• Ostrom QT, Egan KM, Nabors LB, Gerke T, Thompson RC, Olson JJ, LaRocca R, Chowdhary S, Eckel-Passow JE, Armstrong G, Wiencke JK, Bernstein JL, Claus EB, Il'yasova D, Johansen C, Lachance DH, Lai RK, Merrell RT, Olson SH, Sadetzki S, Schildkraut JM, Shete S, Houlston RS, Jenkins RB, Wrensch MR, Melin B, Amos CI, Huse JT, Barnholtz-Sloan JS, Bondy ML. Glioma risk associated with extent of estimated European genetic ancestry in African Americans and Hispanics. International journal of cancer. 2020;146(3):739–48. Epub 2019/04/10. 10.1002/ijc.32318. [DOI] [PMC free article] [PubMed]
- 41.Rollison DE, Utaipat U, Ryschkewitsch C, Hou J, Goldthwaite P, Daniel R, Helzlsouer KJ, Burger PC, Shah KV, Major EO. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. International journal of cancer. 2005;113(5):769–74. Epub 2004/10/23. 10.1002/ijc.20641. [DOI] [PubMed]
- 42.Limam S, Missaoui N, Bdioui A, Yacoubi MT, Krifa H, Mokni M, Selmi B. Investigation of simian virus 40 (SV40) and human JC, BK, MC, KI, and WU polyomaviruses in glioma. Journal of neurovirology. 2020;26(3):347–57. Epub 2020/03/04. 10.1007/s13365-020-00833-4. [DOI] [PubMed]
- 43.Neves AM, Thompson G, Carvalheira J, Trindade JC, Rueff J, Caetano JM, Casey JW, Hermouet S. Detection and quantitative analysis of human herpesvirus in pilocytic astrocytoma. Brain research. 2008;1221:108–14. Epub 2008/06/21. 10.1016/j.brainres.2008.05.009. [DOI] [PubMed]
- 44.Chauvin C, Suh M, Remy C, Benabid AL. Failure to detect viral genomic sequences of three viruses (herpes simplex, simian virus 40 and adenovirus) in human and rat brain tumors. Italian journal of neurological sciences. 1990;11(4):347–57. Epub 1990/08/01. 10.1007/bf02335937. [DOI] [PubMed]
- 45.Garcia-Martinez A, Alenda C, Irles E, Ochoa E, Quintanar T, Rodriguez-Lescure A, Soto JL, Barbera VM. Lack of cytomegalovirus detection in human glioma. Virology journal. 2017;14(1):216. Epub 2017/11/09. 10.1186/s12985-017-0885-3. [DOI] [PMC free article] [PubMed]
- 46.Holdhoff M, Guner G, Rodriguez FJ, Hicks JL, Zheng Q, Forman MS, Ye X, Grossman SA, Meeker AK, Heaphy CM, Eberhart CG, De Marzo AM, Arav-Boger R. Absence of cytomegalovirus in glioblastoma and other high-grade gliomas by real-time PCR, immunohistochemistry, and in situ hybridization. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(12):3150–7. Epub 2016/12/31. 10.1158/1078-0432.ccr-16-1490. [DOI] [PMC free article] [PubMed]
- 47.Strong MJ, Blanchard Et, Lin Z, Morris CA, Baddoo M, Taylor CM, Ware ML, Flemington EK. A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus-tumor association. Acta neuropathologica communications. 2016;4(1):71. Epub 2016/07/13. 10.1186/s40478-016-0338-z. [DOI] [PMC free article] [PubMed]
- 48.Foster H, Piper K, DePledge L, Li HF, Scanlan J, Jae-Guen Y, Boeckh M, Cobbs C. Human cytomegalovirus seropositivity is associated with decreased survival in glioblastoma patients. Neuro-oncology advances. 2019;1(1):vdz020. Epub 2020/07/10. 10.1093/noajnl/vdz020. [DOI] [PMC free article] [PubMed]
- 49.Stragliotto G, Pantalone MR, Rahbar A, Söderberg-Nauclér C. Valganciclovir as add-on to standard therapy in secondary glioblastoma. Microorganisms. 2020;8(10). Epub 2020/09/30. 10.3390/microorganisms8101471. [DOI] [PMC free article] [PubMed]
- 50.Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, 2nd, Healy P, McLendon RE, Friedman AH, Friedman HS, Bigner D, Vlahovic G, Mitchell DA, Sampson JH. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(8):1898–909. Epub 2017/04/16. 10.1158/1078-0432.ccr-16-2057. PubMed PMID: 28411277; PMCID: PMC5559300. [DOI] [PMC free article] [PubMed]
