Abstract
Viral B and C hepatitis are a major current health issue, both diseases having a chronic damaging effect on the liver and its functions. Chronic liver disease can lead to even more severe and life-threatening conditions, such as liver cirrhosis and hepatocellular carcinoma. Recent years have uncovered an important interplay between the liver and the gut microbiome: the gut-liver axis. Hepatitis B and C infections often cause alterations in the gut microbiota by lowering the levels of ‘protective’ gut microorganisms and, by doing so, hinder the microbiota ability to boost the immune response. Treatments aimed at restoring the gut microbiota balance may provide a valuable addition to current practice therapies and may help limit the chronic changes observed in the liver of hepatitis B and C patients. This review aims to summarize the current knowledge on the anato-functional axis between the gut and liver and to highlight the influence that hepatitis B and C viruses have on the microbiota balance, as well as the influence of treatments aimed at restoring the gut microbiota on infected livers and disease progression.
Keywords: Viral B hepatitis, Viral C hepatitis, Gut-liver axis, Immunomodulation, Lipopolysaccharides, Short-chain fatty acids
Core Tip: We have provided an overview of the mechanisms involved in the immunomodulation of the gut-liver axis. We highlight the mechanisms by which hepatitis B virus and hepatitis C virus infections influence the microbiota and how in turn these changes affect the liver pathology. We have also looked at the current treatment options and their influence on the intestinal microflora.
INTRODUCTION
Viral B and C hepatitis are two types of infections with a high rate of morbidity and mortality[1]. Hepatitis B virus (HBV) is a DNA virus belonging to the Hepadna virus, and hepatitis C virus (HCV) is an RNA virus in the Flaviviridae family. These viruses have hepatic tropism, are non-cytopathic with the ability to cause chronic liver inflammation and even liver cirrhosis and hepatocellular carcinoma[2].
Both HBV and HCV may cause similar clinical manifestations. Some patients may be asymptomatic, while others may have mild signs and symptoms from general manifestations (fatigue, fever, loss of appetite) to gastrointestinal symptoms (abdominal pain, nausea, vomiting, jaundice)[3].
The microbiota represents the totality of microbes (bacteria, viruses, fungi, protozoans, and archaea) associated with the human microorganism, while the microbiome consists of all microbes and their genes[4]. The main part of the body colonized by microbes is the gastrointestinal tract, whereas other parts such as skin, airways, vaginal tract, etc. are also colonized, but to a lesser extent. Changes in the microbiota are continuous throughout our life and there are many influencing factors, from type of delivery and breastfeeding, to long-term dietary changes, frequent and prolonged antibiotic treatment or other medications, etc.[5]. There are six bacterial dominant phyla in the gut microbiota: Firmicutes and Bacteroidetes (90%), Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia[6]. The intestinal microbiota is a cornerstone in maintaining the homeostasis of the human body. Firstly, this "organ" provides nutrients and energy from ingested food and, secondly, it is able to produce important metabolites that play a role in maintaining the host's metabolism[7].
The liver can be considered the largest immune organ in the body with a high ability to select and activate immune cells in response to metabolic products in the gut or to signals sent by various pathogens[8]. Recent years have seen advances in our understanding of the human microbiome and its interaction with us as hosts. The gut-liver axis is part of these new discoveries, integrating the microbiome modifications and dysbiosis in hepatic pathologies.
Our review will discuss part of the mechanisms by which the microbiome influences host immunity, as well as the gut-liver axis, with an accent on viral hepatitis B and C.
MICROBIOTA AND THE IMMUNE SYSTEM
Through its products, the human microbiota can influence both the local, enteric, and the systemic immune system, dysbiosis being correlated with several autoimmune, metabolic and neurodegenerative diseases (inflammatory bowel disease progression, rheumatoid arthritis, diabetes, asthma and bones homeostasis)[9-15]. This shows that the microbiota is not only involved in intestinal, but also in systemic and organ specific pathologies. This relationship is bidirectional; systemic modifications can trigger intestinal changes, but also intestinal dysbiosis can trigger and maintain organ dysfunctions. Gut-associated lymphoid tissue (GALT) is an important "immunological organ" of the body that belongs to the gut-mucosal immune system. GALT consists of Peyer's patches, intraepithelial lymphocytes, lamina propria lymphocytes (including dendritic cells) and mesenteric lymph nodes. Activation of this system has the ability to produce various mediators with immunostimulatory or immunosuppressive effect[16].
Some of the products by which the intestinal microflora communicates with the rest of our organism are lipopolysaccharides (LPS), bacterial DNA and RNA, flagellin, short-chain fatty acids (SCFA) such as acetate, propionate and butyrate, tryptophan (Trp) and it’s metabolites, teichoic acid and peptidoglycans and secondary bile acids (BA)[9,17]. These bacterial components and products of the bacterial metabolism are recognized by pattern recognition receptors, which particularly include the toll-like receptors (TLR) family. TLRs are expressed on epithelial and immune cells and are capable of recognizing specific bacterial molecules, triggering specific local protective and immunomodulatory (both pro- and anti-inflammatory) responses[18,19]. TLR activation is an essential element of the innate immune systems fight against the HBV and HCV infections[20,21]. Not all of these pathways were studied directly in connection with HBV and HCV. Therefore, more studies are needed to determine the exact relationship between the bacterial products, the immune system and hepatitis.
We will briefly mention some of the most important of the microbial-produced products and their interaction with the immune system (Figure 1).
Figure 1.
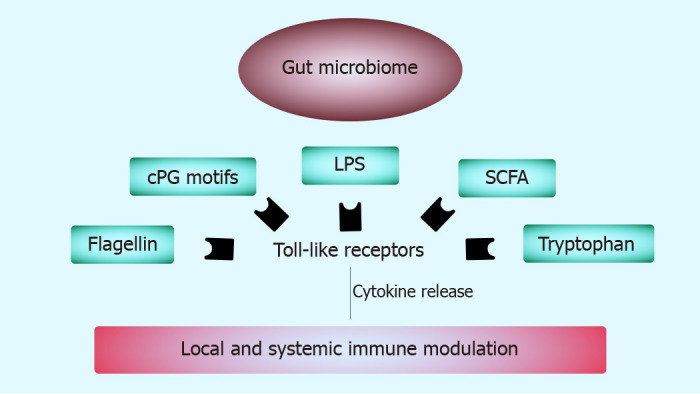
The mechanisms by which the gut microbiome influences the immune system. LPS: Lipopolysaccharides; SCFA: Short-chain fatty acids.
