Abstract
Background
Podocytes, functionally specialized and terminally differentiated glomerular visceral epithelial cells, are critical for maintaining the structure and function of the glomerular filtration barrier. Podocyte injury is considered as the most important early event contributing to proteinuric kidney diseases such as obesity-related renal disease, diabetic kidney disease, focal segmental glomerulosclerosis, membranous nephropathy, and minimal change disease. Although considerable advances have been made in the understanding of mechanisms that trigger podocyte injury, cell-specific and effective treatments are not clinically available.
Summary
Emerging evidence has indicated that the disorder of podocyte lipid metabolism is closely associated with various proteinuric kidney diseases. Excessive lipid accumulation in podocytes leads to cellular dysfunction which is defined as lipotoxicity, a phenomenon characterized by mitochondrial oxidative stress, actin cytoskeleton remodeling, insulin resistance, and inflammatory response that can eventually result in podocyte hypertrophy, detachment, and death. In this review, we summarize recent advances in the understanding of lipids in podocyte biological function and the regulatory mechanisms leading to podocyte lipid accumulation in proteinuric kidney disease.
Key Messages
Targeting podocyte lipid metabolism may represent a novel therapeutic strategy for patients with proteinuric kidney disease.
Keywords: Podocyte, Lipid accumulation, Proteinuric kidney disease, Diabetes, Inflammation
Introduction
Proteinuria is not only a typical sign of kidney damage but also importantly contributes to the progression of chronic kidney disease (CKD) such as obesity-related glomerulopathy (ORG), diabetic kidney disease (DKD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), and minimal change disease (MCD) [1]. Podocyte injury is considered as the most important early event in the pathogenesis of proteinuria and progressive glomerulosclerosis in patients with proteinuric kidney diseases [2]. Although considerable advances have been made in the understanding of mechanisms that trigger podocyte injury, cell-specific and effective treatments are not clinically available.
Emerging evidence has revealed that the disorder of lipid metabolism is linked to renal dysfunction, as well as to several other pathological hallmarks of ORG and DKD [3, 4, 5, 6, 7]. In fact, in addition to obesity and diabetes, ectopic lipid accumulation is common in other kinds of proteinuric kidney diseases such as FSGS, MN, and MCD which has been confirmed in numerous experimental animal and clinical studies [8, 9, 10, 11]. And different types of renal parenchymal cells have different sensitivities to pathologic lipid accumulation, which make different contributions to disease progression. Podocytes are vulnerable to lipid accumulation, resulting in their dysfunction which is defined as lipotoxicity, a phenomenon characterized by mitochondrial oxidative stress, actin cytoskeleton remodeling, insulin resistance, and inflammatory response. Importantly, pathologic lipid accumulation in podocytes is the consequence of the dysregulation of genes or proteins involved in cellular lipid metabolism, which comprises synthesis, uptake, storage, utilization, and cellular export of lipids rather than the amount of circulating lipids [12], indicating the importance of podocyte lipid metabolism in proteinuric kidney diseases. An increasing number of evidence suggests that not only the quantity of lipids but also the type of accumulated lipids may be responsible for cellular damage [4]. In this review, we summarize recent advances in the understanding of the lipids in podocyte biological function and the regulatory mechanisms leading to podocyte lipid accumulation in proteinuric kidney disease. Targeting podocyte lipid metabolism will pave the way to new therapeutic approaches in podocyte injury and proteinuric kidney disease.
Lipids in Podocyte Biological Function
Lipids are not only the key structural components of biological membranes but also potent signal transduction molecules, orchestrating intra- and extracellular signal transmission as well as acting as a source of energy [13]. The structure and function of podocytes largely depend on the integrity of slit diaphragm (SD), a lipid raft-like structure. In addition to sphingolipids, lipids especially cholesterol is enriched 5–8-fold in lipid rafts compared with the rest of the plasma membrane and play an important role in assuring proper localization and function of SD proteins including transmembrane proteins (nephrin), integral membrane proteins (podocin), structural proteins (alpha-actinin-4), signaling adapters (CD2-associated protein), ion channels (transient receptor potential cation channel 6) and other proteins involved in cell signaling. Fatty acids are also essential to form the phospholipid bilayers of the cell membranes and act as phospholipid messengers, transmitting vital intracellular signals. Moreover, large quantities of cholesterol esters and triacylglycerol (esterified fatty acid [FA]) forming a neutral lipid core, surrounded by a phospholipid monolayer, can accumulate in intracellular lipid droplets for storage [8].
Although FAs including saturated FAs (SFAs) and unsaturated FAs, together with glucose and amino acids, are generally used as energy sources for the formation of ATP, the peculiarities of podocyte energy metabolism keep controversy [14]. Regarding this issue, Abe et al. [15] first reported a primary role of mitochondria and, in particular, a high dependency of podocytes on mitochondrial energy transduction and on FAs as a metabolic fuel source in transformed mouse podocyte cell lines, while glycolysis makes a lesser contribution. On the contrary, Ozawa et al. [16] suggested that both glycolytic and oxidative phosphorylation (OXPHOS) pathways contribute to podocyte energy supply, depending on the intracellular sub-localization of mitochondria and on the cell differentiation status. Imasawa et al. [17] further recorded that high-glucose conditions force differentiating human podocyte cell lines to switch from OXPHOS to glycolysis, with consecutive lactic acidosis. Recently, Brinkkoetter et al. [18] provided comprehensive metabolomics data from freshly isolated glomeruli and primary podocytes, showing that under physiologic conditions, podocytes largely rely on metabolizing glucose as fuel to lactate (anaerobic glycolysis) and only to a minor extent on β-oxidation of FAs for ATP production. This baseline glucose preference can shift toward an enhanced FAs β-oxidation for metabolic reprogramming [19]. Yuan et al. [20] indicated that differentiated (mature) murine podocytes can promote both glycolysis and mitochondrial metabolism to meet their augmented energy demands. During the differentiation process, the predominant energy source from anaerobic glycolysis in immature podocytes is shifted to OXPHOS. Chen et al. [21] also showed that high glucose induces marked upregulation of lipogenic gene expression and the decrease of FA oxidation (FAO), which results in the ectopic lipid deposition in podocytes. Collectively, accumulating evidence suggests that the metabolic switch from mitochondrial respiration to glycolysis or vice versa in podocytes is not uniform but rather depends on the cell type and cell environmental context.
Podocyte Lipid Accumulation in Proteinuric Kidney Disease
Podocytes are vulnerable to lipid accumulation. Excessive lipid accumulation in podocytes can lead to lipotoxicity characterized by mitochondrial oxidative stress [22], inflammatory responses [23, 24], actin cytoskeleton remodeling, insulin resistance, and endoplasmic reticulum (ER) stress, which eventually trigger podocyte hypertrophy, autophagy, dedifferentiation, mesenchymal transition, detachment to apoptosis, and death [25]. Podocyte injury is considered as a hallmark of proteinuric kidney diseases such as DKD, FSGS, MN, and MCD.
Normally, lipids are classified into 8 different categories including fatty acyls, glycerolipids, glycerophospholipids (GPs), sphingolipids, sterols, prenol lipids, saccharolipids, and polyketides [26]. Some of them that abnormally accumulate in podocytes mainly contribute to proteinuric kidney disease, including (1) fatty acyls: SFAs, monounsaturated FAs (MUFAs), polyunsaturated FAs (PUFAs), and PUFA-derivatives eicosanoids; (2) glycerolipids: monoacylglycerols, diacylglycerols, and triacylglycerols also termed as triglycerides; (3) GPs: phosphatidylcholine (PC), phosphatidylserine, phosphatidylethanolamine (PE), phosphatidylinositol, cardiolipin (CL), phosphatidylglycerol, and phosphatidic acid; (4) sphingolipids: phosphosphingolipids (sphingomyelin), glycosphingolipids (gangliosides, globosides, and cerebrosides), sulfatides, ceramide (-1-phosphate) (C1P), sphingosine (-1-phosphate) (S1P); and (5) sterol lipids: cholesterol and its derivatives cholesterol esters (Table 1).
Table 1.
