Key Points
Question
What are the incidence rates and risk factors for intraocular inflammation (IOI) and/or retinal vascular occlusion (RO) after brolucizumab treatment for neovascular age-related macular degeneration (AMD) in clinical practice?
Findings
In this cohort study of patient eyes with neovascular AMD treated with brolucizumab, the incidence rate for any form of IOI and/or RO was approximately 2.4%. A history of IOI and/or RO was a key risk factor for IOI and/or RO after brolucizumab treatment initiation.
Meaning
These early findings explore potential risk factors for inflammation-associated adverse events that may occur following real-world treatment with brolucizumab.
This cohort study assesses the real-world incidence of intraocular inflammation, including retinal vasculitis and/or retinal vascular occlusion for patients with neovascular age-related macular degeneration who underwent brolucizumab treatment.
Abstract
Importance
Limited data exist on the real-world safety outcomes of patients with neovascular age-related macular degeneration treated with brolucizumab (Beovu).
Objective
To determine the real-world incidence of intraocular inflammation (IOI), including retinal vasculitis (RV) and/or retinal vascular occlusion (RO), for patients with neovascular age-related macular degeneration who underwent brolucizumab treatment. Additionally, potential risk factors associated with these adverse events were evaluated.
Design, Setting, and Participants
This cohort study included patients with neovascular age-related macular degeneration in the Intelligent Research in Sight (IRIS) Registry and Komodo Healthcare Map. Patients initiating and receiving 1 or more brolucizumab injections from October 8, 2019, to June 5, 2020, with up to 6 months of follow-up were included.
Intervention
Brolucizumab injections.
Main Outcome and Measures
Incidence of IOI (including RV) and/or RO and RV and/or RO and risk stratification for the identified risk factors.
Results
Of 10 654 and 11 161 included eyes (from the IRIS Registry and Komodo Health database, respectively), the median follow-up times were 97 and 95 days. Most eyes switched from another anti–vascular endothelial growth factor agent (9686 of 10 654 [90.9%] and 10 487 of 11 161 [94.0%], respectively), most commonly aflibercept (7160 of 9686 [73.9%] and 7156 of 10 487 [68.2%]), and most were from women (6105 of 10 654 [57.3%] and 6452 of 11 161 [57.8%]). The overall incidence of IOI and/or RO was 2.4% (255 of 10 654 eyes) and 2.4% (268 of 11 161 eyes) for the IRIS and Komodo groups, respectively, and RV and/or RO, 0.6% (59 of 10 654 eyes and 63 of 11 161 eyes), respectively. Patients with a history of IOI and/or RO in the 12 months before brolucizumab initiation had an increased observed risk rate (8.7% [95% CI, 6.0%-11.4%] and 10.6% [95% CI, 7.5%-13.7%]) for an IOI and/or RO event in the 6 months following the first brolucizumab treatment compared with patients without prior IOI and/or RO (2.0% in both data sets). There was an increased estimated incidence rate in women (2.9% [95% CI, 2.5%-3.3%] and 3.0% [95% CI, 2.6%-3.4%]) compared with men (1.3% [95% CI, 1.0%-1.7%] and 1.4% [95% CI, 1.0%-1.7%]), but this risk was not as large as that of a prior IOI and/or RO. Similar findings were observed for patients with RV and/or RO events.
Conclusions and Relevance
The incidence rate of IOI and/or RO was approximately 2.4%. Patient eyes with IOI and/or RO in the 12 months prior to first brolucizumab injection had the highest observed risk rate for IOI and/or RO in the early months after the first brolucizumab treatment. However, given study limitations, the identified risk factors cannot be used as predictors of IOI and/or RO events, and causality with brolucizumab cannot be assessed.
