Abstract
Organisms in the Mycobacterium avium complex (MAC; M. avium, M. intracellulare, and “nonspecific or X” MAC) are emerging pathogens among individual organisms of which significant genetic variability is displayed. The objective of the present study was to evaluate various molecular methods for the rapid and definitive identification of MAC species. Isolates were obtained from both human immunodeficiency virus (HIV)-positive patients and HIV-negative patients with and without known predisposing conditions. The isolates were initially hybridized with nucleic acid probes complementary to the rRNA of the respective mycobacterial species (AccuProbe Culture Confirmation kits for M. avium, M. intracellulare, and MAC species; Gen-Probe). Isolates were also examined by PCR and in some cases by Southern blot hybridization for the insertion element IS1245. Two other techniques included a PCR assay that amplifies the mig gene, a putative virulence factor for MAC, and hsp65 gene amplification and sequencing. This study led to the following observations. Eighty-five percent of the isolates from HIV-positive patients were M. avium and 86% of the isolates from HIV-negative patients were M. intracellulare. Fifteen of the M. avium isolates did not contain IS1245 and 7% of the M. intracellulare isolates were found to carry IS1245. All of the M. avium strains were mig positive, and all of the M. intracellulare strains were mig negative.
Organisms in the Mycobacterium avium complex (MAC) are opportunistic pathogens that can be isolated from soil and water and cause infection and clinical disease in a wide variety of animals and humans (9). Many M. avium infections are seen in patients who are immunocompromised, such as those with AIDS (5). These infections are usually disseminated and have been shown to contribute significantly to the morbidity of AIDS patients (7). Prior to the advent of AIDS, most patients in the United States with MAC pulmonary disease were elderly males who had underlying lung diseases, such as bronchitis, emphysema, prior tuberculosis, and other fibrotic disorders. More recently, MAC pulmonary disease has been diagnosed in an increasing number of women who have no history of underlying lung disorders and who appear to be immunocompetent (10, 17, 18, 24, 25).
The objective of this study was to evaluate various molecular methods for the rapid and definitive identification of MAC species (M. avium, M. intracellulare, and “nonspecific or X” MAC). MAC isolates from the three human populations described above were included in the study. The isolates were initially hybridized with nucleic acid probes complementary to the rRNA of the respective mycobacterial species (AccuProbe Culture Confirmation kits for M. avium, M. intracellulare, and MAC species; Gen-Probe Inc., San Diego, Calif.). These kits are routinely used in clinical mycobacteriology laboratories for the rapid identification of mycobacterial species. Isolates were also examined by PCR and in some cases by Southern blot hybridization for the insertion element IS1245 because it has been suggested to be specific for M. avium (6). Two other techniques included a PCR assay that amplifies the mig gene, a putative virulence factor for MAC, and hsp65 gene amplification and sequencing (16, 23). The combination of these techniques has yielded new information concerning clinical MAC isolates.
MATERIALS AND METHODS
MAC isolates.
A patient profile sheet was obtained for each human isolate. The strains used in this study were obtained from the following sources: Walter Pace, University of Arkansas for Medical Sciences clinical laboratory and the John L. McClellan Memorial Veteran's Hospital clinical laboratory; Richard Wallace and Barbara Brown, Department of Microbiology, University of Texas Health Center at Tyler; Leonid Heifets and Gwen Huitt, National Jewish Center for Immunology and Respiratory Medicine; Richard J. Blinkhorn, MetroHealth Medical Center Clinical Laboratory, Cleveland, Ohio; Hiroe Shiratsuchi, Case Western Reserve University, Cleveland, Ohio; and William Stead, Arkansas State Health Laboratory. The isolates from the United Kingdom were received from Jack Crawford, Centers for Disease Control and Prevention, Atlanta, Ga., and were isolated by Tobin Hellyer, Becton-Dickinson Corp., Baltimore, Md.
In some cases the isolates had been streaked for isolation and single colonies had been selected before being sent to us. All other isolates, upon receipt, were streaked onto 7H11 plates and were examined for colonial morphology in our laboratory. Single colonies were selected and cultivated in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI), two aliquots were frozen, and the remainder of the culture was used for DNA isolation and pulsed-field gel electrophoresis (PFGE).
Testing with Gen-Probe kits.
