Abstract
Early life stress presents an important risk factor for drug addiction and comorbid depression and anxiety through persistent effects on the mesolimbic dopamine pathways1. Using an early life stress model for child neglect (a single 24 h episode of maternal deprivation, MD) in rats, recent published works from our lab show that MD induces dysfunction in the ventral tegmental area2–4 and its negative controller, the lateral habenula (LHb)5–7. MD-induced potentiation of glutamatergic synaptic transmission onto LHb neurons shifts the coordination of excitation/inhibition (E/I) balance towards excitation, resulting in an increase in the overall spontaneous neuronal activity with elevation in bursting and tonic firing, and in the intrinsic excitability of LHb neurons in early adolescent male rats5–7. Here, we explored how MD affects intravenous morphine self-administration (MSA) acquisition and sucrose preference as well as glutamatergic synaptic function in LHb neurons of adult male rats self-administering morphine. We found that MD-induced increases in LHb neuronal and glutamatergic synaptic activity and E/I ratio persisted into adulthood. Moreover, MD significantly reduced morphine intake, triggered anhedonia-like behavior in the sucrose preference test, and was associated with persistent glutamatergic potentiation 24h after the last MSA session. MSA also altered the decay time kinetics of AMPAR currents in LHb neurons of control rats during this time period. Our data highlights that early life stress-induced glutamatergic plasticity in LHb may dampen the positive reinforcing and motivational properties of both natural rewards and opioids, and may contribute to the development of anhedonia and dysphoric states associated with opioids.
Keywords: early life stress, glutamatergic synaptic transmission, lateral habenula, LHb, drug self-administration
Introduction
Exposure to early life stress is a strong predictor for several later-life mental disorders, including substance use disorders, anxiety and depression1. Early life stress may increase the risk of a variety of mental illnesses and addiction through modifications in brain reward circuits and synaptic integration controlling brain dopamine signaling1,3,6,8–10 although the exact mechanistic link between early life stress and this increased vulnerability is still unknown.
Prevalent early life stress rodent models are maternal deprivation (MD, a single prolonged episode of maternal separation) and maternal separation (repeated daily maternal separations) models, in which animals are separated from their mothers early in life. Although the general consensus in the literature is that early life stress increases the risk of addiction, conflicting results have been reported in regards to drug seeking and taking behaviors. Previous work on the effects of early life stress in drug addiction has largely focused on stimulants and alcohol8,11–22 but overlooked opioids, including morphine. A few studies report early life stress effects in the context of opioid dependence and reward using experimenter- administered morphine in morphine-induced sensitization and conditioned place preference23–25. Of importance, maternal separation (using a 4 h daily isolation of pups from litters from postnatal day 1, PND1-PND14) is shown to induce higher sensitivity to the rewarding properties of morphine. In these studies, maternally separated Long–Evans adult male rats demonstrated increased morphine-conditioned place preference and resilience to extinction of drug reward memory, leading to enhanced morphine reinforcement in the conditioned place preference test with intraperitoneal drug administration and in oral self-administration paradigms25–27. Using an established early life stress model of child neglect in rats (a single 24 h episode of MD at PND9), our recent studies have demonstrated that the lateral habenula (LHb) may serve as a critical converging brain region for early life stress-induced dysregulation of reward circuits5–7. The LHb is an emerging anti-reward hub for motivation and decision making that links forebrain limbic structures with midbrain monoaminergic centers and is involved in reward and aversion-related learning associated with avoidance from stressful and aversive situations through the suppression of dopamine and serotonin systems28–31. Not surprisingly, LHb dysfunction contributes to a myriad of cognitive, learning, and affective impairments associated with depression, anxiety, psychosis, and drug addiction32–34. Consistently, we also found that MD induces LHb hyperactivity through glutamatergic synaptic potentiation that shifts the excitation/inhibition (E/I) balance towards excitation, and produces increased behavioral immobility in the forced swim test in adolescent male rats5–7. This indicates that MD-induced modifications in LHb function may promote depressive and anhedonic states. Mu opioid receptor activation is found to suppress LHb activity35, and naloxone-precipitated morphine withdrawal from passive and repeated injections of morphine in mice depresses glutamatergic synapses onto raphe-projecting LHb neurons36. However, the effects of intravenous MSA on LHb glutamatergic function, particularly following MD, are still unknown. Here, we demonstrate that MD alters MSA acquisition and decreases sucrose preference, and is associated with persistent MD-induced LHb glutamatergic synaptic plasticity that supports enhanced E/I ratio and LHb hyperactivity. This persistent MD-induced glutamatergic potentiation in LHb neurons may contribute to anhedonia to natural rewarding stimuli as well as alter the reinforcing and motivational properties of opioids, potentially facilitating opioid-related behaviors in adult male rats.
Materials and Methods
Animals:
All experiments employed male Sprague Dawley rats (sourced from Taconic Inc, and Charles River Laboratories) in experiments conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Uniformed Services University Institutional Animal Care and Use Committee. All rats were received on PND6 with lactating dams and allowed to acclimate undisturbed for ~72h before initiation of the MD procedure. All rats were kept on a 12h dark: 12h light cycle schedule with lights on at 06:00, and all procedures began 3–4 h after the start of the light-cycle (except for SSA and MSA procedure, see below). All animals received ad libitum standard chow and water (except where noted during the MD procedure).
MD Procedure:
MD was performed on male rats at PND9. Half of the rat pups in the litter (randomly selected) were isolated from the dam and their siblings for 24h (MD group). The isolated pups remained together and were placed on a heating pad (34°C) in a separate quiet room and not disturbed for 24h until being returned to their home cage, joining their non-maternally deprived (non-MD) litter mates. The remaining non-MD control group received the same amount of handling as the MD rats. All rats were group-housed (2 per cage, treatment-matched) from weaning at PND28 until sacrifice at the age range of PND70-PND80 with standard housing care and no additional experimenter manipulation prior to sacrifice.
Slice Preparation:
For all electrophysiology experiments, several separate cohorts of non-MD/MD-treated rats were used. As described before6, all rats were anesthetized with isoflurane, decapitated, and brains were quickly dissected and placed into ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 21.4 NaHCO3, 2.5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.0 MgSO4, 11.1 glucose, and 0.4 ascorbic acid and saturated with 95% O2-5% CO2. Briefly, sagittal slices containing LHb were cut at 250 μm and incubated in above prepared ACSF at 34°C for at least 1 h prior to electrophysiological experiments. For patch clamp recordings, slices were then transferred to a recording chamber and perfused with ascorbic-acid free ACSF at 28°C.
Electrophysiology:
Voltage-clamp cell-attached and voltage/current-clamp whole-cell recordings were performed from LHb neurons in LHb-containing slices using patch pipettes (3–6 MOhms) and a patch amplifier (MultiClamp 700B) under infrared-differential interference contrast microscopy. Data acquisition and analysis were carried out using DigiData 1440A, pCLAMP 10 (Molecular Devices), Clampfit, and Mini Analysis 6.0.3 (Synaptosoft, Inc.). Signals were filtered at 3 kHz and digitized at 10 kHz.
To assess LHb spontaneous activity, cells were patch clamped with potassium gluconate-based internal solution (130 mM K-gluconate, 15 mM KCl, 4 mM adenosine triphosphate (ATP)-Na+, 0.3 mM guanosine triphosphate (GTP)-Na+, 1 mM EGTA, and 5 mM HEPES, pH adjusted to 7.28 with KOH, osmolarity adjusted to 275–280 mOsm) in slices perfused with ACSF. Spontaneous neuronal activity and AP firing patterns (tonic, bursting) were assessed in both cell-attached recordings in voltage-clamp mode at V=0 and whole-cell recording in current-clamp mode at I=0 for the duration of the ~1 min recording. Cells that fired less than 2 single action potentials (APs) during this time period were characterized as silent. In general, bursting neurons have regular bursting activity, fire with bursts of 2–3 APs, and exhibit more negative resting membrane potentials and hyperpolarized inter-burst potentials (evident in current-clamp recordings) while tonic neurons exhibit regular or irregular single AP firing with more depolarized resting membrane potentials (evident in current-clamp recordings) as previously described37.