Introduction
Age-related macular degeneration (AMD) is a degenerative eye disease and the leading cause of permanent visual impairment in the US for people aged 65 years and older.1 Neovascular (wet) AMD is characterized by choroidal neovascularization and subretinal, intraretinal, and/or subretinal pigment epithelial fluid, blood, and/or exudates.2 Anti–vascular endothelial growth factor (VEGF) agents are the first-line therapy for treating neovascular AMD.2
Brolucizumab (Beovu) gained US Food and Drug Administration approval for the treatment of neovascular AMD in October 2019.3 Brolucizumab has been evaluated in 2 parallel phase 3 randomized clinical trials: HAWK (N = 1082) and HARRIER (N = 743).4,5 Following Food and Drug Administration approval, there were postmarketing reports of retinal vasculitis (RV), including retinal occlusive vasculitis (RO).6,7,8,9 Novartis initiated an internal review of these reports and established an external safety review committee to provide an unmasked, post hoc review of these cases.10 Together with the safety review committee, Novartis concluded a confirmed safety signal of RV and/or ROs, typically in the presence of intraocular inflammation (IOI), following brolucizumab injections, and patients were instructed to report any change in vision without delay. The safety review committee found similar incidences of IOI to those initially reported by the investigators in the HAWK and HARRIER studies (4.4%)5 and reported incidences of IOIs of any form in 4.6% (50 of 1088 individuals treated with brolucizumab). The safety review committee also reported signs of RV in 3.3% (36 of 1088), concomitant signs of RV and RO in 2.1% (23 of 1088), and IOI associated with losing 15 or more letters of visual acuity at the last visit or the end of the study in 0.7% (8 of 1088). Overall, the rates of moderate or severe visual acuity loss were comparable in patients treated with brolucizumab (7.4%) and aflibercept (7.7%).11 Novartis worked with health authorities to include this new safety information in the product label. Using 2 large, real-world US databases, the key objectives of this retrospective cohort study were (1) to evaluate safety by assessing incidence rates of any form of IOI (including RV) and/or RO (hereafter referred to as IOI and/or RO) and RV and/or RO and (2) to identify potential risk factors and assess risk stratification for any form of IOI and/or RO and RV and/or RO following the initiation of brolucizumab in the treatment of neovascular AMD.
Methods
IRIS Registry and Komodo Healthcare Map
This study used the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry12 and Komodo Healthcare Map. Analyses were conducted in parallel and were identical to the extent possible. The data accessed were deidentified in accordance with the Health Insurance Portability and Accessibility Act privacy rule, and no personal health information was extracted. Therefore, the study did not require informed consent or institutional review board approval.
The IRIS Registry is the first US-based national comprehensive eye disease database.13 Electronic health record data are provided for more than 750 000 patients with neovascular AMD and include information on patient characteristics, treatment patterns, health care utilization, and ocular comorbidities (but does not include information on systemic comorbidities or medications).14 The Komodo Healthcare Map is a nationally representative, open-source, longitudinal claims database that includes open and payer-complete medical and pharmacy claims.15,16 Komodo has information on patient characteristics and medical history (but does not include information on visual acuity) for more than 500 000 patients with neovascular AMD per year.15
Definitions
The study cohorts based on the IRIS Registry and Komodo included patients who initiated and had received at least 1 brolucizumab injection from October 8, 2019, to June 5, 2020 (IRIS Registry) or October 8, 2019, to April 30, 2020 (Komodo) (ie, the index period). The index date was defined as the date of the earliest brolucizumab injection (based on procedure code or electronic health record note). The study period extended from October 8, 2019, to June 5, 2020. The preindex period was 36 months prior to the index date. Given the recent launch of brolucizumab at the time of the analyses, the postindex period was up to 180 days after the index date. Patient eyes included in the study had varying lengths of follow-up depending on when brolucizumab treatment was initiated.
Key Inclusion and Exclusion Criteria
The study included adult patients with neovascular AMD initiating brolucizumab treatment during the corresponding index period. Patients were excluded if they had used brolucizumab prior to the start of the index period. eTable 1 in the Supplement presents further details.
Definition of Observed Adverse Events
The observed adverse events (AEs) for IOI and/or RO and RV and/or RO were identified using International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic codes and were defined as AEs observed in the postindex period (eTable 2 in the Supplement). Patient eyes were stratified into cohorts defined by the occurrence of AEs after the index date:
Cohort 1 (control participants): patient eyes with no AEs of interest reported.
Cohort 2: patient eyes with any form of IOI and/or RO.
Cohort 3: patient eyes with RV and/or RO, which is a subset of cohort 2 (eTable 2 in the Supplement).
Patient eyes with prior IOI and/or RO were analyzed further to assess a true new event after brolucizumab initiation vs a carryover diagnosis. The final cohorts were refined based on further review and new aligned rules to estimate incident events (true new events observed after brolucizumab treatment). The following groups were selected:
Patient eyes with events after the index date, with no IOI and/or RO in the 36 months pre–index period.
Patient eyes with events with different ICD-10 CM codes at a subevent level before and after the index date.
Patient eyes with events with the same ICD-10 CM codes at a subevent level before and after the index date and occurring 2 times or less in the 12 months before the index date.
Patient eyes with nonincident events (the same events observed both before and after brolucizumab treatment initiation) with a visual acuity drop that was moderate or severe (≥3 lines), without substantial visual acuity decline prior to the AE documentation, in the postindex period following the event (from IRIS only).
Outcome Measures
Outcome measures included observed incidence of any form of IOI and/or RO; RV and/or RO; and risk factors identified following risk stratification of baseline clinical and demographic characteristics. Outcomes are presented at the patient-eye level.
