Abstract
Background
The clinical use of common cardiac biomarkers, such as brain natriuretic peptides and troponins, has traditionally been limited to adult populations in the assessment of heart failure and acute coronary syndrome, respectively. While many have discounted the value of these markers in pediatric populations, emerging evidence suggests they may be useful in the diagnosis and prognostication of many cardiac and noncardiac pathologies in neonates, children, and adolescents, and an increasing number of pediatric hospitals are routinely measuring cardiac markers in their clinical practice.
Content
This review summarizes and critically evaluates the current literature regarding the application of cardiac biomarkers for clinical decision-making in the pediatric population. Main potential clinical indications discussed herein include primary cardiac disease, immune-related conditions, and noncardiac disease. Important diagnostic and interpretative challenges are also described in relation to each potential indication.
Summary
Despite a general lack of clinical awareness regarding the value of cardiac biomarkers in pediatrics, there is increasing literature to support their application in various contexts. Cardiac biomarkers should be considered an undervalued resource in the pediatric population with potential value in the diagnosis and prognosis of myocarditis, congenital heart disease, and heart failure, as well as in the assessment of severity and cardiac involvement in immune-related and other systemic conditions. While interpretation remains challenging in pediatrics due to the age- and sex-specific dynamics occurring throughout growth and development, this should not prevent their application. Future research should focus on defining evidence-based cut-offs for specific indications using the most up-to-date assays.
Keywords: cardiac troponin, brain natriuretic peptide, pediatric, clinical application
Introduction
Circulating cardiac biomarkers are integral to the diagnosis, prognosis, and monitoring of cardiac distress in patients with both primary and nonprimary cardiac etiology. The most commonly used cardiac biomarkers in clinical care are B-type natriuretic peptides (i.e., B-type natriuretic peptide (BNP) and N-terminal fragment prohormone B-type natriuretic peptide (NT-proBNP)) and cardiac troponins (cTns). Indeed, the advent of high sensitivity cTn (hs-cTn), BNP, and NT-proBNP assay measurements has revolutionized the management of cardiac disease in adults, providing particular value in the diagnosis of acute coronary syndrome (ACS) and heart failure (HF), respectively, and their differentiation from other conditions with similar clinical presentations. Additional cardiac biomarkers that are similarly useful in the identification of myocardial injury or HF in adults, but often not available clinically, include creatine kinase MB isoform (CK-MB), myoglobin, soluble interleukin 1 receptor-like 1 (ST2), galectin 3, and heart-type fatty acid-binding protein (H-FABP). Despite clear applications in adult populations, the clinical value of cardiac biomarkers in pediatrics is not well understood. Importantly, the clinical presentation and pathophysiological mechanisms underlying common cardiac stressors in children are quite discrepant from adults. For example, the most common clinical indication for measuring cTns in adults is suspicion of myocardial infarction (MI). Due to the extreme rarity of pediatric MI, the clinical value of cTns in children has often been discounted. However, existent and emerging literature points toward different, but perhaps equally valuable, indications for these biomarkers in neonates, children, and adolescents relative to adults. Here, we critically review the current literature investigating potential clinical applications for cardiac biomarker measurement in pediatric populations and discuss possible implications for clinical decision-making and patient care.
Cardiac Biomarker Interpretation: Unique Pediatric Considerations
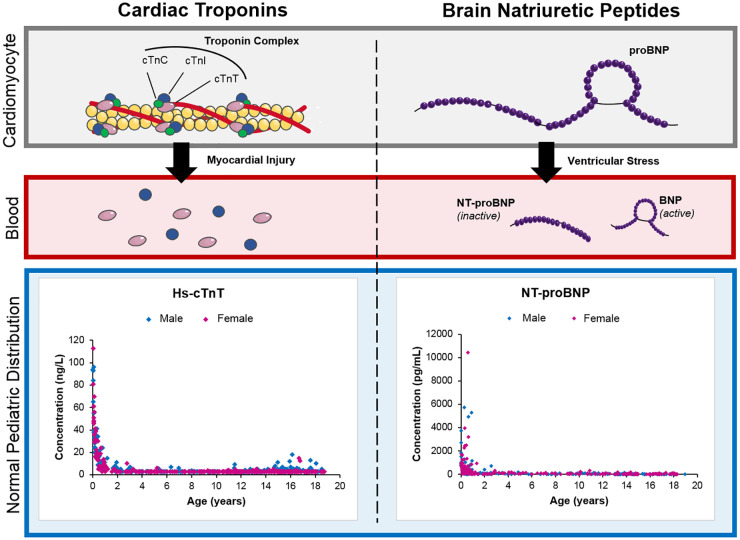
Proper use and interpretation of cardiac biomarkers in a pediatric context require special consideration, as well as a basic understanding of their physiological role, analytical limitations, and underlying concentration pattern throughout childhood and adolescence (Fig. 1).
Fig. 1.
Overview of main cardiac biomarkers and their structures in cardiomyocytes and subsequent release into circulation. Pediatric reference value distributions for hs-cTnT and NT-proBNP are also included (1, 2).
Cardiac Troponins
Tropomyosin protein contains complexes composed of cardiac troponin C (cTnC), cardiac troponin I (cTnI), and cardiac troponin T (cTnT). In healthy individuals, most cTn is bound to the sarcomere with the remainder existing freely in the cytoplasm within cardiac myocytes. In adults, both cTnI and cTnT are of diagnostic and clinical value in the identification of myocardial injury. The clinical and analytical considerations associated with cTnI and cTnT measurement in this context, including assay sensitivity, commutability, and interpretation, have been extensively reviewed elsewhere (3). In brief, the Fourth Universal Definition of MI defines acute myocardial injury as rising or falling patterns in cTn values with at least one value above the assay-specific 99th percentile (4). While cTn values increase under ischemic conditions as a reflection of myocardial injury, the underlying pathophysiological mechanism (e.g., preload-induced mechanical stretch, myocardial cell turnover, and apoptosis) cannot be definitively concluded. An important caveat to the specificity of cTns in the identification of myocardial injury in adults is re-expression of cTnT in injured skeletal muscle, causing false-positive cardiac diagnoses (4). This has not been reported to date for cTnI (4). An appreciation of this is important in the interpretation of increases in cTn in both adults and children.
Evaluating cTns in pediatrics presents unique challenges that are often overlooked in available adult-focused literature. The International Federation for Clinical Chemistry and Laboratory Medicine (IFCC) Committee on Clinical Applications of Cardiac Bio-Markers (C-CB) has developed definitive tables of 99th percentiles for the majority of high-sensitivity, contemporary, and point-of-care cTn assays (Table 1) (10). Unfortunately, these manufacturer-derived percentile cut-offs are based exclusively on adult populations. In the limited literature available, cTnI and cTnT concentrations in healthy children and adolescents have been reported to differ slightly as compared to adults, with some reports indicating slight increases, particularly in the neonatal period (Table 1) (1, 2, 9, 11). These relative increases could be a result of cTn release from cardiomyocytes due to physiological growth (1, 2, 9, 11). While the mechanism behind increased neonatal cTn concentrations is unknown, fetal cTn expression in the skeletal muscle, transient hypoxia at birth, and/or cardiac leakage are proposed to contribute to increased concentrations (12). These concentration dynamics suggest age-specific interpretation may be required within the pediatric population. Age-specific dynamics in cTn have also been observed in adults, with higher concentrations reported in the elderly. Considering age in cTn interpretation is thought to add significant clinical complexity with unknown improvements on clinical outcome. However, in pediatrics, it is clear that cTn interpretation in neonates requires age-specific interpretation (Fig. 1).