- 51.• Herbein G. The human cytomegalovirus, from oncomodulation to oncogenesis. Viruses. 2018;10(8). Epub 2018/08/08. 10.3390/v10080408. [DOI] [PMC free article] [PubMed]
- 52.Hodge JM, Coghill AE, Kim Y, Bender N, Smith-Warner SA, Gapstur S, Teras LR, Grimsrud TK, Waterboer T, Egan KM. Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. International journal of cancer. 2021. Epub 2021/01/12. 10.1002/ijc.33443. [DOI] [PubMed]
- 53.Pergam SA, Limaye AP. Varicella zoster virus (VZV) in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9 Suppl 4(Suppl 4):S108–15. Epub 2010/01/28. 10.1111/j.1600-6143.2009.02901.x. [DOI] [PMC free article] [PubMed]
- 54.Wrensch M, Weinberg A, Wiencke J, Miike R, Sison J, Wiemels J, Barger G, DeLorenze G, Aldape K, Kelsey K. History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. American journal of epidemiology. 2005;161(10):929–38. Epub 2005/05/05. 10.1093/aje/kwi119. [DOI] [PubMed]
- 55.Lee ST, Bracci P, Zhou M, Rice T, Wiencke J, Wrensch M, Wiemels J. Interaction of allergy history and antibodies to specific varicella-zoster virus proteins on glioma risk. International journal of cancer. 2014;134(9):2199–210. Epub 2013/10/16. 10.1002/ijc.28535. [DOI] [PMC free article] [PubMed]
- 56.Amirian ES, Scheurer ME, Zhou R, Wrensch MR, Armstrong GN, Lachance D, Olson SH, Lau CC, Claus EB, Barnholtz-Sloan JS, Il'yasova D, Schildkraut J, Ali-Osman F, Sadetzki S, Jenkins RB, Bernstein JL, Merrell RT, Davis FG, Lai R, Shete S, Amos CI, Melin BS, Bondy ML. History of chickenpox in glioma risk: a report from the glioma international case-control study (GICC). Cancer medicine. 2016;5(6):1352–8. Epub 2016/03/15. 10.1002/cam4.682. [DOI] [PMC free article] [PubMed]
- 57.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. Journal of the National Cancer Institute. 2007;99(20):1544–50. Epub 2007/10/11. 10.1093/jnci/djm170. [DOI] [PubMed]
- 58.Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, Miike R, Barger G, Wrensch M. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer research. 2004;64(22):8468–73. Epub 2004/11/19. 10.1158/0008-5472.can-04-1706. [DOI] [PubMed]
- 59.Schwartzbaum J, Ding B, Johannesen TB, Osnes LT, Karavodin L, Ahlbom A, Feychting M, Grimsrud TK. Association between prediagnostic IgE levels and risk of glioma. Journal of the National Cancer Institute. 2012;104(16):1251–9. Epub 2012/08/03. 10.1093/jnci/djs315. [DOI] [PMC free article] [PubMed]
- 60.Schoemaker MJ, Robertson L, Wigertz A, Jones ME, Hosking FJ, Feychting M, Lönn S, McKinney PA, Hepworth SJ, Muir KR, Auvinen A, Salminen T, Kiuru A, Johansen C, Houlston RS, Swerdlow AJ. Interaction between 5 genetic variants and allergy in glioma risk. American journal of epidemiology. 2010;171(11):1165–73. Epub 2010/05/14. 10.1093/aje/kwq075. [DOI] [PubMed]
- 61.Backes DM, Siddiq A, Cox DG, Calboli FC, Gaziano JM, Ma J, Stampfer M, Hunter DJ, Camargo CA, Michaud DS. Single-nucleotide polymorphisms of allergy-related genes and risk of adult glioma. Journal of neuro-oncology. 2013;113(2):229–38. Epub 2013/03/26. 10.1007/s11060-013-1122-6. [DOI] [PMC free article] [PubMed]
- 62.Disney-Hogg L, Cornish AJ, Sud A, Law PJ, Kinnersley B, Jacobs DI, Ostrom QT, Labreche K, Eckel-Passow JE, Armstrong GN, Claus EB, Il'yasova D, Schildkraut J, Barnholtz-Sloan JS, Olson SH, Bernstein JL, Lai RK, Schoemaker MJ, Simon M, Hoffmann P, Nöthen MM, Jöckel KH, Chanock S, Rajaraman P, Johansen C, Jenkins RB, Melin BS, Wrensch MR, Sanson M, Bondy ML, Houlston RS. Impact of atopy on risk of glioma: a Mendelian randomisation study. BMC medicine. 2018;16(1):42. Epub 2018/03/16. 10.1186/s12916-018-1027-5. [DOI] [PMC free article] [PubMed]