Statistical Analysis
Data were analyzed by Verana Health (IRIS Registry) and Komodo (Komodo Healthcare Map) using Python 3.6.8 (Python Software Foundation). If a patient received brolucizumab in both eyes, both were included in this study. More than 120 baseline demographic and clinical characteristics and outcome variables were tabulated using descriptive statistics and assessed to understand the difference between patient eyes with and without AEs. Continuous variables were summarized by providing the number of observations, means (SDs), and medians (ranges). Categorical variables were summarized by providing counts and percentages (based on the total number of eyes or events), with missing data considered a separate category.
Generalized estimating equations (GEE) based on multivariable models were used to estimate and identify possible risk factors associated with the AEs of interest (any form of IOI and/or RO and RV and/or RO) after the index date and estimate the observed risk rate for identified risk factors. The GEE models were specifically used to account for intereye correlation of data in case of 2 eyes being from the same patient.
The multivariable models included age and sex in addition to other variables evaluated based on their statistical significance from the univariate analyses and their clinical relevance. Considering the limited number of events (especially for the RV and/or RO cohort), the number of variables were restricted to avoid model instability resulting from rare events, separation, collinearity, etc. It was additionally hypothesized that the same covariates model should be applicable to both IOI and/or RO and RV and/or RO, because of the link observed between IOI, RV, and RO in case reports and phase 3 brolucizumab data. This procedure led to a base model for each database analysis. Extended models with additional variables were considered, but their utility was judged to be limited. In total, approximately 40 baseline characteristics were considered for inclusion in the models. The final covariates are shown in eTable 1 in the Supplement. The results of the multivariable models were displayed as odds ratios (ORs) for the occurrence of AEs of interest, based on baseline demographic and clinical characteristics.
The identified risk factors based on their statistical significance from the multivariable models were used to estimate adjusted incidence (risk stratification) of AEs of interest based on selected subgroups of interest. The GEE models for cohorts 2 and 3 were fit and output estimated marginal means based on the models. The estimated means were based on a reference grid consisting of all combinations of factor levels, with each covariate set to its mean. These expected data were log transformed.
Results
Baseline Characteristics
Overall, 10 654 patient eyes (in 9456 patients) and 11 161 patient eyes (in 9261 patients) were treated with brolucizumab and met study criteria in the IRIS Registry and Komodo, respectively. More than 90% of patient eyes had been switched from another anti-VEGF agent (9686 of 10 654 [90.9%] and 10 487 of 11 161 [94.0%], respectively), with aflibercept being the most common immediate prior anti-VEGF agent among eyes that were switched (IRIS, 7160 of 9686 [73.9%]; Komodo, 7156 of 10 487 [68.2%]) (eTable 3 in the Supplement). The median (range) duration of treatment on the prior anti-VEGF agent, among patient eyes that had 1 or more anti-VEGF treatment, was 526 (14-1094) days (IRIS) and 663 (7-1820) days (Komodo). Most eyes belonged to women (IRIS: 6105 of 10 654 [57.3%]; Komodo: 6452 of 11 161 [57.8%]) and were treated unilaterally with brolucizumab (IRIS: 8258 of 10 654 [77.5%]; Komodo: 7361 of 11 161 [66.0%]). Visual acuity when converted to approximate Early Treatment Diabetic Retinopathy Study scores were a mean letter score of 62 (approximate Snellen equivalent, 20/63) at or within 12 months of the index date in the IRIS Registry.
The median (range) follow-up times were 97 (0-180) and 95 (0-180) days in the IRIS Registry and Komodo, respectively. In the IRIS Registry analysis, the last date of follow-up was defined as the date of the last recorded visit or day that the ophthalmology practice that administered the index brolucizumab injection contributed data to the IRIS Registry, whichever occurred later. In the Komodo analysis, the last date of follow-up was defined as the most recent follow-up claim date (ophthalmic or nonophthalmic).
Incidence of Adverse Events
The overall incidence of any form of IOI and/or RO was 2.4% (IRIS: 255 of 10 654; Komodo, 268 of 11 161; Table). Relative to time to event, the median (range) days from the start of brolucizumab treatment to an event of any form of IOI and/or RO was 39 (2-141) days (IRIS) and 56 (2-168) days (Komodo). In the IRIS Registry, 130 patient eyes (51.0%), 97 patient eyes (38.0%), and 28 patient eyes (11.0%), and in Komodo, 126 patient eyes (47.0%), 108 patient eyes (40.3%), and 34 patient eyes (12.7%) had 1, 2, and 3 or more injections, respectively, prior to the first event of any form of IOI and/or RO.
