Abstract
Though asthma and bronchiectasis are two different diseases, their coexistence has been demonstrated in many patients. The aim of the present study is to compare the characteristics of asthmatic patients with and without bronchiectasis and to assess risk factors for the development of this condition. Two hundred and twenty-four moderate-severe asthmatic patients were included. The severity of bronchiectasis was assessed by Reiff and FACED parameters. Logistic regression was used to identify independent factors associated with bronchiectasis. Bronchiectasis was identified in 78 asthma patients. In severe asthma patients, its prevalence was 56.9%. Bronchiectasis was defined as mild in81% of patients using modified Reiff criteria and in 74% using FACED criteria. Asthmatic patients with bronchiectasis had decreasing FEV1, FVC and FEV1/FVC (p = 0.002, 0.005 and 0.014 respectively), presented more frequent asthma exacerbations (p < 0.001) and worse asthma control (ACT 21 vs 16pts, p < 0.001). Factors independently associated with bronchiectasis were older age (42–65 years: OR, 3.99; 95% CI 1.60 to 9.95, P = 0.003; ≥ 65 years: OR, 2.91; 95% CI 1.06 to 8.04, P = 0.039), severe asthma grade (OR, 8.91; 95% CI 3.69 to 21.49; P < 0.001) and frequency of asthma exacerbations (OR, 4.43; 95% CI 1.78 to 11.05; P < 0.001). In patients with severe asthma, age of asthma onset (OR, 1.02; 95% CI 1.01 to 1.04; P = 0.015) and asthma exacerbations (OR, 4.88; 95% CI 1.98 to 12.03; P = 0.001) were independently associated with the development of bronchiectasis. The prevalence of bronchiectasis in severe asthmatic patients is high. Age of asthma onset and exacerbations were independent factors associated with the occurrence of bronchiectasis.
Subject terms: Asthma, Medical research
Introduction
Asthma is a disease characterized by chronic airway inflammation. It is estimated that asthma affects about 339 million people worldwide, and its prevalence is increasing rapidly1,2. Asthma symptoms may be triggered or worsened by many factors: biological factors such as viral or microorganism infections, physiological factors such as exercise and cold air, and environmental triggers such as exposure to cigarettes, pollen, and diesel exhaust particles3–5.
For a long time, bronchiectasis (BQ) was considered an orphan disease6,7. The Spanish guidelines on the Evaluation and Diagnosis of Bronchiectasis in Adults8 define BQ as a heterogeneous chronic airway disease with bronchial dilatation, accompanied by clinical symptoms such as cough, chronic expectoration and recurrent lung infections. Bronchiectasis is now reported to be the third most frequent airway chronic disease, just behind asthma and chronic obstructive pulmonary disease (COPD)1.
The coexistence of asthma and BQ has been reported previously. Indeed, the prevalence of BQ in asthmatic patients ranges from 3% in general asthmatic patients, without specification of asthma severity, to 47–67.5% in patients with severe asthma9–11. In some studies of BQ the prevalence of asthma has also been reported12,13. It seems that there are some underlying correlations between asthma and bronchiectasis; however, the evolution of BQ and its pathophysiology require long term follow-up studies14. Little is known about the impact of BQ on asthma, its clinical manifestation, comorbidities and disease management. The presence of BQ may also trigger asthma exacerbations and may challenge the management of the disease.
The aim of the present study is to compare the characteristics of asthmatic patients with and without BQ and to assess risk factors for the development of BQ, using data consecutively recorded at a specific asthma unit over a one–year period.
Materials and methods
Study population and design
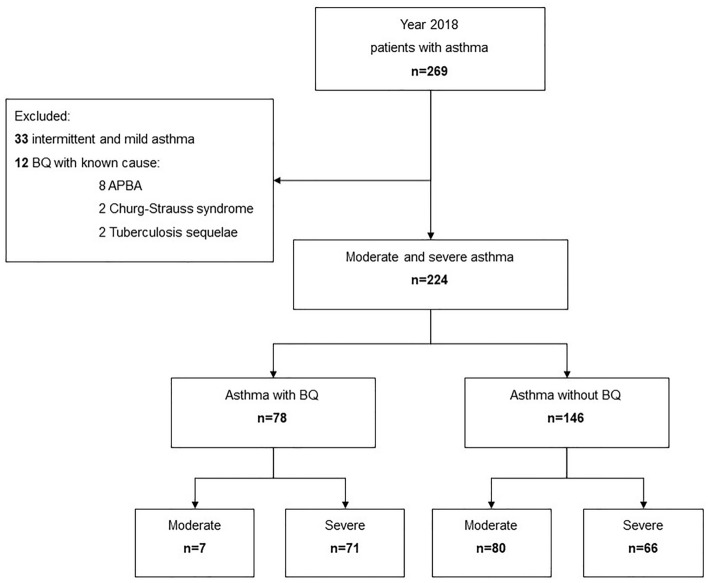
We performed an ambispective study of 269 asthma patients seen at our specialized asthma unit in 2018. Of these, 33 with intermittent and mild asthma, and twelve with known etiology of BQ (eight Churg-Strauss syndrome, two allergic bronchopulmonary aspergillosis, and two sequelae post-tuberculosis) were excluded. The remaining 224 patients with moderate and severe asthma were finally included in the study (Fig. 1).
Figure 1.
Flow chart of the study population. ABPA: allergic bronchopulmonary aspergillosis; BQ: non-fibrosis bronchiectasis.