The excitatory and inhibitory balance (E/I ratio) was recorded with a cesium (Cs)-gluconate–based internal solution in intact synaptic transmission. Patch pipettes were filled with Cs-gluconate internal solution (117 mM Cs-gluconate, 2.8 mM NaCl, 5 mM MgCl2, 2 mM ATP-Na+, 0.3 mM GTP-Na+, 0.6 mM EGTA, and 20 mM HEPES, pH adjusted to 7.28 using CsOH, osmolality adjusted to 275–280 mOsm). Evoked EPSCs and IPSCs from LHb neurons were recorded in the same neuron in drug-free ACSF using a stimulating electrode placed in the stria medullaris. AMPA receptors (AMPAR) EPSCs were recorded at the reversal potential for GABAA receptor (GABAAR) IPSCs (−55 mV), and GABAAR IPSCs were recorded at the reversal potential for AMPAR EPSCs (+10 mV) in the same LHb neuron. EPSCs and IPSCs in each cell were evoked in response to the same stimulus intensity that evoked a near maximal response in each cell. Relative AMPAR EPSC and GABAAR IPSC components of evoked currents were calculated by normalizing to the amplitude of the total evoked EPSC (absolute values) + IPSC for each recording to 1 as previously described38. The E/I ratio was then calculated as EPSC/IPSC amplitude ratio by dividing the average peak amplitude of 10 consecutive sweeps of EPSCs or IPSCs from the same recording. Whole cell recordings of AMPAR-meditated miniature excitatory postsynaptic current (mEPSC) recordings were performed in ACSF perfused with picrotoxin (100 μM), d-APV (50 μM), and tetrodotoxin (TTX, 1 μM), and pipettes filled with Cs-gluconate internal solution similar to E/I ratio recordings. For mEPSCs, LHb neurons were voltage-clamped at −70 mV and recorded over 10 sweeps, each lasting 50 s. The cell series resistance was monitored through all the experiments and if this value changed by more than 10%, data were not included.
Catheter Surgery
Non-MD and MD male rats (PND60-PND63) were anesthetized with a cocktail of ketamine/xylazine (100 mg/kg and 10 mg/kg, i.p.) and a catheter was implanted in the right jugular vein and attached to the back of the animal as described previously39,40. The entry point of the catheter was secured in place with a 0.5 cm × 0.5 cm Mersilene surgical mesh attached to the catheter. Then the catheter composed of 22-gauge stainless steel tubing cemented into place with bell-shaped dental cement with a 1.5 cm × 1.5 cm Marlex surgical mesh was secured under the skin of the back of the animal. The incision was closed using stainless steel wound clips and treated with topical antibiotic ointment. Animals received antibiotic gentamycin sulfate (5–8 mg/kg, i.v.) after the surgery and were monitored until full recovery.
Morphine Self-Administration (MSA)
Eight operant conditioning chambers (Med Associates Inc., St. Albans, VT) were used for intravenous MSA experiments. Each chamber was equipped with two levers, an infusion pump, and a 10 mL glass syringe connected to a fluid swivel (Instech, Plymouth Meeting, PA) by Teflon tubing. After one week recovery from catheter surgery, animals (PND67-PND70) were placed in the operant conditioning chambers and allowed to self-administer either morphine (0.3 mg/kg/infusion, 0.1 mL over 5 sec, MSA) or 0.9% saline (saline self-administration, SSA) on one lever press/injection (Fixed Ratio, FR 1) reinforcement schedule for a 4 h session with a maximum number of 60 infusions a day. Animals were trained with a FR1 schedule of reinforcement for 6 days, and then switched to a FR3 schedule from days 7 to 10. During each infusion (5 sec), a cue light above the active lever was illuminated, and the house light was extinguished for an additional 20 sec (time-out) period after infusion. The lever press responses on the inactive lever and responses to both levers during the time-out period were recorded but had no programmed consequences. Each chamber was equipped with two infrared photo beams that monitored spontaneous locomotor activity of animals during the self-administration session. The numbers of drug-paired lever presses, drug infusions, inactive lever presses and locomotor activity were recorded by the MED-PC®-IV self-administration software.
Sucrose Preference Test
At PND50, non-MD and MD male rats were transferred to a reverse light cycle room (12:12, light: dark, lights off at 0600) and acclimated to the new reverse light cycle for one week. Because rats tend to avoid novel foods41, they may at first avoid sweet solutions used in this assay. To overcome this obstacle, we presented non-MD and MD rats on PND58 with two bottles, one containing 2% sucrose in water and the other containing normal drinking water in their home-cage. We allowed the rats to become accustomed to the sucrose water over a 7-day period. We alternated the water and sucrose bottle positions on each habitation day, in addition to checking for leakage. Prior to the two-bottle choice test, both bottles were removed from the cage and animals underwent 18 h of water deprivation. On the day of testing, all animals needed to be single-housed for accurate measurement of sucrose and water consumption. However, single-housing rats over a long-term period could be stressful42,43. We therefore opted to single-house each rat only for the duration of testing (total of 90 min). On PND70, animals were individually placed in a new cage with bedding, allowed 30 minutes to acclimate to the new cage, and then presented with the two-bottle choice selection for a one-hour test period. After the test, bottles were removed and weighed. Sucrose Preference (% Preference) was calculated using the following formula: % Preference = [(sucrose consumed in g)/(sucrose consumed in g+ water consumed in g)] × 100.
Statistics:
Values are presented as mean ± SEM. The threshold for significance was set at *p < 0.05 for all analyses. All statistical analyses of data were performed using GraphPad Prism 8.4.1. For the effects of MD on E/I ratios and the sucrose preference test, we used unpaired Welch’s t test. For detecting the difference in distribution of silent, tonic or bursting LHb neurons in non-MD and MD rats, we used Chi-square tests. Mini Analysis software was used to detect and measure mEPSC amplitude, charge transfer (area under the curve), decay time constants (Tau) and frequency (inter-event interval) using preset detection parameters of mEPSCs with an amplitude cutoff of 5 pA. Effects of MD and MSA on the mean and cumulative probabilities of mEPSC amplitude, charge transfer, decay time constants (Tau) and frequency data sets were analyzed using two-way ANOVA tests and Kolmogorov-Smirnov tests, respectively. Effects of MD stress and MSA during the acquisition time were analyzed separately for the FR1 and FR3 schedules using 3-way Repeated Measures ANOVA and Mixed-effect (only for locomotor activity with the FR1 schedule) tests for with Tukey’s post-hoc test with MD and MSA as between-group factors and time as the within-group factor.
Results
MD-induced changes in LHb E/I balance and spontaneous activity persisted into adulthood.