Table 1.
Comparison of reference values for common cardiac biomarkers in healthy children and adults as well as key primary cardiac clinical decision limits in adults.
| Physiological reference values |
Clinical decision limits |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Children |
Adults |
Adults |
|||||||
| Analyte | Population (assay, citation) | Upper reference limita | Population (assay, citation) |
Upper reference limita | Ruling out HF in non-acute settings (5) | Ruling out HF in acute settings (5) | Detection of myocardial injury and infarction (4) | ||
|
BNP (pg/mL) |
Residual patient specimens (Biosite, (6)) |
0–30 d: <1585 30–9 d: <1259 3–5 m: <759 6 m–1 y: <263 1–2 y M: <173 3–9 y M: <132 10–14 y M: <120 15–17 y M: <100 |
1–2 y F: <158 3–9 y F: <120 10–14 y F: <115 15–17 y F: <107 |
Healthy adult population (Biosite, (7)) |
45–54 y M: <40 55–64 y M: <52 65–74 y M: <67 75–83 y M: <86 |
45–54 y F: <73 55–64 y F: <93 65–74 y F: <120 75–83 y F: <155 |
Unlikely: <50 |
Unlikely: <100 Possible: 100–400 Very likely: >400 |
NA |
|
NT-proBNP (pg/mL) |
CALIPER healthy cohort (Roche cobas, (2)) |
0–11 m: <3569 1–18 y: <178 |
Framingham Heart Study Generation 3 Cohort (Roche Elecsys, (8)) |
20–24 y M: <42 25–29 y M: <52 30–34 y M: <53 35–39 y M: <69 40–44 y M: <60 45–49 y M: <85 50–54 y M: <104 55–59 y M: <131 |
20–24 y F: <104 25–29 y F: <154 30–34 y F: <134 35–39 y F: <154 40–44 y F: <162 45–49 y F: <186 50–54 y F: <201 55–59 y F: <224 |
Unlikely: <125 |
Unlikely: <300 Possible: <50 y: 300–450 50–75 y: 450–900 >75 y: 900–1800 Very likely: <50 y: >450 50–75 y: >900 >75 y: >1800 |
NA | |
|
hs-cTnI (ng/L) |
CALIPER healthy cohort (Abbott Architect—LoD =1.1 ng/L, (9)) |
1–18 y: <30.9 |
IFCC C-CB Tables (Abbott Architect-LoD = 1.1 ng/L, (10)) |
>18 y M: <34.2 | >18 y F: <15.6 | NA | NA | Myocardial injury: cTn value above the 99th percentile | |
|
hs-cTnT (ng/L) |
CALIPER healthy cohort (Roche cobas— LoD = 3 ng/L, (1)) |
0–5 m: <93 6–11 m: <21 1–18 y M: <14 |
1–18 y F: <11 |
IFCC C-CB Tables (Roche cobas cobas—LoD = 3 ng/L, (10)) |
>18 y M: <16 | >18 y F: <9 | NA | NA | Myocardial infarction: clinical evidence of myocardial ischemia and a rise and/or fall of cTn values >99th percentile |
BNP, brain natriuretic peptide; NT-proBNP, N-terminal fragment prohormone brain natriuretic peptide; hs-cTnI, high sensitivity cardiac troponin I; hs-cTnT, high sensitivity cardiac troponin T; HF, heart failure; d, days; m, months; y, years; M, male; F, female; IFCC, International Federation for Clinical Chemistry and Laboratory Medicine; C-CB, Committee on Clinical Applications of Cardiac Bio-Markers; LoD, limit of detection.
Upper reference limits are denoted as 95th percentiles for BNP, 97.5th percentiles for NT-proBNP, and 99th percentiles for hs-cTnI and hs-cTnT. Manufacturers were selected to be concordant between studies.
Minor sex-specific differences in cTn have also been observed in healthy adolescents, with higher concentrations in males relative to females (1). This observation is consistent with adult findings, although the endorsement of sex-specific 99th percentiles for cTns in the assessment of MI is not consistent across adult-based clinical guidelines (4, 13). Based on available evidence, sex-specific 99th percentiles are strongly encouraged by the laboratory community (13). Taken together, these considerations underscore the potential importance of interpreting cTnI and cTnT concentrations in an age- and sex-specific manner. More large-scale studies are needed to support whether age and sex-stratification is of clinical value. In addition to considering age/sex, differences between the clinical utility of cTnI and cTnT are not well understood, especially in pediatrics. This evidence gap combined with lack of standardization in cTn assays poses clinical challenges and complicates the comparison and interpretation of research study findings, particularly in pediatrics where literature is more limited. In this review, findings from conventional and high sensitivity assays will be notated as cTn in ng/mL and hs-cTn in ng/L, respectively. The isoform of cTn (i.e., cTnT, cTnI) will be specified.
B-Type Natriuretic Peptides
BNPs are released primarily from the cardiac ventricles in response to pressure overload and ventricular wall stretch. The prohormone, proBNP, is released and subsequently cleaved to produce the active hormone, BNP, and an inactive N-terminal fragment, NT-proBNP. Similar to cTn, BNP and NT-proBNP interpretation in children is complicated by unique age and sex dynamics. Pediatric reference concentrations of BNP and NT-proBNP have been reported to be significantly increased at birth, decreasing rapidly during the first few weeks of life, after which concentrations remain largely stable (6, 14, 15). The increased concentration at birth is suspected to be a result of perinatal circulatory changes causing increased ventricular volume and pressure (16). Following the neonatal period, there is a decline in BNP and NT-proBNP concentrations. Some studies have observed slight increases in puberty, suggesting modulation of cardiac natriuretic peptides by endocrine and/or paracrine actions of sex hormones on the renin-angiotensin system (17). However, others have reported no change in adolescence relative to early childhood (2). When comparing pediatric and adult values, BNP and NT-proBNP have been observed to be higher in children and adolescents (Table 1). The mechanism behind such physiological increases is unknown and further research is needed. The influence of sex on BNP and NT-proBNP concentrations is even less clear, and conflicting findings have been reported (14, 15). Furthermore, the clinical utility and performance of BNP and NT-proBNP are largely regarded as comparable in many adult guidelines, with the exception of evaluating patients on angiotensin receptor–neprilysin inhibitors (ARNIs) where NT-proBNP is preferred due to BNP acting as a substrate for neprilysin (5, 18). Their relative benefits in pediatric indications are not clear. Most studies do not provide rationale for selecting BNP vs NT-proBNP and few evaluate comparative clinical utility. Biomarker selection may be related to analytical considerations; NT-proBNP is often seen as more desirable due to its longer half-life, increased stability in vitro, and better concordance between assays compared to BNP (19).