Clinical histories of all patients included and data including anthropometric, smoking habits, comorbidities, asthma history, asthma grade, atopy status and exacerbations were retrospectively reviewed. Variables associated with BQ and microorganism colonization in sputum culture, when they were available, were also reviewed and recorded. Patients were examined and asked about their present medication, and the Asthma Control Test (ACT)15 was administered. The records were checked to establish whether spirometry had been performed during the previous year, or a High-Resolution Computed Tomography (HRCT) during the last three years. If not, or if no data were available, these tests were administered. The study was approved by our hospital’s Ethics Committee (PR(AG)50/2019). All patients provided written informed consent to participate in the study.
Asthma diagnosis and severity
The diagnosis of asthma was made according to GINA16 guidelines and based on clinical symptoms plus one or more complementary tests. All patients had shown reversible airway obstruction on bronchodilator testing, or a positive methacholine challenge test, or variability > 20% in the peak flow recording. Moderate and severe asthma were defined based on the GINA guidelines, which corresponded to patients taking steps 3, 4, and 5 of medication with at least 200 μg of inhaled corticosteroids (budesonide or equivalent doses) plus a long-acting beta-2 agonist16.
Pulmonary function test
Spirometry was performed using a MasterLab instrument (MasterLab, Jaeger, Germany), according to European Respiratory Society (ERS) and American Thoracic Society (ATS) guidelines17. The reference values used were those proposed by the ERS18.
Bronchiectasis diagnosis and severity
Bronchiectasis was identified in accordance with the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) recommendations, by comparing the internal bronchial lumen diameter with the adjacent artery calibre in HRCT images8. Morphological characteristics of BQ, including bronchodilation, bronchial wall thickening, dilatation type and lobe extension were reviewed and assessed according to the modified Reiff score19 in six lobes (18 points in total): 1–6 mild, 7–12 moderate, and ≥ 13 severe.
The FACED parameter20, which incorporates variables such as FEV1, age, pseudomonas colonization, lobe extension and dyspnea, was used for the clinical estimation of patients' status..
HRCT was performed with 1 mm cuts at 10 mm intervals in maximum inspiration. All the imaging features of BQ were interpreted by two pulmonologists working independently. For controversial images, an expert radiologist was consulted and the final decision was made. The degree of the inter-observer agreement was assessed by the Kappa statistic21.
Atopy and smoking status
Patients were considered atopic if they had at least one positive prick test for any of the common environmental allergens. Non-smokers were patients who had never smoked, and ex-smokers were those who had not smoked for at least six months. The number of pack-years was calculated in all cases.
Definition of exacerbations
Episodes of asthma exacerbations during the previous year were recorded. Asthma exacerbations were defined according to GINA guidelines16 which specify asthma attacks or acute asthma exacerbation as episodes of progressive increases in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms, accompanied by reductions in expiratory airflow.
Statistical analysis
Categorical variables were presented as frequencies and percentages. Statistical differences were analysed using the Chi-square test (or Fisher's exact test when appropriate). Continuous variables were presented as means and standard deviations (SD), or as medians with interquartile ranges (IQR) when data were not normally distributed. The Shapiro–Wilk test was used to analyse the distribution of variables. A logistic regression model was used to determine the factors that were independently associated with the outcome, in this case, the presence of BQ. Variables that presented statistically significant differences (p < 0.05) in the bivariate analysis or were considered to be of clinical interest (such as gender) were included as independent variables in the first step. Variables associated with disease management and asthma control test score were not included. A forward stepwise technique (Wald test; removal threshold, p > 0.10) was used to perform this analysis. ORs and 95% CIs were calculated for independent variables. Tolerance and the Variance Inflation Factor (VIF) were examined to avoid multicollinearity among variables. Statistical significance was defined as a two-tailed p ≤ 0.05. The statistical analyses were performed using SPSS (version 25, Chicago, IL).
Ethics approval and consent to participate
The study was approved by the hospital Vall d'Hebron Ethics Committee (Reference number: PR(AG)50/2019). All patients provided written informed consent prior to participating. All methods were performed in accordance with the relevant guidelines and regulations.
Informed consent
All patients provided written informed consent to participate in the study.
Results
The socio-demographic characteristics of patients with and without BQ are shown in Table 1. Asthma patients with BQ were older than patients without BQ (56.2 vs. 49.9 years, p = 0.005). Bronchiectasis was more frequent in middle-aged subjects (42–65 years) (62.8% vs 44.5%, p < 0.05), but this trend was reversed in the younger adult group (< 42 years) (11.5% vs 30.8%, p < 0.05). Furthermore, subjects with BQ had more severe asthma (91% vs 45.2%, p < 0.001) and presented a higher number of exacerbations (median 1 vs 0 episodes, p < 0.001). Significantly lower FVC% pred, FEV1%pred and the ratio of FEV1/FVC were observed in asthma patients with BQ than in those without (89.4% vs 82.8%, p = 0.005; 81.0% vs 73.2%, p = 0.002; 72.7 vs 69.5, p = 0.014 respectively). Subjects with BQ presented more sinusitis (10.3% vs 2.1%, p = 0.018) and nasal polyps (28.2% vs 13.0%, p = 0.005) than those without. Subjects without BQ seemed to be more atopic than those with BQ (63.0% vs 43.6%, p = 0.005). No significant differences were observed in the remaining comorbidities.
Table 1.
