Abstract
In the current study, an eco-friendly management technology to improve young carob (Ceratonia siliqua L.) tree tolerance to water deficit was set up by using single or combined treatments of arbuscular mycorrhizal fungi (AMF) and/or compost (C). Two groups of young carob have been installed: (i) carob cultivated under well-watered conditions (WW; 70% field capacity (FC)) and (ii) where the plants were drought-stressed (DS; 35% FC) during 2, 4, 6, and 8 months. The effect of used biofertilizers on the course of growth, physiological (photosynthetic traits, water status, osmolytes, and mineral content), and biochemical (hydrogen peroxide (H2O2), oxidative damage to lipids (malondialdehyde (MDA), and membrane stability (MS)) traits in response to short- and long-term droughts were assessed. The dual application of AMF and C (C + AMF) boosted growth, physiological and biochemical parameters, and nutrient uptake in carob under WW and DS. After eight months, C + AMF significantly enhanced stomatal conductance by 20%, maximum photochemical efficiency of PSII by 7%, leaf water potential by 23%, chlorophyll and carotenoid by 40%, plant uptake of mineral nutrients (P by 75%, N by 46%, K+ by 35%, and Ca2+ by 40%), concentrations of soluble sugar by 40%, and protein content by 44% than controls under DS conditions. Notably, C + AMF reduced the accumulation of H2O2 and MDA content to a greater degree and increased MS. In contrast, enzyme activities (superoxide dismutase, catalase, peroxidase, and polyphenoloxidase) significantly increased in C + AMF plants under DS. Overall, our findings suggest that the pairing of C + AMF can mediate superior drought tolerance in young carob trees by increasing leaf stomatal conductance, cellular water content, higher solute concentration, and defense response against oxidative damage during the prolonged period of DS.
Subject terms: Arbuscular mycorrhiza, Drought
Introduction
Mediterranean ecosystems are characterized recently by scarce and irregular rainfall year-to-year and prolonged periods of drought—usually lasting for several months—, which alter them and cause several negative impacts1. Drought is becoming one of the most critical environmental stresses outside of plants’ physiological limits and is causing a substantial decline in crop productivity, particularly in arid and semi-arid regions2,3. This constraint affects plants' morphological, physiological, and nutritional traits, including water content, leaf water potential, photosynthetic pigment, stomatal conductance, photochemical efficiency of PSII, and mineral nutrients uptake4. Drought also affects membrane stability, leading to lipid and protein damage due to reactive oxygen species (ROS)5. Nevertheless, drought-inducing detrimental effects on growth and crop productivity depend on several factors: the level of stress experienced, stress length, the rainfall pattern during the growing season, and the soil physical and chemical properties6. Recent widespread of Mediterranean forests and scrublands mortality associated with drought and/or temperature stress will only worsen over the next few decades7, thereby potentially affecting biodiversity, risk of wildfire, nutrient cycling, hydrology, land–atmosphere interactions, and resilience of ecosystems services supplied to humans on a regional to global scale8. The substitution of some species by others more resilient, improving our understanding of the plant capacity to acclimate/adapt to ‘new’ conditions and emergent occurring phenomena—e.g. forest fires and avalanches—and successful forest management strategies should be prioritized to minimize adverse effects.
The carob (Ceratonia siliqua L.) is considered an essential component of the arboreal flora of the Mediterranean basin owing to its drought tolerance capacity and adaptation to degraded and impoverished plants nutrient soils9,10. The carob tree was widely known as the ‘black gold’ due to the numerous health benefits and nutritional value—rich in dietary fibres, minerals, and low in lipids, has abundance in D-pinitol and antioxidants, such as polyphenols and tannins—, thermo-tolerant volatile organic compounds aroma, and livestock feed in agroforestry systems11–13. Carob leaves and pods have numerous functional, pharmaceutical, and cosmetic properties and applications. Recent studies have shown that this plant extracts exhibit antioxidant, antidiarrheal, antibacterial, antifungal, anti-inflammatory, antidiabetic activities, and hepatoprotective and antiproliferative effects14,15. It was reported that the carob tree could be used as a neurodegenerative disorders therapy14 and in the prevention of free radical related diseases as a natural food supplement16. Moreover, the carob tree is generally used in biotechnological fields as a natural bioactive resource for the food industry. Indeed, the locust bean gum derived from the seed coat is used as a food additive (E410), having thickening, stabilizing, flavoring and emulsifying functions17. In addition, the seeds are also used in cosmetic industries (e.g. associated with its antioxidant/preservative function)18.
Traditionally, the carob is an indigenous drought- and temperature-tolerant tree, benefitting thereby the agricultural economy—136,540 tons per year worldwide—, however, the increase in labor cost and the era of ever-lower pricing in the international market gradually resulted in the abandonment of the crop9,19. The drought tolerance of carobs depends on morphological, physiological, and biochemical mechanisms that reduce stress20,21.
To combat climate change-induced drought in Mediterranean crops, management practices and strategies that allow plants to resist abiotic stresses are urgently required and should be exploited to improve agricultural production and protect crops and soil quality22. The long-term sustainable development of natural resource management is increasingly supported by integrating environmental health and economic profitability. The design of integrated biological agroecosystems based on soil nutrient cycling would help maintain an economical production system. Thus, the application of biofertilizers such as organic fertilizers (compost) and beneficial symbiotic microorganisms (i.e. arbuscular mycorrhizal fungi) has emerged as a potential solution to improve soil nutrients and water availability, productivity, and resistance of plants to harsh environmental conditions, particularly in forestry programs23–26. Recently, using organic compost as an alternative to chemical fertilizers has become a global consensus increasingly. It has been demonstrated to be efficient to improve the resilience, yield, and tolerance of plants to harsh conditions26. Compost application could enhance mineral nutrition, soil organic matter content, and soil properties such as water-holding capacity27,28. Previous studies have shown that the application of organic fertilizer in soil enhanced tolerance to environmental stresses through, among others, enhanced microbial activity and soil fungal to the bacterial ratio29. By contributing significantly to the acquisition of nutrient release of the compost through biochemical transformations, microorganisms play an essential role to ensure stable, safe and sustainable agricultural and biomass production30. The symbiotic association between arbuscular mycorrhizal fungi (AMF) and plant roots is one of the most known beneficial interactions in soil31. Thus, they are collecting growing interest as natural fertilizers.
AMF form intimate associations with about 80% of land plant species, with the fungus gaining photosynthate in return for supplying the host plant with mineral nutrients and water32. AMF increase plant drought resistance owing to the well-developed extra-radical mycorrhizal mycelia that proliferate in the bulk soil beyond the rhizosphere and increase the soil volume connected to the plant31. These mycelia's ability to enlarge the absorption range of roots accelerates the water and nutrients uptake, especially the P element, which moves 10 × faster in mycelium than in roots33,34. The mycorrhizal symbiosis can also help the host plants to improve their performance under water stress situations by increasing the stomatal conductance and photosynthesis35. AMF can mitigate the detrimental effect of drought stress by restraining reactive oxygen species (ROS) overproduction in plant cell36,37. Less accumulation of ROS in mycorrhizal plants is owing to the enhancement of non-enzymatic antioxidants (e.g. ascorbate and glutathione) concentrations and/or antioxidant enzyme (e.g. superoxide dismutase, catalase, peroxidases) activities38, and/or AMF-mediated ROS signaling, such as root H2O2 effluxes39. Under drought conditions, plants alter water relations by synthesizing osmolytes to maintain turgidity pressure and cellular functions necessary for the metabolic processes40. One of the crucial biotic soil factors determining the fungal inoculant's success is the indigenous mycorrhizal community. Indeed, different combinations of AMF taxa differentially interact and can exhibit functional complementarity41,42. Therefore, inoculation of compatible AMF—and selecting plant species—could be an effective method for re-vegetation in the arid area. However, the management practices that integrate a better compost application combined with soil microbes' beneficial roles remain poorly investigated 24,43, and the associated regulatory mechanisms remain mostly obscure. In the current study, we attempted to (1) investigate the functionality of compost amendment and/or AMF inoculation on young carob tree to improve its drought tolerance as a high priority for the ideal plant for afforestation, and (2) assess the development of growth, physiological, and biochemical responses involved in carob under the implementation of eco-friendly cultivation practices and subjected to long-term drought. The obtained results will provide a deeper understanding of the mechanisms of carob tolerance to long-term lack of water and pave the way for the theoretical basis for applying emerging technologies and management of crop stress tolerance in the ecologic restoration of the arid forest. Therefore, we hypothesized (1) that the drought-induced reduction of carob trees will be mitigated by native AMF and/or compost. Moreover, we expected (2) that interactive effects of AMF and compost could induce greater growth and stress tolerance of carob through improved photosynthetic apparatus, alleviated oxidative damage, and enhanced ROS generation and scavenging via its modulatory role in the activities of antioxidant enzymes under stressful condition.
Results
Mycorrhizal status and carob growth
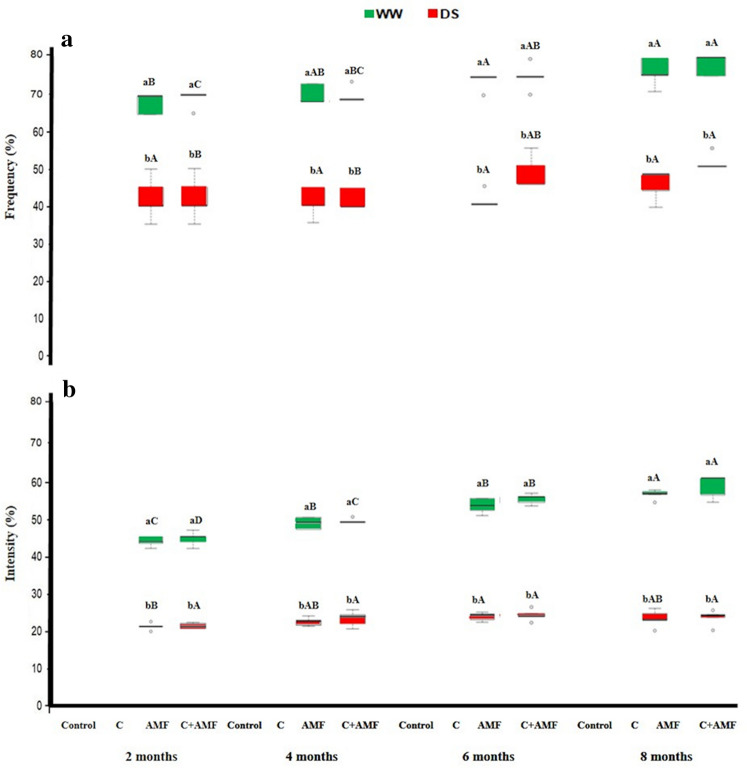
There was no AM colonization detectable in untreated plants (Fig. 1). The frequency and intensity of mycorrhization of young carob tree roots were significantly affected by AMF, compost, drought, and their interactions (Table 1). Plants inoculated with AMF consortium alone or in combination with compost (AMF and C + AMF) yielded higher frequency (F% > 67%) and intensity (I% > 48%) of mycorrhization percentages under WW regime than DS conditions. Indeed, under WW, the intensity of infection was ca. 2 × higher in C + AMF compared to those inoculated and amended plants subjected to DS along with the carob culture. Under DS, mycorrhizal frequency remained stable throughout the 2-, 4-, 6-, and 8-months drought periods in AMF inoculated plants, while the addition of compost increased + 20% the F% in the 2–8 period (Fig. 1). Under DS, mycorrhizal intensity recorded no significant development for all treatments over time except for AMF treatment, which significantly increased in the 2–6 period.
Figure 1.
Effects of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) AMF infection frequency and (b) intensity in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regimes, and the lower case represents the differences within a one-time point.
Table 1.
ANOVA test results for independent variables; (A) AMF, (C) compost, (D) drought, and (T) timing of the measured parameters in carob.
| Parameters | A | C | D | T | A × C | A × D | A × T | C × D | C × T | D × T | A × C × D | A × C × T | A × D × T | C × D × T | A × C × D × T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of mycorrhization (F%) | *** | *** | *** | *** | *** | *** | *** | ** | * | NS | ** | * | * | NS | NS |
| Intensity of mycorrhization (I%) | *** | * | *** | *** | * | *** | *** | NS | NS | *** | NS | NS | NS | NS | NS |
| Shoot Height (SH) | *** | *** | *** | *** | *** | * | *** | * | *** | *** | NS | * | * | NS | * |
| Root Length (RL) | *** | *** | *** | *** | NS | NS | * | NS | NS | *** | NS | NS | NS | NS | NS |
| Shoot Dry Matter (SDM) | *** | *** | *** | *** | * | NS | *** | NS | *** | *** | *** | *** | *** | NS | ** |
| Root Dry Matter (RDM) | *** | *** | *** | *** | NS | ** | *** | *** | NS | *** | ** | * | * | NS | * |
| Leaf water potential (ΨLeaf) | *** | *** | *** | *** | *** | *** | NS | *** | NS | *** | NS | * | * | NS | NS |
| Relative Water Content (RWC) | *** | *** | *** | *** | *** | *** | NS | *** | NS | *** | NS | * | * | NS | NS |
| Stomatal conductance (gs) | *** | *** | *** | *** | ** | * | * | *** | * | ** | * | NS | NS | * | * |
| Chlorophyll fluorescence (Fv/Fm) | *** | *** | *** | NS | *** | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Chlorophyll a (Chl a) | *** | *** | *** | *** | ** | * | NS | *** | NS | *** | *** | ** | ** | NS | *** |
| Chlorophyll b (Chl b) | *** | *** | *** | *** | *** | *** | NS | ** | * | *** | *** | * | ** | * | NS |
| Total Chlorophyll (Chl T) | *** | *** | *** | *** | *** | *** | NS | NS | * | *** | *** | * | ** | * | NS |
| Carotenoid (Car) | *** | *** | *** | *** | *** | *** | *** | *** | *** | NS | * | *** | *** | NS | *** |
| Total soluble sugar (TSS) | *** | *** | *** | *** | NS | * | *** | NS | *** | ** | NS | *** | *** | *** | *** |
| Protein | *** | *** | *** | *** | NS | ** | *** | NS | NS | *** | NS | NS | NS | NS | NS |
| Membrane stability (MS) | *** | *** | *** | *** | *** | *** | NS | NS | NS | *** | * | ** | ** | NS | NS |
| Hydrogen peroxide (H2O2) | *** | *** | *** | *** | *** | NS | *** | ** | ** | *** | NS | *** | *** | *** | *** |
| Malondialdehyde (MDA) | *** | *** | *** | *** | *** | *** | *** | *** | NS | ** | NS | *** | *** | *** | NS |
| Nitrogen (N) | *** | *** | *** | *** | *** | *** | ** | *** | NS | NS | * | NS | NS | NS | * |
| Phosphorus (P) | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** | *** | *** | *** | *** | *** |
| Potassium (K) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Calcium (Ca) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Superoxide dismutase (SOD) | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | *** | *** | ** | ** | *** |
| Catalase (CAT) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** | *** | *** |
| Peroxidase (POX) | *** | *** | *** | *** | *** | *** | NS | *** | NS | * | *** | * | *** | ** | NS |
| Polyphenoloxidase (PPO) | *** | *** | *** | *** | ** | * | *** | NS | *** | NS | NS | NS | NS | NS | NS |
NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
The growth of carob seedlings significantly (P < 0.001) varied depending on the water regime, biofertilizers application, and length of treatments (Table 2). Biofertilizers benefited the shoot and root dry weights, plant height, and root length in young carob under WW and DS conditions. At short-term, 2-month drought stress, the young control carob showed a significant decrease (P < 0.001) on growth parameters including shoot height (SH) by −19.4%, root length (RL) by −17.9%, shoot dry matter (SDM) by −41.4% and root DM (RDM) by −32% than WW regime. This decrease has been exacerbated after eight months of stress to reach ca. −58% for SDM and −41% for RDM than WW plants. The application of AMF and/or organic amendments allowed the growth and development of carob in drought conditions. Following a 6-month drought, the application of C and AMF alone and their combination C + AMF significantly improved SH and RL to a greater extent than in non-inoculated and non-amended plants. Moreover, C + AMF showed the highest SDM and RDM values under WW and DS compared to untreated plants. The carob treated with C + AMF under DS was significantly (P < 0.05) taller with higher biomass than controls. This highest gain in biomass accumulation under DS was more important in shoots (59%, 65%, 70% and 92%) than in roots (12%, 12%, 19%, and 21%) at 2, 4, 6, and 8 months, respectively compared to WW. Under DS conditions, all treatments showed a great improvement throughout the 2-, 4-, 6-, and 8-months drought period. The effect of interactions among all factors (AMF x compost x drought x timing) was significant (P < 0.05) for SH, SDM, and RDM (Table 1).
