Overview
Colorectal cancer is the third most frequently diagnosed cancer in men and women in the United States. In 2002, an estimated 107,300 new cases of colon cancer will occur. During the same year, it is estimated that 48,100 people will die from colon cancer.1 Despite these statistics, mortality from colon cancer has decreased over the past 30 years, possibly because of earlier diagnosis through screening and better treatment modalities.
This article summarizes the National Comprehensive Cancer Network’s clinical practice guidelines for managing colon cancer. The guidelines begin with the clinical presentation of the patient to the primary care physician or gastroenterologist and address diagnosis, pathologic staging, surgical management, adjuvant treatment, management of recurrent and metastatic disease, and patient surveillance.
When reviewing these guidelines, clinicians should note several things. First, these guidelines adhere to the tumor/node/metastasis staging system (Table 1)2,3 Furthermore, all recommendations are classified as category 2A except where noted in the text or on the algorithm (see Categories of Consensus below). The panel unanimously endorses giving priority to treating patients in a clinical trial over standard or accepted therapy. This is especially true for cases of advanced disease and for patients with locally aggressive colorectal cancer who are receiving combined modality treatment.
Table 1.
American Joint Committee on Cancer Tumor/Node/Metastasis Staging System for Colorectal Cancer*
| Primary Tumor (T) | |||||
|---|---|---|---|---|---|
| TX | Primary tumor cannot be assessed | ||||
| T0 | No evidence of primary tumor | ||||
| Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria† | ||||
| T1 | Tumor invades submucosa | ||||
| T2 | Tumor invades muscularis propria | ||||
| T3 | Tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic or perirectal tissues | ||||
| T4 | Tumor directly invades other organs or structures and/or perforates visceral peritoneum‡ | ||||
| Regional Lymph Nodes (N)§ | |||||
| NX | Regional lymph nodes cannot be assessed | ||||
| N0 | No regional lymph node metastasis | ||||
| N1 | Metastasis in one to three regional lymph nodes | ||||
| N2 | Metastasis in four or more regional lymph nodes | ||||
| Distant Metastasis (M) | |||||
| MX | Distant metastasis cannot be assessed | ||||
| M0 | No distant metastasis | ||||
| M1 | Distant metastasis | ||||
| Stage Grouping | |||||
| Stage | T | N | M | Dukes¶ | MAC¶ |
| 0 | Tis | N0 | M0 | - | - |
| I | T1 | N0 | M0 | A | A |
| T2 | N0 | M0 | A | Bl | |
| IIA | T3 | N0 | M0 | B | B2 |
| IIB | T4 | N0 | M0 | B | B3 |
| IIIA | T1-T2 | N1 | M0 | C | Cl |
| IIIB | T3-T4 | N1 | M0 | C | C2/C3 |
| IIIC | Any T | N2 | M0 | C | C1/C2/C3 |
| IV | Any T | Any N | M1 | - | D |
| Histologic Grade (G) | |||||
| GX | Grade cannot be assessed | ||||
| G1 | Well differentiated | ||||
| G2 | Moderately differentiated | ||||
| G3 | Poorly differentiated | ||||
| G4 | Undifferentiated | ||||
This includes cancer cells confined within the glandular basement membrane (intraepithelial) or lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa.
Direct invasion in T4 includes invasion of other segments of the colorectum by way of the serosa (e.g., invasion of the sigmoid colon by a carcinoma of the cecum). Tumor that is adherent to other organs or structures macroscopically is classified T4; however, if no tumor is present in the adhesion microscopically, the classification should be pT3. The V and L substaging should be used to identify the presence or absence of vascular or lymphatic invasion.
A tumor nodule in the pericolorectal adipose tissue of a primary carcinoma without histologic evidence of residual lymph node in the nodule is classified in the pN category as a regional lymph node metastasis if the nodule has the form and smooth contour of a lymph node. If the nodule has an irregular contour, it should be classified in the T category and also coded as V1 (microscopic venous invasion) or as V2 (if it was grossly evident) because there is a strong likelihood that it represents venous invasion.
Dukes B is a composite of better (T3 N0 M0) and worse (T4 N0 M0) prognostic groups, as is Dukes C (Any TN1 M0 and Any T N2 M0). MAC is the modified Astler-Coller classification.
Note: The y prefix is to be used for those cancers that are classified after pretreatment, whereas the r prefix is to be used for those cancers that have recurred.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original material source for this is the AJCC Cancer Staging Manual, 6th Edition (2002) published by Springer-Verlag New York (www.springer-ny.com).
Staging
The sixth edition of the Americal Joint Committee on Cancer’s AJCC Cancer Staging Manual includes several modifications to the colon and rectum staging system. In this version of the staging system, smooth metastatic nodules in the pericolic or perirectal fat are considered lymph node metastasis and should be included in N staging. Irregularly contoured metastatic nodules in the peritumoral fat are considered vascular invasion. Stage II is now subdivided into IIa (if the primary tumor is T3) and IIB (for T4 lesions). Stage III is subdivided into IIIA (T1 to T2, Nl, M0), IIIB (T3 to T4, Nl, M0), and IIIC (any T, N2, M0). In addition, the current staging suggests that the surgeon mark the area of the specimen with the deepest tumor penetration so that the pathologist can directly evaluate the radial margin. The surgeon is encouraged to score the completeness of the resection as follows: 1) R0 for complete tumor resection with all margins negative, 2) R1 for incomplete tumor resection with microscopic involvement of a margin, and 3) R2 for incomplete tumor resection with gross residual tumor not resected.
Risk Assessment
First-degree relatives of patients with newly diagnosed adenomas4 or invasive carcinoma5 are at an increased risk for colorectal cancer. Therefore, colon cancer patients, especially those who are 50 years old or younger and those with suspected hereditary nonpolyposis colon cancer, familial adenomatous polyposis, or attenuated familial adenomatous polyposis, should be counseled regarding their family history, as detailed in the NCCN Colorectal Cancer Screening Clinical Practice Guidelines (in this issue).