Here, we first examined whether MD-induced increases in E/I ratio and LHb neuronal activity persist in adult male rats. We detected an increase in E/I ratio following MD suggesting a shift in the balance between excitation and inhibition in synaptic inputs towards excitation following MD (Figure 1A, Welch’s t test, t(13.46)= 2.30, p < 0.05). Further measurements of the relative AMPAR EPSCs and GABAAR IPSCs in LHb neurons showed that the higher E/I ratios in MD rats are due to changes in relative weights of both excitatory (increased) and inhibitory (decreased) synaptic inputs onto LHb neurons following MD (Figure 1B). We observed that MD increased LHb spontaneous neuronal activity in both cell-attached voltage-clamp and whole-cell current-clamp recordings with intact synaptic transmission (Figure 1C–D, Chi squared test, p < 0.01). Moreover, we found that the percentage of neurons which were spontaneously active in tonic firing was larger in both types of recordings in adult male MD rats compared to control non-MD rats, while MD-induced increases in neuronal bursting were only detected in current-clamp recordings, similar to what we found in early and late adolescent male rats5,7. Figure 1E–F shows examples of silent, and low- and high-frequency tonic and bursting LHb neurons in both voltage- and current-clamp recordings.
Figure 1.
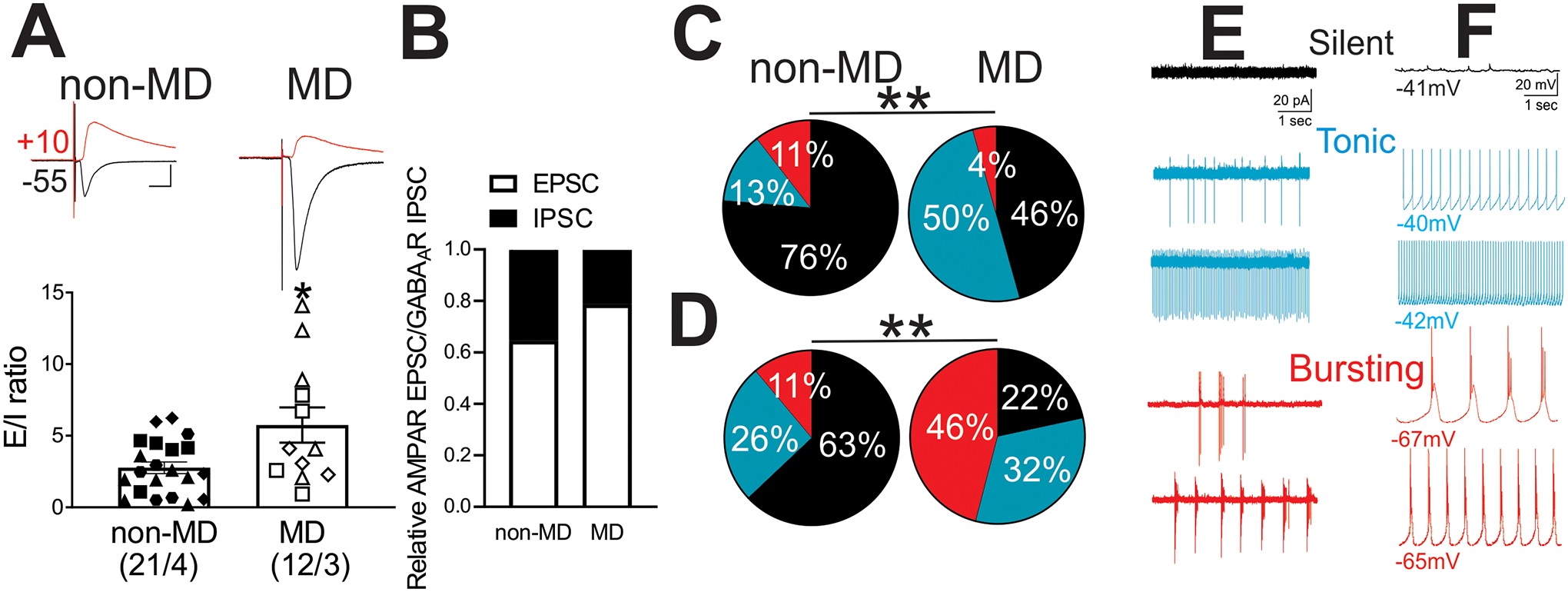
MD increased E/I ratios and spontaneous activity of LHb neurons. A) Summary of E/I ratios of LHb neurons obtained from non-MD (n=21/4) and MD rats (n=12/3) with individual data points and representative traces of evoked EPSCs (black, recorded at −55 mV holding potential) and IPSCs (red, recorded at +10 mV holding potential) in response to electrical stimulation of inputs via the stria medullaris. B) Relative AMPAR/GABAAR components of synaptic currents normalized to the total EPSC+IPSC amplitude for each cell evoked in response to the same stimulus intensity. C-D) Pie charts of voltage-clamp cell-attached recordings (C, V=0 mV, non-MD, n=38/4; MD, n=46/5) and current-clamp whole-cell recordings (D, I=0 pA, non-MD, n=27/4; MD, n=37/5) of spontaneous neuronal activity across non-MD and MD rats. Comparison of the percent distributions of silent (black), tonic (blue), or bursting (red) LHb neurons showed a significant increase in tonic (both C-D) and bursting (only in D) LHb neuronal activity following MD. E-F) Representative traces of E) voltage-clamp cell-attached recordings and F) current-clamp whole-cell recordings of silent (black), and examples of low- and high-frequency tonic (blue) and bursting (red) activities of LHb neurons. *p<0.05, **p<0.01 by unpaired Welch’s t-tests or Chi squared tests. n represents the number of recorded cells/rats, and matching symbols in scatter plots denote cells analyzed from a single rat.
MD altered the acquisition of MSA and reduced sucrose preference in adulthood.
We tested the effects of MD on intravenous MSA acquisition at 0.3 mg/kg/infusion or SSA on a FR1 schedule (morphine/saline delivered after one active lever press) for 6 days followed by a FR3 schedule (morphine/saline delivered after 3 active lever presses) of reinforcement for 4 days in adult male rats. We found that although both non-MD and MD rats readily acquired MSA compared to animals with SSA, MD rats significantly decreased the number of drug-paired lever presses as compared to non-MD rats in FR3 schedule MSA sessions (Figure 2A; 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MD: F(1, 23)= 5.30, p<0.05; effect of MSA: F(1, 23)= 5.34, p<0.05; FR3 schedule: effect of MD: F(1, 23)= 8.37, p<0.01; effect of MSA: F(1, 23)= 43.07, p<0.0001; MD × MSA interaction: F(1, 23)= 9.47, p<0.01). Note that there were no significant effects of MD or MSA on numbers of inactive lever presses among non-MD and MD rats in FR3 schedule sessions (Figure 2B; 3-way Repeated Measures ANOVA tests). MD rats also self-administered lower amounts of morphine as compared to non-MD rats in the FR3 schedule sessions, but not during the FR1 sessions (Figure 2C; 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of time: F(5, 115)= 2.30, p<0.05; MSA × time interaction: F(5, 115)= 5.05, p<0.001; FR3 schedule: effect of MD: F(1, 23)= 9.96, p<0.01; effect of MSA: F(1, 23)= 44.71, p<0.0001; MD × MSA interaction: F(1, 23)= 10.01, p<0.01). In both non-MD and MD rats, MSA increased locomotor activity in FR1 and FR3 schedule sessions (Figure 2D, Mixed-effect analysis and 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MSA: F(1, 23)= 11.56, p<0.01; effect of time: F(3.1, 65.6)= 2.67, p=0.05; MSA × time interaction: F(5, 104)= 2.63, p<0.05; FR3 schedule: effect of MSA: F(1, 23)= 30.67, p<0.0001). In both non-MD and MD rats, MSA also significantly reduced defecation in both FR1 and FR3 schedule sessions (Figure 2E, 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MSA: F(1, 23)= 61.76, p<0.0001; MSA × time interaction: F(5, 115)= 3.08, p<0.05; FR3 schedule: effect of MSA: F(1, 23)= 43.35, p<0.0001). Using separate cohorts of non-MD and MD rats, we also tested the effects of MD on the sucrose preference test and found that MD rats exhibited a significantly lower sucrose consumption compared to non-MD rats, suggesting an anhedonia-like behavior in MD male rats (Figure 2F, Welch’s t-test, t(17)= 2.36, p < 0.05).
