Key Points
Question
Can computer vision algorithms facilitate accurate percent total body surface area (%TBSA) burn computation across the wide body habitus spectrum of the modern population?
Findings
In this cohort study, 3-dimensional image segmentation of 3047 adult laser body scans were integrated into a mobile application that computes %TBSA burn based on exact burn injury pattern, sex, and body habitus. There is wide individual variability in how much each body region contributes to %TBSA, and the tool developed in this study reflects measured body surface areas of adults across the wide body habitus spectrum of the modern population.
Meaning
An intuitive, accurate, and practical mobile application may be an improvement over existing one-size-fits-all models for computing %TBSA burn, a foundational estimate for burn injury management.
This cohort study develops a practical percent total body surface area burn estimation tool that accounts for exact burn injury pattern, sex, and body habitus.
Abstract
Importance
Critical burn management decisions rely on accurate percent total body surface area (%TBSA) burn estimation. Existing %TBSA burn estimation models (eg, Lund-Browder chart and rule of nines) were derived from a linear formula and a limited number of individuals a century ago and do not reflect the range of body habitus of the modern population.
Objective
To develop a practical %TBSA burn estimation tool that accounts for exact burn injury pattern, sex, and body habitus.
Design, Setting, and Participants
This population-based cohort study evaluated the efficacy of a computer vision algorithm application in processing an adult laser body scan data set. High-resolution surface anthropometry laser body scans of 3047 North American and European adults aged 18 to 65 years from the Civilian American and European Surface Anthropometry Resource data set (1998-2001) were included. Of these, 1517 participants (49.8%) were male. Race and ethnicity data were not available for analysis. Analyses were conducted in 2020.
Main Outcomes and Measures
The contributory %TBSA for 18 body regions in each individual. Mobile application for real-time %TBSA burn computation based on sex, habitus, and exact burn injury pattern.
Results
Of the 3047 individuals aged 18 to 65 years for whom body scans were available, 1517 (49.8%) were male. Wide individual variability was found in the extent to which major body regions contributed to %TBSA, especially in the torso and legs. Anterior torso %TBSA increased with increasing body habitus (mean [SD], 15.1 [0.9] to 19.1 [2.0] for male individuals; 15.1 [0.8] to 18.0 [1.7] for female individuals). This increase was attributable to increase in abdomen %TBSA (mean [SD], 5.3 [0.7] to 8.7 [1.8]) among male individuals and increase in abdomen (mean [SD], 4.6 [0.6] to 6.8 [1.7]) and pelvis (mean [SD], 1.5 [0.2] to 2.9 [0.9]) %TBSAs among female individuals. For most body regions, Lund-Browder chart and rule of nines estimates fell outside the population’s measured interquartile ranges. The mobile application tested in this study, Burn Area, facilitated accurate %TBSA burn computation based on exact burn injury pattern for 10 sex and body habitus-specific models.
Conclusions and Relevance
Computer vision algorithm application to a large laser body scan data set may provide a practical tool that facilitates accurate %TBSA burn computation in the modern era.
Introduction
Burns are the 4th leading cause of unintentional injury deaths globally.1 In the US, nearly 500 000 patients present to emergency departments and 50 000 are hospitalized for burns annually.2 Critical burn management decisions, such as hospital admission and burn center referral criteria, initial fluid resuscitation volume, and optimal caloric intake, rely on one foundational estimate: percent total body surface area (%TBSA) burn.3
The Lund-Browder chart4 and rule of nines5 are widely used to estimate %TBSA burns.3 However, both models were derived from a century-old formula based on papier-mâché molds of 12 individuals aged 21 months to 43 years.6,7,8 Many studies report inaccuracy and poor interrater reliability of existing %TBSA burn estimation models, especially for patients with obesity.9,10,11,12,13,14 Both the Lund-Browder chart and rule of nines assume a singular physique applies for all adults and do not account for the modern population’s wide spectrum of body habitus. Inaccurate %TBSA burn estimation has been associated with increased mortality and morbidity.15,16,17 A better way to estimate %TBSA burns for patients of all body habitus is critically needed.
We aimed to develop a model that facilitates rapid, accurate %TBSA burn computation across the range of adult body habitus. Applying computer vision algorithms to the largest available data set, to our knowledge, on adult surface anthropometry laser body scans, we built a mobile application that outputs precise %TBSA burn measurements based on a patient’s specific burn injury pattern, sex, and body habitus. A better tool to measure %TBSA is essential to cultivate renewed strategies to improve burn injury outcomes.
