Abstract
Patients with concomitant features of asthma and chronic obstructive pulmonary disease (COPD) have a heavy disease burden.
Using data collected prospectively in the European Community Respiratory Health Survey, we compared the risk factors, clinical history and lung function trajectories from early adulthood to late sixties of middle-aged subjects with asthma+COPD (n=179), past (n=263) or current (n=808) asthma alone, COPD alone (n=111) or none of these (n=3477).
Interview data and pre-bronchodilator forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were obtained during three clinical examinations in 1991–1993, 1999–2002 and 2010–2013. Disease status was classified in 2010–2013, when the subjects were aged 40–68 years, according to the presence of fixed airflow obstruction (post-bronchodilator FEV1/FVC below the lower limit of normal), a lifetime history of asthma and cumulative exposure to tobacco or occupational inhalants. Previous lung function trajectories, clinical characteristics and risk factors of these phenotypes were estimated.
Subjects with asthma+COPD reported maternal smoking (28.2%) and respiratory infections in childhood (19.1%) more frequently than subjects with COPD alone (20.9% and 14.0%, respectively). Subjects with asthma+COPD had an impairment of lung function at age 20 years that tracked over adulthood, and more than half of them had asthma onset in childhood. Subjects with COPD alone had the highest lifelong exposure to tobacco smoking and occupational inhalants, and they showed accelerated lung function decline during adult life.
The coexistence between asthma and COPD seems to have its origins earlier in life compared to COPD alone. These findings suggest that prevention of this severe condition, which is typical at older ages, should start in childhood.
Short abstract
The coexistence of asthma and COPD is generally diagnosed in older persons. Nonetheless, prevention of this condition should start in childhood. As a priority, maternal and personal smoking avoidance should be encouraged. https://bit.ly/3uQCmIX
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are common chronic respiratory diseases in the general population. Distinguishing between these conditions can be problematic, especially among older adults [1]. In fact, some patients with asthma, in particular smokers, develop fixed airflow obstruction and COPD at older ages [2]. Some patients with COPD show clinical features that are commonly observed in asthma, such as airway hyperresponsiveness (AHR), bronchodilator responsiveness (BDR) and increased eosinophils in blood and airways [3], although the development of asthma is less common among these patients.
In 1995, the American Thoracic Society (ATS) guidelines proposed a non-proportional Venn diagram to describe the overlap of chronic bronchitis, emphysema and asthma [4], and later studies quantified the extension of the overlap [5]. There is growing consensus that the widely used label “asthma–COPD overlap” encompasses heterogeneous phenotypes rather than indicating a stand-alone disease entity [6–10]. The 2020 update of the Global Initiative for Chronic Obstructive Lung Diseases (GOLD) guidelines no longer uses the asthma–COPD overlap label, and the Global Initiative for Asthma (GINA) guidelines replaced it with “asthma+COPD” [2, 3].
Owing to the rising trends in asthma incidence over the last decades [11], and the global increase in life expectancy that is shifting the age of the asthma population upwards [1], the clinical presentation of patients with both asthma and COPD has been increasing. According to a recent meta-analysis, the estimated global prevalence of asthma+COPD is 2.0%, despite large heterogeneity across studies, while the corresponding figures for asthma and COPD alone are 6.2% and 4.9%, respectively [12]. Diagnosis and clinical management of patients with asthma+COPD are challenging, because there is no consensus on how to define this condition [13], and most of the evidence regarding treatment has been extrapolated from trials on patients with either disease alone [7].
Understanding when the risk factors and clinical characteristics of asthma+COPD occur during life can help to identify susceptibility windows when prevention can be more effective [10]. Another important question is whether lung function trajectories associated with these phenotypes vary [7]. While the clinical guidelines state that patients with asthma+COPD have accelerated lung function decline compared to having COPD alone [3], a number of recent studies would question this assumption [14–20].
Given the above knowledge gaps, we classified subjects with asthma alone, COPD alone and asthma+COPD among participants in the third wave of the European Community Respiratory Health Survey (ECRHS) aged 40–68 years. We estimated the risk factors, clinical characteristics and lung function trajectories of these phenotypes in the age range 20–68 years.
