Abstract
PURPOSE
This real-life cohort of patients describes the treatment patterns and compares the overall survival (OS) and hazard risk of utilization of multiple therapies.
MATERIALS AND METHODS
Electronic medical registries of patients with stage III non–small-cell lung cancer (NSCLC) regularly attended in 72 hospitals were included. Univariate and multivariate analyses were conducted to evaluate the primary patients' characteristics leading to better OS and cancer-specific survival.
RESULTS
A total of 3,363 patients with stage III NSCLC followed over 19 years were included in this study. The median age was 66.00 (58.00-72.00) years, 65% male, and 41.2% with squamous cell carcinoma followed by adenocarcinoma (34.6%) and undifferentiated carcinoma (13.1%) in clinical stage T3 (50.3%), T2 (29.3%), and T4 (12.3%). The median survival (in months) was 18.4 (95% CI, 16.9 to 19.5) in patients submitted to radiotherapy plus chemotherapy, 11.2 (95% CI, 10.5 to 12.1) to chemotherapy, 31.5 (95% CI, 25.9 to 37.7) to surgery plus chemotherapy, and 33.8 (95% CI, 28.3 to 47.8) to chemotherapy plus radiotherapy plus surgery. The median cancer-specific survival (in months) was 19.3 (95% CI, 17.9 to 20.9) in patients submitted to radiotherapy plus chemotherapy, 12.1 (95% CI, 11.1 to 12.9) to chemotherapy, 36.9 (95% CI, 29.6 to 43.2) to surgery plus chemotherapy, and 41.3 (95% CI, 32.1 to 61.3) to chemotherapy plus radiotherapy plus surgery. The patients treated with multiple chemotherapy plus radiotherapy followed by surgery had significantly better OS and lower mortality rates than those treated with other treatments (adjusted hazard ratio, 0.55; 95% CI, 0.45 to 0.66; P < .001). At the end of the study, 11.2% and 10.7% of the patients were living with and without cancer, respectively.
CONCLUSION
Our real-life 19-year cohort study has shown that only 30.3% of the total patients with stage III NSCLC have been submitted to standard chemotherapy and radiotherapy treatment. This may show a substantial difference between the recruited clinical trials' patients and the real-life patients' characteristics in daily routine treatment.
INTRODUCTION
Lung cancer is currently the first-ranking cause of cancer-related deaths globally, accounting for 17% and 9% of all cancers in men and women, respectively,1-5 accounting for more than 1.8 million deaths in 2020 (18.0% of the total).
CONTEXT
Key Objective
Real-life stage III lung cancer studies are rare, and the patients followed are almost always in contrast with the best-fitted patients enrolled in clinical trials.
Knowledge Generated
Differences in age, previous treatment, stage, nodal metastasis extension, and comorbidities in the patients enrolled in stage III lung cancer clinical trials limit the applicability of the results in the real-life general population attended in the oncologic hospitals.
Relevance
The optimal treatment remains controversial because of a high degree of heterogeneity among stage III patients and a lack of a universally agreed upon definition of resectability.
The age-standardized incidence rate of lung cancer is 22.4 (31.5 in males and 14.6 in females) per 100,000 people, and the age-standardized mortality rate is 18.0 (22.4 in men and 11.2 in women) per 100,000 persons.4-15
More than 85% of newly diagnosed lung cancer are non–small-cell lung cancer (NSCLC), and nearly 30% of patients are in stage III on admission. Locally advanced stage III NSCLC is the most advanced stage for which cure can still be achieved.16
Patients who receive combined chemotherapy plus radiotherapy treatment had a median progression-free survival of approximately 8 months, whereas the 5-year overall survival (OS) with this modality is 15%.17,18
In the immunotherapy setting, the median progression-free survival increases to 16.8 months (95% CI, 13.0 to 18.1), whereas the median OS and the estimated 4-year OS rates are 47.5 months and 49.6%, respectively.18-20
Stage IIIA NSCLC is a complex disease that includes a resectable small-volume local tumor with metastatic spread to regional lymph nodes to nonoperable large-sized tumors with nodal or pleural involvements.21,22 Most patients are diagnosed when they already have a stage III disease, which is often unresectable.23 Two standard treatment options are offered for patients with stage IIIA–N2 disease: definitive-concurrent chemoradiotherapy to patients who are not suitable for surgery or chemotherapy plus surgery.24,25 However, most resected patients had disease recurrence after the treatment.19,22,26 A lack of a consensus definition of resectability of N2 disease adds to the complexity of the decision-making process.22 However, extended follow-up with real-world data about treatment patterns and clinical outcomes for stage III lung cancer is limited to date. Patients with stage IIIA lung cancer are highly distinct from those included in clinical trials, resulting in limited use of the evidence presented in these studies. In general, patients with stage III lung cancer are elderly and have multicomorbidity, leading to discrepancies in selecting eligible patients to follow the guideline-based treatment and the curative-intent treatment.23,27-30
This study aimed to describe the patterns of treatment and primary clinical patients' characteristics in a real-life 19-year large cohort of patients with stage IIIA lung cancer and leading cancer and all-cause death risk factors to OS in these patients.
MATERIALS AND METHODS
Patients, Clinical Stages, and Cancer Registry
A hospital-based retrospective cohort study including 3,363 patients diagnosed with stage IIIA lung carcinoma (International Classification of Diseases for Oncology 3rd edition 8140/3-TNM-5th, 6th, and 7th edition) between January 2000 and December 2015 and followed-up until December 31, 2019, was conducted. We included the patients with lung cancer stage IIIA, older than 18 years, with confirmed histologic lung cancer. The patients who had undergone treatment for other neoplasms and those with small-cell lung cancer were excluded.
The variables analyzed were sex, age at diagnosis, patients' region (same state of the Cancer Hospital or not same state of the Cancer Hospital), and clinical stage at diagnosis. The histologic cancer type included adenocarcinoma, squamous cell carcinoma, large-cell lung carcinoma, NSCLC (it was not possible to identify histologic subtype after immunohistochemical analysis), undifferentiated carcinoma, and others. Nodal status was confirmed by thorax computed tomography (CT), positron emission tomography-CT, or invasive stage of the mediastinum with mediastinoscopy. This study was conducted on the basis of the six proposed treatment groups of the stage IIIA resectable lung cancer found in our databank as follows: chemotherapy only, surgery plus chemotherapy, radiotherapy plus chemotherapy, radiotherapy plus chemotherapy plus surgery, palliative care, and other treatment, which includes surgery only or radiotherapy only or surgery plus radiotherapy. The OS was obtained by the difference between the date of vital status (death or alive) and the treatment date.