Figure 2.
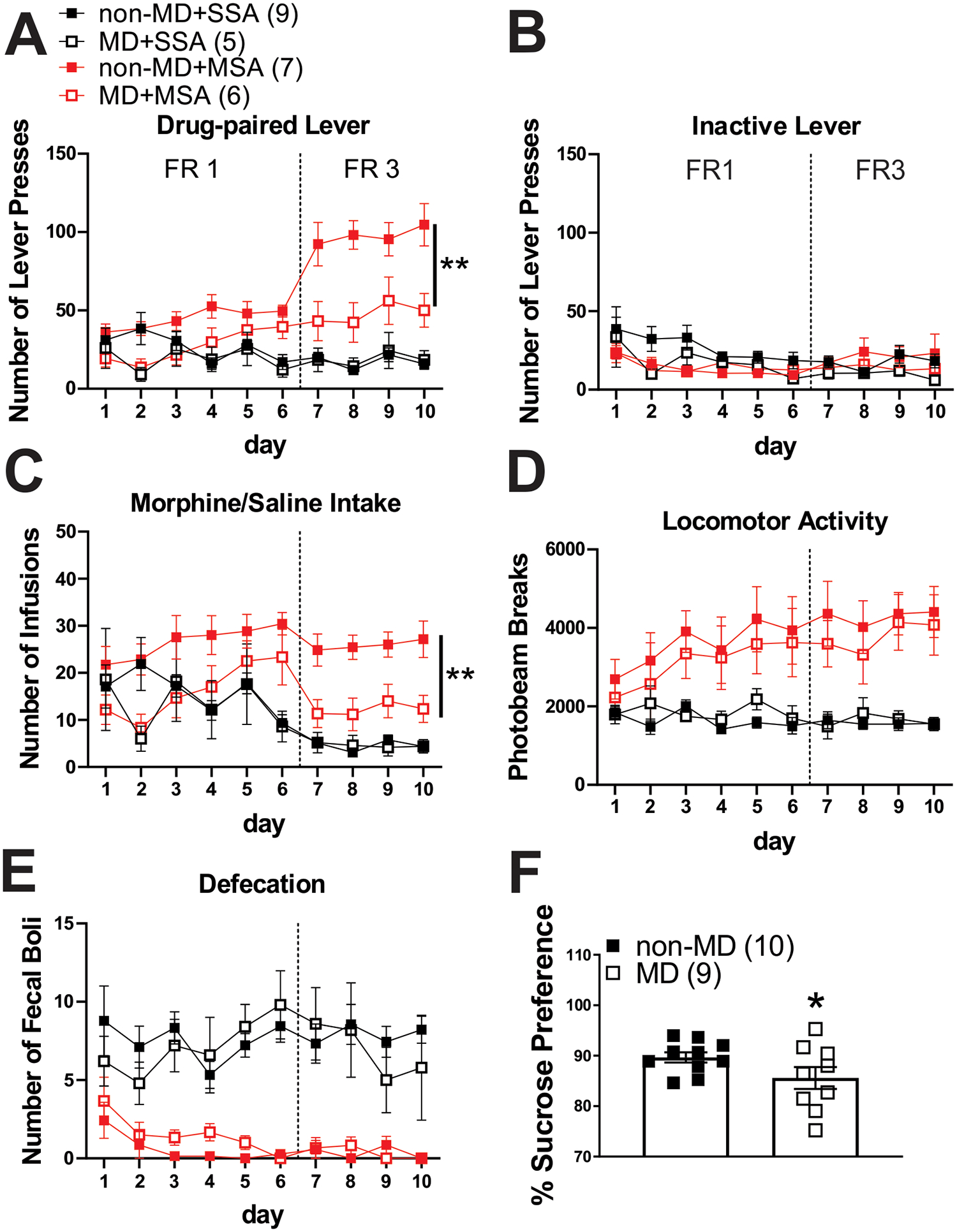
MD decreased morphine intake during FR3 MSA and sucrose preference in the sucrose preference test. A) Average number of drug-paired lever presses, B) inactive lever presses, C) drug infusions, D) locomotor activity, and E) defecation during FR1 and FR3 SSA (black) and MSA (0.3 mg/kg per infusion of morphine, red) sessions. MD significantly decreased drug-paired lever presses and morphine intake in FR3 sessions of MSA. In both non-MD and MD rats, MSA induced locomotor sensitization and constipation (n=5–9/group). F) MD rats consumed less sucrose solution compared to the control non-MD counterparts in the sucrose preference test (n=10/group). *p<0.05, **p<0.01 by 3-way Repeated Measures ANOVA or unpaired Welch’s t tests. n represents the number rats.
Effects of MD on time course of last FR1 and first FR3 SSA/MSA sessions.
To test whether possible motivational deficits in MD rats contributed to lower morphine intake during the FR3 schedule, we then analyzed the time course of cumulative hourly drug-paired lever presses (Figure 3A), drug infusions (Figure 3B) and locomotor activity (Figure 3C) on the last day of the FR1 reinforcement schedule (day 6, when non-MD and MD rats both learned the MSA behavior) and the first day of FR3 (day 7) within the 4 h sessions of SSA and MSA in non-MD and MD rats. We found a significant decrease in drug-paired lever presses (Figure 3A; 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MSA: F(1, 23)= 30.59, p<0.0001; effect of time: F(1.5, 36.3)= 56.72, p<0.0001; MSA × time interaction: F(3, 69)= 12.53, p<0.0001; FR3 schedule: effect of MD: F(1, 23)= 7.22, p<0.01; effect of MSA: F(1, 23)= 23.69, p<0.0001; effect of time: F(1.2, 29.3)= 52.08, p<0.0001; MD × time interaction: F(3, 69)= 5.42, p<0.01; MSA × time interaction: F(3, 69)= 18.59, p<0.0001; MD × MSA interaction: F(1, 23)= 5.78, p<0.05; MD × MSA × time interaction: F(3, 69)= 4.43, p<0.05) and morphine infusions (Figure 3B; 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MSA: F(1, 23)= 27.76, p<0.0001; effect of time: F(1.4, 32.7)= 66.98, p<0.0001; MD × time interaction: F(3, 69)= 2. 93, p<0.05; MSA × time interaction: F(3, 69)= 16.51, p<0.0001; FR3 schedule: effect of MD: F(1, 23)= 7.86, p<0.01; effect of MSA: F(1, 23)= 27.50, p<0.0001; effect of time: F(1.3, 30.6)= 64.15, p<0.0001; MD × time interaction: F(3, 69)= 7.01, p<0.001; MSA × time interaction: F(3, 69)= 24.80, p<0.0001; MD × MSA interaction: F(1, 23)= 7. 38, p<0.01; MD × MSA × time interaction: F(3, 69)= 6.16, p<0.001) in MD rats on the first day of an the FR3 schedule (day 7) during day 7 (FR3) which was not observed on the last day (day 6) of the FR1 schedule. Moreover, we found significant morphine-induced locomotor activity during the FR1 and FR3 schedule of MSA sessions in both non-MD and MD rats (Figure 3C, 3-way Repeated Measures ANOVA tests; FR1 schedule: effect of MSA: F(1, 23)= 14.18, p<0.01; effect of time: F(1.04, 24.04)= 61.65, p<0.0001; MSA × time interaction: F(3, 69)= 10.14, p<0.0001; FR3 schedule: effect of MSA: F(1, 23)= 19.97, p<0.001; MSA × time interaction: F(3, 69)= 15.52, p<0.0001).
