Abstract
Pressure ulcers/injuries are caused by sustained loading and deformation of skin and underlying soft tissues. Prophylactic dressings are recommended as an adjunct to other preventive measures such as repositioning and offloading. The aim of this study was to investigate the effects of prophylactic soft silicone multi‐layered foam dressings on the skin structure and function of the two most common pressure areas, sacrum and heel, with and without loading. An exploratory randomised cross‐over trial using intra‐individual comparisons was conducted. Eight healthy volunteers (mean age 27.5 years) were assigned to three groups and either spent 2.5 hours on a standard hospital mattress lying in supine position with and without dressings or spent 2.5 hours with dressings applied but without loading. Skin temperature, stratum corneum, and epidermal hydration increased in all groups irrespective of wearing a dressing and/or loading. Mean roughness decreased at the heels. Reactive hyperaemia and the release of interleukin 1 alpha were associated with loading only. Results suggest that the occlusive effects of dressings are similar or only slightly greater than those observed with non‐loading or loading without dressings. Thus, a dressing does not cause additional irritation or skin changes during loading but it may reduce the inflammatory response.
Keywords: dressing, heel, pressure ulcer, prevention, sacrum
1. INTRODUCTION
Pressure ulcers (PUs) are severe unwanted skin and soft tissue damages, also called pressure injuries. PUs are caused by sustained loading and deformation of the skin and underlying soft tissues. 1 They predominantly occur near bony prominences. The sacral and heel regions are the most frequently affected skin areas in patients lying in supine position. 2 The exact process of PU development is unknown, but evidence supports at least four pathophysiological pathways: (a) ischaemia caused by capillary occlusion; (b) reperfusion injury; (c) impaired lymphatic function and (d) mechanical deformation injury. 3 , 4 , 5 Direct deformation damage seems to be a rather fast process, whereas ischaemia‐related tissue damages occur later. 5 Ischaemic injuries cause inflammation, which seems to be an important (modifying) variable in the PU development process. 6
The main interventions for PU prevention are repositioning, early mobilisation, and the use of special support surfaces. 1 In addition, the application of dressings on PU predilection sites helps to prevent PU development. 7 In the latest international Pressure Ulcer Prevention and Treatment Guideline, the use of prophylactic dressings is recommended as an adjunct to other preventive measures such as heel offloading. 1 The assumed mode of action of PU preventive dressings includes mechanical cushioning and the reduction of shear loads within soft tissues. 8 , 9
Evidence suggests that the prolonged deformation of the skin during loading leads to various structural and functional changes. 10 , 11 These changes are caused by the mechanical deformation per se and by changes in the microclimate because of the occlusion between the support surface and the skin. 12 , 13 , 14 The direct effects of PU preventive dressings on the skin structure and function of PU predilection areas have only partly been investigated so far. For example, Wert et al 15 investigated the inflammatory response of the skin in healthy participants after combined loading of pressure and shear applied on the volar aspect of both forearms in the absence or presence of three foam dressings. Lechner et al 16 suggest that there are differences regarding the cutaneous response between wearing a dressing and no dressing during loading and that there seem to be differences between different dressings. However, neither the heel skin nor the effects of wearing dressings without loading were investigated. This is important, because it is unknown, so far, whether the effects of occlusion by the prophylactic dressings are enhanced during mechanical deformation.
Wert et al 15 and Lechner et al 16 measured interleukin 1 alpha (IL‐1α) to detect possible inflammatory responses in the skin. IL‐1α is released from keratinocytes in response to mechanical deformation and is considered today as a suitable marker for skin damage because of loading. 15 , 17 , 18 Skin functional parameters such as stratum corneum hydration (SCH) or erythema and structural parameters such as stiffness and roughness are also successfully used in current PU prevention research, because they provide a comprehensive picture of the cutaneous response as a result of deformation and occlusion. 10 , 13 , 17 , 19
Therefore, the overall aim of this study was to investigate the effects of prophylactic soft silicone multi‐layered foam dressings on the skin structure and function of the two most common pressure areas, sacrum and heel, with and without loading.
2. MATERIALS AND METHODS
2.1. Trial design
An exploratory randomised cross‐over trial using intra‐individual comparisons was conducted at the Department of Dermatology and Allergy, Charité Universitätsmedizin Berlin (Germany) between July and October 2016. A clinical situation was simulated, in which the study participants spent 2.5 hours on a standard hospital mattress lying in supine position. The study was approved by the local ethics committee at the Charité Universitätsmedizin Berlin (approval number: EA1/156/16).
2.2. Participants
Healthy male volunteers aged 25 to 35 years, with a body height between 1.75 and 1.85 m and a body weight between 75 and 85 kg were eligible. Being a non‐smoker and being free of any dermatological disease were further inclusion criteria. The skin of the heel and the sacrum had to be intact without any scars.
2.3. Interventions
After giving informed consent, subjects acclimatised under standardised room conditions (temperature 22 ± 2°C, 40%‐60% relative humidity) for 30 minutes with the heel and sacral skin uncovered. After that, subjects moved into the prone position. Using a skin marker, the investigator marked the investigational areas, which were the lateral skin of both heels and the sacral skin area (Figure 1).
FIGURE 1.
Markings of the intervention areas
All included subjects came to the study centre for three visits. After the baseline measurements (ie, the first visit), an opaque randomisation envelope was opened to allocate subjects to one of three intervention groups: group A = loading without dressing, group B = no loading with dressing, and group C = loading with dressing. The soft silicone multi‐layered foam dressings (Mepilex® Border Sacrum and Mepilex® Border Heel) were applied in groups B and C (Figure 2) or the skin was left uncovered (group A), followed by a 2.5 hours loading/non‐loading period.
FIGURE 2.