Table. Overall Estimated Incidence Rates, Time to Event, and Event by Injection Sequence of Any Form of IOI (Including Retinal Vasculitis) and/or RO Up to 6 Months After the First Brolucizumab Injectiona.
| Characteristic | IRIS Registry (n = 10 654 eyes)b | Komodo Healthcare Map (n = 11 161 eyes)b |
|---|---|---|
| Incidence rates, No. (%) | ||
| No IOI, RV, or RO (control) | 10 399 (97.6) | 10 893 (97.6) |
| Any form of IOI and/or ROa | 255 (2.4) | 268 (2.4) |
| RV and/or RO | 59 (0.6) | 63 (0.6) |
| Any form of IOI and/or ROa | ||
| Time or injections to event | ||
| Time to event from first injection, median (range), d | 39 (2-141) | 56 (2-168) |
| Time to event from most recent injection, median (range), d | 28 (1-105) | 28 (0-139) |
| No. of injections to first event, median (range) | 1 (1-5) | 2 (1-4) |
| Proportion of events by brolucizumab injection sequence, No. (%) | ||
| 1 | 130 (51.0) | 126 (47.0) |
| 2 | 97 (38.0) | 108 (40.3) |
| ≥3 | 28 (11.0) | 34 (12.7) |
| RV and/or RO | ||
| Time or injections to event | ||
| Time to event from first injection, median (range), d | 42 (6-140) | 66 (6-168) |
| Time to event from most recent injection, median (range), d | 36 (2-130) | 47 (0-135) |
| No. of injections to first event, median (range) | 1 (1-5) | 1 (1-4) |
| Proportion of events by brolucizumab injection sequence, % | ||
| 1 | 30 (50.9) | 35 (55.6) |
| 2 | 23 (39.0) | 21 (33.3) |
| ≥3 | 6 (10.2) | 7 (11.1) |
Abbreviations: IRIS, Intelligent Research in Sight; IOI, intraocular inflammation; RO, retinal vascular occlusion; RV, retinal vasculitis.
Represents IOI, endophtalmitis, panuveitis, RV, or RO and does not include the infectious type of IOI or endophtalmitis. In patient eyes with up to 6 months of follow-up.
Overall brolucizumab study population: IRIS, 9456 patients and 10 654 patient eyes; Komodo, 9261 patients and 11 161 patient eyes.
The overall incidence of RV and/or RO was 0.6% in both databases (IRIS: 59 of 10 654 eyes; Komodo, 63 of 11 161; Table). The time and injections to event are shown in the Table. In addition, 17 patient eyes in the IRIS (0.2%) and 18 in the Komodo (0.2%) were reported to have RV, 30 (0.3%) and 29 (0.3%), respectively, were reported to have retinal artery occlusion, and 32 (0.3%) and 13 (0.1%), respectively, were reported to have panuveitis.
Risk Factors
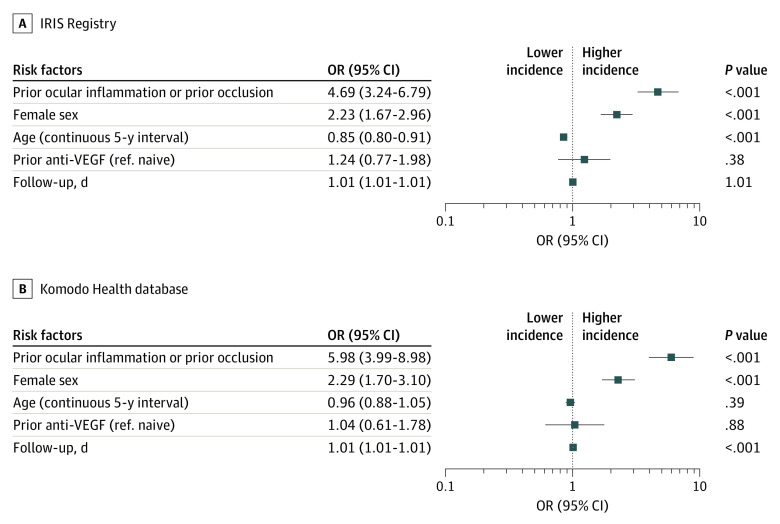
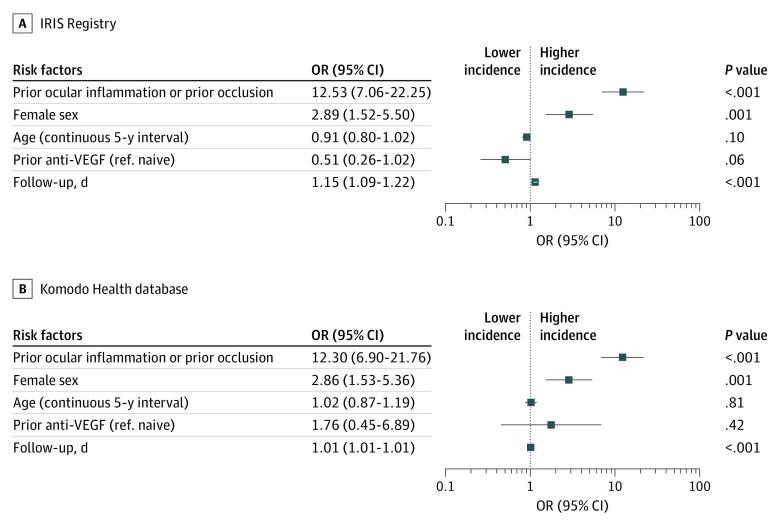
As shown in the forest plots (Figure 1 and Figure 2) for both analyses, prior IOI and/or RO in the preceding 12 months (odds ratios: IRIS, 4.69 [95% CI, 3.24-6.79]; Komodo, 5.98 [95% CI, 3.99-8.98]) and female sex (odds ratios: IRIS, 2.23 [95% CI, 1.67-2.96]; Komodo, 2.29 [95% CI, 1.70-3.10]) were identified as independent risk factors for increased incidence of any form of IOI and/or RO. Age and prior anti-VEGF treatment were not associated with the occurrence of any form of IOI and/or RO. Follow-up days were included only to adjust for different lengths of follow-up time between patient eyes in the studies, and this was not clinically relevant. Based on the multivariable model, the adjusted incidence of any form of IOI and/or RO was 2.2% (IRIS; 95% CI, 1.6%-2.8%) and 2.3% (Komodo; 95% CI, 2.0%-2.6%). eTable 4 in the Supplement shows select univariate analysis results.