Socio-demographic characteristics and clinical data of the study population.
| Moderate and severe asthma | P | Severe asthma | P | |||
|---|---|---|---|---|---|---|
| Without BQ (146) | with BQ (78) | Without BQ (66) | with BQ (71) | |||
| Sociodemographic data | ||||||
| Gender (female) | 94 (64.4%) | 46 (59.0%) | 0.43 | 37 (56.1%) | 43 (60.6%) | 0.59 |
| Age (years) | 49.9 (17.2) | 56.2 (13.1) | 0.005 | 49.7 (17.0) | 56.2 (13.5) | 0.014 |
| Age group* | 0.004 | 0.033 | ||||
| A1, < 42 yrs | 45 (30.8%) | 9 (11.5%) | < 0.001† | 20 (30.3%) | 9 (12.7%) | 0.009† |
| A2, 42–65 yrs | 65 (44.5%) | 49 (62.8%) | 0.364†† | 29 (43.9%) | 43 (60.6%) | 0.078†† |
| A3, ≥ 65 yrs | 36 (24.7%) | 20 (25.6%) | 0.023¶ | 17 (25.8%) | 19 (26.8%) | 0.491 |
| Race (Caucasian) | 141 (96.6%) | 72 (92.3%) | 0.20 | 63 (95.5%) | 65 (91.5%) | 0.50 |
| BMI (kg/m2) | 27.1 (23.6,30.7) | 27.1 (24.4, 29.1) | 0.77 | 27.9 (24.7, 30.7) | 27.3 (24.6, 29.1) | 0.35 |
| Smoking status | 0.24 | 0.42 | ||||
| Non-smoker | 100 (68.5%) | 46 (59.0%) | 43 (65.2%) | 42 (59.2%) | ||
| Current-smoker | 8 (5.5%) | 3 (3.8%) | 4 (6.1%) | 2 (2.8%) | ||
| Ex-smoker | 38 (26.0%) | 29 (37.2%) | 19 (28.8%) | 27 (38.0%) | ||
| Packs-year | 0.0 (0.0, 3.0) | 0.0 (0.0, 15.0) | 0.052 | 0.0 (0.0, 4.0) | 0.0 (0.0, 15.0) | 0.30 |
| Clinical data of asthma & lung function | ||||||
| Atopic asthma | 92 (63.0%) | 34 (43.6%) | 0.005 | 42 (63.6%) | 30 (42.3%) | 0.012 |
| Asthma grade | < 0.001 | NA | ||||
| Moderate | 80 (54.8%) | 7 (9.0%) | ||||
| Severe | 66 (45.2%) | 71 (91.0%) | ||||
| Years of asthma | 17.8 (9.4, 31.8) | 19.4 (9.5, 37.1) | 0.27 | 20.2 (13.3, 40.1) | 20.4 (9.6, 37.3) | 0.57 |
| Age of asthma onset | 26.0 (6.0, 45.1) | 33.7 (16.0, 45.9) | 0.20 | 20.5 (5.5, 37.6) | 33.8 (15.0, 46.0) | 0.024 |
| CAO (yes) | 49 (33.6%) | 22 (28.2%) | 0.41 | 25 (37.9%) | 21 (29.6%) | 0.30 |
| ACT (pts) | 21 (18, 24) | 16 (13, 21) | < 0.001 | 20.0 (17.0, 23.0) | 16.0 (13.0, 21.0) | 0.003 |
| Exacerbation | 0.0 (0.0, 1.0) | 1.0 (0.0, 3.0) | < 0.001 | 0.0 (0.0, 2.0) | 2.0 (0.0, 4.0) | < 0.001 |
| ≥ 3 courses | 8 (5.5%) | 27 (34.6%) | < 0.001 | 8 (12.1%) | 27 (38.0%) | < 0.001 |
| FVC% pred | 89.4 (15.9) | 82.8 (17.7) | 0.005 | 86.8 (16.9) | 81.8 (17.7) | 0.096 |
| FEV1% pred | 81.0 (17.1) | 73.2 (19.6) | 0.002 | 75.8 (17.1) | 72.2 (19.4) | 0.25 |
| FEV1/FVC | 72.7 (9.0) | 69.5 (9.1) | 0.014 | 70.2 (8.5) | 69.5 (9.2) | 0.62 |
| FEV1/FVC ≥ 70 | 86 (58.9%) | 42 (53.8%) | 0.47 | 32 (48.5%) | 34 (47.9%) | 0.94 |
| Comorbidity | ||||||
| NSAIDs allergy | 25 (17.1%) | 13 (16.7%) | 0.93 | 19 (28.8%) | 11 (15.5%) | 0.060 |
| Rhinitis | 50 (34.2%) | 22 (28.2%) | 0.36 | 21 (31.8%) | 21 (29.6%) | 0.78 |
| Sinusitis | 3 (2.1%) | 8 (10.3%) | 0.018 | 1 (1.5%) | 8 (11.3%) | 0.034 |
| Nasal polyps | 19 (13.0%) | 22 (28.2%) | 0.005 | 11 (16.7%) | 20 (28.2%) | 0.11 |
| Obesity | 46 (31.5%) | 18 (23.1%) | 0.18 | 23 (34.8%) | 16 (22.5%) | 0.11 |
| WRA | 25 (17.1%) | 14 (17.9%) | 0.88 | 10 (15.2%) | 12 (16.9%) | 0.78 |
Continuous variables expressed as mean (SD) or median (p25, p75); categorical data expressed as percentage n (%); *Bonferroni adjust method was used to perform comparison between age subgroup, significant p level at 0.017; †, A1 vs A2; ††, A2 vs A3; ¶, A1 vs A3). BMI: body mass index; CAO: childhood asthma onset (18 yrs cut-off); ACT: asthma control test; BQ: non-cystic fibrosis bronchiectasis; WRA, work-related asthma. Significant p values in bold font.
With regard to disease management (Table 2), subjects with BQ were more corticosteroid-dependent (34.6% vs 3.4%, p < 0.001) and had also taken more azithromycin (34.6% vs 6.8%, p < 0.001). In addition, the consumption of long-acting muscarinic receptor antagonists (LAMA) and anti-leukotriene was significantly higher in asthma patients with BQ than in those without (69.2% vs. 26.7%, p < 0.001 and 65.4% vs. 39.0%, p < 0.001 respectively).