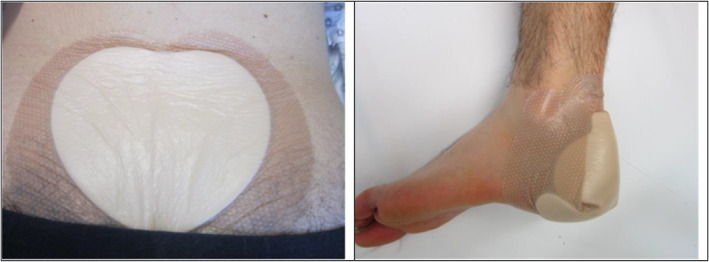
Applied dressings in group B (no loading with dressing) and group C (loading with dressing)
In case of loading (groups A and C), subjects lay supine on a standard hospital mattress (Softline Schaum GmbH & Co. KG, Germany) with no/minimal movement: 2.5 hours were chosen because previous research indicates that skin changes do occur within this period. 14 , 20 Longer periods may have caused unnecessary burden and may have increased the risk for non‐compliance and pain. In the case of non‐loading, the subject remained in prone or lateral positions, ensuring that no mechanical loads were applied at the sacral and heel skin areas.
After the loading/non‐loading period, subjects turned to the prone position again. In the case of interventions B and C, the dressings were removed. Immediately after removing the first follow‐up, measurements were conducted. Measurements were repeated after 20 minutes, including stratum corneum samplings, and again after 2 hours. The total duration of one study visit per subject was ~7 hours. In between the study visits, there were at least 8 days to prevent any carry over effects.
2.4. Outcomes
No distinction between primary, secondary, or other variables was made because of the exploratory design of this study.
2.4.1. Demographic characteristics
At the baseline visits, age (years), BMI (kg/m2), skin phototype according to Fitzpatrick classification, 21 heart rate and blood pressure were recorded.
2.4.2. Skin function parameters
Erythema index (EI), transepidermal water loss (TEWL), skin surface temperature, SCH, and epidermal hydration were measured at baseline, after the 2.5 hours loading phase, after 2.8 hours, and after 4.5 hours. All measurements were performed three times per measurement time, and skin area and the mean values were calculated.
The EI was measured using the Mexameter MX18 (Courage and Khazaka Electronic GmbH, Germany). This is a narrow‐band reflectance spectrophotometer, which uses three specific wavelengths to measure the absorption capacity of the skin, specifically the content of haemoglobin in the skin. The values range from 0 to 999 in arbitrary units (AU). The measurement accuracy is specified by the manufacturer as ±5%.
TEWL was measured with the Tewameter TM 300 (Courage and Khazaka Electronic GmbH, Cologne, Germany) in g/m2/h. Higher TEWL may either indicate skin barrier impairments or increased desorption of accumulated water molecules in the stratum corneum. 22
The skin surface temperature was measured with a skin thermometer based on the infrared technique (Courage & Khazaka electronic GmbH, Germany) and given in °C.
SCH was measured with the Corneometer CM 825 (Courage and Khazaka Electronic GmbH, Germany) with AU ranging from 0 to 120. This measurement is based on differences in the dielectric constant of water and dry tissue in the stratum corneum. The depth of measurement is lower than 20 μm to avoid the influence of the deeper epidermal layers. According to the manufacturer, the accuracy is ±3%. Evidence indicates high reliability of TEWL, temperature, and SCH estimates in a research setting. 23
Finally, epidermal moisture was measured with the Moisture Meter EpiD (Delfin Technologies, Kuopio, Finland). The results were presented in percentages, ranging from 0% to 100%.
2.4.3. Skin structure parameters
Skin surface roughness was measured with the Visioscan VC 98 camera (Courage & Khazaka Electronic GmbH, Germany). During every measurement phase, two duplicate images were taken per skin area. The ultraviolet light source of this device provides high‐resolution images of the skin surface with its greyscale representing different depths. With the corresponding software, the calculation of different roughness parameters is possible because of the distribution of 255 grey levels. The roughness parameter “mean roughness” (Rz) was determined as the mean value of the software output based on both images taken and was reported in μm. The reliability and validity of these two roughness parameters are supported by previous research. 24
Epidermal thickness was determined using optical coherence tomography (OCT) (Thorlabs, Lübeck, Germany). Two OCT images were taken per skin area and time point. The better quality one was chosen for epidermal thickness measurements in mm.
Skin elasticity was measured using Cutometer MPA 580 (Courage and Khazaka Electronic GmbH, Germany). A probe opening of 2 mm diameter and a suction pressure of −450 mBar for 2 seconds were used, as previously described. 10 The parameters for structural stiffness (Uf) and biological elasticity (Ua/Uf) were measured at baseline, after 2.5, 2.8, and 5 hours.
2.4.4. Clinical evaluations
The clinical assessment of erythema was performed via visual inspection of the sacral and heel skin at baseline, after 2.5 hours loading phase, after 2.8 hours, and after 5 hours using a four‐category scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. The whole sacral and heel areas were considered for this assessment. Sacral pain and heel pain were assessed by the subjects' self‐report. Pain was measured on a score of 0 (=no pain) to 10 (=worst pain) at baseline, after 2.5 hours loading phase, 2.8, and 5 hours.
2.4.5. Inflammatory marker
Cyanoacrylate skin surface stripping (CSSS) was conducted to obtain stratum corneum material for IL‐1α and total protein analysis according to a standard operating procedure. 25 CSSS removes ~30% of the stratum corneum. The remaining stratum corneum is left intact. 26 The investigational area comprised 2 cm × 2.5 cm. Two drops of cyanoacrylate glue were applied to the area. The glue was spread evenly on the investigational site with the help of a microscope slide and an adhesive tape was placed on it. A rubber roll was used to improve the adherence and to eliminate air bubbles. After 20 minutes of hardening time, the tape (incl. The adhering glue) was removed quickly from the skin surface, cut to a size based on the markings on the tape, and immediately stored in tubes at −80°C until analysis. 27 Phosphate‐buffered saline with 0.005% Tween 20 was used for protein extraction. IL‐1α was measured by a human IL‐1α enzyme‐linked immunosorbent assay (ELISA) (DuoSet R&D system) and the total protein by a Coomassie Plus protein assay (Thermo Scientific), with subsequent photometric analyses. The IL‐1α was calculated per one μg total protein (pg/μg) in order to adjust the amount of sample uptake to be compared.
2.5. Sample size
Because of the exploratory nature of this trial, a formal sample size calculation was not performed. Eight subjects were considered sufficient to describe possible differences between and within treatment groups A, B, and C.