Methods
Study population
ECRHS is an international cohort study on subjects from the general population aged 20–44 years at enrolment in 1991–1993 [21]. At ECRHS I, a 20% random sample of participants in a postal screening (stage 1) was invited to take part in a clinical assessment (stage 2), along with a sample enriched for subjects with respiratory symptoms. Participants in stage 2 were surveyed again in 1999–2002 at ECRHS II [22] and in 2010–2013 at ECRHS III [23], after a median follow-up time of 9 years (range 4–12 years) and 20 years (range 18–23 years), respectively. Ethical approval was obtained for each centre, and written informed consent was obtained from participants.
Clinical data
At each examination, subjects underwent standardised interviews and pre-bronchodilator spirometry (appendix E1). The maximum forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) repeatable to 150 mL were obtained [24]. Serum levels of total and specific IgE for house dust mite, timothy grass and cat allergens were also measured. At ECRHS I and II, methacholine challenge tests were performed [25]. At ECRHS III, post-bronchodilator spirometry using 200 µg salbutamol (two puffs, dose of 100 µg per puff) was conducted, and fractional exhaled nitric oxide (FENO) was determined with an electrochemical analyser (NIOX MINO; Aerocrine AB, Solna, Sweden) at an expiratory flow rate of 50 mL·s−1 [26].
Definitions
We defined a lifetime history of asthma either as self-reported asthma at ECRHS I, II and/or III; or having AHR in combination with asthma-like symptoms or use of inhaled or oral respiratory medication at ECRHS I and/or II (supplementary table E1). We reconstructed lifetime occupational exposures to vapours, gas, dusts or fumes as described in appendix E1 [27]. We identified the following GOLD-defined key indicators for considering a diagnosis of COPD at ECHRS III [3]: 1) a lifetime history of exposures based on cumulative data from any time point (≥10 pack-years smoked or ≥5 years of low-intensity exposure to occupational agents); 2) key symptoms at ECHRS III (chronic cough, chronic sputum production, dyspnoea or shortness of breath following strenuous activity); 3) early-life risk factors (respiratory infections before age of 5 years or parent had chronic bronchitis, emphysema or COPD).
Disease classification
We assigned subjects to five mutually exclusive groups at the last examination (ECRHS III), when post-bronchodilator lung function measurements were available (supplementary figure E1). All the criteria composing disease definitions were fulfilled at the time of ECRHS III on the basis of data measured at ECRHS III (lung function data, symptoms) or cumulative/past data (history of exposures, history of asthma, early-life respiratory infections). Disease groups were classified as follows: 1) asthma+COPD, defined as post-bronchodilator FEV1/FVC<lower limit of normal (LLN) [28] + at least one GOLD-defined indicator for COPD (lifetime history of exposures, key symptoms and/or early life risk factors) + either lifetime asthma history or marked BDR (increase in FEV1>12% and >400 mL) [2]; 2) COPD alone, defined as post-bronchodilator FEV1/FVC<LLN + at least one GOLD-defined indicator for COPD + neither lifetime asthma history nor marked BDR; 3) current asthma alone, defined as lifetime asthma history + one among asthma-like symptoms, asthma attacks and use of inhaled/oral respiratory medicines in the last 12 months (with or without post-bronchodilator FEV1/FVC<LLN); 4) past asthma alone, defined as lifetime asthma history but no symptoms, attacks or medication (with or without post-bronchodilator FEV1/FVC<LLN); and 5) reference subjects, defined as none of the aforementioned conditions and post-bronchodilator FEV1/FVC≥LLN.
We excluded 18 subjects with post-bronchodilator FEV1/FVC<LLN at ECRHS III who could not be classified in any of the disease groups (no GOLD-defined indicators, no asthma history) (figure 1).
FIGURE 1.