LPS
In Gram-negative bacteria, LPS are an important pathogen-associated molecular pattern and a well-studied microbial marker in connection with bacterial translocation and host systemic responses[22,23]. The outer membrane of gram-negative bacteria consists of LPS, which possess a hydrophobic endotoxin, called lipid A[24]. This component is recognized by TLR4 and via this mechanism it further activates nuclear factor kappa B (NF-κB) and elicits pro-inflammatory effects[25,26]. One type of LPS is Escherichia coli (E. coli) produced LPS. This stimulates TLR4 receptors and triggers the release of pro-inflammatory cytokines. E.coli LPS also increases endotoxin tolerance and decreases the autoimmune activity, protecting against autoimmune diabetes[27]. However, some bacterial species produce LPS molecules with underacylated lipid A that exhibit an immuno-inhibitory effect[28]. These LPS molecules are produced especially by members of the Bacteroidales order and instead of stimulating TLR receptors, they silence the TLR4 signaling and the inflammatory process[29]. LPS induces the upregulation of cluster of differentiation 14 protein (CD14) via the TLR4 pathway, which decreases the relative epithelial resistance and increases its permeability. Increased intestinal permeability allows for more LPS to reach the general circulation, aiding it in reaching different organs and exhibiting a pro-inflammatory effect[30]. This is also true in cases of dysbiosis with an increase in LPS production that is correlated with an increase in tumor necrosis factor alpha (TNF-α), interleukin (IL) 6 and C-reactive protein levels[31,32]. Intestinal dysbiosis caused an LPS-induced inflammatory response in a mice model, while unaltered host microbiota reduced the inflammatory response to LPS in the liver[33]. LPS-induced monocyte activation has been shown to be increased in patients with HBV or HCV[34].
This underlines the ability of LPS and gut lipid metabolism to modulate both intestinal and organ-specific inflammatory response.
SCFA
In the gut, non-digestible carbohydrates are transformed by the microbiota into SCFA such as acetate, propionate and butyrate[35]. Acetate and propionate are produced mainly by Bacteroidetes, while butyrate, the main source of energy for colonocytes, by Firmicutes. A small portion of SCFA that is not metabolized can reach the liver through the portal vein, being used as energy substrates for hepatocytes[36,37]. Certain bacteria such as Butyricimonas and Prevotella have the ability to generate butyrate and propionate, SCFAs with anti-inflammatory effect[38].
SCFA bind to the G-protein coupled free fatty acid receptors (FFA): GPR41 (FFA2) and GPR43 (FFA3)[39,40]. Enteroendocrine and pancreatic β-cells present both GPR41 and GPR43 receptors, while immune cells and adipocytes present mostly GPR41 and peripheral neurons GPR43[41]. This links SCFA production to a multitude of metabolic, neurological and inflammatory mechanisms. Thus, FFA receptors and SCFA production presents therapeutic targets in these diseases[41-43].
In immune cells (leukocytes and neutrophils) SCFA increase the intracellular calcium levels[39,44,45]. This reaction leads to an increased production of reactive oxygen species, as well as an increased neutrophil recruitment and a pro-inflammatory effect[46-48]. GPR41 activation by SCFAs in the gut promotes the function and size of regulatory T cells, protecting against intestinal inflammation[49]. Also, GPR43 was found to be a chemotactic receptor for neutrophils, stimulating their migration towards the source of SCFAs[50,51]. In a mouse model of gout, the intestinal microbiota-produced SCFA determined inflammasome assembly, reactive oxygen species formation and IL-1b production and improved the inflammatory response[52]. Increased SCFA levels determined the production of macrophages and dendritic cells, protecting the lung against allergic inflammation[53]. Also, by activating another G-protein coupled receptor, GPR109A, the microbiota is involved in inflammatory suppression via the NF-κB pathway in normal and colon cancer cells[54].
Another SCFA mechanism involved the inhibition of histone deacetylases (HDAC). By non-competitively inhibiting the activity of HDAC 1 and 2, butyrate causes histone hyperacetylation. By this mechanism, butyrate and other SCFAs are thought to serve as a protective factor against colon cancer, dysbiosis being a risk factor for the development of this disease, as well as other chronic inflammatory diseases[55]. HDAC inhibition also promotes macrophage activity and CD8 T cells and improves anti-cancer therapy[56-59]. Furthermore, class 1 HDACs inhibition is proposed as a target in pulmonary inflammation, due to its contribution in the release of pro-inflammatory cytokines[60]. HDAC inhibition promotes effector and regulatory T-cell differentiation and the production of IL-17, interferon-γ (IFN-γ) and IL-10, contributing to an overall anti-inflammatory effect mediated by SCFAs[61,62].
By increasing acetyl-CoA activity and controlling gene expression, SCFA are involved in plasma B cells metabolism, activity, energy production boosting, and differentiation. During an infection, they support B cells antibody production, decreasing the host susceptibility to pathogens[63].
Therefore, SCFA present both a pro- and anti-inflammatory role[61]. There is still the need for more studies to fully understand the implications of SCFA in inflammatory and immune diseases and determine in which conditions they act as pro-inflammatory or as anti-inflammatory factors.
Trp
The microbiota is involved in the transformation of Trp in indole derivatives, serotonin (5-hydroxytryptamine) and kynurenine[64].
Lactobacilli species can metabolize Trp into indole-3-aldehyde, a ligand for the aryl hydrocarbon receptor (AhR) that is involved in intestinal immunity and the production of IL-22[65,66]. There are only a few species such as Peptostreptococcus russellii and Lactobacillus spp. with the ability to produce AhR ligands[64]. In high fat diets IL-22 can act as an antioxidant and anti-inflammatory agent, protecting the intestinal mucosa and epithelial cells from oxidative and inflammatory stressors[67]. Also, IL-22 is involved in the intestinal mucosa immune response against exterior pathogens[68,69]. However, in patients with inflammatory bowel disease, Il-22 is considered a “two-headed cytokine”: it acts as a mucosal producing and healing agent, but in the chronic form of the disease it is also involved in tumorigenesis, promoting tumoral growth[70-72].
The Trp microbiota metabolite AhR regulates the activation and transcription of several other pathways, including IL-6, cytochrome P450 1A1 (CYP1A1), and 1B1 (CYP1B1), vascular endothelial growth factor A, and prostaglandin G/H synthase 2 and also stimulates innate lymphoid cells and intraepithelial lymphocytes development, mediating their anti-inflammatory effects[73,74]. Other bacteria that interfere with Trp metabolism are E. coli, Lactobacilli and Clostridium sporogenes. The first two possess tryptophanase which converts Trp to indole, while the latter decarboxylates Trp and increases tryptamine production[64].
The microbiota influence on Trp provides intestinal anti-inflammatory effects, but it also poses potential research directions regarding systemic inflammation[75,76].