Additional Cardiac Biomarkers
While cTns, BNP, and NT-proBNP dominate clinical practice and recent literature, other cardiac biomarkers have demonstrated value in the identification of myocardial injury or HF in adults. Specifically, prior to the development and clinical implementation of hs-cTn assays, CK-MB and myoglobin were primarily used for the identification of MI in adults. Given the improved sensitivity and specificity of cTns relative to CK-MB and myoglobin, The Fourth Universal Definition of MI only recommends their measurement when cTn is not available (4). This has led many clinical laboratories to stop offering these assays. Thus, this review will not focus on their application in pediatrics.
In addition to markers of myocardial injury, ST2, galectin-3, and H-FABP have demonstrated value in the prognostication of HF in adults. In contrast to CK-MB and myoglobin, these markers are relatively novel and often not available on routine analyzers, requiring specialized assay methodologies (e.g., ELISA), leading to lack of clinical implementation. Guidelines on the classification of HF in adults do not provide strong recommendations on the clinical application of these markers (5, 18). Unsurprisingly, their role in pediatrics is even less clear with very few publications reporting clinical and normative data. Thus, these cardiac biomarkers will not be a focus in this review, but should be revisited as more literature becomes available.
In summary, as demonstrated in Fig. 1, cTnI, cTnT, BNP, and NT-proBNP concentrations vary by age and sometimes by sex, requiring consideration of these covariates in pediatric test interpretation. An appreciation of this, along with analytical and biochemical differences, is important to keep in mind in test application and interpretation in children and adolescents.
Potential clinical indications for cardiac biomarkers in pediatrics
Primary cardiac disease
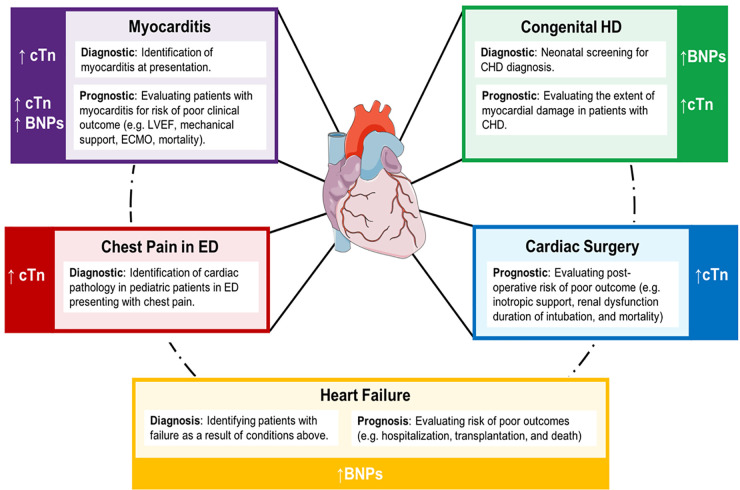
While the overall prevalence of cardiac disease in the pediatric population is much lower when compared to adults, it contributes significantly to pediatric morbidity and mortality with high disease burden. Noninvasive biochemical monitoring may present a clinically useful resource in pediatric cardiology, correlating to severity of congenital heart disease, as well as various acquired heart conditions. However, very few guidelines are available to support their definitive clinical use in certain contexts in pediatrics. A summary of literature regarding potential indications for cardiac biomarkers in pediatric heart diseases is described below (Fig. 2).
Fig. 2.
Summary of potential diagnostic and prognostic indications for traditional cardiac biomarkers in pediatric heart disease. Note: cTn isoforms and BNPs are not differentiated due to insufficient literature to support a clinical advantage of measuring one versus the other.
Emergency Settings
The measurement of cTn in adult patients presenting to the emergency department (ED) with chest pain is routine. In pediatrics, suspicion of MI or other underlying cardiac pathology in patients with chest pain is low, and thus the utility of cTn in this scenario is heavily debated. Few retrospective studies have suggested that both cTnI and cTnT can be useful adjuncts in the differentiation of cardiac involvement in these patients (20–23). In a recent study by Dionne et al., increased cTnT (≥0.1 ng/mL) was observed in 9% of the study population (0 to <20 years, ED patients) and a cardiac diagnosis was made in 60% of those patients (20). The most common cardiac diagnoses observed included myocarditis, cardiomyopathy, and arrhythmia (20), similar to other reports (21–23). Serial cTn measurement was not reported as useful when cTn concentrations were increased at presentation, but was suggested to be of potential value when initial concentrations are not increased and there is ongoing concern about cardiac involvement (20). Other reports did not specifically evaluate the impact of serial testing, but discussed potential value in trending cTn values (21–23). These findings suggest that while there is very low likelihood of MI in the pediatric population, cTn screening in children presenting with chest pain may be useful in the identification of underlying cardiac etiology, especially in the presence of clinical suspicion or abnormal electrocardiogram. It is important to note there are no clinical recommendations supporting the measurement of cTn in children presenting with nonspecific chest pain. Indeed, many argue cTn screening provides minimal benefit in relation to its associated costs and resource utilization (24). Prospective cohort studies are needed to weigh the benefits of cTn screening in pediatric EDs using the most up-to-date high sensitivity assays and standardized serial testing algorithms.
Myocarditis
Myocarditis, an inflammatory disease of the heart muscle, remains a diagnostic and prognostic challenge in the pediatric population. Cardiac biomarkers, in conjunction with imaging and endomyocardial biopsy, may aid in both the diagnosis and prognosis of myocarditis in children. Specifically, cTn measurement has been suggested to have clinical value in the exclusion of myocarditis in children, and several diagnostic cut-offs have been proposed. cTnT diagnostic cut-offs of <0.052 ng/mL (sensitivity: 71%, specificity: 86%) (25) and <0.01 ng/mL (sensitivity: 100%, specificity: 85%) (26) have been reported. In both reports, statistics are based on a single screening test, providing limited information on the value of reassessment (25, 26). Recently, Howard et al. used pediatric ED data to assess the appropriateness of these cut-offs (27). As part of their diagnostic algorithm for myocarditis, the authors found that a cTnT cut-off of 0.01 ng/mL was more sensitive as a single screening test and did not significantly compromise specificity. These proposed cut-offs are much lower than those commonly used to identify myocarditis in adults (>0.1 ng/mL). This could suggest that clinical outcomes are associated with smaller relative increases in cTn concentrations in pediatric populations, further highlighting the need for special considerations in children. In addition to cTnT, significant increases in cTnI have been described in pediatric patients with myocarditis relative to idiopathic dilated cardiomyopathy, suggesting value in the differential diagnosis of these conditions (28). BNP has not demonstrated similar differential capacity, with generally poor diagnostic value (28). Literature regarding NT-proBNP measurement in pediatric myocarditis diagnosis is very limited.