Figure 3.
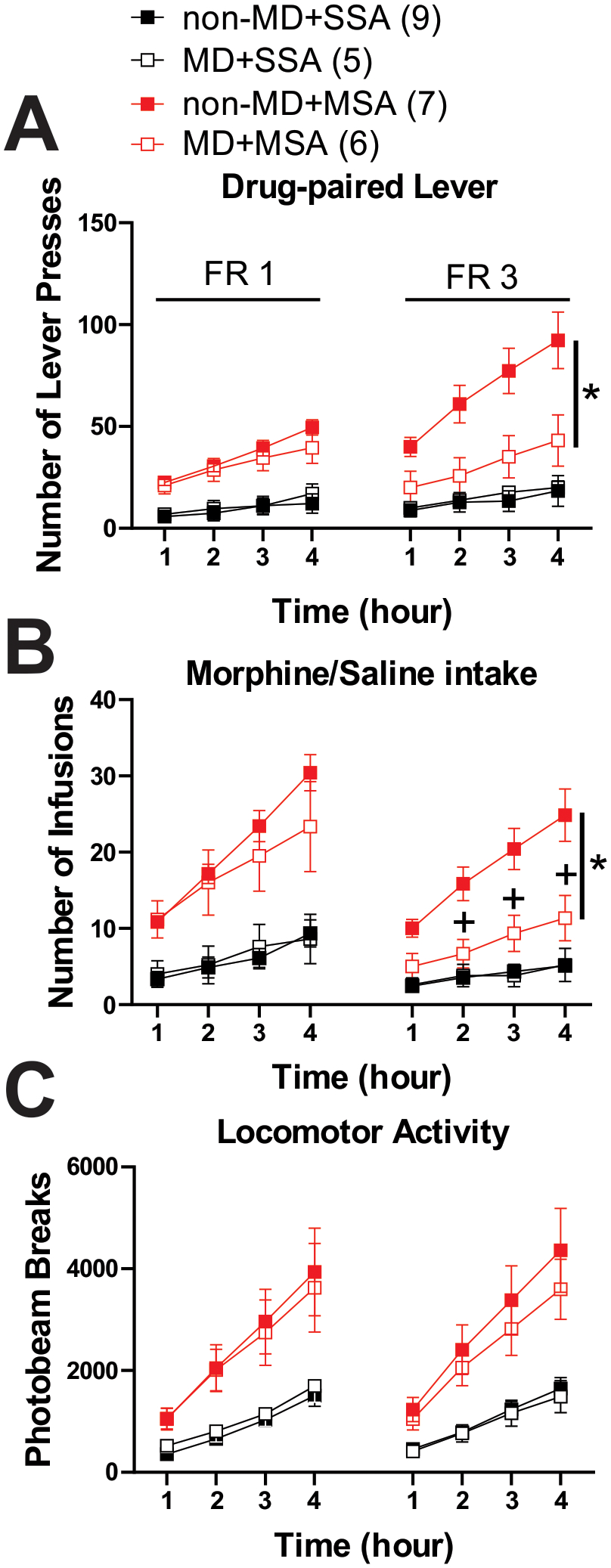
Cumulative hourly time course of final FR1 and first FR3 SSA/MSA sessions. Time course of hourly A) drug-paired lever presses, B) number of infusions, and C) locomotor activity during day 6 (FR1) and day 7 (FR3) of the 4h sessions of SSA (black) and MSA (0.3 mg/kg per infusion of morphine, red) (n=5–9/group). *p<0.05 for interaction effect for MDxMSA, +p<0.05 for post-hoc tests of interaction effect for MDxMSAxtime by 3-way Repeated Measures ANOVA. n represents the number of rats.
MD potentiated glutamatergic synaptic transmission onto LHb neurons in adult male rats.
We recorded AMPAR-mediated mEPSCs in non-MD and MD rats with SSA or MSA at 0.3mg/kg/infusion, one day after the last session of self-administration (Figure 4A–E). Similar to our observations in adolescent MD rats6, glutamatergic synapses onto LHb neurons were potentiated both pre-and post-synaptically in adult MD rats with SSA compared to non-MD rats with SSA (effect of MD). The increase in postsynaptic AMPAR-mediated synaptic function was evident by a significant increase in the cumulative probability of AMPAR-mediated mEPSC amplitude after MD (Figure 4B, Kolmogorov-Smirnov tests, p<0.0001), although the average amplitudes were not significantly different between MD and non-MD rats (Figure 4B, two-way ANOVA tests). Therefore, to provide a more sensitive measurement of postsynaptic glutamatergic plasticity, we also calculated the AMPAR-mediated charge transfer (area under the curve) of mEPSCs. Consistently, we found a significant increase in the mean and cumulative probability of mEPSC charge transfer in MD rats with SSA compared to non-MD rats with SSA (Figure 4C, two-way ANOVA tests; effect of MD: F(1, 75)= 21.54, p<0.0001; Kolmogorov-Smirnov tests, p<0.0001). Given that Kolmogorov-Smirnov tests of the cumulative probabilities of amplitude and charge transfer revealed significant increases in non-MD rats with MSA compared to non-MD rats with SSA with no significant change in the means of these measurements, we also analyzed the decay time constants of mEPSCs which could reveal possible changes in AMPAR kinetics and subunit compositions. Interestingly, we detected changes in Tau decay of mEPSCs of non-MD rats following MSA. Although there was no statistically significant difference in Tau decay means of mEPSCs (only a trend towards a decrease in mean EPSC decay), we observed a significant leftward shift in cumulative probability of mEPSC Tau decay in non-MD rats with MSA compared to non-MD rats with SSA (effect of MSA) (Figure 4D, two-way ANOVA tests; effect of MSA: F(1, 75)= 3.51, p=0.06; Kolmogorov-Smirnov tests, p<0.0001). We also found a significant increase in the cumulative probability of AMPAR-mediated mEPSC frequency and the average frequencies in MD rats with SSA compared to those from non-MD rats with SSA, suggesting an increase in presynaptic glutamate release by MD (Figure 4E, Kolmogorov-Smirnov tests p<0.0001; two-way ANOVA tests; effect of MD: F(1, 75)= 36.86, p<0.0001; effect of MSA: F(1, 75)= 6.577, p<0.01; MDxMSA interaction: F(1, 75)= 5.199, p<0.05). Similar pre- and post-synaptic glutamatergic plasticity was present in MD rats with MSA compared to non-MD SSA rats 24 h after the last morphine intake, suggesting that MD-induced glutamatergic plasticity that persists into adulthood remains intact in MD rats that self-administered morphine (Figure 4B–C,E, 2-way ANOVA and Kolmogorov-Smirnov tests, p=0.06, p<0.05, p<0.0001). Although it should be noted that there was a significant decrease in the mean and cumulative probability of mEPSC frequency in MD rats with MSA compared to MD rats with SSA (Figure 4B–C,E, 2-way ANOVA and Kolmogorov-Smirnov tests, p<0.05, p<0.0001).
Figure 4.
