ABSTRACT
Novel chemotherapeutic agents are developed to treat recurrent/relapsed lymphoid malignancies. Umbralisib, a novel phosphatidylinositol 3-kinase inhibitor with a selective isoform binding, has shown an improved efficacy and safety profile in clinical trials. Immune-mediated colitis, a frequently observed dose-limiting adverse event of phosphatidylinositol 3-kinase inhibitors, has been mostly observed at supratherapeutic doses in the trials, with grade 1 or 2 diarrhea being the most common adverse event at the therapeutic dose (800 mg PO QD). We present a grade-3 colitis that can be attributed to umbralisib-mediated immune toxicity in a patient with chronic lymphocytic leukemia at the therapeutic dose.
INTRODUCTION
Many novel chemotherapeutic agents are currently under development that target different levels in the signal transduction pathways of the cancer cells. Phosphatidylinositol 3-kinase (PI3K) enzymes are involved in cellular proliferation and differentiation. PI3K-δ, a subtype of PI3K enzymes, is largely present on the hematopoietic cells and often abnormally activated in B-cell malignancies, making it an ideal therapeutic target for hematological malignancies especially B-cell malignancies.1 However, their use has been limited, given the frequently occurring immune-mediated adverse events (eg, colitis and transaminitis). Owing to these side effects, there has been a need to develop newer agents with improved selectivity and safety profile. Umbralisib is an oral, once-daily formulation with better selectivity in inhibiting PI3K-δ subtype when compared with the other approved drugs of the same class (duvelisib and idelalisib) likely because of its unique structural properties.2
CASE REPORT
A 61-year-old White woman with a medical history of chronic lymphocytic leukemia (CLL) presented to the emergency department with 3 weeks of worsening diarrhea. She was initially diagnosed with CLL 5 years ago after lymphocytosis was incidentally found on screening laboratory work. Treatment was deferred because the patient was asymptomatic at the time, and she was monitored yearly by a hematologist. After 5 years of uneventful surveillance, a computed tomography scan showed diffuse enlargement of cervical lymph nodes, enlarging spleen, and gastrohepatic, periportal, and retroperitoneal lymphadenopathy. She was subsequently enrolled into a phase 2 clinical study and started on umbralisib in combination with ublituximab and venetoclax. She had an excellent response after 3 full cycles of chemotherapy. Laboratory results showed improvement in leukocytosis (191.4 × 109/L to 6.4 × 109/L), lymphocytosis (96.5%–28.3%), thrombocytopenia (101 × 109/L to 151 × 109/L), and anemia (9.7–12 g/dL). She had a grade 3 neutropenia (not dose-limiting), grade 1 rash, and grade 1 arthralgia.
Three months into chemotherapy, she developed loose bowel movements, at 3–4 episodes per day with associated mild abdominal cramping, bloating, and anorexia. She reported nocturnal diarrhea and diarrhea that persisted with fasting. She tried Imodium, psyllium, and loperamide with no relief. Her diarrhea rapidly and progressively worsened with up to 15–20 watery, nonbloody, large volume stools per day with significant urgency. In the days leading to her hospitalization, she developed nausea, poor oral intake, and had multiple syncopal episodes.
On presentation to the emergency department, her temperature was 37.2°C, heart rate was 105 beats/min, and her blood pressure was 121/73 mm Hg. Mucous membranes were dry. The pulse was good in volume, and the skin pinch test was normal. Abdominal examination showed a soft, nondistended, and nontender abdomen without any organomegaly. The remaining examination was within normal limits. Laboratory studies showed hemoglobin = 12.2 g/dL, white blood cell count = 3,100/mm3 (N = 32.5%, L = 49.7%, M = 15.3%), platelets = 162,000/mm3, hypokalemia (2.7 mEq/L), creatinine = 1.18 mg/dL, lactate dehydrogenase = 366 U/L, and ante meridiem cortisol = 10 μg/dL.
The patient was admitted and started on IV fluids and potassium supplementation. Stool studies came back negative for Clostridium difficile, Shiga toxin, enteric pathogens, rotavirus, norovirus, cytomegalovirus, cryptosporidium, giardia and, ova and parasites. Esophagogastroduodenoscopy demonstrated a mild Schatzki ring at the gastroesophageal junction, a 4 cm hiatal hernia, and a diffuse moderately scalloped mucosa in the entire duodenum (Figure 1). Colonoscopy demonstrated mild erythema and a few aphthous ulcers in the terminal ileum, granularity, and moderate erythema in the entire colon, few ulcers in the rectosigmoid colon, and a few diminutive polyps (Figure 2). The pathology report from the biopsies was suggestive of marked active enteritis and diffusely active colitis likely because of drug-mediated toxicity (Figure 3 and 4).
Figure 1.