Culture confirmation kits (AccuProbe; Gen-Probe) were used to determine the species of each isolate. The M. avium probe kit detects M. avium isolates, the M. intracellulare probe kit detects M. intracellulare isolates, and the MAC probe kit detects M. avium, M. intracellulare, and MAC isolates which are designated X or nonspecific MAC and which are not detected by either species-specific probe (3). Each test was performed by following the manufacturer's instructions and with the inclusion of controls.
Each isolate was tested with the MAC AccuProbe kit to confirm that the isolates belonged to MAC. Isolates were then tested with the M. avium-specific AccuProbe, and all positive isolates were given a number preceded by an “A” to identify them as M. avium isolates. Isolates that were positive with the MAC-specific AccuProbe but negative with the M. avium-specific probe were tested with the M. intracellulare-specific AccuProbe. Isolates that were positive with this probe were given a number preceded by “I” to identify them as M. intracellulare isolates. Isolates that were positive with the MAC-specific probe but that were negative with the M. avium-specific or the M. intracellulare-specific probe were given a number preceded by “X” to identify them as nonspecific MAC isolates.
Preparation of genomic DNAs from mycobacteria.
The following large-scale DNA preparation method was developed in our laboratory. For DNA preparation, mycobacterial cells (10 to 100 ml) were incubated to a Klett reading of 100 to 150, which represents logarithmic-phase growth, and were treated with d-cycloserine (1 mg/ml) for 24 h. After sedimentation in the centrifuge, the cell pellets were heat killed, resuspended in 1 ml of lysing buffer (4 mM Tris HCl [pH 8.0], 4% sodium dodecyl sulfate, 10 mM NaCl, 0.001 mM EDTA, 0.1 mg of proteinase K per ml), and incubated at 37°C overnight or at 68°C for 2 h. The lysate was cooled to room temperature, and the debris was pelleted by centrifugation. The supernatant was extracted two times with phenol-chloroform in phase-lock gel tubes (Five Prime to Three Prime Inc., Boulder, Colo.). After a final extraction in chloroform the aqueous phase was precipitated with 0.3 M sodium acetate (pH 5.5) and 2 volumes of ethanol for 15 min at −70°C. Preparations were centrifuged for 20 min at 4°C, washed one time with 70% ethanol, dried, and reconstituted in TE (Tris-EDTA) buffer. Yields were approximately 200 to 1,000 ng/μl.
PCR assays.
DNA amplification was performed on a GeneAmp PCR system 9600 (Perkin-Elmer, Norwalk, Conn.) in a 50-μl reaction mixture containing 10 pmol of each primer, 1 ng of genomic DNA, 200 μM deoxyribonucleoside triphosphates, 1× PCR buffer (1.5 mM MgCl2 [pH 8.3]; Perkin-Elmer), and 1 U of Taq DNA polymerase (Perkin-Elmer). Occasionally, the organisms were tested directly in the PCR without prior DNA extraction. In this case, a sterile toothpick was used to obtain cells from a colony, and the cells were added directly to 49 μl of the complete PCR mixture. Primers and conditions for IS1245 PCR were as reported and amplify IS1245 and the closely related element IS1311 (6, 20). The primers specific for the mig gene were designed to amplify a 737-bp fragment within the coding region of the gene (16). The mig-specific primer sequences were as follows: mig upper, 5′-CCC GTT CAA CGT CAA CTT CC-3′; mig lower, 5′-GGG CTC GCC GGT CAT CAG GT-3′. The cycling conditions were as follows: initial denaturation at 95°C for 5 min and 30 cycles of 30 s of denaturation at 95°C and 2.0 min of annealing and extension at 68°C, followed by a single 5-min extension at 72°C. The PCR products were electrophoresed on a 2% agarose gel, with a 1-kb DNA ladder used for size determination (Gibco Life Technologies, Grand Island, N.Y.), stained with ethidium bromide, and photographed by using the Eagle Eye II gel documentation system (Stratagene, La Jolla, Calif.).
Fingerprinting with IS1245.