Table 2.
Comorbidities and disease management of the study population.
| Moderate and severe asthma | P | Severe asthma | P | |||
|---|---|---|---|---|---|---|
| without BQ (146) | with BQ (78) | without BQ (66) | with BQ (71) | |||
| Comorbidity | ||||||
| NSAIDs allergy | 25 (17.1%) | 13 (16.7%) | 0.93 | 19 (28.8%) | 11 (15.5%) | 0.060 |
| Rhinitis | 50 (34.2%) | 22 (28.2%) | 0.36 | 21 (31.8%) | 21 (29.6%) | 0.78 |
| Sinusitis | 3 (2.1%) | 8 (10.3%) | 0.018 | 1 (1.5%) | 8 (11.3%) | 0.034 |
| Nasal polyps | 19 (13.0%) | 22 (28.2%) | 0.005 | 11 (16.7%) | 20 (28.2%) | 0.11 |
| Obesity | 46 (31.5%) | 18 (23.1%) | 0.18 | 23 (34.8%) | 16 (22.5%) | 0.11 |
| WRA | 25 (17.1%) | 14 (17.9%) | 0.88 | 10 (15.2%) | 12 (16.9%) | 0.78 |
| Disease management | ||||||
| Cortisone-dependent | 5 (3.4%) | 27 (34.6%) | < 0.001 | 4 (6.1%) | 27 (38.0%) | < 0.001 |
| Budesonide (μg/d) | 1040 (640,1600) | 1280 (640,1600) | 0.37 | 1440 (800,1600) | 1400 (640,1600) | 0.29 |
| ICS dose category | 0.14 | 0.61 | ||||
| Low | 30 (20.5%) | 10 (12.8%) | 5 (7.6%) | 9 (12.7%) | ||
| Med | 42 (28.8%) | 18 (23.1%) | 13 (19.7%) | 14 (19.7%) | ||
| High | 74 (50.7%) | 50 (64.1%) | 48 (72.7%) | 48 (67.6%) | ||
| LAMA | 39 (26.7%) | 54 (69.2%) | < 0.001 | 29 (43.9%) | 53 (74.6%) | < 0.001 |
| Azithromycin | 10 (6.8%) | 26 (33.3%) | < 0.001 | 8 (12.1%) | 26 (36.6%) | < 0.001 |
| Omalizumab | 18 (12.3%) | 15 (19.2%) | 0.16 | 18 (27.3%) | 15 (21.1%) | 0.40 |
| Mepolizumab | 4 (2.7%) | 5 (6.4%) | 0.28 | 4 (6.1%) | 5 (7.0%) | 1.00 |
| Anti-leukotriene | 57 (39.0%) | 51 (65.4%) | < 0.001 | 39 (59.1%) | 48 (67.6%) | 0.30 |
| Theophylline | 2 (1.4%) | 1 (1.3%) | 1.00 | 2 (3.0%) | 1 (1.4%) | 0.61 |
Continuous variables expressed as mean (SD) or median (p25, p75); categorical data expressed as percentage n (%); BQ: non-cystic fibrosis bronchiectasis; NSAIDs, allergy to nonsteroidal anti-inflammatory drugs; LAMA: long-acting muscarinic receptor antagonists; ICS: Inhaled Corticosteroid. Significant p values in bold font.
The prevalence of BQ in severe asthma patients was 56.9% (Fig. 1). A sub-analysis limited to these subjects with severe asthma found similar differences in age, ACT, asthma exacerbation, atopy, sinusitis, cortisone dependency and asthma medication (LAMA and azithromycin). However, the differences in lung function were no longer statistically significant. Furthermore, patients with BQ had a later asthma onset than patients without (median 20.5 years’ vs 33.8 years, p = 0.024) (Tables 1 and 2).
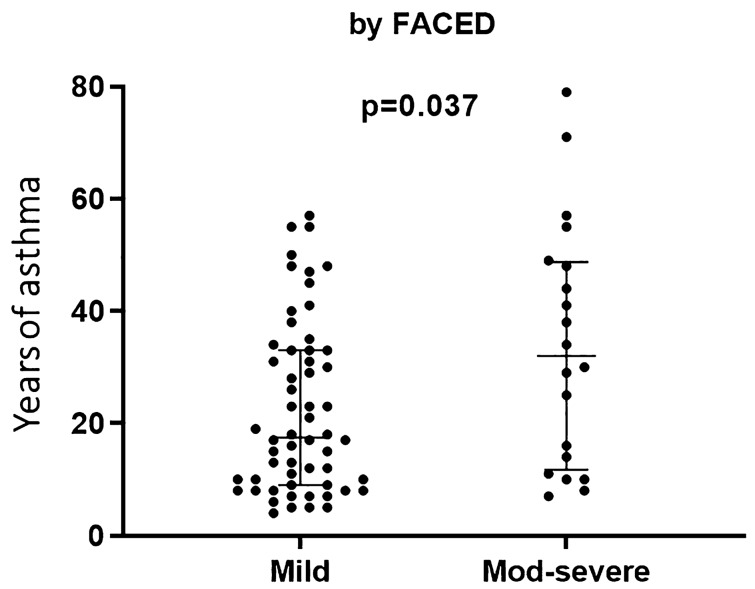
Of the 78 patients with BQ, 81% were classified as mild according to modified Reiff criteria, and 74% according to FACED criteria (Table 3). Patients with mild BQ (according to FACED) had fewer years of asthma evolution than those with moderate and severe BQ (17.2 yrs vs 31.7 yrs, p = 0.037) (Fig. 2). A significant correlation between the FACED score and dyspnea (r = 0.66, p = 0.0001) was observed. Seventy-five patients (96%) had widespread bronchial dilatation, with all six lobes involved. Bronchial wall thickening was moderate in 68% of patients and mild in 27%. The predominant dilatation type was cylindrical (82%). Kappa values (and overall agreement %) for lobe extension, bronchial dilatation, bronchial wall thickening, and dilatation type were 0.83 (98.7%), 0.75 (88.5%), 0.70 (89.7%), and 0.79 (94.9%) respectively.