Figure 1. Identification of Potential Risk Factors at Baseline on Estimated Incidence of Any Form of Intraocular Inflammation (Including Retinal Vasculitis) and/or Retinal Vascular Occlusion, Based on a Multivariable Model.
Retinal vascular occlusion is inclusive of retinal vein occlusion and retinal artery occlusion. Follow-up days variable was used to adjust for varying length of follow-up; graphs are plotted to logarithmic scale. Odds ratio (OR) greater than 1 indicates increased risk of intraocular inflammation and/or retinal vascular occlusion, and an OR less than 1 indicates a decreased risk of intraocular inflammation and/or retinal vascular occlusion. Retinal vascular occlusion represents any form of intraocular inflammation (including retinal vasculitis and retinal vascular occlusion; 255 patient eyes [2.4%] in the Intelligent Research in Sight [IRIS] Registry and 268 patient eyes [2.4%] in the Komodo database). Anti-VEGF indicates anti–vascular endothelial growth factor; ref., reference.
Figure 2. Identification of Potential Risk Factors at Baseline on Estimated Incidence of Retinal Vasculitis and/or Retinal Vascular Occlusion Based on a Multivariable Model.
Retinal vascular occlusion is inclusive of retinal vein occlusion and retinal artery occlusion. The follow-up days variable was used to adjust for varying length of follow-up; graphs are plotted to logarithmic scale, odds ratios (ORs) greater than 1 indicate increased risk of intraocular inflammation and/or retinal vascular occlusion, and ORs less than 1 indicate a decreased risk of intraocular inflammation and/or retinal vascular occlusion. Retinal vascular occlusion represents retinal vasculitis and/or retinal vascular occlusion (59 patient eyes [0.6%] in the Intelligent Research in Sight [IRIS] Registry and 63 patient eyes [0.6%] in the Komodo database). Anti-VEGF indicates anti–vascular endothelial growth factor; ref., reference.
Risk Stratification
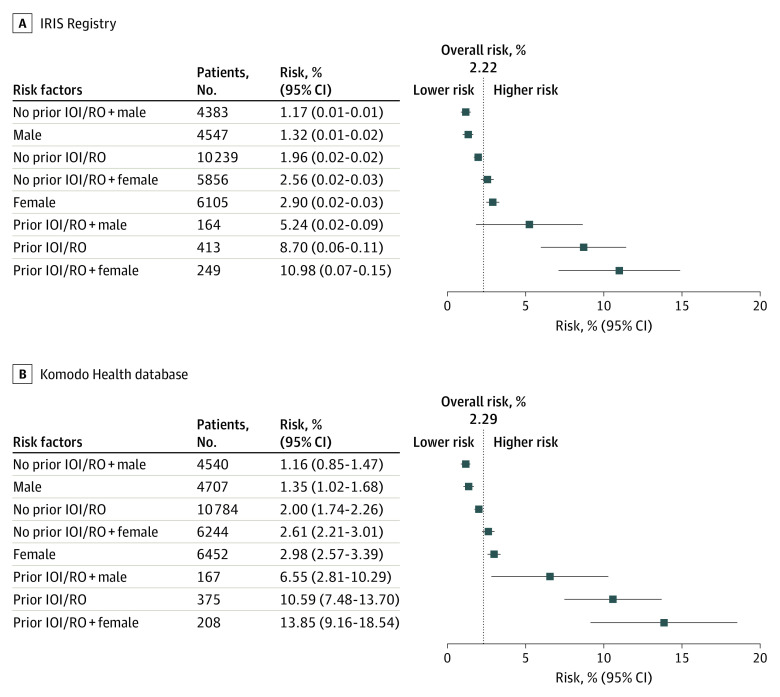
There was an increased estimated incidence rate of IOI and/or RO in patient eyes with prior inflammation and/or prior occlusion (IRIS, 8.7% [95% CI, 6.0%-11.4%]; Komodo, 10.6% [95% CI, 7.5%-13.7%]) (Figure 3). Even though in women there was an increased estimated incidence rate compared with men (IRIS, 2.9% [95% CI, 2.5%-3.3%] vs 1.3% [95% CI, 1.0%-1.7%]; Komodo, 3.0% [95% CI, 2.6%-3.4%] vs 1.4% [95% CI, 1.0%-1.7%]), the magnitude was similar to the overall estimated incidence rate 2.2% (IRIS; 95% CI, 1.6%-2.8%) and 2.3% (Komodo; 95% CI, 2.0%-2.6%) and not as large as seen with prior inflammation and/or prior occlusion (IRIS, 8.7% [95% CI, 6.0%-11.4%]; Komodo, 10.6% [95% CI, 7.5%-13.7%]).
