Abstract
Keratin particles impregnated with amorolfine or clotrimazole in serial doubling dilutions (64 to 0.125 μg/ml) were used to evaluate the activities of these agents against 20 isolates each of Trichophyton mentagrophytes and Trichophyton rubrum in a yeast carbon broth medium incorporating Alamar Blue dye. The proposed MIC with keratin impregnation (MICK) is defined as the lowest concentration of an agent used to impregnate keratin particles that effects a fluorescence-based fungal growth quotient of 0.05 or less. The conventional colorimetric and visual MICs of amorolfine for the dermatophytes, ≤0.03 μg/ml for T. mentagrophytes and ≤0.063 μg/ml for T. rubrum, were approximately half of those of clotrimazole for the same isolates. The superiority of the MICKs of amorolfine for isolates of T. mentagrophytes (2.0 μg/ml; range, 0.5 to 8.0 μg/ml) and T. rubrum (4.0 μg/ml; range, 2.0 to 8.0 μg/ml) over those of clotrimazole (32 μg/ml [range, 8.0 to >64 μg/ml] and 64 μg/ml [range, 16 to >64 μg], respectively) may indicate the strong in vivo antidermatophytic activity of amorolfine as a topical agent. The new antidermatophytic susceptibility testing procedure has potential clinical utility for the in vitro screening of agents for use in the topical treatment of superficial mycoses.
Dermatophytoses, which constitute the majority of superficial fungal infections, are infections of keratinized tissues such as the stratum corneum, nail, and hair by dermatophytic fungi. Some agents with high levels of in vitro antidermatophytic activity show rather poor in vivo effects (5, 16). This difference in the in vivo and in vitro activities of some agents is due to the dependency of the in vivo action on the interaction of drug molecules with tissue components (4, 8, 14, 16). Thus, the efficacy of a topically applied antidermatophytic agent is influenced not only by its antifungal property but also by the ability of the drug molecules to penetrate the keratinized tissue (9).
The effect of the MIC of an agent as determined under in vitro conditions that approximate the situation in vivo would be a better predictor of in vivo efficacy. Merten and Lippold (9) estimated the relative therapeutic potencies of antimycotics against onychomycoses using an efficacy coefficient based on the rate of maximum flux through hoof membrane and an independently determined MIC. Polak (15), using keratin into which solubilized antimycotics were impregnated and placement of the keratin into agar wells, assessed the antifungal activities of agents by measuring the zones of inhibition surrounding the wells in cultures.
In this study, Alamar Blue, a dye that exhibits both fluorescence and colorimetric changes caused by cellular metabolic reduction (12, 18, 19), was used in a quantitative fluorometric assay to determine the activities of keratin-bound amorolfine and clotrimazole against isolates of Trichophyton mentagrophytes and Trichophyton rubrum.
MATERIALS AND METHODS
Antifungal agents.
Amorolfine powder was a gift from Kyorin Pharmaceutical Co. Ltd. (Tokyo, Japan), and clotrimazole powder was purchased from Sigma Chemical Co. (St. Louis, Mo.). Stock solutions (100 mg/ml) of the agents were prepared by dissolving the powders in 100% dimethyl sulfoxide. The stock solutions were frozen at −20°C until use.
Fungal isolates and inoculum preparation.
Twenty isolates each of T. mentagrophytes and T. rubrum were obtained from the culture collection of the Research Institute for Chemobiodynamics, Chiba University, Chiba, Japan. Homogeneous suspensions of conidia and hyphal fragments in 0.85% saline were prepared from 7-day cultures on potato dextrose agar slants (Difco Laboratories, Detroit, Mich.). The optical densities of the suspensions were read at 530 nm and were adjusted to 0.15 to 0.17 to yield 0.6 × 106 to 1.4 × 106 and 0.7 × 106 to 1.2 × 106 CFU of T. mentagrophytes and T. rubrum per ml, respectively (3).
Broth microdilution antifungal susceptibility testing.
The MICs of the antidermatophytic agents were determined by modifying the broth microdilution antifungal susceptibility test procedure recommended by a subcommittee of the National Committee for Clinical Laboratory Standards for antifungal susceptibility testing of filamentous fungi (3). Briefly, the test was performed with RPMI 1640 medium supplemented with l-glutamine but without sodium bicarbonate (Life Technologies, Grand Island, N.Y.). The medium was buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma).