2.6. Randomisation and blinding
Computerised random lists were created by a statistician not involved in the study design. Three interventions resulted in six possible orders of interventions. Sequentially numbered opaque sealed envelopes containing the group assignment were prepared by a data manager who was not involved in any study preparation or procedures. The batch of sequentially numbered envelopes was stored at the study centre. Envelopes were opened subsequently after confirming eligibility and provision of informed consent.
Because of the nature of the intervention, subjects, study assistants, and researchers were not blinded. The data manager was blinded.
2.7. Statistical methods
Demographic characteristics were described using numbers, proportions, frequencies, means, and SDs. Because of the small sample size, baseline and follow‐up estimates are given as medians and are compared descriptively. The Friedman test was used to compare medians/ranks from baseline to the end of visit per intervention group. All P values are regarded as descriptive, indicating possible strengths of associations.
3. RESULTS
3.1. Participants
In total, n = 8 healthy male subjects were included in this study. Demographic characteristics are shown in Table 1. The mean age was 27.5 years and the mean BMI was 23.7 kg/m2.
TABLE 1.
Sample characteristics at baseline (n = 8)
| Age in years | |
| Mean (SD) | 27.5 (1.5) |
| Median (IQR) | 27 (27.0‐28.8) |
| Body mass index in kg/m2 | |
| Mean (SD) | 23.7 (1.8) |
| Median (IQR) | 23.1 (22.3‐24.9) |
| Skin phototype, n | |
| I | 2 |
| II | 6 |
| Heart rate in beats per minute | |
| Mean (SD) | 76 (7.5) |
| Median (IQR) | 77.5 (72‐83) |
| Systolic blood pressure in mm Hg | |
| Mean (SD) | 119 (12.2) |
| Median (IQR) | 116 (110‐129) |
| Diastolic blood pressure in mm Hg | |
| Mean (SD) | 77 (6.5) |
| Median (IQR) | 75 (71‐80) |
3.2. Outcomes
The results for the heel skin are shown in Table 2. During loading (groups A and C) or wearing a dressing also (group B), the skin temperature increased from baseline to immediately after intervention and then decreased again. These changes were similar in all three groups.
TABLE 2.
Comparisons between the three interventional groups (n = 8) at both heels
| Intervention groups | |||
|---|---|---|---|
| A | B | C | |
| Loading | Yes | No | Yes |
| Dressing | No | Yes | Yes |
| Skin temperature °C (Median) | |||
| Baseline | 28.2 | 26.1 | 27.8 |
| After 2.5 hours | 29.7 | 28.4 | 29.5 |
| After 3 hours | 27.2 | 26.5 | 27.2 |
| After 4.5 hours | 25.3 | 24.0 | 25.2 |
| P value a | .027 | .003 | .034 |
| Stratum corneum hydration (AU) (Median) | |||
| Baseline | 24.8 | 17.3 | 19.3 |
| After 2.5 hours | 35.1 | 31.6 | 37.2 |
| After 3 hours | 28.2 | 21.0 | 22.3 |
| After 4.5 hours | 31.1 | 20.4 | 23.5 |
| P value a | .031 | .003 | .002 |
| Epidermal hydration (AU) (Median) | |||
| Baseline | 33.4 | 24.3 | 28.9 |
| After 2.5 hours | 36.9 | 35.5 | 38.7 |
| After 3 hours | 35.5 | 26.9 | 32.4 |
| After 4.5 hours | 31.0 | 25.5 | 29.2 |
| P value a | .054 | .003 | <.001 |
| Transepidermal water loss in g/m2/h (Median) | |||
| Baseline | 21.4 | 20.1 | 18.0 |
| After 2.5 hours | 57.6 | 49.6 | 52.7 |
| After 3 hours | 19.2 | 16.7 | 17.6 |
| After 4.5 hours | 17.8 | 14.3 | 14.2 |
| P value a | .001 | .002 | .001 |
| Erythema index (AU) (Median) | |||
| Baseline | 259 | 251 | 241 |
| After 2.5 hours | 320 | 325 | 330 |
| After 3 hours | 295 | 257 | 321 |
| After 4.5 hours | 266 | 235 | 287 |
| P value a | .041 | .047 | <.001 |
| Structural stiffness (Median) (Uf, mm) | |||
| Baseline | 0.12 | 0.10 | 0.12 |
| After 2.5 hours | 0.13 | 0.14 | 0.15 |
| After 3 hours | 0.13 | 0.11 | 0.14 |
| After 4.5 hours | 0.13 | 0.10 | 0.10 |
| P value a | .717 | .062 | .086 |
| Biological elasticity (Median) (Ua/Uf, mm) | |||
| Baseline | 0.74 | 0.81 | 0.79 |
| After 2.5 hours | 0.74 | 0.84 | 0.80 |
| After 3 hours | 0.80 | 0.80 | 0.77 |
| After 4.5 hours | 0.76 | 0.85 | 0.75 |
| P value a | .199 | .290 | .369 |
| Roughness (Median) (Rz) | |||
| Baseline | 36.5 | 40.3 | 41.3 |
| After 2.5 hours | 30.5 | 29.0 | 30.3 |
| After 3 hours | 27.5 | 30.0 | 29.0 |
| After 4.5 hours | 26.8 | 26.0 | 28.8 |
| P value b | .003 | .005 | .004 |
| Stratum corneum thickness (Median) (mm) | |||
| Baseline | 0.28 | 0.36 | 0.36 |
| After 2.5 hours | 0.35 | 0.33 | 0.41 |
| After 3 hours | 0.33 | 0.41 | 0.42 |
| After 4.5 hours | 0.32 | 0.35 | 0.32 |
| P value a | .466 | .041 | .419 |
| Epidermal thickness (Median) (mm) | |||
| Baseline | 0.52 | 0.64 | 0.63 |
| After 2.5 hours | 0.59 | 0.57 | 0.65 |
| After 3 hours | 0.55 | 0.67 | 0.67 |
| After 4.5 hours | 0.60 | 0.57 | 0.60 |
| P value a | .886 | .165 | .615 |
| Thickness of living epidermal layers without SC (Median, SD) (mm) | |||
| Baseline | 0.25 | 0.26 | 0.26 |
| After 2.5 hours | 0.26 | 0.24 | 0.28 |
| After 3 hours | 0.24 | 0.26 | 0.26 |
| After 4.5 hours | 0.25 | 0.27 | 0.24 |
| P value a | .704 | .080 | .563 |
| Interleukin‐1 alpha (Median) (pg/μg) | |||
| Baseline | 13.1 | 11.6 | 10.7 |
| After 3 hours | 17.5 | 12.0 | 13.3 |
Friedman test.