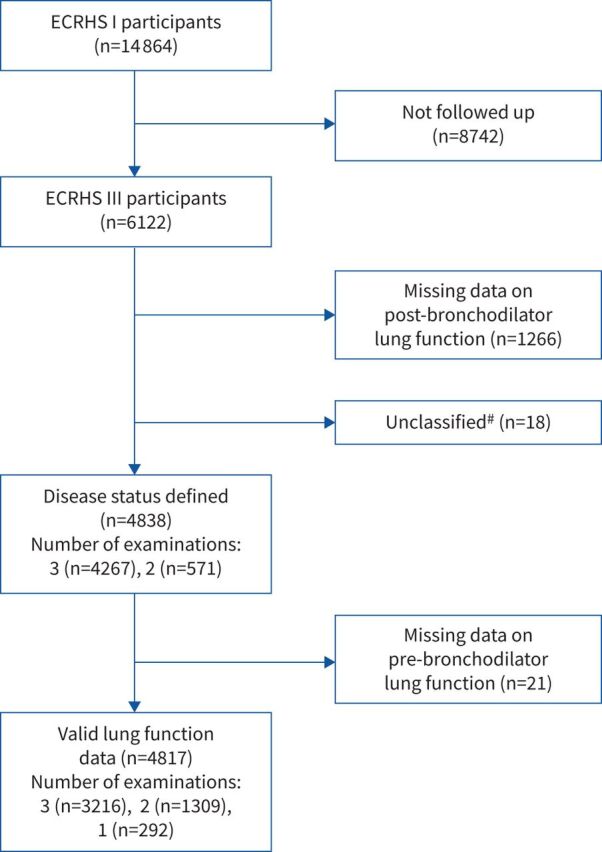
Study flowchart. #: subjects with post-bronchodilator forced expiratory volume in 1 s/forced vital capacity <lower limit of normal at European Community Respiratory Health Survey III who could not be classified in any of the disease groups (no Global Initiative for Chronic Obstructive Lung Diseases-defined indicators, no asthma history).
Statistical analysis
For the subjects classified in disease groups at ECRHS III (age 40–68 years), we described the distribution of characteristics using mean±sd, median (Q1–Q3) or number (%), and tested overall differences across the groups using Kruskal–Wallis or Chi-squared/exact tests as appropriate. We then estimated adult-life trajectories of these phenotypes from the minimum age of 20 years at ECRHS I to the maximum age of 68 years at ECRHS III. We modelled indicators that were available for all the three time points, including two risk factors (proportion exposed to active or passive smoking), two self-reported outcomes (proportion reporting dyspnoea (ever having trouble with breathing) or medical examinations (seen by a general practitioner/specialist in the last 12 months, supplementary table E2)); and three lung function outcomes (average FEV1 (L), FVC (L) and % FEV1/FVC). We used generalised estimating equations with Poisson outcome distribution and log link function for binary outcomes, or a Gaussian outcome distribution and identity link function for quantitative outcomes. The models included a robust variance estimator and the following independent variables: sex, age, disease status, disease status×age (interaction term), ECRHS sample (random versus symptomatic), spirometer type (only for lung function outcomes) and height (only for FEV1 and FVC). Age2 and disease status×age2 were also tested sequentially and included to account for non-linearity in trajectories if they improved model fitting (α=0.10). We set unstructured within-subject correlations to account for repeated measurements. We deleted missing data on adjustment variables list-wise. Each subject contributed available information from any time point. We used STATA software, release 16.1 (StataCorp, College Station, TX, USA).
Sensitivity analyses
We also estimated lung function trajectories: 1) modelling FEV1 and FVC % predicted; 2) only considering subjects with three valid lung function measurements; and 3) after excluding subjects with post-bronchodilator FEV1/FVC>LLN in combination with FEV1 or FVC<LLN at ECRHS III, an indicator of preserved ratio impaired spirometry (PRISm) (measurements of total lung capacity and residual volume were not available). Moreover, 4) we replicated the analyses after applying disease definitions based on the GOLD fixed cut-off for persistent obstruction (FEV1/FVC<0.70) (appendix E1).