Table 3.
Assessment of bronchiectasis by radiological and clinical parameters.
| Comprehensive parameter | Individual parameter | ||
|---|---|---|---|
| FACED | Lobe extension with lingula included | ||
| 4 lobes involved | 3 (4%) | ||
| 6 lobes involved | 75 (96%) | ||
| Mild | 58 (74%) | Bronchial wall thickening* (predominant) | |
| Moderate | 18 (23%) | Mild | 21 (27%) |
| Severe | 2 (3%) | Moderate | 53 (68%) |
| Modified Reiff | Severe | 4 (5%) | |
| Dilatation type (predominant) | |||
| Mild | 63 (81%) | Cylindrical | 64 (82%) |
| Moderate | 13 (17%) | Varicose | 12 (15%) |
| Severe | 2 (3%) | Cystic | 2 (3%) |
Left part: systemic parameters. Right part: individual parameters. * Bronchial wall thickening by comparing with the adjacent artery diameter: mild < 0.5 A; moderate (0.5–1) A; severe > 1 A. Modified Reiff score: 1–6 pts mild, 7–12 pts moderate and ≥ 13 pts severe. FACED score: 0–2 pts mild, 3–4 pts moderate and 5–7 pts severe bronchiectasis.
Figure 2.
Asthma-year according to bronchiectasis severity assessed by FACED. Data expressed as median (IQR). Years of asthma 17.2 (9.1, 32.5) in mild vs 31.7 (12.0, 48.3) in moderate and severe bronchiectasis, p = 0.037.
Potentially pathogenic bacteria were identified in 16 subjects (21%) with BQ, including P. aeruginosa (five cases), H. influenzae (three), S. pneumonia (three), S. aureus (two), K. pneumonia (one), M. catarrhalis (one) and P. putida (one).
The odds ratios and 95% confidence intervals of variables related to BQ in all asthma patients and in severe asthma patients are shown in Table 4 and in Figs. 3 and 4 respectively. In the entire group, age, severe asthma grade and asthma exacerbations (≥ 3) were independently associated with the presence of BQ, and in the severe asthma group, age of asthma onset and ≥ 3 exacerbations were independently associated with this outcome.
Table 4.
Univariate and multivariate regression analysis of appearance of bronchiectasis in the study population.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Moderate and severe asthma patients | ||||||
| Age < 42 yrs | 0.002 | 0.012 | ||||
| 42–65 yrs | 3.77 | 1.68–8.44 | 0.001 | 3.99 | 1.60–9.95 | 0.003 |
| ≥ 65 yrs | 2.78 | 1.13–6.84 | 0.026 | 2.91 | 1.06–8.04 | 0.039 |
| Severe asthma | 12.29 | 5.30–28.54 | < 0.001 | 8.91 | 3.69–21.49 | < 0.001 |
| Severe asthma patients | ||||||
| Age of asthma onset | 1.02 | 1.00–1.04 | 0.026 | 1.02 | 1.01–1.04 | 0.015 |
| Exacerbations ≥ 3 | 4.45 | 1.84–10.74 | 0.001 | 4.88 | 1.98–12.03 | 0.001 |
OR: odds ratio. CI: confidence interval. The multivariate model is the final model after stepwise removal of covariates.
Figure 3.
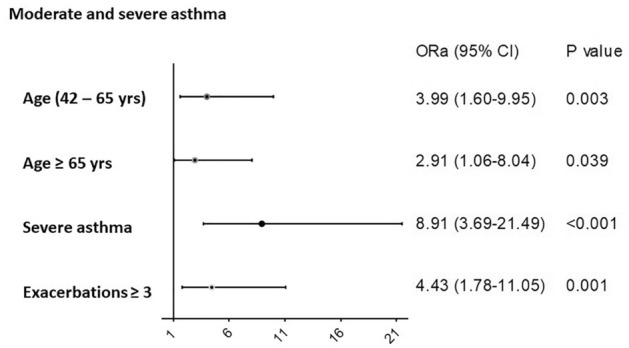
Factors associated with bronchiectasis in all subjects (moderate and severe asthma). ORa: adjusted odds ratio. CI: confidence interval. Model: χ2 = 73.64, p < 0.001. Model adjusted by gender, atopy, sinusitis, nasal polyps, FVC% and FEV1%. Logistic regression was used to perform the analysis.
Figure 4.
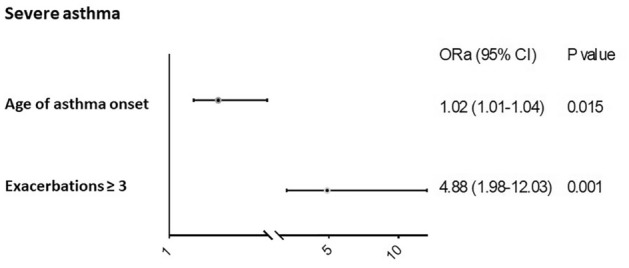
Factors associated with bronchiectasis in severe asthma patients. ORa: adjusted odds ratio. CI: confidence interval. Model: χ2 = 18.82, p < 0.001. Model adjusted for gender, age group, atopy and sinusitis. Logistic regression was used to perform the analysis.
Discussion
The present study found a high percentage of BQ in asthma patients, especially in those with severe asthma. In severe asthma patients, age of asthma onset and exacerbations were independently associated with the occurrence of BQ.