For fingerprinting with IS1245 (6), isolated genomic DNA (500 ng) was digested with the restriction enzyme PvuII, electrophoresed on a 0.7% gel, alkaline transferred to Hybond (Amersham Pharmacia Biotech, Piscataway, N.J.), probed with a 427-bp fragment of IS1245 produced by PCR, and labeled with [32P]dCTP with the Random Primed DNA Labeling Kit (Boehringer Mannheim, Mannheim, Germany). The gel images were saved in tif format and were analyzed by using the PFGE program described below.
PFGE and analysis.
Whole cellular DNA for the experiments was prepared essentially as described by Zhang et al. (26). Bacterial DNA slices were restricted with the low-frequency-cleavage restriction endonuclease XbaI and in some cases with SpeI (New England Biolabs, Beverly, Mass.). In order to detect large plasmids, whole cellular DNA was electrophoresed without prior digestion with a restriction endonuclease. PFGE of the digested or undigested DNA samples was performed in a contour-clamped homogeneous electric field on the CHEF-DR II system (Bio-Rad Laboratories, Richmond, Calif.) in 0.8% low-endosmosis agarose gels (Pulsed Field Certified Agarose; Bio-Rad Laboratories) at a ramped pulse time from 5 to 30 s for 24 h. DNA size standards consisting of concatemers of bacteriophage lambda DNA (Bio-Rad Laboratories) were included in each gel.
After electrophoresis, the gels were stained with ethidium bromide, destained in distilled water, and visualized on a UV transilluminator (Stratagene), and a tif image of each gel was stored on a diskette. The profiles were scanned and analyzed with the Molecular Analyst Fingerprinting software (Bio-Rad Laboratories). This package enables conversion of the gel image, normalization to a universal standard, analysis of the macrorestriction patterns, and cluster analysis of the PFGE or IS1245 patterns. By using the hierarchic unweighted pair group average method as a clustering algorithm and the Jaccard coefficient of similarity, dendrograms for the isolates from each subtype were created.
hsp65 sequencing.
The hsp65 gene encodes a 65-kDa heat shock protein and has been used to identify species in the MAC complex (11, 21–23). Amplification and sequencing of the 441-bp segment of the hsp65 gene were performed for selected strains, as described elsewhere (23).
RESULTS
Strain identification with Gen-Probe kits.
An integral aspect of this study was identification of MAC isolates from human immunodeficiency virus (HIV)-positive and HIV-negative patients to the species level. The most common method of species identification of MAC organisms is by use of the 16S ribosomal gene sequence, which is the basis of the Gen-Probe assay. We identified 92 M. avium isolates 75 of which were from HIV-positive patients and 17 of which were from HIV-negative patients; 57 M. intracellulare isolates, 7 of which were from HIV-positive individuals and 50 of which were from HIV-negative patients; and 12 nonspecific or X isolates, 5 of which were from HIV-positive patients and 7 of which were from HIV-negative patients.
IS1245 fingerprinting.
During this study all isolates were checked for the presence of the insertion element IS1245 by PCR (6) (Table 1). Fourteen M. avium isolates (15%) did not contain IS1245 by PCR. These isolates were checked for the presence of IS1245 by Southern blot hybridization, with the same negative results (data not shown).
TABLE 1.
Summary of IS1245 and mig gene data for all isolates
| GenProbe result | No. of isolates
|
|||
|---|---|---|---|---|
| IS1245
|
mig gene
|
|||
| Positive | Negative | Positive | Negative | |
| M. avium | 78 | 13 | 92 | 0 |
| M. intracellulare | 4 | 53 | 0 | 57 |
| MAC X isolate | 7 | 5 | 9 | 3 |
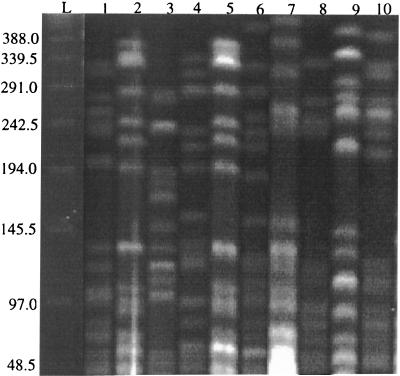
Three of the IS1245-negative M. avium isolates were from HIV-positive patients in the United Kingdom. The remaining 11 isolates were from Cleveland, and all but 1 of these isolates were from HIV-positive patients. To verify that the Cleveland isolates were not identical, they were digested with XbaI, electrophoresed on a single pulsed-field gel, and analyzed (Fig. 1). The results showed that two of the isolates (isolates A67 and A73) shared identical patterns, while the remainder of the isolates had unique patterns. When A67 and A73 were run after SpeI digestion, there was a single band difference which has been determined to be a large plasmid in A73 (data not shown). Therefore, these isolates would be considered identical or essentially the same.