In addition to the diagnosis of myocarditis in children, current evidence supports the value of cardiac biomarkers in patient prognosis. Markers of cardiac injury, such as creatine kinase (CK), CK-MB, and/or cTn, have been shown to correlate with poor clinical outcomes in children with myocarditis, including the need for extracorporeal membrane oxygenation (ECMO) support and higher risk of mortality (29, 30). NT-proBNP and BNP also have demonstrated value in the prediction of low left ventricular ejection fraction, need for mechanical circulatory support, and risk of cardiac arrest in children with myocarditis (31, 32). Thus, monitoring various cardiac biomarkers may provide both diagnostic and prognostic value in the setting of pediatric myocarditis, particularly given existing challenges in rapid patient identification.
Congenital Heart Disease
Congenital heart disease (CHD) is the most common form of congenital anomaly worldwide, with complex CHDs representing the most severe form of illness. The clinical utility of cardiac biomarkers in the context of CHD relates mainly to prognosis, although BNP has been proposed as a neonatal screening tool for the identification of CHD (33). Focusing on prognosis, CHDs are commonly associated with anatomical abnormalities, volume and pressure overload, cyanosis, and pulmonary hypertension (PH). These pathophysiological stressors can result in myocardial damage due to the exertion of direct pressure and stretching of myocardial cells. Thus, there is an anticipated role for markers of cardiac injury in assessing the extent of myocardial damage in pediatric patients with CHD. Indeed, increases in hs-cTnI have been observed in children with atrial septal defects (ASDs) and to a greater extent in ventricular septal defects (VSDs) as a potential indicator of silent myocardial damage (34). Stratification of pediatric CHD patients based on hs-cTnT increases (>14 ng/L) has also been shown to predict higher incidence of cardiovascular events upon hospital admission (35). Markers of cardiac injury thus should be considered when assessing myocardial damage and prognosis in pediatric patients with CHD.
Another important consideration in the prognosis of pediatric patients with CHD is the identification of PH and/or HF. PH is characterized by increased blood pressure combined with arterial resistance in the pulmonary vasculature. NT-proBNP, BNP, cTnI, and cTnT have all been shown to correlate with measures of PH in pediatric CHD patients, suggesting they may be useful in the differentiation of CHD patients with and without PH (36). In addition, both BNP and NT-proBNP have demonstrated clinical value in the diagnosis of HF in patients with CHD, as discussed in detail below.
Cardiac Surgery
Predicting postoperative outcomes in pediatric cardiac surgery is key in reducing adverse events, hospital stays, and the need for reoperation. The value of cTns in postoperative evaluation, particularly in neonatal and pediatric CHD patients, is well appreciated in the literature (37–41). Indeed, a formative prospective study by Immer et al. showed that cTnI concentrations >25 ng/mL in children at 4 h and >35 ng/mL at 24 h after admission to the intensive care unit (ICU) postsurgery, reliably predicted the need for increased inotropic support, renal dysfunction, and duration of intubation (41). The validity of these findings is supported by more recent studies evaluating persistent postoperative pediatric cTn increases, indicating value in assessing risk of mortality as well as low cardiac output syndrome (37–40). There is conflicting evidence to suggest that other cardiac markers, such as BNP, possess similar clinical utility (40). High pediatric preoperative concentrations of BNP have been shown to be an independent predictor of prolonged ICU stay (38). Conversely, the use of BNP as a prognostic marker for readmission and mortality in children was refuted by both Gupta et al. and Parker et al. (42, 43), suggesting further investigation is warranted.
Heart Failure
The clinical manifestations of HF in infants and children have been reported as diverse and dissimilar to adults, with primary causes relating to conditions discussed above, including dilated cardiomyopathy, myocarditis, and CHD. As most pediatric patients with new-onset HF are diagnosed late in disease pathogenesis in a state of severe decompensation, facilitating early diagnosis and accurate prognostication through laboratory testing is of great interest (44). In this regard, BNP and NT-proBNP have demonstrated utility in identifying HF in pediatric patients presenting with nonspecific symptoms of respiratory and noncardiac disease. A study by Auerbach et al. concluded that a BNP concentration >140 pg/mL in pediatric outpatients with mild-to-moderately symptomatic HF can be used to identify children at higher risk for worse outcomes, including hospitalization, transplantation, and death (45). As notated in Table 1, this cut-off is substantially higher than that proposed to rule out HF as unlikely in adults in ambulatory settings (>50 pg/mL). Few guidelines for the management of pediatric HF are available. The Canadian Cardiovascular Society published a guideline on the Presentation, Diagnosis, and Management of Heart Failure in Children in 2013 (44). In this guideline, BNP and/or NT-proBNP measurement is strongly recommended in the evaluation of acute HF in children. However, unlike their corresponding adult recommendations, specific quantitative cut-offs are not provided. This lack of recommended quantitative cut-offs presents challenges in the clinical implementation of this guideline in pediatric healthcare centers and further highlights the lack of clinical appreciation for these markers in pediatrics.
In summary, there is evidence suggesting advantage of the application of cardiac biomarkers in the assessment of pediatric heart conditions, especially in myocarditis, CHD, cardiac surgery, and HF (Fig. 2). However, despite available literature, there is limited clinical guidance to support the routine measurement of these parameters in children and adolescents with primary heart conditions. Future work should focus on more extensive outcome studies in pediatrics using up-to-date analytical assays in a prospective fashion.
Infectious, autoimmune, & rheumatic disease
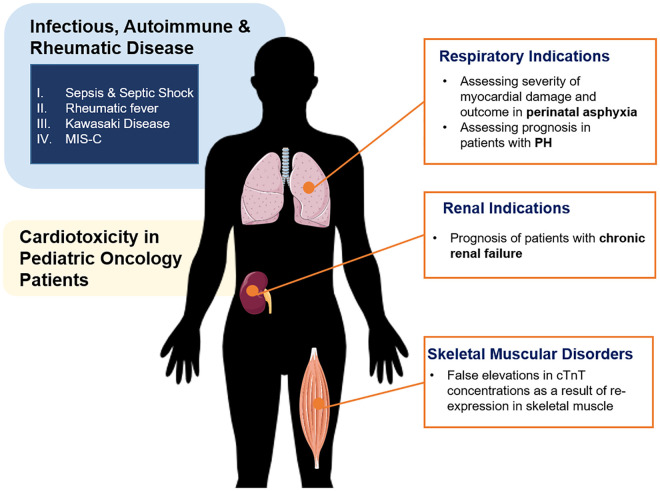
The literature regarding the clinical utility of cardiac biomarker assessment in pediatric patients is not limited to evaluating primary cardiac disease. Indeed, some studies suggest utility in prognosis and monitoring of several immune-related conditions with secondary cardiac manifestations, including infectious, autoimmune, and rheumatic disease, as reviewed below and presented in Fig. 3.
Fig. 3.
Summary of potential diagnostic and prognostic indications for cardiac biomarkers in noncardiac conditions in children and adolescents.