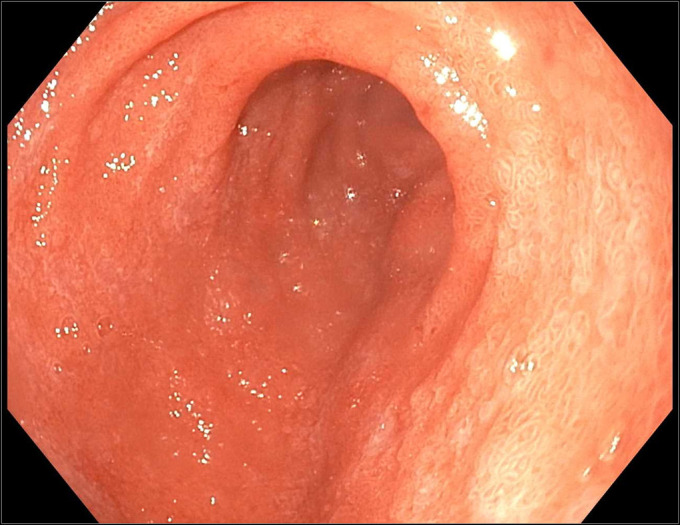
Esophagogastroduodenoscopy demonstrating a diffuse moderately scalloped mucosa in the duodenum.
Figure 2.
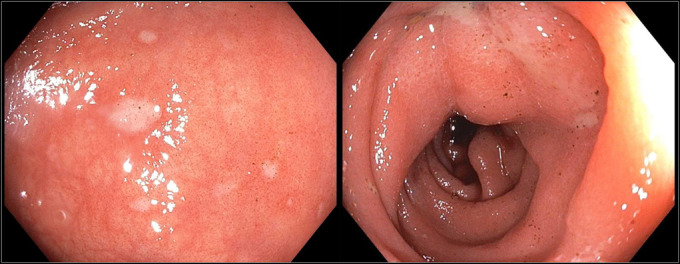
(A) Rectal ulcerations and (B) sigmoid colon showing erythematous colon mucosa during a colonoscopy.
Figure 3.
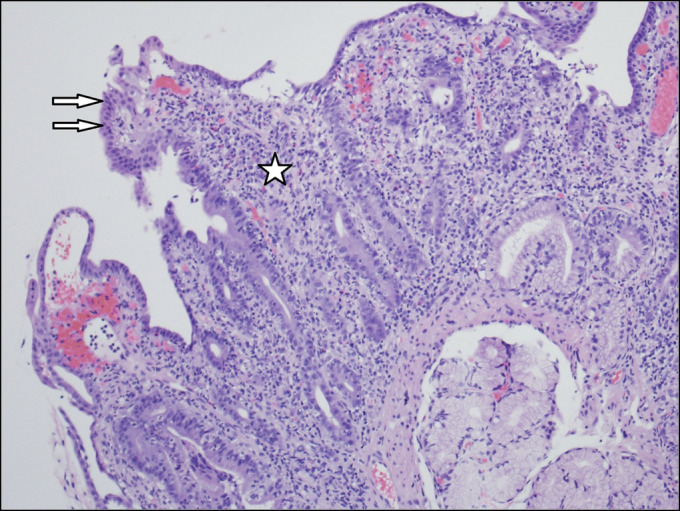
Duodenal biopsy demonstrating architectural distortion with villous blunting (arrows) and marked increase in lamina propria inflammation (*). The infiltrate is mixed with lymphocytes, plasma cells, eosinophils, and polymorphonuclear leukocytes.
Figure 4.
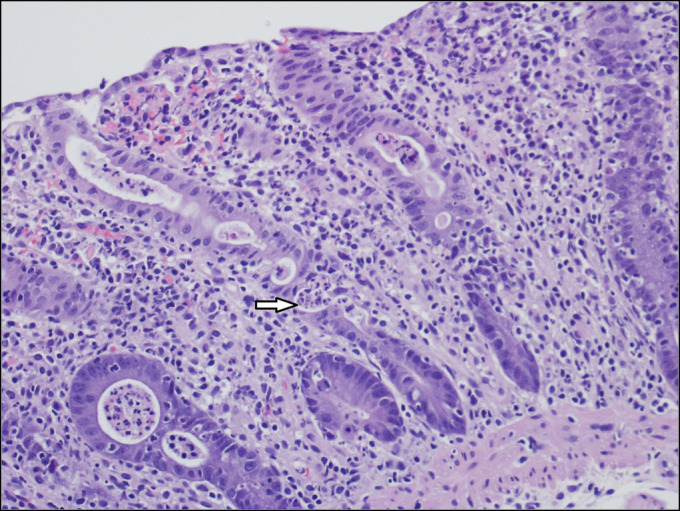
Colonic biopsy demonstrating marked inflammatory infiltrate with prominence of polymorphonuclear leukocytes including cryptitis and crypt abscesses (arrow).