Table 2.
Growth parameters (SH: Shoot Height, RL: Root Length, SDM: Shoot Dry Matter, RDM: Root Dry matter) in carob under different water status (WW: well-watered, DS: drought stress) and/or biofertilizers (C: compost, AMF: arbuscular mycorrhizal fungi, C + AMF: compost and AMF consortium combination) after 2, 4, 6, and 8 months of treatments’ application.
| SH (cm) | RL (cm) | SDM (g) | RDM (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WW | DS | WW | DS | WW | DS | WW | DS | ||
| 2 months | Control | 24.2 ± 0.7 aD | 19.5 ± 0.8 bC | 19.6 ± 1.8 a-dB | 16.1 ± 1.3 eC | 2.9 ± 0.5 a-cD | 1.7 ± 0.3 dD | 2.5 ± 0.6 bcB | 1.7 ± 0.2 dB |
| C | 25.2 ± 1.0 aC | 20.6 ± 1.0 bC | 20.2 ± 1.3 a-cC | 17.4 ± 0.8 deC | 3.2 ± 0.5 abD | 2.3 ± 0.4 cdD | 3.8 ± 0.1 aC | 1.8 ± 0.2 cdB | |
| AMF | 24.9 ± 1.0 aD | 20.1 ± 0.8 aD | 21.0 ± 1.5 abC | 17.9 ± 1.3 cdeC | 3.6 ± 0.2 aD | 2.6 ± 0.5 bcD | 2.9 ± 0.5 abC | 1.6 ± 0.4 dB | |
| C + AMF | 24.8 ± 1.4 aC | 20.2 ± 0.7 bC | 22.2 ± 1.0 aC | 19.1 ± 1.0 b-dC | 3.7 ± 0.3 aD | 2.8 ± 0.2 bcD | 2.8 ± 0.6 abA | 1.9 ± 0.2 cdC | |
| 4 months | Control | 32.7 ± 0.7 bC | 23.9 ± 0.6 dB | 24.4 ± 1.3 aA | 19.3 ± 1.0 cAB | 5.0 ± 0.2 bC | 2.5 ± 0.2 eC | 3.1 ± 0.1 bB | 1.9 ± 0.2 cB |
| C | 33.8 ± 1.2 abB | 25.7 ± 1.4 cdB | 25.5 ± 0.9 aB | 20.8 ± 1.4 bcB | 5.1 ± 0.5 bC | 3.4 ± 0.2 dC | 4.1 ± 0.2 aB | 1.8 ± 0.2 cB | |
| AMF | 34.8 ± 1.0 aC | 26.1 ± 0.5 cC | 25.4 ± 0.4 aB | 21.2 ± 0.4 bcB | 5.1 ± 0.2 bC | 3.3 ± 0.2 dC | 4.0 ± 0.1 aB | 1.9 ± 0.2 cB | |
| C + AMF | 33.3 ± 0.8 abB | 25.7 ± 0.9 cdB | 26.3 ± 1.6 aB | 22.1 ± 1.6 bB | 6.4 ± 0.2 aC | 4.2 ± 0.2 cC | 3.9 ± 0.5 aA | 2.1 ± 0.5 cC | |
| 6 months | Control | 47.3 ± 3.6 bB | 31.2 ± 0.6 dA | 25.8 ± 0.5 cA | 20.0 ± 0.5 fAB | 8.0 ± 0.7 bB | 3.5 ± 0.3 dB | 3.8 ± 0.4 bA | 2.3 ± 0.2 cAB |
| C | 52.3 ± 0.6 aA | 35.8 ± 1.1 cA | 26.7 ± 0.9 bcAB | 21.5 ± 0.9 efAB | 9.0 ± 0.6 bB | 5.6 ± 0.3 cB | 4.8 ± 0.1 aA | 2.4 ± 0.2 cA | |
| AMF | 53.1 ± 0.6 aB | 37.5 ± 1.1 cB | 29.1 ± 0.9 aA | 23.1 ± 0.9 deAB | 8.6 ± 0.6 bB | 5.4 ± 0.3 cB | 4.9 ± 0.1 aA | 2.6 ± 0.2 cA | |
| C + AMF | 53.0 ± 2.9 aA | 38.4 ± 0.9 cA | 29.0 ± 2.0 abA | 24.4 ± 2 cdA | 11.2 ± 0.8 aB | 6.0 ± 0.2 cB | 5.4 ± 0.9 aB | 2.7 ± 0.4 cB | |
| 8 months | Control | 52.4 ± 0.7 bA | 30.4 ± 0.8 eA | 26.0 ± 0.2 bcA | 21.1 ± 0.9 dA | 10.7 ± 0.4 cA | 4.5 ± 0.3 fA | 4.4 ± 0.3 cA | 2.6 ± 0.5 dA |
| C | 54.6 ± 1.1 abA | 36.2 ± 0.7 dA | 28.0 ± 1.1 abA | 23.7 ± 1.9 cA | 13.4 ± 1.1 bA | 7.1 ± 0.4 eA | 5.0 ± 0.4 bcA | 2.6 ± 0.2 dA | |
| AMF | 55.5 ± 1.0 aA | 39.2 ± 0.7 cA | 29.7 ± 0.9 aA | 23.9 ± 1.2 cA | 13.7 ± 0.7 bA | 7.9 ± 0.4 deA | 5.3 ± 0.6 bA | 2.9 ± 0.4 dA | |
| C + AMF | 56.1 ± 1.8 aA | 39.6 ± 1.9 cA | 29.9 ± 0.7 aA | 25.5 ± 1.5 cA | 15.2 ± 0.3 aA | 8.7 ± 0.4 dA | 6.5 ± 0.4 aB | 3.2 ± 0.1 dA | |
Means (± standard error) within the same parameter, followed by different letters, are significantly different among treatments at P ≤ 0.05. The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
Water relations and mineral uptake efficiency
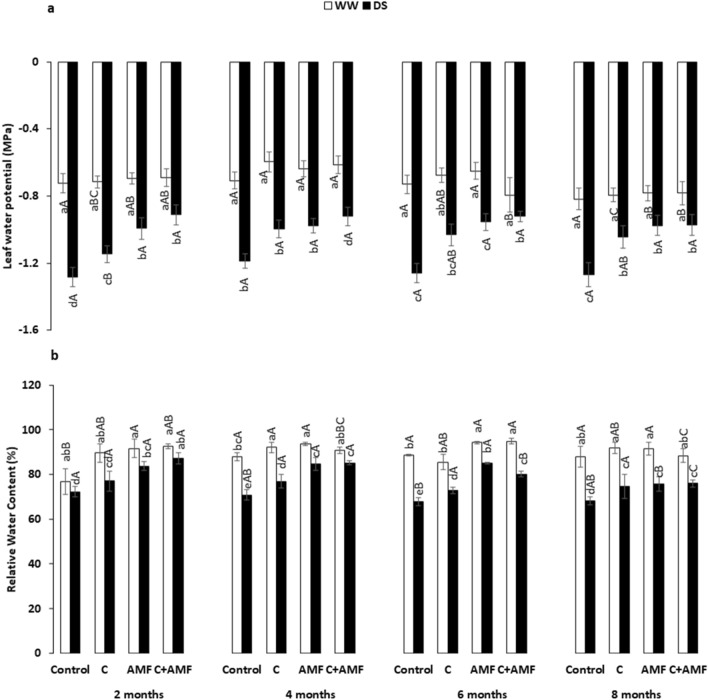
The leaf water potential (ΨLeaf) (Fig. 2a) and relative water content (RWC) (Fig. 2b) significantly (P < 0.05) decreased with the severity of drought in untreated plants. Under WW, there was no significant effect of compost and/or AMF treatments on ΨLeaf, while both treatments single or combined resulted in higher RWC in the short term. Under DS, in contrast, both treatments' effect was mainly significant than untreated plants, with higher values being observed in C + AMF treated plants. The dual application C + AMF increased ΨLeaf by ca. 30, 22, 27 and 23% and RWC by ca. 21, 20, 18 and 11% after 2, 4, 6 and 8 months, respectively, compared to the control plants under DS conditions. There was a significant interaction (P < 0.05) among the three main factors AMF x drought x timing on ΨLeaf and RWC (Table 1).
Figure 2.
Influence of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) leaf water potential (ΨLeaf) and (b) relative water content (RWC) in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regimes, and the lower case represents the differences within a one-time point.
Overall, foliar nutrients concentrations were more significant in young carob inoculated with the AMF assemblage and/or amended with compost, independently of the water regime and the observation time (Tables 1 and 3). Indeed, from short-term, AMF and C + AMF treated plants displayed the highest foliar P, N, K, and Ca concentrations under DS (Table 3). These promotion effects induced by the biofertilizers were observed all along the experiment period. After 8 months, irrespective of water regimes, C + AMF showed a positive synergistic effect on foliar N, P, K, and Ca with the maximum increase (20, 65, 92 and 83%, respectively, under WW and 46, 75, 35 and 40%, respectively, under DS) compared with those untreated plants. The stressed plants did not show any significant variation for each treatment over time. The interactions among AMF, compost, drought, and timing on the nutrients content were significant at P < 0.05 (Table 1).
Table 3.
Carob shoots mineral content subjected to different water status (WW: well-watered, DS: drought stress) and/or biofertilizers (C: compost, AMF: arbuscular mycorrhizal fungi, C + AMF: compost and AMF consortium combination) after 2, 4, 6, and 8 months of treatments’ application.