Sepsis & Septic Shock
The underlying immunopathology of various infectious diseases can result in cardiac dysfunction in severe cases. Current literature supports the measurement of cardiac biomarkers to assist in patient risk assessment and outcome prediction in various infectious diseases in children, particularly sepsis. Studies have shown that cTnI and BNP concentrations measured upon hospital admission are associated with myocardial dysfunction among septic pediatric patients (46, 47). These cardiac biomarkers have also been indicated as useful prognostic measures in the assessment of sepsis severity. In a prospective observational study, Fenton et al. observed increased cTnI (>0.1 ng/mL) in more than 50% of children with septic shock upon hospital admission, which was associated with severity of illness (48). More recently, Zhang et al. reported cTnI and BNP measured one day after admission were significantly associated with the severity of sepsis in 120 pediatric patients (49). Compared to children with sepsis, Wu et al. observed significantly higher BNP concentrations in children with septic shock, as well as among nonsurvivors relative to survivors (47). NT-proBNP concentrations have also been shown to be useful in differentiating HF in children with sepsis, as well as in predicting in-hospital mortality (50, 51). The mechanisms behind increases in cTns and BNPs in septic neonatal and pediatric patients remain unclear, although many groups postulate direct inflammatory injury to myocytes results in cardiomyocyte necrosis, leading to increased cTn concentrations. Increased BNP concentrations may also be attributed to severity of illness, including extent of inflammation and renal damage, rather than underlying cardiomyopathy.
Rheumatic Disease
Cardiac involvement in pediatric onset rheumatic diseases has prompted investigation into the clinical utility of cardiac biomarkers in patient assessment. Specifically, acute rheumatic fever (ARF) is a major cause of HF in children and continues to be an important public health concern, particularly in developing countries. It is estimated that approximately 60% of ARF patients develop rheumatic carditis, progressing to rheumatic heart disease and constituting the main cause of morbidity and mortality (52). Despite known cardiac involvement in ARF, several studies investigating the concentrations of prominent cardiac injury markers such as cTnI, cTnT, and CK-MB in pediatric patients with rheumatic carditis compared to ARF patients without active carditis (53–55), patients with scarlet fever (55), and healthy control children (56) have found no clinically significant differences. However, Ozdemir et al. did report a slight statistically significant increase in cTnT concentrations in 28 pediatric patients with ARF active carditis in comparison to 32 healthy control children, although this observed increase did not surpass reported normal limits (57). It is speculated that this lack of prominent change, specifically in cTnI and cTnT concentrations, suggests rheumatic carditis results from connective tissue involvement leading to valve malfunction, rather than direct myocyte damage (54, 55). Conversely, NT-proBNP concentrations have been reported to be significantly increased in children with rheumatic carditis compared both to children with quiescent rheumatic heart disease (58) and healthy control children (58–60). Therefore, while cTnI, cTnT, and CK-MB concentrations likely have limited value in the diagnosis or prognosis of rheumatic carditis in children, NT-proBNP could present a useful adjunct in this regard.
Kawasaki Disease
Kawasaki disease (KD) is an acute systemic vasculitis that primarily affects children under 5 years of age and is considered by many to be the most prominent cause of pediatric acquired heart disease. Currently, the diagnosis of KD is based on the presentation of clinical features and lacks a specific diagnostic biomarker. However, several studies have reported significantly increased concentrations of NT-proBNP in infants and children diagnosed with KD compared to febrile controls, as reviewed elsewhere (61). Following an early prospective study by Dahdah and colleagues, which concluded NT-proBNP to be a better marker of KD compared to BNP, particularly in incomplete cases, the majority of research in this field has focused on NT-proBNP (62).
Importantly, NT-proBNP has been suggested to play a role in supporting the diagnosis of KD, even in the hyper-acute stage of disease when fever has not been present long enough for a definitive diagnosis based on clinical criteria alone (63). Moreover, some studies have proposed NT-proBNP may be of clinical value in the identification of KD patients at risk for developing coronary artery lesions (64, 65) or progression to KD Shock Syndrome (66). Despite promising findings, many researchers stress that NT-proBNP is insufficient to be used as a stand-alone test in the diagnosis of KD. NT-proBNP testing is also not supported in the American Heart Association clinical diagnostic guidelines for KD, as it may not be sufficient to differentiate KD, and clear cut-off points have not yet been defined (67). Research investigating other prominent cardiac biomarkers in KD diagnosis and prognosis are conflicting and do not yet support routine use in clinical practice.
It is also important to note that cardiac biomarkers may assist in the identification and assessment of pediatric patients with multisystem inflammatory syndrome in children (MIS-C), the novel hyperinflammatory condition with KD-like features associated with coronavirus disease 2019 (COVID-19). Cardiovascular involvement in patients with MIS-C has been reported as common. Indeed, a recent US study of 185 MIS-C patients reported 73% and 50% of patients presented with increased BNP and cTn concentrations, respectively (68). The clinical utility of these markers in the assessment of this novel condition should continue to be evaluated as new data emerge.
Other indications
Similar to the immune-related diseases described above, many pathophysiological conditions originating from other organs can ultimately lead to cardiac distress. Thus, cardiac biomarkers have been investigated in the prognostication of various noncardiac conditions (Fig. 3). Specifically, hypoxemia-related myocardial dysfunction is estimated to occur in approximately 30% of neonates, with increased incidence in preterm infants. Increases in cTns are estimated to be useful in evaluating the severity of myocardial damage and outcome in perinatal asphyxia (69). In addition, myocardial stress is common in children with chronic kidney disease (CKD). Although renal transplantation may improve the cardiac damage associated with CKD, it possesses its own cardiac risks requiring monitoring. The assessment of NT-proBNP to identify cardiac strain in patients with compromised renal function can be challenging, due to its physiological clearance by the kidney. However, studies have demonstrated good correlations between NT-proBNP concentrations and reliable echocardiographic markers of cardiac strain, suggesting it could be a useful adjunct in assessing pediatric patients with renal insufficiency (70).
An additional application for pediatric cardiac biomarkers is their use in monitoring cardiotoxicity in pediatric patients after cancer treatment. Anticancer drugs, particularly anthracyclines, can elicit cardiotoxic effects in pediatric cancer patients and lead to irreversible cardiovascular damage, as well as cardiovascular disease (71). BNP and NT-proBNP concentrations have shown to be increased in childhood cancer patients post-treatment with anthracyclines, compared to concentrations pretreatment or in healthy controls, suggesting cardiotoxic effects (72). The risk of developing acute, but not late-onset, anthracycline-related left ventricular dysfunction has been indicated by an increase in BNP or NT-proBNP; however, sensitivity is poor (72). More evidence is required to determine the accuracy of cTnI and cTnT as a marker for cardiotoxicity and resulting cardiac damage in children.
One final consideration of importance in the interpretation of pediatric cardiac biomarkers is their evaluation in musculoskeletal disorders. Increases in cTnT have been observed in pediatric patients with skeletal muscle damage, including Pompe disease, with no evidence of cardiac etiology (73). It is estimated that cTnT is re-expressed in skeletal muscle tissue in these cases, causing a potentially false “cardiac positive” interpretation (73). It is important to take this into consideration when evaluating children with musculoskeletal disorders and avoid unnecessary cardiac testing and interventions (73).