Figure 3. Model-Based Estimation of Incidence Rate for Any Form of Intraocular Inflammation (IOI; Including Retinal Vasculitis) and/or Retinal Vascular Occlusion (RO) Based on Identified Risk Factors at Baseline.
Retinal vascular occlusion is inclusive of retinal vein occlusion and retinal artery occlusion. Based on 12-month history data, in patient eyes with up to 6 months of follow-up. Estimates for risk (95% CIs) were adjusted for age, prior anti–vascular endothelial growth factor treatment and length of follow-up. IOI indicates intraocular inflammation; IRIS, Intelligent Research in Sight.
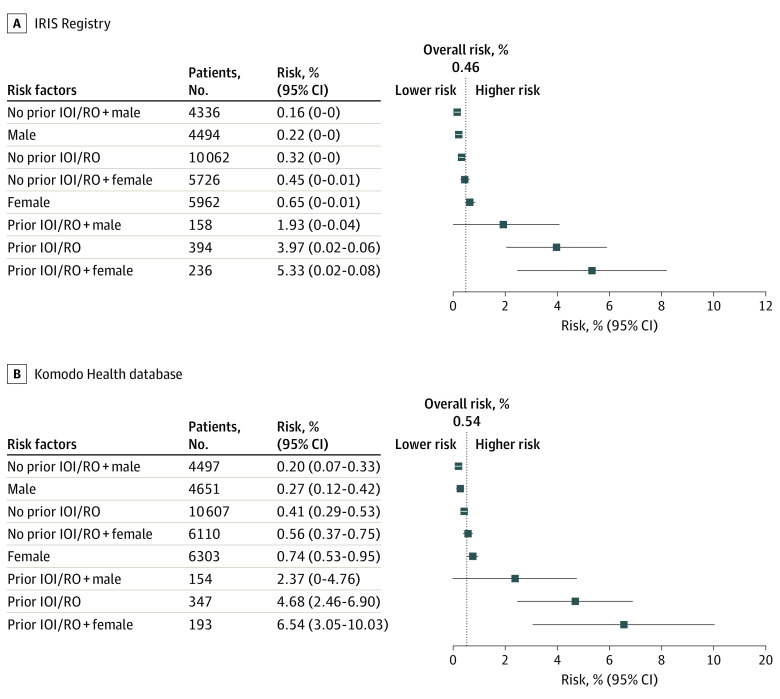
The secondary analysis of RV and/or RO found the overall estimated incidence was 0.5% (IRIS; 95% CI, 0.2%-0.8%) and 0.5% (Komodo; 95% CI, 0.4%-0.7%) (Figure 4). An increased estimated incidence rate was observed in patient eyes with prior inflammation and/or prior occlusion.
Figure 4. Model-Based Estimation of Incidence Rate for Retinal Vasculitis and/or Retinal Vascular Occlusion (RO), Based on Identified Risk Factors at Baseline.
Retinal vascular occlusion is inclusive of retinal vein occlusion and retinal artery occlusion. Based on 12-month history data, in patient eyes with up to 6 months of follow-up. Estimates for risk (95% CIs) were adjusted for age, prior anti–vascular endothelial growth factor treatment, and length of follow-up. IOI indicates intraocular inflammation; IRIS, Intelligent Research in Sight.
Discussion
This study reports early safety data following real-world brolucizumab treatment, building on other recent reports of the real-world safety of brolucizumab.16,17,18,19 The incidence rate for any form of IOI and/or RO was approximately 2.4%. Patients with IOI and/or RO in the 12 months prior to the first brolucizumab injection had the highest estimated incidence rate for an event of any form of IOI and/or RO among patient eyes in the 6 months following the first brolucizumab treatment. Similar findings were observed for the subgroup of RV and/or RO.