| P (mg g−1 DW) | N (mg g−1 DW) | K (mg g−1 DW) | Ca (mg g−1 DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WW | DS | WW | DS | WW | DS | WW | DS | ||
| 2 months | Control | 0.81 ± 0.01 eD | 0.84 ± 0.02 deC | 1.92 ± 0.09 aBC | 1.14 ± 0.09 bC | 1.43 ± 0.04 hD | 2.40 ± 0.04 eC | 4.15 ± 0.59 dD | 3.99 ± 0.24 dD |
| C | 0.84 ± 0.02 deC | 0.87 ± 0.01 dC | 1.94 ± 0.33 aC | 1.72 ± 0.18 aD | 2.06 ± 0.06 gD | 2.21 ± 0.02 fD | 4.21 ± 0.44 dD | 4.29 ± 0.32 dD | |
| AMF | 0.87 ± 0.01 dD | 1.18 ± 0.01 bC | 1.94 ± 0.34 aC | 1.85 ± 0.06 aD | 2.61 ± 0.02 dD | 3.70 ± 0.01 aD | 6.86 ± 0.07 aD | 6.02 ± 0.34 bcD | |
| C + AMF | 0.99 ± 0.03 cD | 1.50 ± 0.02 aD | 2.00 ± 0.12 aC | 1.92 ± 0.33 aD | 2.86 ± 0.02 cB | 3.31 ± 0.02 bD | 6.92 ± 0.05 aD | 6.38 ± 0.05 bA | |
| 4 months | Control | 0.99 ± 0.01 eC | 1.04 ± 0.03 dB | 2.07 ± 0.10 bcB | 1.44 ± 0.21 dB | 1.82 ± 0.06 fC | 3.41 ± 0.06 bBC | 6.57 ± 0.61 cC | 6.26 ± 0.19 deC |
| C | 1.02 ± 0.02 deB | 1.20 ± 0.01 cB | 2.23 ± 0.14 bB | 1.98 ± 0.13 cC | 2.38 ± 0.06 eC | 2.76 ± 0.03 dC | 7.43 ± 0.23 cC | 6.56 ± 0.07 dC | |
| AMF | 1.06 ± 0.02 dC | 1.69 ± 0.02 abB | 2.41 ± 0.06 aB | 2.18 ± 0.10 bcC | 2.95 ± 0.04 cC | 3.97 ± 0.02 aC | 8.31 ± 0.20 abC | 7.50 ± 0.12 cC | |
| C + AMF | 1.67 ± 0.04 abC | 1.75 ± 0.03 aC | 2.20 ± 0.14 bBC | 2.35 ± 0.15 abBC | 3.33 ± 0.01 bB | 4.02 ± 0.02 aC | 8.77 ± 0.23 aC | 7.80 ± 0.35 bA | |
| 6 months | Control | 1.11 ± 0.04 fB | 1.20 ± 0.02 eA | 2.17 ± 0.14 bAB | 1.79 ± 0.15 cA | 2.16 ± 0.02 hB | 3.58 ± 0.11 deB | 8.36 ± 0.30 fB | 10.00 ± 0.12 eB |
| C | 1.42 ± 0.03 dA | 1.48 ± 0.05 dA | 2.31 ± 0.17 a-cAB | 2.15 ± 0.03 bB | 2.89 ± 0.02 gB | 3.40 ± 0.05 fB | 10.41 ± 0.33 dB | 10.69 ± 0.16 dB | |
| AMF | 1.72 ± 0.03 cB | 1.81 ± 0.05 bA | 2.61 ± 0.14 aAB | 2.37 ± 0.03 bB | 3.73 ± 0.06 cdB | 4.29 ± 0.04 bB | 12.86 ± 0.12 bB | 11.84 ± 0.48 cB | |
| C + AMF | 1.82 ± 0.01 bB | 1.94 ± 0.03 aB | 2.41 ± 0.10 abB | 2.48 ± 0.03 aB | 3.90 ± 0.03 cB | 4.68 ± 0.02 aB | 13.64 ± 0.27 aB | 13.66 ± 0.22 aA | |
| 8 months | Control | 1.22 ± 0.03 gA | 1.21 ± 0.03 gA | 2.39 ± 0.18 bA | 1.90 ± 0.06 cA | 2.29 ± 0.04 gA | 3.89 ± 0.04 eA | 10.41 ± 0.08 fA | 13.40 ± 0.19 eA |
| C | 1.46 ± 0.02 fA | 1.53 ± 0.02 eA | 2.48 ± 0.06 bA | 2.33 ± 0.13 bA | 3.75 ± 0.03 fA | 3.88 ± 0.02 eA | 16.66 ± 0.12 dA | 16.54 ± 0.20 dA | |
| AMF | 1.92 ± 0.03 cA | 1.87 ± 0.03 dA | 2.76 ± 0.03 aA | 2.73 ± 0.06 aA | 4.31 ± 0.08 dA | 4.90 ± 0.02 bA | 17.63 ± 0.12 cA | 17.47 ± 0.58 cA | |
| C + AMF | 2.01 ± 0.03 bA | 2.12 ± 0.06 aA | 2.87 ± 0.13 aA | 2.78 ± 0.14 aA | 4.39 ± 0.02 cA | 5.27 ± 0.05 aA | 20.07 ± 0.19 aA | 18.74 ± 0.17 bA | |
Means (± standard error) within the same parameter, followed by different letters, are significantly different among treatments at P ≤ 0.05. The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
Stomatal conductance, efficiency of photosystem II, and photosynthetic pigments
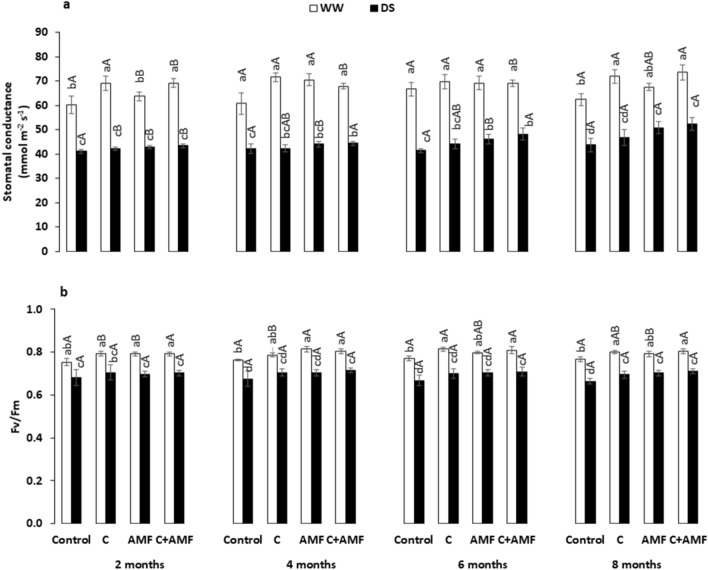
DS significantly decreased (P < 0.001) stomatal conductance (gs) (Fig. 3a) and quantum efficiency of photosystem II (Fv/Fm) (Fig. 3b), being reductions particularly pronounced in the absence of biofertilizers. These parameters increased significantly by C + AMF under DS conditions after 4, 6, and 8 months by ca. 5, 17, and 20%, respectively, for gs and 6, 6, and 7%, respectively, for Fv/Fm compared to the untreated carob. The interaction among AMF, compost, drought, and timing significantly affected gs (P < 0.05).
Figure 3.
Influence of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) stomatal conductance; gs and (b) chlorophyll fluorescence (Fv/Fm) in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regimes, and the lower case represents the differences within a one-time point.
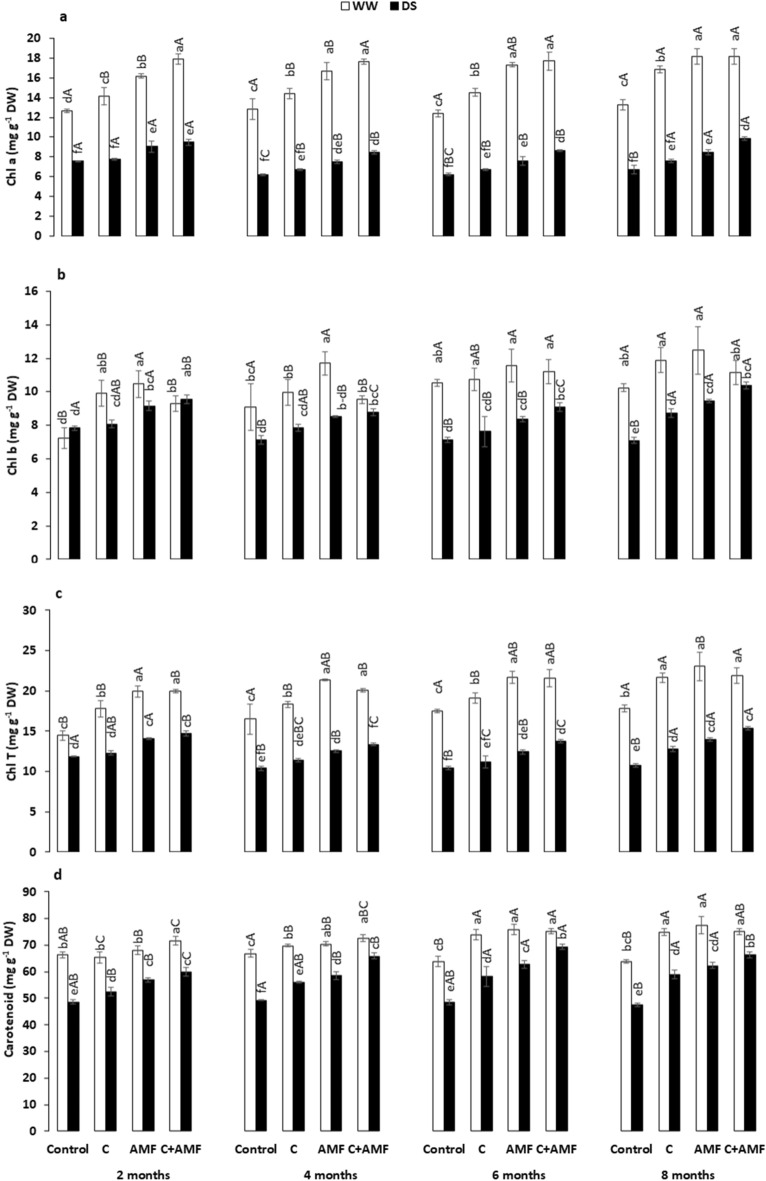
Photosynthetic pigments concentrations decreased significantly (P < 0.001) after drought imposition, independently of the presence/absence of biofertilizers and timing (Fig. 4, Table 1). Under WW, the applied preparations increased the total chlorophyll and carotenoids concentrations in carob leaves, compared with the controls (Fig. 4c and d). The applied biofertilizers (AMF alone and the combination C + AMF) also increased the Chl “a”, “b”, total chlorophylls, and carotenoids content compared to non-inoculated and non-amended plants during an early stage in the first 2 months of the study and the following months throughout the year (Fig. 4a-d). Under DS, the highest level of pigments was yielded in plants treated with C + AMF, where Chl “a” (25, 37, 39 and 47%), “b” (22, 23, 46 and 46%), Chl “T” (24, 28, 32 and 43%), and carotenoids (23, 34, 43, and 45%) concentrations showed an upward trend after 2, 4, 6, and 8 months, respectively. Under DS conditions, these physiological attributes presented no significant difference over time for each treatment except C + AMF, which recorded a high improvement for all these traits, and control treatment showed a significant decrease for photosynthetic pigments throughout the 2-, 4-, 6-, and 8-months. The interaction among AMF, compost, and drought had a significant effect on Chl “a”, “b”, and total Chl (P < 0.001).
Figure 4.
Effects of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) chlorophyll-a; chl ‘a’, (b) chlorophyll-b; chl ‘b’, (c) total chlorophyll; chl ‘T’, and (d) carotenoid in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
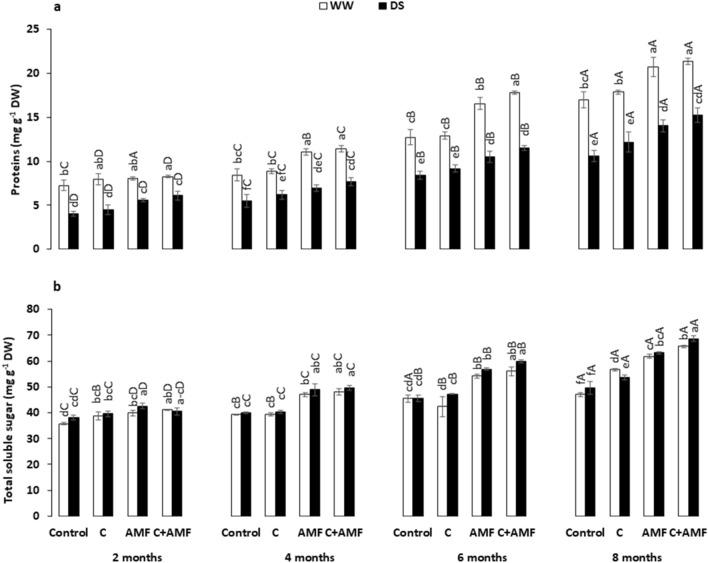
Total soluble sugar and protein content
Under WW conditions, there was no effect of compost on the protein concentrations (Fig. 5a). The application of either AMF and the combination C + AMF treatments increased leaf protein concentrations under both WW—evident from month 4— and DS—from month 2—. The highest values of this parameter under water stress recorded in C + AMF reached ca. 50, 40, 36 and 45%, than respectively untreated plants after 2, 4, 6 and 8 months. The same trend of the protein concentration was observed in the TSS (Fig. 5b), which was increased by AMF and C + AMF applications. The highest amount of TSS was ca. 6, 25, 32, and 40% relative to the control plants after 2, 4, 6 and 8 months. Moreover and for each treatment, these parameters were significantly improved during the experiment period. The interaction among AMF, compost, drought, and timing were significant on TSS (P < 0.001, Table 1).
Figure 5.
Influence of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) proteins and (b) total soluble sugars in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
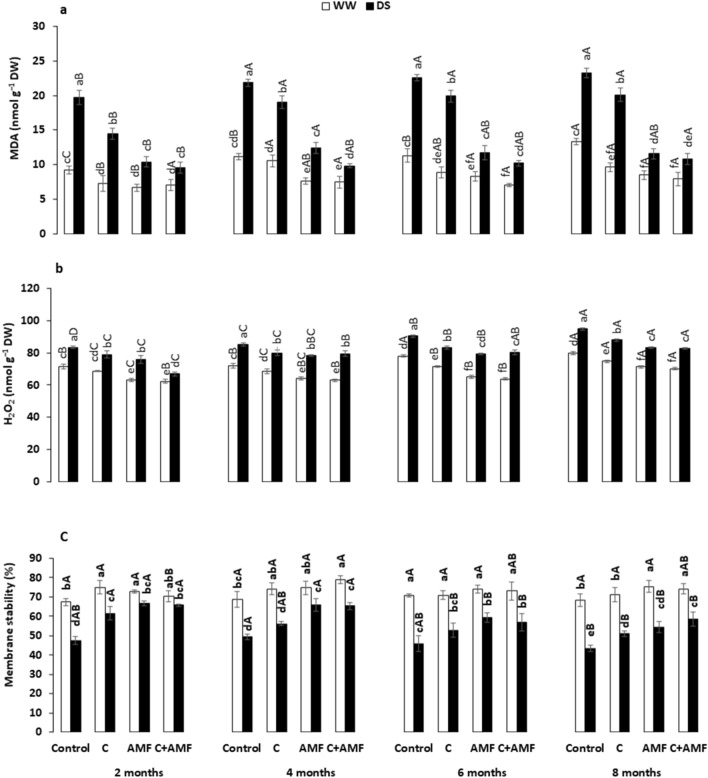
MDA and H2O2 content and membrane stability
Hydrogen peroxide (H2O2) and malondialdehyde (MDA) accumulation, and membrane stability (MS) are indicators of oxidative stress in plant tissues (Fig. 6). As presented in Fig. 6a and b, both MDA and H2O2 concentration significantly increased (P < 0.05) under DS compared to WW regime conditions. Under WW, membrane integrity values exceeded 67% regardless of the applied treatment during all the experiment periods (Fig. 6c). Under DS, in contrast, the membrane integrity steeply decreased below 50% in non-inoculated plants (notably in control plants) compared to AMF-inoculated plants and plants treated with C + AMF, showing values > 56% throughout the stress periods (Fig. 6c). H2O2 and MDA content increased significantly under DS conditions in control plants throughout the 2-, 4-, 6-, and 8-months. Upon applying AMF and/or compost, the H2O2 and MDA content decreased in the leaves under DS.
Figure 6.