Flagellin
The locomotive bacterial flagella contain flagellin, which is recognized by the host TLR5. Via the TLR pathways, flagellin is involved in several immunological mechanisms, both locally, in the gut, but also systemic, inducing the release of pro-inflammatory molecules[77]. In a study administering purified flagellin in mice, there was a decreased microbial dysbiosis, as well as an amelioration of IL-10 deficiency-induced colitis[78]. This shows that flagellin presenting bacterial species could pose a beneficial effect in chronic inflammatory diseases. However, in patients with inflammatory bowel diseases there have been observed higher concentrations of flagellin, putting into question its supposed protective role[79]. Also, flagellin has been observed to be a potent TLR5/NF-κB activator, promoting inflammation in intestinal epithelial cells[80]. Via the same TLR5/NF-κB mechanism, flagellin could also promote the attachment and development of viral molecules, supporting viral infections via the intestine[81].
Bacterial CpG motifs
Bacterial DNA contains unmethylated CpG dinucleotides that are recognized by the immune system and produce an immunostimulatory effect[82,83]. These bacterial CpG motifs are recognized by TLR9 receptors and, depending on their localization, they exhibit several effects. Apical TLR9 activation inhibits NF-κB activation, while basolateral receptors stimulate NF-κB activation and the subsequent inflammatory pathways[84].
INFLAMMATION AND B AND C HEPATITIS
Many extrahepatic changes (metabolic, cardiovascular, autoimmune, renal) have been correlated with chronic HCV infection. This statement is supported by a prospective cohort study in which patients with chronic HCV infection (with HCV RNA detected in the serum) had a high risk of death due to liver or non-liver disease (cardiovascular and renal disease) compared to uninfected patients (without serum HCV RNA) or with patients presenting HCV antibodies[85].
Inflammatory cytokines are normally released in response to various stimuli, including viral infection. This limits cellular stress and cell damage[86]. HCV infection is associated with an immune activation status that can further influence the levels of inflammatory markers (Il-6, TNF-α, iNOS, COX-2, IL-1), which are correlated with various extrahepatic diseases[87,88]. In HBV-infected patients there is an increase in Il-8, IL-29 and COX-2. Under normal conditions, adult hepatocytes do not express COX-2, but in chronic inflammatory diseases, the expression of this isoenzyme increases. Furthermore, IL-8 activates the extracellular signal-regulated kinase and c-Jun N-terminal kinase signaling pathways, which are also involved in inflammatory processes[86].
In infected hepatocytes with HCV, the production of type 1 and 3 interferons is blocked by the action of the viral NS3/4A protease. This protease may also influence the innate immune adaptor molecules mitochondrial antiviral signaling proteins with an effect on the intracellular antiviral defense system. In an experimental study on hepatic macrophages the first activated factor in liver macrophages with HCV infection has been shown to be TNF-α that further activates NF-κB and increases IL-1β. Adding to this, the HCV core protein also activates the NLRP3 inflammasome. The hepatic inflammatory environment is ensured by the activity of the NLRP3 inflammasome, phospholipase-C and IL-1β. Thus, NLRP3 inflammasome and IL-1β can be considered as target of treatment in HCV-induced liver disease[89].
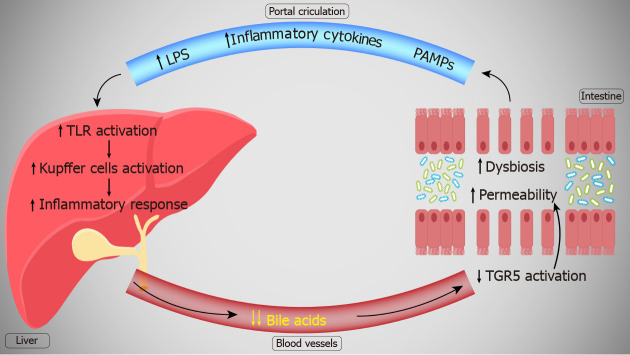
THE GUT-LIVER AXIS
The gut microbiome can interact tightly with the liver via the so-called gut-liver axis. Blood from the intestine, rich in microbiota-derived molecules, reaches the liver via the portal vein. In the liver, these molecules are recognized by TLRs pattern recognition receptors, mediating their effect on the liver tissue[90]. Related to liver pathologies, the gut microbiota is particularly involved in liver fibrosis and cirrhosis, hepatic cancers, alcoholic and non-alcoholic fatty liver disease, autoimmune hepatitis, primary sclerosing and primary biliary cholangitis as well as viral hepatitis[91-96]. Some of the most studied components that affect liver pathologies are represented by LPS and SCFAs.
LPS produced by the microbiota are scarcely found in the normal liver, being cleared by Kupffer cells and not causing any damage[97]. However, in alcoholic liver disease, because of an increase intestinal permeability, an increased amount of LPS reached the liver[96]. LPS binds to TLR4, causing an excessive release of pro-inflammatory cytokines IL-1 and TNF-α[33,98]. Also, LPS can upregulate the expression of the cluster of differentiation 14 (CD14) receptor on Kupffer cells[99]. This could potentially make the liver more sensitive to LPS toxicity, as CD14 is vital for Kupffer cells LPS activation[100]. Kupffer cells activation produces a pro-inflammatory state, increasing the levels of NF-κB, TNF-α and IL-1. This leads to liver injury and disease progression, dysbiosis favoring the chronic inflammatory state[101].
SCFA such as acetate, propionate and butyrate may have a protective effect on liver diseases progression. High levels of butyrate restore the intestinal microbiota in cases of dysbiosis, reducing the intestinal permeability and thus the levels of endotoxins reaching the liver via the portal circulation. This attenuated the histological aspect of steatohepatitis livers, reducing the levels of TNF-α, IL-1, IL-6 and IFN-γ pro-inflammatory cytokines, as well as the expression of TLR4 receptors[102]. In an experimental study by Endo et al[103], administering probiotics, aimed at increasing butyrate levels, significantly improved non-alcoholic fatty liver disease progression, reducing the inflammation and oxidative stress. This clearly shows that intestinal-produced metabolites can influence the immune and inflammatory state of the liver. Dysbiosis and an increased intestinal permeability allows for the gut-liver balance to change, causing a pro-inflammatory state of the liver and contributing to disease progression[104,105]. Pathogen-associated molecular patterns (bacterial antigens and products) such as LPS and viral RNAs activate TLR4 on Kupffer cells and other immune cells. Thus, the innate immune response is induced.