Methods
Study Population
The study used high-resolution surface anthropometry laser body scans of 3047 adults from the Civilian American and European Surface Anthropometry Resource (CAESAR) data set.18,19 We analyzed 3-dimensional meshes derived from standardized point clouds with 12 500 vertices and 25 000 faces for interindividual comparison.
The study used a publicly available body scan data set and did not meet criteria for Stanford University Institutional Review Board review or need for informed consent.
Body Region Segmentation
We used Open3D and PyVista Python libraries for computational analysis.20,21 After instance segmentation on 3D point clouds for each laser body scan from the CAESAR data set,18 we verified accurate surface construction using ball pivoting algorithm of 54 846 meshes representing 18 body regions (eFigure 1 in the Supplement). We computed the total body volume and TBSA of each individual, and each body region’s contributory %TBSA.
Representative Body Models
We generated 10 representative body models (5 male, 5 female) that a patient’s body habitus could be approximated to match. First, we calculated individuals’ TBSA-to-volume ratios. Because volume increases faster than surface area, TBSA/volume decreases with increasing body habitus.22 Next, individuals were divided into subgroups by TBSA/volume standard deviations (less than −2σ, very large body habitus; −2σ to −1σ, large body habitus; −1σ to 1σ, average body habitus; 1σ to 2σ, small body habitus; greater than 2σ, very small body habitus). The 10 representative body models corresponded to individuals with average TBSA/volume within each subgroup.
We compared the models’ body region %TBSA measurements with rule of nines and Lund-Browder chart estimates. One-way ANOVA compared body region %TBSA variations across body habitus subgroups. All analyses were conducted using Python 3.8.0.
Mobile Application
We developed a mobile application to facilitate model validation and bedside use. Users can select 1 of 10 body models that best represents a patient’s sex and body habitus. Intuitive features allow precise 360° mapping of patients’ second- and greater-degree burn injuries. Model-specific %TBSA burn measurements are updated in real time.
Results
Individual Variation in Body Region %TBSAs
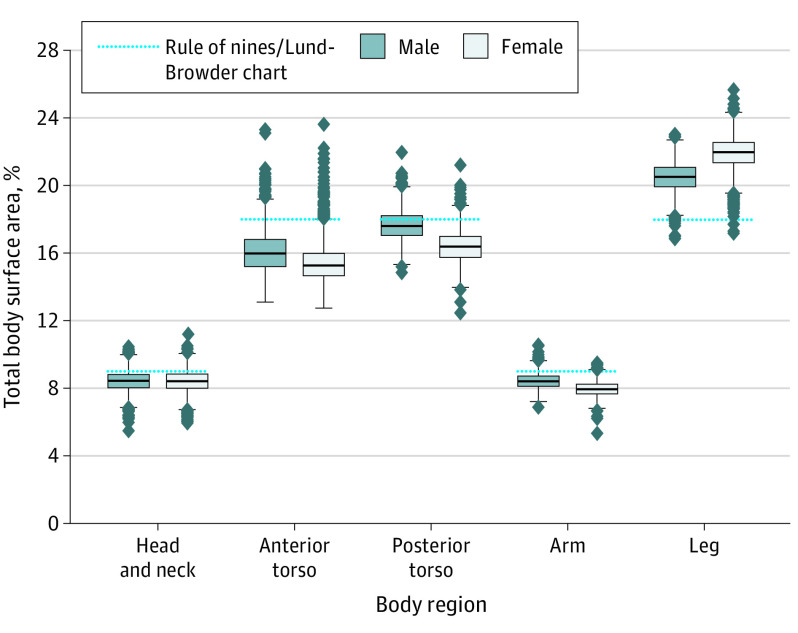
Of 3047 adults aged 18 to 65 years in this data set, 1517 (49.8%) were male. Race and ethnicity data were not available for analysis, as the study had access to body mesh data for computation only. We examined contributory %TBSAs of 5 major body regions: head and neck, anterior torso, posterior torso, arms, and legs (Figure 1). Widest %TBSA variations were in the anterior torso, posterior torso, and legs. Rule of nines and Lund-Browder chart estimates for legs, head and neck, anterior torso, and arms fell outside the middle 50% of the study population’s respective contributory %TBSAs.
Figure 1. Individual Variation in 5 Major Body Regions’ Contributory Percent Total Body Surface Areas.