Effects of water regimes (WW: well-watered; open bars and DS: drought stress; filled bars) on (a) malondialdehyde; MDA, (b) hydrogen peroxide; H2O2 and (c) membrane stability in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
The lowest concentrations of MDA were observed in the leaves of treated plants with AMF and/or compost, these values being halved in C + AMF plants than control throughout 8 months’ stress. The interaction among AMF, drought, and timing significantly affected MDA and H2O2 (P < 0.001; Table 1).
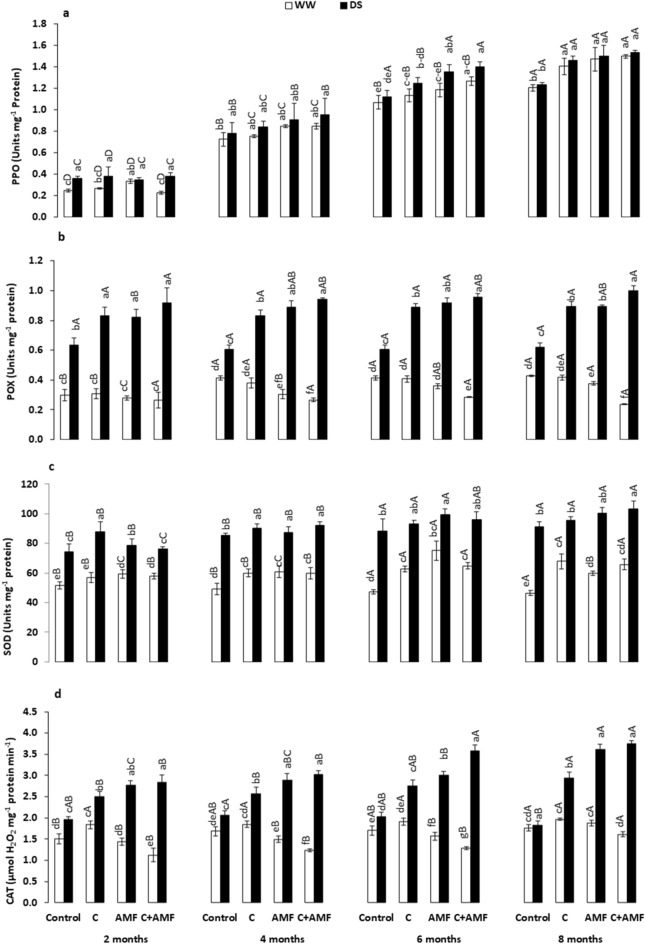
Antioxidant enzymes content
Antioxidant enzymes are essential for scavenging ROS and for promoting plants resistance to droughts stresses. The present study studied the antioxidant enzymes activities on young carob plants under short- and long-term droughts conditions (Fig. 7). The obtained results showed that the activities of antioxidant enzymes (SOD, CAT, POX, and PPO) were significantly increased by biofertilizer treatments under water stress conditions. Under DS, the highest level of antioxidant enzymes was yielded in plants treated with C + AMF, where SOD (3, 8, 9 and 13%), CAT (45, 46, 76 and 104%), PPO (5, 23, 25 and 25%), and POX (45, 56, 58, and 61%) concentration showed an upward trend after 2, 4, 6, and 8 months, respectively. The significant increase in enzymatic antioxidants by applying AMF combined with compost suggested the role of these biofertilizers in young carob responses and their involvement in reducing oxidative damage induced by water stress over time. DS-treated plants showed a significant increase for each treatment over time, except POX values found not significant.
Figure 7.
Effects of water regimes (WW: well-watered; open bars and DS: drought (a) polyphenoloxidase (PPO), (b) peroxidase (POX), (c) superoxide dismutase (SOD), and (d) catalase (CAT) activity in control carob plants and plants amended with compost (C) and/or assemblage of indigenous arbuscular mycorrhizal fungi (AMF). Data are mean ± SE (n = 5). Means followed by the same letters within the same time point are not significantly different at P < 0.05 (Tukey’s HSD). The upper case represents the differences within WW or DS water regime, and the lower case represents the differences within a one-time point.
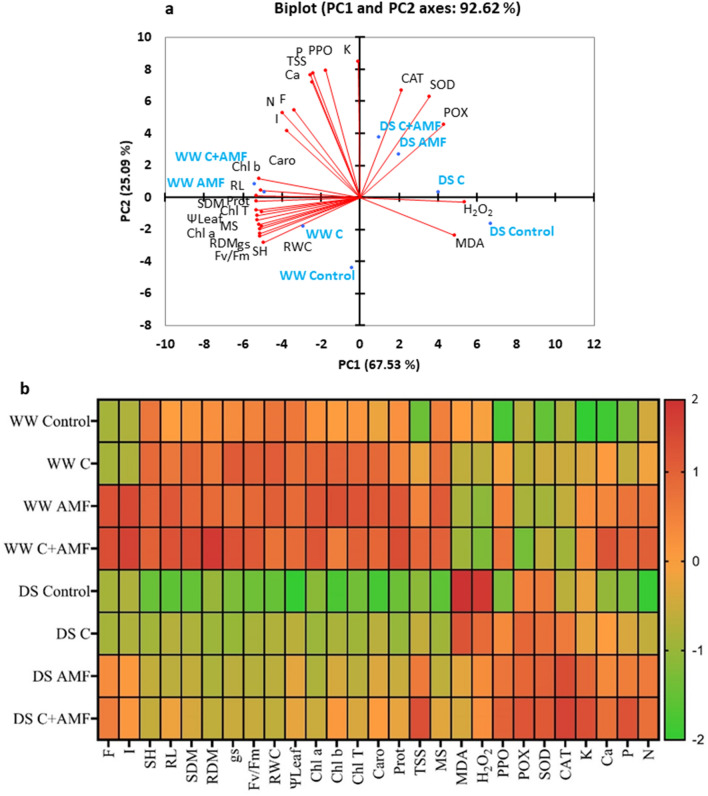
Principal Component Analysis (PCA) and Heat mapping for growth, physiological, and biochemical traits of carob
Principal component analysis (PCA) considered all the analyzed parameters of the different treatments under both water regimes. Two main components accounted for 92.6% of the variability observed in the data, with 67.5% for PC1 and 25.1% for PC2 (Fig. 8a). Under the WW condition, PCA analysis showed a positive correlation among the AMF and/or compost for growth, physiological and water parameters, photosynthetic pigments, and protein content, which were highly correlated to each other. The analysis also confirmed the negative impact of DS conditions on these parameters. The biplot revealed the positive correlation linking control DS treatment and MDA and H2O2 content. Under DS conditions, PCA showed a clear positive correlation between the antioxidant system (SOD, CAT, and POX) and osmolytes (P, N, K, Ca and TSS) and the AMF application alone or combined with compost.
Figure 8.
(a) Principal component and (b) Heat map analysis of the studied traits in young carob grown under WW (well-watered; open bars) and DS (drought conditions; filled bars) and treated or not (control) with AMF an/or compost. AMF: arbuscular mycorrhizal fungi; C: compost; F: frequency of mycorrhization; I: intensity of mycorrhization; SH: shoot height; RL: root length; SDM: shoot dry matter; RDM: root dry matter; ΨLeaf: Leaf water potential; RWC: relative water content; gs: stomatal conductance; Fv/Fm: chlorophyll fluorescence; Chl a: chlorophyll a; Chl b: chlorophyll b; Chl T: total chlorophyll; Car: Carotenoid; TSS: total soluble sugar; Prot: Protein; MS: membrane stability; H2O2: hydrogen peroxide; MDA: malondialdehyde; N: nitrogen; P: phosphorus; K: potassium; Ca: calcium; SOD: superoxide dismutase activity; CAT: catalase activity; POX: peroxidase activity and PPO: polyphenol oxidase activity.
The heat map indicated the relative performance of the treated plants in both well-watered and water stress conditions. According to the color scale, the green darker color represents the highest values, while the darker red bands represent the lower values of carob seedlings under both conditions (Fig. 8b). SH, RL, SDM and RDM values showed moderate trends under DS conditions in the inoculated and composted plants compared to the control plants under normal conditions. Physiological parameters (gs, Fv/Fm, Chl a, b, T and carotenoids) showed a considerable variation between C + AMF carob seedlings under normal and stress conditions, but no significant change was found between C + AMF plants under DS and control plants under WW conditions. Under DS, leaf water potential and stress markers (MDA and H2O2) showed a contrasting effect under stress conditions in the control plants, as they had higher values than C + AMF carob seedlings under water stress conditions. In addition, enzyme activities (SOD, CAT, PPO, and POX) showed the highest intensities with increasing water deficit and were remarkably high in C + AMF carob plants than controls, showing thereby their direct involvement in the drought stress response mechanisms. The organic (proteins and sugars) and non-organic (P, N, Ca, and N) osmolytes increased following the stress conditions. This indicates that the osmolyte content depends mainly on the growing conditions (presence/absence of stress and/or biofertilizers), and this stress increases the accumulation of osmolytes in the leaves, as shown in Fig. 8b.
Discussion
We investigated herein the effect of beneficial AMF and compost on growth, mineral uptake, physiology, and metabolites and ROS accumulation of Ceratonia siliqua cultivated under WW and DS conditions. The present study confirms AMF inoculation and compost addition effects on plant growth under water stress conditions. Furthermore, we suggest that the dual application of AMF and compost provides superior plant growth in the long term compared to AMF or compost applied separately. The improvement in growth and biomass accumulation when AMF and compost were applied in combination follows the previous studies25,44,45. The dual application of both biofertilizers improves plant growth and biomass accumulation, and water stress tolerance over time44. Bona et al.46 reported that inoculated strawberry plant by commercial AMF containing R. intraradices, G. ageratum, G. viscosum, C. etunicatum, and C. claroideum with organic amendment had higher growth than untreated plants. Likely, the improvement in plant growth observed with the dual application of AMF and compost may be due to the capacity of the AMF to acquire nutrients and water for the plants under DS conditions47–49. Besides, AMF symbiosis can increase the uptake of certain nutrients such as P and N, which directly benefits plant growth when organic fertilizers are used50. Phosphorus influences photosynthesis, biosynthesis of protein and phospholipids, membrane transport and cell division, which led to increased roots and shoots development, plant height, and dry biomass accumulation51. Phosphorous’s regulatory function in photosynthesis and carbohydrate metabolism of leaves can be considered one of the major factors limiting plant growth52. Previous studies have revealed the importance of using low doses of compost in combination with symbiotic microorganisms48,53,54. The addition of low dose compost, considered the optimal dose for plant growth with AMF, provides a reliable and predictable supply of nutrient “slow-release”, thereby allowing the AMF colonization of roots and stimulating the functioning of the complex compost-AMF, especially in soils with low nutrients55–57. These biostimulants (compost-AMF), applied individually or in combination, can improve crop production in less fertile soil systems. In contrast, an excessive application of compost amendment (≥ 20%) can hinder plant yield by, among other, altering/reducing microbial communities58,59.
The enrichment of soils low in organic matter by compost-derived substances provides many biological functions60. It can release and store nutrients and growth for plants, promote biological activity as a source of energy for microorganisms, and positively affect the structuring, physicochemical properties, and aeration of the soil28,61. As suggested by Roldán et al.62, the combined treatment of root mycorrhizal inoculation and soil amendment positively affect aggregate stability owing to the polysaccharides’ role. The greater uptake of nutrients led to enhanced root and shoot development, plant height, and dry matter accumulation under DS conditions63,64. It is interesting to note that compost and AMF had a generally synergetic effect on plant growth and AMF colonization under DS25.