FIG. 1.
PFGE patterns of IS1245-negative M. avium isolates from Cleveland. Lane L, 48.5-kbp bacteriophage lambda concameter ladder (numbers on the right are in kilobase pairs); lane 1, A54; lane 2, A67; lane 3, A71; lane 4, A72; lane 5, A73; lane 6, A76; lane 7, A84; lane 8, A94; lane 9, A95; lane 10, A97.
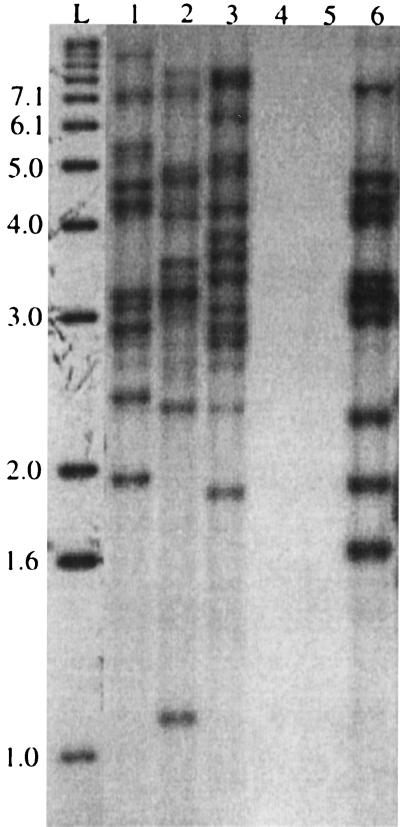
It was verified by PCR and probing of a Southern blot that four isolates of M. intracellulare (7%) contained IS1245 or its related element IS1311. The IS1245 patterns of all four isolates were different (Fig. 2). Isolate I4 was isolated from an HIV-negative patient in Cleveland, isolates I33 and I36 were isolated from HIV-positive patients in Arkansas, and isolate I40 was isolated from an HIV-negative patient in Arkansas. The PFGE patterns of these isolates were also unique (data not shown).
FIG. 2.
IS1245 patterns of five M. intracellulare isolates. Lane L, ladder (numbers on the left are in kilobase pairs); lane 1, I40; lane 2, I36; lane 3, I33; lane 4, I14; lane 5, I7; lane 6, I4. Lanes 4 and 5 contain M. intracellulare strains that were negative for IS1245 by PCR.
hsp65 sequencing.
hsp65 sequencing was used as a secondary method to verify that the 13 M. avium isolates that did not contain IS1245 were M. avium (Table 2). All of the isolates had an M. avium type; 10 of the isolates had the most common M. avium types (types 65.1 and 65.2), and the remaining 3 isolates were of type 65.4 (23). The isolates with identical PFGE patterns, isolates A67 and A73, were of hsp65 type 65.34. The four M. intracellulare isolates that contained IS1245 were all typed as M. intracellulare by hsp65 sequencing. Eight of the X isolates had an hsp65 allele, indicating that they were M. avium, three were typed as M. intracellulare, and one had the 65.29 allele, considered “other” in the MAC group (Table 3).
TABLE 2.
hsp65 alleles for IS1245-negative M. avium isolates and IS1245-positive M. intracellulare isolates
| Gen-Probe result |
hsp65 allele
|
|
|---|---|---|
| M. avium | M. intracellulare | |
| A5 | 65.1 | |
| A6 | 65.1 | |
| A71 | 65.1 | |
| A78 | 65.1 | |
| A8 | 65.2 | |
| A54 | 65.2 | |
| A72 | 65.2 | |
| A76 | 65.2 | |
| A79 | 65.2 | |
| A95 | 65.2 | |
| A67 | 65.34 | |
| A73 | 65.34 | |
| A97 | 65.34 | |
| I4 | 65.3 | |
| I40 | 65.9 | |
| I33 | 65.9 | |
| I36 | 65.9 | |
TABLE 3.