Colon Cancer Treatment Version 1.2003.

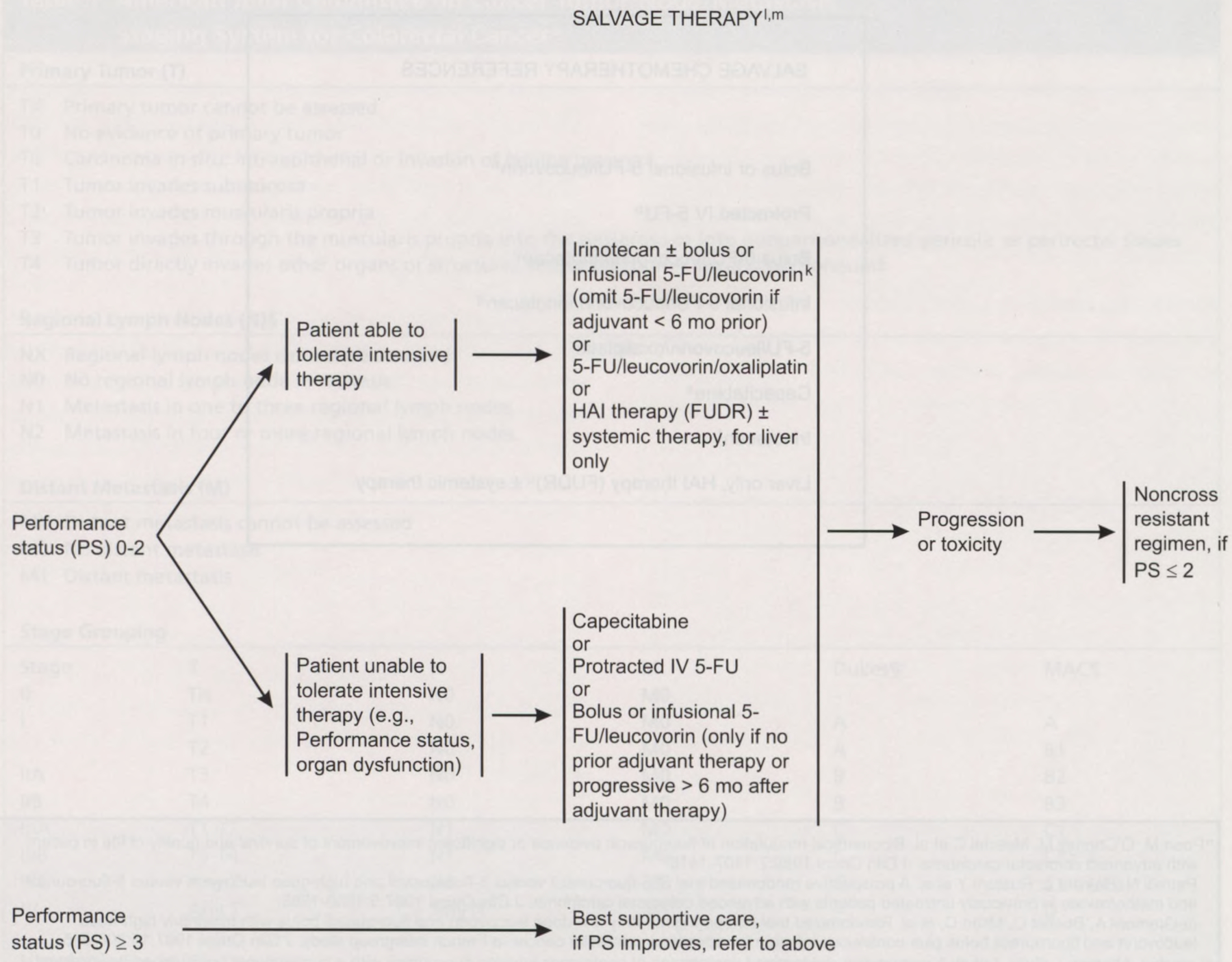

aAll patients with colon cancer should be counseled for family history. Patients with suspected hereditary non-polyposis colon cancer (HNPCC), familial adenomatous polyposis (FAP) and attenuated FAP, see the NCCN Colorectal Screening Guidelines (page 72).
bConsider more extensive colectomy for patients with strong family history of colon cancer or young age (< 50 y).
See the NCCN Colorectal Screening Guidelines (page 72).
cA randomized trial demonstrates that a laparoscopic colectomy is more costly and time to recovery is equivalent to colectomy. Laparoscopic colectomy cannot be recommended as an alternative option at this time.
aAll patients with colon cancer should be counseled for family history. Patients with suspected hereditary non-polyposis colon cancer (HNPCC), familial adenomatous polyposis (FAP) and attenuated FAP see the
dA minimum of 6 lymph nodes need to be examined to clearly establish stage II (T 3–4, NO) colon cancer.
ePatients considered to be NO but who have < 12 nodes examined are suboptimally staged and may be considered in the high risk group.
fThere are insufficient data to recommend the use of molecular markers to determine adjuvant therapy.
gAt this time, combination regimens including irinotecan, oxaliplatin and capecitabine cannot be recommended as standard adjuvant therapy.
hThere are no data supporting the routine use of CT scans of the abdomen or chest x-rays, but some institutions agree these are indicated in certain clinical situations.
iIf patient a potential candidate for surgical resection of isolated metastases.
jWhen considering hepatic artery infusion (HAI) therapy.
kA recent trial demonstrated an increased early 60-day mortality of bolus 5-FU/leucovorin/irinotecan. Careful monitoring of all regimens is required. Rothenberg M, Meropol N, Poplin E et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol 2001;19:3801–3807.
lCombinations of 5-FU/leucovorin therapy with either irinotecan or oxaliplatin should be strongly considered in optimal treatment strategies. The exact sequence has not yet been defined.
mFor chemotherapy references, see Salvage Chemotherapy References, page 48).
nPoon M, O’Connell M, Moertel C et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patient with advanced colorectal carcinoma. J Clin Oncol 1989;7:1407–1418.