Future Directions: At the Heart of the Matter
This critical review summarizes potential clinical indications for cardiac biomarkers in pediatrics, which are predominantly ordered in adults. While the use of these biomarkers is often discounted in pediatric practice, current literature suggests a multitude of possible applications in both cardiac and noncardiac conditions. However, it is important to note that the interpretation and comparison of available literature is complicated by the use of different analytical methodologies and study populations. Very few studies examine both BNP and NT-proBNP as well as cTnI and cTnT, as most literature is retrospective and based on the assays in clinical use at that institution. Thus, the advantages of selecting cTnI vs cTnT or BNP vs NT-proBNP in certain clinical indications is difficult to ascertain. Additionally, many studies use manufacturer-derived cut-offs, which are based exclusively on adults, to define increases in these biomarkers. Indeed, the assessment of these markers in children and adolescents is complicated by physiological changes of these markers with age or sex, necessitating the development and use of multiple age- and/or sex-stratified cut-off values. This is particularly true for neonates wherein increased concentrations are physiologically prevalent and unique applications are possible.
Despite these interpretive limitations, current literature suggests that cardiac biomarkers have potential utility in the diagnosis and prognosis of myocarditis, CHD, cardiac surgery, and HF, as well as the assessment of severity and cardiac involvement in immune-related and other systemic conditions. However, the majority of data discussed in this review reports correlations between increased cardiac biomarkers and clinical outcome based on retrospective chart review. There is a critical evidence gap in prospective studies demonstrating the specific functionality of these biomarkers in preventing unnecessary hospitalization and medical intervention and/or improving time to appropriate treatment and clinical outcome. While these studies are challenging to complete in the pediatric population, particularly given low patient incidence, they are urgently needed to support evidence-based guidelines and appropriate clinical implementation of select cardiac biomarkers in the assessment of both cardiac and noncardiac conditions.
Further, in addition to knowing when to order these tests, another important question that remains to be answered is how often they should be ordered. In the context of diagnosis, rising and falling values of cTn in ED assessment of adult MI is paramount. Exactly how this translates to pediatric practice is unclear, particularly as underlying pathophysiological stressors causing increased cTn in children presenting to the ED are vastly different from those in adults. Although some literature has suggested retesting in acute settings when initial concentrations of cTn are not increased (20), there is no consensus. Unlike cTn, serial measurements of BNP or NT-proBNP to reduce hospitalizations or mortality in the context of adult HF are not recommended due to insufficient data. Unsurprisingly, the value of serial BNP or NT-proBNP measurement in children and adolescents is even less clear. Without this information, it is difficult to provide guidance to clinicians on proper utilization of these tests in pediatrics, warranting further prospective study.
In conclusion, this review provides novel perspective and raises important considerations for the increasing application of cardiac biomarkers in neonates, children, and adolescents, highlighting a population that is often overlooked in cardiac biomarker research. While it is clear cardiac biomarkers have potential value in the assessment of several conditions with cardiac and noncardiac etiology, additional research is urgently needed to establish evidence-based cut-offs and usage guidelines in different clinical contexts to optimize utilization and standardize interpretation of cardiac biomarkers in children and adolescents.
Nonstandard Abbreviations
- BNP
B-type natriuretic peptide
- NT-proBNP
N-terminal fragment prohormone B-type natriuretic peptide
- cTn
cardiac troponin
- hs-cTn
high sensitivity cardiac troponin
- ACS
acute coronary syndrome
- HF
heart failure
- CK-MB
creatine-kinase MB isoform
- ST2
soluble interleukin 1 receptor-like 1
- H-FABP
heart-type fatty acid-binding protein
- MI
myocardial infarction
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- IFCC
International Federation for Clinical Chemistry and Laboratory Medicine
- C-CB
Committee on Clinical Applications of Cardiac Bio-Markers
- ARNI
angiotensin receptor-neprilysin inhibitor
- ED
emergency department
- CK
creatine kinase
- ECMO
extracorporeal membrane oxygenation
- CHD
congenital heart disease
- PH
pulmonary hypertension
- ASD
atrial septal defect
- VSD
ventricular septal defect
- ICU
intensive care unit
- ARF
acute rheumatic fever
- KD
Kawasaki disease
- MIS-C
multisystem inflammatory syndrome in children
- COVID-19
coronavirus disease 2019
- CKD
chronic kidney disease
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
None declared.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
M.K. Bohn, Canadian Institutes of Health Research (CIHR) Doctoral Award.
Expert Testimony
None declared.
Patents
None declared.
References
- 1.Bohn MK, Higgins V, Kavsak P, Hoffman B, Adeli K.. High-sensitivity generation 5 cardiac troponin T sex- and age-specific 99th percentiles in the CALIPER cohort of healthy children and adolescents. Clin Chem 2019;65:589–91. [DOI] [PubMed] [Google Scholar]
- 2.Lam E, Higgins V, Zhang L, Chan MK, Bohn MK, Trajcevski K, et al. Normative values of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in children and adolescents: a study from the CALIPER cohort. J Appl Lab Med 2021;6:344–53. [DOI] [PubMed] [Google Scholar]
- 3.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J, IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–64. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017;33:1342–433. [DOI] [PubMed] [Google Scholar]
- 6.Soldin SJ, Soldin OP, Boyajian AJ, Taskier MS.. Pediatric brain natriuretic peptide and N-terminal pro-brain natriuretic peptide reference intervals. Clin Chim Acta 2006;366:304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82. [DOI] [PubMed] [Google Scholar]
- 8.Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 2011;108:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavsak PA, Rezanpour A, Chen Y, Adeli K.. Assessment of the 99th or 97.5th percentile for cardiac troponin I in a healthy pediatric cohort. Clin Chem 2014;60:1574–6. [DOI] [PubMed] [Google Scholar]
- 10.Collinson PO, Saenger AK, Apple FS, Ifcc C-CB.. High sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin Chem Lab Med 2019;57:623–32. [DOI] [PubMed] [Google Scholar]