In addition to aggressive hydration and electrolytes correction, she was started on high-dose IV methylprednisolone (30 mg twice daily) and her chemotherapy was discontinued. The patient showed significant improvement in her diarrhea after receiving 5 days of IV steroids, and she was discharged on prednisone 40 mg with a slow taper. The patient developed diarrhea when the steroid was tapered from 20 to 10 mg. The dose of steroids was increased to 20 mg with an even slower taper at 2 weeks interval. The patient had a complete resolution of her diarrhea and tapered off without issue. At the time of case report submission, the patient has been off the steroids for more than 2 months without recurrence of diarrhea.
DISCUSSION
Umbralisib has shown excellent results in its efficacy and safety profile in the early studies done so far. It has proven to be a unique PI3K inhibitor with an improved safety profile and can be used in different combination chemotherapies, without causing additional adverse events, to induce remission in cases of CLL, small cell lymphoma, and other B-cell non-Hodgkin lymphoma. It has demonstrated fewer occurrences of immune-mediated toxicities across the phase 1 and 2 randomized controlled trials when compared with the currently approved idelalisib and duvelisib, although the mechanism is not completely understood. Besides inhibiting PI3K, umbralisib also shows inhibition of casein kinase-1ε (CK1ε), which is involved in the activation of the c-Myc oncogene pathway and the regulation of the Wnt5a pathway. Both of these pathways are known to play a role in PI3K-induced colitis.3 Early studies hypothesize that the inhibition of CK-1ε prevents the depletion of regulatory T-cells, which could theoretically cause immune suppression leading to fewer immune-mediated adverse events.4
An open-label phase 1 study that included a total of 90 patients showed that the most common treatment-related adverse events were diarrhea (in 39 [43%] of 90 patients), nausea (38 [42%]), and fatigue (28 [31%]). The grade 3 or 4 adverse events that were seen included neutropenia (in 12 [13%] patients), anemia (8 [9%]), and thrombocytopenia (6 [7%]). This demonstrates the improved safety profile of umbralisib compared with the approved first-generation drugs. Umbralisib especially showed a lower occurrence of grade ≥ 3 transaminitis (3%) or colitis (2%) when compared with idelalisib (grade ≥ 3 transaminitis = 13%–25% and grade ≥ 3 diarrhea or colitis = 9%–16%) and duvelisib (grade ≥ 3 transaminitis = 41%; grade ≥ 3 diarrhea = 22%). The frequency of diarrhea seen with umbralisib (43%) was similar to its counterparts (43%–54%), but most of the episodes were of grade 1 severity and resolved without any need of change in the dosing.5
A phase 2 study for the combination of umbralisib and ublituximab (U2) with 75 patients showed that most of the chemotherapy-related adverse events were of grade 1 or 2 severity. The occurrence of severe adverse events was especially low. Immune-mediated colitis and transaminitis were seen in 2%–3% of the patients, respectively. The combination overall showed lower rates of other immune and nonimmune adverse events commonly seen with PI3K inhibitors.2 Although ublituximab, a monoclonal antibody that targets CD20, may contribute to the diarrhea, none had grade 3 or 4 diarrhea as reported in a phase 1/2 trial.6 In addition, patient's diarrhea was not correlated in any manner with monthly infusion of ublituximab.
In conclusion, umbralisib is a novel PI3K/CK inhibitor that offers a unique advantage in its use as compared to the traditional PI3K inhibitors in its safety profile. Early studies have shown it to have an improved efficacy with fewer side effects, although long-term follow-up is needed to evaluate further. Most cases of colitis reported during the clinical trials occurred at supratherapeutic doses (1,200 mg), but our case shows the development of immune-mediated colitis at the recommended therapeutic dose (800 mg PO OD). Although umbralisib does show promising results, long-term clinical studies will further help to assess its safety profile.
DISCLOSURES
Author contributions: S. Bajaj wrote the manuscript and reviewed the literature. SM Barrett and Y. Bi wrote the manuscript, reviewed the literature, and revised the manuscript for intellectual content. RE Nakhleh provided pathology images. B. Brahmbhatt revised the manuscript for intellectual content Y. Bi is the article guarantor..
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Suryansh Bajaj, Email: suryanshbajaj26@gmail.com.
Stevi M. Barrett, Email: Barrett.Stevi@mayo.edu.
Raouf E. Nakhleh, Email: Nakhleh.raouf@mayo.edu.
Bhaumik Brahmbhatt, Email: Brahmbhatt.bhaumik@mayo.edu.
REFERENCES
- 1.Umbralisib inhibits PI3Kδ with less toxicity than previous inhibitors. Cancer Discov. 2018;8(4):382. [DOI] [PubMed] [Google Scholar]
- 2.Lunning M, Vose J, Nastoupil L, et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(21):1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visentin A, Frezzato F, Severin F, et al. Lights and shade of next-generation Pi3k inhibitors in chronic lymphocytic leukemia. Onco Targets Ther. 2020;13:9679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davids MS, Kim HT, Nicotra A, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: A multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burris HA, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: An open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19(4):486–96. [DOI] [PubMed] [Google Scholar]
- 6.Sawas A, Farber CM, Schreeder MT, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol. 2017;177(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