One‐way repeated‐measures ANOVA.
SCH increased in all three groups. A substantially higher increase was observed in both dressing groups, independent of loading (group B [median]: 17.3 AU [baseline] to 31.6 AU [after 2.5 hours]; group C [median]: 19.3 AU [baseline] to 37.2 AU [after 2.5 hours]). An increase in epidermal hydration was also observed in all groups. The increase was higher in both dressing groups, compared with the no dressing group.
After loading and/or wearing a dressing, the TEWL increased. This increase was slightly higher in the loading group A, but overall differences between the groups were only minor.
After 2.5 hours loading and/or wearing a dressing, erythema increased in all groups and decreased after dressing removal and/or offloading. The highest EI was observed in group C (baseline 241 AU to 330 AU after loading). Compared with both loading groups (A and C), the erythema index in group B decreased substantially faster at 3 and 4.5 hours.
Clinical signs of erythema increased in all three groups independently on both heels. The highest increases were observed in both loading groups compared with no‐loading. After loading, the erythematous response also remained longer.
The structural stiffness (Uf) of the heel skin decreased in both dressing groups B and C irrespectively from loading and returned to baseline at 3 and 4.5 hours. There was no change in the loading only group A. Minor increases of elasticity (Ua/Uf) were observed in group B.
Based on Visioscan images, the mean roughness (Rz) was calculated. The mean roughness decreased in all three intervention groups. The highest decrease was observed in both dressing groups B and C with and without loading.
The visual inspection of Visioscan images of the skin surface topography changed in all three groups. At baseline, the images showed pronounced ridges. After loading of the heel skin with and without dressing, the ridges were more indistinctly visible and the scales were smaller (Figure 3).
FIGURE 3.
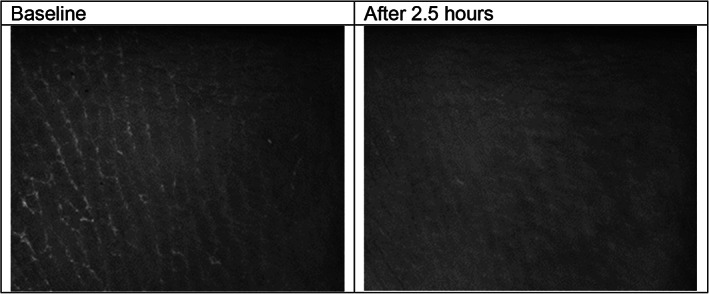
Visioscan images of one subject
The median IL‐1α concentration per one μg total protein increased after loading in groups A and C. The highest increase was observed for group A (median: 13.1 pg/μg [baseline] to 17.5 pg/μg [after 2.5 hours]) followed by the dressing group C (Table 2). Pain was reported in one subject on both heels to the end of loading without dressing.
The results for the sacral skin area are presented in Table 3. The skin surface temperature increased in all intervention groups in a comparable way. Also, the SCH increased in all three groups but was substantially higher in group C (SCH median: 33.2 AU [baseline] to 47.8 AU [after 2.5 hours]). Median epidermal hydration was also higher in both loading groups A and C and it also took longer to return to baseline.
TABLE 3.
Comparisons between the three interventional groups (n = 8) at the sacrum
| Intervention | |||
|---|---|---|---|
| A | B | C | |
| Loading | Yes | No | Yes |
| Dressing | No | Yes | Yes |
| Skin temperature °C (Median) | |||
| Baseline | 29.4 | 29.2 | 29.9 |
| After 2.5 hours | 31.0 | 30.3 | 31.1 |
| After 3 hours | 30.1 | 29.4 | 29.7 |
| After 4.5 hours | 29.7 | 29.4 | 29.6 |
| P value a | <.001 | .076 | .022 |
| Stratum corneum hydration (AU) (Median) | |||
| Baseline | 27.7 | 27.3 | 33.2 |
| After 2.5 hours | 38.2 | 40.4 | 47.8 |
| After 3 hours | 37.7 | 37.9 | 46.4 |
| After 4.5 hours | 35.2 | 35.8 | 40.3 |
| P value a | .019 | .016 | .136 |
| Epidermal hydration (AU) (Median) | |||
| Baseline | 39.7 | 40.7 | 41.7 |
| After 2.5 hours | 49.3 | 44.8 | 49.4 |
| After 3 hours | 46.0 | 45.8 | 50.5 |
| After 4.5 hours | 45.8 | 44.7 | 48.8 |
| P value a | .006 | .271 | .006 |
| Transepidermal water loss in g/m a /h (Median) | |||
| Baseline | 9.2 | 9.3 | 9.1 |
| After 2.5 hours | 15.1 | 13.6 | 18.0 |
| After 3 hours | 8.6 | 8.7 | 10.4 |
| After 4.5 hours | 11.1 | 9.2 | 7.9 |
| P value a | .003 | .001 | .001 |
| Erythema index (AU) (Median) | |||
| Baseline | 171 | 185 | 186 |
| After 2.5 hours | 229 | 173 | 226 |
| After 3 hours | 196 | 193 | 207 |
| After 4.5 hours | 155 | 172 | 195 |
| P value a | .001 | .789 | .070 |
| Structural stiffness (Median) (Uf, mm) | |||
| Baseline | 0.49 | 0.50 | 0.47 |
| After 2.5 hours | 0.51 | 0.49 | 0.51 |
| After 3 hours | 0.50 | 0.45 | 0.51 |
| After 4.5 hours | 0.48 | 0.48 | 0.50 |
| P value a | .187 | .369 | .583 |
| Biological elasticity (Median) (Ua/Uf, mm) | |||
| Baseline | 0.93 | 0.91 | 0.91 |
| After 2.5 hours | 0.90 | 0.93 | 0.90 |
| After 3 hours | 0.92 | 0.92 | 0.89 |
| After 4.5 hours | 0.93 | 0.91 | 0.90 |
| P value a | .054 | .348 | .985 |
| Roughness (Median) (Rz) | |||
| Baseline | 50.8 | 54.5 | 48.5 |
| After 2.5 hours | 45.5 | 51.3 | 48.8 |
| After 3 hours | 52.8 | 54.5 | 49.5 |
| After 4.5 hours | 49.5 | 47.5 | 47.5 |
| P value a | .495 | .110 | .522 |
| Epidermal thickness (Median) (mm) | |||
| Baseline | 0.11 | 0.12 | 0.10 |
| After 2.5 hours | 0.12 | 0.10 | 0.09 |
| After 3 hours | 0.10 | 0.10 | 0.10 |
| After 4.5 hours | 0.11 | 0.11 | 0.11 |
| P value a | .647 | .080 | .127 |
| Interleukin‐1 alpha (Median) (pg/μg) | |||
| Baseline | 5.7 | 8.1 | 8.7 |
| After 3 hours | 7.1 | 7.9 | 7.7 |
Friedman test.