Ethical Statement
The local research ethics committee previously approved this study, registered under protocol 49258615.4, and informed consent was not required.
Statistical Analysis
Descriptive analysis using central tendency, absolute and relative frequencies, and dispersion measures was performed. OS and cancer-specific survival (CSS) curves were constructed using the Kaplan-Meier method. The log-rank test was used to assess differences between curves, followed by multiple pairwise comparisons using the Sidák multiple-comparison method.31,32 Univariate Cox regression analysis was used to assess the association between clinical and demographics characteristics and the survival end points. Variables with P < .20 in the univariate analysis were included in the Cox regression analysis with the backward elimination method (P < .05 to stay). Unadjusted and adjusted hazard ratios (HRs) and their 95% CI were reported.
RESULTS
Patients', Clinical Stages, and Treatment Characteristics
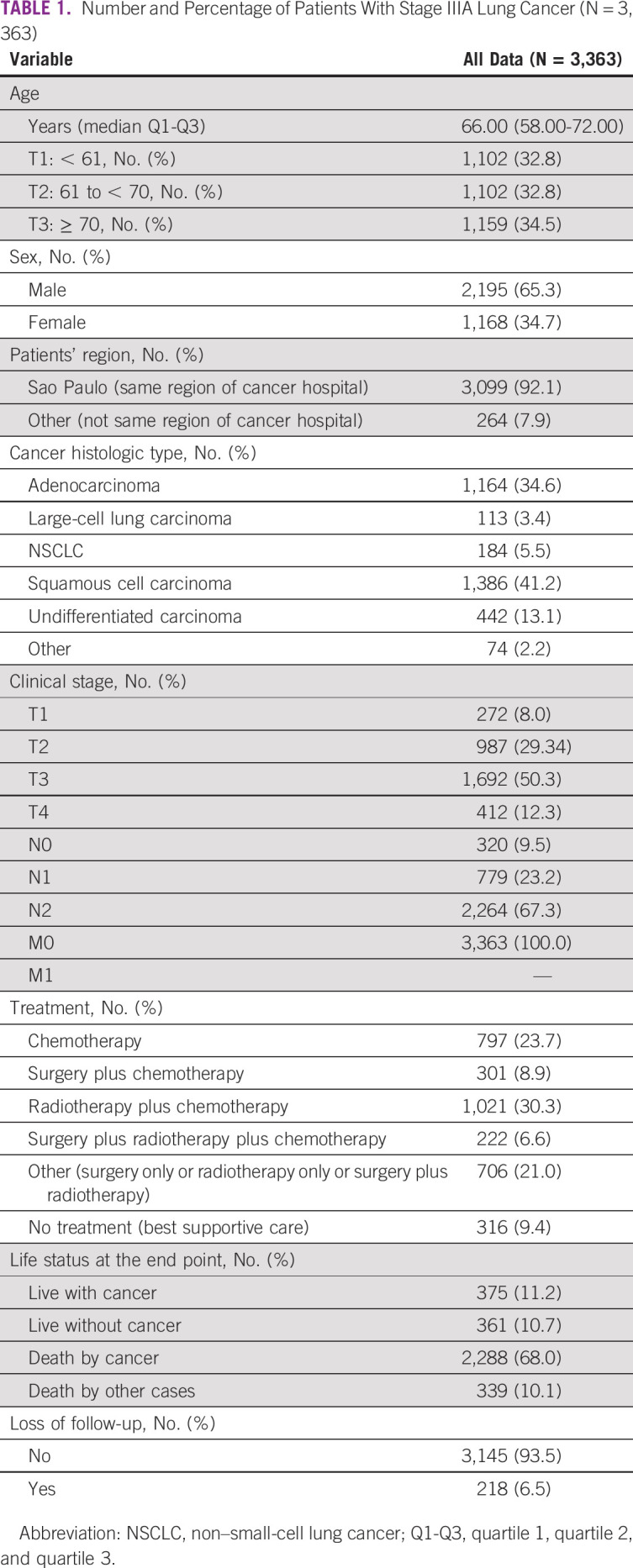
The median age was 66 years (95%CI, 58 to 72). A total of 32.8% of patients were up to 61 years of age, 65.3% were male, and 92.1% of patients were referred from the same region (same state). Most patients were diagnosed with squamous cell carcinoma (41.2%) in clinical stages T3 (50.3%), T2 (29.34%), T4 (12.3%), and T1 (8.0%). The most prevalent treatment was radiotherapy plus chemotherapy (30.3%), followed by chemotherapy (23.7%), surgery plus chemotherapy (8.9%), and surgery plus radiotherapy plus chemotherapy (6.6%). The loss of follow-up in 19 years was about 6.5% (218 patients; Table 1).
TABLE 1.