The liver is influenced by the intestine through the portal circulation, while the intestine is influenced by the liver through the released mediators and hepatic bile flow. It is known that increased intestinal permeability contributes to systemic inflammation and disease progression[106]. BA and other mediators such as immunoglobulin A (IgA) regulate the gut-liver axis. IgA influences the homeostasis of the intestinal microbiota, preventing bacterial translocation. BA modulate the intestinal barrier and have antimicrobial activity. Several enzymes involved in BA synthesis are regulated by the microbiota. However, some secondary BA (e.g., deoxycholic acid) resulting from intestinal biotransformation produce microbial dysbiosis and increase the intestinal permeability[107].
TGR5 is a G-protein-coupled BA receptor involved in the anti-inflammatory immune response, energy homeostasis, metabolic pathways and in pathologies such as diabetes and obesity[108]. In the intestine, TGR5 is involved in regulating the colonic motility and the intestinal permeability via the farnesoid X receptor — cAMP pathway[109,110]. Moreover, TGR5 activation stimulates mucosal proliferation and protects against mucosal injuries[111]. In liver pathologies, the levels of BA are significantly decreased, leading to a reduced activation of TGR5 in the gut[112,113]. In a mouse model with TGR5 silencing, there was a significant reduction in gut epithelial cellularity, with histological abnormalities and distortions and an increased intestinal permeability[114]. BA and TGR5 activation are therefore necessary for a normal functioning of the intestine and the gut-blood barrier. BA administration is beneficial for viral hepatic diseases. In a HBV model, TGR5 agonists administration suppressed the infection[115]. BA and TGR5 agonists pose as potential treatment options for viral hepatitis[116].
Decreased BA quantities in virus hepatitis could be responsible for the increased intestinal permeability and the subsequent increase in LPS and other endotoxins. This in turn favors the progression of the liver pathology, creating a vicious circle where the liver pathology creates an environment that further promotes the liver pathology (Figure 2). Future studies should determine the exact mechanism by which liver diseases influence the intestinal permeability and lead to the production of dysbiosis.
Figure 2.
The gut-liver axis in liver diseases. TGR5: G-protein-coupled bile acid receptor; PAMPs: Pathogen-associated molecular patterns; LPS: Lipopolysaccharides; TLR: Toll-like receptor.
THE GUT MICROBIOTA-VIRAL B AND C HEPATITIS
The presence of the HBV or HCV infection can lead to intestinal dysbiosis[117]. Some of the microbial changes present in patients with HBV and HCV-related liver diseases are shown in Table 1.
Table 1.
Microbiota changes in different studies regarding hepatic B and C virus
|
|
Changes of gut microbiota in patients vs healthy subjects |
Ref. |
| Type of HBV infection | ||
| Chronic HBV infection | ↓ Bacteroidetes and Firmicutes; ↑ Proteobacteria and Actinobacteria | Chen et al[117] |
| ↑ Bifidobacterium dentium; ↓ Bifidobacterium catenulatum and longum | Xu et al[118] | |
| ↑ Veillonellaceae; ↓ Lachnospiraceae, Rikenellaceae, Ruminococcaceae | Wang et al[119] | |
| HBV liver cirrhosis | ↓↓↓ Bacteroidetes and Firmicutes; ↑↑↑ Proteobacteria and Actinobacteria | Chen et al[117] |
| Decompensated HBV cirrhosis | ↓ Bifidobacteria/Enterobacteriaceae ratio; ↑ Enterobacteriaceae; ↓ Firmicutes (F.prausnitzii, Clostridium clusters XI and XIVab, Bifidobacterium); ↓ Bacteroidetes | Lu et al[120] |
| HBV related hepatocellular carcinoma | ↓ Proteobacteria; ↑ Prevotella, Phascolarctobacterium, Anaerotruncus; ↑ Proteus, Veillonella, Prevotella 2, Barnesiella and Ruminococcaceae spp. | Liu et al[121] |
| Type of HCV infection | ||
| Chronic HCV infection without cirrhosis | ↑ Veillonella spp., Lactobacillus spp., Streptococcus spp. and Alloprevotella spp.; ↓ Bilophila spp., Clostridium IV spp., Clostridium XlVb spp., Mitsuokella spp. and Vampirovibrio spp.; No changes: Akkermansia spp., Bifidobacterium spp., Escherichia/Shigella spp., Haemophilus spp., Micrococcus spp. and Weissella spp. | Heidrich et al[122] |
| Chronic HCV infection with cirrhosis | ↑↑↑ Veillonella spp., Lactobacillus spp., Streptococcus spp. and Alloprevotella spp.; ↓↓↓ Bilophila spp., Clostridium IV spp., Clostridium XlVb spp., Mitsuokella spp. and Vampirovibrio spp.; ↑↑↑ Akkermansia spp., Bifidobacterium spp., Escherichia/Shigella spp., Haemophilus spp., Micrococcus spp. and Weissella spp. | Heidrich et al[122] |
| Stage 4 HCV infection (cirrhosis) | ↓ Firmicutes; ↑ Prevotella, Faecalibacterium (F. prausnitzii); ↑ Acinetobacter; ↑ Veillonella | Aly et al[123] |
HBV: Hepatitis B virus; HCV: Hepatitis C virus.
These studies showed significant differences in the composition of the intestinal microbiota between patients with B or C hepatitis with or without cirrhosis present. A healthy gut microbiota means a gut microbiota with great diversity and the ability to react to changes. Thus, B and C viruses can cause changes and can shape the gut microbiota in different directions[122].
Nowadays, the treatment of B and C hepatitis is well established by international guidelines[124-126]. The main question is: does the treatment of B or C hepatitis influence the diversity and abundance of the intestinal microbiota? And if so, are these changes helping in preventing or halting the evolution of the disease? A part of the studies looking into the microbial changes caused by HBV and HCV treatments are presented in Table 2.
Table 2.
Microbial changes as a result of several treatments in viral B and C hepatitis
| Drug |
Type of study |
Changes in gut microbiota |
Ref. |
| Entecavir | Experimental (mice) | ↑ Lachnospiraceae, Akkermansia, Alistipes, Escherichia, Shigella, Oscillibacter, Bilophila | Li et al[127] |
| Clinical | ↑ Clostridium sensu stricto 1, Erysipelotrichaceae UCG-007, Intestinibacter; ↓ Streptococcus, Atopobium, and Murdochiella | Lu et al[128] | |
| Direct antiviral agents in patients with HCV infection | Clinical | ↑ Phylum Firmicutes, genera Lachnospira | Pérez-Matute et al[129] |
| Direct antiviral agents in patients with HCV-related liver cirrhosis | Clinical | ↓ Enterobacteriaceae, Staphylococcus and Veillonellaceae | Ponziani et al[130] |
HCV: Hepatitis C virus.