In this study, no mycorrhizal structure was observed in the roots of non-inoculated carob seedlings, but the native mycorrhizal consortium successfully infected the AMF and C + AMF young carob. Several studies showed that root colonization decreased in the host plants under drought stress65,66. A short-term soil drought did not appear to discourage root colonization20, whereas a long-term soil drought considerably decreased root colonization and hyphal growth67–69. It is worth noting that the native soil composition and host plant genotype are also considered important in the recruitment of microbiome by the host plant. The functional profile of the microbial communities might be correlated with changes in community composition at the species level, suggesting that ‘suitability phenomena’ between the host plant and its native microbial communities, rather than a “generalistic” inoculum, may be functionally driven rather than driven by phylogeny70. Previous studies reported the effectiveness of native inoculum, which promotes and maintains plant growth despite the low colonization percentages under abiotic stresses22,26,57. Essahibi et al.72 reported that long-term drought stress decrease root colonization in carob seedlings inoculated with Rhizophagus intraradices, R. fasciculatus and Funneliformis mosseae. Furthermore, several studies showed that environmental conditions such as soil nutrition, moisture, temperature, and host genotype and age directly influence root and leaf microbial communities73–79. Our results showed that the application of green waste compost in sandy soil favored the spore production of the native AMF consortium, thereby improving the infectious propagules of carob plants. This may be related to the inherent capacity of the used consortium to sporulate or favorable interaction, due to C availability, with the carob host plant. Chaiyasen et al.78 showed that inoculation with indigenous AMF consisting of Claroideoglomus etunicatum, C. etunicatum, and Funneliformis mosseae in sandy soil amended with green waste compost containing 214 mg/Kg P improved spore numbers and the infectious propagule production in maize. Our results indicated that compost addition enhanced root colonization after long-term (after six months) compared to plants treated with AMF alone under DS. These results were consistent with previous studies reporting that compost and/organic amendment addition most often positively affected AMF growth under DS25,78,80. Several research had confirmed that water status in dry soil strongly altered AMF spore survival and germination and inhibited the spread of hyphae67,81,82. AMF exclusively found in the native habitat of wild relatives of crop plants comprise representatives of Glomus, Rhizophagus, Funneliformis, Acaulospora, Entrophospora, Gigaspora, and Scutellospora. The genus Glomus and Glomus related genera are known for its maximum germination82, and greater absorption of minerals and water under soil drying conditions66 and its capabilities to produce bioactive compounds (e.g. lysophosphatidylcholine) eliciting overexpression of fatty acid biosynthesis genes involved in the enhancement of AMF-host plant interactions83. Species including R. intraradices, F. mosseae, R. clarus, R. diaphanum can be found as indigenous soil inhabitants. Their exclusive presence in the native soil may be associated with their ability to improve the expression levels of nutrient transporter genes in fungal absorptive hyphae to increase mineral nutrients availability and translocation to the host plant84, thereby contributing to nutrient cycling in the undisturbed native soils85. These characteristics may ultimately affect seedling emergence, root development, and plant performance under (un) favorable conditions86. AMF facilitate seedlings establishment by enabling them to get access to limiting nutrients87. The diversity and abundance of Glomus species in soil, and their diversity in metabolic traits, makes Glomeraceae a potentially important family in soil nutrient cycling88,89. The genus Acaulospora, which belongs to the phylum Acaulosporaceae, encompasses strictly aerobic chemo-organotrophs that are adapted to acidic conditions90. Acaulospora members are generally known to produce a lot of mycelium but were slow to colonize the host plants likely because they mainly colonized from spores, as did the members of the Gigasporaceae91. Several studies indicate that extraradical mycelium (ERM) is highly infective in the Glomeraceae and Acaulosporaceae whereas members of the Gigasporaceae regenerate mostly from spores92. Gigasporaceae can live and reproduce with a small portion of C available resources93. Scutellospora and Gigaspora—have thick and strong hyphae—were likely able to colonize a new substrate area by themselves without the support of adjacent hyphae94. The healing process in these two fungi is presented as the most probable means of maintaining the viability of the hyphae in adverse conditions95,96. These AMF genera, when re-introduced into forestry soils, were able to establish and survive in the rhizosphere of carob and provided additional life-support functions (growth, tolerance) for the plants under DS. The organic amendments increased the mycorrhizal root colonization and hyphal length in soil62. The compost is rich in humic acid, which was reported to stimulate AMF hyphal network97. Medina et al.98 showed an increase in hyphal length and AMF activity grown in compost-free soil. In contrast, low root colonization of plants grown in soils non-amended with compost owing to the lack of suitable organic substrates25. This limitation may be due to reduced mycorrhizal hyphal growth, spore germination, and carbohydrate supplement from host plants under DS99. In the present study, compost maintained moderate levels of P, N, K, and water status. Therefore, the improvement in root colonization in the combination of compost and AMF is owing to the beneficial effects of compost on hyphae growth and spore germination under stressful conditions80. Besides, the positive effect of compost on plant growth and photosynthesis, thus possibly increasing carbohydrates transfer to AMF, indirectly improves mycorrhizal growth in roots. The dynamics of root colonization in the present study showed low mycorrhizal infection in the carob roots at early growth period (2 months after drought application) than late growth period (8 months after drought application). The AM fungal spore composition, therefore, might be affected by the altered soil, as well as the root mycorrhizal fungal groups, because of plant age or the need to beneficial partners in response to stresses and environmental stimuli. In particular, pioneering studies suggest that plants adopt the “cry-for-help” strategy by changing their root exudation chemistry to recruit beneficial microorganisms to resist (a)biotic stresses100,101. Other works on root colonization carried out on trees elucidated mechanisms of the establishment of the tree root microbiome and of tree root selection. Recently, Fracchia et al.102, indicated that the composition of the final root microbiome in host trees Populus relies on a series of colonization stages characterized by the dominance of different fungal guilds and bacterial community members over time. Pepe et al.103 reported on a time-course basis study that AMF life cycle in the soil, where fungal ERM, growing from both living and dead roots, represents a long-term survival structure functional to the prompt establishment of mycorrhizal symbioses and to the maintenance of soil biological fertility. Also, their data revealed that viability and functionality of certain AMF including F. mosseae and R. irregulare extraradical hyphae were uncoupled from host plant lifespan103. The overall growth dynamic of the microbiome, correlations between the soil properties and the spore densities, as well as the soil properties and root colonizations of chronosequence tree developing remain to be elucidated. Marupakula et al.104 revealed that the full microbiome is likely subjected to a complex dynamic during the colonization process of the roots of pines. The presence of compost, spore germination and mycorrhizal establishment, and plant photosynthesis and root growth, which favored mycorrhizal infection in the roots of older carob seedlings than in the younger ones. Previous studies have shown that the dynamics of mycorrhizal colonization of plant roots is related to soil properties and spore density and varies with plant species, mycorrhizal structures, AMF communities, period of growth, and seasonal conditions105,106.
Under DS, enhanced uptake nutrients are generally regarded as the essential benefit that AMF provide to their host plant107. Moreover, compost is a commonly used organic amendment to increase some nutrients and water storage capacity47,108. The dual application of compost and AMF has been suggested to ensure high uptake nutrients for plants48,58, especially under abiotic constraints such as water deficit62. In the present study, the AMF-compost combination application allowed a high P, K, N, and Ca uptake in the stressed plants than the control under WW conditions. This result corroborates Caravaca et al.96 and Zou et al.109, indicating that the nutrient uptake is more critical in inoculated plants under DS than WW conditions.
Furthermore, the amended soil with compost increased the leaf nutrient contents over the long term in mycorrhizal carob under DS than WW conditions. Ngo and Cavagnaro110 reported that applying organic amendment significantly increases the contents of available nutrients in the soil. Kohler et al.53 indicated that the synergistic relationships between AMF and compost affect the micronutrient elements' mobility in the rhizosphere and improve their bioavailability. Compost can improve soil productivity by increasing water and nutrient status for some limiting nutrients25,47. Compost is an excellent biofertilizer due to its high concentrations of significant macro- (e.g. N, P and K) and micro-nutrients111. Since compost is rich in organic and carbon matter, the transformation of these elements through soil microorganisms' mineralization process could release mineral nutrients in the soil. Moreover, AMF can also help increase the nutrients uptake by external mycelium via establishing an underground network that links the different plants and allows the transfer of these nutrients among them63,112. Under drought stress, the hyphal network still helps host plants sustain greater nutritional uptake67. Nutrient limitations have been overcome after AMF and/or compost addition, which helps the carob tree mitigate the adverse effects of droughts25.
In our study, C + AMF carob seedlings possessed significantly higher water status (leaf water potential and relative water content) under both WW and DS conditions, and the combination C + AMF was considerably higher, among the other treatments, under severe DS than WW conditions. These results were in line with improving the root colonization under WW and DS conditions. Wu et al.67 reported that mycorrhizal hyphae are essential in uptaking and transporting water from the soil to the host plant under DS conditions. However, AMF hyphae are likely to replace aquaporin activity in inoculated plants under water stress conditions113. Zou et al.68 revealed that the root aquaporins showed no changes or down-regulation under DS conditions. Mycorrhization has also improved soil water efficiency, contributing to better water and nutrient absorption to the host plant114. Li et al.115 have shown that organic amendments enhance soil water holding capacity, porosity, and water infiltration rate. In our study, the water status and nutrient content were more critical in plants grown under C + AMF treatment, particularly under long-term stress conditions. These results can be related to the AMF and compost roles in improving the nutrient and water status of the plant under DS. Previous studies demonstrated that the increase in water uptake by AMF resulted from nutrient increase via soil organic amendment26,116. The results obtained are consistent with those of Ortuño et al.80, suggesting that the combination of C + AMF improves water absorption under DS conditions. Moreover, C + AMF provides a higher root hydraulic conductivity contributing to the efficient water absorption by plants. The application of organic amendment and AMF (via hyphal networks) improves the formation of large soil macroaggregates, increasing the available volume of the soil solution under DS conditions117. Hashem et al.116 reported that the higher water uptake in C + AMF plants improves the water movement to evaporation surfaces resulting in increased stomatal opening.
Under DS conditions, the dehydration avoidance by C + AMF plants results from a balance between the capacity of water absorption, stomatal movements, and water distribution in plant tissues118. Haffani et al.119 showed that stomatal closure is another mechanism the plants use to reduce water loss to confront water stress. Our experimental conditions showed that in prolonged DS, stomatal conductance (gs) was significantly reduced in stressed plants. Under these conditions, the combination of AMF and compost contributed to more significant increases in gs. The higher gs in carob trees can be due to several factors, such as increased hydraulic conductance80, increased root-fungi absorptive area, enhancing osmotic adjustment80,118, and/or improved mycorrhizal colonization44. Our data revealed that higher mycorrhizal colonization and osmotic adjustment in plants subject to C + AMF treatment helped improve the plant's water status, as demonstrated by the less negative ΨLeaf values. Plants grown in the presence of AMF and compost often show higher gs under water stress over time80,120. This improvement of physiological traits (higher water status and stomatal opening) in plants amended with compost and inoculated by AMF can lead to an increase in efficiency of photosystem II (PSII) (Fv/Fm) under DS. In this study, the combination of AMF with compost increased Fv/Fm in plants exposed to long-term water stress conditions compared to the control. This result demonstrates that the application of AMF can maintain a normal utilization of light energy in plants photochemical processes under DS. Similarly, some studies have demonstrated AMF inoculation's capacity to increase the maximum quantum yield of PSII under DS20,24,121. Gong et al.122 have reported that two Glomus species significantly increased the Fv/Fm in S. davidii compared to non-inoculated plants under DS conditions. AMF inoculation under DS improved the energy cycling between the reaction center and the chloroplast pool, and it enhanced the efficiency of excitation energy capture by chloroplasts and decreased the injury caused to the photosystem reaction centres123,124.
DS has been reported to affect the photosynthetic apparatus's pigment composition and varies across species due to adaptation to different environments4,125. Differences in short- and long-term pigment pool size responses might contribute to intraspecific variation in photosynthetic C assimilation and photoprotective mechanisms under drought126. Our data showed that the AMF-Compost combination protects the chlorophyll and carotenoids against degradation. The application of compost in soil significantly improved the chlorophyll content in plants under DS conditions127,128. Similarly, the role of AMF to reduce the negative effect of DS by improving chlorophyll and carotenoid content has been demonstrated2,129. Therefore, the beneficial effect of AMF-compost application may be related to compost in improving root colonization. Osmolytes and mineral content increases may, at least partly, mediate the overall increase in chlorophyll content under DS of non-inoculated plants growing in amended soil. Nutrient concentrations were higher in C + AMF treatment than in control, regardless of the stress length and water regime conditions. High water potential to open stomata results in increased C assimilation and higher chlorophyll content under DS40. The beneficial effect of these biofertilizers on plant physiology is not related only to better acquisition of water and nutrients, photosynthesis and chlorophyll content but also to increased carbohydrates and proteins130.
Several studies have reported a positive effect of carbohydrates in regulating the osmotic potential of cells which, in turn, improves water absorption under unfavorable condition131,132. Our data indicated that the concentrations of soluble sugars and protein in treated plants, particularly those amended with C + AMF, significantly increased during all periods of WW and DS. These results agree with several studies using AMF and/or compost under the same conditions26,133. AMF-inoculated carob accumulated more sugar content during the prolonged DS conditions, likely to maintain high hydration and turgor level, which maintain overall physiological activities under harsh environments4,72. Indeed, the carbohydrates accumulation in DS conditions reduced the osmotic potentials in host cells40. The leaf sugar content in inoculated carob was higher than non-inoculated plants, confirming Abbaspour et al.40 findings suggesting that the natural physiological metabolism of non-inoculated plants was less active than amended plants during WW and DS conditions. Under DS, the higher content of soluble proteins in mycorrhizal plants grown in soil amended with compost may explain the strengthening of the non-enzymatic antioxidant defense system by AMF66.
Under DS, the membrane lipids are highly susceptible to reactive oxygen species (ROS) oxidation134,135. The ROS created in plants under DS breaks up lipid membranes, producing MDA, and decreasing membrane stability (MS) in Ceratonia siliqua L cells136. In the present study, the DS elevated MDA content and reduced MS, while AMF inoculation decreased MDA generation and increased carob MS, especially in amended soils throughout the whole year experiment. These results suggest that the C + AMF combination may suppress the high intracellular H2O2 levels and have a vital protective role in reducing cell membrane damage (increase in MS) under DS. Similar results were observed by Duo et al.26 in Festuca arundinacea. Abbaspour et al.40 showed a positive correlation between the low H2O2 and MDA content and water stress tolerance in amended and inoculated plants.
The drought tolerance of carob has been attributed to the induction of the antioxidant enzyme system. The present study showed that water stress significantly increased antioxidant enzymes (SOD, CAT, POX, and PPO) in the carob seedlings over the time. This activity was increased more significantly in treated plants, particularly those grown with the combination of AMF and compost under DS. Similar results were reported previously for different species24,133. In addition, Anli et al.24 showed a significant increase of POX and PPO in plants inoculated with AMF and/or amended with compost than controls under DS. Dumanović et al.137 reported that greater antioxidant activities favour the removal of reactive oxygen species (ROS), which leads to greater drought resistance in plants. Kirova et al.138 and Torun139 demonstrated that SOD and POX activities were increased in plants and related to protection against oxidative damage due to DS. The SOD enzyme transforms superoxide into hydrogen peroxide (H2O2), which is then converted to O2 and water by the antioxidant enzymes CAT and POX140. Under DS, the activities of SOD and CAT can prevent or decrease the formation of this oxide141. Our results showed that the carob seedlings inoculated and/or amended with compost increased antioxidant activities (SOD, CAT, PPO, and POX) over the DS period drought, consistent with the increment in peroxidation levels141. Several studies revealed that compost alone or combined with AMF minimized the effects of abiotic stress by increasing antioxidant enzymes and decreasing MDA and H2O275. A recent study72 reported that the inoculation of carob seedlings with three AMF species, namely Rhizophagus intraradices, R. fasciculatus, and Funneliformis mosseae promoted its capacity to tolerate water stress by improving water and nutrients uptake, stomatal conductance, membrane stability, osmotic adjustment and antioxidant system. On the other hand, Jadrane et al.136 suggested that AMF inoculum applied at 2 g of mycorrhized root fragments can improve carob drought resistance by improving cell membrane stability and elasticity by activating the antioxidant defense system.
Overall, our results revealed that the carob tree drought tolerance mechanism is progressively improved over time in treated plants, particularly plants grown in AMF and compost. The carob growth has been increased by promoting the physiological traits, nutrient contents, antioxidant defense system, and a decrease in stress markers (H2O2 and MDA) in C + AMF plants, resulting in significant protection of the photosynthetic machinery membrane stability under DS conditions.
Conclusions
Water deficits drastically impact carob growth, affecting biomass production, mineral nutrient acquisition, water status, photosynthetic pigment content, and cell membrane stability. Under DS conditions, the application of indigenous AMF with green-made compost can reduce water stress's detrimental effects. The applied biofertilizers mitigated the water stress-induced changes by improving the antioxidant defence system, thus preserving cells components from oxidative damage. The mechanisms of action for these biofertilizers in maintaining physiological functions are probably related to improved water relations, mediation of photosynthesis and ionic homeostasis parameters, higher membrane stability, and lower lipid peroxidation under water deficit. At the cellular level, biofertilizers-mediated drought tolerance allowed biochemical protection measures to be activated in carob seedlings in response to drought to avoid the negative consequences of stress-induced ROS and to withstand a prolonged period of water stress. Hence, the management of natural resources as AMF and compost assured plant growth and establishment under prolonged water limitation, providing a valuable tool for the recovery of degraded forest.