Petrelli N, Herrera L, Rustum Y et al. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol 1987;5:1559–1565.
deGramont A, Bosset C, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808–815.
oLokich J, Ahlgren J, Gullo J et al. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metast colorectal carcinoma: a Mid-Atlantic Oncology Program Study. J Clin Oncol 1989;7:425–432.
PSaltz L, Cox J, Blanke C et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000;343:905–914.
qDouillard J, Cunningham D, Roth A et al. Irinotecan combined with fluorouracil compared with fluorouracil alons as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. The Lancet 2000;355:1041–1047.
rdeGramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000:18:2938–2947.
sVanCutsem E, Twelves C, Cassidy J et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 2001;19:4097–4106.
tCunningham D, Glimelius B. A phase III study of irinotecan (CPT-11 ) versus best supportive care in patients with metastatic colorectal cancer who have failed 5-fluorouracil therapy. V301 Study Group. Semin Oncol 1999;26:6–12.
uKemeny N, Ron I. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol 1999;26:524–535.
Clinical Presentation and Treatment
Managing Polypoid Cancer
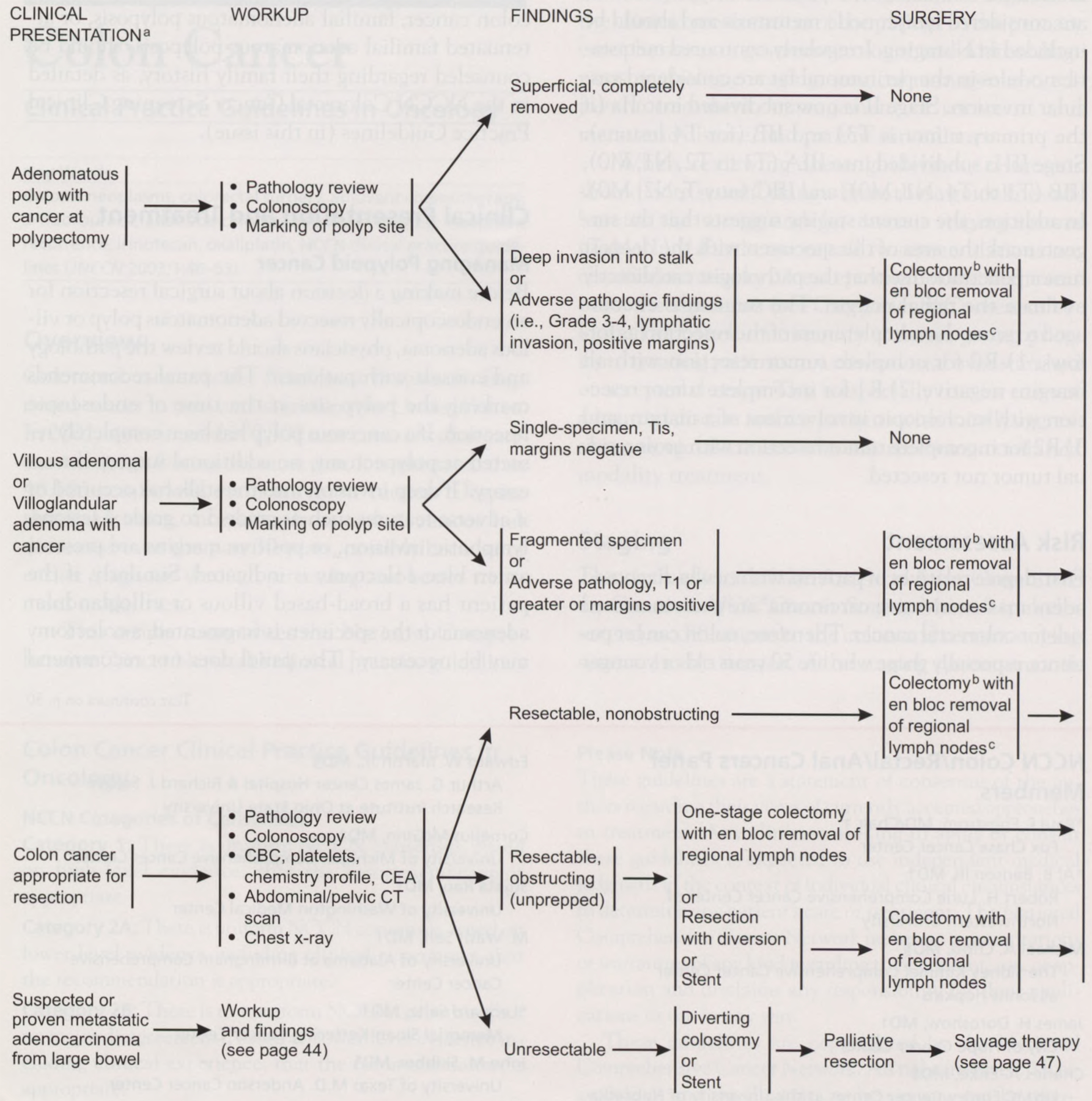
Before making a decision about surgical resection for an endoscopically resected adenomatous polyp or villous adenoma, physicians should review the pathology and consult with patients.6 The panel recommends marking the polyp site at the time of endoscopic resection. If a cancerous polyp has been completely resected at polypectomy, no additional surgery is necessary.7 If deep invasion into the stalk has occurred or if adverse features such as grade 3 to grade 4 lesions, lymphatic invasion, or positive margins are present, an en bloc colectomy is indicated. Similarly, if the patient has a broad-based villous or villoglandular adenoma or the specimen is fragmented, a colectomy may be necessary.7 The panel does not recommend laparoscopic surgery as an option at this time. A randomized trial demonstrated that a laparoscopic colectomy is more costly and that the patient’s recovery time is equivalent to a standard colectomy.8 All patients who have resected polyps should undergo total colonoscopy to exclude other synchronous polyps, as well as appropriate follow-up surveillance endoscopy.9 No adjuvant chemotherapy is indicated for patients with stage 1 lesions.