Lipid and lipid metabolites that contribute to podocyte lipotoxicity
| Class | Model | Outcome | Ref. |
|---|---|---|---|
| Saturated FAs | |||
| Palmitic acid (16:0)* | MPC, DKD mice | ↑ Podocyte injury and apoptosis, mitochondrial dysfunction, oxidative stress, ER stress, insulin resistance; ↑ albuminuria | [27–32] |
| Stearic acid (18:0)* | CKD patients | Decreased abundance in plasma associate with declining renal function | [33] |
| Unsaturated FAs | |||
| Oleic acid (18:1)* | MPC, DKD mice | ↓ Podocytes cell death and loss, ER stress, oxidative stress; ↑ proteasome expression; ↓ albuminuria, histologic changes | [28, 34] |
| Arachidonic acid (20:4)* | HPC | ↑ Actin cytoskeleton remodeling, podocyte injury | [35] |
| Eicosapentaenoic acid (20:5)* | MPC | ↓ Podocyte apoptosis, inflammation | [36] |
| Glycerolipids | |||
| Triglyceride | CKD, metabolic syndrome mice | ↑ Podocyte apoptosis; ↑ albuminuria, histologic changes | [37] |
| Phospholipids | |||
| Phosphatidylcholine | MPC, DKD mice | Associate with podocyte injury and DKD | [38, 39] |
| Phosphatidylethanolamine | MPC, DKD mice | Associate with podocyte injury and DKD | [38, 39] |
| Cardiolipin | HPC, DKD mice | ↑ Podocyte injury, mitochondrial dysfunction; ↑ albuminuria, histologic changes | [25] |
| Phosphosphingolipids | |||
| Sphingomyelin | DKD mice | ↑ Actin cytoskeleton remodeling, podocyte injury; ↑ albuminuria, histologic changes | [40] |
| Glycosphingolipids | |||
| Ganglioside | HPC, MPC, DKD mice | ↑ Actin cytoskeleton remodeling, podocyte injury; ↑ albuminuria, histologic changes | [41, 42, 43] |
| Other sphingolipids | |||
| Ceramide | DKD mice | ↑ Mitochondrial dysfunction, inflammation, insulin resistance, podocyte injury | [42, 44, 45] |
| Sphingosine | Primary MPC, podocytopathy and associated NS mice (Asah1fl/fl/Podo-Cre mice) | ↓ Exosome release of podocytes, podocyte injury; ↓ urinary exosome excretion | [46, 47] |
| Sphingosine-1-phosphate | CKD mice | ↑ Albuminuria, histological changes; ↑ oxidative stress, mitochondrial dysfunction | [46, 48] |
| Ceramide-1-phosphate | HPC, DKD mice | ↓ Inflammation, insulin resistance, podocyte apoptosis; ↓ albuminuria, histologic changes | [25, 40] |
| Sterol lipid | |||
| Cholesterol | HPC, HN mice | ↑ Podocyte apoptosis; ↑ albuminuria, histologic changes | [49, 50] |
| Cholesterol esters | HPC, DKD mice, AS mice | ↑ Podocyte injury; ↑ albuminuria, histologic changes | [51] |
Ref., reference; MPC, mouse podocytes; DKD, diabetic kidney disease; CKD, chronic kidney disease; ER, endoplasmic reticulum; HPC, human podocytes; NS, nephrotic syndrome; KO, knockout; Podo, podocyte; HN, hypertensive nephropathy; AS, Alport syndrome. * Number of carbon atoms: number of C = C double bonds.
Free Cholesterol and Cholesterol Ester
Cellular cholesterol homeostasis is highly regulated by cholesterol synthesis at the ER membrane, cholesterol uptake, and efflux (Fig. 1), all processes are tightly regulated and adapted to cellular needs. However, excess cholesterol accumulation in podocytes can perturb the SD, adversely affect podocyte function, and induce proteinuric kidney diseases [52, 53, 54].
Fig. 1.
Metabolism of cholesterol and FFAs and their regulatory mechanisms in podocytes. a Circulating LDL is the major source for cholesterol uptake via LDLR or CXCL16. LDL, and its receptor complexes are internalized by endocytosis and transport to the lysosome for degradation, resulting in the release of free cholesterol. NPC1/2 transports free cholesterol from lysosomes to the ER and then to the plasma membrane. The efflux of free cholesterol to HDL acceptors is mediated by ABCA1 and ABCG1/8 in the presence of extracellular APO proteins. Also, free cholesterol delivered into ER combines with FFAs to form cholesterol ester via SOAT1. At last, the cholesterol esters will be involved in the lipid droplets for storage. Cholesterol ester can be converted back to free cholesterol via NCEH. Cholesterol synthesis is primarily controlled by HMGCR. During cholesterol deficits, SREBP is transported to the Golgi apparatus and cleaved, allowing its translocation to the nucleus to regulate expression of cholesterol genes. Cholesterol synthesis is also regulated by other nuclear receptors and transcription factors such as LXR, FXR, PPARs, and NFAT. PTEN interferes with the endocytosis of LDL and SIRT6 contributes the export of free cholesterol by ABCG1. SIRT1, which is downregulated by JAML, inhibits the activity of SREBP1 and its target genes. b FFAs enter cells by endocytosis and transport via CD36, FATP, and FABP. Cellular FFAs are converted to fatty acyl-CoA which is transported into mitochondria via CPT1 and converted into acetyl-CoA by β-oxidation. In addition, acetyl-CoA is also derived from glucose uptake through GLUT4. Glucose is converted to pyruvate, which enters the mitochondria to form acetyl-CoA. Excess acetyl-CoA entering cytoplasm can be converted to PA by ACC and FASN activity, then extended to form SA in the ER. FFAs are commonly esterified to form TG, PL, and CE. SCD-1 converts SFAs to MUFAs that are incorporated into TG and stored in lipid droplet. DGAT is a microsomal enzyme that catalyzes the final step in TG synthesis. FFAs synthesis is controlled by SREBP-1c. Besides, ChREBP, LXR, FXR, and PPARs are also important nuclear receptors and transcription factors involving in fat synthesis and metabolism. Furthermore, VEGF-B can induce the expression of FATP, increasing FFAs uptake. SIRT3 deletion aggravates FAO dysfunction. AMPK plays a central role in controlling FFAs metabolism through modulating the downstream ACC and CPT1. JAML deficiency decreases the expression of SREBP1 and its target genes through SIRT1 activation. LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; CXCL16, c-x-c motif ligand 16; NPC1/2, Niemann-Pick C1 and C2; ER, endoplasmic reticulum; HDL, high-density lipoprotein; ABCA1, ATP-binding cassette transporters subfamily A member 1; ABCG1/8, ATP-binding cassette transporters subfamily G member 1 or 8; APO, apolipoproteins; FFAs, free fatty acids; SOAT, sterol O-acyltransferase; NCEH, neutral cholesterol esterhydrolase; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; SREBP, sterol-regulatory element binding protein; LXR, liver X receptor; FXR, farsenoid X receptor; PPARs, peroxisome proliferators-activated receptors; NFAT, nuclear factor of activated T cells; PTEN, phosphatase and tensin homolog; SIRT, NAD-dependent protein deacetylase sirtuin; JAML, junctional adhesion molecule-like protein; CD36, scavenger receptor B2; FATP, fatty acid transport protein; FABP, fatty acid binding proteins; CPT1, carnitine O-palmitoyltransferase 1; GLUT4, glucose transporter type 4; TCA, tricarboxylic acid; PA, palmitic acid; ACC, acetyl-CoA carboxylase; FASN, fatty acid synthase; SA, stearic acid; PL, phospholipid; TG, triglycerides; CE, cholesterol ester; SCD-1, stearoyl-CoA desaturase 1; SFAs, saturated fatty acids; MUFA, monounsaturated fatty acid; DGAT, diacylglycerol O-acyltransferases; ChREBP, carbohydrate-responsive element-binding protein; VEGF-B, vascular endothelial growth factor B; FAO, fatty acid oxidation; AMPK, adenosine 5′-monophosphate-activated protein kinase.