Entecavir increases the abundance of the genus Clostridium sensu stricto 1 which has been associated with large and extra-large HDL particles and also with a decreased risk of cardiovascular disease[131]. Increased lipid content in the liver and steatosis can result in the development of inflammation and, over time, cirrhosis, and can also increase oxidative stress[132]. Genus Intestinibacter along with genus Escherichia, Shigella can be considered as a major contributor to NAFLD progression. Increases in the abundance of Intestinibacter have been correlated with severe intestinal disorders in humans and are recognized as a biomarker of the onset of Crohn's disease[133].
In a study by Pérez-Matute et al[129], it was shown that the use of direct antiviral agents in patients with chronic HCV infection could only restore the intestinal bacterial changes in those patients with a lower degree of fibrosis (F0-1). The data highlight a strong relationship between the liver and the intestine and suggest that mild intestinal changes caused by liver damage could possibly be counteracted with the appropriate drugs.
Blautia, Coprococcus, Dorea, Lachnospira, Oribacterium, Roseburia and L-Ruminococcus were detected in the human intestine as the main genera belonging to the Lachnospiraceae family[134]. Lachnospiraceae is considered a "good" family of bacteria, having a beneficial role in host homeostasis. The bacteria belonging to this family can convert carbohydrates into SCFA in the gut[135]. Decreasing the abundance of Lachnospiraceae leads to decreased SCFA production and thus increases the pH of the colon. This change increases the production of ammonia and its absorption in the intestine[136].
Direct-acting antivirals (DAA) treatment in cirrhotic patients appears to have a positive impact on changes in the intestinal microbiota, as well as fibrosis and inflammation, but without a positive impact on the function of the intestinal barrier. DAA has greatly reduced the abundance of Enterobacteriaceae, Staphylococcus, and Veillonellaceae[130]. The abundance of the Enterobacteriaceae family, belonging to the Proteobacteria phylum, depends on the amount of oxygen that crosses the intestinal barrier. The abundance of Enterobacteriaceae is elevated after the oxygen level increases and can aggravate intestinal inflammation. Members of this family cannot degrade complex carbohydrates (as Clostridia and Bacteroidia do); they are only involved in the passive transport of oligosaccharides. This disadvantage may explain the lower abundance of Enterobacteriaceae compared to Clostridia and Bacteroidia in the healthy distal intestine[137]. Veillonellaceae belonging to Firmicutes phylum, is one of the main microbial taxa associated with the severity of fibrosis in non-obese patients. This family has the ability to produce propionate, one of the most important SCFAs and has been associated with chronic liver disease[138]. The LPS and SCFA metabolites produced by intestinal Veillonella stimulate the release of cytokines (Il-6, IL-10, TNF-α) in human peripheral blood mononuclear cells and thus have a negative impact on liver pathology and host inflammation[139].
GUT MICROBIOTA-TARGET OF TREATMENT
Although standard therapy for B and C viral hepatitis is well established and presented in clinical guidelines, many dietary supplements, including pre-, pro-, and symbiotic agents, are being studied to reduce the toxicity of standard therapy (side effects) or to increase their effect. Also, fecal microbiota transplantation (FMT) is one of the methods that can manipulate the composition of the intestinal microbiota. It has the ability to strengthen the intestinal barrier, reduce intestinal permeability and also improve host immunity[140]. There are various routes of administration for FMT: nasogastric tube, upper endoscopy or colonoscopy, retention enema, etc. The route of administration depends on the characteristics of the disease. For example, good results have been obtained after duodenal administration in metabolic disease[141].
There are only a few studies that support the effect of certain probiotics in viral B or C hepatitis.
Oo et al[142] studied the long-term (36-mo) effect of probiotic heat-treated strain Enterococcus faecalis FK-23 in patients with HCV infection. This probiotic may change the microbiota in these patients and may have an important role of decreased ALT in serum.
In patients with HBV-induced liver cirrhosis, the role of a probiotic (Clostridium butyricum combined with Bifidobacterium infantis) has been studied in the treatment of minimal hepatic encephalopathy. The results claim that the probiotic modulates the intestinal barrier and thus can lower the level of ammonia and can improve cognition[143].
CONCLUSION
Most of the microbiota-derived components elicit an immunomodulatory effect, both pro- and anti-inflammatory. Alteration of the host microbiome produces an unbalance of these factors, leading to negative effects both locally in the intestine, as well as at distance in other organs. Therefore, we can conclude that by its factors, the host microbiota is an important determinant in the hosts immune response modulation. Future experimental and clinical studies are needed to determine the exact mechanisms of these changes, as well as the exact conditions in which the microbiota can serve as a protective factor.
Currently, the intestinal microbiota is a target of treatment for various diseases in humans. Future studies should focus on the effects and efficacy of treatments aimed at restoring the gut microbial environment (prebiotics, probiotics, symbiotics, fecal transplant) and their exact relationship with liver pathologies. By understanding the natural communication pathways between the liver and the gut, in both health and disease, we could potentially formulate better therapies aimed at reducing the effects of the chronic inflammatory response on the progression of liver diseases.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review started: March 17, 2021
First decision: August 9, 2021
Article in press: November 2, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chi G, Sira AM, Wang L S-Editor: Gao CC L-Editor: Filipodia P-Editor: Gao CC
Contributor Information
Maria Adriana Neag, Department of Pharmacology, Toxicology and Clinical Pharmacology, Iuliu Hatieganu University of Medicine and Pharmacy of Cluj-Napoca, Cluj-Napoca 400337, Romania.
Andrei Otto Mitre, Faculty of Medicine, Iuliu Hatieganu University of Medicine and Pharmacy of Cluj-Napoca, Cluj-Napoca 400012, Romania. andrei.otto.mitre@elearn.umfcluj.ro.
Adrian Catinean, Department of Internal Medicine, Iuliu Hatieganu University of Medicine and Pharmacy of Cluj-Napoca, Cluj-Napoca 400006, Romania.
Anca Dana Buzoianu, Department of Pharmacology, Toxicology and Clinical Pharmacology, Iuliu Hatieganu University of Medicine and Pharmacy of Cluj-Napoca, Cluj-Napoca 400337, Romania.
