ABSTRACT
Tofacitinib is an oral Janus kinase inhibitor. Although it contributes to the induction and maintenance of clinical remission of patients with moderate-to-severe ulcerative colitis, various malignancies have been reported after the use of this small molecule. We report a rare case of biopsy-proven Kaposi sarcoma in a patient with complex biological-resistant ulcerative colitis after 2 years of treatment with tofacitinib. Kaposi sarcoma lesions spontaneously regressed after tofacitinib was discontinued. Given the concern of potential risk of malignancy associated with this agent, we believe that specialists should be aware of this rare but serious possible adverse event.
INTRODUCTION
Kaposi sarcoma (KS) is a rare vascular malignancy caused by KS herpesvirus (also known as human herpesvirus 8). There are 4 variant forms of KS: classic, AIDS-related, endemic (African), and iatrogenic KS.1–3 The iatrogenic form commonly occurs in organ transplant patients and patients on long-term immunosuppressive agents. Recently, there has been an increasing number of reported cases of iatrogenic KS associated with biologic therapies in patients with inflammatory bowel disease.4,5 Tofacitinib is an oral Janus kinase (JAK) inhibitor that is approved to induce and maintain remission in patients with moderately to severely active ulcerative colitis (UC).6 The immunosuppressive effect of tofacitinib has raised a concern about adverse events, particularly malignancies.7 We report a case of a patient with a history of moderate-to-severe steroid-dependent UC who developed biopsy-proven KS after receiving tofacitinib for 2 years, which regressed spontaneously after discontinuation of its use.
CASE REPORT
A 61-year-old man from Canada with an unremarkable medical history was diagnosed with refractory moderate-to-severe extensive UC in 2016. His blood test results for hepatitis B virus, hepatitis C virus, and HIV were negative. He failed infliximab and vedolizumab treatment in the first 2 years of postdiagnosis and partially responded to ustekinumab in the third year. His clinical symptoms were not much improved during the ustekinumab treatment at the dose of 90 mg every 4 weeks, with 6–7 bowel movements per day with bloody mucous stool and abdominal pain. He also required 15–20 mg per day of oral prednisone and 4.8 g per day of mesalazine combined with hydrocortisone enema at that time to reduce his UC symptoms. Laboratory tests revealed a fecal calprotectin level of 332 μg/g and C-reactive protein of 1.8 mg/L. His hemoglobin level and serum albumin were within the normal range. Flexible sigmoidoscopy revealed diffuse erythematous mucosa and small erosions (Mayo subscore 2) (Figure 1). Stool Clostridium difficile toxin polymerase chain reaction and tissue immunohistochemistry for cytomegalovirus were negative. Given the inadequate response, ustekinumab was discontinued accordingly.
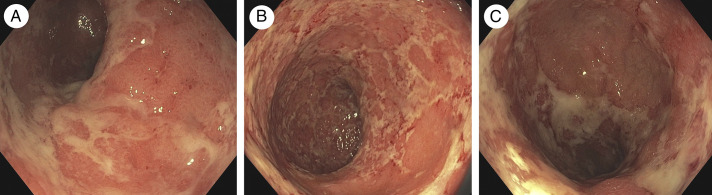
Figure 1.
Sigmoidoscopy findings of active ulcerative colitis show marked erythematous mucosa, lack of a vascular pattern, and small erosions (Mayo endoscopic subscore 2) during ustekinumab therapy from (A) sigmoid colon, (B) rectosigmoid, and (C) rectum.
In October 2018, 10 mg of tofacitinib twice daily for 8 weeks regimen was initiated in combination with 40 mg of prednisone to induce clinical remission. Two doses of recombinant herpes zoster vaccination were completed before the first dose of tofacitinib. After 2 months of tofacitinib treatment, his UC symptoms significantly improved. Tofacitinib was continued in the maintenance therapy at the dose of 5 mg BID, and prednisone was gradually tapered down until discontinuation within 10 weeks after starting. He had 3–4 formed stools per day without mucous, bloody stool, or urgency. Fecal calprotectin level and C-reactive protein decreased to 199 μg/g and 0.3 mg/L, respectively.