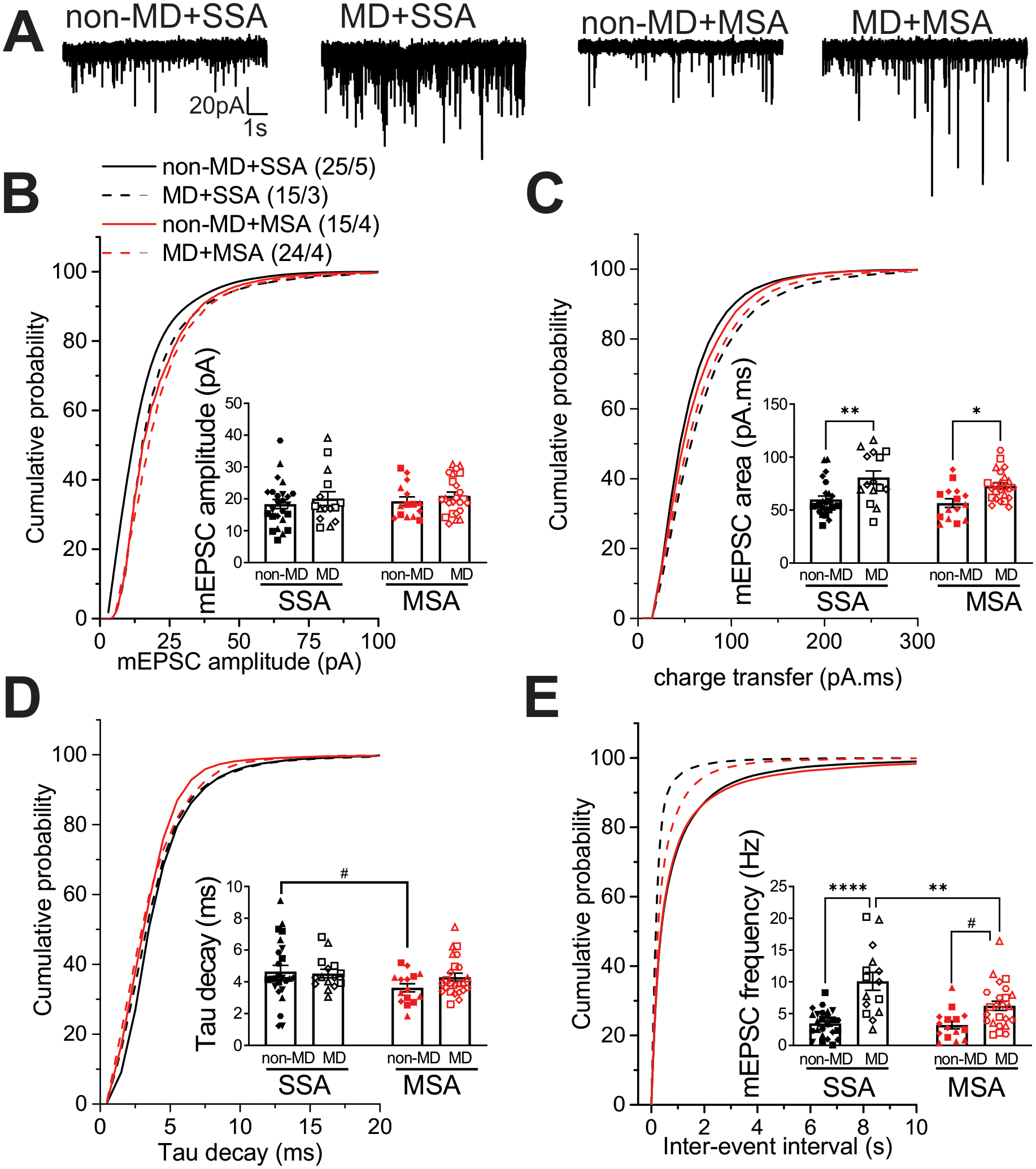
Effects of intravenous SSA or MSA 24h after the last morphine intake at LHb glutamatergic synapses from non-MD and MD rats. A) Representative AMPAR-mediated mEPSC traces from non-MD and MD rats (calibration bars, 20pA/1 s), average and cumulative probability plots of mEPSC B) amplitude, C) charge transfer (area under the curve), D) decay time constants (Tau) and E) frequency (inter-event interval) in non-MD and MD rats (non-MD+SSA: n = 25/5, MD+SSA: n=15/3, non-MD+MSA: n=15/4, MD+MSA: n=24/4). MD potentiated glutamatergic synapses onto LHb neurons but this potentiation was significantly decreased 24h following MSA in MD rats. MSA changed the kinetics of AMPAR mEPSCs in non-MD rats. #p=0.06, *p<0.05, **p<0.01, ****p<0.0001 by 2-Way ANOVA or Kolmogorov–Smirnov tests. n represents the number of recorded cells/rats, and matching symbols in scatter plots denote cells analyzed from a single rat.
Discussion
Here, we used intravenous MSA, which incorporates decision-making processes and reward circuitry to a greater extent than passive administration studies to assess how MD altered volitional morphine intake during MSA and LHb glutamatergic synaptic function following cessation of morphine intake 24h after the last MSA session in adult male rats. We provided evidence that MD-induced pre- and post-synaptic potentiation at glutamatergic synapses onto LHb neurons as well as increased E/I ratios (due to increased excitatory drive and decreased inhibitory drive to LHb neurons) supporting LHb hyperactivity5–7 persisted into adulthood in male MD rats.
Sucrose anhedonia and altered MSA behavior following MD
Consistent with our earlier observation of increased immobility in the forced swim test in late adolescent MD rats5, we found that adult MD rats also showed anhedonia-like behavior in the sucrose preference test, suggesting that a single prolonged MD stress may promote depressive- and anhedonic-like states in Sprague Dawley male rats. This is in agreement with other early life stress studies using MD/maternal separation procedures in Sprague Dawley rats that also trigger increased immobility in the forced swim test and a loss of preference for sucrose as a natural reward44–46. In contrast, a maternal separation procedure that increased oral morphine self-administration behavior and preference in separated Long-Evans male rats also showed enhanced sucrose preference in the sucrose preference test25. Regardless of the differences in rat strains and early life stress models, it is notable that the sucrose preference test in this study was performed differently from the commonly used sucrose preference test in the literature. The sucrose preference test protocol in this study25 lasted for 90 days and was modified to measure preference to a very low concentration of sucrose (0.025% sucrose) that induced a preference of 70% in both control non-separated and separated rats as compared to higher concentrations of sucrose commonly used for the sucrose preference test including the 2% sucrose used in our MD study which also induced a high preference in their rats25. Nevertheless, this study revealed that maternal separation in Long-Evans male rats induced an initial decline (detected from days 11 to 34 which was also present in non-separated controls) and then a long-lasting increase (from days 47 to 54 and on day 90 only in separated rats) in sucrose preference25 suggesting that maternal separation stress may initially diminish but then accentuate the rewarding properties and perception of natural rewarding stimuli following long-term exposures.
Interestingly, the same maternal separation model increased oral consumption and preference for morphine and amphetamine, but not cocaine and ethanol in Long-Evans male rats, suggesting that the early life stress maternal separation rat model is highly suitable to detect vulnerability to opioids26. In our study, both non-MD and MD rats self-administered morphine, suggesting that rats perceived morphine as rewarding compared to saline administration. Although, morphine induced locomotor sensitization and constipation during MSA acquisition in both non-MD and MD rats, MD rats took significantly less morphine compared to non-MD rats during FR3 schedules. Specifically, this was evident in the time course of cumulative hourly intake where there was no difference between non-MD and MD rats in the last day of FR1 MSA sessions when both groups learned the MSA behavior, MD rats exhibited a significant decrease in drug-paired lever presses and morphine infusions during the first day of the FR3 schedule which continued on for the remaining days of FR3 (Figure 2C) in MD-MSA groups. This indicates a possible reduction in motivation of MD rats in acquiring morphine during FR3 when the task became harder. The presence of morphine-induced locomotor stimulation as well as the observed constipation in both non-MD and MD rats also supports the view that morphine is still producing central and peripheral effects in MD rats.