References
- 1.Karnsakul W, Schwarz KB. Hepatitis B and C. Pediatr Clin North Am. 2017;64:641–658. doi: 10.1016/j.pcl.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoletti A, Le Bert N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver. 2018;12:497–507. doi: 10.5009/gnl17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardee M. Diagnosis and Management of Hepatitis B and C. Nurs Clin North Am. 2019;54:277–284. doi: 10.1016/j.cnur.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Philips CA, Augustine P, Yerol PK, Ramesh GN, Ahamed R, Rajesh S, George T, Kumbar S. Modulating the Intestinal Microbiota: Therapeutic Opportunities in Liver Disease. J Clin Transl Hepatol. 2020;8:87–99. doi: 10.14218/JCTH.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front Immunol. 2020;11:700. doi: 10.3389/fimmu.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catinean A, Neag MA, Muntean DM, Bocsan IC, Buzoianu AD. An overview on the interplay between nutraceuticals and gut microbiota. PeerJ. 2018;6:e4465. doi: 10.7717/peerj.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16 Suppl 1:S21–S26. doi: 10.5604/01.3001.0010.5672. [DOI] [PubMed] [Google Scholar]
- 8.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152:1–12. doi: 10.1111/imm.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. 2018;56:154–162. doi: 10.1007/s12275-018-7548-y. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm Behav. 2012;62:286–294. doi: 10.1016/j.yhbeh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 13.D'Amelio P, Sassi F. Gut Microbiota, Immune System, and Bone. Calcif Tissue Int. 2018;102:415–425. doi: 10.1007/s00223-017-0331-y. [DOI] [PubMed] [Google Scholar]
- 14.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26:69–74. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valentini M, Piermattei A, Di Sante G, Migliara G, Delogu G, Ria F. Immunomodulation by gut microbiota: role of Toll-like receptor expressed by T cells. J Immunol Res. 2014;2014:586939. doi: 10.1155/2014/586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ma Z, Cao Q, Xiong Y, Zhang E, Lu M. Interaction between Hepatitis B Virus and Toll-Like Receptors: Current Status and Potential Therapeutic Use for Chronic Hepatitis B. Vaccines (Basel) 2018;6 doi: 10.3390/vaccines6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashfaq UA, Iqbal MS, Khaliq S. Role of Toll-Like Receptors in Hepatitis C Virus Pathogenesis and Treatment. Crit Rev Eukaryot Gene Expr. 2016;26:353–362. doi: 10.1615/CritRevEukaryotGeneExpr.2016017455. [DOI] [PubMed] [Google Scholar]
- 22.Moon MS, Quinn G, Townsend EC, Ali RO, Zhang GY, Bradshaw A, Hill K, Guan H, Hamilton D, Kleiner DE, Koh C, Heller T. Bacterial Translocation and Host Immune Activation in Chronic Hepatitis C Infection. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammad S, Thiemermann C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front Immunol. 2020;11:594150. doi: 10.3389/fimmu.2020.594150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 26.Neag MA, Catinean A, Muntean DM, Pop MR, Bocsan CI, Botan EC, Buzoianu AD. Probiotic Bacillus Spores Protect Against Acetaminophen Induced Acute Liver Injury in Rats. Nutrients. 2020;12 doi: 10.3390/nu12030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M DIABIMMUNE Study Group, Xavier RJ. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coats SR, Do CT, Karimi-Naser LM, Braham PH, Darveau RP. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 Lipopolysaccharide binding site. Cell Microbiol. 2007;9:1191–1202. doi: 10.1111/j.1462-5822.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 29.d'Hennezel E, Abubucker S, Murphy LO, Cullen TW. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems. 2017;2 doi: 10.1128/mSystems.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18:3461–3469. doi: 10.3892/etm.2019.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front Cell Infect Microbiol. 2019;9:409. doi: 10.3389/fcimb.2019.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suriguga S, Luangmonkong T, Mutsaers HAM, Groothuis GMM, Olinga P. Host microbiota dictates the proinflammatory impact of LPS in the murine liver. Toxicol In Vitro. 2020;67:104920. doi: 10.1016/j.tiv.2020.104920. [DOI] [PubMed] [Google Scholar]
- 34.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T, Douek DC. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230, 1230.e1. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang L, Schnabl B. Gut Microbiota in Liver Disease: What Do We Know and What Do We Not Know? Physiology (Bethesda) 2020;35:261–274. doi: 10.1152/physiol.00005.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7:11–20. doi: 10.21037/hbsn.2017.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 40.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 41.Tang C, Offermanns S. FFA2 and FFA3 in Metabolic Regulation. Handb Exp Pharmacol. 2017;236:205–220. doi: 10.1007/164_2016_50. [DOI] [PubMed] [Google Scholar]
- 42.Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21:173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 43.Säemann MD, Böhmig GA, Zlabinger GJ. Short-chain fatty acids: bacterial mediators of a balanced host-microbial relationship in the human gut. Wien Klin Wochenschr. 2002;114:289–300. [PubMed] [Google Scholar]
- 44.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naccache PH, Faucher N, Caon AC, McColl SR. Propionic acid-induced calcium mobilization in human neutrophils. J Cell Physiol. 1988;136:118–124. doi: 10.1002/jcp.1041360115. [DOI] [PubMed] [Google Scholar]
- 46.Mårtensson J, Holdfeldt A, Sundqvist M, Gabl M, Kenakin TP, Björkman L, Forsman H, Dahlgren C. Neutrophil priming that turns natural FFA2R agonists into potent activators of the superoxide generating NADPH-oxidase. J Leukoc Biol. 2018;104:1117–1132. doi: 10.1002/JLB.2A0318-130RR. [DOI] [PubMed] [Google Scholar]
- 47.Björkman L, Mårtensson J, Winther M, Gabl M, Holdfeldt A, Uhrbom M, Bylund J, Højgaard Hansen A, Pandey SK, Ulven T, Forsman H, Dahlgren C. The Neutrophil Response Induced by an Agonist for Free Fatty Acid Receptor 2 (GPR43) Is Primed by Tumor Necrosis Factor Alpha and by Receptor Uncoupling from the Cytoskeleton but Attenuated by Tissue Recruitment. Mol Cell Biol. 2016;36:2583–2595. doi: 10.1128/MCB.00161-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundqvist M, Christenson K, Holdfeldt A, Gabl M, Mårtensson J, Björkman L, Dieckmann R, Dahlgren C, Forsman H. Similarities and differences between the responses induced in human phagocytes through activation of the medium chain fatty acid receptor GPR84 and the short chain fatty acid receptor FFA2R. Biochim Biophys Acta Mol Cell Res. 2018;1865:695–708. doi: 10.1016/j.bbamcr.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 2011;6:e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Häsler R, Nikolaus S, Fölsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 52.Vieira AT, Macia L, Galvão I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, Nicoli JR, Harper JL, Teixeira MM, Mackay CR. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015;67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- 53.