Managing Invasive Colon Cancer
Patients who present with invasive colon cancer require a complete staging workup, including pathologic tissue review, total colonoscopy, a complete blood count, platelets, chemistry profile, a carcinoembryonic antigen (CEA) determination, a chest radiograph, and a computed tomographic (CT) scan of the abdomen and pelvis. For resectable colon cancer, the surgical procedure of choice is colectomy with an en bloc removal of the regional lymph nodes.10 For resectable colon cancer that is causing obstruction, resection with diversion followed by colectomy or stent insertion followed by colectomy is also recommended. If the cancer is unresectable, a diverting colostomy followed by a palliative resection should be considered.
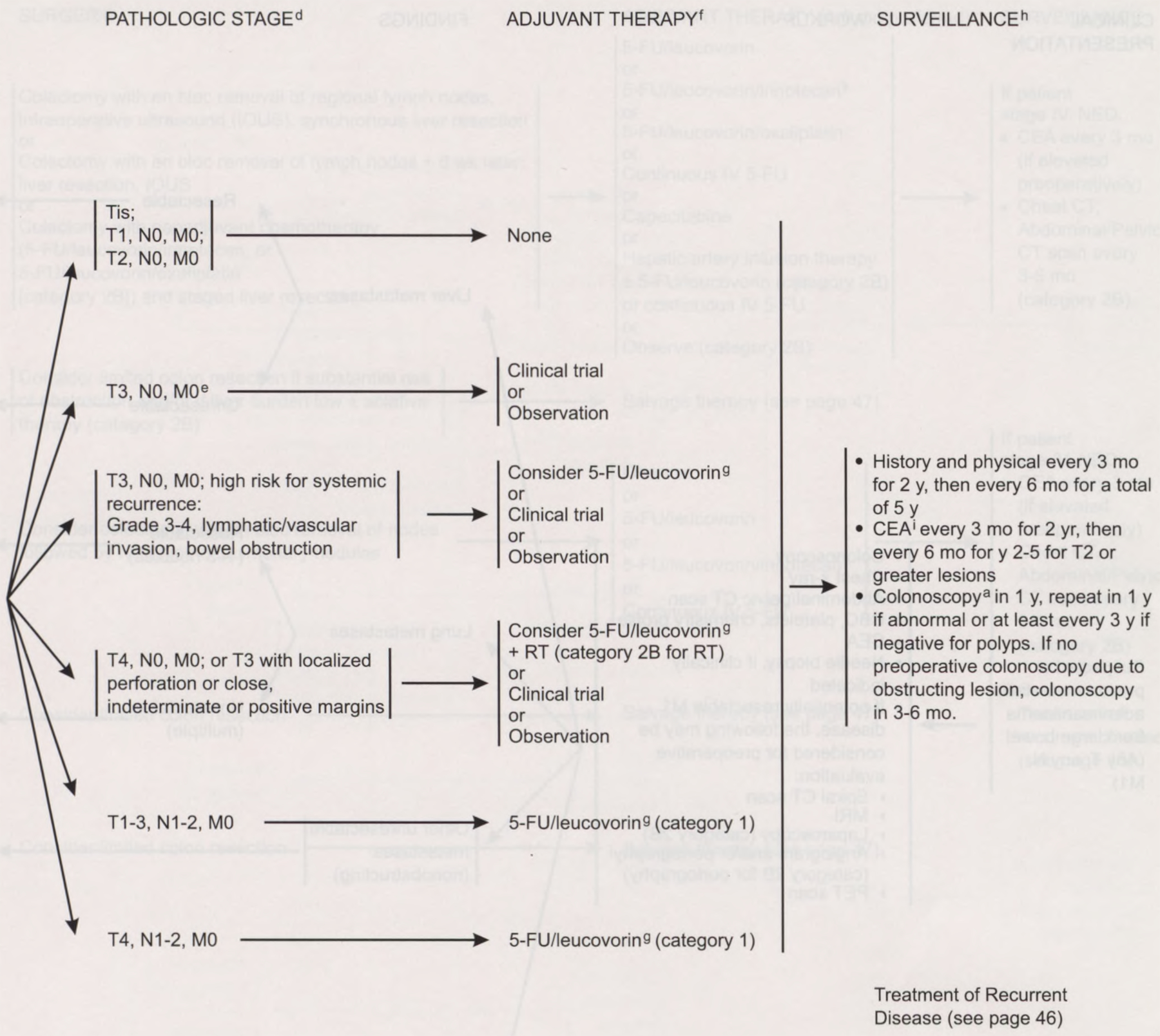
The panel recommends 6 months of 5-fluorouracil (5-FU) plus leucovorin adjuvant chemotherapy for patients with stage III (node-positive) colon carcinoma (category 1).11 Adjuvant chemotherapy is not considered standard for stage II colon cancer. Intergroup Trial 0035 demonstrated a trend toward a decreased recurrence rate with chemotherapy versus surgery alone, but no survival advantage resulted.12 The International Multicentre Pooled Analysis of B2 Colon Cancer Trials included data on 1,016 patients with stage II cancer randomized to receive 5-FU plus leucovorin or observation. The event-free survival rates were 76% and 73%, respectively (5-year hazard ratio, 0.83; 90% confidence interval, 0.72 to 1.07);13 however, patients with T3 colon tumors with no metastases and with high-risk factors for systemic recurrence, including grade 3 or 4 lesions, lymphatic/vascular invasion, or bowel obstruction, may be considered for adjuvant chemotherapy with 5-FU/leucovorin.11 These patients should be entered, if possible, in a clinical trial. These patients may also be observed. Postoperative chemotherapy with radiation (category 2B for radiation) might benefit patients who have T3 tumors with localized perforation or close, indeterminate, or positive margins or patients with T4, N0 colon cancer.