Variable Body Region %TBSAs by Body Habitus
The study population’s TBSA-to-volume ratios appeared to follow a normal distribution (eFigure 2 in the Supplement). For male and female individuals, head and neck %TBSA decreased with larger body habitus (Table). Anterior torso %TBSA increased with increasing body habitus (mean [SD], 15.1 [0.9] to 19.1 [2.0] for male individuals; 15.1 [0.8] to 18.0 [1.7] for female individuals). This increase was attributable to increase in abdomen %TBSA (mean [SD], 5.3 [0.7] to 8.7 [1.8]) among male individuals and increase in abdomen (mean [SD], 4.6 [0.6] to 6.8 [1.7]) and pelvis (mean [SD], 1.5 [0.2] to 2.9 [0.9]) %TBSAs among female individuals. Fist, upper and lower arms, lower legs, and feet %TBSAs remained relatively constant across body habitus categories.
Table. Contributory Percent Total Body Surface Areas of Body Regions, Delineated by Sex and Body Habitus (Total Body Surface Area to Volume Ratios).
| Body part | Body habitus, mean (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Very small | Small | Average | Large | Very large | ||||||
| Male (n = 34) | Female (n = 20) | Male (n = 192) | Female n = 216) | Male (n = 1055) | Female (n = 1066) | Male (n = 199) | Female (n = 180) | Male (n = 37) | Female (n = 55) | |
| Head and neck | 9.6 (0.7) | 9.7 (0.4) | 9.1 (0.6) | 9.1 (0.4) | 8.4 (0.6) | 8.4 (0.6) | 7.6 (0.6) | 7.5 (0.5) | 6.9 (0.6) | 6.9 (0.6) |
| Upper arm | 3.5 (0.4) | 3.6 (0.8) | 3.6 (0.4) | 3.8 (0.8) | 3.7 (0.4) | 3.9 (0.8) | 3.7 (0.5) | 4.0 (0.7) | 3.6 (0.5) | 4.1 (0.7) |
| Lower arm | 3.4 (0.2) | 2.9 (0.2) | 3.4 (0.2) | 2.9 (0.2) | 3.4 (0.2) | 2.9 (0.3) | 3.5 (0.3) | 2.9 (0.4) | 3.3 (0.3) | 2.8 (0.3) |
| Hand | 1.4 (0.1) | 1.4 (0.1) | 1.4 (0.1) | 1.4 (0.1) | 1.3 (0.1) | 1.3 (0.1) | 1.2 (0.1) | 1.2 (0.1) | 1.1 (0.1) | 1.1 (0.1) |