In all intervention groups, TEWL values increased after 2.5 hours and returned to baseline after offloading and/or dressing removal. The highest increase was observed in the loading groups with A and C.
An increase of the EI was observed only in the loading groups A and C. EI in intervention B remained similar over time. Clinical signs of erythema were also visible in the intervention‐groups with loading (groups A and C) only.
The structural stiffness (Uf) and elasticity (Ua/Uf) of the skin showed slight variations over time without clear trends or differences between groups. The roughness (Rz) decreased in groups A and B but remained stable in intervention group C.
The epidermal thickness was based on the analysis of the OCT images. There seemed to be minor decreases of epidermal thickness in groups B and C and slight increases in group A. Overall differences were small.
There was an increase of IL‐1 alpha to total protein values after 2.5 hours loading without dressing A. Pain was reported in one subject in the intervention group A immediately after loading.
4. DISCUSSION
4.1. Limitations
The sample size of n = 8 is too low to allow general conclusions but was considered sufficient for an exploratory study. All results and conclusions are to be regarded as hypothesis generating. The subjects were requested to stay in position for 2.5 hours, which was ensured by the continuous presence of a study assistant or researcher. However, minor slight movements may have taken place unnoticed. Because of the limited skin area at the heels, both heels were needed for performing all the measurements including stratum corneum sampling. The comparability between right and left heels may be limited but this was unlikely as indicated by similar baseline values and substantial evidence supporting complete symmetry of skin structure and function across the human body. 22
The demographic sample characteristics are not representative of populations at PU risk. However, the decision to include young and healthy males at this exploratory stage was based on the following reason: skin structure and function are influenced by age and sex. Ageing always leads to an increase of biological variability. This is well documented for the human skin including chronic micro‐inflammation (“inflammaging”), which may interfere with IL‐1α measurements. 18 Reactivity of aged skin is reduced in general, which does not mean that the skin is less vulnerable. 28 Because the overall objective was to explore group differences in principle, it was important to reduce variability as much as possible. Hormonal rhythms also affect the skin physiology, therefore females were excluded.
For skin surface temperature measurements, we used a skin thermometer (Courage & Khazaka electronic GmbH, Germany) that enables point measurements. The use of devices providing a spatial distribution of temperature values over a skin area provides usually more details. 29 In our study, we performed repeat measurements over time on very small standardised skin areas at the heel and sacrum. In addition, empirical evidence supports high reliability of this method even when using one single reading only. Therefore, the point measurements in this trial were considered appropriate. 30
Another possible limitation is the necessary dressing removal before skin measurements can be performed. When dressings are removed, there is a rapid change of skin parameters. Therefore, all measurements were conducted immediately after offloading and/or dressing removal. The use of sensors between the dressing and the skin surface is a possible alternative, but the sensors will also interact with the skin and the dressing performance.
4.2. Interpretation
Various skin structure and function measurements have been conducted, leading to numerous results. Therefore, in Table 4, there is an overall summary of the observed effects of the interventions at the heel and sacral skin immediately after offloading and/or dressing removal after 2.5 hours. An upwardly pointing arrow indicates an increase, and a downwardly pointing arrow indicates a decrease in the respective parameter. Two arrows indicate that the change (Δ) was highest in this group(s). The question marks in brackets indicate that the differences were only minor.
TABLE 4.
Summary of skin changes on both heels and sacrum immediately after loading and/or dressing removal
| Intervention at heels | |||
|---|---|---|---|
| Loading | Yes | No | Yes |
| Dressing | No | Yes | Yes |
| Skin surface temperature | ↑ | ↑ | ↑ |
| SCH | ↑ | ↑ | ↑↑ |
| Epidermal hydration | ↑ | ↑↑ | ↑↑ |
| TEWL | ↑ | ↑ | ↑ |
| Erythema index | ↑ | ↑ | ↑↑ |
| Clinical signs of erythema | ↑↑ | ↑ | ↑↑ |
| Structural stiffness (Uf) | ↑ (?) | ↑↑ | ↑ |
| Biological elasticity (Ua/Uf) | ↑ (?) | ↑ | ↑ (?) |
| Roughness (Rz) | ↓ | ↓↓ | ↓↓ |
| Stratum corneum thickness | ↑ | → | ↑ |
| Epidermal thickness | ↑ | → | ↑ |
| IL‐1 alpha | ↑↑ | → | ↑ |
| Self‐reported pain | One subject | None | None |
| Intervention at the sacrum | |||
|---|---|---|---|
| Skin surface temperature | ↑ | ↑ | ↑ |
| SCH | ↑ | ↑ | ↑ |
| Epidermal hydration | ↑↑ | ↑ | ↑ |
| TEWL | ↑ | ↑ | ↑ |
| Erythema index | ↑ | → | ↑ |
| Clinical signs of erythema | ↑↑ L | → | ↑ |
| Structural stiffness (Uf) | ↑ | ↓ | ↑ |
| Biological elasticity (Ua/Uf) | → | → | → |
| Roughness (Rz) | ↓ | ↓ | → |
| Epidermal thickness | ↑ (?) | → | → |
| IL‐1 alpha | ↑ | → | → |
| Self‐reported pain | One subject | None | None |
Abbreviations: SCH, stratum corneum hydration; TEWL, transepidermal water loss.