Results
There were 14 864, 9251 and 6122 participants, respectively, in ECRHS I, II and III who underwent clinical examinations, from 23 centres (supplementary table E3). The median time intervals between ECRHS I and II and between ECRHS II and III were 8.8 years (range 4.3–11.7 years) and 11.5 years (range 8.5–15.6 years), respectively. Compared to ECRHS I participants, the subjects followed up at ECRHS III were on average older at baseline (34.3 versus 33.6 years), less likely to have a low level of education (12.4% versus 15.8%), less likely to be current smokers (20.5% versus 23.4%) and had higher FEV1 (99.6% versus 98.8% predicted) (supplementary table E4). Disease status was classified at ECRHS III for 4838 individuals (figure 1), of whom 4267 attended all three examinations, and 3216 had valid lung function measurements at three time points. Subjects assigned to disease groups had similar characteristics compared to the larger sample of ECRHS III participants (supplementary table E4). There were 295 subjects with a post-bronchodilator FEV1/FVC<LLN; of these, 290 fulfilled at least one GOLD-defined indicator of COPD (supplementary table E5), and five were classified as having past (n=1) or current (n=4) asthma alone. There were 1248 subjects with a lifetime history of asthma, of whom 1045 reported having ever had asthma at ECRHS I, II and/or III.
Characteristics of participants at disease classification (ECRHS III)
At ECRHS III, the proportion of women in the asthma+COPD group was similar to the reference group (∼50%), and intermediate between COPD (42.3%) and past/current asthma alone (>60%) (table 1). A low education level was more common in the asthma+COPD group. Obesity was more frequent among subjects with current asthma alone (31.9%) and asthma+COPD (27.7%) than with COPD alone (18.9%). The subjects with asthma+COPD were as physically active as the subjects with current asthma alone (∼43%) and more active than the COPD group (30.6%). Among non-smokers, FENO levels were highest in the group with asthma+COPD, whereas among smokers the highest level was seen in subjects with past asthma. A lifetime history of asthma was reported by 177 of the subjects with asthma+COPD (98.9%). Subjects with asthma+COPD were more likely to report a childhood asthma onset (53.4%) than subjects with past or current asthma alone (41.2% and 38.4%, respectively), and less likely to report a late asthma onset (10.8% versus 12.1% and 16.8%, respectively). Both asthma-like symptoms and GOLD-defined key COPD symptoms were more frequent among subjects with asthma+COPD than among subjects with COPD alone. Allergic outcomes were more common among subjects with the asthmatic phenotypes compared to COPD alone or reference subjects, while total serum IgE levels were highest in the group with asthma+COPD. Emergency room or hospital admissions for breathing problems were most frequent in subjects with asthma+COPD. Subjects with asthma+COPD were more likely to report use of inhaled or oral respiratory medication (72.9%) than subjects with current asthma (61.2%) or COPD alone (10.8%) at ECRHS III, but also at the other examinations (supplementary table E6).
TABLE 1.