A series of doubling dilutions (128 to 0.002 μg/ml) of the stock solutions of the agents were prepared in 2× RPMI 1640 medium (20.8 g/liter). One hundred microliters of each dilution was then mixed with an equal volume of a 1:50 dilution of the fungal suspension (approximately 2 × 104 CFU/ml) in 20% (vol/vol) Alamar Blue dye (Sensititre/Alamar, Westlake, Ohio) in sterile distilled water. A final volume of 200 μl of the reaction mixture contained 104 CFU of fungus per ml and the agents at concentrations ranging from 64 to 0.001 μg/ml. A drug-free medium was inoculated and was used as a growth control. The blank medium was free of drug and fungus.
The microdilution plates were incubated at 30°C for 96 h, and the endpoints were read as the MICVIS and MICCOL. The MICVIS is defined as the lowest concentration of an agent at which there is no visually observable growth in broth, and the MICCOL is defined as the lowest concentration of an agent that prevents the development of a red color in broth. Fungal growth activity was also measured by fluorescence determination at excitation and emission wavelengths of 544 and 590 nm, respectively, by using a microplate fluorescence reader (Fluoroskan Ascent Spectrofluorometer; Labsystems, Helsinki, Finland). Growth was expressed as a quotient calculated in the following manner: (fluorescence intensity in dilution broth − fluorescence intensity in blank medium)/(fluorescence intensity in growth control broth − fluorescence intensity in blank medium).
Keratin impregnation antidermatophytic susceptibility test.
A quantitative susceptibility test method based on the keratin-penetrating powers of agents was developed by using amorolfine and clotrimazole as the test drugs against 20 isolates each of T. mentagrophytes and T. rubrum.
Keratin particles were prepared as described by Negi et al. (10). Briefly, scrapings of the stratum corneum from healthy human soles were cut into pieces, suspended in water, and homogenized with Polytron (Brinkmann, Lucerne, Switzerland). The keratin particles, which were repeatedly washed in distilled water until the optical density of the wash solution was less than 0.01 at 280 nm, were used after lyophilization. One hundred milligrams of the particles was suspended in 1.0 ml of a series of doubling dilutions (64 to 0.125 μg/ml) of the stock solutions of amorolfine or clotrimazole in water, and the suspensions were kept for 1 h at 32°C to allow penetration of the keratin by the drug molecules. The keratin particles were then precipitated, washed twice in 1 ml of distilled water, and dried in vacuo. The keratin was dispensed in 5-mg amounts into wells of a flat-bottom and lidded 24-well plate (Iwaki Glass, Tokyo, Japan) and were sterilized overnight in ethylene oxide gas at 37°C.
Four hundred microliters of filter-sterilized yeast carbon base medium (14.625 g/liter; Difco Laboratories) and 50 μl each of fungal suspension (approximately 106 CFU/ml) and Alamar Blue indicator were added to each well. The final volume of the 500-μl reaction mixture contained approximately 104 CFU of fungus per ml. Broths incorporating drug-free keratin served as growth controls. Blanks were inoculated with 50 μl of sterile 0.85% saline in place of the fungal suspension. The plates were incubated at 30°C for 96 h, during which time the cultures were agitated continually. One hundred microliters of culture medium was then transferred to a 96-well microtiter plate (Becton Dickinson and Co., Lincoln Park, N.J.), and the fluorescence was measured as described above. Fungal growth was expressed as a quotient: (fluorescence intensity in broth containing drug-treated keratin particles − fluorescence intensity in blank medium)/(fluorescence intensity in broth containing drug-free keratin particles − fluorescence intensity in blank medium). The MIC with keratin impregnation (MICK) is defined as the lowest concentration of an agent used to impregnate keratin particles that effects a fluorescence-based fungal growth quotient of 0.05 or less in assay broth.