A comparable skin surface temperature increase was observed in all three groups on both skin areas. This was expected because the prolonged close contact of the skin with the support surface and/or dressing leads to local accumulation of heat. Baseline heel and sacral skin surface temperatures and the increases after loading are very similar to previous results. 11 , 16 This indicates that the obtained temperature estimates are reproducible and that the application of a dressing to the heel and sacral skin during loading does not make a difference in terms of heat accumulation. In other words, the dressing does not seem to contribute to an “extra” increase in local skin temperature. In addition, the results show that the dressing alone without loading produces occlusive conditions leading to heat (and moisture) accumulation, which is very well supported by the literature. 31
The study results indicate that all three interventions increased the hydration of the stratum corneum and possibly also of the entire epidermis. Corneometer SCH readings cover approximately the first 10 μm from the skin surface. Therefore, the top layer of the thick heel stratum corneum was measured. Baseline SCH of heels and sacrum were comparable to previous results 11 and SCH increases were similar between groups but slightly larger in group C. This supports the previous interpretation that the dressing leads to an accumulation of water at both skin areas irrespective of loading. There was also an SCH increase in group A at both skin areas, indicating moisture accumulation between heel and sacral skin and the cotton cover of the mattress. It is well known that the water transport properties of the material of the cover and/or textile play important roles for local water accumulation during loading. 20 The results from the Moisture Meter EpiD support the previous interpretation. The measurement depth of this device is ~0.5 mm. The measured stratum corneum thickness at the heels in the sample ranged from 0.3 to 0.4 mm. Therefore, the readings mostly contain information about the entire stratum corneum hydration and to a lesser degree of the living epidermal cells. Similar, to the SCH increases, the estimates at the heels were slightly higher in group C. Taken together with the SCH readings, this provides evidence that the dressings lead to a slightly higher hydration of the entire heel stratum corneum compared with the loading‐only group, but these differences were minor. The hydration reduced to baseline again after loading with/without dressing in all three groups and at both skin areas, which may be explained by desorption, indicating that the skin rapidly adapts to the environment. 12
The measured thickness of the epidermis at the sacrum was ~0.10 to 0.12 mm, which is the typical epidermal thickness of patterned skin, 24 , 32 and the Moisture Meter EpiD captures the hydration of ~0.5 mm depth. Therefore, dermal influences at the sacrum are highly likely and difficult to interpret.
Baseline TEWL estimates are comparable to previous research for the heels and sacrum. 11 , 14 , 22 There was a substantial and similar TEWL increase in all three groups, but it seemed to be slightly higher in the loading groups at both skin areas, which returned to baseline ~30 minutes after the intervention. The increased TEWL values indicate the increased evaporation of “excess” water, which was accumulated before indicated by the higher SCH. However, the increased TEWL values may also indicate changes in the skin barrier either because of loading and/or because of the dressing alone. TEWL is a very sensitive parameter for skin barrier changes and mechanical deformation is known to damage the stratum corneum. 22 Both SCH and TEWL are also directly related to stratum corneum temperature. 12 Therefore, these physical relationships need to be considered when interpreting results.
In both loading groups at both skin areas, there was an increase of erythema shown by the instrumental measurements and clinical evaluations, which was expected as well. During compression/deformation, there is a decrease of local blood flow followed by reactive hyperaemia (eg, References 33 and 34). The results indicate indeed that the erythema at the heels in both loading groups (A, C) was higher compared with the dressing group without loading (B). The erythema at the heels in group B might be explained by the increase in skin temperature. There is a direct relationship between skin perfusion and skin temperature irrespective of the degree of loading or deformation. 35 At the sacral area, there was no erythema in group B, indicating that the application of a dressing without loading does not alter perfusion at this skin area. Erythema seemed to be the most pronounced at the heels in group C. The recovery took longer compared with groups A and B. Maybe there are accumulating effects of deformation and occlusion when using dressings. Erythema results in general were higher at the heels compared with the sacral skin, which may indicate stronger mechanical deformation as a result of the heel anatomy.
Based on the visual inspection of the Visioscan images, the skin surface topography changed in all three groups at the heels. Baseline images showed more or less pronounced ridges of the heel skin with whitish appearing scales next to the ridges. After loading with and/or without dressing, the ridges became partly indistinct and visible scales became smaller. This indicates the increasing hydration of the stratum corneum during the interventions, which is supported by the increased SCH estimates. In addition, in both dressing groups, imprints of the dressing were visible immediately after dressing removal in approximately half of the subjects at the heels. The direct contact of the skin with the dressing and the water accumulation within the stratum corneum (including the softening of the stratum corneum indicated by decreased stiffness [Uf]) may be responsible for that. Most probably, the moisture accumulation is more important than the loading because the imprints were observed in both groups only at the heels.
These qualitative observations are supported by the roughness measurements Rz. There was a decrease in skin surface roughness in all three groups at the heels. This again supports the interpretation that the hydration of the stratum corneum was much higher under the dressing irrespectively from loading. In contrast to the skin barrier and hydration measurements, the skin surface roughness seems to take much longer to return to baseline again. The baseline values and the decrease of Rz of heel skin after loading are supported by previous research. 10 The roughness estimate Rz at the sacral skin was also slightly decreased in groups A and B but remained unchanged in group C. This finding is supported by the visual evaluation and previous research. 16 The impact of loading with and without dressing and the application of the dressing per se on the skin surface at the sacrum seem to be minor.
The study results impressively show that prolonged loading of heel and sacral skin causes an increase in IL‐1α in the stratum corneum. Increased release of this IL in the stratum corneum initiates the inflammatory cascade in the skin. 36 Thus, the results support an association between mechanical deformation and cutaneous inflammation.