Although we have favored the idea that MD-induced motivational deficits underlie decreased sucrose and morphine intake, it is also possible that MD could result in increased sensitivity to low doses of morphine. This warrants investigating the effects of MD on a morphine dose-response curve for MSA given that MD also increased locomotor sensitization to morphine. Of note, it has been documented that brief maternal separation (e.g., 15-min daily separations of the litter from the dam, often referred to as “handling,”) may reduce the rewarding effects of drugs of abuse while longer maternal separation/MD procedures may enhance the reinforcing properties of drugs including opioids in adulthood18,23,25,26,47,48. Thus, differences between predictable (repeated maternal separation and MD) and unpredictable (our single prolonged MD and limited bedding and nesting) stressors as well as the duration of MD/ maternal separation in early life stress models may confer resistance or vulnerability to drugs of abuse and directly impact the outcomes in terms of addictive behaviors as well as comorbid depression and mood phenotypes.
LHb glutamatergic plasticity following MD and MSA
Given that LHb hyperactivity and glutamatergic potentiation in LHb neurons underlie negative affective states and motivational deficits associated with depression and drug withdrawal49, we also predicted that the lower morphine intake in MSA may be associated with LHb glutamatergic dysfunction that induces overall reduction in motivational states. An LHb-mediated reduction in motivational states following MD could not only underlie MD-induced sucrose anhedonia but also reduce the drive of MD rats to exert efforts in acquiring the morphine reward. Similar to our observations in adolescent MD rats6, glutamatergic synapses onto LHb neurons were still potentiated pre-and post-synaptically in adult MD rats self-administering saline. The postsynaptic glutamatergic plasticity remained intact in LHb of MD rats with MSA 24h following their last morphine intake. Although morphine was able to significantly decrease part of the MD-induced presynaptic potentiation, the increased presynaptic glutamate remained significantly higher compared to control non-MD rats self-administering saline/morphine. Consistently, it has been shown that synaptic transmission from the LHb to the rostromedial tegmental nucleus, a nucleus that suppresses dopamine neuronal activity and signaling, increases during transitions to immobility in the forced swim test to escape this aversive context. Activation of this LHb circuit also decreases motivation of rats to work harder to receive sucrose reward in a progressive ratio schedule of operant appetitive task suggesting a critical role for the LHb in regulation of motivation33. Therefore, it is possible that MD-induced LHb glutamatergic plasticity and LHb hyperactivity could increase the excitatory drive from the LHb to the rostromedial tegmental nucleus and underlie motivational deficits in MD rats. Therefore, it is necessary to employ similar circuit-based studies of LHb circuits combined with a progressive ratio schedule in sucrose self-administration and MSA procedures in the future to further assess the effect of MD on motivation.
Interestingly, we also detected an alteration in postsynaptic AMPAR-mediated glutamatergic kinetics in LHb neurons of non-MD MSA rats compared to non-MD SSA rats 24h following the last MSA. MSA induced a change in AMPAR decay kinetics (decreases in Tau) that was specific for non-MD groups suggesting a possible accumulation of GluA2 lacking AMPARs [i.e., calcium-permeable (CP)-AMPARs] at glutamatergic synapses onto LHb neurons of non-MD rats 24h following MSA. It has been shown that entopeduncular nucleus and lateral hypothalamus glutamatergic transmission onto VTA-projecting LHb neurons are mainly mediated by CP-AMPARs that display strong inward rectification and have faster decay kinetics50,51. We previously reported that MD does not affect the rectification index of AMPAR-mediated EPSCs (e.g., an alteration in this index indicates a change in CP-AMPAR accumulation at glutamatergic synapses) while still pre- and post-synaptically potentiating glutamatergic synapses in LHb neurons6 an observation that is also evident in both MD+SSA and MD+MSA groups. Enhanced glutamate release in VTA-projecting LHb neurons has been shown to contribute to the learned helplessness rodent models of depression51. On the other hand, maternal separation in mice decreases postsynaptic GABABR signaling arising from entopeduncular nucleus GABAergic inputs which then contributes to maternal separation-induced LHb hyperexcitability and the lack of motivation of mice to avoid an aversive context (higher rates of failures in escapable foot-shocks)9. Although it is yet to be known whether maternal separation in mice also induces glutamatergic plasticity in LHb neurons, deep brain stimulation in mouse LHb, which is known to reduce glutamate release in LHb, also ameliorates maternal separation-induced LHb hyperexcitability and reduces failures in escapable foot-shocks. Therefore, we assume that projection-specific reduction of GABAergic transmission in addition to glutamatergic potentiation by MD could contribute to the increase in E/I ratios we have observed in adult MD rats and that deep brain stimulation in rat LHb may also reverse MD-induced LHb hyperactivity as well as sucrose anhedonia and behavioral immobility. A recent report further suggests that naloxone-precipitated morphine withdrawal in mice reduces glutamatergic transmission onto raphe-projecting LHb neurons through habenular cytokine signaling52. Therefore, it is possible that habenular microglial adaptations may occur 24h after the last MSA in MD rats to reduce presynaptic glutamate release onto LHb neurons in a projection-specific manner but it should be noted that morphine withdrawal in mice diminishes the number of postsynaptic AMPARs without any change in AMPAR composition or glutamate release. Overall, pre/post-synaptic glutamatergic and GABAergic plasticity induced by MD and MSA could engage independent LHb neuronal populations and sub-circuits and contribute to modulation of the hedonic effects of natural reward and morphine or more likely encode motivational deficits following MSA and MD.
It should be noted that LHb activity is also linked to aversive properties of drugs of abuse such as cocaine. While passive injections of cocaine in mice have been shown to potentiate AMPAR-mediated glutamatergic plasticity in LHb neurons50,53,54, cocaine exhibits a bi-phasic response in LHb with an initial inhibition followed by delayed excitation of a subset of LHb neurons in rats which mediates cocaine’s initial rewarding effects and delayed aversive properties55. Therefore, it is possible that MD-induced modifications in a specific subpopulation of LHb neurons mediates aversive properties of morphine and also contribute to the reduction of morphine intake in MD rats.
Concluding Remarks
Given that our MD rat model is a single prolonged and unpredictable early life stressor and is associated with anhedonia-like behaviors as well as changes in MSA acquisition, it may represent a valid early life stress model for investigation of potential therapeutic interventions for treatment of opioid use disorder and comorbid mood disorders in patients with a history of childhood neglect and abuse. Our recent discoveries of the neuromodulatory regulation of LHb neuronal excitability and synaptic transmission by intra-LHb corticotropin-releasing factor and dynorphin/kappa opioid receptor signaling and their dysregulation by MD6,7 further highlight the possible involvements of some of the critical and less-studied synaptic and molecular mechanisms underlying early life stress-induced neuromodulation within LHb circuits that could affect the reinforcing and motivational properties of opioids and promote opioid seeking behaviors.
Acknowledgments
The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the Government of the United States. This work was supported by the National Institute of Drugs of Abuse (NIH/NIDA) Grant#R01 DA039533 to FN. The funding agency did not contribute to writing this article or deciding to submit it. We would like to thank Dr. Brian Cox for his helpful and critical review of the manuscript and the support of the Rat Behavior Core at USU.