In podocytes, circulating unoxidized or oxidized low-density lipoprotein (LDL) is the major source for cholesterol uptake into cells via the LDL receptor or c-x-c motif ligand 16, a scavenger receptor [55]. LDL and its receptor complexes are internalized by endocytosis and transport to the lysosome for degradation, resulting in the release of free cholesterol. The proteins Niemann-Pick C1 and C2 have major roles in the transport of free cholesterol from lysosomes to the ER and then to the plasma membrane. It is reported that the downregulation of phosphatase and tensin homolog in podocytes causes the inactivation of the actin depolymerizing factor cofilin-1 by increasing its phosphorylation. This response stimulates the formation of filamentous actin and promotes cholesterol endocytosis, resulting in cholesterol accumulation in podocytes [56].
The efflux of free cholesterol to high-density lipoprotein (HDL) acceptors is mediated by ATP-binding cassette (ABC) transporters such as ABC subfamily A member 1 (ABCA1) and subfamily G member 1 or 8 (ABCG1/8) in the presence of extracellular lipid-poor apolipoproteins (APO proteins) [8, 53]. The increase in cellular free cholesterol is converted to cholesterol ester via ER enzyme sterol-O-acetyltransferase-1 (SOAT1) leading to the formation of cholesterol-enriched lipid droplets. Cholesterol ester can be converted back to free cholesterol via neutral cholesterol esterhydrolase 1 (NCEH).
The accumulation of excess free cholesterol has been demonstrated to be toxic to cells. APOL1, an essential component of HDL3 and highly expressed in podocytes, is the highest risk genetic variant of kidney disease and the main cause of glomerulosclerosis in the African-American population [57]. Human podocytes treated with the sera from the patients with DKD showed increased cholesterol accumulation in association with a reduction of ABCA1 [58]. Pedigo et al. [49] demonstrated that local TNF expression causes free cholesterol-dependent podocyte apoptosis in FSGS and DKD through a dual mechanism that requires a reduction in ABCA1-mediated cholesterol efflux and decreased cholesterol esterification by SOAT1 activity. Consistently, cholesterol accumulation was detected in podocytes from angiotensin II-infused mice, which was associated with ABCG1-mediated cholesterol efflux by epigenetic modification [50] (Table 1). However, some controversial studies suggested that free cholesterol is not cytotoxic to podocytes. They found that in diabetic ob/ob mice, podocyte-specific deletion of Abca1 enhances podocyte injury which is not attributed to an accumulation of free cholesterol but to the mitochondrial membrane phospholipid CL accumulation. That is because an additional genetic deletion of Soat1 that prevents cholesterol esterification had no further detrimental effect on glomerular injury [25].
It is known that cholesterol esterification and storage in lipid droplets are essential to maintain proper levels of free cholesterol. However, whether cholesterol ester accumulation is toxic and contributes to renal disease is still unclear [13]. Recent studies showed that blocking cholesterol esterification via SOAT1 inhibition in podocytes attenuates lipotoxicity-induced podocyte injury in DKD and Alport's syndrome in association with ABCA1-mediated cholesterol efflux [51].
Cholesterol synthesis is primarily controlled by the rate-limiting enzyme, 3-hydroxy-3-methyl-glutaryl-CoA reductase. The expression of genes encoding enzymes such as 3-hydroxy-3-methyl-glutaryl-CoA reductase and SOAT1 is regulated by members of the sterol-regulatory element-binding protein (SREBP) family of transcription factors. In addition, other nuclear receptors and transcription factors such as liver X receptor (LXR), farsenoid X receptor (FXR), peroxisome proliferators-activated receptors (PPARs), and nuclear factor of activated T cells are also involved in cholesterol synthesis and metabolism. Recently, our studies found that junctional adhesion molecule (JAM)-like protein (JAML) (Amica1), a novel member of JAM family, mediates podocyte lipid metabolism by regulating SREBP1 and its target genes involved in FAs and cholesterol synthesis. Mechanistically, histone deacetylase-mediated epigenetic processes in the regulation of lipid metabolism have attracted much attention. SIRT1 is one member of SIRTs (class III histone deacetylase) that are highly conserved NAD+-dependent deacetylases. SIRT1 not only serves as an important energy status sensor but also protects cells against metabolic stresses. In our studies, we further demonstrated SIRT1 links JAML to SREBP1 signaling, which is consistent with the previous studies, showing that SIRT1 can regulate SREBP-1 expression and activity [38].
Saturated Fatty Acids and Triglycerides
Cellular FA homeostasis is regulated by synthesis, uptake, storage, and β-oxidation. Numerous studies have indicated that excessive free FA (FFA) accumulation plays a key role in podocyte injury and proteinuria [59]. FFA and triglyceride syntheses are controlled by SREBP-1c which targets lipogenic enzymes including acetyl-CoA carboxylase (ACC) and FA synthase (FASN). Furthermore, carbohydrate response element-binding protein (ChREBP) is an important transcription factor for cellular fat synthesis. ChREBP deficiency alleviated diabetes-associated renal lipid accumulation by inhibiting mTORC1 activity, suggesting that the reduction of ChREBP is a potential therapeutic strategy to treat DKD [21]. In addition, other nuclear transcription factor receptors including LXR, FXR, and PPARs are also involved in FA synthesis and metabolism.
Disturbed transport and oxidation of FFAs, paralleled by an impaired antioxidant response, damages podocyte structure and leads to glomerulopathy during the early stage of DKD. FFAs can be transported into cells by CD36 (also known as scavenger receptor B2) [60], FA transport protein (FATP), FA-binding proteins (FABP), or via the assistance of vascular endothelial growth factor B (VEGF-B) [39]. CD36 is the main receptor for FFAs uptake in podocytes. CD36-dependent uptake of palmitic acid led to a dose-dependent increase in the levels of mitochondrial reactive oxygen species (ROS), depolarization of mitochondria, ATP depletion, and apoptosis [27, 60]. Podocyte-specific expression of FABP correlates with proteinuria in diabetic db/db mice and enhances FFAs induced podocyte injury [61, 62]. Falkevall et al. [39] suggested that VEGF-B promotes FFA accumulation in the glomeruli of DKD mice via upregulation of FATP4. Inhibiting VEGF-B signaling in DKD mouse models reduces renal lipotoxicity and re-sensitizes podocytes to insulin signaling.
The SFAs (also called nonesterified FA, NEFA) including palmitic acid and stearic acid, together with the MUFAs such as oleic acid account for 70–80% of plasma FFAs [63]. Podocytes are highly susceptible to damage from SFAs [27, 28, 29, 30, 31, 32, 33]. By contrast, MUFAs can attenuate palmitic acid-induced lipotoxicity [28, 34]. Stearoyl-CoA desaturase (SCD)-1, which converts SFAs to MUFAs, is upregulated in podocytes in biopsy samples from patients with DKD and ameliorates ER stress and podocyte injury [64]. PUFA such as arachidonic acid, as a major metabolite of phospholipase A2 group IB, regulates the actin bungling remodeling and contributes to the podocyte injury [35]. However, eicosapentaenoic acid and docosahexaenoic acid help lag the progression of CKD [36].
Cellular FFAs are commonly esterified to form 3 main classes of esters: triglycerides, phospholipids, and cholesterol esters or transported into mitochondria for β-oxidation and ATP production. Uptake of triglycerides-rich very low-density lipoprotein (VLDL) by podocytes is increased in CKD. Increased triglyceride accumulation leads to podocyte apoptosis and glomerulosclerosis [37]. Diacylglycerol O-acyltransferase (DGAT) is a microsomal enzyme that catalyzes the final step in triglycerides synthesis. Nephrotic syndrome has been reported to cause the upregulation of hepatic DGAT-1 expression and activity, which may lead to associated hypertriglyceridemia [65]. Kampe et al. [29] have found that inhibition of carnitine palmitoyltransferase (CPT1), the rate-limiting enzyme of FAO and downstream target of AMP-activated protein kinase (AMPK), augments palmitic acid-induced podocyte death. Moreover, SIRT3 deletion aggravates FAO dysfunction, resulting in increased apoptosis of kidney tissues and aggravated renal injury [66]. In our studies, we found JAML deficiency has lower neutral lipid deposition in glomeruli from mice with DKD and in high glucose-treated podocytes, which is result from the decrease in the expression of transcriptional factor SREBP1 and its targets, such as FASN, ACC1, and SCD1. In this process, we showed that SIRT1 activation correlates with the increase in AMPK activity and the reduction of SREBP1 [38].
Glycerophospholipids and Sphingolipids
More recently, GPs and sphingolipids have also been implicated in the pathogenesis of glomerular diseases [38, 39, 41, 46, 67] (Table 1). A specific modification of GPs is shown to correlate with kidney deterioration in diabetic subjects and mice [68]. Ducasa et al. [25] found that podocyte-specific Abca1 deficiency leads to the accumulation of mitochondrial CL, which promotes podocyte injury in DKD. Pharmacologic induction of ABCA1 ameliorated podocyte injury and decreased CL oxidation [25].
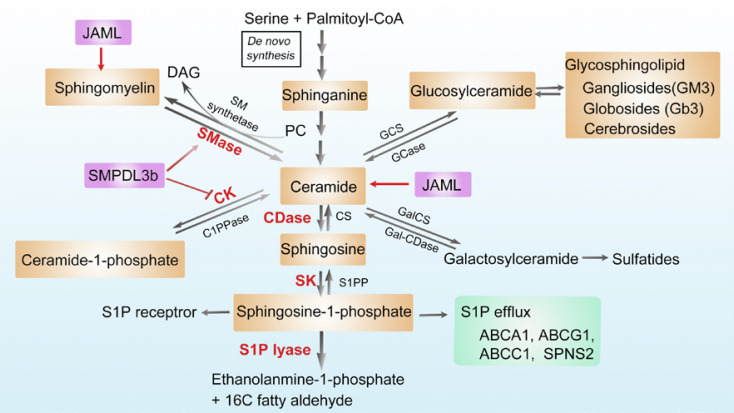
Moreover, intracellular accumulation of SLs or metabolites in the form of ceramide, sphingosine, S1P, sphinganine, sphingomyelin, C1P, and glycosphingolipids (gangliosides GM3 and cerebrosides) in podocytes may contribute to cellular dysfunction and the progression of proteinuric kidney disease [41, 46, 47] (Fig. 2). Ceramide represents the centerpiece of the sphingolipid metabolic pathway. Ceramide accumulation in podocytes induces mitochondrial damage through ROS production in patients with DKD [44]. Podocyte-specific deletion of the main catalytic subunit of acid ceramidase resulted in ceramide accumulation in glomeruli and development of nephrotic syndrome [45]. Acid sphingomyelinase overexpression also leads to ceramide accumulation and glomerular sclerosis in mice [42].
Fig. 2.
Metabolism of sphingolipids and their regulatory mechanisms in podocytes. Ceramide is at the center of sphingolipid metabolism and can be generated through multiple pathways, including de novo synthesis, hydrolysis of sphingomyelin, or the recycling of other complex sphingolipids. First, in the de novo synthesis pathway, ceramide is formed from the condensation of L-serine and palmitoyl-CoA on the surface of the ER. Second, sphingomyelin can be hydrolyzed by sphingomyelinase to generate ceramide. SM synthetase can attach a PC head group onto ceramide to form sphingomyelin. Third, ceramide can be phosphorylated via CK to form ceramide-1-phosphate, another bioactive lipid. Ceramide, in turn, can be generated from ceramide-1-phosphate through the action of C1PPase. Fourth, ceramide can be further catabolized by CDase to sphingosine, which is then phosphorylated to S1P by SK. The efflux of S1P can be mediated by its transporters such as SPNS2, ABCA1, ABCG1, and ABCC1. Circulating S1P can bind to various S1P receptors and modulate MAPK signaling and small GTPase activity. Sphingosine can be recycled back for the generation of ceramide by CS, and S1P can either be dephosphorylated by S1PP or be irreversible cleaved by S1P lyase to form ethanolamine-1-phosphate and C16 fatty aldehyde. Fifth, ceramide can also be glycosylated to form the glucosylceramide or galactosylceramide, which can be converted back to ceramide by hydrolyzation. Glucosylceramide can be further converted into complex glycosphingolipids, namely gangliosides (GM3), globosides (Gb3), and cerebrosides. Overexpression of SMPDL3b blocks CK activity and the conversion of ceramide to C1P, leading to the decrease in the levels of C1P. SMPDL3b is also a protein with homology to ASMase. Decreased expression of SMPDL3b may lead to decreased ASMase activity and accumulation of sphingomyelin. JAML can enhance high glucose induced the acumulation of ceramides and sphingomyelin. ER, endoplasmic reticulum; SM, sphingomyelin; SMase, sphingomyelinase; PC, phosphatidylcholine; DAG, diacylglycerol; C1PPase, ceramide-1-phosphate phosphatase; CK, ceramide kinase; CDase, ceramidase; SPNS2, Sphingolipid Transporter 2; ABCA1, ATP-binding cassette transporters subfamily A member 1; ABCG1, ATP-binding cassette transporters subfamily G member 1; ABCC1, ATP-binding cassette transporters subfamily C member 1; MAPK, mitogen-activated protein kinase; GTPase, guanosine triphosphate phosphatase; CS, ceramide synthase; SK, sphingosine kinase; S1PP, spingosine-1-phosphate phosphatase; GCS, glucosylceramide synthase; GCase, glycosylceramidase; GalCS, galactosylceramide synthase; Gal-CDase, galactosylceramidase; SMPDL3b, sphingomyelinase-like phosphodiesterase 3b; JAML, junctional adhesion molecule-like protein; ASMase, acid sphingomyelinase.
Ceramide can be further catabolized to sphingosine by ceramidases. Then, sphingosine is phosphorylated to S1P by sphingosine kinase. In addition, ceramide kinase catalyzes the formation of another bioactive lipid, C1P. Studies have showed that increased expression of S1P is associated with increased ROS production and renal injury in CKD [48]. S1P lyase is reported to play a role in the development of proteinuria in mice [69]. Genetic mutations in the gene coding for S1P lyase are associated with severe podocyte injury and nephrotic syndrome in humans [70, 71]. Moreover, diabetic db/db mice have less total level of C1P in kidney cortices. Consistently, exogenous administration of C1P protects from DKD progression [40, 72]. Falkevall et al. [39] demonstrated that reducing VEGF-B signaling in db/db mice prevents renal lipotoxicity by reducing the accumulation of neutral lipid, GPs, and sphingolipids. Our recent studies have also indicated that JAML not only enhances high glucose-induced the accumulation of FFAs and cholesterol ester but also GPs (such as PC and phosphatidylethanolamine), sphingolipids (such as ceramides), which contributes to podocyte injury by lipidomics analysis. However, the mechanisms by which JAML regulates GPs and sphingolipids need to be further investigated [38].
In addition, other sphingolipids such as glycosphingolipid, especially gangliosides, also contribute to DKD development. Gangliosides GM3, GD3, and O-acetylated disialosyllactosylceramide are the most abundant gangliosides in the kidney. 9-O-acetylated disialosyllactosylceramide is a podocyte-specific ganglioside [41, 42]. In podocytes, GM3 located in lipid raft domains of the SD, binding with the soluble vascular endothelial growth factor receptor, is of very importance to the maintenance of actin cytoskeleton and the prevention of proteinuria [43]. Sphingomyelinase-like phosphodiesterase 3b (SMPDL3b), a lipid raft enzyme, was previously reported to modulate podocyte injury in the context of DKD or FSGS [73]. Studies have indicated that SMPDL3b-positive podocytes are decreased in renal biopsies of patients with recurrence of FSGS. It was found that decreased expression of SMPDL3b leads to decreased acid-sphingomyelinase activity and the accumulation of sphingomyelin, contributing to the pathogenesis of FSGS. In addition, human podocytes treated with the sera from patients with FSGS had decreased SMPDL3b, which was associated with actin cytoskeleton remodeling and apoptosis [74]. However, unlike FSGS, SMPDL3b expression was upregulated in glomeruli from patients with DKD and in human podocytes treated with DKD sera. Notably, overexpression of SMPDL3b resulted in decreased C1P levels in human podocytes in vitro and in the kidney cortex of mice with DKD in vivo [40]. Podocyte-specific Smpdl3b-deficiency had restored levels of C1P in the kidney cortex and protected from DKD. These results indicate that SMPDL3b may play different roles in the different pathogenesis of kidney disease.
LDs That Store Intracellular Cholesterol Esters and Triglycerides
Lipid droplets (LDs) are storage organelles consisting of a neutral lipid core (cholesterol ester and triglyceride) surrounded by a phospholipid monolayer and a set of LD-specific proteins. Whether LDs exert cytoprotective or cytotoxic effects in podocytes remains unknown. They are commonly considered a cytoprotective mechanism [13]. Some studies showed that they directly contact other organelles such as mitochondria, endosomes, Golgi complex, and peroxisomes, which are critical to buffer the levels of toxic lipid species [75]. In addition, LDs can act as a protective reservoir for unfolded proteins and toxins by preventing interactions with other cellular compartments [76]. Also, they have the potential to reduce podocyte toxicity, autophagic flux, and cell death by scavenging and storing the disease-associated APOL1 risk variants G1 and G2 [77]. Furthermore, LDs protect mitochondria by sequestering FAs and thus prevent an abnormal flux of FAs into acylcarnitine, which at high levels is toxic to mitochondria [78]. Recently, they have been shown to be innate immune hubs that integrate cell metabolism and host defense, highlighting a positive role of intracellular lipids as key regulators of cell function and survival [79]. However, some studies observed that LDs accumulate in glomeruli of renal biopsy samples from diabetic patients, which coincides with the presence of oxidative stress markers [80]. Therefore, LDs are very active scavenging organelles that can coordinate the function of other organelles in response to stress by modulating storage and lipolysis across different subcellular compartments [81].
Therapeutic Strategies
Statins
Statins are a group of drugs (such as lovastatin and simvastatin) that inhibit the synthesis of cholesterol and promote the production of LDL-binding receptors in the liver resulting in a usually marked decrease in the level of LDL and a modest increase in the level of HDL circulating in blood plasma. A recent meta-analysis demonstrated that although statins reduces proteinuria and mortality, this effect was not sufficient to slow the clinical progression of non-end-stage CKD [82]. However, all available clinical guides suggest that controlling LDL cholesterol is a part of the multi-target approach in the treatment of DKD [83, 84]. Therefore, other therapies are needed to lower cellular toxic lipid levels (Table 2).
Table 2.
Therapeutic strategies
| Agent | Category | Pathway | Disease model | Outcome | Ref. |
|---|---|---|---|---|---|
| Statin | Lipid-lowering drug | HMG-CoA reductase | CKD | ↓ Proteinuria, histologic changes, oxidative stress/apoptosis, and podocyte injury | [82, 84] |
|
| |||||
| Fenofibrate | Fibrate | PPARa agonist | HFD | ↓ Albuminuria, histologic changes, oxidative stress/fibrosis, and lipid accumulation | [85] |
|
| |||||
| Fenofibrate | Fibrate | PPARa agonist/AMPK-PGC-1-axis | db/db mice | ↓ Albuminuria, histologic changes, inflammation/oxidative stress, apoptosis/fibrosis, and lipid accumulation | [86] |
|
| |||||
| AdipoRon | Adiponectin receptor agonist | AMPK/PPARa pathway | db/db mice | ↓ UACR, oxidative stress/apoptosis/fibrosis, and lipid accumulation | [7, 87] |
|
| |||||
| JNJ 39933673 | SGLT2 inhibitor | ChREBP-β, SREBP-1 | db/db mice | ↓ UACR, histological changes, inflammation/fibrosis, lipid accumulation, and podocyte injury | [88] |
|
| |||||
| Dapagliflozin | SGLT2 inhibitor | SREBP-1c | WD-induced obesity mice | ↓ UACR, histologic changes, fibrosis, lipid accumulation, and podocyte injury | [88–90] |
|
| |||||
| Obeticholic acid (INT-747) | FXR agonist | Glutathione metabolism pathway | HFD + uninephrectomy | ↓ UACR, histologic changes, oxidative stress/apoptosis, and lipid accumulation | [91] |
|
| |||||
| INT-777 | Selective TGR5 agonist | TGR5 | db/db mice, DIO mice | ↓ Proteinuria, histologic changes, inflammation/fibrosis, FA accumulation, podocyte injury | [92] |
|
| |||||
| INT-767 | FXR/TGR5 dual agonist | SREBP-1 pathway; TGF-β pathway | db/db mice, STZ-treated mice and DIO mice | ↓ Proteinuria, histologic changes, fibrosis, lipid accumulation, and podocyte injury | [93] |
|
| |||||
| Neutralizing monoclonal VEGF-B antibody | VEGF-B antagonism | VEGF-B signaling | Podo-VegfB KO; db/db mice; double KO; HFD or STZ-treated mice | ↓ UACR, histologic changes, inflammation, lipid accumulation, and podocyte injury | [39] |
|
| |||||
| Cyclosporin A2/2-hydroxypropyl-β-cyclodextrin | Calcineurin inhibitor/cholesterol chelator | TNF/NFAT/ABCA1/SOAT1 signaling | Podo-Abca1 KO, double, triple, and inducible KO mice | ↓ UACR, histologic changes, inflammation/oxidative stress/apoptosis, cholesterol accumulation, and podocyte injury | [49, 58] |
|
| |||||
| A30/elamipretide | ABCA1 inductor/cardiolipin peroxidase inhibitor | Mitochondrial dysfunction pathway | Podo-Abca1 KO; Soat KO, db/db and BTBR ob/ob mice | ↓ UACR, histologic changes, oxidative stress/mitochondrial dysfunction, lipid accumulation, and podocyte injury | [25] |
|
| |||||
| Sandoz 58-035 | SOAT1 inhibitor | ABCA1/SOAT1 signaling | db/db Soat KO mice; Col4a3 KO (AS) mice | ↓ UACR, histologic changes, cholesterol accumulation, and podocyte injury | [51] |
|
| |||||
| Ceramide-1-phosphate | Lipid supplementation | SMPDL3b/C1P/IR/Cav-1/Akt signaling | Podo-Smpdl3 KO, double KO, and db/db mice | ↓ UACR, histologicchanges, lipid accumulation, podocyte injury | [40] |
|
| |||||
| Rituximab | CD20 monoclonal antibody | SMPDL3b, ASMase | Patients with recurrent FSGS, human podocytes treated with serum from patients | ↓ UACR, histologic changes, lipid accumulation, and podocyte injury | [74] |
|
| |||||
| Berberine | Flavonoid | Drp1 | db/db mice | ↓ UACR, histologic changes, oxidative stress, mitochondrial dysfunction, lipid accumulation, and podocyte injury | [32] |
Ref., reference; HMG-CoA, β-hydroxy-β-methylglutaryl-coenzyme A; CKD, chronic kidney disease; PPAR, peroxisome proliferator-activated receptor; HFD, high-fat diet; AMPK, AMP-activated protein kinase; PGC-1, peroxisome proliferator activated receptor-gamma coactivator-1; db/db, leptin receptor-deficient; UACR, urinary albumin creatinine ratio; SGLT2, sodium-glucose cotransporter 2; ChREBP, carbohydrate-responsive element-binding protein; SREBP, sterol regulatory element-binding protein; WD, western diet; FXR, farnesoid X receptor; TGR5, G protein-coupled bile acid receptor; DOI mice, diet-induced obesity mice; TGF-β, transforming growth factor beta; STZ, streptozotocin; Podo, podocyte; VEGF-B, vascular endothelial growth factor B; SOAT1, sterol O-acyltransferase 1; KO, knockout; TNF, tumor necrosis factor; NFAT, nuclear factor of activated T-cells; SOAT1, sterol O-acyltransferase 1; ABCA1, ATP, binding cassette A1; AS, Alport syndrome; BTBR, black tan and brachyury; ob/ob, leptin-deficient; SMPDL3b, sphingomyelin phosphodiesterase acid-like 3b; C1P, ceramide-1-phosphate; IR, insulin receptor; Cav-1, caveolin-1; Akt, protein kinase B; ASMase, acid-sphingomyelinase; FSGS, focal segmental glomerulosclerosis; Drp1, dynamin-related protein 1.
PPAR Agonists
PPAR α/γ agonists are recognized as a potential therapy to treat renal lipotoxicity and DKD [94]. For example, fenofibrate, a potent PPARα agonist, improves albuminuria, inhibits intrarenal lipid accumulation, and prevents apoptosis and oxidative stress [86]. In mice fed with high-fat diet, fenofibrate can reduce oxidative stress and lipid accumulation in glomeruli and prevent the development of albuminuria and glomerular injury [85].
Adiponectin Receptor Agonists
Adiponectin exerts favorable effects in diabetes mellitus and metabolic syndrome through its anti-inflammatory, antifibrotic, and antioxidant effects. It mediates FA metabolism by inducing AMPK phosphorylation and increasing PPARα expression through binding to its receptors, adipoR1, and adipoR2, respectively, which in turn activates PPARγ coactivator 1α. Moreover, adiponectin potently stimulates ceramidase activity associated with its 2 receptors and enhances ceramide catabolism and the formation of its antiapoptotic metabolite, S1P [95]. Recent studies have shown adipoRon, an orally active synthetic adiponectin receptor agonist, is able to reduce lipotoxicity and podocyte injury by activating the Ca2+/liver kinase B1-AMPK/PPARα pathway in type 2 diabetes-associated DKD [7]. In addition, adipoRon can lower cellular ceramide levels by activation of acid ceramidase, which hydrolyzes ceramide to form sphingosine leading to an increase in S1P, and finally decrease renal potoxicity, inflammation, and insulin resistance in diabetic mice [87].
Sodium-Glucose Cotransporter-2 Inhibitors
The sodium-glucose cotransporters (SGLTs) are a family of glucose transporters that contribute to renal glucose reabsorption. Studies from SGLT1 and SGLT2 knockout mice have indicated that 97% of proximal tubular glucose transport is mediated via SGLT2 and only 3% by SGLT1 [96]. SGLT2 inhibitors are a new class of antidiabetic drugs with promising effects on the treatment of patients with DKD [97] or nondiabetic CKD [89]. Through glucosuria, SGLT2 inhibitors reduce body weight and body fat and shift substrate utilization from carbohydrates to lipids and, possibly, ketone bodies. It has been reported that in db/db mice, JNJ 39933673, a selective SGLT2 inhibitor prevented the development of DKD and modulated renal lipid metabolism, at least in part through inhibition of the transcriptional factor ChREBP-β and SREBP1. Subsequently, their target genes pyruvate kinase, SCD-1, and DGAT1 expression were decreased [88]. Moreover, dapagliflozin, a highly selective inhibitor of renal SGLT2, prevented podocyte injury, glomerular pathology, and renal fibrosis in the kidney from Western diet-fed mice by reducing renal lipid accumulation [90].
Bile Acid Receptors Agonists
Bile acids, as signaling molecules, can also regulate glucose and lipid homeostasis as well as energy expenditure by activating bile acids receptors. Two major receptors for bile acids have been identified as follows: the nuclear receptor FXR and the membrane-bound, bile acid-activated G protein-coupled receptor TGR5 (GPBAR1 or GPR131). Previous studies indicated that FXR agonist obeticholic acid (INT-747) attenuated renal injury, renal lipid accumulation, and lipid peroxidation in uninephrectomized obese mice [91]. Moreover, TGR5 was downregulated by high glucose and/or FAs in ORG and DKD. A selective TGR5 agonist INT-777 reduced proteinuria, podocyte injury, mesangial expansion, fibrosis, and CD68 macrophage infiltration in the kidney. INT-777 also induced mitochondrial biogenesis and prevented oxidative stress and lipid accumulation, thus establishing a protective role of TGR5 in the inhibition of ORG and DKD [92]. Resent results showed that the dual FXR/TGR5 agonist INT-767 combined effects of both singular activation of FXR and TGR5, improved proteinuria and prevented podocyte injury, inhibited the progression of DKD and ORG [93].
VEGF-B Signaling Inhibition
VEGF-B, through binding with receptors such as VEGFR1 and neuropilin-1, induces the expression of the FA transport proteins FATP3 and FATP4, increasing lipid accumulation. Reducing of VEGF-B signaling or administration of neutralizing VEGF-B antibodies decreases lipid accumulation in podocytes and has renoprotective effects in type 1 and type 2 diabetes mice [39, 98].
Others
2-Hydroxypropyl-β-cyclodextrin has shown the protective effects on podocytes by preventing cholesterol accumulation mediated by ABCA1 upregulation [49, 58]. ABCA1 deficiency was associated with the selective accumulation of CL and subsequent podocyte injury in DKD. Cardiolipin peroxidase inhibitor, elamipretide, reversed DKD progression, with improvements in podocyte number, mesangial expansion, and mitochondrial morphology [25]. Sandoz 58-035, an SOAT1 inhibitor, prevented cholesterol ester accumulation in podocytes by increasing ABCA1 expression and may treat renal disease associated with DKD and Alport syndrome [51]. Exogenous C1P replacement was sufficient to restore the insulin-mediated prosurvival signaling pathway in human podocytes and to normalize urinary albumin levels in mice with DKD [40]. Rituximab, a monoclonal antibody directed against CD20 on B lymphocytes, also prevented recurrent FSGS by modulating podocyte function in an SMPDL-3b-dependent manner [74]. Moreover, berberine, a kind of isoquinoline alkaloid present in Chinese herbal medicines, protected glomerular podocytes via inhibiting dynamin-related protein-1-mediated mitochondrial fission and dysfunction [32]. Besides aforementioned promising treatments, there are many other therapeutic targets have been evaluated in different preclinical models, such as LXRα agonists, pan-TGFβ neutralizing antibodies, NF-κB inhibitors, and fibroblast growth factor-21 therapy [94].
Conclusion
In this review, we summarize recent advances in the understanding of lipids in podocyte biological function and the regulatory mechanisms leading to podocyte lipid accumulation in proteinuric kidney disease. We also provide evidence showing the therapeutic effects of rebalancing the podocyte lipid metabolism on podocyte injury and proteinuric kidney disease. Collectively, pharmacologic targeting of podocyte lipid metabolism will pave the way to new therapeutic approaches in podocyte injury and for the treatment of DKD and other proteinuric kidney diseases.
Conflict of Interest Statement
Dr. Fan Yi is an associate editor of Kidney Diseases.
Funding Sources
This study was supported by the National Science Fund for Distinguished Young Scholars to F.Y. (81525005); the National Natural Science Foundation of China (91949202, 82090020, 82090024, 81770676, 82070753); and Shandong Provincial Natural Science Foundation, China (ZR2019ZD40).
Author Contributions
Y.S. wrote the manuscript; S.C. and Y.H. prepared the figures and the tables; and F.Y. decided on the topics and wrote the manuscript.
References
- 1.Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015 Sep;11((9)):535–45. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016 Jun;89((6)):1221–30. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 3.de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014 May;2((5)):417–26. doi: 10.1016/S2213-8587(14)70065-8. [DOI] [PubMed] [Google Scholar]
- 4.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014 Mar;55((3)):561–72. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M, Wang XX, Magomedova L, John R, Rasheed A, Santamaria H, et al. Liver X receptors preserve renal glomerular integrity under normoglycaemia and in diabetes in mice. Diabetologia. 2014 Feb;57((2)):435–46. doi: 10.1007/s00125-013-3095-6. [DOI] [PubMed] [Google Scholar]
- 6.D'Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016 Aug;12((8)):453–71. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, et al. The adiponectin receptor agonist adipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol. 2018 Apr;29((4)):1108–27. doi: 10.1681/ASN.2017060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte: new perspectives offer new opportunities. Nat Rev Nephrol. 2014 Jul;10((7)):379–88. doi: 10.1038/nrneph.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara S, Kobayashi N, Sakamoto K, Ueno T, Manabe S, Takashima Y, et al. Podocyte injury-driven lipid peroxidation accelerates the infiltration of glomerular foam cells in focal segmental glomerulosclerosis. Am J Pathol. 2015 Aug;185((8)):2118–31. doi: 10.1016/j.ajpath.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitrofanova A, Molina J, Varona Santos J, Guzman J, Morales XA, Ducasa GM, et al. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 2018 Dec;94((6)):1151–9. doi: 10.1016/j.kint.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Yu Z, Zhao S, Qu Z, Sun W, Jiang Y. Lipid metabolism participates in human membranous nephropathy identified by whole-genome gene expression profiling. Clin Sci. 2019 Jun 14;133((11)):1255–69. doi: 10.1042/CS20181110. [DOI] [PubMed] [Google Scholar]
- 12.Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol. 2016 Mar 15;310((6)):F433–45. doi: 10.1152/ajprenal.00375.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer-Schwesinger C. The ins-and-outs of podocyte lipid metabolism. Kidney Int. 2020 Nov;98((5)):1087–90. doi: 10.1016/j.kint.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Imasawa T, Rossignol R. Podocyte energy metabolism and glomerular diseases. Int J Biochem Cell Biol. 2013 Sep;45((9)):2109–18. doi: 10.1016/j.biocel.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol. 2010 Aug;299((2)):C464–76. doi: 10.1152/ajpcell.00563.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa S, Ueda S, Imamura H, Mori K, Asanuma K, Yanagita M, et al. Glycolysis, but not mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci Rep. 2015 Dec 18;5:18575. doi: 10.1038/srep18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imasawa T, Obre E, Bellance N, Lavie J, Imasawa T, Rigothier C, et al. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J. 2017 Jan;31((1)):294–307. doi: 10.1096/fj.201600293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Özel C, et al. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep. 2019 Apr 30;27((5)):1551–66.e5. doi: 10.1016/j.celrep.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li SY, Park J, Qiu C, Han SH, Palmer MB, Arany Z, et al. Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy. JCI Insight. 2017 Jul 20;2((14)) doi: 10.1172/jci.insight.92930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Q, Miao J, Yang Q, Fang L, Fang Y, Ding H, et al. Role of pyruvate kinase M2-mediated metabolic reprogramming during podocyte differentiation. Cell Death Dis. 2020 May 11;11((5)):355. doi: 10.1038/s41419-020-2481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Mu L, Yang Z, Du C, Wu M, Song S, et al. Carbohydrate response element-binding protein regulates lipid metabolism via mTOR complex1 in diabetic nephropathy. J Cell Physiol. 2021 Jan;236((1)):625–40. doi: 10.1002/jcp.29890. [DOI] [PubMed] [Google Scholar]
- 22.Ge M, Fontanesi F, Merscher S, Fornoni A. The vicious cycle of renal lipotoxicity and mitochondrial dysfunction. Front Physiol. 2020;11:732. doi: 10.3389/fphys.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L, et al. Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol. 2015 Dec;52((6)):1045–56. doi: 10.1007/s00592-015-0753-9. [DOI] [PubMed] [Google Scholar]
- 24.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017 Jun 7;12((6)):983–97. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A, et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest. 2019 Jul 22;129((8)):3387–400. doi: 10.1172/JCI125316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, et al. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res. 2009 Apr;50((Suppl)):S9–14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Rui HL, Yang M, Sun LJ, Dong HR, Cheng H. CD36-mediated lipid accumulation and activation of NLRP3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm. 2019;2019:3172647. doi: 10.1155/2019/3172647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 2010 Oct;299((4)):F821–9. doi: 10.1152/ajprenal.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampe K, Sieber J, Orellana JM, Mundel P, Jehle AW. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am J Physiol Renal Physiol. 2014 Feb 15;306((4)):F401–9. doi: 10.1152/ajprenal.00454.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun YB, Qu X, Howard V, Dai L, Jiang X, Ren Y, et al. Smad3 deficiency protects mice from obesity-induced podocyte injury that precedes insulin resistance. Kidney Int. 2015 Aug;88((2)):286–98. doi: 10.1038/ki.2015.121. [DOI] [PubMed] [Google Scholar]
- 31.Lee E, Choi J, Lee HS. Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J Cell Physiol. 2017 Dec;232((12)):3209–17. doi: 10.1002/jcp.25867. [DOI] [PubMed] [Google Scholar]
- 32.Qin X, Zhao Y, Gong J, Huang W, Su H, Yuan F, et al. Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics. 2019;9((6)):1698–713. doi: 10.7150/thno.30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang ZH, Chen H, Vaziri ND, Mao JR, Zhang L, Bai X, et al. Metabolomic signatures of chronic kidney disease of diverse etiologies in the rats and humans. J Proteome Res. 2016 Oct 7;15((10)):3802–12. doi: 10.1021/acs.jproteome.6b00583. [DOI] [PubMed] [Google Scholar]
- 34.Lee HS, Suh JY, Kang BC, Lee E. Lipotoxicity dysregulates the immunoproteasome in podocytes and kidneys in type 2 diabetes. Am J Physiol Renal Physiol. 2021 Apr 1;320((4)):F548–58. doi: 10.1152/ajprenal.00509.2020. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Pan Y, Wu Y, Lin S, Dai B, Chen H, et al. Excessive arachidonic acid induced actin bunching remodeling and podocyte injury via a PKA-c-Abl dependent pathway. Exp Cell Res. 2020 Mar 15;388((2)):111808. doi: 10.1016/j.yexcr.2019.111808. [DOI] [PubMed] [Google Scholar]
- 36.Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid accumulation and chronic kidney disease. Nutrients. 2019 Mar 28;11((4)):722. doi: 10.3390/nu11040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HS. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol Histopathol. 2011 Dec;26((12)):1599–610. doi: 10.14670/HH-26.1599. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 2020 Dec 1;32((6)):1052–62.e8. doi: 10.1016/j.cmet.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 2017 Mar 7;25((3)):713–26. doi: 10.1016/j.cmet.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun. 2019 Jun 19;10((1)):2692. doi: 10.1038/s41467-019-10584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merscher S, Fornoni A. Podocyte pathology and nephropathy − sphingolipids in glomerular diseases. Front Endocrinol. 2014;5:127. doi: 10.3389/fendo.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savas B, Astarita G, Aureli M, Sahali D, Ollero M. Gangliosides in podocyte biology and disease. Int J Mol Sci. 2020 Dec 17;21((24)):21. doi: 10.3390/ijms21249645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012 Oct 12;151((2)):384–99. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 44.Woo CY, Baek JY, Kim AR, Hong CH, Yoon JE, Kim HS, et al. Inhibition of ceramide accumulation in podocytes by myriocin prevents diabetic nephropathy. Diabetes Metab J. 2020 Aug;44((4)):581–91. doi: 10.4093/dmj.2019.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Kidd J, Kaspar C, Dempsey S, Bhat OM, Camus S, et al. Podocytopathy and nephrotic syndrome in mice with podocyte-specific deletion of the Asah1 gene: role of ceramide accumulation in glomeruli. Am J Pathol. 2020 Jun;190((6)):1211–23. doi: 10.1016/j.ajpath.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitrofanova A, Drexler Y, Merscher S, Fornoni A. Role of sphingolipid signaling in glomerular diseases: focus on DKD and FSGS. J Cell Signal. 2020 Sep;1((3)):56–69. doi: 10.33696/Signaling.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Huang D, Bhat OM, Poklis JL, Zhang A, Zou Y, et al. Abnormal podocyte TRPML1 channel activity and exosome release in mice with podocyte-specific Asah1 gene deletion. Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Feb;1866((2)):158856. doi: 10.1016/j.bbalip.2020.158856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhat OM, Yuan X, Li G, Lee R, Li PL. Sphingolipids and redox signaling in renal regulation and chronic kidney diseases. Antioxid Redox Signal. 2018 Apr 1;28((10)):1008–26. doi: 10.1089/ars.2017.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest. 2016 Sep 1;126((9)):3336–50. doi: 10.1172/JCI85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Q, Hu J, Yang Y, Chen Z, Feng J, Zhu Z, et al. Sirt6 deficiency aggravates angiotensin II-induced cholesterol accumulation and injury in podocytes. Theranostics. 2020;10((16)):7465–79. doi: 10.7150/thno.45003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Ducasa GM, Mallela SK, Kim JJ, Molina J, Mitrofanova A, et al. Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome. Kidney Int. 2020 Nov;98((5)):1275–85. doi: 10.1016/j.kint.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tufro A. Cholesterol accumulation in podocytes: a potential novel targetable pathway in diabetic nephropathy. Diabetes. 2013 Nov;62((11)):3661–2. doi: 10.2337/db13-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merscher S, Pedigo CE, Mendez AJ. Metabolism, energetics, and lipid biology in the podocyte − cellular cholesterol-mediated glomerular injury. Front Endocrinol. 2014;5:169. doi: 10.3389/fendo.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu CC, Wang GH, Lu J, Chen PP, Zhang Y, Hu ZB, et al. Role of podocyte injury in glomerulosclerosis. Adv Exp Med Biol. 2019;1165:195–232. doi: 10.1007/978-981-13-8871-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutwein P, Abdel-Bakky MS, Schramme A, Doberstein K, Kämpfer-Kolb N, Amann K, et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol. 2009 Jun;174((6)):2061–72. doi: 10.2353/ajpath.2009.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y, Wang C, Zhou X, Li Y, Ma Y, Zhang R, et al. Downregulation of PTEN promotes podocyte endocytosis of lipids aggravating obesity-related glomerulopathy. Am J Physiol Renal Physiol. 2020 Mar 1;318((3)):F589–99. doi: 10.1152/ajprenal.00392.2019. [DOI] [PubMed] [Google Scholar]
- 57.Freedman BI, Limou S, Ma L, Kopp JB. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis. 2018 Nov;72((5 Suppl 1)):S8–16. doi: 10.1053/j.ajkd.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013 Nov;62((11)):3817–27. doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung JJ, Huber TB, Gödel M, Jarad G, Hartleben B, Kwoh C, et al. Albumin-associated free fatty acids induce macropinocytosis in podocytes. J Clin Invest. 2015 Jun;125((6)):2307–16. doi: 10.1172/JCI79641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Okamura DM, Lu X, Chen Y, Moorhead J, Varghese Z, et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat Rev Nephrol. 2017 Dec;13((12)):769–81. doi: 10.1038/nrneph.2017.126. [DOI] [PubMed] [Google Scholar]
- 61.Chen HM, Zheng CX, Gao Q, Ge YC, Liu ZH. Heart-type fatty acid binding protein is associated with proteinuria in obesity. PLoS One. 2012;7((9)):e45691. doi: 10.1371/journal.pone.0045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Q, Sarkar A, Chen Y, Xu B, Zhu X, Yuan Y, et al. Overexpression of heart-type fatty acid binding protein enhances fatty acid-induced podocyte injury. Exp Ther Med. 2018 Feb;15((2)):2054–61. doi: 10.3892/etm.2017.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sieber J, Jehle AW. Free fatty acids and their metabolism affect function and survival of podocytes. Front Endocrinol. 2014;5:186. doi: 10.3389/fendo.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD, et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol. 2013 Sep;183((3)):735–44. doi: 10.1016/j.ajpath.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaziri ND, Kim CH, Phan D, Kim S, Liang K. Up-regulation of hepatic acyl CoA: diacylglycerol acyltransferase-1 (DGAT-1) expression in nephrotic syndrome. Kidney Int. 2004 Jul;66((1)):262–7. doi: 10.1111/j.1523-1755.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Li CM, Ye ZC, Huang J, Li Y, Lai W, et al. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J Cell Mol Med. 2020 May;24((9)):5109–21. doi: 10.1111/jcmm.15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao YY, Vaziri ND, Lin RC. Lipidomics: new insight into kidney disease. Adv Clin Chem. 2015;68:153–75. doi: 10.1016/bs.acc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, et al. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res. 2014 Jul;55((7)):1375–85. doi: 10.1194/jlr.M049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schumann J, Grevot A, Ledieu D, Wolf A, Schubart A, Piaia A, et al. Reduced activity of sphingosine-1-phosphate lyase induces podocyte-related glomerular proteinuria, skin irritation, and platelet activation. Toxicol Pathol. 2015 Jul;43((5)):694–703. doi: 10.1177/0192623314565650. [DOI] [PubMed] [Google Scholar]
- 70.Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest. 2017 Mar 1;127((3)):912–28. doi: 10.1172/JCI89626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest. 2017 Mar 1;127((3)):942–53. doi: 10.1172/JCI90171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallela SK, Mitrofanova A, Merscher S, Fornoni A. Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Dec;1864((12)):158517. doi: 10.1016/j.bbalip.2019.158517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015 Jan;26((1)):133–47. doi: 10.1681/ASN.2013111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011 Jun 1;3((85)):85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019 Mar;20((3)):137–55. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell. 2006 Jun;17((6)):2674–83. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chun J, Zhang JY, Wilkins MS, Subramanian B, Riella C, Magraner JM, et al. Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc Natl Acad Sci U S A. 2019 Feb 26;116((9)):3712–21. doi: 10.1073/pnas.1820414116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, et al. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell. 2017 Jul 10;42((1)):9–21.e5. doi: 10.1016/j.devcel.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bosch M, Sánchez-Álvarez M, Fajardo A, Kapetanovic R, Steiner B, Dutra F, et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020 Oct 16;370((6514)):370. doi: 10.1126/science.aay8085. [DOI] [PubMed] [Google Scholar]
- 80.Kiss E, Kränzlin B, Wagenblaβ K, Bonrouhi M, Thiery J, Gröne E, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am J Pathol. 2013 Mar;182((3)):727–41. doi: 10.1016/j.ajpath.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 81.Fornoni A, Merscher S. Lipid metabolism gets in a JAML during kidney disease. Cell Metab. 2020 Dec 1;32((6)):903–5. doi: 10.1016/j.cmet.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z, Wu P, Zhang J, Wang S, Zhang G. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: a meta-analysis. Pharmacol Res. 2016 Mar;105:74–83. doi: 10.1016/j.phrs.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Summary of revisions: standards of medical care in diabetes-2020. Diabetes Care. 2020 Jan;43((Suppl 1)):S4–6. doi: 10.2337/dc20-Srev. [DOI] [PubMed] [Google Scholar]
- 84.Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Podocytopathies. Nat Rev Dis Primers. 2020 Aug 13;6((1)):68. doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011 Apr;79((8)):871–82. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 86.Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS One. 2014;9((5)):e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi SR, Lim JH, Kim MY, Kim EN, Kim Y, Choi BS, et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism. 2018 Aug;85:348–60. doi: 10.1016/j.metabol.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017 Mar 31;292((13)):5335–48. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight. 2018 Aug 9;3((15)):e98720. doi: 10.1172/jci.insight.98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in Western diet induced obesity mice. Int J Mol Sci. 2018 Jan 3;19((1)):137. doi: 10.3390/ijms19010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gai Z, Gui T, Hiller C, Kullak-Ublick GA. Farnesoid X receptor protects against kidney injury in uninephrectomized obese mice. J Biol Chem. 2016 Jan 29;291((5)):2397–411. doi: 10.1074/jbc.M115.694323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. 2016 May;27((5)):1362–78. doi: 10.1681/ASN.2014121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang XX, Wang D, Luo Y, Myakala K, Dobrinskikh E, Rosenberg AZ, et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol. 2018 Jan;29((1)):118–37. doi: 10.1681/ASN.2017020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Opazo-Rios L, Mas S, Marin-Royo G, Mezzano S, Gomez-Guerrero C, Moreno JA, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci. 2020 Apr 10;21((7)):2632. doi: 10.3390/ijms21072632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim Y, Park CW. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int J Mol Sci. 2019 Apr 10;20((7)):1782. doi: 10.3390/ijms20071782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017 Feb;60((2)):215–25. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380((24)):2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 98.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012 Oct 18;490((7420)):426–30. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]