In the preliminary studies, the effect on the fungal growth quotient of varying the impregnation time of the keratin particles in the agent solution, the fungal inoculum size, and the cultural incubation period was investigated by using keratin particles impregnated in 4 μg of amorolfine or clotrimazole per ml. An isolate of T. mentagrophytes (IFM 45794) was used as the test fungus. The time of impregnation of the keratin in the agents was set at 1 h, the inoculum size was set at 104 CFU/ml, and the incubation period was set at 96 h. When any one of these parameters was varied, the other two parameters were fixed at the following settings: keratin drug impregnation periods of 10, 60, and 120 min; fungal inoculum sizes of 103, 104, and 105 CFU/ml; and cultural incubation times of 72, 96, and 120 h.
RESULTS
Determination of MICCOLs and MICVISs.
The MICCOLs and MICVISs of amorolfine and clotrimazole for the isolates of T. mentagrophytes and T. rubrum are presented in Table 1. Intraspecific variations within narrow ranges of drug concentrations were observed in the MICCOLs and MICVISs of both agents. The MICCOL was generally lower than the corresponding MICVIS by 1 to 3 dilutions for each drug-fungus pair. The MICCOLs and MICVISs of amorolfine were lower than those of clotrimazole for the same test isolates.
TABLE 1.
MICs and MICKs for amorolfine and clotrimazole for 20 isolates each of T. mentagrophytes and T. rubrum
| Fungus | Drug | MICVIS (μg/ml)
|
MICCOL (μg/ml)
|
MICK (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC range | Modal MICVISa | MICVIS90b | MIC range | Modal MICCOL | MICCOL90c | MIC range | Modal MICK | MICK90d | ||
| T. mentagrophytes | Amorolfine | 0.004–0.063 | 0.030 | 0.063 | 0.002–0.030 | 0.015 | 0.015 | 0.5–8.0 | 2.0 | 4.0 |
| Clotrimazole | 0.030–0.125 | 0.063 | 0.125 | 0.008–0.063 | 0.03 | 0.063 | 8.0–>64 | 32.0 | >64 | |
| T. rubrum | Amorolfine | 0.004–0.063 | 0.015 | 0.063 | 0.001–0.030 | 0.004 | 0.015 | 2.0–8.0 | 4.0 | 8.0 |
| Clotrimazole | 0.015–0.125 | 0.030 | 0.125 | 0.002–0.063 | 0.030 | 0.030 | 16.0–>64 | 64 | >64 | |
The most frequent MIC among the isolates.
MICVIS90, MICVIS at which 90% of the isolates are inhibited.
MICCOL90, MICCOL at which 90% of the isolates are inhibited.
MICK90, MICK at which 90% of isolates are inhibited.
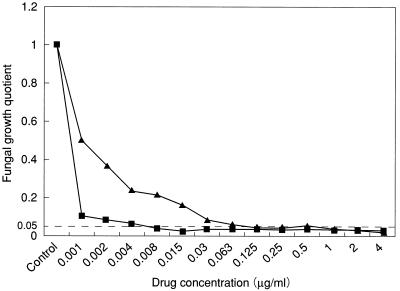
The fungal growth quotients of the MICVISs by the broth dilution method were within the range of 0.030 to 0.049 and 0.028 to 0.042 for amorolfine and clotrimazole, respectively, indicating a more than 95% reduction in fungal growth activity relative to that in the growth control broth. On this basis, a fungal growth inhibition quotient of 0.05 was adopted as the inhibition threshold in the subsequent keratin impregnation antidermatophytic susceptibility test. Figure 1 shows the curves relating the fungal growth quotients for an isolate of T. mentagrophytes (IFM 45828) to the corresponding drug concentrations. The dotted line, indicating the 0.05 threshold of inhibition, intersects the curves at the MICVISs of amorolfine and clotrimazole, 0.008 and 0.125 μg/ml, respectively.
FIG. 1.
Curves relating the fluorescence-based fungal growth quotient for an isolate of T. mentagrophytes (IFM 45828) to concentrations of amorolfine (■) and clotrimazole (▴) in the conventional broth microdilution antifungal susceptibility test. The dotted line indicates the 0.05 fungal growth quotient inhibition threshold.
Determination of MICK.