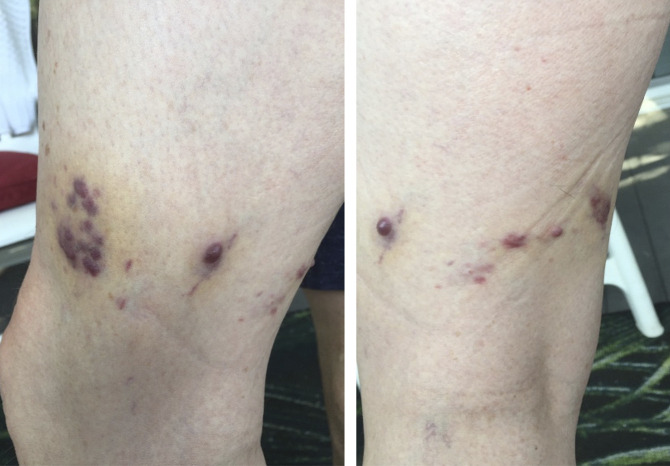
In July 2020, 2 years after the initiation of tofacitinib, he developed small purple skin nodules on his left leg (Figure 2). Skin biopsies demonstrated KS, confirmed by the presence of cellular proliferation of neoplastic spindle cells. After a discussion with the patient and the oncologist about the potential role of tofacitinib, it was discontinued. In an attempt to maintain clinical remission, vedolizumab was reintroduced to replace tofacitinib because it provides a potentially safer mechanism of action.
Figure 2.
Photographs and characteristics of the skin lesions (Kaposi sarcoma).
Two months after stopping tofacitinib, the KS lesions spontaneously regressed without any specific treatment. His UC symptoms were controlled with vedolizumab and the low dose of prednisone at 7.5 mg per day in the maintenance therapy. On the follow-up, fecal calprotectin remained stable at 213 μg/g.
DISCUSSION
Tofacitinib shows promising clinical benefits in patients with moderate-to-severe UC. The impact of tofacitinib on the risk of malignancies has been controversial in real-world data. Various malignancies have been reported after its use in case reports and meta-analyses.3,7,8 In a recent cohort of UC clinical studies, including data from 1,157 patients with UC treated with tofacitinib for up to 6–8 years, the risk of malignancies have remained stable over the time. The incidence rates of malignancies excluding nonmelanoma skin cancer (NMSC) and NMSC were 0.75 (95% confidence interval [CI], 0.46–1.16) and 0.73 (95% CI, 0.44–1.13) per 100 person-year (PY), respectively. In addition, there was no specific type of cancer reported.9,10
A systematic review and meta-analysis including 82 studies showed a significant increase in the risk of herpes zoster infection among patients exposed to JAK inhibitors (relative risk 1.57; 95% CI, 1.04–2.37). Other adverse events of JAK inhibitors were also reported as incidence rates of serious infections (2.8/100 PY), major cardiovascular events (0.48/100 PY), and deep vein thrombosis or pulmonary embolism (0.31/100 PY)11 as per the Food and Drug Administration warning.
In patients with inflammatory bowel disease, iatrogenic KS was reported in patients who received long-term immunosuppressive therapy (systemic corticosteroid, thiopurine, and methotrexate) and antitumor necrosis factor agents.4,5,12,13 Recently, the colonic KS was reported in a patient with UC treated with vedolizumab.14 Of note, in a large series of consecutive patients with KS, 27% of the 137 patients were iatrogenic (age 60 years, males 54%), including patients on immunosuppressants or immunodeficiencies (19), posttransplant cases (10), and underlying malignant diseases (8). The percentage of iatrogenic KS in published consecutive case series was between 9% and 27%.15
To the best of our knowledge, this is the first reported case of tofacitinib-associated KS in a patient with UC, which spontaneously regressed after discontinuation of the drug, clearly suggesting a causative association. It might hypothetically be the effect of tofacitinib on the inhibition of the JAK/STAT pathway that predisposes to various viral infections, such as KS herpesvirus, respiratory syncytial virus, and coronavirus.16 It has been revealed that signaling transduction of various cytokines serving as a major inhibitory role in tumorigenesis (eg, interleukin and STAT) was decreased, which escalated the risk of some malignancies, including KS.7,17 However, multiple factors (genetic, long-term systemic corticosteroid, immunologic, and environment) are certainly involved as well.
In most cases of iatrogenic KS, it can spontaneously resolve when the immunosuppressive therapy is altered, reduced, or discontinued, as was the case in our patient.18 Nevertheless, the risk of KS exists in our case owing to vedolizumab and continuing steroid use. The close monitoring of recurrent KS and cancer surveillance are still needed. In summary, although tofacitinib contributed to the induction of clinical remission and the maintenance of patients with UC, it could be a causative agent in triggering the development of KS in our patient. This represents a clinically serious side effect, and treating clinicians should be aware of the possibility of malignancies occurring in association with this drug.