Managing Stage IV Disease
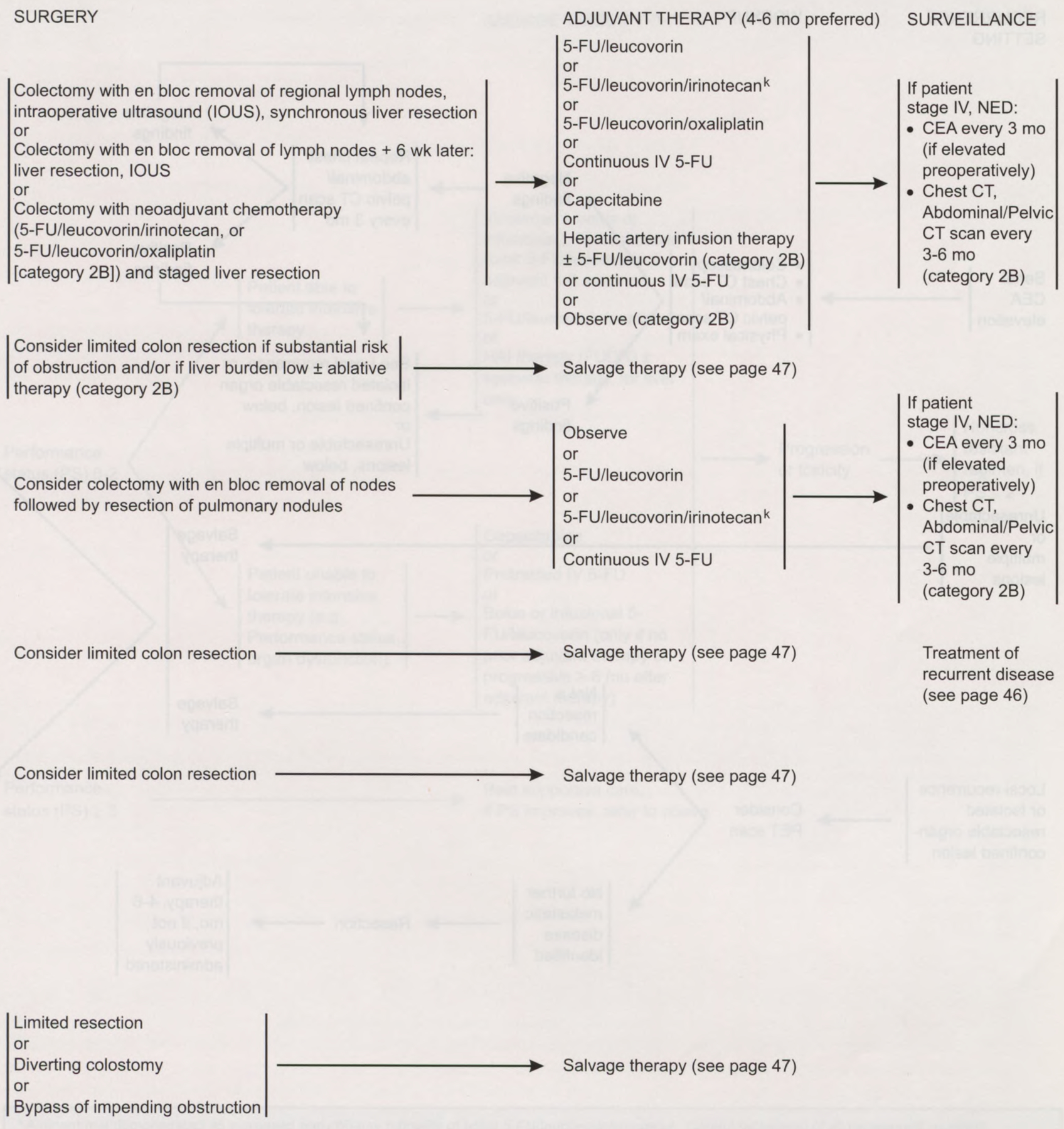
Patients with stage IV colon cancer can present with a lesion in the colon and evidence of metastatic disease in the liver. If a patient is a candidate for surgery, the panel recommends colectomy followed by liver resection14 or colectomy with neoadjuvant chemotherapy and a staged liver resection. Limited colon resection should be considered if the patient has a substantial risk of obstruction or if the liver burden is low (category 2B).
Patients who have completely resected liver metastases should be offered 4 to 6 months of adjuvant chemotherapy. A completed trial demonstrated that the combination of fluorodeoxyuridine by hepatic artery infusion plus systemic chemotherapy was superior to systemic chemotherapy alone following hepatic resection.15 Additional options for adjuvant therapy include systemic 5-FU plus leucovorin,16 5-FU plus leucovorin plus oxaliplatin,17 continuous-infusion 5-FU,18 or 5-FU plus leucovorin plus irinotecan.19,20 Intraperitoneal chemotherapy is considered investigational, given the absence of evidence that it prolongs life.21 Patients with unresectable liver metastases should receive salvage therapy. Patients may also be observed (category 2B).
Metastatic disease can also occur in the lung. Patients with one to three nodules in their lungs who can undergo resection should be considered for colectomy with an en bloc removal of nodes followed by resection of pulmonary nodules. A biologic waiting period of up to 2 months can distinguish patients who would be more likely to benefit from metastasectomy because of indolent disease. Adjuvant therapy recommended for patients with lung metastases includes 5-FU plus leucovorin, 5-FU plus leucovorin plus irinotecan, continuous infusion of 5-FU, or observation. If a patient cannot undergo resection or has multiple lesions, a limited colon resection should be considered followed by salvage therapy.