IS1245, mig, and hsp65 allele data for MAC X strains
| Gen-Probe result | PCR resulta
|
hsp65 allele
|
|||
|---|---|---|---|---|---|
| IS1245 | mig | M. avium | M. intracellulare | Other (MAC X) | |
| X1 | Neg | Pos | 65.1 | ||
| X5 | Neg | Pos | 65.29 | ||
| X6 | Pos | Pos | 65.1 | ||
| X7 | Pos | Pos | 65.1 | ||
| X8 | Pos | Pos | 65.2 | ||
| X10 | Pos | Pos | 65.10 | ||
| X11 | Pos | Pos | 65.34 | ||
| X12 | Pos | Pos | 65.1 | ||
| X16 | Pos | Pos | 65.1 | ||
| X9 | Neg | Neg | 65.7 | ||
| X14 | Neg | Neg | Isolate 1373 | ||
| X17 | Neg | Neg | 65.7 | ||
Pos, positive; Neg, negative.
mig PCR.
The only virulence factor identified and well characterized in MAC isolates is the mig gene (14, 16). The mig PCR results indicated that all 92 of the M. avium isolates contained this gene and that none of the 57 M. intracellulare isolates contained mig (Table 1). Therefore, mig appears to be a marker for differentiation of these two species. The isolates designated X varied in their possession of the mig gene. The eight X isolates identified as M. avium and the one isolate identified as “other” by hsp65 sequencing were mig positive, and of these nine isolates, seven contained IS1245 (Table 3). The three X isolates which were negative for mig were also negative for IS1245 and were identified as M. intracellulare by their hsp65 sequence.
DISCUSSION
The objective of this study was to use various molecular techniques to define the species of MAC isolated from three patient populations. The most commonly used method for species identification of MAC isolates is based on the ribosomal 16S sequence and is the principle of the Gen-Probe AccuProbes. Generally, the MAC-specific AccuProbe test is used to identify an organism as a member of this group. Most clinical laboratories do not use the species-specific kits to identify the particular species because the kits are expensive and the treatment is the same for each type of MAC infection. Therefore, the exact species causing infection is not known.
Each isolate was identified to the species level with the Gen-Probe AccuProbes. Eighty-five percent of the isolates from HIV-positive patients were identified as M. avium. The isolates from HIV-negative patients were predominately M. intracellulare (86%) and were from individuals with the clinical manifestations of cavitary disease and nodulation or bronchiectasis and a few patients with invasive disease. Thirteen of the HIV-negative patients had no known predisposing factor. The leading predisposing factor for the majority of the other HIV-negative patients was bronchiectasis, with smoking being the next most common factor. It is not clear if bronchiectasis precedes MAC infection or is the result of this infection (12). The few studies performed to look for immune dysfunction in these patients have not identified immune dysfunction in any of the patients at this time (8, 12).
Twelve of the isolates were positive only with the MAC-specific AccuProbe, which defined these strains as being neither M. avium nor M. intracellulare but MAC strain X or nonspecific. When the hsp65 genes of these strains were sequenced, all but one could be identified as either M. avium or M. intracellulare (23). Therefore, many isolates that would not be recognized as M. avium or M. intracellulare with the species-specific Gen-Probe kits can be given a species identification by hsp65 sequencing (Table 3). This is due to the increased variability of the hsp65 gene compared to that of the 16S gene and therefore its greater power of discrimination. hsp65 sequencing was especially useful as a secondary species identification tool for verification that the M. avium isolates without IS1245 were in fact M. avium and that the M. intracellulare isolates with IS1245 were in fact M. intracellulare. There have been two other reports of the identification of M. avium strains without IS1245, but other than tests with Gen-Probe no other method of identification was performed with the strains (1, 19). We do not know the significance of the fact that all of the M. avium isolates that lacked IS1245 were isolated from patients in Great Britain or the Cleveland area. We are continuing our collection of MAC from various geographical sites in order to determine the extent to which M. avium isolates lack the element and M. intracellulare isolates carry the IS1245 element. It was interesting to find that many isolates of both M. avium and M. intracellulare contained large plasmids (100 to 300 kb) (data not shown), which, if found to be conjugative, could explain the passage of IS1245 between species (4, 15). Therefore, it appears that the presence of the IS1245 insertion element does not necessarily identify an isolate as M. avium, as has been suggested in other reports (2, 6, 19).