Number and Percentage of Patients With Stage IIIA Lung Cancer (N = 3,363)
CSS by Patients' Treatment
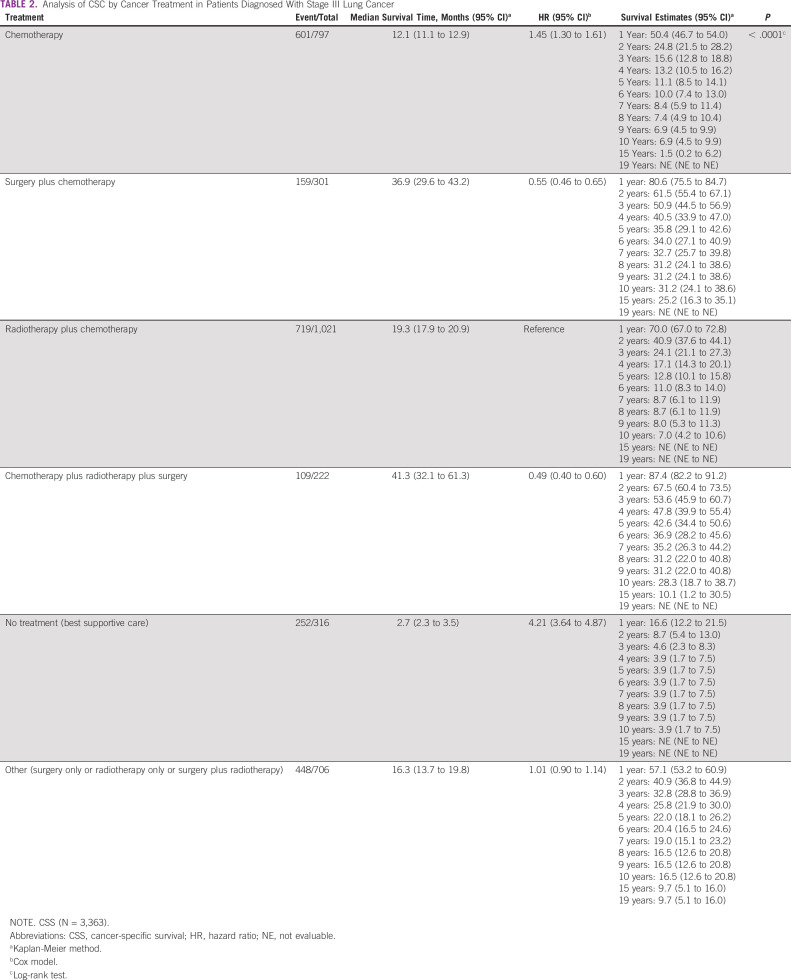
The cumulative 5-year CSS rate, considering only deaths by cancer, was 11.1% (95% CI, 8.5 to 14.1) in chemotherapy only, 35.8% (95% CI, 29.1 to 42.6) in surgery plus chemotherapy treatment, 12.8% (95% CI, 10.1 to 15.8) in radiotherapy plus chemotherapy treatment, 42.6% (95% CI, 34.4 to 50.6) in the chemotherapy plus radiotherapy plus surgery treatment, 3.9% (95% CI, 1.7 to 7.5) in the patients in palliative care, and 22.0% (95% CI, 18.1 to 26.2) in those submitted to other treatments (surgery only or radiotherapy only or surgery plus radiotherapy; P < .0001; Table 2).
TABLE 2.
Analysis of CSC by Cancer Treatment in Patients Diagnosed With Stage III Lung Cancer
The median survival time in patients grouped by treatment type (in months) was 12.1 (95% CI, 11.1 to 12.9) to chemotherapy, 36.9 (95% CI, 29.6 to 43.2) to surgery plus chemotherapy, 19.3 (95% CI, 17.9 to 20.9) to radiotherapy plus chemotherapy, 41.3 (95% CI, 32.1 to 61.3) to chemotherapy plus radiotherapy plus surgery, 2.7 (95% CI, 2.3 to 3.5) in patients with palliative care and, 16.3 (95% CI, 13.7 to 19.8) in patients submitted to other treatments (surgery only or radiotherapy only or surgery plus radiotherapy; Table 2).
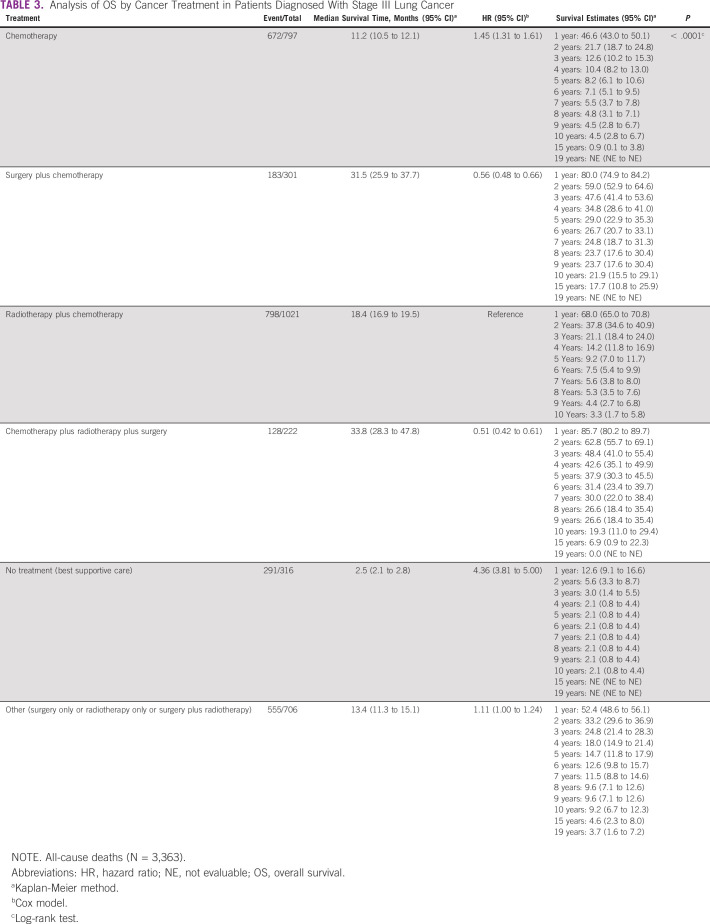
OS by Patients' Treatment: All-Cause Deaths
Grouped by cancer treatment, the 5-year cumulative OS rate, considering all-cause deaths was 8.2% (95% CI, 6.1 to 10.6) in chemotherapy only, 29.0% (95% CI, 22.9 to 35.3) in surgery plus chemotherapy treatment, 9.2% (95% CI, 7.0 to 11.7) in radiotherapy plus chemotherapy treatment, 37.9% (95% CI, 30.3 to 45.5) in the chemotherapy plus radiotherapy plus surgery treatment, 2.1% (95% CI, 0.8 to 4.4) in the patients with palliative care, and 14.7% (95% CI, 11.8 to 17.9) in the patients with other treatments (P < .0001).
The median survival time (in months) in patients grouped by treatment was 11.2 (95% CI, 10.5 to 12.1) in chemotherapy, 31.5 (95% CI, 25.9 to 37.7) in surgery plus chemotherapy, 18.4 (95% CI, 16.9 to 19.5) in radiotherapy plus chemotherapy, 33.8 (95% CI, 28.3 to 47.8) in the chemotherapy plus radiotherapy plus surgery, 2.5 (95% CI, 2.1 to 2.8) in patients with palliative care, and 13.4 (95% CI, 11.3 to 15.1) in patients treated with other treatment (Table 3).
TABLE 3.