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 54.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012–1017. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 56.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, Davis SP, Lobera M, Nolan MA, Letai A. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods DM, Sodré AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J. HDAC Inhibition Upregulates PD-1 Ligands in Melanoma and Augments Immunotherapy with PD-1 Blockade. Cancer Immunol Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCaw TR, Goel N, Brooke DJ, Katre AA, Londoño AI, Smith HJ, Randall TD, Arend RC. Class I histone deacetylase inhibition promotes CD8 T cell activation in ovarian cancer. Cancer Med. 2021;10:709–717. doi: 10.1002/cam4.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Su X, Liu R, Pan Y, Fang J, Cao L, Feng C, Shang Q, Chen Y, Shao C, Shi Y. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene. 2021;40:1836–1850. doi: 10.1038/s41388-020-01636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanfield BA, Purves T, Palmer S, Sullenger B, Welty-Wolf K, Haines K, Agarwal S, Kasotakis G. IL-10 and class 1 histone deacetylases act synergistically and independently on the secretion of proinflammatory mediators in alveolar macrophages. PLoS One. 2021;16:e0245169. doi: 10.1371/journal.pone.0245169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee N, Kim WU. Microbiota in T-cell homeostasis and inflammatory diseases. Exp Mol Med. 2017;49:e340. doi: 10.1038/emm.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim M, Qie Y, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11:1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 67.Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, Denman S, Begun J, Florin TH, Perkins A, Cuív PÓ, McGuckin MA, Hasnain SZ. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 69.Mühl H, Bachmann M. IL-18/IL-18BP and IL-22/IL-22BP: Two interrelated couples with therapeutic potential. Cell Signal. 2019;63:109388. doi: 10.1016/j.cellsig.2019.109388. [DOI] [PubMed] [Google Scholar]
- 70.Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–474. doi: 10.1007/s00535-017-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laurence A, O'Shea JJ, Watford WT. Interleukin-22: a sheep in wolf's clothing. Nat Med. 2008;14:247–249. doi: 10.1038/nm0308-247. [DOI] [PubMed] [Google Scholar]
- 72.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zelante T, Iannitti RG, Fallarino F, Gargaro M, De Luca A, Moretti S, Bartoli A, Romani L. Tryptophan Feeding of the IDO1-AhR Axis in Host-Microbial Symbiosis. Front Immunol. 2014;5:640. doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr. 2020;11:709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Honko AN, Mizel SB. Effects of flagellin on innate and adaptive immunity. Immunol Res. 2005;33:83–101. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 78.Tran HQ, Ley RE, Gewirtz AT, Chassaing B. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat Commun. 2019;10:5650. doi: 10.1038/s41467-019-13538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cook L, Lisko DJ, Wong MQ, Garcia RV, Himmel ME, Seidman EG, Bressler B, Levings MK, Steiner TS. Analysis of Flagellin-Specific Adaptive Immunity Reveals Links to Dysbiosis in Patients With Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2020;9:485–506. doi: 10.1016/j.jcmgh.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tallant T, Deb A, Kar N, Lupica J, de Veer MJ, DiDonato JA. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benedikz EK, Bailey D, Cook CNL, Gonçalves-Carneiro D, Buckner MMC, Blair JMA, Wells TJ, Fletcher NF, Goodall M, Flores-Langarica A, Kingsley RA, Madsen J, Teeling J, Johnston SL, MacLennan CA, Balfe P, Henderson IR, Piddock LJV, Cunningham AF, McKeating JA. Bacterial flagellin promotes viral entry via an NF-kB and Toll Like Receptor 5 dependent pathway. Sci Rep. 2019;9:7903. doi: 10.1038/s41598-019-44263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 83.Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955–962. [PubMed] [Google Scholar]
- 84.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 85.Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 86.Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, Chen M, Liu Y, Zhu Y, Wu J. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187:4844–4860. doi: 10.4049/jimmunol.1100998. [DOI] [PubMed] [Google Scholar]
- 87.Babiker A, Hassan M, Muhammed S, Taylor G, Poonia B, Shah A, Bagchi S. Inflammatory and cardiovascular diseases biomarkers in chronic hepatitis C virus infection: A review. Clin Cardiol. 2020;43:222–234. doi: 10.1002/clc.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen WC, Tseng CK, Chen BH, Lin CK, Lee JC. Grape Seed Extract Attenuates Hepatitis C Virus Replication and Virus-Induced Inflammation. Front Pharmacol. 2016;7:490. doi: 10.3389/fphar.2016.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antushevich H. Interplays between inflammasomes and viruses, bacteria (pathogenic and probiotic), yeasts and parasites. Immunol Lett. 2020;228:1–14. doi: 10.1016/j.imlet.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohamadkhani A. On the potential role of intestinal microbial community in hepatocarcinogenesis in chronic hepatitis B. Cancer Med. 2018 doi: 10.1002/cam4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sehgal R, Bedi O, Trehanpati N. Role of Microbiota in Pathogenesis and Management of Viral Hepatitis. Front Cell Infect Microbiol. 2020;10:341. doi: 10.3389/fcimb.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Usami M, Miyoshi M, Yamashita H. Gut microbiota and host metabolism in liver cirrhosis. World J Gastroenterol. 2015;21:11597–11608. doi: 10.3748/wjg.v21.i41.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 97.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955–965. doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 99.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su GL, Goyert SM, Fan MH, Aminlari A, Gong KQ, Klein RD, Myc A, Alarcon WH, Steinstraesser L, Remick DG, Wang SC. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol. 2002;283:G640–G645. doi: 10.1152/ajpgi.00253.2001. [DOI] [PubMed] [Google Scholar]
- 101.Grabherr F, Grander C, Effenberger M, Adolph TE, Tilg H. Gut Dysfunction and Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne) 2019;10:611. doi: 10.3389/fendo.2019.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanduzzi Zamparelli M, Rocco A, Compare D, Nardone G. The gut microbiota: A new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterol J. 2017;5:944–953. doi: 10.1177/2050640617705576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossetto A, De Re V, Steffan A, Ravaioli M, Miolo G, Leone P, Racanelli V, Uzzau A, Baccarani U, Cescon M. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers (Basel) 2019;11 doi: 10.3390/cancers11111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chopyk DM, Grakoui A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology. 2020;159:849–863. doi: 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Corrigendum to "Targeting the gut-liver axis in liver disease" [J Hepatol 67 (2017) 1084-1103] J Hepatol. 2018;68:1336. doi: 10.1016/j.jhep.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Jacinto S, Fang S. Essential roles of bile acid receptors FXR and TGR5 as metabolic regulators. Animal Cells Syst (Seoul) . 2014;18:359–364. [Google Scholar]