| Chest | 8.4 (0.9) | 8.8 (0.8) | 8.4 (0.7) | 9.0 (0.7) | 8.4 (0.8) | 8.8 (0.8) | 8.2 (0.9) | 8.5 (0.9) | 8.2 (0.8) | 8.4 (1.4) |
| Abdomen | 5.3 (0.7) | 4.6 (0.6) | 5.4 (0.7) | 4.6 (0.8) | 5.9 (1.0) | 4.9 (0.8) | 7.2 (1.5) | 5.9 (1.4) | 8.7 (1.8) | 6.8 (1.7) |
| Pelvis | 1.5 (0.2) | 1.5 (0.2) | 1.5 (0.3) | 1.5 (0.2) | 1.6 (0.2) | 1.6 (0.3) | 1.7 (0.3) | 2.0 (0.8) | 1.8 (0.5) | 2.9 (0.9) |
| Back | 12.0 (0.6) | 11.7 (1.0) | 12.1 (1.0) | 11.5 (1.0) | 12.2 (1.2) | 11.7 (1.1) | 12.3 (1.3) | 11.6 (1.2) | 12.6 (1.4) | 11.7 (1.5) |
| Buttocks | 4.9 (0.8) | 3.8 (0.4) | 4.8 (0.6) | 4.1 (0.6) | 4.8 (0.8) | 4.2 (0.6) | 4.8 (0.9) | 4.3 (0.9) | 5.3 (1.0) | 4.8 (1.2) |
| Upper leg | 11.7 (1.0) | 13.0 (1.0) | 11.8 (1.1) | 13.4 (1.1) | 11.7 (1.1) | 13.5 (1.2) | 11.5 (1.1) | 13.2 (1.3) | .8 (1.3) | 12.7 (1.6) |
| Lower leg | 6.6 (0.4) | 6.7 (0.4) | 6.8 (0.42) | 6.8 (0.5) | 6.8 (0.4) | 6.7 (0.5) | 6.9 (0.4) | 6.7 (0.5) | 6.9 (0.5) | 6.7 (0.7) |
| Foot | 2.0 (0.3) | 2.1 (0.3) | 2.0 (0.3) | 2.0 (0.3) | 1.9 (0.3) | 1.9 (0.3) | 1.8 (0.3) | 1.7 (0.3) | 1.7 (0.3) | 1.6 (0.2) |
Comparing Model Measurements With Existing Estimates
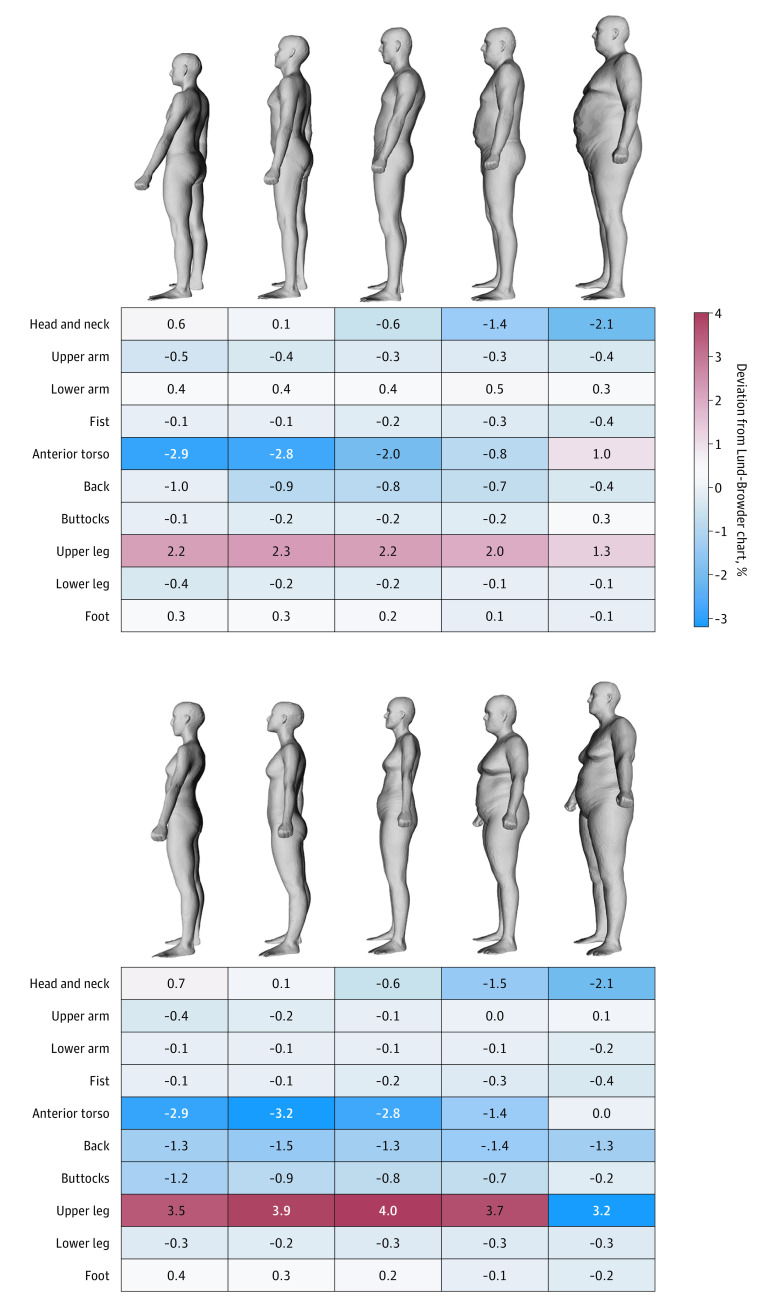
Figure 2 represents deviations between subgroups’ body region %TBSA measurements and Lund-Browder chart estimates. With increasing body habitus, we found increasing deviations in head and neck and anterior torso estimates (Lund-Browder chart overestimates by up to 2.1% and 3.2%, respectively). The greatest deviation with the Lund-Browder chart was a 4% underestimation of upper leg %TBSA among female individuals with average body habitus.
Figure 2. Deviation of Measured Body Region Percent Total Body Surface Areas From Lund-Browder Chart Estimates Among 10 Sex–Body Habitus Subgroups.
Visual body models represent anthropometry laser body scans of patients with average total body surface area/volume within each subgroup.