Analysis of OS by Cancer Treatment in Patients Diagnosed With Stage III Lung Cancer
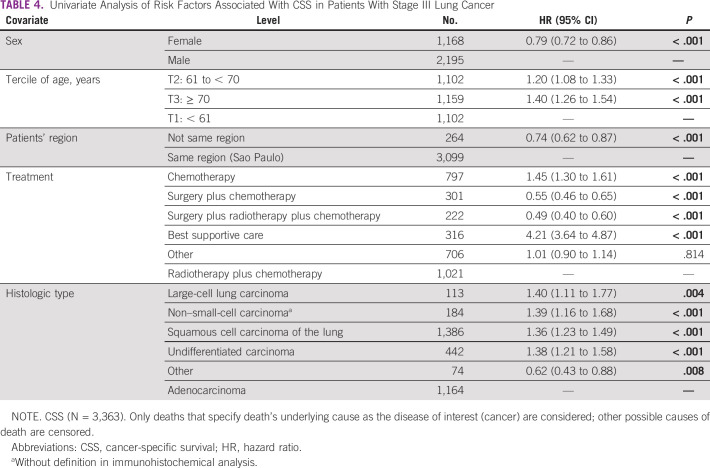
Univariate Analysis: CSS
Considering only the death by cancer, the risk of death in the univariate analysis was decreased in the female patients, HR = 0.79 (95% CI, 0.72 to 0.86; P < 0,01), but increased in patients age 61-70 years, HR = 1.20 (95% CI, 1.08-1.33; P < 0,01), and those age 70 years and older, HR = 1.40 (95% CI, 1.26 to 1.54; P < 0,01). Patients from other states had also decreased risk of cancer death, HR = 0.74 (95% CI, 0.62 to 0.87; P < .001), similar to those treated with surgery plus chemotherapy with or without radiotherapy. All histologic types increase the risk of death by cancer than adenocarcinoma (Table 4).
TABLE 4.
Univariate Analysis of Risk Factors Associated With CSS in Patients With Stage III Lung Cancer
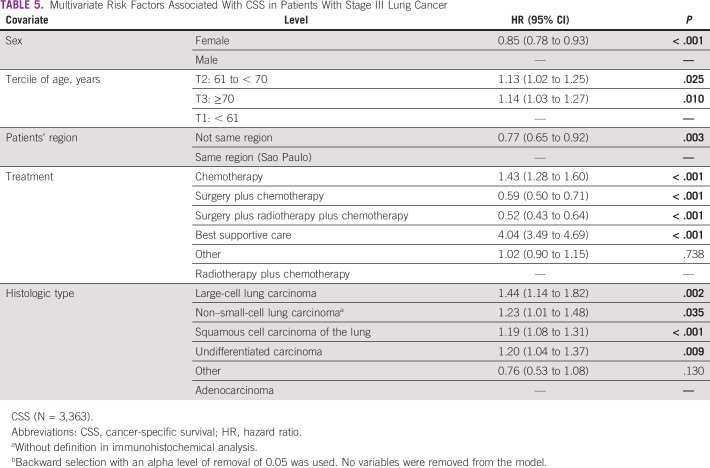
Multivariate Analysis: CSS
In the Cox multiple regression analysis, the independent risk factors associated with CSS were sex, age, patients' region, the treatment, and the histologic cancer type. The female sex had a lower risk of death than male, adjusted HR (adjHR) = 0.85 (95% CI, 0.78 to 0.93; P < .001), and patients age 70 years or older and those age 61 to 70 years were at increased risk of all-cause death, adjHR = 1.14 (95% CI, 1.03 to 1.27; P = .01) and adjHR = 1.13 (95% CI, 1.02 to 1.25; P = .025), respectively.
Patients from other states are at lower risk of death, adjHR = 0.77 (95% CI, 0.65 to 0.92; P = .003), than those from the same region of the oncologic hospital. The patients who underwent surgery plus chemotherapy with or without radiotherapy had decreased risk of all-cause death than other treatments. All histologic types increase the risk of death by cancer than adenocarcinoma (Table 5).
TABLE 5.
Multivariate Risk Factors Associated With CSS in Patients With Stage III Lung Cancer
Univariate Analysis: OS and All-Cause Deaths
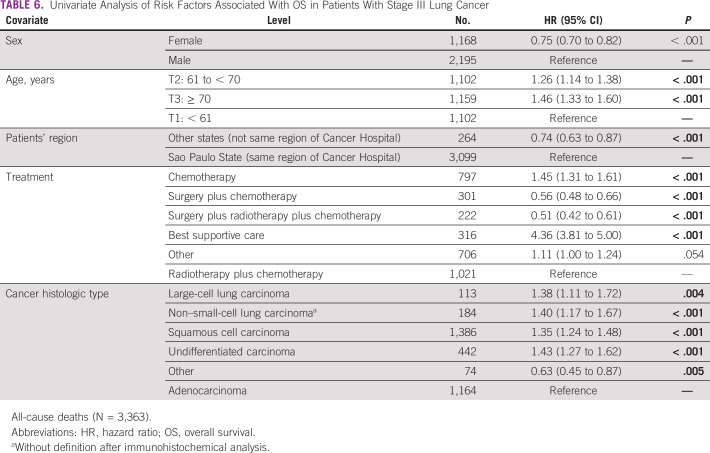
In the univariate analysis, the risk of all-cause death was HR = 0.75 (95% CI, 0.70 to 0.82; P < .001), 1.26 (95% CI, 1.14 to 1.38; P < .001), 1.46 (95% CI, 1.33 to 1.60; P < .001), and 0.74 (95% CI, 0.63 to 0.87; P < .001) respectively, related to female patients, patients age between 61 and 70 years, patients age older than 70 years, and patients from other states (Table 6).
TABLE 6.