Footnotes
Data Sharing
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Shepard RD, Nugent FS. Early Life Stress- and Drug-Induced Histone Modifications Within the Ventral Tegmental Area. Front Cell Dev Biol. 2020;8:588476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard RD, Gouty S, Kassis H, et al. Targeting histone deacetylation for recovery of maternal deprivation-induced changes in BDNF and AKAP150 expression in the VTA. Experimental neurology. 2018;309:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Authement ME, Kodangattil JN, Gouty S, et al. Histone Deacetylase Inhibition Rescues Maternal Deprivation-Induced GABAergic Metaplasticity through Restoration of AKAP Signaling. Neuron. 2015;86(5):1240–1252. [DOI] [PubMed] [Google Scholar]
- 4.Shepard RD, Langlois LD, Authement ME, Nugent FS. Histone deacetylase inhibition reduces ventral tegmental area dopamine neuronal hyperexcitability involving AKAP150 signaling following maternal deprivation in juvenile male rats. J Neurosci Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepard RD, Langlois LD, Browne CA, Berenji A, Lucki I, Nugent FS. Ketamine Reverses Lateral Habenula Neuronal Dysfunction and Behavioral Immobility in the Forced Swim Test Following Maternal Deprivation in Late Adolescent Rats. Front Synaptic Neurosci. 2018;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Authement ME, Langlois LD, Shepard RD, et al. A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress. Science signaling. 2018;11(520). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons SC, Shepard RD, Gouty S, et al. Early life stress dysregulates kappa opioid receptor signaling within the lateral habenula. Neurobiology of Stress. 2020;13:100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton JL, Molet J, Regev L, et al. Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol Psychiatry. 2018;83(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchenio A, Lecca S, Valentinova K, Mameli M. Limiting habenular hyperactivity ameliorates maternal separation-driven depressive-like symptoms. Nature communications. 2017;8(1):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena CJ, Kronman HG, Walker DM, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science (New York, NY. 2017;356(6343):1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gracia-Rubio I, Martinez-Laorden E, Moscoso-Castro M, Milanes MV, Laorden ML, Valverde O. Maternal Separation Impairs Cocaine-Induced Behavioural Sensitization in Adolescent Mice. PLoS One. 2016;11(12):e0167483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis DD, Kuhar MJ. Frequency of maternal licking and grooming correlates negatively with vulnerability to cocaine and alcohol use in rats. Pharmacology, biochemistry, and behavior. 2008;90(3):497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73(3):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the neurosciences. 2000;11(4):383–408. [DOI] [PubMed] [Google Scholar]
- 15.Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7261–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor RM, Moloney RD, Glennon J, Vlachou S, Cryan JF. Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology. 2015;99:168–176. [DOI] [PubMed] [Google Scholar]
- 17.Bolton JL, Ruiz CM, Rismanchi N, et al. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress. 2018;8:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology. 1999;141(2):123–134. [DOI] [PubMed] [Google Scholar]
- 19.Lewis CR, Staudinger K, Scheck L, Olive MF. The Effects of Maternal Separation on Adult Methamphetamine Self-Administration, Extinction, Reinstatement, and MeCP2 Immunoreactivity in the Nucleus Accumbens. Frontiers in psychiatry. 2013;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahng JW, Ryu V, Yoo SB, Noh SJ, Kim JY, Lee JH. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience. 2010;171(1):144–152. [DOI] [PubMed] [Google Scholar]
- 21.Martini M, Valverde O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology (Berl). 2012;219(1):149–158. [DOI] [PubMed] [Google Scholar]
- 22.Lu YG, Wang L, Chen JL, et al. Projections from lateral habenular to tail of ventral tegmental area contribute to inhibitory effect of stress on morphine-induced conditioned place preference. Brain research. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002;27(4):518–533. [DOI] [PubMed] [Google Scholar]
- 24.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology. 2001;157(3):305–312. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci. 2005;25(18):4453–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol. 2006;17(8):715–724. [DOI] [PubMed] [Google Scholar]
- 27.Naudon L, Piscitelli F, Giros B, Di Marzo V, Dauge V. Possible involvement of endocannabinoids in the increase of morphine consumption in maternally deprived rat. Neuropharmacology. 2013;65:193–199. [DOI] [PubMed] [Google Scholar]
- 28.Schultz W Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nature neuroscience. 2014;17(9):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hikosaka O The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuno-Perez A, Tchenio A, Mameli M, Lecca S. Lateral Habenula Gone Awry in Depression: Bridging Cellular Adaptations With Therapeutics. Frontiers in neuroscience. 2018;12:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proulx CD, Aronson S, Milivojevic D, et al. A neural pathway controlling motivation to exert effort. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(22):5792–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graziane NM, Neumann PA, Dong Y. A Focus on Reward Prediction and the Lateral Habenula: Functional Alterations and the Behavioral Outcomes Induced by Drugs of Abuse. Front Synaptic Neurosci. 2018;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis EB, Fields HL. Mu Opioid Receptor Actions in the Lateral Habenula. PLoS One. 2016;11(7):e0159097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentinova K, Tchenio A, Trusel M, et al. Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nature Neuroscience. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. [DOI] [PubMed] [Google Scholar]
- 38.Otaka M, Ishikawa M, Lee BR, et al. Exposure to cocaine regulates inhibitory synaptic transmission in the nucleus accumbens. J Neurosci. 2013;33(16):6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le T, Xia M, Jia M, et al. Association between initial morphine intake and body weight change, acoustic startle reflex and drug seeking in rats. Psychopharmacology. 2014;231(23):4569–4577. [DOI] [PubMed] [Google Scholar]
- 40.Nishida KS, Park TY, Lee BH, Ursano RJ, Choi KH. Individual differences in initial morphine sensitivity as a predictor for the development of opiate addiction in rats. Behavioural brain research. 2016;313:315–323. [DOI] [PubMed] [Google Scholar]
- 41.Neath KN, Limebeer CL, Reilly S, Parker LA. Increased liking for a solution is not necessary for the attenuation of neophobia in rats. Behavioral neuroscience. 2010;124(3):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manouze H, Ghestem A, Poillerat V, et al. Effects of Single Cage Housing on Stress, Cognitive, and Seizure Parameters in the Rat and Mouse Pilocarpine Models of Epilepsy. eNeuro. 2019;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner PV, Sunohara-Neilson J, Ovari J, Healy A, Leri F. Effects of single compared with pair housing on hypothalamic-pituitary-adrenal axis activity and low-dose heroin place conditioning in adult male Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2014;53(2):161–167. [PMC free article] [PubMed] [Google Scholar]
- 44.Bai M, Zhang L, Zhu X, Zhang Y, Zhang S, Xue L. Comparison of depressive behaviors induced by three stress paradigms in rats. Physiol Behav. 2014;131:81–86. [DOI] [PubMed] [Google Scholar]
- 45.Valvassori SS, Varela RB, Arent CO, et al. Sodium Butyrate Functions as an Antidepressant and Improves Cognition with Enhanced Neurotrophic Expression in Models of Maternal Deprivation and Chronic Mild Stress. Current neurovascular research. 2014. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Zhu X, Bai M, Zhang L, Xue L, Yi J. Maternal deprivation enhances behavioral vulnerability to stress associated with miR-504 expression in nucleus accumbens of rats. PLoS One. 2013;8(7):e69934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. The Journal of pharmacology and experimental therapeutics. 2006;317(3):1210–1218. [DOI] [PubMed] [Google Scholar]
- 48.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neuroscience and biobehavioral reviews. 2003;27(1–2):45–55. [DOI] [PubMed] [Google Scholar]
- 49.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21(5):277–295. [DOI] [PubMed] [Google Scholar]
- 50.Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J Neurosci. 2012;32(36):12641–12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Piriz J, Mirrione M, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470(7335):535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valentinova K, Tchenio A, Trusel M, et al. Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nature neuroscience. 2019;22(7):1053–1056. [DOI] [PubMed] [Google Scholar]
- 53.Meye FJ, Valentinova K, Lecca S, et al. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nature neuroscience. 2015;18(3):376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jhou TC, Good CH, Rowley CS, et al. Cocaine Drives Aversive Conditioning via Delayed Activation of Dopamine-Responsive Habenular and Midbrain Pathways. J Neurosci. 2013;33(17):7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]