The baseline demographic and clinical characteristics of patients treated with brolucizumab differ from those in real-world studies that evaluated other anti-VEGF agents. Many other studies evaluated only patients who were naive to treatment, compared with less than 10% of patient eyes naive to treatment in this analysis.20 The most common therapy immediately prior to brolucizumab was aflibercept (68% to 74%).
Data from the current study suggests that prior IOI and/or prior occlusion is a key risk factor for any form of IOI and/or RO, with the highest observed risk rate compared with the other characteristics available and analyzed in this study. Reoccurrence of ocular inflammation is not unprecedented. For example, patients with uveitis are at a much greater risk for future inflammation events.21,22,23 It should be noted that IOI and RO are uncommon events, and little is known about the pathogenesis of these events in many cases.24
We observed an increased risk rate in women over men, although the magnitude paralleled the overall observed risk rate and was not as large as seen with prior IOI and/or RO. For those patients with prior IOI and/or RO, the observed risk rate was higher for women rather than men, although the confidence intervals were wide and overlapped. In addition, a male patient with no history of prior IOI and/or RO had the lowest observed risk rate for all forms of IOI and/or RO or RV and/or RO among the identified combination patient characteristics.
Limitations
There are certain limitations with these types of retrospective analyses using real-world data. Although these analyses were prespecified, they were noninterventional and retrospective, as opposed to a clinical trial in which data are collected prospectively. Limitations include a lack of access to patient medical records and imaging and the use of ICD codes to identify observed events, which do not provide information on diagnostic technique and can only constitute a proxy for the event of interest. In addition, the severity of the conditions of interest remained unknown. Thus, in the absence of medical records or imaging, a causality link of events with brolucizumab treatment cannot be confirmed. Other risk factors and confounders are not available in the data set. An additional limitation was the median follow-up of 3 months, compared with 2 years for the HAWK and HARRIER trials,4,5 which may explain the lower overall incidence rate of IOI and/or RO observed in this study compared with HARRIER and HAWK (2.4% vs 4.4%). Thus, a longer follow-up in real world would be needed to assess the long-term safety of brolucizumab. While the results from the IRIS Registry and Komodo were similar, the degree of patient overlap could not be determined. The combination of the low number of events and the absence of robust discriminatory risk factors that can clearly distinguish patients with the risk factor from those without means that the risk factors identified in these analyses cannot be used as a independent variable for the occurrence of IOI and/or RO in routine clinical practice. Patients with a prior incidence of AEs are likely to be more closely monitored for subsequent AEs; thus, new incidences of any IOI and/or RO might be detected more readily. It is unknown whether initiating brolucizumab treatment in patients without prior IOI and/or RO in the past 12 months would reduce the incidence of AEs of interest observed following exposure to brolucizumab.
There may have been a truncation bias introduced in the IRIS Registry analyses because of the potential for the end of the index period and the end of the study period to coincide for some patients. This coincidence could reduce the follow-up window for observation of AEs if brolucizumab treatment was initiated later in the index period. Cases of AEs may occur months after brolucizumab initiation, which would not be captured by this study, resulting in an underestimation. Moreover, early 2020 coincided with the first wave of COVID-19 cases in the US, which led to decreased clinic attendance of patients with AMD for follow-up anti-VEGF injections.25,26,27 Estimates of AEs could be subject to bias as a result of patient failure to attend follow-up appointments before the end of the study period. In defining the boundary conditions of the analysis, we attempted to balance this by providing enough time for patient follow-up and obtaining early insights into the prevalence of AEs following brolucizumab injection.
Conclusions
In conclusion, data from the IRIS Registry and Komodo Healthcare Map provided insights into the safety of patients with neovascular AMD who initiated brolucizumab. Patient eyes with IOI and/or RO in the 12 months prior to the first brolucizumab injection had the highest estimated incidence rate for an event of any form of IOI and/or RO in the 6 months after first brolucizumab treatment. Similar findings were observed among patients with RV and/or RO events. Additional studies with longer follow-up intervals are needed to assess the long-term safety of brolucizumab treatment. These results represent early findings that explore potential risk factors for inflammation-associated AEs that may occur following real-world treatment with brolucizumab. However, because of study limitations, the identified risk factors cannot be used as predictors of IOI and/or RO events and the causality with brolucizumab cannot be assessed.
eTable 1. IRIS Registry and Komodo Healthcare Map Inclusion and Exclusion Criteria
eTable 2. Adverse Events Definitions
eTable 3. Baseline patient/eye characteristics (overall cohort) at Index.
eTable 4. Baseline patient/eye characteristics by cohorts
References
- 1.Centers for Disease Control and Prevention . Common eye disorders. Updated June 3, 2020. Accessed October 15, 2020. https://www.cdc.gov/visionhealth/basics/ced/index.html
- 2.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology. 2020;127(1):1-P65. doi: 10.1016/j.ophtha.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Novartis Pharmaceuticals Corporation . Beovu package insert. Published 2020.