Preliminary studies showed that the growth quotient of T. mentagrophytes IFM 45794 in cultures with amorolfine-impregnated keratin particles decreased in correlation to an increase in the keratin impregnation period (0.071, 0.047, and 0.042 for 10, 60, and 120 min, respectively), the fungal inoculum size (0.037, 0.028, and 0.019 for 103, 104, and 105 CFU/ml, respectively), and the incubation time (0.046, 0.013, and 0.004 for 72, 96, and 120 h, respectively). The data for clotrimazole are not shown. The MICKs of the agents, derived from the growth quotients under the conditions of 1 h of keratin impregnation, an inoculum size of 104 CFU/ml, and a 96-h incubation period are presented in Table 1. The most frequent MICKs of amorolfine (modal MICK) for isolates of T. mentagrophytes and T. rubrum (2.0 and 4.0 μg/ml, respectively) were considerably lower than the corresponding modal MICKs of clotrimazole (32 and 64 μg/ml, respectively) (Table 1). The MICKs of clotrimazole for two and five isolates of T. mentagrophytes and T. rubrum, respectively, were higher than 64 μg/ml, the maximum concentration of the agents used for keratin impregnation.
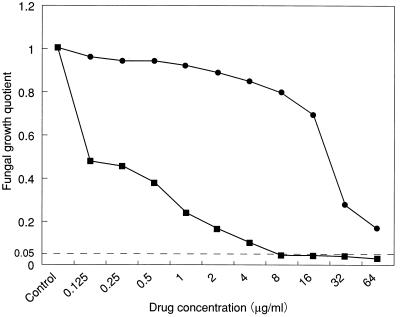
Figure 2 shows the curves relating the fungal growth quotients of an isolate of T. mentagrophytes (IFM 45828) to the corresponding drug concentrations used for the impregnation of the keratin particles. The inhibition threshold line intersects the curve at the MICK of amorolfine (8 μg/ml) for the isolate. Keratin particles impregnated with 64 μg of clotrimazole per ml effected a growth quotient of 0.173 for the isolate.
FIG. 2.
Curves relating the fluorescence-based fungal growth quotient of an isolate of T. mentagrophytes (IFM 45828) to the concentrations of amorolfine (■) and clotrimazole (●) in the keratin-impregnation antifungal susceptibility test. The dotted line indicates the 0.05 fungal growth quotient inhibition threshold.
DISCUSSION
In this study, a novel susceptibility testing method was developed and compared with the broth microdilution method for the evaluation of the antifungal activities of amorolfine and clotrimazole against isolates of T. mentagrophytes and T. rubrum.
The MICCOLs of both agents for the dermatophytes were lower than the corresponding MICVISs. The fungal growth at 96 h in the presence of 1 or 2 higher dilutions compared with the MICVIS, although visible as a mycelial mat at the bottom of the wells, failed to effect a color change in the broth. Similar variable results in colorimetric and visual MICs have been reported by other workers (3, 6, 13, 19). In our study, the discrepancy between the MICCOL and MICVIS for each drug-fungus pair was not greater than 3 dilutions and thus was not considered significant, in line with the study conducted by the National Committee for Clinical Laboratory Standards (3).
In view of the dose-response relationship between the fungal growth quotient and the corresponding drug concentration (Fig. 1), we adopted a standard quotient of 0.05 as the threshold of inhibition for the determination of MICK. Tellier et al. (17) had earlier proposed a 90% reduction in the optical density of the reduced tetrazolium salt as a colorimetric MIC for antifungal susceptibility testing of yeasts.
The fungal growth quotient, and consequently the MICK, was dependent on experimental parameters such as the impregnation time of the keratin particles, the inoculum size, and the incubation period. These parameters, in addition to the composition of the medium, have been known to affect the intensities of the in vitro antifungal actions of agents by the conventional broth dilution method (3, 11, 14). The reduction in the growth quotient that correlated with an increased inoculum size or incubation time resulted from more rapid fungal growth in the control broth than in the test broth. The MICK of an agent would also depend on the type of the keratin used in the test, with the values obtained with drug-impregnated hard keratin (hair) differing from those obtained with soft keratin (stratum corneum) according to their different porosities for the agent molecules. The maximum flux of an agent into keratin is also known to depend on the surface area of the particle exposed to impregnation (9).