- 109.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ji CG, Xie XL, Yin J, Qi W, Chen L, Bai Y, Wang N, Zhao DQ, Jiang XY, Jiang HQ. Bile acid receptor TGR5 overexpression is associated with decreased intestinal mucosal injury and epithelial cell proliferation in obstructive jaundice. Transl Res. 2017;182:88–102. doi: 10.1016/j.trsl.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Wang X, Xie G, Zhao A, Zheng X, Huang F, Wang Y, Yao C, Jia W, Liu P. Serum Bile Acids Are Associated with Pathological Progression of Hepatitis B-Induced Cirrhosis. J Proteome Res. 2016;15:1126–1134. doi: 10.1021/acs.jproteome.5b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan LT, Wang LL, Yao J, Yang YT, Mao XR, Yue W, Mao YW, Zhou W, Chen QF, Chen Y, Duan ZP, Li JF. Total bile acid-to-cholesterol ratio as a novel noninvasive marker for significant liver fibrosis and cirrhosis in patients with non-cholestatic chronic hepatitis B virus infection. Medicine (Baltimore) 2020;99:e19248. doi: 10.1097/MD.0000000000019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito K, Okumura A, Takeuchi JS, Watashi K, Inoue R, Yamauchi T, Sakamoto K, Yamashita Y, Iguchi Y, Une M, Wakita T, Umezawa K, Yoneda M. Dual Agonist of Farnesoid X Receptor and Takeda G Protein-Coupled Receptor 5 Inhibits Hepatitis B Virus Infection In Vitro and In Vivo. Hepatology. 2021;74:83–98. doi: 10.1002/hep.31712. [DOI] [PubMed] [Google Scholar]
- 116.Chen W, Liu J, Gluud C. Bile acids for viral hepatitis. Cochrane Database Syst Rev. 2003:CD003181. doi: 10.1002/14651858.CD003181. [DOI] [PubMed] [Google Scholar]
- 117.Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. doi: 10.3389/fmicb.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu M, Wang B, Fu Y, Chen Y, Yang F, Lu H, Xu J, Li L. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb Ecol. 2012;63:304–313. doi: 10.1007/s00248-011-9925-5. [DOI] [PubMed] [Google Scholar]
- 119.Wang J, Wang Y, Zhang X, Liu J, Zhang Q, Zhao Y, Peng J, Feng Q, Dai J, Sun S, Zhao L, Zhang Y, Hu Y, Zhang M. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front Microbiol. 2017;8:2222. doi: 10.3389/fmicb.2017.02222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 121.Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. doi: 10.1186/s13099-018-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heidrich B, Vital M, Plumeier I, Döscher N, Kahl S, Kirschner J, Ziegert S, Solbach P, Lenzen H, Potthoff A, Manns MP, Wedemeyer H, Pieper DH. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int. 2018;38:50–58. doi: 10.1111/liv.13485. [DOI] [PubMed] [Google Scholar]
- 123.Aly AM, Adel A, El-Gendy AO, Essam TM, Aziz RK. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016;8:42. doi: 10.1186/s13099-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Ghany MG, Morgan TR AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members: EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 127.Li X, Wu S, Du Y, Yang L, Li Y, Hong B. Entecavir therapy reverses gut microbiota dysbiosis induced by hepatitis B virus infection in a mouse model. Int J Antimicrob Agents. 2020;56:106000. doi: 10.1016/j.ijantimicag.2020.106000. [DOI] [PubMed] [Google Scholar]
- 128.Lu YX, He CZ, Wang YX, Ai ZS, Liang P, Yang CQ. Effect of Entecavir on the Intestinal Microflora in Patients with Chronic Hepatitis B: A Controlled Cross-Sectional and Longitudinal Real-World Study. Infect Dis Ther. 2021;10:241–252. doi: 10.1007/s40121-020-00355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pérez-Matute P, Íñiguez M, Villanueva-Millán MJ, Recio-Fernández E, Vázquez AM, Sánchez SC, Morano LE, Oteo JA. Short-term effects of direct-acting antiviral agents on inflammation and gut microbiota in hepatitis C-infected patients. Eur J Intern Med. 2019;67:47–58. doi: 10.1016/j.ejim.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, Reddel S, Calvani R, Marzetti E, Sanguinetti M, Gasbarrini A, Pompili M. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301–1311. doi: 10.1111/apt.15004. [DOI] [PubMed] [Google Scholar]
- 131.Vojinovic D, Radjabzadeh D, Kurilshikov A, Amin N, Wijmenga C, Franke L, Ikram MA, Uitterlinden AG, Zhernakova A, Fu J, Kraaij R, van Duijn CM. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat Commun. 2019;10:5813. doi: 10.1038/s41467-019-13721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel V, Joharapurkar A, Kshirsagar S, Sutariya B, Patel M, Pandey D, Patel H, Ranvir R, Kadam S, Patel D, Bahekar R, Jain M. Coagonist of GLP-1 and glucagon decreases liver inflammation and atherosclerosis in dyslipidemic condition. Chem Biol Interact. 2018;282:13–21. doi: 10.1016/j.cbi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Sarkar S, Kimono D, Albadrani M, Seth RK, Busbee P, Alghetaa H, Porter DE, Scott GI, Brooks B, Nagarkatti M, Nagarkatti P, Chatterjee S. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:8742. doi: 10.1038/s41598-019-45009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8 doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S, Liu S, Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34:1368–1376. doi: 10.1111/jgh.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, Guan M, Zhao X, Li X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm Biol. 2018;56:325–332. doi: 10.1080/13880209.2018.1479868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, Kang H, Park JH, Kim JH, Lee DH, Lee S, Kim W, Ko G. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11:4982. doi: 10.1038/s41467-020-18754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deng YD, Peng XB, Zhao RR, Ma CQ, Li JN, Yao LQ. The intestinal microbial community dissimilarity in hepatitis B virus-related liver cirrhosis patients with and without at alcohol consumption. Gut Pathog. 2019;11:58. doi: 10.1186/s13099-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang Y, Cai Y. Gut microbiota and hepatitis-B-virus-induced chronic liver disease: implications for faecal microbiota transplantation therapy. J Hosp Infect. 2017;96:342–348. doi: 10.1016/j.jhin.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 141.Cammarota G, Ianiro G, Bibbò S, Gasbarrini A. Gut microbiota modulation: probiotics, antibiotics or fecal microbiota transplantation? Intern Emerg Med. 2014;9:365–373. doi: 10.1007/s11739-014-1069-4. [DOI] [PubMed] [Google Scholar]
- 142.Oo KM, Lwin AA, Kyaw YY, Tun WM, Fukada K, Goshima A, Shimada T, Okada S. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci Microbiota Food Health. 2016;35:123–128. doi: 10.12938/bmfh.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596–3604. doi: 10.1177/0300060518776064. [DOI] [PMC free article] [PubMed] [Google Scholar]