Univariate Analysis of Risk Factors Associated With OS in Patients With Stage III Lung Cancer
Multivariate Analysis: OS and All-Cause Deaths
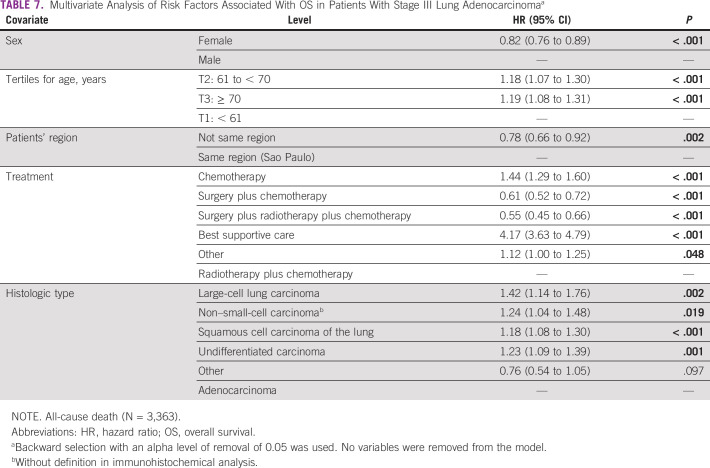
In the Cox multiple regression analysis, the independent risk factors related to all-cause deaths included sex, age, patients' region, the proposed treatment, and the histologic cancer type. The female patients have a lower risk of death than male patients, adjHR = 0.82 (95% CI, 0.76 to 0.89; P < .001), and patients age 70 years or older were at increased risk of all cause death from adjHR = 1.19 (95% CI, 1.08 to 1.31; P < .001).
Likewise, patients from other states are at a lower risk of death, adjHR = 0.78 (95% CI, 0.66 to 0.92; P = .002), than those from the same region of the oncologic hospital. The patients who underwent surgery plus chemotherapy with or without radiotherapy had decreased risk of all-cause death than other therapy. The patients with large-cell lung carcinoma were those with an increased risk of all-cause death compared with patients with adenocarcinoma, adjHR = 1.42 (95% CI, 1.14 to 1.76; P = .002; Table 7).
TABLE 7.
Multivariate Analysis of Risk Factors Associated With OS in Patients With Stage III Lung Adenocarcinomaa
Figure 1 shows the cumulative OS rate between sex, histologic type of lung cancer, patient's treatment, and patients' region regarding specific cancer death and all-cause deaths.
FIG 1.
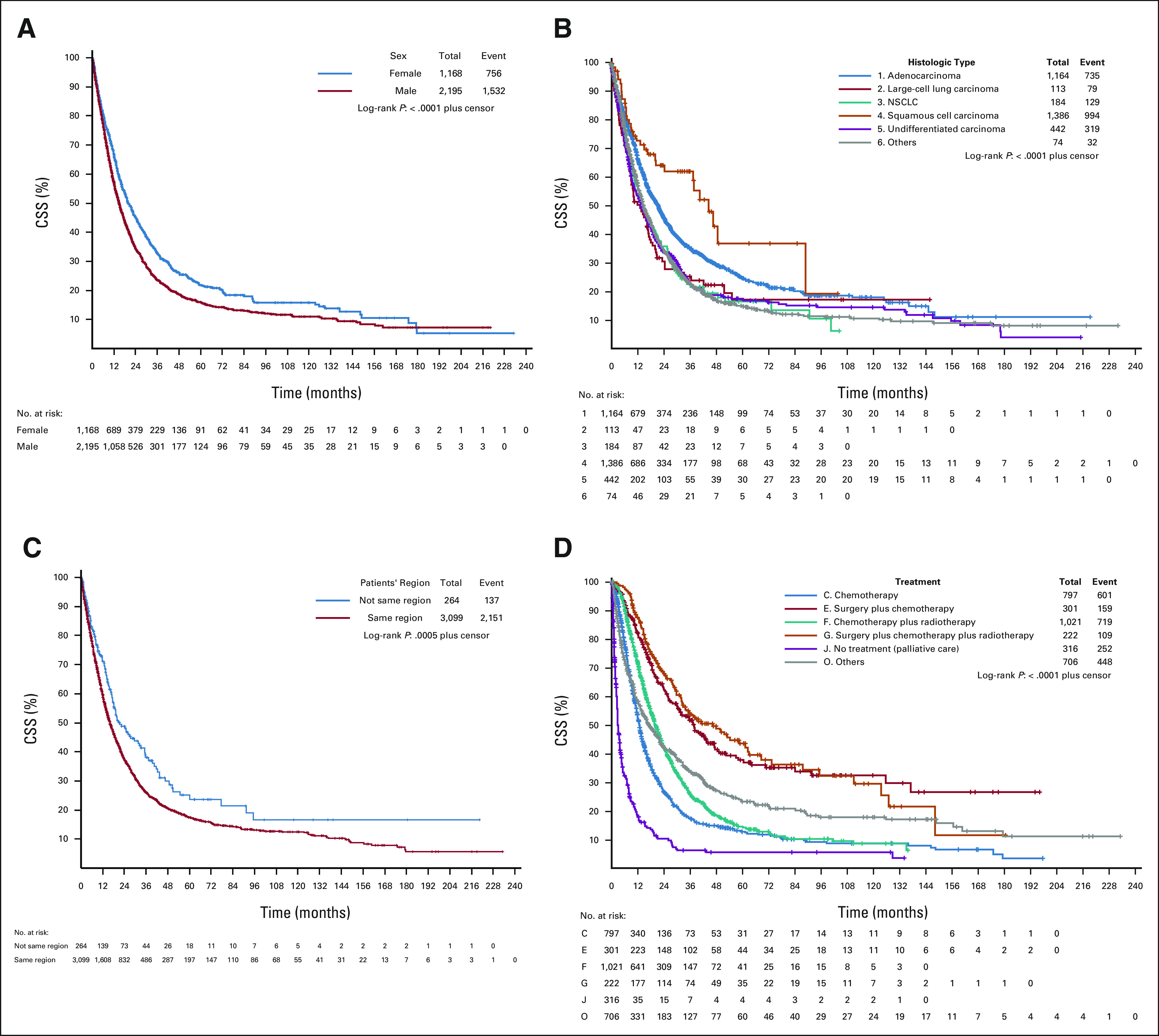
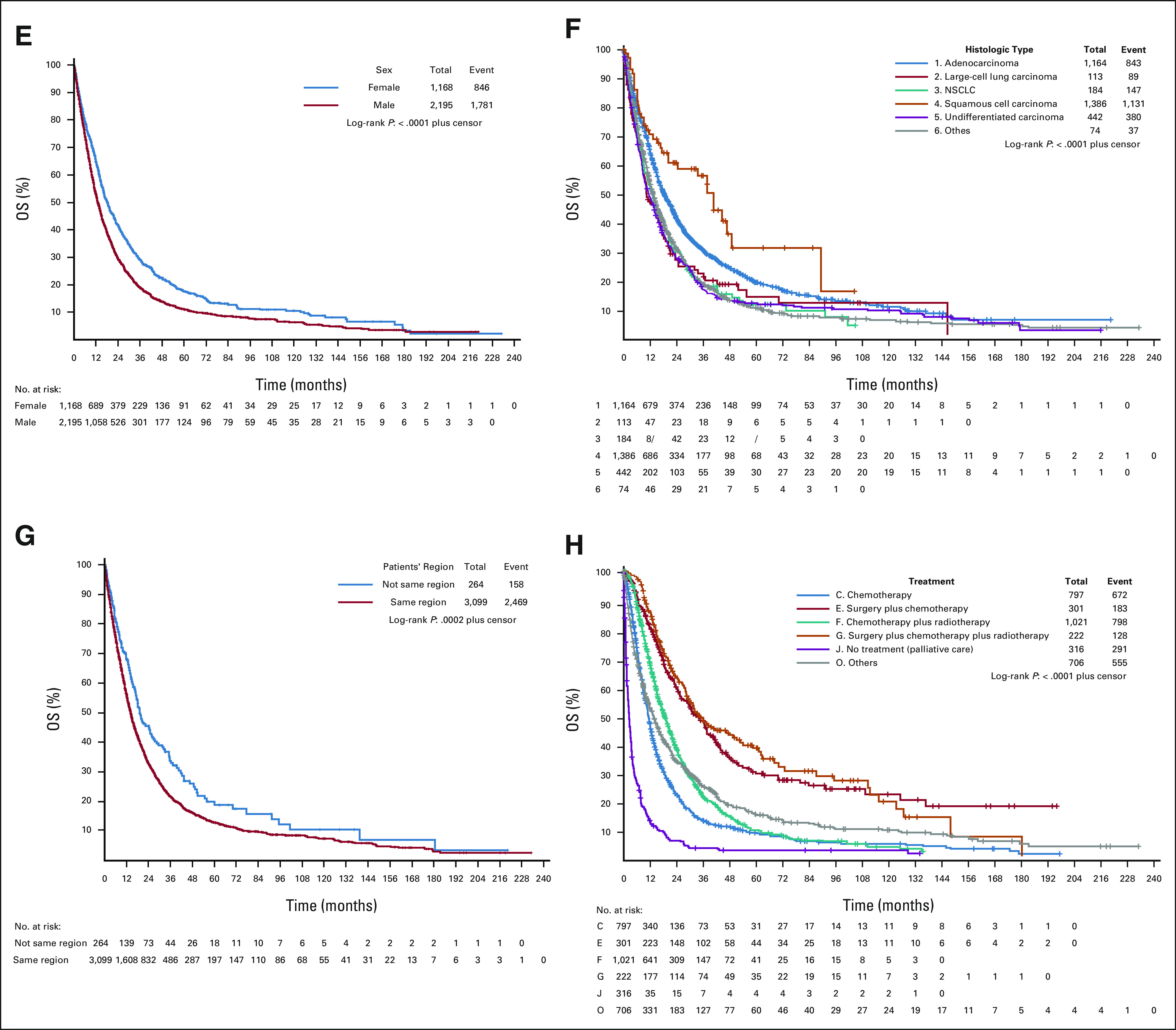
Cumulative OS rate (in months) between sex, histologic type of lung cancer, treatment, and patients' region regarding cancer and all-cause deaths. (A). Survival analysis by sex, cancer deaths. (B) Survival analysis by histologic cancer type, cancer deaths. (C) Survival analysis by patients' region, cancer deaths. (D) Survival analysis by treatment, all cancer deaths. (E) Survival analysis by sex, all-cause deaths. (F) Survival analysis by histologic cancer type, all-cause deaths. (G) Survival analysis by patients' region, all-cause deaths. (H) Survival analysis by treatment, all-cause deaths. NSCLC, non–small-cell lung cancer; OS, overall survival.
DISCUSSION
This real-life observational study has provided additional valuable evidence of the patients' profile treated in reference cancer centers as well cancer and all causes of mortality. To our knowledge, this is the first study that followed a large real-life cohort of 3,363 patients diagnosed with stage IIIA lung cancer for 19 years. Our results showed that 9.4% of the patients had received the best supportive care, and 54.1% were offered therapies not recommended for stage IIIA