For patients with other nonobstructing unresectable metastases, a limited colon resection should be considered followed by salvage therapy. Recommended surgery for patients with an impending obstruction includes limited resection, a diverting colostomy, or a bypass of impending obstruction. Surgery should be followed with salvage therapy.
Salvage Therapy
If a patient with metastatic disease and good performance scores progresses while on 5-FU–based therapy or if a metastasis develops within 6 months of the patient completing 5-FU–based adjuvant chemotherapy, treatment with bolus irinotecan (CPT-11) or 5-FU/leucovorin/oxaliplatin is recommended as a possible second-line approach.17,22 For patients with good performance scores who have not previously received chemotherapy, options include 5-FU plus leucovorin, 5-FU/leucovorin/irinotecan,19,20 capecitabine, protracted infusional 5-FU, or 5-FU/leucovorin/oxaliplatin.17 Individuals receiving bolus 5-FU/leucovorin/irinotecan should be carefully monitored because an increase in 60-day mortality caused by gastrointestinal toxicity has been reported in these patients.23 If recurrence is confined to the liver, liver-directed therapy should be considered.24 Metastatic cancer patients with performance 3 or 4 should receive the best supportive care.
Post-treatment Surveillance
Post-treatment surveillance of colon cancer patients remains controversial.25 Oncologists perform surveillance in these patients to assess therapeutic complications, discover a recurrence that is potentially resectable for cure, identify new metachronous neoplasms at a preinvasive stage, and reassure the patient. For successfully treated patients who have no known residual disease, the panel recommends a history and a physical examination every 3 months for the first 2 years and then every 6 months for the next 5 years.
For T2 or greater lesions, a CEA test is recommended at baseline and every 3 months for 2 years and then every 6 months for the next 2 to 5 years if the clinician determines that the patient is a potential candidate for aggressive curative surgery.26–28 Colonoscopy is indicated within 1 year of resection (or 3 to 6 months postoperatively) and is repeated annually if neoplastic polyps are noted; if the colon is free of polyps, colonoscopic surveillance at least every 3 years is recommended.9 No data exist to justify or refute routine monitoring with periodic chest films or serial CT scans of the abdomen and pelvis.
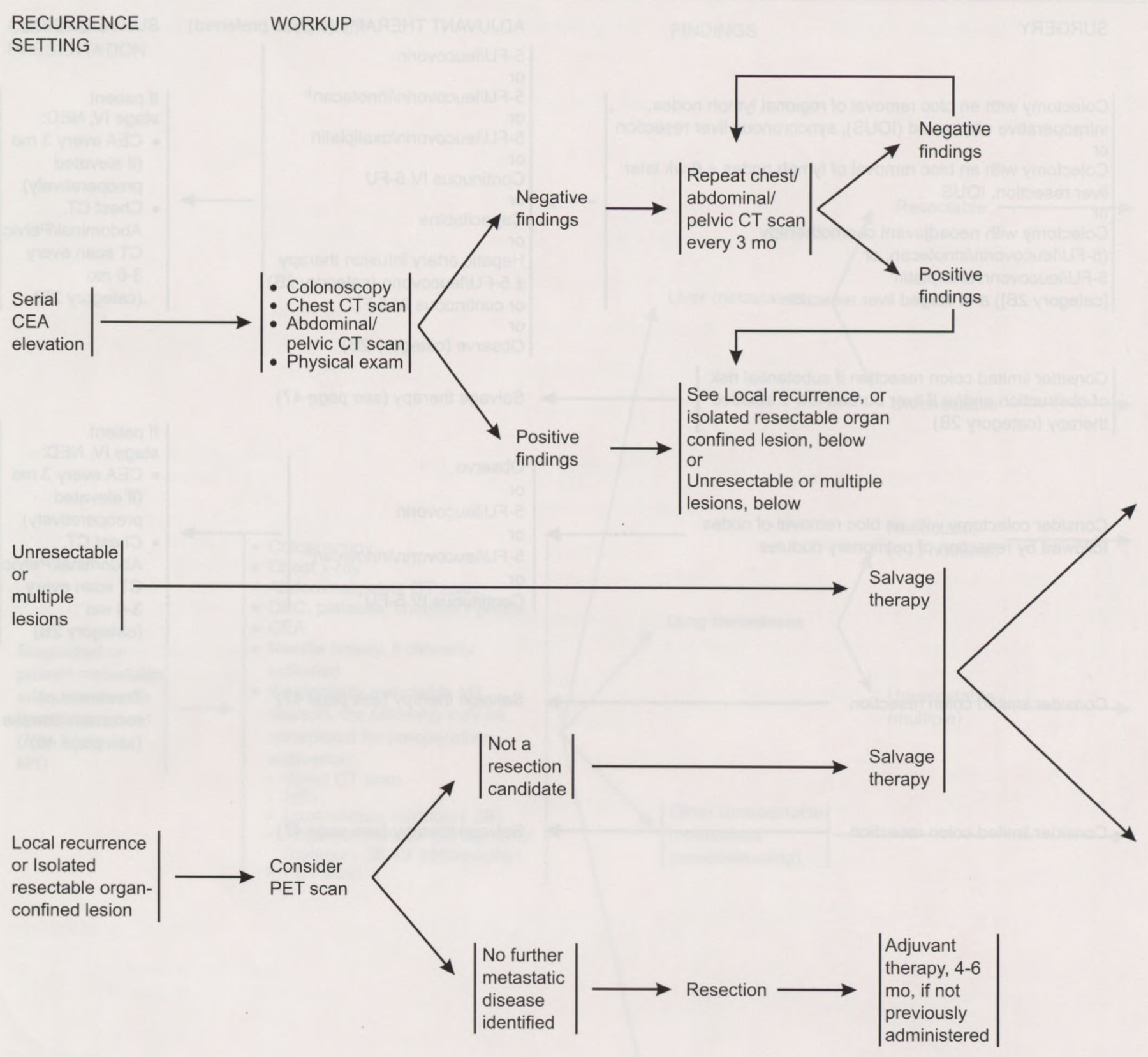
Managing Recurrent or Progressing Metastasis
Managing patients with an elevated CEA level after resection should include colonoscopy; chest, abdominal, and pelvic CT scans; and a careful physical examination. If imaging study results are normal in the face of a rising CEA, repeat scans are indicated every 3 months if symptoms occur. The panel does not recommend a “blind abdominal exploration” for patients whose workup for an elevated CEA level is negative.29 The panel considered, but does not recommend, the use of anti-CEA-radiolabeled scintigraphy.30 Positron emission tomography should be considered before surgical resection for patients with a suspected recurrence or if an isolated, resectable, organ-confined lesion is detected.31 When recurrent disease is identified at the site of the anastomosis in the bowel, curative surgery may be possible. Likewise, isolated lesions in the liver or lung may be resected for cure.