Mobile Application
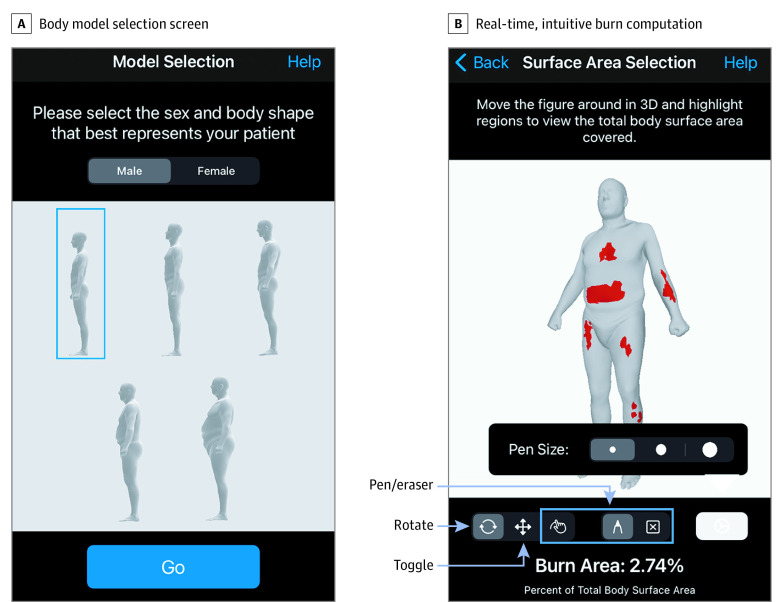
Our mobile application, Burn Area, is depicted in Figure 3. After selecting a body model that best matches the patient’s sex and body habitus, clinicians can replicate exact burn injury patterns to compute %TBSA burn.
Figure 3. Burn Area User Interface.
A, Body model selection screen; B, Real-time, intuitive percent total body surface area burn computation.
Discussion
In this cohort study, we used the largest, to our knowledge, available adult laser body scan data set to compute sex and body habitus–specific body region %TBSAs. Estimates from existing %TBSA burn models (rule of nines and Lund-Browder chart) fell outside measured interquartile ranges of most body regions and do not capture heterogenous body habitus of the modern population. We developed an intuitive mobile application that facilitates rapid beside %TBSA burn measurement for the modern era.
Despite the critical importance of accurate %TBSA burn estimation for burn injury management (eg, hospital admission and specialized burn center referral criteria23 and fluid and caloric need calculations24,25,26,27,28), body surface area measurements on 12 individuals underlie existing estimation models. The original formula from DuBois and DuBois in 19156 was based on papier-mâché molds of 5 individuals.6 The linear formula underwent modification in 1916 after papier-mâché molds of 7 additional individuals had been added, including 2 children aged 21 months and 12 years and an adult who had lost both legs.7 Based on revised formula application to 33 individuals (no new measurements were made),8 Berkow29 designed a table delineating contributory %TBSA of several body regions in 1924. Both Lund-Browder charts (1944) and the rule of nines (1951) were derived from Berkow’s summary table.4,5
Many studies report inaccuracy and poor interrater reliability of existing %TBSA burn estimation models, especially for noncontiguous burns.30,31,32 Existing models frequently overestimate %TBSA burn, begetting excessive fluid resuscitation and inappropriate referrals.14,31,32,33,34 Existing models’ estimates are particularly erroneous for patients with obesity, who experience higher rates of morbidity and mortality after burn injury.9,10,35,36,37,38 With nearly half of all US adults projected to be obese by 2030,39 a one-size-fits-all model for estimating %TBSA burn is inadequate. Moreover, existing models only outline how much major body regions (eg, arms) as a whole contribute to %TBSA. However, burn injuries are rarely limited to a singular whole-body region. With burn injuries that span multiple body regions or consist of discontinuous patches, the potential for erroneous %TBSA burn estimation compounds.
Despite known limitations, existing estimation models are used because a more practical and accurate alternative has not been available.3,30,40 Two mobile applications, MoBurnZA and BurnMed, have attempted to facilitate real-time %TBSA burn estimation. However, MoBurnZA is a mobile replication of the Lund-Browder chart,41 and BurnMed delineates 2 adult body models (male and female).42 There were previous efforts to develop and validate a %TBSA estimation model based on body habitus and burn injury pattern (BurnCase 3D),43,44,45 but the software was designed to facilitate data collection and a practical and reliable %TBSA estimation tool remains critically needed. To our knowledge, our tool is unique in reflecting measured body surface areas of modern adults across the wide body habitus spectrum.