Tests for the detection of the mig gene by PCR were performed because it is the only well-characterized virulence factor identified in MAC (14, 16). The role of this gene has not been elucidated, but the gene has been demonstrated to enhance the growth of organisms residing inside the macrophage (14). It was interesting that all our isolates identified as M. avium contain this gene but that the M. intracellulare isolates did not. This finding also held true for the environmental and animal isolates that were examined, but those findings are not included in this report. These results suggest that the detection of the mig gene may be a simple and useful way of differentiating M. avium and M. intracellulare. The presence of this gene in M. avium strains but not M. intracellulare strains suggests that these two species may differ in their virulence mechanisms, which has also been suggested by Maslow et al. (13). The mig gene may be part of a pathogenicity island which is not easily transferred between these two species. Further studies in this regard may reveal the virulence mechanisms used by each species when causing human infections.
In summary, hsp65 sequencing is a useful method for identification of MAC isolates whose species cannot be identified by the AccuProbe Gen-Probe assays. IS1245 is not universally found in all M. avium strains and can be present in M. intracellulare strains. The presence of the mig gene appears to be specific for M. avium strains and may indicate differences in virulence mechanisms between M. avium and M. intracellulare organisms.
ACKNOWLEDGMENTS
This work was funded by an NIH Institutional Development Award Program, Program for the Study of Opportunistic Infections (PAR-96-012).
We thank Chris Hemphill and Suzhannah Mayo for excellent technical assistance. We thank Vivian Jonas and Gen-Probe Inc. for providing some of the species-specific AccuProbe kits. We also express our gratitude to those who sent us the MAC isolates and the patient profile information.
REFERENCES
- 1.Bauer J, Anderson A B, Askgaard D, Giese S B, Larsen B. Typing of clinical Mycobacterium avium complex strains cultured during a 2-year period in Denmark by using IS1245. J Clin Microbiol. 1999;37:600–605. doi: 10.1128/jcm.37.3.600-605.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bono M, Jemmi T, Bernasconi C, Burki D, Telenti A, Bodmer T. Genotypic characterization of Mycobacterium avium strains recovered from animals and their comparison to human strains. Appl Environ Microbiol. 1995;61:371–373. doi: 10.1128/aem.61.1.371-373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bull T J, Shanson D C. Evaluation of a commercial chemiluminescent gene probe system “AccuProbe” for the rapid differentiation of mycobacteria, including “MAIC X”, isolated from blood and other sites, from patients with AIDS. J Hosp Infect. 1992;21:143–149. doi: 10.1016/0195-6701(92)90034-j. [DOI] [PubMed] [Google Scholar]
- 4.Crawford J T, Bates J H. Analysis of plasmids in Mycobacterium avium-intracellulare isolates from persons with acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;134:659–661. doi: 10.1164/arrd.1986.134.4.659. [DOI] [PubMed] [Google Scholar]
- 5.Ellner J J, Goldberger M J, Parenti D M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991;163:1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero C, Bernasconi C, Burki D, Bodmer T, Telenti A. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J Clin Microbiol. 1995;33:304–307. doi: 10.1128/jcm.33.2.304-307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horsburgh C R, Selik R M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS) Am Rev Respir Dis. 1989;139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Huang J H, Oefner P J, Adi V, Ratnam K, Ruoss S J, Trako E, Kao P N. Analyses of the NRAMP1 and IFN-gR1 genes in women with Mycobacterium avium-intracellulare pulmonary disease. Am J Respir Crit Care Med. 1998;157:377–381. doi: 10.1164/ajrccm.157.2.9706012. [DOI] [PubMed] [Google Scholar]
- 9.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iseman M D. M. avium complex: an emerging respiratory pathogen. J Respir Dis. 1995;16:950–962. [Google Scholar]
