Abstract
This case series study assesses whether a fourth dose of a SARS-CoV-2 messenger RNA (mRNA)–based vaccine is associated with improved anti–SARS-CoV-2 antibody response in solid organ transplant recipients in France.
Introduction
Anti–SARS-CoV-2 antibodies have been detected in up to approximately 70% of solid organ transplant recipients who were given 3 doses of the SARS-CoV-2 vaccine.1,2 In France, it has been allowed to offer a fourth dose on a case-by-case basis.3 We assessed whether a fourth dose of the SARS-CoV-2 vaccine is associated with improved anti–SARS-CoV-2 antibody concentrations in solid organ transplant recipients in France.
Methods
This case series study was conducted from July 1, 2021, to August 5, 2021. A fourth dose of the messenger RNA-based BNT162b2 vaccine (Pfizer-BioNTech) was given to the 37 solid organ transplant recipients, including 5 (13.5%) who had a weak response to the previous 3 doses (antibody concentration <14 binding antibody units [BAU]/mL)4 and 31 (83.8%) who had no response to the 3 previous doses. All participants provided oral informed consent and received approval of the medical staff (Table). According to French law (Loi Jardé), anonymous retrospective studies do not require institutional review board approval. This study followed the reporting guideline for case series.
Table. Clinical and Biological Characteristics of Solid Organ Transplant Recipients According to Humoral Response 1 Month After 3 Doses of mRNA-Based Vaccine.
| Characteristic | All patients (N = 37) | Patients seronegative before dose 4a | P value | |
|---|---|---|---|---|
| Remained seronegative (n = 19) | Became seropositive (n = 13) | |||
| Gender, No. (%) | ||||
| Male | 20 (54.0) | 12 (63.2) | 6 (46.2) | .26 |
| Female | 17 (46.0) | 5 (26.3) | 7 (53.8) | |
| Age, mean (SEM), y | 60 (14) | 58 (16) | 60 (14) | .76 |
| Type of organ transplant, No. (%) | ||||
| Kidney | 25 (67.6) | 11 (57.9) | 13 (100) | .03 |
| Heart | 5 (13.5) | 4 (21.1) | 0 | |
| Liver | 4 (10.8) | 4 (21.1) | 0 | |
| Pancreas | 3 (8.1) | 0 | 0 | |
| Rejection in the year before vaccination, No. | 0 | 0 | 0 | NA |
| Time between vaccine and transplant, mean (SD), mo | 109 (84) | 79 (66) | 161 (97) | .007 |
| Induction therapy, No. (%) | ||||
| No | 12 (32.4) | 9 (47.4) | 2 (15.4) | .12 |
| Yes | 25 (67.6) | 10 (52.6) | 11 (84.6) | .12 |
| Anti–IL-2 receptor blockers | 15 (60.0) | 6 (60.0) | 6 (54.5) | .99 |
| Polyclonal antibodies | 10 (40.0) | 4 (40.0) | 5 (45.5) | .99 |
| Type of immunosuppressive regimen, No. (%) | ||||
| Calcineurin-inhibitors | 32 (86.5) | 17 (89.5) | 10 (76.9) | .37 |
| Tacrolimus | 30 (93.8) | 17 (100) | 8 (80.0) | .13 |
| Ciclosporin A | 2 (6.3) | 0 (0) | 2 (20.0) | .13 |
| Mycophenolic acid | 32 (86.5) | 15 (78.9) | 12 (92.3) | .62 |
| mTOR inhibitors | 9 (24.3) | 4 (21.1) | 4 (30.8) | .68 |
| Steroids | 34 (91.9) | 17 (89.5) | 12 (92.3) | .99 |
| Count before vaccination, mean (SD), per mm3 | ||||
| Neutrophils | 6413 (1892) | 6763 (2255) | 5714 (1402) | .14 |
| Lymphocytes | 1298 (742) | 1126 (527) | 1609 (906) | .07 |
| CD4+ T cells | 370 (211)b | 351 (197)c | 374 (144)d | .45 |
| CD8+ T cells | 393 (261)b | 452 (271)c | 342 (201)d | .23 |
| CD19+ T cells | 61 (59)b | 74 (71)c | 59 (44)d | .88 |
| NK cells | 202 (115)b | 228 (133)c | 126 (52)d | .11 |
| eGFR before vaccination, mean (SD), mL/min/1.73 m2 | 45 (21) | 42 (20) | 45 (22) | .71 |
| Neutrophil count before dose 4, mean (SD), per mm3 | 5357 (2070) | 5647 (2227) | 6046 (1438) | .57 |
| Lymphocyte count before dose 4, mean (SD), per mm3 | 1193 (711) | 947 (427) | 1431 (866) | .04 |
| eGFR before dose 4, mean (SD), mL/min/1.73 m2 | 45 (20) | 43 (19) | 45 (23) | .81 |
Abbreviations: eGFR, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation); IL-2, interleukin-2; mTOR, mammalian target of rapamycin; NA, not applicable; NK, natural killer.