There seemed to be an increase of IL‐1α after loading at the sacrum. This is supported by a recent study by Wert et al 16 who showed reduced IL‐1α releases during mechanical stimulation when wearing a dressing compared with no dressing. Interestingly, median differences between baseline and post‐loading at the heels and sacrum were higher in group A compared with group C, indicating a possible protective effect of the dressing during loading. This finding is also supported by previous research. Disregarding biological variation, a dressing alone does not initiate inflammation without loading (group B). This indicates that increased hydration of the stratum corneum as a result of the dressing is not associated with inflammation, but mechanical deformation is.
A number of similarities between heel and sacral skin have been observed, indicating similar skin responses to either loading and/or dressing wear. In both situations, the dressing created occlusive conditions on the skin surface leading to moisture accumulation and temperature increases. This caused changes in the skin surface topography and stiffness properties. Interestingly, these changes were more or less similar for loaded and unloaded skin, indicating that the dressing does not lead to an “extra” occlusion during loading.
On the other hand, most cutaneous responses were much more pronounced at heel compared with sacral skin. This may be partly explained by structural and functional differences. Compared with sacral skin, heel skin is, for example, much stiffer, has a much higher TEWL, and the stratum corneum is drier. For the first time, empirical evidence is provided that the baseline IL‐1α levels are much higher in heel skin compared with sacral skin. This supports the idea that heel skin and underlying soft tissues behave differently compared with sacral skin and that PU pathogenesis is slightly different. 2 , 37 Because of the extremely thick stratum corneum of the heel, the particular connective strengths of the corneocytes, and the abundance of sweat glands, heel stratum corneum may be especially prone to moisture accumulation and all associated consequences. This stronger deformation and the higher levels of IL‐1α may be the reason for a higher susceptibility to inflammation. Finally, differences in the loading and deformation intensities between the heel and the sacral regions may also contribute to different skin responses.
5. CONCLUSIONS
This study characterises skin responses to dressing application with and without loading of the two most important PU predilection sites in a comprehensive way. The results suggest that a dressing contributes to occlusion of the skin, which is similar or slightly increased compared with non‐loading or loading without dressings. Thus, a dressing does not cause additional irritation or skin changes during loading. However, whether these microclimate changes are clinically relevant is unclear at the moment. It is well known that an increase in the skin surface temperature and stratum corneum hydration goes along with an increased PU risk. However, maybe the dressing compensates these unwanted effects between the skin‐dressing interface by providing additional friction (and shear) reduction at the outer dressing surface in contact with the support surface and within inner dressing materials. This may explain the clinical benefit shown in clinical trials (eg, References 38 and 39). At the same time, a possible “cushioning” effect seems to be minor, because the erythematous response was similar after loading irrespective of the dressing. Prolonged loading of heel and sacral skin leads to increases of IL‐1α in the stratum corneum, which is clearly linked to “early” inflammation. The results indicate that the dressing may reduce the inflammatory response.
CONFLICT OF INTEREST
Jan Kottner received honoraria from Mölnlycke Health Care. The other authors declared no conflicts of interest.
ACKNOWLEDGEMENT
This study was sponsored by Mölnlycke Health Care (402 52 Gothenburg, Sweden).
Lichterfeld‐Kottner A, Vogt A, Tomova‐Simitchieva T, Blume‐Peytavi U, Kottner J. Effects of loading and prophylactic dressings on the sacral and heel skin: An exploratory cross‐over trial. Int Wound J. 2021;18:909–922. 10.1111/iwj.13596
Funding information Mölnlycke Health Care
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. European Pressure Ulcer Advisory Panel NPUAPaPPPIA . Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. EPUAP, NPUAP & PPPIA; 2019. [Google Scholar]
- 2. Lechner A, Lahmann N, Neumann K, Blume‐Peytavi U, Kottner J. Dry skin and pressure ulcer risk: a multi‐center cross‐sectional prevalence study in German hospitals and nursing homes. Int J Nurs Stud. 2017;73:63‐69. [DOI] [PubMed] [Google Scholar]
- 3. Berlowitz DR, Brienza DM. Are all pressure ulcers the result of deep tissue injury? A review of the literature. Ostomy Wound Manage. 2007;53:34‐38. [PubMed] [Google Scholar]
- 4. Kottner J, Balzer K, Dassen T, Heinze S. Pressure ulcers: a critical review of definitions and classifications. Ostomy Wound Manage. 2009;55:22‐29. [PubMed] [Google Scholar]
- 5. Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng. 2015;43:297‐305. [DOI] [PubMed] [Google Scholar]
- 6. Bhargava A, Chanmugam A, Herman C. Heat transfer model for deep tissue injury: a step towards an early thermographic diagnostic capability. Diagn Pathol. 2014;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore ZEH, Webster J. Dressings and topical agents for preventing pressure ulcers. Cochrane Db Syst Rev. 2018;12:CD009362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Call E, Pedersen J, Bill B, et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J. 2015;12:408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy A, Frank MB, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24:1‐11. [DOI] [PubMed] [Google Scholar]
- 10. Dobos G, Gefen A, Blume‐Peytavi U, Kottner J. Weight‐bearing‐induced changes in the microtopography and structural stiffness of human skin in vivo following immobility periods. Wound Repair Regen. 2015;23:37‐43. [DOI] [PubMed] [Google Scholar]
- 11. Kottner J, Dobos G, Andruck A, et al. Skin response to sustained loading: a clinical explorative study. J Tissue Viability. 2015;24:114‐122. [DOI] [PubMed] [Google Scholar]