Materials and methods
Biological material and application of biofertilizers
Scarified carob seeds with sulfuric acid were germinated in Petri dishes on moist filter paper and were incubated at 28 °C for five days and then rinsed and immersed in sterile distilled water for 24 h. The uniform-looking seedlings were transplanted, one plant per pot, in 5 kg plastic pots filled with sterilized forestry soil (at 0.11 MPa and 121 °C for 2 h), containing 1% organic matter, 0.6% total organic carbon (TOC), 8 mg/Kg available P, 900 mg/Kg N, 2356 mg/Kg Ca, and 568 mg/g available K, 8.2 pH, and 0.14 mS/cm electrical conductivity. The soil texture used was: 51% sand, 19% clay, and 30% loam.
We extracted and isolated the native AMF community comprising AMF spores from the rhizospheric soil field of five different regions in Morocco (Taounate, Taza, Errachidia, Zagora, and Essaouira). All spores were extracted from the respective inoculum using the wet sieving and sucrose centrifugation method142 and were examined ad libitum to confirm AMF identity and viability as per Souza143 and Kachkouch et al.144. The number of AMF spores detected in this inoculum was 165 spores/100 g of the soil sample. The inoculum contains a mixture of 26 species classified into 8 genera Glomeromycota (Glomus, Rhizophagus, Funneliformis, Acaulospora, Entrophospora, Gigaspora, Claroideoglomus, and Scutellospora) and 6 families (Glomeraceae, Acaulosporaceae, Entrophosporaceae, Diversisporaceae, Claroideoglomeraceae and Gigasporaceae ), and 2 orders (Glomerales et Diversisporales): Rhizophagus proliferus (= Glomus proliferum), Claroideoglomus etunicatum (= Glomus etunicatum), Rhizophagus clarus, Rhizophagus diaphanus (= Glomus diaphanum), Rhizophagus intraradices, Funneliformis mosseae, F. geosporum, F. constrictum (= Glomus constrictum), Diversispora epigaea Glomus sp. 1, Glomus sp. 2, Glomus sp. 3, Glomus sp. 4, Glomus sp. 5, Acaulospora denticulata, A. spinosa, A. kentinensis, Acaulospora sp. 1, Acaulospora sp. 2, Acaulospora sp. 3, Acaulospora sp. 4, Entrophospora sp. 1, Gigaspora sp. 1, Gigaspora sp. 2, Gigaspora sp. 3, and Scutellospora sp. 1.
We produced the compost locally from green waste as described previously by Meddich et al.145. The physicochemical and microbiological properties of the compost are 6.87 pH, 2.5 mg/g Olsen available P, 800 mg K /Kg, and 1900 mg Ca /Kg, 306.5 g C /kg organic, 551.7 g/kg organic matter, 21.9 g N /kg, 14 C/N ratio, and 490 g/kg ash content.
Experimental design
The experiment consisted of four treatments: (i) Control: not inoculated and compost-free treatment, (ii) AMF: plants inoculated with 60 g of AMF inoculum containing about 100 spores and 2 g of mycorrhizal roots fragments (with high AMF infection frequency (96%) and intensity (60%)) containing hyphae, vesicles, and spores, (iii) C: plants treated with 175 g/plant of compost alone (5% W/W with respect to culture soil), and (iv) C + AMF: plants treated with a combination of AMF and compost. Young carobs were arranged in a fully randomized block design, and there were 30 biological replicates per treatment. To standardize the background non-AMF microbial community within each pot, all pots received microbial filtrate (300 mL) made of equal parts of extraneous extraction solution (i.e., without AM fungal spores) from the AMF consortium (including the forest soil before sterilization).
The young carobs were watered and maintained at 70% field capacity (FC) for four months. Soil moisture was measured for all pots using a TDR meter (Delta UK Ltd., Clacton-on Sea, UK) in the morning and evening of each day. The amount of needed water under different water conditions was calculated according to the measured soil water content, soil bulk density, soil moisture maximum field capacity, and soil weight. Then, young carobs were subjected to two water regimes: 70% FC (WW; well-watered) and 35% FC (DS; severe drought stress) conditions. Plants were maintained for 12 months in semi-controlled conditions with natural light (photon flux density ranged from 500 to 750 µmol m−2 s−1), average temperature 23 °C (day/night air temperatures of 28/18 ± 4 °C), and average relative humidity 70%. Pots were rearranged within the greenhouse every week to reduce any spatial effects, and plant material harvest was carried out every two months.
The design of the experiment was factorial with the following different factors:
Factor1: the application of AMF and/or compost, factor 2: the water stress (well-watered or severe stress conditions), and factor 3: treatments (AMF and/or compost and/or drought) timing (2, 4, 6, and 8 months).
The experimental research on carob plants, including the collection of plant material, has been conducted in accordance with local, national, and international guidelines and legislation.
Plant harvest, mineral nutrient content, leaf water potential kinetics, and fungal colonization
After 2, 4, 6, and 8 months, fully expanded leaves of plants cultured in the absence or presence of biofertilizers and-or drought stress were harvested at the end of the light period, snap-frozen, and ground to a fine powder in liquid nitrogen with a pestle and mortar for the subsequent analyses. Carob seedlings' growth performance was assessed by measuring the shoot height, root length, and shoot and root dry matters (DM; obtained after drying samples at 80 °C until the weight remained constant). The dry shoot samples were then ground to a fine powder using a pulverising mill before measuring the total N, P, K, and Ca. Total N content was measured according to the method described by Jones146. Available P was determined according to Olsen and Sommers method147. The amount of K and Ca content was measured by flame spectrophotometer (AFP100) as described by Wolf (1982).
Leaf water potential (ΨLeaf) was measured using a pressure chamber (Model 600-EXP Super Pressure Chamber, PMS instrument, Albany, OR, USA) at predawn (06:00–08:00 h). Measurements were performed on fully developed leaves from the upper part of the five plants' stemper treatment.
To quantify the mycorrhizal colonization in each harvest, root samples were carefully washed, cleared with 10% KOH at 90 °C for 2 h, acidified with 5% lactic acid for 20 min, and stained with 0.05% (w/v) Trypan blue for 30 min at 90 °C148. Total arbuscular and vesicular colonization were assessed using the gridline-intersect method with at least 100 intersects per sample149.
Relative water content kinetics
Leaf relative water content (RWC%) was measured according to Barrs and Weatherley150. Leaf discs (1 cm2) were cut out from the third leaf of the apex (most expanded leaf), weighed to obtain fresh leaf weight (FW), and immediately hydrated by floating on deionized water to full turgidity for 24 h under darkness at 4 °C. Then, the samples were immediately weighted to determine the maximum turgidity (TW). Subsequently, these discs were dried at 70 °C until the weight was constant to determine the dry matter (DM). The leaf RWC was measured using the following formula:
Stomatal conductance, chlorophyll fluorescence and photosynthetic pigments kinetics
Stomatal conductance (gs) was measured on mature leaves using a portable steady-state diffusion porometer (Leaf Porometer LP1989, Decagon Device, Inc., Washington, USA). Five measurements per treatment were made on the abaxial side per plant (between 09:30–11:00 a.m).
Chlorophyll fluorescence (Fv/Fm) was measured in dark-acclimated (using leaf clips for 30 min) youngest fully expanded leaves using a hand-held fluorometer (Opti-sciences OSI 30p, Hudson, NY, USA). The measured Fv/Fm corresponded to the quantum yields (Fv/Fm = (Fm− F0)/Fm), where Fm and F0 are the maximum and initial quantum yields of dark-adapted leaves, respectively.
The chlorophyll a, b, total chlorophyll, and carotenoid concentration was determined according to the method described by Arnon151. Photosynthetic pigments were extracted from the frozen leaf powder using 80% (v/v) cold acetone. The extracted material was centrifuged at 10,000 × g at 4 °C for 10 min, and the supernatant was retained. Supernatant absorbance was read at 480, 645, and 663 nm using a UV–visible spectrophotometer (UV-3100PC spectrophotometer, VWR).
Total soluble sugars and proteins quantification kinetics
The total soluble sugar (TSS) content was measured in 0.1 g of frozen samples homogenized with 4 mL of ethanol (80%). The resulted supernatant was mixed with 0.25 mL of phenol (5%) and 1.25 mL of concentrated sulfuric acid. The absorbance was measured at 485 nm in a UV-3100PC spectrophotometer, according to Dubois et al.152. Protein concentration was determined by the Pierce 660 nm Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) using bovine serum albumin (BSA) as a standard.
Malondialdehyde and hydrogen peroxide content
Malondialdehyde (MDA) was determined according to Savicka and Škute153 method. In brief, lipid peroxides were extracted from leaves with 10 mL of 0.1% (w/v) trichloroacetic acid (TCA). After centrifugation (18,000 × g for 20 min), the chromogen was formed by mixing 1 mL of supernatant with 2.5 mL thiobarbituric acid (TBA). The mixture was incubated at 95 °C for 30 min, and the reaction stopped by placing the tubes in an ice bath. The chromogen formed was measured at 450, 532, and 600 nm.
MDA content was calculated as follows:
Hydrogen peroxide (H2O2) content in leaves was determined according to Velikova et al.154. The frozen leaf subsamples were mixed with 5 mL of 10% (w/v) TCA and then centrifuged for 15 min at 15,000 × g at 4 °C. The supernatant (0.5 mL) was recovered and then mixed with 0.5 mL of potassium phosphate buffer (10 mM, pH 7.0), and 1 mL of iodic potassium (1 M) was added. After 1 h of incubation in the dark at room temperature, the absorbance at 390 nm was recorded and plotted against a standard H2O2 curve.
Determination of membrane stability kinetics
Membrane stability (MS%) was determined after each harvest by measuring the electrolyte leakage according to Shanahan et al.155. Briefly, leaf discs were washed with deionized water to remove any surface-adhered electrolytes. Samples were placed in individual stoppered vials containing 10 mL of distilled water and kept at 25 °C on a shaker (100 rpm) for 6 h. The initial electrical conductivity (EC1) was measured using a conductivity meter (Hannah Instruments HI8820N). Then, the leaf discs were placed in a thermostatic water bath at 95 °C for 15 min, and the second reading (EC2) was determined after cooling the solutions to 25 °C. The Membrane Stability (MS) was calculated as:
Determination of antioxidant enzymes kinetics
Fresh leaf (0.1 g) ground with liquid nitrogen was homogenized in a cold mortar with 4 mL of 1 M phosphate buffer (pH 7) and 5% polyvinylpolypyrrolidone. The mixture was centrifuged at 18,000 g for 15 min at 4 °C. Then, the supernatant obtained was used to measure the antioxidant enzyme activities156. Briefly, superoxide dismutase (SOD) activity was assayed by Beyer and Fridovich157 method. One unit of SOD activity was defined as the ability to inhibit 50% of the photochemical reduction of p-nitroblue-tetrazolium (NBT) at 25 °C. The SOD activity was expressed at unit min−1 mg protein−1. The catalase (CAT) activity was measured by monitoring the decrease in absorbance at 240 nm for 3 min following the consumption of H2O2 substrate at 240 nm for 3 min158. Peroxidase (POX) activity was measured for 3 min using the method described by Tejera-Garcıa et al. (2004). The reaction mixture was composed of K2HPO4/KH2PO4 (100 mM) buffer K2HPO4/KH2PO4 (pH 6.5), guaiacol (40 mM), H2O2 (10 mM) and enzyme extract (0.1 mL). The results obtained were expressed at units mg of protein−1. Polyphenoloxidase (PPO) activity was evaluated by monitoring the oxidation of catechols at 410 nm, according to Gauillard et al.159. The mixture used contains K2HPO4/KH2PO4 buffer (100 mM, pH 6), catechol (50 mM) and an enzyme extract. The activity of PPO was expressed at units mg of protein−1.
Statistical analysis
Data are presented as mean ± SE (standard error) of five independent biological replicates. All results were subjected to a multifactorial analysis of variance (MANOVA) using SPSS 23.0 software (IBM, Armonk, NY, USA) for the tested factors (AMF; A, Compost; C, drought; D, and time of the four harvests; T) and their interactions. Mean comparisons were carried out using Tukey’s honest significant difference test using a significance level of 5% (p ≤ 0.05). Principal component analysis (PCA) was performed using XLSTAT-software v. 2016, and Heatmap was performed using software GraphPad® Prism v9.0 to categorize various growth, physiological, and biochemical characteristics of carob plants under the two water regimes (DS and WW) that illustrate the distinction between the variables and the different treatments applied.
Supplementary Information
Acknowledgements
This research was supported by Socially Responsible Projects, Cadi Ayyad University (Marrakech, Morocco) UCAM/RSU 2018 to M.A., Grant-in-Aid for Early-Career Scientists to MB (JSPS KAKENHI Grant Number 20K15425), and by a Grant for Promotion of KAAB Projects (Niigata University) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author contributions
M.B, S.W, and A.M designed and supervised the research. A.B performed the experiments and carried out the analysis. M.A-E-M, M.A, R.B-L, and Y.A contributed to analytic tools. A.B, M.B, M.A-E-M, and A.M interpreted the data. A.D, C. E-M, and T.M contributed to the conception and design of the study. M.B and A.B wrote the manuscript. M.B, A.B, A.M, and M.A-E-M revised and finalized the paper. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marouane Baslam, Email: mbaslam@gs.niigata-u.ac.jp.
Abdelilah Meddich, Email: a.meddich@uca.ma.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02018-3.