- 4.Yannuzzi NA, Freund KB. Brolucizumab: evidence to date in the treatment of neovascular age-related macular degeneration. Clin Ophthalmol. 2019;13:1323-1329. doi: 10.2147/OPTH.S184706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugel PU, Koh A, Ogura Y, et al. ; HAWK and HARRIER Study Investigators . HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. doi: 10.1016/j.ophtha.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 6.Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi: 10.1016/j.ajoc.2020.100680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18:100687. doi: 10.1016/j.ajoc.2020.100687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4(4):269-279. doi: 10.1177/2474126420930863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345-1359. doi: 10.1016/j.ophtha.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 10.Holzner J. Subspecialty news: a label change for Beovu, AI for AMD, clinical trial data, and more. Published July 1, 2020. Accessed October 4, 2021. https://www.retinalphysician.com/issues/2020/july-august-2020/subspecialty-news-a-label-change-for-beovu,-ai-for
- 11.Mones J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050-1059. doi: 10.1016/j.ophtha.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 12.Parke DW II, Rich WL III, Sommer A, Lum F. The American Academy of Ophthalmology’s IRIS® Registry (Intelligent Research in Sight Clinical Data): a look back and a look to the future. Ophthalmology. 2017;124(11):1572-1574. doi: 10.1016/j.ophtha.2017.08.035 [DOI] [PubMed] [Google Scholar]
- 13.Rao P, Lum F, Wood K, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS Registry. Ophthalmology. 2018;125(4):522-528. doi: 10.1016/j.ophtha.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Ophthalmology . IRIS Registry. Published 2020. Accessed November 5, 2020. https://www.aao.org/iris-registry/about.
- 15.Komodo Health . Homepage. Published 2020. Accessed November 5, 2020. https://www.komodohealth.com/
- 16.Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441-448. doi: 10.1001/jamaophthalmol.2020.7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter SD, Saba NJ. Real-world efficacy and safety of brolucizumab. Invest Ophthalmol Vis Sci. 2021;62(8):456. [Google Scholar]
- 18.Hamou SJ, Raimondo CD, Weber P, Woods BC. A retrospective study on the use of brolucizumab for the treatment of NVAMD: a 1-year private practice experience. Invest Ophthalmol Vis Sci. 2021;62(8):286. [Google Scholar]
- 19.Aziz AA, Khanani AM, London N, et al. Real world efficacy and safety of brolucizumab in neovascular AMD: the REBEL study. Invest Ophthalmol Vis Sci. 2021;62(8):451. [Google Scholar]
- 20.Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS One. 2015;10(7):e0133968. doi: 10.1371/journal.pone.0133968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natkunarajah M, Kaptoge S, Edelsten C. Risks of relapse in patients with acute anterior uveitis. Br J Ophthalmol. 2007;91(3):330-334. doi: 10.1136/bjo.2005.083725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun D, Getahun D, Chiu VY, et al. Population-based frequency of ophthalmic adverse events in melanoma, other cancers, and after immune checkpoint inhibitor treatment. Am J Ophthalmol. 2021;224:282-291. doi: 10.1016/j.ajo.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Grunwald L, Newcomb CW, Daniel E, et al. ; Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study . Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118(10):1911-1915. doi: 10.1016/j.ophtha.2011.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and/or vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519-527. doi: 10.1016/j.oret.2020.09.020 [DOI] [PubMed] [Google Scholar]
- 25.Wasser LM, Weill Y, Brosh K, et al. The impact of COVID-19 on intravitreal injection compliance. SN Compr Clin Med. 2020;1-4. doi: 10.1007/s42399-020-00614-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahimzadeh M, Muniraju R, Izadi S.. Effect of COVID-19 pandemic on anti-VEGF treatment of medical retinal conditions—an audit. The Physician. 2021;6(3):1-9. [Google Scholar]
- 27.Sekeroglu MA, Kilinc Hekimsoy H, Horozoglu Ceran T, Doguizi S. Treatment of neovascular age related macular degeneration during COVID-19 pandemic: the short term consequences of unintended lapses. Eur J Ophthalmol. 2021;11206721211010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. IRIS Registry and Komodo Healthcare Map Inclusion and Exclusion Criteria
eTable 2. Adverse Events Definitions
eTable 3. Baseline patient/eye characteristics (overall cohort) at Index.
eTable 4. Baseline patient/eye characteristics by cohorts