lung cancer like chemotherapy, best supportive care, or other treatment. Otherwise, one third of patients were treated with chemotherapy plus radiotherapy, as indicated in the recent guidelines. Additionally, 15.5% of patients were treated with the standard treatment, as indicated in the recent guidelines that include chemotherapy plus surgery or chemotherapy plus surgery plus radiotherapy. In line with the literature, these last had better OS than those who had received the standard treatment chemotherapy plus radiotherapy.16,17 The main factor associated with better survival was the inclusion of surgery in the multimodal treatment. This finding may have been associated with the patient's selection mode, where those with lesser tumor extension were selected to the surgical group in the multimodal treatment. Also, when it is mentioned as a better prognosis in the surgery subgroup and with the smaller tumors, we might have an omitted variable bias, as although all tumors were IIIA stage, lymph nodes were not accessed, and there was not a correlation between T stage (or a tumor cutoff measure) and surgery in this cohort. This finding must be evaluated carefully, mainly because of the commonly existent possible selection bias in retrospective studies.
Considering the territorial extension of our country and the economic disparities between regions, the referral process of cancer treatment leads patients from a distant region where there are not specialized lung cancer hospitals to be promptly referred to our hospital network. By contrast, the patients who live nearest to our hospital network have some other alternatives to the treatment, and have been referred only with more advanced stages or symptoms. Olesen et al have observed that the referral process from GP to cancer hospitals may have been a possible cause of delays and the worsening in clinical stages of lung cancer.33 Van der Linden et al have also observed that most patients with less-advanced clinical stages are those referred from other hospitals for specialized treatment.13
In our study, chemotherapy plus radiotherapy was given to 30.3% of the patients. Similar data were found in a study conducted by De Ruysscher et al,34 where 41% of patients with nonresectable stage III lung cancer would be eligible for concurrent chemotherapy plus radiotherapy on the basis of age or comorbidity. Ouwens et al35 found that 39% of patients with stage III lung cancer were treated with chemotherapy plus radiotherapy. The loss of performance status was probably the leading cause of 21% of patients submitted to other treatment and 23.7% submitted to chemotherapy only.