References
- 1.Gómez-Muñoz B, Magid J, Jensen LS. Nitrogen turnover, crop use efficiency and soil fertility in a long-term field experiment amended with different qualities of urban and agricultural waste. Agric. Ecosyst. Environ. 2017;240:300–313. [Google Scholar]
- 2.Abdel-Salam E, Al-Atar A, El-Sheikh MA. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018;25:1772–1780. doi: 10.1016/j.sjbs.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarwat M, Tuteja N. Hormonal signaling to control stomatal movement during drought stress. Plant Gene. 2017;11:143–153. [Google Scholar]
- 4.Baslam M, Qaddoury A, Goicoechea N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees Struct. Funct. 2014;28:161–172. [Google Scholar]
- 5.Mathur S, Singh R, Anjana T. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2018;139:227–238. doi: 10.1007/s11120-018-0538-4. [DOI] [PubMed] [Google Scholar]
- 6.Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Peñuelas J, et al. Assessment of the impacts of climate change on Mediterranean terrestrial ecosystems based on data from field experiments and long-term monitored field gradients in Catalonia. Environ. Exp. Bot. 2018;152:49–59. [Google Scholar]
- 8.Anderegg WRL, Kane JM, Anderegg LDL. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013;3:30–36. [Google Scholar]
- 9.Correia PJ, Gama F, Pestana M, Martins-Loução MA. Tolerance of young (Ceratonia siliqua L.) carob rootstock to NaCl. Agric. Water Manag. 2010;97:910–916. [Google Scholar]
- 10.Ozturk M, Dogan Y, Sakcali MS, Doulis A, Karam F. Ecophysiological responses of some maquis (Ceratonia siliqua L., Olea oleaster Hoffm. & Link, Pistacia lentiscus and Quercus coccifera L.) plant species to drought in the east Mediterranean ecosystem. J. Environ. Biol. 2010;31:233–245. [PubMed] [Google Scholar]
- 11.Goulas V, Stylos E, Chatziathanasiadou MV, Mavromoustakos T, Tzakos AG. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016;17:1–20. doi: 10.3390/ijms17111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papaefstathiou E, Agapiou A, Giannopoulos S, Kokkinofta R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018;6:2151–2161. doi: 10.1002/fsn3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krokou A, Kokkinofta R, Stylianou M, Agapiou A. Decoding carob flavor aroma using HS–SPME–GC–MS and chemometrics. Eur. Food Res. Technol. 2020;246:1419–1428. [Google Scholar]
- 14.Lakkab I, et al. Phytochemistry, bioactivity: Suggestion of Ceratonia siliqua L. as neurodegenerative disease therapy. J. Complement. Integr. Med. 2018;15:2–10. doi: 10.1515/jcim-2018-0013. [DOI] [PubMed] [Google Scholar]
- 15.Roseiro LB, Tavares CS, Roseiro JC, Rauter AP. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013;44:119–126. [Google Scholar]
- 16.Rtibi K, et al. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017;93:522–528. doi: 10.1016/j.biopha.2017.06.088. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen A, et al. Re-evaluation of locust bean gum (E 410) as a food additive. EFSA J. 2017;15:e04646. doi: 10.2903/j.efsa.2017.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben Ayache S, et al. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021;351:129263. doi: 10.1016/j.foodchem.2021.129263. [DOI] [PubMed] [Google Scholar]
- 19.FAOSTAT. Statistics Division of the Food and Agricultural Organization of the United Nations. (2019).
- 20.Boutasknit A, et al. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in)organic adjustments. Plants. 2020;9:80. doi: 10.3390/plants9010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Essahibi A, et al. Responsiveness of carob (Ceratonia siliqua L.) plants to arbuscular mycorrhizal symbiosis under different phosphate fertilization levels. J. Plant Growth Regul. 2019;38:1243–1254. [Google Scholar]
- 22.Ait-El-Mokhtar, M. et al. Vulnerability of oasis agriculture to climate change in Morocco. In Impacts of Climate Change on Agriculture and Aquaculture. IGI Global 76–106 (2020). 10.4018/978-1-7998-3343-7.ch004.
- 23.Ait-El-Mokhtar M, et al. Infectivity of the palm groves arbuscular mycorrhizal fungi under arid and semi-arid climate and its edaphic determinants towards efficient ecological restoration. Rhizosphere. 2020;15:100220. [Google Scholar]
- 24.Anli M, et al. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020;11:1560. doi: 10.3389/fpls.2020.516818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armada E, Portela G, Roldán A, Azcón R. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma. 2014;232–234:640–648. [Google Scholar]
- 26.Duo LA, Liu CX, Zhao SL. Alleviation of drought stress in turfgrass by the combined application of nano-compost and microbes from compost. Russ. J. Plant Physiol. 2018;65:419–426. [Google Scholar]
- 27.Boutasknit A, et al. Potential Effect of horse manure-green waste and olive pomace-green waste composts on physiology and yield of garlic (Allium sativum L.) and Soil Fertility. Gesunde Pflanzen. 2020;72:285–295. [Google Scholar]
- 28.Scotti R, Bonanomi G, Scelza R, Zoina A, Rao MA. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015;15:333–352. [Google Scholar]
- 29.Lin W, et al. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE. 2019;14:1–16. doi: 10.1371/journal.pone.0217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alguacil M, Caravaca F, Diaz-Vivancos P, Hernández JA, Roldan A. Effect of arbuscular mycorrhizae and induced drought stress on antioxidant enzyme and nitrate reductase activities in Juniperus oxycedrus L. grown in a composted sewage sludge-amended semi-arid soil. Plant Soil. 2006;279:209–218. [Google Scholar]
- 31.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Luo W, Li Y, Wang G, Li G. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Biores. Technol. 2018;250:853–859. doi: 10.1016/j.biortech.2017.08.136. [DOI] [PubMed] [Google Scholar]
- 33.Smith SE, Read DJ. Mycorrhizal Symbiosis. Academic Press; 1997. pp. 379–408. [Google Scholar]
- 34.Bitterlich M, Franken P, Graefe J. Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front. Plant Sci. 2018;9:301. doi: 10.3389/fpls.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augé RM, Toler HD, Saxton AM. Mycorrhizal stimulation of leaf gas exchange in relation to root colonization, shoot size, leaf phosphorus and nitrogen: A quantitative analysis of the literature using meta-regression. Front. Plant Sci. 2016;7:1084. doi: 10.3389/fpls.2016.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Meng P, Feng H, Wang C. Effects of arbuscular mycorrhizal fungi on growth and physiological performance of catalpa bungei C.A.Mey. under drought stress. Forests. 2020;11:1–29. [Google Scholar]
- 37.Nath M, et al. Reactive oxygen species generation-scavenging and signaling during Plant-Arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016;7:1–7. doi: 10.3389/fpls.2016.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begum N, Ahanger MA, Zhang L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020;176:104088. [Google Scholar]
- 39.Huang YM, Zou YN, Wu QS. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep42335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012;169:704–709. doi: 10.1016/j.jplph.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Jansa J, Smith FA, Smith SE. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008;177:779–789. doi: 10.1111/j.1469-8137.2007.02294.x. [DOI] [PubMed] [Google Scholar]
- 42.Sikes BA, Powell JR, Rillig MC. Deciphering the relative contributions of multiple functions within plant–microbe symbioses. Ecology. 2010;91:1591–1597. doi: 10.1890/09-1858.1. [DOI] [PubMed] [Google Scholar]
- 43.Ait-El-Mokhtar M, et al. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020;4:131. [Google Scholar]
- 44.Fernández DA, Roldán A, Azcón R, Caravaca F, Bååth E. Effects of Water Stress, Organic amendment and mycorrhizal inoculation on soil microbial community structure and activity during the establishment of two heavy metal-tolerant native plant species. Microb. Ecol. 2012;63:794–803. doi: 10.1007/s00248-011-9972-y. [DOI] [PubMed] [Google Scholar]
- 45.Cozzolino V, Di Meo V, Monda H, Spaccini R, Piccolo A. The molecular characteristics of compost affect plant growth, arbuscular mycorrhizal fungi, and soil microbial community composition. Biol. Fertil. Soils. 2016;52:15–29. [Google Scholar]
- 46.Bona E, et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza. 2015;25:181–193. doi: 10.1007/s00572-014-0599-y. [DOI] [PubMed] [Google Scholar]
- 47.Medina A, Azcón R. Effectiveness of the application of arbuscular mycorrhiza fungi and organic amendments to improve soil quality and plant performance under stress conditions. J. Soil Sci. Plant Nutr. 2010;10:354–372. [Google Scholar]
- 48.Cavagnaro TR. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Biol. Biochem. 2014;78:38–44. [Google Scholar]
- 49.Abud-Archila M, et al. Growth and fruit chemical characteristics of blackberry (Rubus Fruticosus) cultivated with vermicompost, glomus mosseae and phosphate rock. Compost. Sci. Util. 2018;26:225–231. [Google Scholar]
- 50.Perner H, Schwarz D, Bruns C, Mäder P, George E. Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and flowering of pelargonium plants. Mycorrhiza. 2007;17:469–474. doi: 10.1007/s00572-007-0116-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Liu H, Tao P, Chen H. Comparative proteomic analyses provide new insights into low phosphorus stress responses in maize leaves. PLoS ONE. 2014;9:2014. doi: 10.1371/journal.pone.0098215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griebenow S, Zuniga-feest A, Kleinert A, Valentine A. Photosynthetic metabolism during phosphate limitation in a legume from the Mediterranean-type Fynbos ecosystem. J. Plant Physiol. 2019;243:153051. doi: 10.1016/j.jplph.2019.153051. [DOI] [PubMed] [Google Scholar]
- 53.Kohler J, Caravaca F, Azcón R, Díaz G, Roldán A. The combination of compost addition and arbuscular mycorrhizal inoculation produced positive and synergistic effects on the phytomanagement of a semiarid mine tailing. Sci. Total Environ. 2015;514:42–48. doi: 10.1016/j.scitotenv.2015.01.085. [DOI] [PubMed] [Google Scholar]
- 54.Yang W, et al. Compost addition enhanced hyphal growth and sporulation of arbuscular mycorrhizal fungi without affecting their community composition in the soil. Front. Microbiol. 2018;9:1–13. doi: 10.3389/fmicb.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Laouane R, Meddich A, Bechtaoui N, Oufdou K, Wahbi S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago Sativa to Salt Stress. Gesunde Pflanzen. 2019;71:135–146. [Google Scholar]
- 56.El Maaloum S, et al. Effect of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria consortia associated with phospho-compost on phosphorus solubilization and growth of tomato seedlings (Solanum lycopersicum L.) Commun. Soil Sci. Plant Anal. 2020;51:622–634. [Google Scholar]
- 57.Meddich A, Ait El Mokhtar M, Wahbi S, Boumezzough A. Evaluation of the mycorrhizal potential in relation with the physico-chemical properties of soils in Moroccan palm groves (Marrakech and Tafilalet) Cahiers Agric. 2017;26:45012. [Google Scholar]
- 58.El Amerany F, Rhazi M, Wahbi S, Taourirte M, Meddich A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2020;261:109015. [Google Scholar]
- 59.Anli M, et al. Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera ‘Boufgouss’. Plant Biosyst. 2020;3504:1–14. [Google Scholar]
- 60.Hussain A, et al. Integrated application of organic amendments with alcaligenes sp. az9 improves nutrient uptake and yield of maize (Zea mays) J. Plant Growth Regul. 2020;2020:1–16. doi: 10.1007/s00344-020-10067-7. [DOI] [Google Scholar]
- 61.Zhen Z, et al. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE. 2014;9:2014. doi: 10.1371/journal.pone.0108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roldán A, Carrasco L, Caravaca F. Stability of desiccated rhizosphere soil aggregates of mycorrhizal Juniperus oxycedrus grown in a desertified soil amended with a composted organic residue. Soil Biol. Biochem. 2006;38:2722–2730. [Google Scholar]
- 63.Ghorchiani M, Etesami H, Alikhani HA. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018;258:59–70. [Google Scholar]
- 64.Lim SS, et al. Nitrogen, carbon, and dry matter losses during composting of livestock manure with two bulking agents as affected by co-amendments of phosphogypsum and zeolite. Ecol. Eng. 2017;102:280–290. [Google Scholar]
- 65.Baslam M, Goicoechea N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza. 2012;22:347–359. doi: 10.1007/s00572-011-0408-9. [DOI] [PubMed] [Google Scholar]
- 66.Meddich A, Jaiti F, Bourzik W, Asli AE, Hafidi M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera) Sci. Hortic. 2015;192:468–474. [Google Scholar]
- 67.Wu F, et al. Effects of long-term fertilization on AM fungal community structure and Glomalin-related soil protein in the Loess Plateau of China. Plant Soil. 2011;342:233–247. [Google Scholar]
- 68.Zou Y, Wu H, Giri B, Wu Q, Ku K. Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiol. Biochem. 2019;144:292–299. doi: 10.1016/j.plaphy.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Huang YM, et al. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 2014;5:1–7. doi: 10.3389/fmicb.2014.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davison J, et al. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS ONE. 2012;7:e41938. doi: 10.1371/journal.pone.0041938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ait-El-Mokhtar M, et al. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019;253:429–438. [Google Scholar]
- 72.Essahibi A, Benhiba L, Ait Babram M, Ghoulam C, Qaddoury A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.) Trees. 2018;32:87–97. [Google Scholar]
- 73.Wagner MR, et al. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016;7:12151. doi: 10.1038/ncomms12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahbouki S, et al. Evaluation of arbuscular mycorrhizal fungi and vermicompost supplementation on growth, phenolic content and antioxidant activity of prickly pear cactus (Opuntia ficus-indica) Plant Biosyst. 2021;2021:1–19. [Google Scholar]
- 75.Ben-Laouane R, et al. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms. 2020;8:1–27. doi: 10.3390/microorganisms8111695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akhter A, Hage-Ahmed K, Soja G, Steinkellner S. Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycopersici. Front. Plant Sci. 2015;6:1–13. doi: 10.3389/fpls.2015.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boutasknit A, et al. Improvement of garlic growth, physiology, biochemical traits, and soil fertility by Rhizophagus irregularis and compost. Gesunde Pflanzen. 2020;2020:1–12. doi: 10.1007/s10343-020-00533-3. [DOI] [Google Scholar]
- 78.Chaiyasen A, Chaiya L, Douds DD, Lumyong S. Influence of host plants and soil diluents on arbuscular mycorrhizal fungus propagation for on-farm inoculum production using leaf litter compost and agrowastes. Biol. Agric. Hortic. 2017;33:52–62. [Google Scholar]
- 79.IJdo M, Cranenbrouck S, Declerck S. Methods for large-scale production of AM fungi: Past, present, and future. Mycorrhiza. 2011;21:1–16. doi: 10.1007/s00572-010-0337-z. [DOI] [PubMed] [Google Scholar]
- 80.Ortuño MF, Lorente B, Hernández JA, Sánchez-Blanco MJ. Mycorrhizal inoculation on compost substrate affects nutritional balance, water uptake and photosynthetic efficiency in Cistus albidus plants submitted to water stress. Braz. J. Bot. 2018;41:299–310. [Google Scholar]
- 81.Wu HH, Zou YN, Rahman MM, Ni QD, Wu QS. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep42389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giovannetti, M., Avio, L. & Sbrana, C. Fungal spore germination and pre-symbiotic mycelial growth–physiological and genetic aspects. in Arbuscular Mycorrhizas: Physiology and Function (eds. H, K. & Y, K.) 3–32 (2010). doi:10.1007/978-90-481-9489-6.