Summary
The NCCN Colon/Rectal/Anal Cancers Guidelines panel believes that a multidisciplinary approach is necessary for managing colorectal cancer. The panel endorses the concept that treating patients in a clinical trial has priority over standard or accepted therapy.
The recommended surgical procedure for resectable colon cancer is an en bloc resection. For patients with stage III disease, 5-FU–based adjuvant therapy is recommended. A patient who has metastatic disease in the liver or lung should be considered for surgical resection if he or she is a candidate for surgery and if surgery can extend survival. Surgery should be followed by adjuvant chemotherapy.
The panel advocates a conservative post-treatment surveillance program for colon carcinoma patients. Serial CEA determinations are appropriate if the patient is a candidate for aggressive surgical resection should recurrence be detected. Abdominal and pelvic CT scans should be used only when there are clinical indications of possible recurrence. Patients whose disease progresses during 5-FU-based therapy should be treated with bolus irinotecan. Patients who progress on irinotecan are candidates for 5-FU/leucovorin/oxaliplatin therapy17 or should be encouraged to participate in a phase I or phase II clinical trial.
Colon Cancer Clinical Practice Guidelines in Oncology.
NCCN Categories of Consensus
Category 1: There is uniform NCCN consensus, based on high-level evidence, that the recommendation is appropriate.
Category 2A: There is uniform NCCN consensus, based on lower-level evidence including clinical experience, that the recommendation is appropriate.
Category 2B: There is nonuniform NCCN consensus (but no major disagreement), based on lower-level evidence including clinical experience, that the recommendation is appropriate.
Category 3: There is major NCCN disagreement that the recommendation is appropriate.
Footnotes
All recommendations are category 2A unless otherwise noted.
Clinical trials: The NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Publisher's Disclaimer: These guidelines are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult these guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network makes no representations or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way.
At the beginning of each panel meeting to develop NCCN guidelines, panel members disclosed financial support they have received, in the form of research support or advisory committee membership, from the following: Pharmacia, Roche, Schering-Plough Corporation, Eli Lilly and Company, Bristol-Myers Squibb, Sanofi-Synthelabo, Digene Corporation, Medtronic Corp, RITA Medical Inc, Fujisawa, Imclone, and Medimmune. The panel did not regard any of these potential conflicts of interest as sufficient reason to disallow participation in panel deliberations by any member.
Contributor Information
Paul F. Engstrom, Fox Chase Cancer Center.
AI B. Benson, III, Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Michael A. Choti, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.
James H. Doroshow, City of Hope Cancer Center.
Charles A. Enke, UNMC Eppley Cancer Center at the University of Nebraska Medical Center.
Charles Fuchs, Dana-Farber Cancer Institute.
James Helm, H. Lee Moffitt Cancer Center & Research Institute at the University of South Florida.
Krystyna Kiel, Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Kirk Ludwig, Duke Comprehensive Cancer Center.
Edward W. Martin, Jr., Arthur G. James Cancer Hospital & Richard J. Solove Research Institute at Ohio State University.
Cornelius McGinn, University of Michigan Comprehensive Cancer Center.
Sujata Rao, University of Washington Medical Center.
M. WasifSaif, University of Alabama at Birmingham Comprehensive Cancer Center.
Leonard Saltz, Memorial Sloan-Kettering Cancer Center.
John M. Skibber, University of Texas M.D. Anderson Cancer Center.
Alan P. Venook, UCSF Comprehensive Cancer Center.
Timothy J. Yeatman, H. Lee Moffitt Cancer Center & Research Institute at the University of South Florida.