Limitations
Our study has several limitations. The CAESAR database does not include surface area measurements of children. A recent study46 showed that 94% of initial %TBSA burn estimates were inaccurate among children referred to burn centers. Mirroring our study, foundational formulae of pediatric %TBSA burn deserve reevaluation. Second, our study population included North American and European adults. The CAESAR project enrolled racially and ethnically diverse subjects to ensure laser body scans would reflect the modern population, but geographical body habitus differences may limit generalizability of our tool. Third, prospective and controlled evaluation is needed to assess clinical utility—that is, whether burn injury management using our tool’s %TBSA burn estimates improves outcomes. For the average-weight US male individual (89 kg), a 5% overestimation of %TBSA burn may lead to 1.8 L fluid overresuscitation within the first 24 hours per the Parkland formula. Several studies have shown that overresuscitation is associated with increased odds of adverse outcomes, including pneumonia, acute respiratory distress syndrome, and mortality.17,47 However, if the foundational formulae for %TBSA burn estimation are inaccurate, derivative formulae may require reevaluation. For example, the Parkland formula, frequently used to calculate initial fluid resuscitation volumes, was devised using Lund-Browder chart %TBSA burn estimates.24 Implications of inputting our model’s %TBSA burn measurements into the many derivative formulae require thoughtful consideration.
Future studies should first confirm low interuser variability in %TBSA burn estimation (selecting the most representative body model and mirroring an accurate injury pattern). Next, stepwise evaluations should compare how using our tool vs existing tools may affect outcomes of clinical decisions that rely on %TBSA burn, such as decisions to admit or transfer patients to specialty burn centers, initial fluid resuscitation volumes, and caloric intake calculations.
Conclusions
Computer vision algorithm application to laser body scans of more than 3000 adults generated an accurate, intuitive, and practical tool for rapid %TBSA burn computation. In contrast to existing one-size-fits-all models, our application computes real-time %TBSA burn based on sex, body habitus, and exact burn injury patterns. We hope our practical tool will facilitate clinical utility studies and reliable %TBSA computation in the modern era.
eFigure 1. Eighteen segmented body regions
eFigure 2. Total body surface area to volume ratios of 3047 adults
References
- 1.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723-1730. doi: 10.1056/NEJMra1109343 [DOI] [PubMed] [Google Scholar]
- 2.US Centers for Disease Control and Prevention . Ambulatory Health Care Data: NAMCS and NHAMCS Web Tables. Accessed February 18, 2021. https://www.cdc.gov/nchs/ahcd/web_tables.htm
- 3.Greenhalgh DG. Management of burns. N Engl J Med. 2019;380(24):2349-2359. doi: 10.1056/NEJMra1807442 [DOI] [PubMed] [Google Scholar]
- 4.Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352-358. https://ci.nii.ac.jp/naid/20001139318/en/ [Google Scholar]
- 5.Wallace AB. The exposure treatment of burns. Lancet. 1951;1(6653):501-504. doi: 10.1016/S0140-6736(51)91975-7 [DOI] [PubMed] [Google Scholar]
- 6.DuBois D, DuBois EF. Fifth paper the measurement of the surface area of man. Arch Intern Med. 1915;XV(5_2):868-881. doi: 10.1001/archinte.1915.00070240077005 [DOI] [Google Scholar]
- 7.Sawyer M, Stone R, DuBois EF. Clinical calorimetry: ninth paper further measurements of the surface area of adults and children. Arch Intern Med. 1916;XVII(6_2):855-862. doi: 10.1001/archinte.1916.00080130002001 [DOI] [Google Scholar]
- 8.DuBois D, DuBois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(6_2):863-871. doi: 10.1001/archinte.1916.00080130010002 [DOI] [Google Scholar]
- 9.Neaman KC, Andres LA, McClure AM, Burton ME, Kemmeter PR, Ford RD. A new method for estimation of involved BSAs for obese and normal-weight patients with burn injury. J Burn Care Res. 2011;32(3):421-428. doi: 10.1097/BCR.0b013e318217f8c6 [DOI] [PubMed] [Google Scholar]