- 63.Besson C, Amiel C, Le-Pendeven C, Plancoulaine S, Bonnardel C, Ranque B, Abbed K, Brice P, Ferme C, Carde P, Hermine O, Raphael M, Bresson JL, Nicolas JC, Gessain A, Dethe G, Abel L. Strong correlations of anti-viral capsid antigen antibody levels in first-degree relatives from families with Epstein-Barr virus-related lymphomas. J Infect Dis. 2009;199(8):1121–7. Epub 2009/03/17. 10.1086/597424. [DOI] [PubMed]
- 64.Willemsen G, van Beijsterveldt TC, van Baal CG, Postma D, Boomsma DI. Heritability of self-reported asthma and allergy: a study in adult Dutch twins, siblings and parents. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2008;11(2):132–42. Epub 2008/03/26. 10.1375/twin.11.2.132. [DOI] [PubMed]
- 65.Fagnani C, Annesi-Maesano I, Brescianini S, D'Ippolito C, Medda E, Nisticò L, Patriarca V, Rotondi D, Toccaceli V, Stazi MA. Heritability and shared genetic effects of asthma and hay fever: an Italian study of young twins. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2008;11(2):121–31. Epub 2008/03/26. 10.1375/twin.11.2.121. [DOI] [PubMed]
- 66.Waage J, Standl M, Curtin JA, Jessen LE, Thorsen J, Tian C, Schoettler N, Flores C, Abdellaoui A, Ahluwalia TS, Alves AC, Amaral AFS, Antó JM, Arnold A, Barreto-Luis A, Baurecht H, van Beijsterveldt CEM, Bleecker ER, Bonàs-Guarch S, Boomsma DI, Brix S, Bunyavanich S, Burchard EG, Chen Z, Curjuric I, Custovic A, den Dekker HT, Dharmage SC, Dmitrieva J, Duijts L, Ege MJ, Gauderman WJ, Georges M, Gieger C, Gilliland F, Granell R, Gui H, Hansen T, Heinrich J, Henderson J, Hernandez-Pacheco N, Holt P, Imboden M, Jaddoe VWV, Jarvelin MR, Jarvis DL, Jensen KK, Jónsdóttir I, Kabesch M, Kaprio J, Kumar A, Lee YA, Levin AM, Li X, Lorenzo-Diaz F, Melén E, Mercader JM, Meyers DA, Myers R, Nicolae DL, Nohr EA, Palviainen T, Paternoster L, Pennell CE, Pershagen G, Pino-Yanes M, Probst-Hensch NM, Rüschendorf F, Simpson A, Stefansson K, Sunyer J, Sveinbjornsson G, Thiering E, Thompson PJ, Torrent M, Torrents D, Tung JY, Wang CA, Weidinger S, Weiss S, Willemsen G, Williams LK, Ober C, Hinds DA, Ferreira MA, Bisgaard H, Strachan DP, Bønnelykke K. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nature genetics. 2018;50(8):1072–80. Epub 2018/07/18. 10.1038/s41588-018-0157-1. [DOI] [PMC free article] [PubMed]
- 67.Tang J, Shao W, Dorak MT, Li Y, Miike R, Lobashevsky E, Wiencke JK, Wrensch M, Kaslow RA, Cobbs CS. Positive and negative associations of human leukocyte antigen variants with the onset and prognosis of adult glioblastoma multiforme. Cancer Epidemiol Biomarkers Prev. 2005;14(8):2040–4. Epub 2005/08/17. 10.1158/1055-9965.EPI-05-0136. [DOI] [PubMed]
- 68.Song W, Ruder AM, Hu L, Li Y, Ni R, Shao W, Kaslow RA, Butler M, Tang J. Genetic epidemiology of glioblastoma multiforme: confirmatory and new findings from analyses of human leukocyte antigen alleles and motifs. PLoS One. 2009;4(9):e7157. Epub 2009/09/24. 10.1371/journal.pone.0007157. [DOI] [PMC free article] [PubMed]
- 69.Zhang C, de Smith AJ, Smirnov IV, Wiencke JK, Wiemels JL, Witte JS, Walsh KM. Non-additive and epistatic effects of HLA polymorphisms contributing to risk of adult glioma. J Neurooncol. 2017;135(2):237–44. Epub 2017/07/20. 10.1007/s11060-017-2569-7. [DOI] [PMC free article] [PubMed]
- 70.Kachuri L, Francis SS, Morrison ML, Wendt GA, Bosse Y, Cavazos TB, Rashkin SR, Ziv E, Witte JS. The landscape of host genetic factors involved in immune response to common viral infections. Genome Med. 2020;12(1):93. Epub 2020/10/29. 10.1186/s13073-020-00790-x. [DOI] [PMC free article] [PubMed]