Though asthma and BQ are two different diseases, their coexistence has been demonstrated in many patients. In 2012, in a study applying HRCT Khadadah et al.22 reported the presence of BQ in 28.6% of persistent moderate asthma patients. In patients with persistent severe asthma, the presence of BQ is higher, ranging between 35%-80% according to the study10,11,23–27. In agreement with our results, in a large cohort of asthma patients Oguzulgen et al.9 recently reported that most asthma patients with BQ had severe persistent asthma (49.0%). In paediatric populations with severe asthma, approximately one-third of children had BQ, which was especially frequent in older children28. The differences observed between the studies may have been due to the study design or to the heterogeneity of the populations and comorbidities. Moreover, the widespread use of HRCT has increased the rate of identification of radiological bronchial dilatation and other airway abnormalities. Indeed, data from the US suggest that the number of annual HRCT procedures performed rose from 3 million in 1980 to 81.2 million in 201429.
In the present study, asthmatic patients with bronchiectasis presented more frequent asthma exacerbations and worse asthma control. As we mentioned above, the coexistence of BQ and asthma has been observed in many patients, but few studies have been undertaken to investigate the relationship between the two diseases and the effect of BQ on exacerbations30. One such study, by Kang et al.31, found that BQ may be a risk factor for asthma exacerbation; these authors suggested that HRCT could be considered to search for concurrent BQ in uncontrolled asthma patients. In fact, BQ may be a terminal status in some patients with chronic airway inflammation, and early detection of the condition is recommended when patients present these features. Crimi et al.32 also advocate the use of HRCT to rule out BQ in patients with severe asthma and frequent exacerbations. A 5-year follow-up study reported a mortality rate of 20% in adults with BQ33. Bronchiectasis challenges asthma control and may aggravate the evolution of the condition.
Although asthma severity is an independent risk factor for BQ, the question of whether it is a cause of BQ remains unclear. In mild to moderate predominant BQ, asthma was the cause in 5.4% of cases34. Moreover, a Finnish study suggested that one-fourth of cases with BQ could be attributed to asthma13. The British guidelines also recommend considering asthma as the cause of BQ in the absence of any other etiological explanation35.
The present study found that, in the overall group, the factors independently associated with the occurrence of BQ were older age, asthma severity and frequent asthma exacerbations, while in patients with severe asthma the independently associated factors were age of asthma onset and frequent asthma exacerbations. In line with the results of the present study, Kang et al.31 found that annual incidence of asthma exacerbations, and emergency room visits due to asthma exacerbations were higher in patients with both asthma and BQ than in those with asthma alone, although the fact that the study population comprised mostly mild to moderate asthma patients may have influenced the results. In a cohort of moderate to severe asthma patients using the NOPES score (NOPES: nitric oxide, pneumonia, expectoration, and severity), Padilla-Galo et al. found these risk factors to be independently associated with BQ36.
We found that patients with asthma and BQ were more likely to present with sinusitis and nasal polyps. The association of sinusitis, nasal polyps and intolerance to aspirin, known as Samter's Triad Syndrome37, is a common phenomenon in patients with asthma and a relationship between asthma severity and nasal polyps has been reported38, but the pathology of BQ and its interaction with other nasal airway diseases requires further exploration. Nonetheless, in patients with BQ, nasal polyps and chronic sinusitis are quite frequent and are also characterized by early onset39,40. In fact, chronic sputum expectoration is common in patients with BQ, and mucus hypersecretion has been associated with rhinosinusitis and nasal polyps41. The present study also found that asthma patients with BQ were less atopic. In other research, atopy was significantly more prevalent in patients with asthma than in patients with chronic rhinosinusitis and nasal polyps37. Likewise, atopic dermatitis is less frequent in subjects with BQ than in those without (2% vs 11%, OR 0.188)10.
Bronchial wall thickening and bronchial dilatation are very common in severe asthma patients42–44, but few studies provide systematic quantification scores of these abnormalities in asthma patients. The present study describes the characteristics and severity of BQ, mainly finding its grade to be mild according to the criteria of Reiff19 and FACED20. Our results are consistent with the study by Padilla-Galo et al.36 with regard to bronchiectasis severity, since those authors recorded a mean FACED score of 1.45 pts, which also corresponds to mild grade disease. In contrast, they observed a mild predominant grade of bronchial wall thickening, whereas we found a notable proportion of patients with moderate predominant wall thickening. This difference may be attributed to the different degrees of asthma severity analysed in the two studies. In our study, 82% of patients presented cylindrical dilatation, similar to the rate reported by Padilla-Galo et al. (92.9%).
This study has several limitations. First, is a single-center study carried out at a clinic specialized in severe asthma. Larger studies should now be carried out in different settings to corroborate the data obtained. Secondly, the fact that the HRCT was carried out in some patients during the last three years may have introduced a bias. Third, in HRCT, low-dose volumetric acquisition protocol, with 1 mm cuts at 10 mm intervals in maximum inspiration was performed. In this sense, continuous 1 mm slices have a higher sensitivity than conventional HRCT images. Moreover, the bronchoarterial ratio could be influenced by aging. In this sense, the normal bronchoarterial ratio in up to 20% of healthy subjects older than 65 years overlaps with the ratio considered to represent bronchiectasis8. In our series, 53 patients (24%) were older than 65 years and in this group this radiological criterion may be present, which could alter the results. Finally, the characteristics of the study design do not allow us to differentiate whether the cause of the reduced FEV1 is asthma or the presence of BQ. However, we observed that patients with asthma and BQ have a lower FEV1 than those without BQ, which could mean that there is a synergistic effect between both conditions.