Participants’ characteristics at the time of disease classification (ECRHS III)
| Reference subjects | Past asthma alone | Current asthma alone | Asthma+COPD | COPD alone | Overall p-value | |
| Subjects n | 3477 | 263 | 808 | 179 | 111 | |
| Female sex | 1763/3477 (50.7) | 167/263 (63.5) | 492/808 (60.9) | 89/179 (49.7) | 47/111 (42.3) | <0.001 |
| Age years | 54.1±7.1 | 54.1±7.1 | 54.1±7.1 | 54.5±7.0 | 55.3±6.5 | 0.578 |
| Low education level (completed before age 16) | 375/3473 (10.8) | 40/262 (15.3) | 115/805 (14.3) | 34/178 (19.1) | 18/110 (16.4) | <0.001 |
| BMI kg·m−2 | 27.0± 4.7 | 27.0±4.8 | 28.2±5.6 | 27.4±5.3 | 26.5±5.2 | <0.001 |
| Obesity (BMI≥30 kg·m−2) | 764/3456 (22.1) | 56/263 (21.3) | 256/802 (31.9) | 49/177 (27.7) | 21/111 (18.9) | <0.001 |
| Physical activity (exercising for ≥1 h and ≥2 times a week) | 1483/3459 (42.9) | 127/262 (48.5) | 345/802 (43.0) | 75/176 (42.6) | 34/111 (30.6) | 0.038 |
| Post-bronchodilator FEV1<LLN | 120/3477 (3.4) | 10/263 (3.8) | 67/808 (8.3) | 96/179 (53.6) | 41/111 (36.9) | <0.001# |
| Post-bronchodilator FVC<LLN | 139/3477 (4.0) | 6/263 (2.3) | 60/808 (7.4) | 27/179 (15.1) | 7/111 (6.3) | <0.001 |
| BDR mL | 57.5 (-8.8–129.4) | 99.7 (14.4–172.6) | 92.6 (21.6–171.6) | 126.9 (48.8–236.9) | 103.8 (17.8–176.9) | <0.001# |
| BDR % | 1.9 (-0.3–4.2) | 3.5 (0.6–6.4) | 3.5 (0.8–6.6) | 5.9 (2.0–12.7) | 4.0 (0.9–7.2) | <0.001# |
| BDR>12% and >200 mL | 71/3385 (2.1) | 10/258 (3.9) | 53/788 (6.7) | 43/177 (24.3) | 11/108 (10.2) | <0.001# |
| Marked BDR>12% and >400 mL | 25/3385 (0.7) | 1/258 (0.4) | 19/788 (2.4) | 19/177 (10.7) | -¶ | <0.001# |
| FENO ppb | ||||||
| Non-current smokers | 18.0 (13.0–25.0) | 18.0 (13.0–27.0) | 21.0 (14.0–33.0) | 22.0 (13.0–35.0) | 17.5 (14.0–25.0) | <0.001 |
| Current smokers | 11.0 (8.0–16.0) | 14.0 (11.0–19.0) | 11.0 (8.0–16.0) | 9.5 (6.0–13.0) | 11.0 (7.0–16.0) | 0.008 |
| FENO ≥25 ppb | ||||||
| Non-current smokers | 691/2728 (25.3) | 60/221 (27.2) | 254/650 (39.1) | 52/127 (40.9) | 13/46 (28.3) | <0.001 |
| Current smokers | 51/566 (9.0) | 4/29 (13.8) | 14/128 (10.9) | 32/46 (6.5) | 7/62 (11.3) | 0.774 |
| Childhood asthma onset (<18 years) + | -¶ | 75/182 (41.2) | 258/672 (38.4) | 79/148 (53.4) | -¶ | 0.004 |
| Late asthma onset (>40 years) + | -¶ | 22/182 (12.1) | 113/672 (16.8) | 16/148 (10.8) | -¶ | 0.084 |
| Asthma-like symptoms§, last 12 months | 1106/3413 (32.4) | -¶ | 719/806 (89.2) | 153/177 (86.4) | 75/111 (67.6) | <0.001 |
| Key COPD symptoms ƒ , last 12 months | 1001/3398 (29.5) | 39/256 (15.2) | 631/801 (78.8) | 151/175 (86.3) | 63/111 (56.8) | <0.001# |
| Chronic cough/sputum production | 347/3426 (10.1) | 15/259 (5.8) | 209/792 (26.4) | 62/173 (35.8) | 27/110 (24.6) | <0.001# |
| MRC dyspnoea score>1 | 506/2753 (18.4) | 31/214 (14.5) | 223/630 (35.4) | 56/143 (39.2) | 38/85 (44.7) | <0.001 |
| Hay fever | 921/3462 (26.6) | 125/262 (47.7) | 471/803 (58.7) | 93/178 (52.3) | 17/111 (15.3) | <0.001 |
| Eczema, ever | 1327/3456 (38.4) | 123/262 (47.0) | 466/803 (58.0) | 93/176 (52.8) | 42/110 (38.2) | <0.001 |
| Allergic sensitisation ## | 638/3316 (19.2) | 109/251 (43.4) | 376/771 (48.8) | 80/171 (46.8) | 20/107 (18.7) | <0.001 |
| Total serum IgE kU·L−1 | 24.5 (10.2–59.9) | 30.2 (11.8–69.0) | 45.5 (17.4–118.7) | 72.6 (21.7–193.1) | 29.7 (12.0–72.7) | <0.001 |