- 12. Kottner J, Black J, Call E, Gefen A, Santamaria N. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech (Bristol, Avon). 2018;59:62‐70. [DOI] [PubMed] [Google Scholar]
- 13. Pfannes EKB, Blume‐Peytavi U, Kottner J. Patterns and associations of structural and functional cutaneous responses during loading at heel and sacral skin in aged females: a reanalysis of clinical study data. J Tissue Viability. 2018;27:123‐129. [DOI] [PubMed] [Google Scholar]
- 14. Tomova‐Simitchieva T, Lichterfeld‐Kottner A, Blume‐Peytavi U, Kottner J. Comparing the effects of 3 different pressure ulcer prevention support surfaces on the structure and function of heel and sacral skin: an exploratory cross‐over trial. Int Wound J. 2018;15:429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Wert LA, Schoonhoven L, Stegen J, et al. Improving the effect of shear on skin viability with wound dressings. J Mech Behav Biomed Mater. 2016;60:505‐514. [DOI] [PubMed] [Google Scholar]
- 16. Lechner A, Rancan F, Hadam S, Vogt A, Blume‐Peytavi U, Kottner J. Comparing the effects of three different multilayer dressings for pressure ulcer prevention on sacral skin after prolonged loading: an exploratory crossover trial. Wound Repair Regen. 2021;29(2):270–279. [DOI] [PubMed] [Google Scholar]
- 17. Bader DL, Worsley PR. Technologies to monitor the health of loaded skin tissues. Biomed Eng Online. 2018;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hemmes B, de Wert LA, Brink PRG, Oomens CWJ, Bader DL, Poeze M. Cytokine IL1alpha and lactate as markers for tissue damage in spineboard immobilisation. A prospective, randomised open‐label crossover trial. J Mech Behav Biomed Mater. 2017;75:82‐88. [DOI] [PubMed] [Google Scholar]
- 19. Lechner A, Kottner J, Coleman S, et al. Outcomes for Pressure Ulcer Trials (OUTPUTs) project: review and classification of outcomes reported in pressure ulcer prevention research. Br J Dermatol. 2020. 10.1111/bjd.19304. [DOI] [PubMed] [Google Scholar]
- 20. Schario M, Tomova‐Simitchieva T, Lichterfeld A, et al. Effects of two different fabrics on skin barrier function under real pressure conditions. J Tissue Viability. 2017;26:150‐155. [DOI] [PubMed] [Google Scholar]
- 21. Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Arch Dermatol. 1988;124:869‐871. [DOI] [PubMed] [Google Scholar]
- 22. Akdeniz M, Gabriel S, Lichterfeld‐Kottner A, Blume‐Peytavi U, Kottner J. Transepidermal water loss in healthy adults: a systematic review and meta‐analysis update. Br J Dermatol. 2018;179:1049‐1055. [DOI] [PubMed] [Google Scholar]
- 23. Elban F, Hahnel E, Blume‐Peytavi U, Kottner J. Reliability and agreement of skin barrier measurements in a geriatric care setting. J Tissue Viability. 2020;29:269‐276. [DOI] [PubMed] [Google Scholar]
- 24. Trojahn C, Schario M, Dobos G, Blume‐Peytavi U, Kottner J. Reliability and validity of two in vivo measurements for skin surface topography in aged adults. Skin Res Technol. 2015;21:54‐60. [DOI] [PubMed] [Google Scholar]
- 25. Pfannes EK, Hadam S, Doge N, Fimmel S, Blume‐Peytavi U, Vogt A. Mini‐zone cyanoacrylate skin surface stripping: a new method for non‐invasive sampling of scalp material. Exp Dermatol. 2016;25:555‐556. [DOI] [PubMed] [Google Scholar]
- 26. Vogt ACB, Hadam S, Stieler KM, Lademann J, Schaefer H. 40nm, but not 750 or 1,500nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 2006;126:1316‐1322. [DOI] [PubMed] [Google Scholar]
- 27. Vogt AMB, Costagliola D, Bonduelle O, Hadam S, Schaefer G. Transcutaneous anti‐influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J Immunol. 2008;180:1482‐1489. [DOI] [PubMed] [Google Scholar]
- 28. Kottner J, Beeckman D, Vogt A, Blume‐Peytavi U. Skin health and integrity. In: Gefen A, ed. Innovations and Emerging Technologies in Wound Care. Cambridge, MA: Elsevier; 2020:183‐196. [Google Scholar]
- 29. Amrani G, Peko L, Hoffer O, Ovadia‐Blechman Z, Gefen A. The microclimate under dressings applied to intact weight‐bearing skin: infrared thermography studies. Clin Biomech (Bristol, Avon). 2020;75:104994. [DOI] [PubMed] [Google Scholar]
- 30. Kottner J, Blume‐Peytavi U. Reliability and agreement of instrumental skin barrier measurements in clinical pressure ulcer prevention research. Int Wound J. 2021. 10.1111/iwj.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cavallini M, Gazzola R, Vaienti L. Effects of adhesive dressings on stratum corneum conductance. Skin Res Technol. 2012;18:241‐244. [DOI] [PubMed] [Google Scholar]
- 32. Mlosek RK, Malinowska S, Sikora M, et al. The use of high frequency ultrasound imaging in skin moisturization measurement. Skin Res Technol. 2013;19:169‐175. [DOI] [PubMed] [Google Scholar]
- 33. Choi WJ, Wang H, Wang RK. Optical coherence tomography microangiography for monitoring the response of vascular perfusion to external pressure on human skin tissue. J Biomed Opt. 2014;19:056003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herrman EC, Knapp CF, Donofrio JC, Salcido R. Skin perfusion responses to surface pressure‐induced ischemia: implication for the developing pressure ulcer. J Rehabil Res Dev. 1999;36:109‐120. [PubMed] [Google Scholar]
- 35. Patel S, Knapp CF, Donofrio JC, Salcido R. Temperature effects on surface pressure‐induced changes in rat skin perfusion: implications in pressure ulcer development. J Rehabil Res Dev. 1999;36:189‐201. [PubMed] [Google Scholar]
- 36. de Jongh CM, Verberk MM, Spiekstra SW, Gibbs S, Kezic S. Cytokines at different stratum corneum levels in normal and sodium lauryl sulphate‐irritated skin. Skin Res Technol. 2007;13:390‐398. [DOI] [PubMed] [Google Scholar]
- 37. Kottner J, Gefen A, Lahmann N. Weight and pressure ulcer occurrence: a secondary data analysis. Int J Nurs Stud. 2011;48:1339‐1348. [DOI] [PubMed] [Google Scholar]
- 38. Hahnel E, El Genedy M, Tomova‐Simitchieva T, et al. The effectiveness of two silicone dressings for sacral and heel pressure ulcer prevention compared with no dressings in high‐risk intensive care unit patients: a randomized controlled parallel‐group trial. Br J Dermatol. 2020;183:256‐264. [DOI] [PubMed] [Google Scholar]
- 39. Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi‐layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