Comparison between patients who were seronegative before the fourth dose and who either remained seronegative or became seropositive after the fourth dose.
Data are for 26 patients.
Data are for 14 patients.
Data are for 7 patients.
The first 2 doses were given 1 month apart, the third dose was administered a mean (SD) of 57 (17) days after the second dose, and the last dose was given a mean (SD) of 65 (9) days after the third dose. Anti–SARS-CoV-2 spike protein total antibody concentrations were assessed using the Wantaï enzyme-linked immunosorbent assay test. Neutralizing antibody (NAb) titers were assessed using a live virus neutralization assay. Enzyme-linked immunospot assay measuring interferon (IFN)–γ produced by specific SARS-CoV-2 T cells was performed for 14 patients (eMethods in the Supplement). Proportions were compared using the Fisher exact test. Quantitative variables were compared by the Student t test or the Mann-Whitney test. A 2-sided P value <.05 was considered to be statistically significant. All statistical analyses were performed with Prism Software v8.1 (GraphPad).
Results
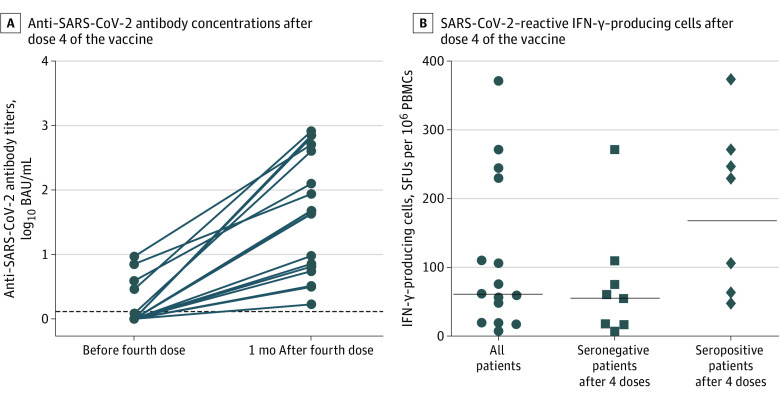
Of 37 patients included in this case series study, 20 (54.0%) were male, with a mean (SEM) age of 60 (14) years. Anti–SARS-CoV-2 antibodies were detected in 5 of 37 patients (13.5%) before dose 4 and in 18 of 37 patients (48.6%) 1 month later (P = .002). Among the 5 patients who were seropositive before dose 4, the median antibody concentration increased from 4 BAU/mL (range, 1-9 BAU/mL) to 402 BAU/mL (range, 87-508 BAU/mL) at 4 weeks after dose 4 (P < .001) (Figure, A). Neutralizing antibody titers increased from a median of 8 IU/mL (range, 2-32 IU/mL) to 16 IU/mL (range, 4-32 IU/mL) (P = .07).
Figure. Anti–SARS-CoV-2 Antibody Concentrations and Number of SARS-CoV-2–Reactive IFN-γ–Producing Cells After a Fourth Dose of the SARS-CoV-2 Messenger RNA (mRNA) BNT162b2 Vaccine.