Conclusions
Our results suggest that, in severe asthma patients, the presence of bronchiectasis is associated with age of asthma onset and the number of exacerbations. Our findings draw attention to the impact of BQ in asthma patients and should encourage clinical professionals to improve early detection measures and interventions for BQ in severe asthma patients, in order to improve patients’ quality of life and reduce the economic burden of this disease. Further studies are needed to confirm our results and to focus on the underlying mechanisms, for example via an assessment of the proinflammatory cytokines involved in this complex entity, and thus to be able to offer immunophenotype-based precision treatment.
Abbreviations
- ACT
Asthma Control Test
- ATS
American Thoracic Society
- BQ
Bronchiectasis
- COPD
Chronic Obstructive Pulmonary Disease
- ERS
European Respiratory Society (ERS)
- FEV1
Forced Expiratory Volume in 1 Second.
- FVC
Forced Expiratory Volume in 1 Second.
- GINA
Global Initiative for Asthma
- HRCT
High-Resolution Computed Tomography
- IQR
Interquartile Ranges
- LAMA
Long-Acting Muscarinic Receptor Antagonists
- NOPES
Nitric oxide, Pneumonia, Expectoration, Severity.
- OR
Odds Ratio
- SD
Standard deviations (SD)
- SEPAR
Spanish Society of Pulmonology and Thoracic Surgery
- VIF
Variance Inflation Factor
Author contributions
Conceptualization: M.J.C., X.M. Formal analysis: D.M. Funding acquisition: M.J.C., X.M. Methodology: D.M., C.R.M., I.O., D.V.P. Project administration: X.M. Writing + original draft: D.M., M.J.C., X.M. Writing + review & editing: D.M., M.J.C., X.M., D.V.P., C.R., I.O.
Funding
The study was partially supported by FIS PI15/01900 (Fondo Europeo de Desarrollo Regional (FEDER) and Fundacio Catalana de Pneumología (FUCAP). MJC is supported by the Miguel Servet program of the Instituto de Salud Carlos III (MSII17/00025). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
Not available in order to preserve participant confidentiality.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network, 2018.
- 3.GINA. Global Strategy For Asthma Management and Prevention. Glob Initiat Asthma [Internet]. 2017 [cited 2020 Feb 4];http://ginasthma.org/2017-gina-report-global-strat.
- 4.Cullinan P, Muñoz X, Suojalehto H, Agius R, Jindal S, Sigsgaard T, et al. Occupational lung diseases: From old and novel exposures to effective preventive strategies. Lancet Respir. Med. 2017;5(5):445–455. doi: 10.1016/S2213-2600(16)30424-6. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Simón D, Muñoz X, Gómez-Ollés S, de Homdedeu M, Untoria M-D, Cruz M-J. Effects of diesel exhaust particle exposure on a murine model of asthma due to soybean. PLoS ONE. 2017;12(6):e0179569. doi: 10.1371/journal.pone.0179569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker AF. Bronchiectasis. N Engl. J. Med. 2002;346(18):1384–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 7.Aliberti S, Polverino E, Chalmers JD, Altenburg J, Shteinberg M, Goeminne PC, et al. The European multicentre bronchiectasis audit and research collaboration (EMBARC) ERS Clinical Research Collaboration. Eur. Respir. J. (2018), Vol. 52, No. 5. [DOI] [PubMed]
- 8.Martinez-Garcia MÁ, Máiz L, Olveira C, Giron RM, de la Rosa D, Blanco M, et al. Spanish Guidelines on the Evaluation and Diagnosis of Bronchiectasis in Adults. Arch Bronconeumol [Internet]. 2017;(xx). [DOI] [PubMed]
- 9.Oguzulgen IK, Kervan F, Ozis T, Turktas H. The impact of bronchiectasis in clinical presentation of asthma. South Med. J. [Internet]. 2007;100(5):468–471. doi: 10.1097/SMJ.0b013e31802fa16f. [DOI] [PubMed] [Google Scholar]
- 10.Coman I, Pola-Bibián B, Barranco P, Vila-Nadal G, Dominguez-Ortega J, Romero D, et al. Bronchiectasis in severe asthma: Clinical features and outcomes. Ann. Allergy Asthma Immunol. 2018;120(4):409–413. doi: 10.1016/j.anai.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Dimakou K, Gousiou A, Toumbis M, Kaponi M, Chrysikos S, Thanos L, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin. Respir. J. 2018;12(3):1212–1218. doi: 10.1111/crj.12653. [DOI] [PubMed] [Google Scholar]
- 12.Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann. Am. Thorac. Soc. 2015;12(12):1764–1770. doi: 10.1513/AnnalsATS.201507-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäntylä J, Mazur W, Törölä T, Bergman P, Saarinen T, Kauppi P. Asthma as aetiology of bronchiectasis in Finland. Respir. Med. 2019;152(April):105–111. doi: 10.1016/j.rmed.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz X, Álvarez-Puebla MJ, Arismendi E, Arochena L, Ausín M del P, Barranco P, et al. Estudio de los mecanismos implicados en la génesis y evolución del asma (proyecto MEGA): creación y seguimiento a largo plazo de una cohorte de pacientes asmáticos. Arch. Bronconeumol. (2018). [DOI] [PubMed]
- 15.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Gina. Global strategy for asthma management and prevention [Internet]. (2019).