- 11.Kapur V, Li L-L, Hamrick M R, Plikaytis B B, Shinnick T M, Telenti A, Jacobs W R, Jr, Banerjee A, Cole S, Yuen K Y, Clarridge III J E, Kreisworth B N, Musser J M. Rapid Mycobacterium species assignment and unambiguous identification of mutations associated with antimicrobial resistance in Mycobacterium tuberculosis by automated DNA sequencing. Arch Pathol Lab Med. 1995;119:131–138. [PubMed] [Google Scholar]
- 12.Kubo K, Yamazaki Y, Masubuchi T, Takamizawa A, Yamamoto H, Koizumi T, Fujimoto K, Matsuzawa Y, Honda T, Hasegawa M, Sone S. Pulmonary infection with Mycobacterium avium-intracellulare leads to air trapping distal to the small airways. Am J Respir Crit Care Med. 1998;158:979–984. doi: 10.1164/ajrccm.158.3.9802042. [DOI] [PubMed] [Google Scholar]
- 13.Maslow J N, Dawson D, Carlin E A, Holland S M. Hemolysin as a virulence factor for systemic infection with isolates of Mycobacterium avium complex. J Clin Microbiol. 1999;37:445–446. doi: 10.1128/jcm.37.2.445-446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer M, von Grünberg P W R, Knoop T, Hartmann P, Plum G. The macrophage-induced gene mig as a marker for clinical pathogenicity and in vitro virulence of Mycobacterium avium complex strains. Infect Immun. 1998;66:4549–4552. doi: 10.1128/iai.66.9.4549-4552.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picardeau M, Vincent V. Characterization of large linear plasmid in mycobacteria. J Bacteriol. 1997;179:2753–2756. doi: 10.1128/jb.179.8.2753-2756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plum G, Brenden M, Clark-Curtiss J E, Pulverer G. Cloning, sequencing, and expression of the mig gene of Mycobacterium avium, which codes for a secreted macrophage-induced protein. Infect Immun. 1997;65:4548–4557. doi: 10.1128/iai.65.11.4548-4557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prince D S, Peterson D D, Steiner R M, Gottlieb J E, Scott R, Israel H L, Figueroa W G, Fish J E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;32:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 18.Reich J M, Johnson R E. Mycobacterium avium complex pulmonary disease. Incidence, presentation, and response to therapy in a community setting. Am Rev Respir Dis. 1991;143:1381–1385. doi: 10.1164/ajrccm/143.6.1381. [DOI] [PubMed] [Google Scholar]
- 19.Ritacco V, Kremer K, van der Laan T, Pijnenburg J E M, de Haas P E W, van Soolingen D. Use of IS901 and IS1245 in RFLF typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. Int J Tuberc Lung Dis. 1998;3:242–251. [PubMed] [Google Scholar]
- 20.Roiz M, Palenque E, Guerrero C, Garcia M. Use of restriction fragment length polymorphism as a genetic marker for typing Mycobacterium avium strains. J Clin Microbiol. 1995;33:1389–1391. doi: 10.1128/jcm.33.5.1389-1391.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinnick T. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson D S, Pan X, Kline M W, McKinney R E, Jr, Yogev R, Lewis L L, Brady M, McSherry G D, Dankner W M, Musser J M. Genetic diversity among Mycobacterium avium complex strains recovered from children with and without human immunodeficiency virus infection. J Infect Dis. 1998;178:776–782. doi: 10.1086/515364. [DOI] [PubMed] [Google Scholar]
- 23.Swanson D S, Kapur V, Stockbauer K, Pan X, Frothingham R, Musser J M. Subspecific differentiation of Mycobacterium avium complex strains by automated sequencing of a region of the gene (hsp65) encoding a 65-Kilodalton heat shock protein. Int J Syst Bacteriol. 1997;47:414–419. doi: 10.1099/00207713-47-2-414. [DOI] [PubMed] [Google Scholar]
- 24.Wallace R J., Jr Mycobacterium avium complex lung disease in women: now an equal opportunity disease. Chest. 1994;105:6–7. doi: 10.1378/chest.105.1.6. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 25.Wallace R J, Jr, Zhang Y, Brown B A, Dawson D, Murphy D T, Wilson R, Griffith D E. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235–1244. doi: 10.1164/ajrccm.158.4.9712098. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wallace R J, Jr, Mazurek G H. Genetic differences between BCG substrains. Tubercle Lung Dis. 1995;76:43–50. doi: 10.1016/0962-8479(95)90579-0. [DOI] [PubMed] [Google Scholar]