In real-life oncologic treatment, the multidisciplinary medical staffs' perception of the patient's performance status has been a factor taken into consideration in the choice of treatment to be given. Different from clinical trials, in general, PS0/1 are accepted, but these patients are not selected; the prescriber should carefully look at all inclusion criteria of the approval-treatment study so that we can drop the disparities between the clinical benefit of the clinical trials and the clinical benefit of the real-world evidence, once those inclusion criteria will be applied precisely to the sample population that is designed. These common scenarios may explain our finding that 54.1% of patients were not treated with standard therapy (chemotherapy, other treatments, and best supportive care). In our study, < 10% of all referred patients only received the best supportive care. The reasons for best supportive care included poor performance status and patient's or family's choices. Our real-life scenario may be considered the final junction of all strengthens to diagnose and start as fast as possible the curative-intent treatment to more than 90% of patients. In some studies, nearly half of all patients with advanced NSCLC did not receive any systemic therapy because of a poor performance status.36
The multivariate risk factors associated with OS in patients with stage III lung cancer have shown that the multimodal treatment surgery plus radiotherapy plus chemotherapy or chemotherapy plus surgery significantly decreased the risk of death. These multimodal therapies, including surgery, also show a better median survival time than the standard chemotherapy plus radiotherapy treatment. The management of stage III NSCLC is undergoing rapid evolution, and the multiple-therapy treatment on the basis of surgery is an endorsed alternative.27,37 The optimal treatment remains controversial because of a high degree of heterogeneity among stage III patients and a lack of a universally agreed definition on resectability.22
Since our study includes an extensive period of data collection, a significant number of patients who have undergone chemotherapy plus radiotherapy did not benefit from recent or more advanced therapeutical tools like immunotherapy. When treated with chemotherapy and radiotherapy for patients deemed unresectable, survival time could be more effective, as reported by Gray et al19 in the PACIFIC 3-year OS study, showing the average OS time of 57 months with immunotherapy use in patients with stage III NSCLC. Still, compared with the PACIFIC trial three-year OS patients', our study has shown worse global survival rates in the therapeutical modalities that include surgical approach as indicated in the international guidelines. A recent study validated another strength, ie, the potential of immunotherapy to significantly prolong progression-free survival and overall survival among patients with stage III NSCLC. Antonia et al26 has shown a 24-month OS rate of 66.3% (95% CI, 61.7 to 70.4) and median survival time of 28.3 months, in contrast with our study that has shown 34.4% (95% CI, 32.2 to 36.6) in male and 45.2% (95% CI, 42.1 to 48.3) in female patients. These data have shown the potential of immunotherapy in the treatment scenario of patients with stage IIIA NSCLC (Data Supplement).
Nearly a third of our patients were suitable for chemotherapy and radiotherapy treatment, and the OS and median survival time are similar or even better than in some stage III lung cancer clinical trials. Curran et al evaluated whether sequential or concurrent chemotherapy is the optimal combination strategy in a three-arm phase III trial. The results have shown that the median survival times were 14.6, 17.0, and 15.6 months for a sequential arm and two concurrent therapies regimens, respectively.38 In our study, the median survival time in the chemotherapy and radiotherapy combined strategy was 19.3 months (95% CI, 17.9 to 20.9) to radiotherapy plus chemotherapy considering cancer-specific death, and 5-year OS was 12.8% (95% CI, 10.1 to 15.8).
Differential response rates may be explained by the differences in patterns of clinical outcomes to systemic therapy associated with different pathologic subtypes.23 In our study, the 5-year OS ranged from 23.4% (95% CI, 20.3 to 26.6) in adenocarcinoma, 15.9% (95% CI, 12.0 to 20.4) in undifferentiated carcinoma, 15.7% (95% CI, 8.0 to 25.9) in large-cell lung carcinoma, 15.1% (95% CI, 9.0 to 22.6) in NSCLC, and 13.3% (95% CI, 11.1 to 15.7) in squamous cell carcinoma. Similar to those found in the PACIFIC trial, where the squamous cell carcinoma was the most prevalent histologic type (45.7%), our study has also shown 41.2% of patients with squamous cell carcinoma.19
Some limitations of this study include the lack of availability of chemotherapy schemes and radiotherapy dosages, and unfortunately, in our study, we also could not distinguish the rate of patients referred to sequential or concurrent chemoradiotherapy treatment.
Since this study started about 20 years ago, internal differences in therapeutic modalities were not available, and the drugs of choice used in the chemotherapy and the radiation dosage used in the radiotherapy scheme were not able to be compared. More recent drugs and equipment may be responsible for some differences between our study and more recent publications. In the same way, the frequency of positron emission tomography-CT and invasive mediastinal staging was not evaluated. We did not have complete data on whether all operations were complete and performed according to the International Association for the Study of Lung Cancer guidelines.
Consequently, we did not analyze the number of dissected mediastinal lymph nodes or the surgical margins for each patient, affecting survival. In addition, the study relies on data already collected, and therefore any systematic errors occurring during the chart abstraction process cannot be captured.
In conclusion, our real-life cohort study, with lengthy follow-up, has shown that only 30.3% of the total patients with stage III NSCLC have been submitted to standard chemotherapy and radiotherapy treatment. Nearly 54.1% of patients have not been treated with the standard treatment, according to the guidelines. This may show a substantial difference between the recruited clinical trials' patients and the real-life patients' characteristics attended in the daily routine treatment. Our study has also evidenced that the patients submitted to the multimodal treatment, including surgery, were well selected, bearing in mind the survival benefit regarding the chemotherapy plus radiotherapy treatment.
ACKNOWLEDGMENT
All the authors would like to thank the administrative staff of the Epidemiology Directorate of Oncocentro Foundation of Sao Paulo (FOSP) for the data acquisition and cancer registry of the State of Sao Paulo (SISRHC).
SUPPORT
The Oncocentro Foundation of Sao Paulo (FOSP) partially granted this article. This sponsor did not influence the writing in any way.