- 83.Vijayakumar V, et al. Integrated multi-omics analysis supports role of lysophosphatidylcholine and related glycerophospholipids in the Lotus japonicus-Glomus intraradices mycorrhizal symbiosis. Plant Cell Environ. 2016;39:393–415. doi: 10.1111/pce.12624. [DOI] [PubMed] [Google Scholar]
- 84.Giovannini L, et al. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy. 2020;10:1–14. [Google Scholar]
- 85.Bender SF, Conen F, Van der Heijden MGA. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol. Biochem. 2015;80:283–292. [Google Scholar]
- 86.Pankaj U, Kurmi A, Lothe NB, Verma RK. Influence of the seedlings emergence and initial growth of palmarosa (Cymbopogon martinii (Roxb.) Wats. Var. Motia Burk) by arbuscular mycorrhizal fungi in soil salinity conditions. J. Appl. Res. Med. Aromat. Plants. 2021;24:100317. [Google Scholar]
- 87.Van Der Heijden MGA. Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol. Lett. 2004;7:293–303. [Google Scholar]
- 88.Buade R, Chourasiya D, Prakash A, Sharma MP. Changes in arbuscular mycorrhizal fungal community structure in soybean rhizosphere soil assessed at different growth stages of soybean. Agricultural Research. 2021;10:32–43. [Google Scholar]
- 89.Kammadavil Sahodaran N, Arun AK, Ray JG. Native arbuscular mycorrhizal fungal isolates (Funneliformis mosseae and Glomus microcarpum) improve plant height and nutritional status of banana plants. Exp. Agric. 2019;55:924–933. [Google Scholar]
- 90.Bindell M, et al. Arbuscular mycorrhizal fungal communities associated with switchgrass (Panicum virgatum L.) in the acidic, oligotrophic pine barrens ecosystem. Grass Research. 2021;1:1–10. [Google Scholar]
- 91.Goss, M. J., Carvalho, M. & Brito, I. Functional diversity of mycorrhiza and sustainable agriculture: management to overcome biotic and abiotic stresses. in Functional Diversity of Mycorrhiza and Sustainable Agriculture: Management to Overcome Biotic and Abiotic Stresses 1–231 (2017).
- 92.Morton JB, Bever JD, Pfleger FL. Taxonomy of Acaulospora gerdemannii and Glomus leptotichum, synanamorphs of an arbuscular mycorrhizal fungus in Glomales. Mycol. Res. 1997;101:625–631. [Google Scholar]
- 93.Diop T, Becard G, Piche Y. Long-term in vitro culture of an endomycorrhizal fungus, Gigaspora margarita, on Ri T-DNA transformed roots of carrot. Symbiosis (Philadelphia, PA) 1992;12:249–259. [Google Scholar]
- 94.De Souza FA, Declerck S. Mycelium development and architecture, and spore production of Scutellospora reticulata in monoxenic culture with Ri T-DNA transformed carrot roots. Mycologia. 2003;95:1004–1012. [PubMed] [Google Scholar]
- 95.De La Providencia IE, De Souza FA, Fernández F, Delmas NS, Declerck S. Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol. 2005;165:261–271. doi: 10.1111/j.1469-8137.2004.01236.x. [DOI] [PubMed] [Google Scholar]
- 96.Caravaca F, et al. Establishment of two ectomycorrhizal shrub species in a semiarid site after in situ amendment with sugar beet, rock phosphate, and Aspergillus niger. Microb. Ecol. 2005;49:73–82. doi: 10.1007/s00248-003-0131-y. [DOI] [PubMed] [Google Scholar]
- 97.Pinos NQ, Louro Berbara RL, Elias SS, van Tol de Castro TA, García AC. Combination of humic substances and arbuscular mycorrhizal fungi affecting corn plant growth. J. Environ. Qual. 2019;48:1594–1604. [Google Scholar]
- 98.Medina A, Jakobsen I, Vassilev N, Azcón R, Larsen J. Fermentation of sugar beet waste by Aspergillus niger facilitates growth and P uptake of external mycelium of mixed populations of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2007;39:485–492. [Google Scholar]
- 99.Zhang F, Zou Y, Wu Q. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018;229:132–136. [Google Scholar]
- 100.Rolfe SA, Griffiths J, Ton J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019;49:73–82. doi: 10.1016/j.mib.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Lilleskov EA, Bruns TD. Root colonization dynamics of two ectomycorrhizal fungi of contrasting life history strategies are mediated by addition of organic nutrient patches. New Phytol. 2003;159:141–151. doi: 10.1046/j.1469-8137.2003.00794.x. [DOI] [PubMed] [Google Scholar]
- 102.Fracchia F, et al. Colonization of naive roots from Populus tremula × alba involves successive waves of fungi and bacteria with different trophic abilities. Appl. Environ. Microbiol. 2021;87:1–21. doi: 10.1128/AEM.02541-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pepe A, Giovannetti M, Sbrana C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018;8:2–11. doi: 10.1038/s41598-018-28354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marupakula S, et al. Bacterial microbiomes of individual ectomycorrhizal Pinus sylvestris roots are shaped by soil horizon and differentially sensitive to nitrogen addition. Environ. Microbiol. 2017;19:4736–4753. doi: 10.1111/1462-2920.13939. [DOI] [PubMed] [Google Scholar]
- 105.Han X, et al. Dynamics of arbuscular mycorrhizal fungi in relation to root colonization, spore density, and soil properties among different spreading stages of the exotic plant threeflower beggarweed (Desmodium triflorum) in a Zoysia tenuifolia lawn. Weed Sci. 2019;67:689–701. [Google Scholar]
- 106.Bencherif K, Boutekrabt A, Dalpé Y, Lounès-Hadj Sahraoui A. Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl. Soil Ecol. 2016;107:182–190. [Google Scholar]
- 107.Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013;4:1–16. doi: 10.3389/fpls.2013.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hosseinzadeh SR, Amiri H, Ismaili A. Effect of vermicompost fertilizer on photosynthetic characteristics of chickpea (Cicer arietinum L.) under drought stress. Photosynthetica. 2016;54:87–92. [Google Scholar]
- 109.Zou YN, Huang YM, Wu QS, He XH. Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+ influxes in trifoliate orange roots under drought stress. Mycorrhiza. 2015;25:143–152. doi: 10.1007/s00572-014-0598-z. [DOI] [PubMed] [Google Scholar]
- 110.Ngo HTT, Cavagnaro TR. Interactive effects of compost and pre-planting soil moisture on plant biomass, nutrition and formation of mycorrhizas: A context dependent response. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-017-18780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rady MM, Semida WM, Hemida KA, Abdelhamid MT. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int. J. Recycl. Org. Waste Agric. 2016;5:311–321. [Google Scholar]
- 112.Aroca R, et al. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013;170:47–55. doi: 10.1016/j.jplph.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 113.Quiroga G, Erice G, Aroca R, Chaumont F, Ruiz-Lozano JM. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019;167:103821. [Google Scholar]
- 114.Egerton-warburton LM, Querejeta JI, Allen MF. Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J. Exp. Bot. 2007;58:1473–1483. doi: 10.1093/jxb/erm009. [DOI] [PubMed] [Google Scholar]
- 115.Li Z, Schneider RL, Morreale SJ, Xie Y, Li C. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in decertified soils of Ningxia, China. Geoderma. 2018;310:143–152. [Google Scholar]
- 116.Hashem A, et al. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019;26:614–624. doi: 10.1016/j.sjbs.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hueso S, Hernández T, García C. Resistance and resilience of the soil microbial biomass to severe drought in semiarid soils : The importance of organic amendments. Appl. Soil. Ecol. 2011;50:27–36. [Google Scholar]
- 118.Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012;58:186–191. [Google Scholar]
- 119.Haffani S, Mezni M, Ben Nasri M, Chaibi W. Comparative leaf water relations and anatomical responses of three vetch species (Vicia narbonensis L., V. sativa L. and V. villosa Roth.) to cope with water stress. Crop Pasture Sci. 2017;68:691–702. [Google Scholar]
- 120.Rao DE, Chaitanya KV. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016;60:201–218. [Google Scholar]
- 121.Pan J, et al. Effect of arbuscular mycorrhizal aungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline Soil. Appl. Sci. 2020;10:945. [Google Scholar]
- 122.Gong M, Tang M, Chen H, Zhang Q, Feng X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013;44:399–408. [Google Scholar]
- 123.Bagheri V, Shamshiri MH, Shirani H, Roosta HR. Effect of mycorrhizal inoculation on ecophysiological responses of pistachio plants grown under different water regimes. Photosynthetica. 2011;49:531–538. [Google Scholar]
- 124.Wu QS, Xia RX, Zou YN. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008;44:122–128. [Google Scholar]
- 125.Li F, Deng J, Nzabanita C, Li Y, Duan T. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis. 2019;79:151–161. [Google Scholar]
- 126.Junker LV, et al. Variation in short-term and long-term responses of photosynthesis and isoprenoid-mediated photoprotection to soil water availability in four Douglas-fir provenances. Sci. Rep. 2017;7:1–16. doi: 10.1038/srep40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bashir A, et al. Chemosphere Application of co-composted farm manure and biochar increased the wheat growth and decreased cadmium accumulation in plants under different water regimes. Chemosphere. 2020;246:125809. doi: 10.1016/j.chemosphere.2019.125809. [DOI] [PubMed] [Google Scholar]
- 128.Teodoro M, et al. Application of co-composted biochar significantly improved plant- growth relevant physical/chemical properties of a metal contaminated soil. Chemosphere. 2020;242:125255. doi: 10.1016/j.chemosphere.2019.125255. [DOI] [PubMed] [Google Scholar]
- 129.Baslam M, Garmendia I, Goicoechea N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown Lettuce. J. Agric. Food Chem. 2011;59:5504–5515. doi: 10.1021/jf200501c. [DOI] [PubMed] [Google Scholar]
- 130.Zaferanchi S, Salmasi SZ, Yahya S, Lisar S. Influence of organics and bio fertilizers on biochemical properties of Calendula officinalis L. Int. J. Hortic. Sci. Technol. 2019;6:125–136. [Google Scholar]
- 131.Zahoor R, et al. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017;137:73–83. [Google Scholar]
- 132.Tomasella M, Petrussa E, Petruzzellis F, Nardini A, Casolo V. The Possible Role of Non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2020;21:144. doi: 10.3390/ijms21010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mo Y, et al. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016;7:644. doi: 10.3389/fpls.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:53. [Google Scholar]
- 135.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:1–26. [Google Scholar]
- 136.Jadrane I, et al. Inoculation with selected indigenous mycorrhizal complex improves Ceratonia siliqua’s growth and response to drought stress. Saudi J. Biol. Sci. 2021;28:825–832. doi: 10.1016/j.sjbs.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V. The significance of reactive oxygen species and antioxidant defense system in plants: A Concise Overview. Front. Plant Sci. 2021;11:1–13. doi: 10.3389/fpls.2020.552969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirova E, Pecheva D, Simova-Stoilova L. Drought response in winter wheat: protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021;43:1–11. [Google Scholar]
- 139.Torun H. Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol. Plant. 2019;165:169–182. doi: 10.1111/ppl.12798. [DOI] [PubMed] [Google Scholar]
- 140.Rani B, Madan S, Sharma KD, Pooja Kumar A. Influence of arbuscular mycorrhiza on antioxidative system of wheat (Triticum aestivum) under drought stress. Indian J. Agric. Sci. 2018;88:289–295. [Google Scholar]
- 141.Kusvuran S, Dasgan HY. Effects of drought stress on physiological and biochemical changes in Phaseolus vulgaris L. Legum. Res. 2017;40:55–62. [Google Scholar]
- 142.Daniels, B. A. & Skipper, H. Methods for the recovery and quantitative estimation of propagules from soil. In N. C. Schenck (Ed.), Methods and Principles of Mycorrhizal Research 133–151 (1982).
- 143.Souza, T. Spores: A special tool to survive. Handbook of Arbuscular Mycorrhizal Fungi. Cham: Springer International Publishing. 1–153 (2015). 10.1007/978-3-319-24850-9.
- 144.Kachkouch W, et al. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Olea europaea in three regions of Morocco (Tafilalt, Zagora and Taounate) Int. J. Pure Appl. Biosci. 2014;2:178–195. [Google Scholar]
- 145.Meddich A, Elouaqoudi F, Khadra A, Bourzik W. Valorization of green and industrial waste by composting process. J. Rev. Compos. Adv. Mater. 2016;26:451–469. [Google Scholar]
- 146.Jones J. Kjeldahl Method for Nitrogen (N) Determination. Micro-Macro Publishing Inc; 1991. [Google Scholar]
- 147.Olsen, S. & Sommers, L. Phosphorus. In: Page, A. (Ed.), Methods of soil analysis: Part 2. Chemical and microbiological properties. American Society of Agronomy 403–430 (1982).
- 148.Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970;55:18–29. [Google Scholar]
- 149.Trouvelot A. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. Physiol. Genet. Asp. Mycorrhizae. 1986 doi: 10.1177/004057368303900411. [DOI] [Google Scholar]
- 150.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. [Google Scholar]
- 151.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 153.Savicka M, Škute N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.) Ekologija. 2010;56:26–33. [Google Scholar]
- 154.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- 155.Shanahan JF, Edwards IB, Quick JS, Fenwick JR. Membrane Thermostability and heat tolerance of spring wheat. Crop Sci. 1990;30:247. [Google Scholar]
- 156.Tejera García NA, Olivera M, Iribarne C, Lluch C. Partial purification and characterization of a non-specific acid phosphatase in leaves and root nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004;42:585–591. doi: 10.1016/j.plaphy.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 157.Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 158.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 159.Gauillard F, Richard-Forget F, Nicolas J. New sprectrophotometric assay for polyphenol oxidase activity. Anal. Biochem. 1993;215:59–65. doi: 10.1006/abio.1993.1554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.