DISCLOSURES
Author contributions: P. Wetwittayakhlang wrote the manuscript, reviewed the literature, and is the article guarantor. PA Golovics, W. Afif, and T. Bessissow revised the final manuscript. PL Lakatos supervised and revised the final manuscript.P. Wetwittayakhlang has been a speaker and/or advisory board member for Takeda, Pfizer, Janssen, Ferring, A. Menerini, and MSD. T. Bessissow has acted as a speaker or advisor for Abbvie, BMS, Ferring, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, and Takeda. PL Lakatos has been a speaker and/or advisory board member for AbbVie, Amgen, Arena Pharmaceuticals, Fresenius Kabi, Genetech, Gilead, Janssen, Merck, Mylan, Pharmacosmos, Pfizer, Roche, Takeda, Tillots, and Viatris.
Financial disclosure: PL Lakatos has received unrestricted research grants: AbbVie, MSD, and Pfizer.
Informed consent was obtained for this case report
Contributor Information
Panu Wetwittayakhlang, Email: wet.panu@gmail.com.
Petra A. Golovics, Email: golovics.petra@gmail.com.
Waqqas Afif, Email: waqqasafif@gmail.com.
Talat Bessissow, Email: talat.bessissow@gmail.com.
REFERENCES
- 1.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruocco E. Kaposi's sarcoma: etiology and pathogenesis, inducing factors, causal associations, and treatments: facts and controversies. Clin Dermatol. 2013;31:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis JR, Lee EB, Kaplan IV, et al. Tofacitinib, an oral Janus kinase inhibitor: Analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stasi E, De Santis S, Cavalcanti E, Armentano R. Iatrogenic Kaposi sarcoma of the terminal ileum following short-term treatment with immunomodulators for Crohn disease: A case report. Medicine (Baltimore). 2019;98(20):e15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pioche M, Boschetti G, Cotte E, et al. Human Herpesvirus 8-associated colorectal Kaposiʼs Sarcoma occurring in a drug-induced immunocompromised patient with refractory ulcerative colitis: Report of a new case and review of the literature. Inflamm Bowel Dis. 2013;19(2):E12–5. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman P, Cohen SB. Malignancy and Janus kinase inhibition. Rheum Dis Clin North Am. 2017;43(1):79–93. [DOI] [PubMed] [Google Scholar]
- 8.Xie W, Yang X, Huang H, Gao D, Ji L, Zhang Z. Risk of malignancy with non-TNFi biologic or tofacitinib therapy in rheumatoid arthritis: A meta-analysis of observational studies. Semin Arthritis Rheum. 2020;50(5):930–7. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Ciorba MA, Rogler G, et al. Sa1763 - tofacitinib for the treatment of ulcerative colitis: Analysis of malignancy rates from the octave clinical program. Gastroenterology. 2018;154(6):S-385–6. [Google Scholar]
- 10.Sandborn WJ, Panés J, D'Haens GR, et al. S0703 Tofacitinib for the treatment of ulcerative colitis: Up to 6.8 Years of Safety data from global clinical trials. J Am Coll Gastroenterol. 2020;115:S353–4. [Google Scholar]
- 11.Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: A Systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–73.e12. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Peláez M, Fernández-García MS, Gutiérrez-Corral N, et al. Kaposi's sarcoma: An opportunistic infection by human herpesvirus-8 in ulcerative colitis. J Crohns Colitis. 2010;4(5):586–90. [DOI] [PubMed] [Google Scholar]
- 13.Shah N, Lidofsky S, Laskiewicz L. Colorectal Kaposi Sarcoma in an immunosuppressed ulcerative colitis patient. J Gastrointest Surg. 2018;22(7):1301–2. [DOI] [PubMed] [Google Scholar]
- 14.Papa V, Giustiniani MC, Lopetuso LR, Papa A. Human herpesvirus 8-associated colonic Kaposi's sarcoma during vedolizumab treatment in ulcerative colitis: A case report and review of the literature. BMC Gastroenterol. 2020;20(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baykal C, Atci T, Buyukbabani N, Kutlay A. The spectrum of underlying causes of iatrogenic Kaposi's Sarcoma in a large Series: A retrospective study. Indian J Dermatol. 2019;64(5):392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee A, Goswami RP, Chatterjee M. Network theoretic analysis of JAK/STAT pathway and extrapolation to drugs and viruses including COVID-19. Sci Rep. 2021;11(1):2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MS, Jones T, Song DY, Jang JH, Jung JU, Gao SJ. Exploitation of the complement system by oncogenic Kaposi's Sarcoma-associated Herpesvirus for cell survival and persistent infection. PLoS Pathog. 2014;10(9):e1004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moosa MR. Kaposi's sarcoma in kidney transplant recipients: A 23-year experience. QJM. 2005;98(3):205–14. [DOI] [PubMed] [Google Scholar]