A, The dotted line represents the cutoff value. B, The numbers of cells reactive to overlapping peptide pools spanning SARS-CoV-2 structural protein S (pools S1 and S2) are shown. The numbers of SARS-CoV-2–reactive IFN-γ–producing cells in the 3 patients who had a low antibody concentration before the fourth dose were 17.5, 47.5, and 110 spot-forming units (SFUs) per 106 peripheral blood mononuclear cells (PBMCs). BAU indicates binding antibody unit.
Among the 31 patients who were seronegative before dose 4, 13 (41.9%) became seropositive (median antibody concentration, 9.5 BAU/mL [range, 1.7-658 BAU/mL]) at 4 weeks after dose 4; 6 patients (19.4%) had antibody concentrations greater than 14 BAU/mL, and 2 (6.5%) had antibody concentrations greater than 140 BAU/mL (Figure, A). Among these patients, the median NAb titer was 8 IU/mL (range, 2-32 IU/mL).
At 4 weeks after dose 4, antibody concentrations were significantly higher among patients who had detectable antibodies before dose 4 than among those who had no response. However, Nab titers at 4 weeks after dose 4 did not differ between responders and nonresponders to 3 doses.
Overall, at 4 weeks after dose 4, 32 of 37 patients (86.5%) had antibody concentrations less than 140 BAU/mL (a threshold providing 12.4% protection among health care workers4) and all 37 patients (100%) had NAb titers less than 64 IU/mL. No breakthrough infection was observed during follow-up.
At 4 weeks after D4, the number of SARS-CoV-2–reactive IFN-γ–producing cells was 61.25 spot-forming units (SFUs) per 106 peripheral blood mononuclear cells (PBMCs) (range, 2.5-372.5 SFUs per 106 PBMCs), with 167.5 SFUs per 106 PBMCs (range, 47.5-372.5 SFUs per 106 PBMCs) among seropositive patients and 55 SFU per 106 PBMC (range, 2.5-110 SFUs per 106 PBMC) among seronegative patients (Figure, B).
No serious adverse event or acute rejection was observed after dose 4. One kidney transplant recipient presented with a recurrence of IgA nephropathy. Four patients presented with fatigue and myalgia. One patient indicated gastrointestinal symptoms.
Discussion
In this case series study, our findings were similar to those of the study by Alejo et al,5 in which a fourth dose of SARS-CoV-2 vaccine was associated with slightly improved humoral response among patients with a weak response after 3 doses and with no improvement among those with no response after 3 doses. Neutralizing antibody titers and cellular response were low in both groups. In a study6 of 22 healthy volunteers (median age, 59 years), among all individuals, antibody concentrations at 1 month after 2 doses of BNT162b2 vaccine were greater than 140 BAU/mL (median, 1309 BAU/mL [range, 457-7605 BAU/mL]) and NAb titers were greater than 64 IU/mL (median 128 [range, 64-512 IU/mL]). In another study of 20 healthy volunteers (median age, 55 years), the number of SARS-CoV-2–reactive IFN-γ–producing cells at 1 month after 2 doses of BNT162b2 vaccine was 542 SFUs per 106 PBMCs (range, 0-1669 SFUs per 106 PBMCs) (Jacques Izopet, Pharm D, PhD, unpublished data). A limitation of our study was the small number of patients. Other strategies should be tested for solid organ transplant recipients.
eMethods.
References
- 1.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661-662. doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244-1246. doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Société Francophone de Transplantation. Renaloo. Accessed July 21, 2021. https://renaloo.com/wp-content/uploads/2021/07/20210717-mailing-SFT-4edose.pdf
- 4.Dimeglio C, Herin F, Martin-Blondel G, Miedougé M, Izopet J. Antibody titers and protection against a SARS-CoV-2 infection. J Infect. Published online September 20, 2021.doi: 10.1016/j.jinf.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021. doi: 10.1097/TP.0000000000003934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimeglio C, Herin F, Da-Silva I, et al. Heterologous ChAdOx1-S/BNT162b2 vaccination: neutralizing antibody response to SARS-CoV-2. Clin Infect Dis. Published online August 12, 2021. doi: 10.1093/cid/ciab705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.