- 10.Butz DR, Collier Z, O’Connor A, Magdziak M, Gottlieb LJ. Is palmar surface area a reliable tool to estimate burn surface areas in obese patients? J Burn Care Res. 2015;36(1):87-91. doi: 10.1097/BCR.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 11.Livingston EH, Lee S. Percentage of burned body surface area determination in obese and nonobese patients. J Surg Res. 2000;91(2):106-110. doi: 10.1006/jsre.2000.5909 [DOI] [PubMed] [Google Scholar]
- 12.Livingston EH, Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281(3):E586-E591. doi: 10.1152/ajpendo.2001.281.3.E586 [DOI] [PubMed] [Google Scholar]
- 13.Pham C, Collier Z, Gillenwater J. Changing the way we think about burn size estimation. J Burn Care Res. 2019;40(1):1-11. doi: 10.1093/jbcr/iry050 [DOI] [PubMed] [Google Scholar]
- 14.Harish V, Raymond AP, Issler AC, et al. Accuracy of burn size estimation in patients transferred to adult burn units in Sydney, Australia: an audit of 698 patients. Burns. 2015;41(1):91-99. doi: 10.1016/j.burns.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Liu NTM, Fenrich CA, Serio-Melvin MLR, Peterson WC, Cancio LC, Salinas J. The impact of patient weight on burn resuscitation. J Trauma Acute Care Surg. 2017;83(1)(suppl 1):S112-S119. doi: 10.1097/TA.0000000000001486 [DOI] [PubMed] [Google Scholar]
- 16.Rae L, Pham TN, Carrougher G, et al. Differences in resuscitation in morbidly obese burn patients may contribute to high mortality. J Burn Care Res. 2013;34(5):507-514. doi: 10.1097/BCR.0b013e3182a2a771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayampanathan AA. Systematic review and meta-analysis of complications and outcomes of obese patients with burns. Burns. 2016;42(8):1634-1643. doi: 10.1016/j.burns.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 18.Civilian American and European Surface Anthropometry Resource Project (CAESAR) . Digitally defining the human body. Accessed February 18, 2021. https://www.sae.org/standardsdev/tsb/cooperative/caesar.htm
- 19.Yang Y, Yu Y, Zhou Y, Du S, Davis J, Yang R. Semantic parametric reshaping of human body models. Presented at: 2nd International Conference on 3D Vision; December 8-11, 2014; Tokyo, Japan. Accessed December 27, 2020. doi: 10.1109/3DV.2014.47 [DOI] [Google Scholar]
- 20.Zhou Q-Y, Park J, Koltun V. Open3D: a modern library for 3D data processing. Accessed January 17, 2021. https://arxiv.org/abs/1801.09847
- 21.Sullivan CB, Kaszynski AA. PyVista: 3D plotting and mesh analysis through a streamlined interface for the Visualization Toolkit (VTK). J Open Source Softw. 2019;4(37):1450. doi: 10.21105/joss.01450 [DOI] [Google Scholar]
- 22.Harris LK, Theriot JA. Surface Area to Volume Ratio: A Natural Variable for Bacterial Morphogenesis. Trends Microbiol. 2018;26(10):815-832. doi: 10.1016/j.tim.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Burn Association/American College of Surgeons . Guidelines for the operation of burn centers. J Burn Care Res. 2007;28(1):134-141. doi: 10.1093/jbcr/28.1.134 [DOI] [PubMed] [Google Scholar]
- 24.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968;150(3):874-894. doi: 10.1111/j.1749-6632.1968.tb14738.x [DOI] [PubMed] [Google Scholar]
- 25.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Carnegie Institution of Washington; 1919. [Google Scholar]
- 26.Curreri PW, Luterman A. Nutritional support of the burned patient. Surg Clin North Am. 1978;58(6):1151-1156. doi: 10.1016/S0039-6109(16)41683-X [DOI] [PubMed] [Google Scholar]
- 27.Allard JP, Jeejheebhoy KN, Whitwell J, Pashutinski L, Peters WJ. Factors influencing energy expenditure in patients with burns. J Trauma. 1988;28(2):199-202. doi: 10.1097/00005373-198802000-00012 [DOI] [PubMed] [Google Scholar]
- 28.Milner EAR, Cioffi WG, Mason AD, McManus WF, Pruitt BA Jr. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994;37(2):167-170. doi: 10.1097/00005373-199408000-00001 [DOI] [PubMed] [Google Scholar]
- 29.Berkow SG. A method of estimating the extensiveness of lesions (burns and scalds) based on surface area proportions. Arch Surg. 1924;8(1):138-148. doi: 10.1001/archsurg.1924.01120040149006 [DOI] [PubMed] [Google Scholar]