- 71.Hammer C, Begemann M, McLaren PJ, Bartha I, Michel A, Klose B, Schmitt C, Waterboer T, Pawlita M, Schulz TF, Ehrenreich H, Fellay J. Amino acid variation in HLA class II Proteins is a major determinant of humoral response to common viruses. Am J Hum Genet. 2015;97(5):738–43. Epub 2015/10/13. 10.1016/j.ajhg.2015.09.008. [DOI] [PMC free article] [PubMed]
- 72.• Ostrom QT, Edelson J, Byun J, Han Y, Kinnersley B, Melin B, Houlston RS, Monje M, Walsh KM, Amos CI, Bondy ML. Partitioned glioma heritability shows subtype-specific enrichment in immune cells. Neuro-oncology. 2021. Epub 2021/03/21. 10.1093/neuonc/noab072. [DOI] [PMC free article] [PubMed]
- 73.Martinez-Lage M, Lynch TM, Bi Y, Cocito C, Way GP, Pal S, Haller J, Yan RE, Ziober A, Nguyen A, Kandpal M, O'Rourke DM, Greenfield JP, Greene CS, Davuluri RV, Dahmane N. Immune landscapes associated with different glioblastoma molecular subtypes. Acta neuropathologica communications. 2019;7(1):203. Epub 2019/12/10. 10.1186/s40478-019-0803-6. [DOI] [PMC free article] [PubMed]
- 74.• Dejaegher J, Solie L, Hunin Z, Sciot R, Capper D, Siewert C, Van Cauter S, Wilms G, van Loon J, Ectors N, Fieuws S, Pfister SM, Van Gool SW, De Vleeschouwer S. DNA methylation based glioblastoma subclassification is related to tumoral T-cell infiltration and patient survival. Neuro-oncology. 2021;23(2):240–50. Epub 2020/11/02. 10.1093/neuonc/noaa247. [DOI] [PMC free article] [PubMed]
- 75.Wiencke JK, Koestler DC, Salas LA, Wiemels JL, Roy RP, Hansen HM, Rice T, McCoy LS, Bracci PM, Molinaro AM, Kelsey KT, Wrensch MR, Christensen BC. Immunomethylomic approach to explore the blood neutrophil lymphocyte ratio (NLR) in glioma survival. Clinical epigenetics. 2017;9:10. Epub 2017/02/12. 10.1186/s13148-017-0316-8. [DOI] [PMC free article] [PubMed]
- 76.Demers PA, Vaughan TL, Schommer RR. Occupation, socioeconomic status, and brain tumor mortality: a death certificate-based case-control study. Journal of occupational medicine: official publication of the Industrial Medical Association. 1991;33(9):1001–1006. [PubMed] [Google Scholar]
- 77.Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PloS one. 2013;8(4):e60910. Epub 2013/04/16. 10.1371/journal.pone.0060910. [DOI] [PMC free article] [PubMed]
- 78.Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer causes & control : CCC. 2015;26(2):179–85. Epub 2014/11/26. 10.1007/s10552-014-0496-x. [DOI] [PubMed]
- 79.Khanolkar AR, Ljung R, Talbäck M, Brooke HL, Carlsson S, Mathiesen T, Feychting M. Socioeconomic position and the risk of brain tumour: a Swedish national population-based cohort study. Journal of epidemiology and community health. 2016;70(12):1222–8. Epub 2016/06/22. 10.1136/jech-2015-207002. [DOI] [PubMed]
- 80.•• Cote DJ, Ostrom QT, Gittleman H, Duncan KR, CreveCoeur TS, Kruchko C, Smith TR, Stampfer MJ, Barnholtz-Sloan JS. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer. 2019;125(19):3390–400. Epub 2019/06/18. 10.1002/cncr.32328. [DOI] [PMC free article] [PubMed]
- 81.• Francis SS, Wang R, Enders C, Prado I, Wiemels JL, Ma X, Metayer C. Socioeconomic status and childhood central nervous system tumors in California. Cancer causes & control : CCC. 2021;32(1):27–39. Epub 2020/10/29. 10.1007/s10552-020-01348-3. [DOI] [PubMed]
- 82.• Erdmann F, Hvidtfeldt UA, Sørensen M, Raaschou-Nielsen O. Socioeconomic differences in the risk of childhood central nervous system tumors in Denmark: a nationwide register-based case-control study. Cancer causes & control : CCC. 2020;31(10):915–29. Epub 2020/08/09. 10.1007/s10552-020-01332-x. [DOI] [PMC free article] [PubMed]
- 83.Strachan DP. Hay fever, hygiene, and household size. BMJ (Clinical research ed). 1989;299(6710):1259–60. Epub 1989/11/18. 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed]