Abstract
Reliable diagnostics are a major challenge for the detection and treatment of Helicobacter pylori (H. pylori) infection. Currently at the forefront are non-invasive urea breath test (UBT) and stool antigen test (SAT). Polymerase chain reaction (PCR) is not endorsed due to nonspecific primers and the threat of false-positives. The specificity of DNA amplification can be achieved by nested PCR (NPCR), which involves two rounds of PCR. If the primers are properly designed for the variable regions of the 16S rRNA gene, it is not difficult to develop an NPCR assay for the unambiguous identification of H. pylori. Elaborate NPCR for a 454 bp amplicon was validated on 81 clinical biopsy, stool, and saliva samples, each from the same individuals, and compared with available H. pylori assays, namely histology, rapid urease test, SAT, and 13C-UBT. The assay was much more sensitive than simple PCR, and it was equally sensitive in biopsy samples as the 13C-UBT test, which is considered the gold standard. In addition, it is sufficiently specific because sequencing of the PCR products exclusively confirmed the presence of H. pylori-specific DNA. However, due to the threshold and lower abundance, the sensitivity was much lower in amplifications from stool or saliva. Reliable detection in saliva also complicates the ability of H. pylori to survive in the oral cavity aside from and independent of the stomach. The reason for the lower sensitivity in stool is DNA degradation; therefore, a new NPCR assay was developed to obtain a shorter 148 bp 16S rRNA amplicon. The assay was validated on stool samples from 208 gastroenterological patients and compared to SAT results. Surprisingly, this NPCR revealed the presence of H. pylori in twice the number of samples as SAT, indicating that many patients are misdiagnosed, not treated by antibiotics, and their problems are interpreted as chronic. Thus, it is unclear how to properly diagnose H. pylori in practice. In the first approach, SAT or UBT is sufficient. If samples are negative, the 148 bp amplicon NPCR assay should be performed. If problems persist, patients should not be considered negative, but due to threshold H. pylori abundance, they should be periodically tested. The advantage of NPCR over UBT is that it can be used universally, including questionable samples taken from patients with achlorhydria, receiving proton pump inhibitors, antibiotics, bismuth compound, intestinal metaplasia, or gastric ulcer bleeding.
Keywords: Chronic diseases, Helicobacter pylori, Diagnostics, Nested polymerase chain reaction, DNA sequencing, Detection limit
Core Tip: Polymerase chain reaction (PCR) is not endorsed for Helicobacter pylori (H. pylori) diagnostics due to nonspecific primers and the threat of false-positives. However, a nested PCR that is as specific and equally sensitive in biopsy samples as the 13C-urea breath test was developed. Due to the threshold of H. pylori abundance and the ability to survive in the oral cavity, it is not suitable for saliva samples. Despite DNA degradation in stool samples, nested PCR for a shorter 148 bp amplicon identified twice the number of positive samples as stool antigen test, indicating that many patients are misdiagnosed, not treated by antibiotics, explaining why their problems are interpreted as chronic.
INTRODUCTION
Chronic diseases, such as cardiovascular disease (CVD) and cancer, are the leading causes of death worldwide. In the United States alone, they account for 70% of deaths per year, and CVD and cancer account for over 50% of all deaths each year. Additionally, diseases of the joints, such as arthritis, Parkinson’s disease, and Alzheimer´s disease, reduce the quality of life for the elderly, and the treatment consumes enormous resources (reviewed in[1,2]). In 2005, 133 million Americans had at least one chronic disease. The economic cost was estimated at $1.3 trillion (sic) per year[1]. Of the 12.7 million new cases of cancer in 2008, about 2 million were attributed to infectious agents, such as human papilloma virus, hepatitis B virus, hepatitis C virus, and Helicobacter pylori (H. pylori)[1].
There is clear evidence that H. pylori is a major cause of chronic disease in the gastrointestinal tract (GIT). This helical, gram-negative, microaerophilic bacterium is the most successful human pathogen. The route of infection is not fully understood, but it is transmitted between sexual partners and relatives due to gastroesophageal reflux, often in childhood by an oral–oral or oral–fecal route. The infection persists and remains with the host for life[3]. In most cases, the infection causes mild gastritis, which remains mostly asymptomatic. In approximately 10%–20% of infected people, H. pylori causes stomach and duodenal ulcers. The chronic state increases the risk of developing duodenal and gastric cancer. Thus, since 1994, the International Agency for Research on Cancer has classified H. pylori as a "group 1 (definite carcinogen)" alongside asbestos and benzopyrene[4-6]. Patients with stomach cancer have a poor prognosis. After lung, breast, colorectal, and prostate cancers, stomach cancer is the fifth most common malignancy in the world, and is the third-leading cause of cancer death in both sexes (723000 deaths in 2012, 8.8%)[7]. The high mortality rate is related to early metastatic expansion through the lymphatic system. Since the discovery of H. pylori as the causative agent, its eradication has reduced the incidence of ulcers to almost zero. Additionally, a decrease in the incidence of stomach cancer has been recorded in most European countries over the last decade. Despite this trend, the incidence remains high. In some areas of Eastern Europe, it is more than 20 per 100000 inhabitants, while it is approximately 7 per 100000 in Central and Western Europe, and approximately 2 per 100000 in North America[3,7-10]. Recently, H. pylori infection has also been associated with a number of extragastric diseases, such as idiopathic thrombocytopenic purpura, iron deficiency anemia, vitamin B12 deficiency, insulin resistance, and metabolic syndrome[4,10,11].
There are several clinical tests for H. pylori identification used differentially, depending on the method of medical examination and considering country-specific preferences. The Maastricht V/Florence Consensus Report recommends only the 13C-urea breath test (UBT), the stool antigen test (SAT), or endoscopy for consistent identification[4,10].
The 13C-UBT detects H. pylori indirectly by measuring the activity of bacterial urease in the stomach. The principle is based on the hydrolysis of orally administered 13C- or 14C-labelled urea that is hydrolyzed into ammonia and CO2, which diffuse into the blood and are exhaled through the lungs. The increase in 13C-labelled CO2 in breath specimens (analyzed before and 30 min after the consumption of the urea) is the proof of urease activity and can be measured by mass spectrometry or by the less expensive infrared spectroscopy and laser-assisted ratio analysis. False-negative test results can occur if the patient has received a proton-pump inhibitor (PPI) two weeks before the examination or antibiotics four weeks before. Bleeding similarly affects the diagnostic reliability of the UBT, and therefore, the assay should be accomplished only when bleeding is suppressed. Corpus-predominant gastritis can also be the reason for false-negatives[11].
SAT relies on the recognition of H. pylori antigens in stool. Two types of SAT, the enzyme immunoassay (EIA) and immunochromatography assay (ICA), have been used for H. pylori detection. Either polyclonal or monoclonal antibodies are used, but monoclonal antibody-based and EIA-based tests provide more accurate and reliable data. The accuracy can be affected if the patient has taken PPIs, antibiotics, or N-acetylcysteine, or has bleeding ulcers. False-negative results may also occur when the H. pylori count is low, also due to the use of antibiotics, bismuth, and PPIs. The SAT is a fast, simple, and inexpensive test that is also useful in epidemiological studies and screening programs[11].
Both tests have good sensitivity and specificity, as well as excellent performance if a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) is used[4,10-12]. Nevertheless, extensive study has shown that 3% of cases examined by UBT and 9% of patients tested by H. pylori-specific SAT should be taken as false-negatives[13].
In cases where the patient's medical condition requires endoscopy, H. pylori infection is examined in gastric biopsies by a rapid urease test (RUT), histology, or cultivation. RUT depends on the ability of H. pylori to secrete the enzyme urease. In the mixture containing urea, phenol red or other pH indicators and stomach tissue samples, urea is decomposed into ammonia and carbon dioxide. The presence of ammonia increases the pH, which changes the color of the indicator. The sensitivity and specificity of this method is considered to be > 90%. Patients with achlorhydria and those treated with PPIs, antibiotics, or bismuth compounds may have false-negative results. The test is also not very reliable in patients with intestinal metaplasia or gastric ulcer bleeding[11].
In histology, the biopsy sample is usually stained with Giemsa to identify pathogens and by hematoxylin and eosin to visualize inflammatory cells. If these stains provide inconclusive images, toluidine blue, acridine orange, and Warthin-Starry silver staining can be beneficial. H. pylori is unevenly distributed in the mucus layer; therefore, biopsy samples used to be taken from different parts of the stomach. The sensitivity is 80%–95%, and the specificity is 99%–100%. The diagnostic accuracy of histological examination affects many factors, such as the skill and experience of the gastroenterologist performing the sampling and the pathologist observing the biopsy specimens, the staining technique adopted, the use of PPIs or antibiotics, and the bleeding of peptic ulcers[11,12].
The culture of H. pylori from gastric biopsy samples is performed only in specialized laboratories, as it is not a routine technique. However, it is rather useful for the detection of antibiotic susceptibility and for scientific research. Sensitivity and specificity are considered to be about 70%–80% and 100%, respectively, but in our hands, it is possible to cultivate H. pylori from only about 8% of positive samples[11,14].
Serology, the main approach to detect bacterial infections in blood, is a controversial topic. The ability to recognize active infections of H. pylori relies on age, the clinical conditions of the infection, the antigen used for antibody preparation in the ELISA kit, and the prevalence of infection. Serology has a high negative predictive value; despite its low accuracy, it is cost-effective and due to the availability and simplicity, it is commonly used in epidemiological studies[10,11].
Approaches involving DNA amplification have not been widely accepted in medical practice, due to their higher price in comparison to SAT and UBT and the associated technical demands. The other objections are doubts concerning accuracy, as alterations in the primer binding site may produce false-negatives; on the other hand, nonspecific primers may generate false-positives[12,13,15,16]. This opinion comes from the article by Sugimoto et al[17], who examined 26 various PCR reactions with diverse primers, designed to number of different H. pylori genes including 16S rRNA. DNA from biopsy and saliva specimens was amplified and compared to the results from cultivation and histological examination[17]. They concluded that none of the amplification systems were consistent in terms of specificity or sensitivity with classical tests and all provided false-positives.
In addition, multiple cases of positive results in PCR assays were reported without confirmation by other methods[16-19]. Furthermore, there are considerable doubts about interpreting PCR from a single gene, although the reliability can be unambiguously verified by sequencing[15,16]. Despite the ability to detect a few H. pylori cells or DNA molecules, the PCR approach was not even accepted for proving the eradication by antibiotics, which is again associated with the danger of the identification of cell debris[16]. These misbeliefs are still held, despite the recent meta-analysis reporting that the sensitivity and specificity of stool PCR tests are similar to those of other diagnostic methods[20].
Several PCR modifications, such as real-time PCR, allow the rapid detection and quantification of target DNA[21-24]. However, the disadvantages of real-time PCR include the high equipment cost, the high levels of technical skill required, the increased chance of false-negative results due to operator error resulting from improper assay development, and improper data analysis[21,25,26].
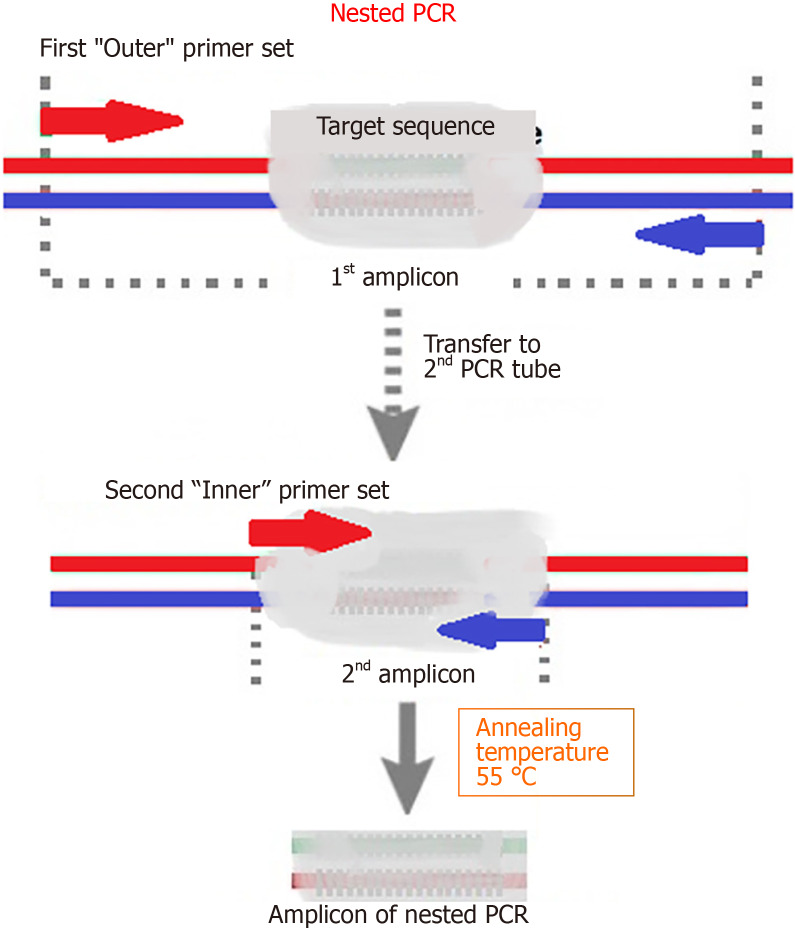
The alternative of choice is nested PCR (NPCR), which includes two rounds of PCR reactions. The first reaction amplifies a larger DNA region that is used as a template in the second reaction, which amplifies a narrower sub-region (Figure 1)[15,27].
Figure 1.
Nested polymerase chain reaction. Nested polymerase chain reaction involves two amplification reactions. The first round targeted a larger DNA region, and the second targeted a narrower sub-region of the products of the first round that were used as a template. PCR: Polymerase chain reaction.
Due to the two sets of primers, NPCR is more specific, and DNA can be amplified from samples with a smaller number of target molecules than simple PCR[27-30]. However, this method is prone to spray contamination and false-positives[27,28]. Nevertheless, NPCR has the potential to become the gold standard in diagnostics when sampling difficulties due to the patchy distribution of H. pylori and the recurrent incidence of false-positives are properly addressed[15]. The potential of NPCR is reinforced by the detection of antibiotic-resistant mutations in 23S rRNA in stool samples with no reports of false-positives[31,32].
We have been involved in H. pylori diagnostics for a while, and during four master’s degree theses and one dissertation, we found that simple PCR was much more sensitive than histology and that many samples that were considered negative were actually positive by NPCR performed in our laboratory. However, before designing this assay, 17 different NPCRs available for H. pylori detection were evaluated from the point of view of efficiency and selectivity. In most of them, serious limitations and mistakes were found in the design of primers. The first major drawback was the non-specificity of primers, especially at the 3´ ends, which can be proved by the BlastN comparison if the Helicobacter TaxId is excluded. This is typical for oligos designed to amplify ribosomal RNAs and protein genes. A lack of specificity was confirmed in two cases from PCR product sequences by the authors themselves[30,33]. The common cause is a mismatch at the 3´ ends of the primer, resulting from polymorphisms found in the fliI, hpaA, hsp60, ureA, ureC/glmM genes. Frequently, alterations in the melting temperature (Tm) are greater than the accepted 4 °C. Sporadically, primer oligos are very short and their Tm is consequently low. These differences should have an impact on the efficiency of the amplification and could cause a failure in the amplification of positive samples. This issue is profound for the housekeeping gene glmM, which was used to confirm qPCR results[34], where the detection rate was only about half of that in the 16S rRNA-positive samples[35-37]. In another two NPCR assays, specificity was assessed through an experiment[31,38] on the Helicobacter-free stool samples and the products targeted to the 16S rRNA gene were sequenced. Their GenBank comparison showed 97% or more identity to the unrelated bacteria Actinomyces naeslundii, Bifidobacterium pseudocatenulatum, and Varibaculum cambriense[38] and to Bacteroides salanitronis in the case of 23S rRNA amplification[31]. An identity of 97% or higher is the taxonomic criterion that allows isolates to be assigned to the same bacterial species[39]. Apparently, almost all published NPCR systems are not specific or sensitive enough to spot low-density infection in complex specimens. Only ureA gene amplification systems passed the BlastN in silico test[40,41], but the ureA product is not critical for the H. pylori persistence in the stomach[42], although it is indispensable for colonization of the GIT in mice[43]. Furthermore, the ureA gene PCR systems were not examined on composite samples, and the PCR products were not sequenced.
In conclusion, nonspecific primers that amplify the DNA of other biological species are major pitfalls in PCR diagnostics. This current state results from inappropriate primer design, mostly from 30 years ago, and the recurrent use of outdated primers. At that time, only a limited number of sequences were stored in GenBank, so primer design was focused only on unique genes, such as urease genes.
RELIABLE DNA DIAGNOSTICS
Primer design
Many sequences are currently available for various strains, related organisms, and organisms from natural ecological niches, with many for entire genomes. In addition, most of the bioinformatic software offers the option of primer design. However, it is not difficult to design them using common sense.
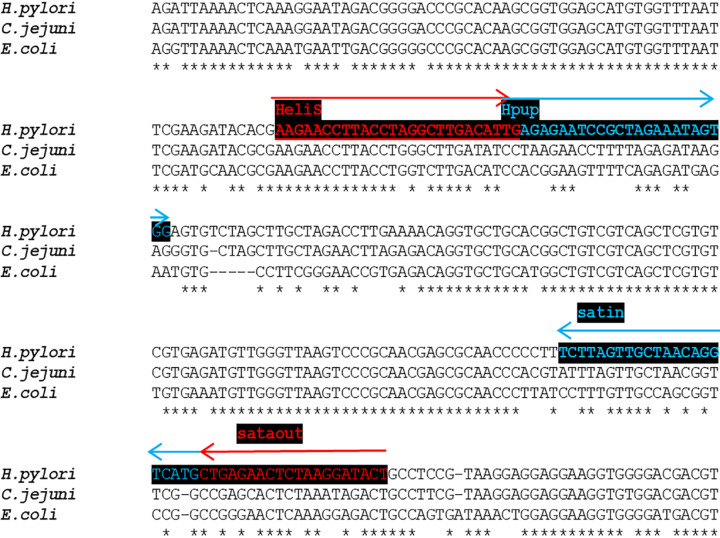
The first task is gene selection. Various housekeeping genes are considered, but due to habits in taxonomy and clinical microbiology, especially with regard to the identification of new species, our attention should be focus on the 16S rRNA gene for the small ribosomal subunit. This RNA gene present in any bacteria contains conserved regions that are used in metagenomic studies for the universal amplification of bacterial DNA[39,44,45]. In addition, species- or genus-specific hypervariable sections can be found in the 16S rRNA sequence, which favors this gene for primer design[46,47]. Sufficiently selective primers can be designed in the regions discriminating Helicobacter from other known bacteria. To select a suitable region, the H. pylori 16S rDNA sequence was compared to the corresponding genes from representative stool resident (Escherichia coli) and representative of the closely related genera (Campylobacter jejuni). If these DNAs are aligned, several unique H. pylori regions useful for primer design can be found (Figure 2).
Figure 2.
Design of Helicobacter pylori-specific primers for shorter 148-bp 16S rRNA amplicon. Alignment of Helicobacter pylori (amplified region) to other bacterial species. Selective primers marked in red, and blue were designed in the regions with a high divergence of Helicobacter sequence.
First pick primers were modified for a significant mismatch at the 3′ end, in order to keep the GC content below 50%. Primer length was then trimmed to maintain the Tm at around 55 °C, which can be easily calculated in many programs. These parameters are important to prevent the amplification of false priming sites and to improve efficiency. Primer specificity can be assessed simply in silico by BlastN comparison with other GenBank sequences. Good primers match precisely 100 times or more within the Helicobacter genus, but exhibit a strong divergence at the 3′ end of other bacteria. This can be done by the exclusion of Helicobacter TaxId from the task. According to these principles, two primer sets were selected: External primers for the amplification of a 497 bp region and internal primers for the amplification of a 454 bp fragment. However, the excellent performance of any PCR assay requires the optimization of amplification conditions, such as annealing temperature, the concentration of magnesium and the number of cycles (25–45)[28].
Sensitivity and the limit of detection
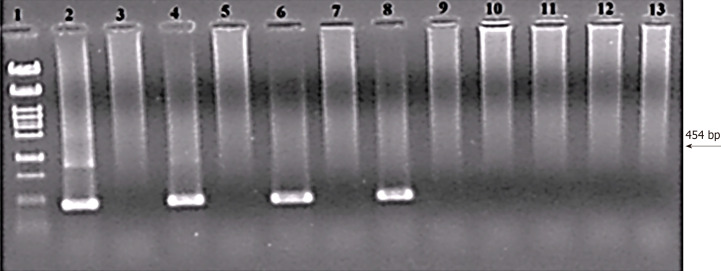
The Achilles heel of all identification methods is the absence of a detection limit, which should be understood as the minimal number of cells or DNA copies that can be consistently identified. This can be determined by adding (‘spiking’) a known number of cells directly into the PCR reaction (‘colony PCR’) or to the spare samples that previously tested negative. The detection limit for H. pylori cells was as low as 0.5 cells in a PCR vial (Figure 3)[28].
Figure 3.
Threshold value of the nested polymerase chain reaction assay for Helicobacter pylori detection in cell suspension (colony polymerase chain reaction). Lines: 1: Size marker λ/Pst1; 2: 500; 3: Negative control (NC); 4: 50; 5: NC; 6: 5; 7: NC; 8: 0.5; 9: NC; 10: 0.05; 11: NC; 12: 0.005; 13: NC. Numbers express cell counts in the polymerase chain reaction (PCR) reaction. External primers HeliS/HeliN. Internal primers Hpup/Hpdown. Size of PCR product is 454 bp[28,53]. Each sample was tested by PCR separately in two independent experiments, always with the same result. Separated on 2% agarose in TBE.
This value expresses the smallest DNA amount that can be theoretically amplified, as the H. pylori genome contains duplicate 16S rRNA genes[48]. Nevertheless, in reality, when samples are spiked with H. pylori culture, the detection threshold is roughly ten times less sensitive; it contained approximately 10 cells in a PCR vial that requires more than 1–5 × 103 cells per g or mL of biopsy, saliva, or stool specimen[28]. This is apparently the consequence of reduced DNA yield from silica columns. However, due to the unknown elution volumes, these data cannot be compared to those of other studies[17,49]. Nonetheless, the detection limit does not rely on the DNA isolation kit or the enzyme used[28].
Solo PCR is significantly less effective since approximately ten times more cells are needed in the amplification reaction for consistent identification. Another parameter that is extremely important but omitted from almost all diagnostic works is the concentration of target molecules in the analyzed samples. Their actual abundance is possible to determine from dilutions of the sample solutions. The lowest detectable density should be the same for particular NPCRs and specimens as the known threshold limits. The number of target DNA copies in the sample can be estimated by multiplying this value by the dilution factor. The density of H. pylori in stomach biopsies was found to be in the range 0.5–2.5 × 104 cells/g, while in saliva and feces it corresponded to 5 × 103 cells/g or 1 × 103 cells/mL, respectively. This was at least 5- to 25-fold lower than that in stomach mucosa[28].
Reliability of NPCR assays in different specimens
NPCR was validated in biopsy, stool, and saliva samples from the same individuals and compared to other detection methods[28]. Overall, 39.5% of patients were positive for H. pylori by UBT considered the gold standard, but only 21% by histopathology, 18.5% by RUT, and 27.2% by immunochromatographic SAT, while 39.5% of the biopsy samples were positive by NPCR (Table 1)[28]. Biopsy specimens were subjected to evaluation by simple PCR (second amplification reaction from NPCR), but only 29.6% were positive (Table 1).
Table 1.
Helicobacter pylori positivity by different diagnostic tests
|
Patients
|
13C-UBT
|
Histology (biopsy)
|
RUT (biopsy)
|
SAT (stool)
|
PCR1 (biopsy)
|
NPCR (biopsy)
|
NPCR (stool)
|
NPCR (saliva)
|
| 5 | + | + | + | + | + | + | + | + |
| 3 | + | + | + | + | + | + | + | - |
| 7 | + | + | + | + | + | + | - | - |
| 2 | + | - | - | + | + | + | + | - |
| 5 | + | - | - | + | + | + | - | - |
| 2 | + | + | - | - | + | + | - | - |
| 6 | + | - | - | - | - | + | - | - |
| 2 | + | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | + | - | - |
| 3 | - | - | - | - | - | - | - | + |
| 44 | - | - | - | - | - | - | - | - |
| Total 81 | 32 | 17 | 15 | 22 | 24 | 32 | 10 | 8 |
Simple polymerase chain reaction (PCR).
Hpup/HPdown primers; 37 cycles; PCR products sequenced. GenBank database comparisons confirmed the DNA sequence origin as Helicobacter pylori. Plus indicates a positive result, minus indicates a negative result[43]. RUT: Rapid urease test; SAT: Stool antigen; PCR: Polymerase chain reaction; NPCR: Nested PCR.
As expected, NPCR was more sensitive than simple PCR. In addition to NPCR, samples that were positive in all other H. pylori tests were also positive using UBT. The H. pylori origin of PCR products was confirmed by DNA sequencing and their comparison revealed that they belong to at least 32 different strains. The sensitivity of histology, RUT, SAT, and simple PCR tests was lower, so these differences were interpreted as false-negatives (Tables 1 and 2)[28].
Table 2.
Sensitivity and specificity of diagnostic tests
|
|
Histology (biopsy)
|
RUT (biopsy)
|
SAT (stool)
|
PCR (biopsy)
|
NPCR (biopsy)
|
| Sensitivity (%) | 53.1 | 46.9 | 68.8 | 75 | 100 |
| Specificity (%) | 100 | 100 | 100 | 100 | 95.6 |
| Positive predictive values (%) | 100 | 100 | 100 | 100 | 93.8 |
| Negative predictive values (%) | 74.6 | 72.1 | 81.5 | 84.6 | 91.3 |
Sensitivity and specificity related urea breath test[43]. RUT: Rapid urease test; SAT: Stool antigen; PCR: Polymerase chain reaction; NPCR: Nested PCR.
Are saliva specimens reliable?
There is an increasing demand for non-invasive diagnostics to circumvent the discomfort of the endoscopic examination required to collect samples[16,17]. The oral cavity is as suitable for a H. pylori reservoir as the stomach in adults[18], as are inflamed teeth (pulp) in children[18], but this is still a controversial issue[15,35,50]. It remains unclear whether H. pylori colonizes the oral cavity residentially, transiently, or at all[35]. Therefore, saliva and feces from 81 individuals were examined for the presence of H. pylori-specific DNA by simple and nested PCR. Simple PCR did not provide specific PCR products from any samples, but NPCR revealed a positive rate of about 12% in stool and 10% in saliva samples. Sequencing confirmed the correct origin, demonstrating high specificity of NPCRs, because several hundred diverse bacteria can be found in saliva[51]. The variability or identity of microbial populations can be distinguished simply by DNA polymorphism. Sequence comparisons of stool and saliva sources confirmed identical strains in the GIT and oral cavity in only three of the eight H. pylori-positive samples. Different strains in the stomach and saliva were found in two cases. However, in three individuals, H. pylori was identified exclusively in saliva/the oral cavity, but not in stool samples. Apparently, this pathogen can persist in the oral cavity, aside from and independent of the stomach, which was already reported in adolescents[52]. Nevertheless, NPCR of saliva samples appears to be a reproducible, consistent assay because the H. pylori 16S rRNA gene can be repeatedly amplified from any positive specimen. To find out how sampling could affect the results, we took advantage of the willingness of one SAT- and saliva-positive volunteer who was keen to provide samples throughout the day. However, besides one sample, which was only positive when the DNA concentration in the reaction was increased to maximum, all other daily saliva assays were negative. This outcome can be explained by variables but especially by the insufficient occurrence of bacteria in the samples. Therefore, saliva cannot be considered as a reliable source to confirm the presence of H. pylori in the stomach. This conclusion regarding the consistent detection also supports a 50-fold lower (threshold) abundance in the oral cavity in comparison to the stomach[28,53].
The detection of H. pylori DNA in stool
Stool contains several thousand different species of bacteria. Nevertheless, we only amplified H. pylori DNA from stool samples using NPCR. These data again demonstrate the specificity of NPCR[54]. According to the SAT test, 22 samples were positive but only 10 were positive by NPCR. When stool samples were spiked with dilutions of H. pylori culture, the SAT limit was ≥ 2–5 × 105/g, which is 100 times less than that of the NPCR assay[28]. This shows strong inconsistencies between the detection limits and detection capabilities of SAT and NPCR. This paradox could be caused by the breakdown of intact H. pylori cells and its DNA in the digestive system. During digestion, DNA from food components is degraded to only about 200 bp fragments[55] which are much smaller than the NPCR product (454 bp). Despite our efforts, we were unable to determine which antigen was used to produce antibody components of the immunochromatographic SAT kits. Hypothetically, the SAT test could be more sensitive if antibodies were prepared against secreted antigens such as urease, CagA, VacA or surface antigens, which are not extensively degraded in the stool. To explain the SAT/NPCR paradox, we designed a new NPCR that allows the amplification of a shorter 148 bp segment of the 16S rRNA gene. SAT and NPCR for the 148 bp amplicon showed that only about 30% of 106 volunteers and 203 gastroenterological patients were positive by SAT, but 60% by short NPCR[53,56]. The origin of the PCR product was confirmed by DNA sequencing, indicating a sensitivity for SAT of only 50%.
A comparison of SAT and NPCR indicates that many patients are misdiagnosed. They have health problems and host H. pylori, but are diagnosed as negative. They have been using proton pump inhibitors for a long time and see a physician regularly, but their problems become chronic. Gastroenterologists are aware of this phenomenon and often consider alternative pathogens[57]. We and others[51] have not identified any another pathogens, even by metagenomic analysis, and the most plausible explanation is the insufficient sensitivity of H. pylori tests due to the threshold of abundance[53,56,58].
Pitfalls of NPCR and H. pylori diagnostics
The major drawback of NPCR is false-positives due to the spray effect, as the tubes are opened after the first PCR to add aliquots to the second amplification reaction[27,59,60]. To avoid contamination, instead of a single negative control, we included two negative controls after each sample and analyzed the samples in triplicate. Only cases with a signal in the sample and without the signal in the negative control were considered positive.
This arrangement is good for the amplification of longer fragments (400–500 bp) but not for the amplification of shorter DNA (100–200 bp). Testing for a short 148 bp amplicon is not routine. The rules of the forensic laboratory and a number of rules, especially for pipetting, must be followed, not all of which can be reported in the protocol. Apparently, this assay cannot be used in practice in medical laboratories for the routine analysis of tens or hundreds of samples. The major source of the spray effect and thus of false-positives is the opening of tubes containing DNA amplified in the first reaction. However, it is possible to simplify NPCR so that it can take place in a single tube according to the rules described in previous studies[61-63]. Moreover, the assay can be modified for real-time PCR using both SYBR Green and TaqMan detection. Preliminary data are promising, and even the SYBR Green variant was shown to be more robust and as sensitive as the 148 bp amplicon NPCR assay. This modification has the potential for use in medical practice in the future. However, there are several other emerging methods, such as CRISPR-based detection, new imaging techniques, and novel fluorescent methods in histology[10,64,65].
CONCLUSION
The diagnosis of H. pylori can be divided into two basic categories. The first includes culture, RUT, and UBT and relies more on the good physiological state of metabolically active bacteria than on their abundance. However, this feature cannot be neglected; although in the case of UBT, it involves the extent of stomach colonization. However, these methods have a number of limitations, as errors can occur in patients receiving PPIs, antibiotics, and bismuth compounds or those with intestinal metaplasia or gastric ulcer bleeding. The second category involves PCR, NPCR, SAT, histology, and partial RUT, and relies strictly on cell abundance and the scale of their degradation. RUT and histology likely require bacterial loads of at least 104[10-64], an abundance that can be reached only in some biopsy samples[28]. For other methods, cell debris is sufficient for the identification of H. pylori, despite the threshold of occurrence in stool.
Generally, the fundamental problems in medical research are methods, their use, and their interpretation. The stumbling block is the common effort to detect various analytes (antigens, antibodies, pathogens) at levels around the detection limit, but the results are interpreted as data from an area of high confidence. The attempt to find the nature of the problem is then replaced by statistics and the comparison of inaccurate results. Its massive and improper use is the reason why half-true data are accumulated, complicating the solution to the problem[66]. Whether or not this is the case, medicine attempts to solve the problem via meta-analysis of the already published results (perhaps it is the only science to do so). The problem is significant in the identification of H. pylori.
The most common objection about the use of PCR is concerns about false-negatives that could be caused by polymorphism and the risk of false-positive results that could occur if non-specific primers are used[12,14,16].
One source of these misbeliefs is the phenomenon known as the 'gold standard'. This is a reliable method, which is usually histological examination together with urease or breath tests in the case of H. pylori detection. The gold standard implies unanimously positive samples in all tests. Furthermore, the terms sensitivity and specificity are used, given as percentages[14]. The sensitivity expresses the percentage of samples in which the presence of H. pylori is detected compared to the gold standard, with 100% indicating that all samples identified as positive by the gold standard are identified as positive in the new test. The second concept, specificity, expresses the ability of the new test to accurately select samples in which H. pylori is absent. When a new test identifies samples as positive which were negative by the gold standard, they are considered false-positives. The fact that the new test might have a better detection threshold and is more sensitive, as is the case for NPCR, is disregarded. Samples are simply considered false-positives. However, the origin of the amplified DNA can be confirmed by DNA sequencing and comparison with databases such as GenBank. If the identity is > 97%, the isolates are considered to be the same bacterial species. This criterion is generally used in taxonomy and molecular biology[39,67], but for unknown reasons, it is ignored in medicine.
Apparently, the most promising H. pylori DNA detection method is the one-vial modification of short-amplicon NPCR. In addition to sensitivity and specificity, another advantage is that it can be used to verify the presence of H. pylori in questionable samples from patients that are SAT-negative but with achlorhydria, those receiving PPIs, antibiotics, or bismuth compounds, or in those with intestinal metaplasia or gastric ulcer bleeding, although all symptoms indicate H. pylori infection.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: March 20, 2021
First decision: July 3, 2021
Article in press: September 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovakia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keikha M, Lee SW S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
Contributor Information
Pavol Sulo, Department of Biochemistry, Comenius University, Bratislava 842 15, Slovakia. pavol.sulo@uniba.sk.
Barbora Šipková, Department of Biochemistry, Comenius University, Bratislava 842 15, Slovakia.
References
- 1.Gargano LM, Hughes JM. Microbial origins of chronic diseases. Annu Rev Public Health. 2014;35:65–82. doi: 10.1146/annurev-publhealth-032013-182426. [DOI] [PubMed] [Google Scholar]
- 2.Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. 2015;39:567–591. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leja M, Grinberga-Derica I, Bilgilier C, Steininger C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter. 2019;24 Suppl 1:e12635. doi: 10.1111/hel.12635. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 5.Ansari S, Yamaoka Y. Current understanding and management of Helicobacter pylori infection: an updated appraisal. F1000Res. 2018;7 doi: 10.12688/f1000research.14149.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong IW. Climate change: Impact on health and infectious diseases globally. In Current trends and concerns in infectious diseases. Canada: Springer, 2020: 165-190. [Google Scholar]
- 7.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 8.Bauer B, Meyer TF. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers. 2011;2011:1–23. [Google Scholar]
- 9.Khatoon J, Rai RP, Prasad KN. Role of Helicobacter pylori in gastric cancer: Updates. World J Gastrointest Oncol. 2016;8:147–158. doi: 10.4251/wjgo.v8.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, McInnes MD, Spijker R, Van den Bruel A Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev . 2021;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, Babazadeh A, Koppolu V, Vasigala VR, Nouri HR, Ebrahimpour S. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38:55–66. doi: 10.1007/s10096-018-3414-4. [DOI] [PubMed] [Google Scholar]
- 12.Miftahussurur M, Yamaoka Y. Diagnostic Methods of Helicobacter pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. Biomed Res Int. 2016;2016:4819423. doi: 10.1155/2016/4819423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Gurusamy KS. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl U. User-Oriented Control of Personal Information Security in Communication Systems. Springer. 1997 [Google Scholar]
- 15.Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20:12847–12859. doi: 10.3748/wjg.v20.i36.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvet X. Diagnosis of Helicobacter pylori Infection in the Proton Pump Inhibitor Era. Gastroenterol Clin North Am. 2015;44:507–518. doi: 10.1016/j.gtc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto M, Wu JY, Abudayyeh S, Hoffman J, Brahem H, Al-Khatib K, Yamaoka Y, Graham DY. Unreliability of results of PCR detection of Helicobacter pylori in clinical or environmental samples. J Clin Microbiol. 2009;47:738–742. doi: 10.1128/JCM.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail H, Morgan C, Griffiths P, Williams J, Jenkins G. A Newly Developed Nested PCR Assay for the Detection of Helicobacter pylori in the Oral Cavity. J Clin Gastroenterol. 2016;50:17–22. doi: 10.1097/MCG.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 19.Nomura R, Ogaya Y, Matayoshi S, Morita Y, Nakano K. Molecular and clinical analyses of Helicobacter pylori colonization in inflamed dental pulp. BMC Oral Health. 2018;18:64. doi: 10.1186/s12903-018-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khadangi F, Yassi M, Kerachian MA. Review: Diagnostic accuracy of PCR-based detection tests for Helicobacter Pylori in stool samples. Helicobacter. 2017;22 doi: 10.1111/hel.12444. [DOI] [PubMed] [Google Scholar]
- 21.Klein D. Quantification using real-time PCR technology: applications and limitations. Trends Mol Med. 2002;8:257–260. doi: 10.1016/s1471-4914(02)02355-9. [DOI] [PubMed] [Google Scholar]
- 22.Mikula M, Dzwonek A, Jagusztyn-Krynicka K, Ostrowski J. Quantitative detection for low levels of Helicobacter pylori infection in experimentally infected mice by real-time PCR. J Microbiol Methods. 2003;55:351–359. doi: 10.1016/s0167-7012(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 23.Mishra KK, Srivastava S, Dwivedi PP, Prasad KN, Ayyagari A. UreC PCR based diagnosis of Helicobacter pylori infection and detection of cag A gene in gastric biopsies. Indian J Pathol Microbiol. 2002;45:31–37. [PubMed] [Google Scholar]
- 24.Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol. 2014;20:9299–9313. doi: 10.3748/wjg.v20.i28.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valasek MA, Repa JJ. The power of real-time PCR. Adv Physiol Educ. 2005;29:151–159. doi: 10.1152/advan.00019.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kralik P, Ricchi M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front Microbiol. 2017;8:108. doi: 10.3389/fmicb.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu G, Fadrosh D, Goedert JJ, Ravel J, Goldstein AM. Nested PCR Biases in Interpreting Microbial Community Structure in 16S rRNA Gene Sequence Datasets. PLoS One. 2015;10:e0132253. doi: 10.1371/journal.pone.0132253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Šeligová B, Lukáč Ľ, Bábelová M, Vávrová S, Sulo P. Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter. 2020;25:e12680. doi: 10.1111/hel.12680. [DOI] [PubMed] [Google Scholar]
- 29.Germani Y, Dauga C, Duval P, Huerre M, Levy M, Pialoux G, Sansonetti P, Grimont PA. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H. felis in a human gastric biopsy. Res Microbiol. 1997;148:315–326. doi: 10.1016/S0923-2508(97)81587-2. [DOI] [PubMed] [Google Scholar]
- 30.Silva DG, Tinoco EM, Rocha GA, Rocha AM, Guerra JB, Saraiva IE, Queiroz DM. Helicobacter pylori transiently in the mouth may participate in the transmission of infection. Mem Inst Oswaldo Cruz. 2010;105:657–660. doi: 10.1590/s0074-02762010000500009. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi N, Rimbara E, Kato A, Tanaka A, Tokunaga K, Kawai T, Takahashi S, Sasatsu M. Detection of mixed clarithromycin-resistant and -susceptible Helicobacter pylori using nested PCR and direct sequencing of DNA extracted from faeces. J Med Microbiol. 2007;56:1174–1180. doi: 10.1099/jmm.0.47302-0. [DOI] [PubMed] [Google Scholar]
- 32.Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613–629. doi: 10.1038/s41575-021-00449-x. [DOI] [PubMed] [Google Scholar]
- 33.Yamada R, Yamaguchi A, Shibasaki K. Detection and analysis of Helicobacter pylori DNA in the gastric juice, saliva, and urine by nested PCR. Oral Sci Int. 2008;5:24–34. [Google Scholar]
- 34.Gastli N, Allain M, Lamarque D, Abitbol V, Billoët A, Collobert G, Coriat R, Terris B, Kalach N, Raymond J. Diagnosis of Helicobacter pylori Infection in a Routine Testing Workflow: Effect of Bacterial Load and Virulence Factors. J Clin Med. 2021;10 doi: 10.3390/jcm10132755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao X, Jakubovics NS, Bächle M, Buchalla W, Hiller KA, Maisch T, Hellwig E, Kirschneck C, Gessner A, Al-Ahmad A, Cieplik F. Colonization of Helicobacter pylori in the oral cavity - an endless controversy? Crit Rev Microbiol. 2021;47:612–629. doi: 10.1080/1040841X.2021.1907740. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Muñoz LJ, González-Díaz CA, Muñoz-Escobar A, Tovar-Ayona BJ, Aguilar-Anguiano LM, Vargas-Olmos R, Sánchez-Monroy V. Prevalence of Helicobacter pylori from the oral cavity of Mexican asymptomatic children under 5 years of age through PCR. Arch Oral Biol. 2017;73:55–59. doi: 10.1016/j.archoralbio.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Lu JJ, Perng CL, Shyu RY, Chen CH, Lou Q, Chong SK, Lee CH. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J Clin Microbiol. 1999;37:772–774. doi: 10.1128/jcm.37.3.772-774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto K, Ohashi H, Takakura A, Itoh T. Current status of Helicobacter contamination of laboratory mice, rats, gerbils, and house musk shrews in Japan. Curr Microbiol. 2000;41:161–166. doi: 10.1007/s002840010111. [DOI] [PubMed] [Google Scholar]
- 39.Rosselló-Móra R. Towards a taxonomy of Bacteria and Archaea based on interactive and cumulative data repositories. Environ Microbiol. 2012;14:318–334. doi: 10.1111/j.1462-2920.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 40.Kisa O, Albay A, Mas MR, Celasun B, Doganci L. The evaluation of diagnostic methods for the detection of Helicobacter pylori in gastric biopsy specimens. Diagn Microbiol Infect Dis. 2002;43:251–255. doi: 10.1016/s0732-8893(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 41.Ogaya Y, Nomura R, Watanabe Y, Nakano K. Detection of Helicobacter pylori DNA in inflamed dental pulp specimens from Japanese children and adolescents. J Med Microbiol. 2015;64:117–123. doi: 10.1099/jmm.0.079491-0. [DOI] [PubMed] [Google Scholar]
- 42.Tsuda M, Karita M, Morshed MG, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debowski AW, Walton SM, Chua EG, Tay AC, Liao T, Lamichhane B, Himbeck R, Stubbs KA, Marshall BJ, Fulurija A, Benghezal M. Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection. PLoS Pathog. 2017;13:e1006464. doi: 10.1371/journal.ppat.1006464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Wiley, 1991: 115-175. [Google Scholar]
- 45.Sontakke S, Cadenas MB, Maggi RG, Diniz PP, Breitschwerdt EB. Use of broad range16S rDNA PCR in clinical microbiology. J Microbiol Methods. 2009;76:217–225. doi: 10.1016/j.mimet.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Castelino M, Eyre S, Moat J, Fox G, Martin P, Ho P, Upton M, Barton A. Optimisation of methods for bacterial skin microbiome investigation: primer selection and comparison of the 454 versus MiSeq platform. BMC Microbiol. 2017;17:23. doi: 10.1186/s12866-017-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schriefer AE, Cliften PF, Hibberd MC, Sawyer C, Brown-Kennerly V, Burcea L, Klotz E, Crosby SD, Gordon JI, Head RD. A multi-amplicon 16S rRNA sequencing and analysis method for improved taxonomic profiling of bacterial communities. J Microbiol Methods. 2018;154:6–13. doi: 10.1016/j.mimet.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oxley AP, Powell M, McKay DB. Species of the family Helicobacteraceae detected in an Australian sea lion (Neophoca cinerea) with chronic gastritis. J Clin Microbiol. 2004;42:3505–3512. doi: 10.1128/JCM.42.8.3505-3512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diouf A, Martinez-Gomis J, Miquel M, Quesada M, Lario S, Sixou M. [Comparison of four different primer sets for detection of Helicobacter pylori in gastric biopsies and oral samples by using real-time PCR] Pathol Biol (Paris) 2009;57:30–35. doi: 10.1016/j.patbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Yee JKC. Are the view of Helicobacter pylori colonized in the oral cavity an illusion? Exp Mol Med. 2017;49:e397. doi: 10.1038/emm.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeshita T, Kageyama S, Furuta M, Tsuboi H, Takeuchi K, Shibata Y, Shimazaki Y, Akifusa S, Ninomiya T, Kiyohara Y, Yamashita Y. Bacterial diversity in saliva and oral health-related conditions: the Hisayama Study. Sci Rep. 2016;6:22164. doi: 10.1038/srep22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aksit Bıcak D, Akyuz S, Kıratlı B, Usta M, Urganci N, Alev B, Yarat A, Sahin F. The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints. BMC Oral Health. 2017;17:67. doi: 10.1186/s12903-017-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Šeligová B. DNA diagnostics for reliable and universal identification of Helicobacter pylori. Dissertation Thesis, Comenius University in Bratislava. Bratislava: Faculty of Natural Sciences, 2020. [Google Scholar]
- 54.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzi A, Raddadi N, Sorlini C, Nordgrd L, Nielsen KM, Daffonchio D. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: implications for horizontal gene transfer and the biosafety of GMOs. Crit Rev Food Sci Nutr. 2012;52:142–161. doi: 10.1080/10408398.2010.499480. [DOI] [PubMed] [Google Scholar]
- 56.Abrahamovská M. Pitfalls of Helicobacter pylori identification in medical practice. M. Sc. Thesis, Comenius University in Bratislava. Bratislava: Faculty of Natural Sciences, 2020. [Google Scholar]
- 57.Gantuya B, El-Serag HB, Matsumoto T, Ajami NJ, Oyuntsetseg K, Azzaya D, Uchida T, Yamaoka Y. Gastric Microbiota in Helicobacter pylori-Negative and -Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers (Basel) 2019;11 doi: 10.3390/cancers11040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13:1–22. doi: 10.1080/19490976.2021.1909459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strom CM, Rechitsky S. Use of nested PCR to identify charred human remains and minute amounts of blood. J Forensic Sci. 1998;43:696–700. [PubMed] [Google Scholar]
- 60.Butler JM. Low-level DNA testing: issues, concerns, and solutions. Adv Top Forensic DNA Typing Methodol. 2012;2:311–346. [Google Scholar]
- 61.Moser DA, Neuberger EW, Simon P. A quick one-tube nested PCR-protocol for EPO transgene detection. Drug Test Anal. 2012;4:870–875. doi: 10.1002/dta.1348. [DOI] [PubMed] [Google Scholar]
- 62.da Silva MA, Pedrosa Soares CR, Medeiros RA, Medeiros Z, de Melo FL. Optimization of single-tube nested PCR for the diagnosis of visceral leishmaniasis. Exp Parasitol. 2013;134:206–210. doi: 10.1016/j.exppara.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Sun Y, Chen J, Li J, Xu Y, Jin H, Xu N, Yin R, Hu G. Novel approach based on one-tube nested PCR and a lateral flow strip for highly sensitive diagnosis of tuberculous meningitis. PLoS One. 2017;12:e0186985. doi: 10.1371/journal.pone.0186985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dore MP, Pes GM. What Is New in Helicobacter pylori Diagnosis. An Overview. J Clin Med. 2021;10 doi: 10.3390/jcm10102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu E, Jin S, Xiao Z, Chen Q, Wang Q, Liu H, Xie C, Chen C, Li Z, Han S. CRISPR-based detection of Helicobacter pylori in stool samples. Helicobacter. 2021:e12828. doi: 10.1111/hel.12828. [DOI] [PubMed] [Google Scholar]
- 66.Ottiwet O, Chomvarin C, Chaicumpar K, Namwat W, Mairiang P. Nested polymerase chain reaction for detection of Helicobacter pylori in gastric biopsy specimens. Southeast Asian J Trop Med Public Health. 2010;41:1423–1431. [PubMed] [Google Scholar]
- 67.Louca S, Mazel F, Doebeli M, Parfrey LW. A census-based estimate of Earth's bacterial and archaeal diversity. PLoS Biol. 2019;17:e3000106. doi: 10.1371/journal.pbio.3000106. [DOI] [PMC free article] [PubMed] [Google Scholar]