- 30.Parvizi D, Kamolz L-P, Giretzlehner M, et al. The potential impact of wrong TBSA estimations on fluid resuscitation in patients suffering from burns: things to keep in mind. Burns. 2014;40(2):241-245. doi: 10.1016/j.burns.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 31.Wachtel TL, Berry CC, Wachtel EE, Frank HA. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns. 2000;26(2):156-170. doi: 10.1016/S0305-4179(99)00047-9 [DOI] [PubMed] [Google Scholar]
- 32.Chong HP, Quinn L, Jeeves A, et al. A comparison study of methods for estimation of a burn surface area: Lund and Browder, e-burn and Mersey Burns. Burns. 2020;46(2):483-489. doi: 10.1016/j.burns.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 33.Armstrong JR, Willand L, Gonzalez B, Sandhu J, Mosier MJ. Quantitative analysis of estimated burn size accuracy for transfer patients. J Burn Care Res. 2017;38(1):e30-e35. doi: 10.1097/BCR.0000000000000460 [DOI] [PubMed] [Google Scholar]
- 34.Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. 2007;28(3):382-395. doi: 10.1097/BCR.0B013E318053D3A1 [DOI] [PubMed] [Google Scholar]
- 35.Ghanem AM, Sen S, Philp B, Dziewulski P, Shelley OP. Body mass index (BMI) and mortality in patients with severe burns: is there a “tilt point” at which obesity influences outcome? Burns. 2011;37(2):208-214. doi: 10.1016/j.burns.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 36.Sheridan RL, Rue LWI III, McManus WF, Pruitt BA Jr. Burns in morbidly obese patients. J Trauma. 1992;33(6):818-820. doi: 10.1097/00005373-199212000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Gottschlich MM, Mayes T, Khoury JC, Warden GD. Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. J Am Diet Assoc. 1993;93(11):1261-1268. doi: 10.1016/0002-8223(93)91952-M [DOI] [PubMed] [Google Scholar]
- 38.Purdue GF, Hunt JL, Lang ED. Obesity: a risk factor in the burn patient. J Burn Care Rehabil. 1990;11(1):32-34. doi: 10.1097/00004630-199001000-00007 [DOI] [PubMed] [Google Scholar]
- 39.Ward ZJ, Bleich SN, Cradock AL, et al. Projected US state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 40.Blumetti J, Hunt JL, Arnoldo BD, Parks JK, Purdue GF. The Parkland formula under fire: is the criticism justified? J Burn Care Res. 2008;29(1):180-186. doi: 10.1097/BCR.0b013e31815f5a62 [DOI] [PubMed] [Google Scholar]
- 41.Wallis LA, Fleming J, Hasselberg M, Laflamme L, Lundin J. A smartphone app and cloud-based consultation system for burn injury emergency care. PLoS One. 2016;11(2):e0147253. doi: 10.1371/journal.pone.0147253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg H, Klaff J, Spjut A, Milner S. A mobile app for measuring the surface area of a burn in three dimensions: comparison to the Lund and Browder assessment. J Burn Care Res. 2014;35(6):480-483. doi: 10.1097/BCR.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 43.Haller HL, Dirnberger J, Giretzlehner M, Rodemund C, Kamolz L. “Understanding burns”: research project BurnCase 3D—overcome the limits of existing methods in burns documentation. Burns. 2009;35(3):311-317. doi: 10.1016/j.burns.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 44.Dirnberger J, Giretzlehner M, Ruhmer M, Haller H, Rodemund C. Modelling human burn injuries in a three-dimensional virtual environment. Stud Health Technol Inform. 2003;94:52-58. [PubMed] [Google Scholar]
- 45.Parvizi D, Giretzlehner M, Dirnberger J, et al. The use of telemedicine in burn care: development of a mobile system for TBSA documentation and remote assessment. Ann Burns Fire Disasters. 2014;27(2):94-100. [PMC free article] [PubMed] [Google Scholar]
- 46.Goverman J, Bittner EA, Friedstat JS, et al. Discrepancy in initial pediatric burn estimates and its impact on fluid resuscitation. J Burn Care Res. 2015;36(5):574-579. doi: 10.1097/BCR.0000000000000185 [DOI] [PubMed] [Google Scholar]
- 47.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245(4):622-628. doi: 10.1097/01.sla.0000252572.50684.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Eighteen segmented body regions
eFigure 2. Total body surface area to volume ratios of 3047 adults