| Cat owner | 712/3468 (20.5) | 51/262 (19.5) | 141/805 (17.5) | 30/177 (17.0) | 19/111 (17.1) | 0.263 |
| Dog owner | 652/3465 (18.8) | 49/262 (18.7) | 181/806 (22.5) | 47/178 (26.4) | 15/110 (13.6) | 0.01 |
| Mould in the house, ever | 724/3429 (21.1) | 43/260 (16.5) | 209/792 (26.4) | 32/176 (18.2) | 29/110 (26.4) | 0.001 |
| Mould in the house, last 12 months | 499/3426 (14.6) | 27/258 (10.5) | 136/786 (17.3) | 21/176 (11.9) | 21/110 (19.1) | 0.031 |
| Emergency room/hospital admission for breathing problems since the last survey | 131/3451 (3.8) | 15/261 (5.8) | 101/805 (12.6) | 38/177 (21.5) | 4/110 (3.6) | <0.001 |
| Heart disease (angina, heart attack, coronary heart disease) | 96/3448 (2.8) | 5/261 (1.9) | 36/804 (4.5) | 6/175 (3.4) | 8/111 (7.2) | 0.009 |
Data are presented as n/N with available data (%), mean±sd or median (Q1–Q3), unless otherwise indicated. COPD: chronic obstructive pulmonary disease; BMI: body mass index; FEV1: forced expiratory volume in 1 s; LLN: lower limit of normal; FVC: forced vital capacity; BDR: bronchodilator responsiveness; FENO: fractional exhaled nitric oxide; MRC: Medical Research Council. #: this characteristic (or a closely related one) was considered for disease definition; ¶: 0% frequency forced by disease definitions; +: information on age at first asthma attack was only collected for subjects who reported to have ever had asthma; §: wheeze, nocturnal chest tightness, breathlessness after activity/at rest/at night-time; ƒ: chronic cough, chronic sputum production, dyspnoea, shortness of breath following strenuous activity; ##: having specific IgE >0.35 kU·L−1 for at least one of house dust mite, timothy grass or cat allergens.
Childhood and lifelong characteristics of participants
Subjects with asthma+COPD most frequently reported maternal smoking in childhood (28.2%), followed by those with past asthma and current asthma alone (table 2). Subjects with asthma+COPD were more likely to have parents with asthma (25.3%) or respiratory infections in childhood (19.1%) and less likely to have parents with COPD (28.7%) than subjects with COPD alone (20.8%, 14.0% and 34.3%, respectively). Lifelong cumulative exposure to tobacco smoking or occupational inhalants was lower among subjects with asthma+COPD than COPD alone. Subjects with asthma+COPD were most likely to have a history of AHR (89.6%) and high total serum IgE (73.3%).
TABLE 2.
Early-life and lifelong exposure to risk factors and clinical characteristics
| Reference subjects | Past asthma alone | Current asthma alone | Asthma+ COPD | COPD alone | Overall p-value | |
| Subjects n | 3477 | 263 | 808 | 179 | 111 | |
| Respiratory infections in childhood | 287/3287 (8.7) | 40/250 (16.0) | 134/747 (17.9) | 31/162 (19.1) | 15/107 (14.0) | <0.001# |
| Maternal smoking in childhood | 725/3440 (21.1) | 69/260 (26.5) | 203/794 (25.6) | 49/174 (28.2) | 23/110 (20.9) | 0.007 |
| Cat in childhood | 1581/3471 (45.6) | 113/263 (43.0) | 367/805 (45.6) | 80/177 (45.2) | 58/111 (52.3) | 0.604 |
| Dog in childhood | 1495/3473 (43.1) | 105/263 (39.9) | 356/805 (44.2) | 77/177 (43.5) | 46/111 (41.4) | 0.803 |
| Parental asthma | 302/3235 (9.3) | 48/248 (19.4) | 190/754 (25.2) | 40/158 (25.3) | 21/101 (20.8) | <0.001 |