- 11.Apple FS, Wu AHB, Sandoval Y, Sexter A, Love SA, Myers G, et al. Sex-specific 99th percentile upper reference limits for high sensitivity cardiac troponin assays derived using a universal sample bank. Clin Chem 2020;66:434–44. [DOI] [PubMed] [Google Scholar]
- 12.Caselli C, Cangemi G, Masotti S, Ragusa R, Gennai I, Del RS, et al. Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a high sensitive immunoassay method: High sensitive troponin I in pediatric age. Clin Chim Acta 2016;458:68–71. [DOI] [PubMed] [Google Scholar]
- 13.Apple FS, Collinson PO, Kavsak PA, Body R, Ordóñez-Llanos J, Saenger AK, et al. Getting cardiac troponin right: Appraisal of the 2020 European Society of Cardiology guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation by the International Federation of Clinical Chemistry and Laboratory Medicine Committee on Clinical Applications of Cardiac Bio-Markers. Clin Chem 2021;67:730–5. [DOI] [PubMed] [Google Scholar]
- 14.Mir TS, Flato M, Falkenberg J, Haddad M, Budden R, Weil J, et al. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and gender. Pediatr Cardiol 2006;27:73–7. [DOI] [PubMed] [Google Scholar]
- 15.Koch A, Singer H.. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart 2003;89:875–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannarino S, Garofoli F, Mongini E, Cerbo RM, Codazzi AC, Tzialla C, et al. BNP concentrations and cardiovascular adaptation in preterm and full-term newborn infants. Early Hum Dev 2010;86:295–8. [DOI] [PubMed] [Google Scholar]
- 17.de Bold AJ, Ma KK, Zhang Y, de Bold ML, Bensimon M, Khoshbaten A.. The physiological and pathophysiological modulation of the endocrine function of the heart. Can J Physiol Pharmacol 2001;79:705–14. [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–61. [DOI] [PubMed] [Google Scholar]
- 19.Farnsworth CW, Bailey AL, Jaffe AS, Scott MG.. Diagnostic concordance between NT-proBNP and BNP for suspected heart failure. Clin Biochem 2018;59:50–5. [DOI] [PubMed] [Google Scholar]
- 20.Dionne A, Kheir JN, Sleeper LA, Esch JJ, Breitbart RE.. Value of troponin testing for detection of heart disease in previously healthy children. J Am Heart Assoc 2020;9:e012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thankavel PP, Mir A, Ramaciotti C.. Elevated troponin levels in previously healthy children: value of diagnostic modalities and the importance of a drug screen. Cardiol Young 2008;24:283–9. [DOI] [PubMed] [Google Scholar]
- 22.Harris TH, Gossett JG.. Diagnosis and diagnostic modalities in pediatric patients with elevated troponin. Pediatr Cardiol 2016;37:1469–74. [DOI] [PubMed] [Google Scholar]
- 23.Brown JL, Hirsh DA, Mahle WT.. Use of troponin as a screen for chest pain in the pediatric emergency department. Pediatr Cardiol 2012;33:337–42. [DOI] [PubMed] [Google Scholar]
- 24.Liesemer K, Casper TC, Korgenski K, Menon SC.. Use and misuse of serum troponin assays in pediatric practice. Am J Cardiol 2012;110:284–9. [DOI] [PubMed] [Google Scholar]
- 25.Soongswang J, Durongpisitkul K, Nana A, Laohaprasittiporn D, Kangkagate C, Punlee K, et al. Cardiac troponin T: a marker in the diagnosis of acute myocarditis in children. Pediatr Cardiol 2005;26:45–9. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg MA, Green-Hopkins I, Alexander ME, Chiang VW.. Cardiac troponin T as a screening test for myocarditis in children. Pediatr Emerg Care 2012;28:1173–8. [DOI] [PubMed] [Google Scholar]
- 27.Howard A, Hasan A, Brownlee J, Mehmood N, Ali M, Mehta S, et al. Pediatric myocarditis protocol: an algorithm for early identification and management with retrospective analysis for validation. Pediatr Cardiol 2020;41:316–26. [DOI] [PubMed] [Google Scholar]
- 28.Suthar D, Dodd DA, Godown J.. Identifying non-invasive tools to distinguish acute myocarditis from dilated cardiomyopathy in children. Pediatr Cardiol 2018;39:1134–8. [DOI] [PubMed] [Google Scholar]
- 29.Chang YJ, Hsiao HJ, Hsia SH, Lin JJ, Hwang MS, Chung HT, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One 2019;14:e0214087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe T, Tsuda E, Miyazaki A, Ishibashi-Ueda H, Yamada O.. Clinical characteristics and long-term outcome of acute myocarditis in children. Heart Vessels 2013;28:632–8. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Gonzalez M, Sanchez-Codez MI, Lubian-Gutierrez M, Castellano-Martinez A.. Clinical presentation and early predictors for poor outcomes in pediatric myocarditis: a retrospective study. World J Clin Cases 2019;7:548–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casadonte JR, Mazwi ML, Gambetta KE, Palac HL, McBride ME, Eltayeb OM, et al. Risk factors for cardiac arrest or mechanical circulatory support in children with fulminant myocarditis. Pediatr Cardiol 2017;38:128–34. [DOI] [PubMed] [Google Scholar]
- 33.Cantinotti M, Giovannini S, Murzi B, Clerico A.. Diagnostic, prognostic and therapeutic relevance of B-type natriuretic hormone and related peptides in children with congenital heart diseases. Clin Chem Lab Med 2011;49:567–80. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto M, Kuwata S, Kurishima C, Kim JH, Iwamoto Y, Senzaki H.. Cardiac biomarkers in children with congenital heart disease. World J Pediatr 2015;11:309–15. [DOI] [PubMed] [Google Scholar]
- 35.Abiko M, Inai K, Shimada E, Asagai S, Nakanishi T.. The prognostic value of high sensitivity cardiac troponin T in patients with congenital heart disease. J Cardiol 2018;71:389–93. [DOI] [PubMed] [Google Scholar]
- 36.Kayali S, Ertugrul I, Yoldas T, Kaya O, Ozgür S, Orün UA, et al. Sensitive cardiac troponins: could they be new biomarkers in pediatric pulmonary hypertension due to congenital heart disease? Pediatr Cardiol 2018;39:718–25. [DOI] [PubMed] [Google Scholar]
- 37.Su JA, Kumar SR, Mahmoud H, Bowdish ME, Toubat O, Wood JC, et al. Postoperative serum troponin trends in infants undergoing cardiac surgery. Semin Thorac Cardiovasc Surg 2019;31:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Piaya M, Abarca E, Soler V, Coca A, Cruz M, Villagra F, et al. Levels of N-terminal-pro-brain natriuretic peptide in congenital heart disease surgery and its value as a predictive biomarker. Interact Cardiovasc Thorac Surg 2011;12:461–6. [DOI] [PubMed] [Google Scholar]
- 39.Giordano R, Cantinotti M, Arcieri L, Poli V, Pak V, Murzi B.. Arterial switch operation and plasma biomarkers: analysis and correlation with early postoperative outcomes. Pediatr Cardiol 2017;38:1071–6. [DOI] [PubMed] [Google Scholar]
- 40.Carmona F, Manso PH, Vicente WVA, Castro M, Carlotti APCP.. Risk stratification in neonates and infants submitted to cardiac surgery with cardiopulmonary bypass: a multimarker approach combining inflammatory mediators, N-terminal pro-B-type natriuretic peptide and troponin I. Cytokine 2008;42:317–24. [DOI] [PubMed] [Google Scholar]
- 41.Immer FF, Stocker F, Seiler AM, Pfammatter JP, Bachmann D, Printzen G, et al. Troponin-I for prediction of early postoperative course after pediatric cardiac surgery. J Am Coll Cardiol 1999;33:1719–23. [DOI] [PubMed] [Google Scholar]