AUTHOR CONTRIBUTIONS
Conception and design: Fernando Conrado Abrão, Frederico Rafael Moreira, Igor Renato Louro Bruno de Abreu, Marcelo Giovanni, Riad Naim Younes
Collection and assembly of data: Fernando Conrado Abrão, Igor Renato Louro Bruno de Abreu, Marcelo Giovanni Marciano
Data analysis and interpretation: Fernando Conrado Abrão, Frederico Rafael Moreira, Igor Renato Louro Bruno de Abreu, Riad Naim Younes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Fitzmaurice C, Abate D, Abbasi N, et al. :Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol 5:1749–17682019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin H-T, Liu F-C, Wu C-Y, et al. :Epidemiology and survival outcomes of lung cancer: A population-based study. Biomed Res Int 2019:19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck N, Hoeijmakers F, van der Willik EM, et al. :National comparison of hospital performances in lung cancer surgery: The role of case mix adjustment. Ann Thorac Surg 106:412–4202018 [DOI] [PubMed] [Google Scholar]
- 4.Romaszko A, Romaszko AM, Doboszyńska A:Multiple primary lung cancer: A literature review. Adv Clin Exp Med 27:725–7302018 [DOI] [PubMed] [Google Scholar]
- 5.Cheng T-YD, Cramb SM, Baade PD, et al. The International Epidemiology of Lung Cancer: Latest trends, disparities, and tumor characteristics. J Thorac Oncol 11:1653–16712016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Cancer Observatory. https://gco.iarc.fr/ [Google Scholar]
- 7.Araujo LH, Baldotto C, de Castro G, et al. :Lung cancer in Brazil [in Portuguese]. J Bras Pneumol 44:55–642018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekine I, Shintani Y, Shukuya T, et al. :A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci 111:1685–16912020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, et al. :Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–4242018 [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Colombet M, Soerjomataram I, et al. :Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–3872018 [DOI] [PubMed] [Google Scholar]
- 11.Vos T, Lim SS, Abbafati C, et al. :Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 396:1204–12222020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allemani C, Matsuda T, Di Carlo V, et al. :Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023–10752018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Linden N, Bongers ML, Coupé VMH, et al. :Treatment patterns and differences in survival of non-small cell lung cancer patients between academic and non-academic hospitals in the Netherlands. Clin Lung Cancer 18:e341–e3472017 [DOI] [PubMed] [Google Scholar]
- 14.Hanna N, Johnson D, Temin S, et al. :Systemic therapy for stage IV non–small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3484–35152017 [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen MM, Silverstein SC, Quinn M, et al. :Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 112:156–1642017 [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt WEE, Pöttgen C, Gauler TC, et al. :Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 33:4194–42012015 [DOI] [PubMed] [Google Scholar]
- 17.Pless M, Stupp R, Ris HB, et al. :Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomized trial. Lancet 386:1049–10562015 [DOI] [PubMed] [Google Scholar]
- 18.Antonia SJ, Villegas A, Daniel D, et al. :Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 377:1919–19292017 [DOI] [PubMed] [Google Scholar]
- 19.Gray JE, Villegas A, Daniel D, et al. :Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC—Update from PACIFIC. J Thorac Oncol 15:288–2932020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faivre-Finn C, Vicente D, Kurata T, et al. :Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—An update from the PACIFIC trial. J Thorac Oncol 16:860–8672021 [DOI] [PubMed] [Google Scholar]
- 21.Agulnik J, Kasymjanova G, Pepe C, et al. :Understanding clinical practice and survival outcomes in patients with unresectable stage III non-small-cell lung cancer in a single centre in Quebec. Curr Oncol 27:e459–e4662020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putora PM, Leskow P, McDonald F, et al. :International guidelines on stage III N2 non-small cell lung cancer: Surgery or radiotherapy? ERJ Open Res 6:00159–020192020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinod SK, Wai E, Alexander C, et al. :Stage III non-small-cell lung cancer: Population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol 7:1155–11632012 [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke N, Roqué i Figuls M, Farré Bernadó N, et al. :Concurrent chemo-radiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 6:CD002140.2010 [DOI] [PubMed] [Google Scholar]
- 25.Aupérin A, Le Péchoux C, Rolland E, et al. :Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28:2181–21902010 [DOI] [PubMed] [Google Scholar]
- 26.Antonia SJ, Villegas A, Daniel D, et al. :Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342–23502018 [DOI] [PubMed] [Google Scholar]
- 27.Albain KS, Swann RS, Rusch VW, et al. :Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomized controlled trial. Lancet 374:379–3862009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson BD, Shroff GS, Truong MT, et al. :Spectrum of lung adenocarcinoma. Semin Ultrasound CT MRI 40:255–2642019 [DOI] [PubMed] [Google Scholar]
- 29.Uhlig J, Case MD, Blasberg JD, et al. :Comparison of survival rates after a combination of local treatment and systemic therapy vs systemic therapy alone for treatment of stage IV non-small cell lung cancer. JAMA Netw Open 2:e199702.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Castro J, Tagliaferri P, de Lima VCC, et al. :Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl) 26:e12734.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X:Survival Analysis: Models and Applications. Bethesda, MD, Wiley; 2012 [Google Scholar]
- 32.Brocklebank JC, Dickey DA, Choi B:SAS for Forecasting Time Series(ed 3).Cary, NC: SAS Institute; 2018 [Google Scholar]
- 33.Olesen F, Hansen RP, Vedsted P:Delay in diagnosis: The experience in Denmark. Br J Cancer 101:S5–S82009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Ruysscher DKM, Botterweck A, Dirx M, et al. :Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: A prospective, population-based study. Ann Oncol 20:98–1022009 [DOI] [PubMed] [Google Scholar]
- 35.Ouwens MMMTJ, Hermens RRPMG, Termeer RAR, et al. :Quality of integrated care for patients with non-small cell lung cancer: Variations and determinants of care. Cancer 110:1782–17902007 [DOI] [PubMed] [Google Scholar]
- 36.Brule SY, Al-Baimani K, Jonker H, et al. :Palliative systemic therapy for advanced non-small cell lung cancer: Investigating disparities between patients who are treated versus those who are not. Lung Cancer 97:15–212016 [DOI] [PubMed] [Google Scholar]
- 37.van Meerbeeck JP, Kramer GWPM, Van Schil PEY, et al. :Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 99:442–4502007 [DOI] [PubMed] [Google Scholar]
- 38.Curran WJ, Paulus R, Langer CJ, et al. :Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 103:1452–14602011 [DOI] [PMC free article] [PubMed] [Google Scholar]