References
- 1.Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23–47. [DOI] [PubMed] [Google Scholar]
- 2.Greene FL, Page DL, Fleming, et al. AJCC cancer staging manual, 6th ed. New York: Springer-Verlag, 2002. [Google Scholar]
- 3.Greene FL, Stewart AK, Norton JH. A new TNM staging strategy for node-positive (stage III) colon cancer 2002. Am Surg 2002;236:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahsan H, Neugut Al, Garbowski GC, et al. Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Ann Intern Med 1998;128:900–905. [DOI] [PubMed] [Google Scholar]
- 5.Bonelli L, Martines H, Conio M, et al. Family history of colorectal cancer as a risk factor for benign and malignant tumors of the large bowel: a case-control study. Int J Cancer 1988;41:513–517. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HS, Deppisch LM, Gourley WK, et al. Endoscopically removed malignant colorectal polyps, clinical pathologic correlations. Gastroenterology 1995;108:1657–1665. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz AJ, Winawer SJ. Management of colorectal polyps. CA Cancer J Clin 1997;47:93–112. [DOI] [PubMed] [Google Scholar]
- 8.Weeks JC, Nelson H, Gelber S, et al. Short-term quality of life outcomes following laparoscopic-assisted colectomy vs. open colectomy for colon cancer: a randomized trial. JAMA 2002;287:321–328. [DOI] [PubMed] [Google Scholar]
- 9.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594–642. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AM. Surgical considerations in patients with cancer of the colon and rectum. Semin Oncol 1991;18:381–387. [PubMed] [Google Scholar]
- 11.Moore HCF, Haller DG. Adjuvant therapy of colon cancer. Semin Oncol 1999;26:545–555. [PubMed] [Google Scholar]
- 12.Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 Colon Cancer. J Clin Oncol 1995; 13:2936–2943. [DOI] [PubMed] [Google Scholar]
- 13.International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol 1999;17:1356–1363. [PubMed] [Google Scholar]
- 14.Fong Y, Salo J. Surgical therapy of hepatic colorectal metastases. Semin Oncol 1999;26:514–523. [PubMed] [Google Scholar]
- 15.Kemeny N, Huang Y, Cohen A, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 16.Buroker TR, O’Connell MJ, Wieand HS, et al. Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 1994;12:14–20. [DOI] [PubMed] [Google Scholar]
- 17.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 18.Leichman CG, Leichman L, Spears CP, et al. Prolonged continuous infusion of fluorouracil with weekly bolus leucovorin: a phase II study in patients with disseminated colorectal cancer. J Natl Cancer Inst 1993;85:41–44. [DOI] [PubMed] [Google Scholar]
- 19.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000;343:905–914. [DOI] [PubMed] [Google Scholar]
- 20.Salz LB, Locker PK, Piroha N, et al. Weekly irinotecan (CPT-11) leucovorin (LV) and fluorouracil (FU) is superior to daily x5 LV/FU in patients with previously untreated metastatic colorectal cancer [abstract]. Proc ASCO 1999;18:233a. [Google Scholar]
- 21.Markman M Intraperitoneal chemotherapy in the management of colon cancer. Semin Oncol 1999;26;536–539. [PubMed] [Google Scholar]
- 22.Rothenberg ML, Blanke CD. Topoisomerase I inhibitors in the treatment of colorectal cancer. Semin Oncol 1999; 26:632–639. [PubMed] [Google Scholar]
- 23.Rothenberg ML, Meropol NJ, Poplin PA, et al. Mortality associated with irinotecan plus bolus fluouracil/leucovorin: summary finding of an independent panel. J Clin Oncol 2001;19:3801–3807. [DOI] [PubMed] [Google Scholar]
- 24.Kemeny NE, Ron IG. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol 1999;26: 524–535. [PubMed] [Google Scholar]
- 25.Safi F, Link KH, Berger HG. Is follow-up of colorectal cancer patients worthwhile? Dis Colon Rectum 1993;36: 636–644. [DOI] [PubMed] [Google Scholar]
- 26.Benson AB III, Desch CE, Flynn PJ, et al. 2000 Update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol 2000;18:3586–3588. [DOI] [PubMed] [Google Scholar]
- 27.Desch CE, Benson AB III, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol 1999;17:1312–1321. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald JS. Carcinoembryonic antigen screening: pros and cons. Semin Oncol 1999;26:556–560. [PubMed] [Google Scholar]
- 29.Martin EN, Minton JP, Carey LC. CEA-directed second-look surgery in the asymptomatic patients after primary resection of colorectal carcinoma. Ann Surg 1985;202:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffat FL Jr, Pinsky CM, Hammershaimb L, et al. Clinical utility of external immunoscintography with IMMU-4 technetium-99m Fab1 antibody fragment in patients undergoing surgery for carcinoma of colon and rectum: results of a pivotal, phase II trial. J Clin Oncol 1996;14:2295–2305. [DOI] [PubMed] [Google Scholar]
- 31.Akhurst T, Larson SM. Positron emission tomography imaging of colorectal cancer. Semin Oncol 1999;26:577–583. [PubMed] [Google Scholar]
Recommended Reading
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first line treatment for metastatic colorectal cancer: a multicenter randomized trial. Lancet 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- Green FL. Laparoscopic management of colorectal cancer. CA Cancer J Clin 1999;49:221–228. [DOI] [PubMed] [Google Scholar]
- Guillem JG, Cohen AM. Current issues in colorectal cancer surgery. Semin Oncol 1999;26:505–513. [PubMed] [Google Scholar]
- Kodner IJ, Gilley MT, Shemesh EL, et al. Radiation therapy as definitive treatment for selected invasive rectal cancer. Surgery 1993;114:850–857. [PubMed] [Google Scholar]
- Minsky BD. Adjuvant therapy of rectal cancer. Semin Oncol 1999;26:540–544. [PubMed] [Google Scholar]
- Minsky BD, Cohen AM, Enker WE, et al. Sphincter preservation in rectal cancer by local excision and post-operative radiation therapy. Cancer 1991;67:908–914. [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Niedzwiecki D, O’Connell MJ, et al. Recommendations for caution with irinotecan, fluorouracil, and leucovorin for colorectal cancer. N Engl J Med 2001;345:144–145. [DOI] [PubMed] [Google Scholar]
- Tempero M, Brand R, Haldeman K, et al. New imaging techniques in colorectal cancer. Semin Oncol 1995;22:448–471. [PubMed] [Google Scholar]
- Tepper JE, O’Connell MJ, Petroni GR, et al. Adjuvant postoperative fluorouracil-modulated chemotherapy combined with pelvic radiation therapy for rectal cancer: initial results of intergroup U114.J Clin Oncol 1997;15:2030–2039. [DOI] [PubMed] [Google Scholar]
- Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation with pre-operative radiation therapy (RT) and coloanal anastomoses: long term follow up. Int J Radiar Oncol Biol Phys 1998;42:51–57. [DOI] [PubMed] [Google Scholar]
- Willett CG, Compton CC, Shelleto PC, et al. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer 1994;73:2716–2720. [DOI] [PubMed] [Google Scholar]
- Willett CG, Fung CY, Kaufman DS, et al. Postoperative radiation therapy for high-risk colon carcinoma. J Clin Oncol 1993;11:1112–1117. [DOI] [PubMed] [Google Scholar]