- 42.Parker DM, Everett AD, Stabler ME, Leyenaar J, Vricella L, Jacobs JP, et al. The association between cardiac biomarker NT-proBNP and 30-day readmission or mortality after pediatric congenital heart surgery. World J Pediatr Congenit Hear Surg 2019;10:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta RK, Zheng H, Cui Y, Qu J, Li L, Liang H, et al. Change in N-terminal pro B-type natriuretic peptide levels and clinical outcomes in children undergoing congenital heart surgery. Int J Cardiol 2019;283:96–100. [DOI] [PubMed] [Google Scholar]
- 44.Kantor PF, Lougheed J, Dancea A, McGillion M, Barbosa N, Chan C, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol 2013;29:1535–52. [DOI] [PubMed] [Google Scholar]
- 45.Auerbach SR, Richmond ME, Lamour JM, Blume ED, Addonizio LJ, Shaddy RE, et al. BNP levels predict outcome in pediatric heart failure patients post hoc analysis of the Pediatric Carvedilol Trial. Circ Hear Fail 2010;3:606–11. [DOI] [PubMed] [Google Scholar]
- 46.Raj S, Killinger JS, Gonzalez JA, Lopez L.. Myocardial dysfunction in pediatric septic shock. J Pediatr 2014;164:72–7. [DOI] [PubMed] [Google Scholar]
- 47.Wu JR, Chen IC, Dai ZK, Hung JF, Hsu JH.. Early elevated B-type natriuretic peptide levels are associated with cardiac dysfunction and poor clinical outcome in pediatric septic patients. Acta Cardiol Sin 2015;31:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenton KE, Sable CA, Bell MJ, Patel KM, Berger JT.. Increases in serum levels of troponin I are associated with cardiac dysfunction and disease severity in pediatric patients with septic shock. Pediatr Crit Care Med 2004;5:533–8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Luo Y, Nijiatijiang G, Balati K, Tuerdi Y, Liu L.. Correlations of changes in brain natriuretic peptide (BNP) and cardiac troponin I (cTnI) with levels of C-reactive protein (CRP) and TNF-α in pediatric patients with sepsis. Med Sci Monit 2019;25:2561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samransamruajkit R, Uppala R, Pongsanon K, Deelodejanawong J, Sritippayawan S, Prapphal N.. Clinical outcomes after utilizing surviving sepsis campaign in children with septic shock and prognostic value of initial plasma NT-proBNP. Indian J Crit Care Med 2014;18:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin CW, Tang W, Wen F, Chen JJ, Zeng XL, Chen Z.. Diagnostic accuracy of NT-proBNP for heart failure with sepsis in patients younger than 18 years. PLoS One 2016;11:e0147930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Prim 2016;2:15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavli V, Canbal A, Şaylan B, Saritaş T, Meşe T, Atlihan F.. Assessment of myocardial involvement using cardiac troponin-I and echocardiography in rheumatic carditis in Izmir, Turkey. Pediatr Int 2008;50:62–4. [DOI] [PubMed] [Google Scholar]
- 54.Alehan D, Ayabakan C, Hallioglu O.. Role of serum cardiac troponin T in the diagnosis of acute rheumatic fever and rheumatic carditis. Heart 2004;90:689–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta M, Lent RW, Kaplan EL, Zabriskie JB.. Serum cardiac troponin I in acute rheumatic fever. Am J Cardiol 2002;89:779–82. [DOI] [PubMed] [Google Scholar]
- 56.Oran B, Çoban H, Karaaslan S, Atabek E, Gürbilek M, Erkul I.. Serum cardiac troponin-I in active rheumatic carditis. Indian J Pediatr 2001;68:943–4. [DOI] [PubMed] [Google Scholar]
- 57.Ozdemir O, Oguz D, Atmaca E, Sanli C, Yildirim A, Olgunturk R.. Cardiac troponin T in children with acute rheumatic carditis. Pediatr Cardiol 2011;32:55–8. [DOI] [PubMed] [Google Scholar]
- 58.Kotby AA, El-Shahed GS, Elmasry OA, El-Hadidi IS, El Shafey RNS.. N-terminal proBNP levels and tissue doppler echocardiography in acute rheumatic carditis. ISRN Pediatr 2013;:970394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esen F, Argun M, Pamukçu Ö, Ozyurt A, Baykan A, Sezer S, et al. Importance of N-terminal pro-brain natriuretic peptide in monitoring acute rheumatic carditis. Cardiol Young 2014;25:110–4. [DOI] [PubMed] [Google Scholar]
- 60.Çimen Ö, Oran B, Çimen D, Baysal T, Karaaslan S, Ünal E, et al. Release of N-terminal pro-brain natriuretic peptide in children with acute rheumatic carditis. Cardiol Young 2010;20:297–301. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez-Gonzalez M, Perez-Reviriego AA, Castellano-Martinez A, Cascales-Poyatos HM.. N-terminal pro-brain natriuretic peptide as biomarker for diagnosis of Kawasaki disease. Biomark Med 2019;13:307–23. [DOI] [PubMed] [Google Scholar]
- 62.Dahdah N, Siles A, Fournier A, Cousineau J, Delvin E, Saint-Cyr C, et al. Natriuretic peptide as an adjunctive diagnostic test in the acute phase of Kawasaki disease. Pediatr Cardiol 2009;30:810–7. [DOI] [PubMed] [Google Scholar]
- 63.Kwon H, Lee JH, Jung JY, Kwak YH, Kim DK, Jung JH, et al. N-terminal pro-brain natriuretic peptide can be an adjunctive diagnostic marker of hyper-acute phase of Kawasaki disease. Eur J Pediatr 2016;175:1997–2003. [DOI] [PubMed] [Google Scholar]
- 64.Kaneko K, Yoshimura K, Ohashi A, Kimata T, Shimo T, Tsuji S.. Prediction of the risk of coronary arterial lesions in Kawasaki disease by brain natriuretic peptide. Pediatr Cardiol 2011;32:1106–9. [DOI] [PubMed] [Google Scholar]
- 65.Jung JY, Ham EM, Kwon H, Kwak YH, Kim DK, Lee JH, et al. N-terminal pro-brain natriuretic peptide and prediction of coronary artery dilatation in hyperacute phase of Kawasaki disease. Am J Emerg Med 2019;37:468–71. [DOI] [PubMed] [Google Scholar]
- 66.Qiu H, Li C, He Y, Weng F, Shi H, Pan L, et al. Association between left ventricular ejection fraction and Kawasaki disease shock syndrome. Cardiol Young 2019;29:178–84. [DOI] [PubMed] [Google Scholar]
- 67.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135:e927–99. [DOI] [PubMed] [Google Scholar]
- 68.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa S, Zecca E, De RG, De LD, Barbato G, Pardeo M, et al. Is serum troponin T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr 2007;96:181–4. [DOI] [PubMed] [Google Scholar]
- 70.Rinat C, Becker-Cohen R, Nir A, Feinstein S, Algur N, Ben-Shalom E, et al. B-type natriuretic peptides are reliable markers of cardiac strain in CKD pediatric patients. Pediatr Nephrol 2012;27:617–25. [DOI] [PubMed] [Google Scholar]
- 71.Lipshultz SE, Sambatakos P, Maguire M, Karnik R, Ross SW, Franco VI, et al. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol 2014;132:391–9. [DOI] [PubMed] [Google Scholar]
- 72.Michel L, Mincu RI, Mrotzek SM, Korste S, Neudorf U, Rassaf T, et al. Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer—a meta-analysis. ESC Hear Fail 2020;7:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wens SCA, Schaaf GJ, Michels M, Kruijshaar ME, van Gestel TJM, In’TGroen S, et al. Elevated plasma cardiac troponin T Levels caused by skeletal muscle damage in Pompe disease. Circ Cardiovasc Genet 2016;9:6–13. [DOI] [PubMed] [Google Scholar]