The considerably superior MICK of amorolfine for the dermatophytes over that of clotrimazole, relative to the closer MICCOLs and MICVISs of the two agents, was due as much to the potent antifungal activity of the agent as to its ability to penetrate and bind to keratin. In a related study, an efficacy coefficient calculated from independently determined values of the maximum flux of the agent in bovine hoof membrane and the MICs also pointed to amorolfine as the most effective of 10 antimycotics against onychomycoses (9). On the basis of the conventional MICs obtained in our study, the in vivo efficacies of both agents in the topical treatment of dermatophytosis would be equal, which is in opposition to the considerable superiority of amorolfine, as indicated by the MICK. The increasing numbers of reports on the successful treatment of refractile fungal nail infections with amorolfine-containing lacquer are in keeping with the MICK index (1, 2, 7).
In conclusion, the keratin impregnation antidermatophytic susceptibility testing method is quantitative and objective, and the MICK may be a useful index of the in vivo potency of a topical agent relative to those of other antimycotics.
REFERENCES
- 1.Cohen P R, Scher R K. Topical and surgical treatment of onychomycosis. J Am Acad Dermatol. 1994;31:S74–S77. doi: 10.1016/s0190-9622(08)81273-x. [DOI] [PubMed] [Google Scholar]
- 2.Downs A M, Lear J T, Archer C B. Scytalidium hyalinum onychomycosis successfully treated with 5% amorolfine nail lacquer. Br J Dermatol. 1999;40:555. doi: 10.1046/j.1365-2133.1999.02739.x. [DOI] [PubMed] [Google Scholar]
- 3.Espinel-Ingrof A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinell-Ingroff A, Kerkering T M, Goldson P R, Shadomy S. Comparison of broth macrodilution and microdilution antifungal susceptibility. J Clin Microbiol. 1991;29:1089–1094. doi: 10.1128/jcm.29.6.1089-1094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galgiani J N. Antifungal susceptibility tests. Antimicrob Agents Chemother. 1987;31:1867–1870. doi: 10.1128/aac.31.12.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haria M, Bryson H M. Amorolfine. A review of its pharmacological properties and therapeutic potential in the treatment of onychomycosis and other superficial fungal infection. Drugs. 1995;49:103–120. doi: 10.2165/00003495-199549010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hawser S P, Norris H, Jessup C J, Ghannoum M A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertin D, Lippold B C. In vitro permeability of the human nail and of a keratin membrane from bovine hooves: prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J Pharm Pharmacol. 1997;49:866–872. doi: 10.1111/j.2042-7158.1997.tb06127.x. [DOI] [PubMed] [Google Scholar]
- 10.Negi M, Tsuboi R, Matsui T, Ogawa H. Isolation and characterization of proteinase from Candida albicans: substrate specificity. J Invest Dermatol. 1984;83:32–36. doi: 10.1111/1523-1747.ep12261656. [DOI] [PubMed] [Google Scholar]
- 11.Odds F C. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J Antimicrob Chemother. 1980;6:749–761. doi: 10.1093/jac/6.6.749. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Bushelman B, Bale M J, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G. Multicenter comparison of a colorimetric microdilution broth method with the reference macrodilution method for in vitro susceptibility testing of yeast isolates. Diagn Microbiol Infect Disease. 1994;19:9–13. doi: 10.1016/0732-8893(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 14.Polak A. Antifungal activity in vitro of Ro 14-4767/002, a phenylpropyl-morpholine. Sabouraudia. 1983;21:205–213. [PubMed] [Google Scholar]
- 15.Polak A. Kinetics of amorolfine in human nails. Mycoses. 1993;36:101–103. doi: 10.1111/j.1439-0507.1993.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 16.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellier R, Krajden M, Grigoriew G A, Campbell I. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–1625. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiballi R N, He X, Zarins L T, Revanka S G, Kauffman C A. Use of a colorimetric system for yeast susceptibility testing. J Clin Microbiol. 1995;33:915–917. doi: 10.1128/jcm.33.4.915-917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.To W K, Fothergill A W, Rinaldi M G. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing for yeast isolates. J Clin Microbiol. 1995;33:2660–2664. doi: 10.1128/jcm.33.10.2660-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]