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Europ. Respirat. J. Europ. Respir. Soc. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: Limited value in distinguishing between idiophatic and specific types. Am. J. Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-García MA, De Gracia J, Relat MV, Girón RM, Carro LM, De La Rosa CD, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 2014;43(5):1357–1367. doi: 10.1183/09031936.00026313. [DOI] [PubMed] [Google Scholar]
- 21.Diederich S, Jurriaans E, Flower CDR. Interobserver variation in the diagnosis of bronchiectasis on high-resolution computed tomography. Eur. Radiol. 1996;6(6):801–806. doi: 10.1007/BF00240675. [DOI] [PubMed] [Google Scholar]
- 22.Khadadah M, Jayakrishnan B, Muquim A, Roberts O, Sinan T, Maradny N. High resolution computed tomography in asthma. Oman Med. J. 2012;27(2):145–150. doi: 10.5001/omj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Clemente M, Enríquez-Rodríguez AI, Iscar-Urrutia M, Escobar-Mallada B, Arias-Guillén M, López-González FJ, et al. Severe asthma and bronchiectasis. J. Asthma. 2019;20:1–5. doi: 10.1080/02770903.2019.1579832. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Nam Jin K, Cho SH, Hyun Lee C, Kang HR. Severe Asthma Phenotypes Classified by Site of Airway Involvement and Remodeling via Chest CT Scan. J. Investig. Allergol. Clin. Immunol. [Internet]. 2018;28(5):0. [DOI] [PubMed]
- 25.Menzies D, Holmes L, McCumesky G, Prys-Picard C, Niven R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy Eur. J. Allergy Clin. Immunol. 2011;66(5):679–685. doi: 10.1111/j.1398-9995.2010.02542.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009;136(6):1521–1528. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paganin F, Séneterre E, Chanez P, Daurés JP, Bruel JM, Michel FB, et al. Computed tomography of the lungs in asthma: Influence of disease severity and etiology. Am. J. Respir. Crit. Care Med. 1996;153(1):110–114. doi: 10.1164/ajrccm.153.1.8542102. [DOI] [PubMed] [Google Scholar]
- 28.Lo D, Maniyar A, Gupta S, Gaillard E. High prevalence of bronchiectasis on chest CT in a selected cohort of children with severe Asthma. BMC Pulm. Med. 2019;19(1):136. doi: 10.1186/s12890-019-0900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goeminne PC, De SA. Bronchiectasis: How to be an orphan with many parents. Eur. Respir. J. 2016;47(1):10–13. doi: 10.1183/13993003.01567-2015. [DOI] [PubMed] [Google Scholar]
- 30.Mao B, Yang J-WW, Lu H-WW, Xu J-FF. Asthma and bronchiectasis exacerbation. Eur. Respir. J. 2016;47(6):1680–1686. doi: 10.1183/13993003.01862-2015. [DOI] [PubMed] [Google Scholar]
- 31.Kang HR, Choi GS, Park SJ, Song YK, Kim JM, Ha J, et al. The effects of bronchiectasis on asthma exacerbation. Tuberc. Respir. Dis. (Seoul). 2014;77(5):209–214. doi: 10.4046/trd.2014.77.5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: A dangerous liaison? Curr. Opin. Allergy Clin. Immunol. 2019;19(1):46–52. doi: 10.1097/ACI.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 33.Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: A prospective cohort analysis. Respir. Med. 2014;108(2):287–296. doi: 10.1016/j.rmed.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Guan WJ, Gao YH, Xu G, Lin ZYMZ, Tang Y, Li HM, et al. Aetiology of bronchiectasis in Guangzhou, southern China. Respirology. 2015;20(5):739–748. doi: 10.1111/resp.12528. [DOI] [PubMed] [Google Scholar]
- 35.Pasteur MC, Bilton D, Hill AT. British thoracic society guideline for non-CFbronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 36.Padilla-Galo A, Olveira C, de Rota-Garcia FL, Marco-Galve I, Plata AJ, Alvarez A, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: A study in 398 patients. Respir. Res. 2018;19(1):43. doi: 10.1186/s12931-018-0746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens WW, Peters AT, Hirsch AG, Nordberg CM, Schwartz BS, Mercer DG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. Pract. 2017;5(4):1061–1070. doi: 10.1016/j.jaip.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceylan E, Gencer M, San I. Nasal polyps and the severity of asthma. Respirology. 2007;12(2):272–276. doi: 10.1111/j.1440-1843.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 39.Guilemany JM, Angrill J, Alobid I, Centellas S, Pujols L, Bartra J, et al. United airways again: High prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy. 2009;64(5):790–797. doi: 10.1111/j.1398-9995.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 40.Shteinberg M, Nassrallah N, Jrbashyan J, Uri N, Stein N, Adir Y. Upper airway involvement in bronchiectasis is marked by early onset and allergic features. ERS Monogr. 2018;4(1). [DOI] [PMC free article] [PubMed]
- 41.Martínez-Rivera C, Crespo A, Pinedo-Sierra C, García-Rivero JL, Pallarés-Sanmartín A, Marina-Malanda N, et al. Mucus hypersecretion in asthma is associated with rhinosinusitis, polyps and exacerbations. Respir. Med. 2018;135:22–28. doi: 10.1016/j.rmed.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Richards JC, Lynch D, Koelsch T, Dyer D. Imaging of Asthma. Vol. 36, Immunology and Allergy Clinics of North America. W.B. Saunders; 2016. p. 529–45. [DOI] [PubMed]
- 43.Ash SY, Diaz AA. The role of imaging in the assessment of severe asthma. Vol. 23, Current Opinion in Pulmonary Medicine. Lippincott Williams and Wilkins; 2017. p. 97–102. [DOI] [PMC free article] [PubMed]
- 44.Wang D, Luo J, Du W, Zhang LL, He LX, Liu CT. A morphologic study of the airway structure abnormalities in patients with asthma by high-resolution computed tomography. J. Thorac. Dis. 2016;8(10):2697–2708. doi: 10.21037/jtd.2016.09.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available in order to preserve participant confidentiality.