| Parental COPD | 589/3157 (18.7) | 47/243 (19.3) | 189/735 (25.7) | 45/157 (28.7) | 34/99 (34.3) | <0.001# |
| History of heavy smoking | 1046/2713 (38.6) | 69/213 (32.4) | 238/672 (35.4) | 71/136 (52.2) | 67/83 (80.7) | <0.001# |
| History of occupational exposures | 1286/3286 (39.1) | 101/250 (40.4) | 302/767 (39.4) | 71/157 (45.2) | 54/100 (54.0) | 0.026# |
| History of AHR ¶ | 146/2006 (7.3) | 137/205 (66.8) | 422/596 (70.8) | 103/115 (89.6) | 7/48 (14.6) | <0.001# |
| History of high total IgE + | 924/2601 (35.5) | 106/216 (49.1) | 394/665 (59.3) | 107/146 (73.3) | 33/82 (40.2) | <0.001 |
| History of allergic sensitisation § | 1052/2638 (39.9) | 142/228 (62.3) | 484/689 (70.3) | 105/150 (70.0) | 32/87 (36.8) | <0.001 |
Data are presented as n/N with available data (%), unless otherwise indicated. COPD: chronic obstructive pulmonary disease; AHR: airway hyperresponsiveness; ECRHS: European Community Respiratory Health Survey. #: this characteristic (or a closely related one) was considered for disease definition; ¶: having a 20% decrease of forced expiratory volume in 1 s at a methacholine dose ≤1 mg at ECRHS I and/or II; +: having total IgE >100 kU·L−1 at ECRHS I, II and/or III; §: having specific IgE >0.35 kU·L−1 for at least one among house dust mite, timothy grass and cat allergens at ECRHS I, II and/or III.
Adult-life trajectories
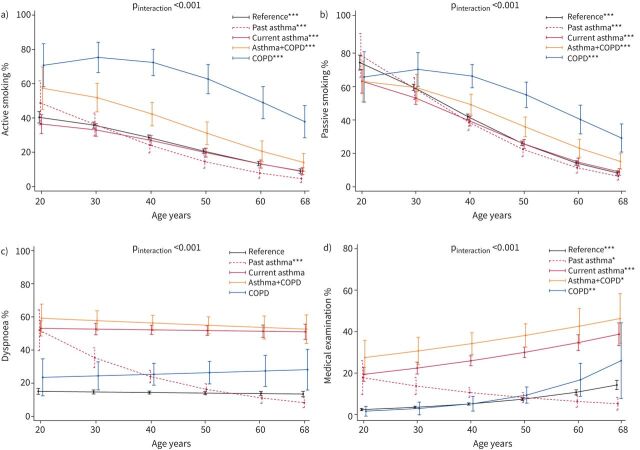
In the group with asthma+COPD, the lifetime prevalence of active smoking from age 20 to 68 years was midway between the subjects with COPD and the other less exposed groups (figure 2a). We estimated a monotonic age-related decline in the proportion of smokers for all groups except for COPD, in which it reached the maximum around age 30 years before declining. The proportion of subjects exposed to passive smoking was similar between the disease groups during early adulthood (60–70%), and then it followed trajectories similar to those of active smoking at older ages (figure 2b).
FIGURE 2.
Predicted trajectories for the proportion of subjects reporting a) active smoking, b) passive smoking, c) dyspnoea or d) having been seen by a physician during the last 12 months as a function of disease group and age. Number of subjects contributing data=4838. pinteraction obtained by Wald test (null hypothesis: true trajectories do not vary by disease group). The vertical lines represent 95% confidence intervals. Quantitative/indicator independent variables were set equal to the mean/proportion calculated over the set of subjects included. p-values are for the test of significance of the age-related trend within a disease group (null hypothesis: true proportion is constant across ages). *: p<0.05; **: p<0.01; ***: p<0.001.
No statistically significant variation in the prevalence of dyspnoea was found with ageing, except for a decrease among subjects with past asthma alone, consistent with their inactive disease status at ECRHS III (figure 2c). All groups except for past asthma showed an age-related increase in the proportion of subjects seen by a physician in the previous 12 months, which became steeper after age 50 years in the group with COPD alone (figure 2d).
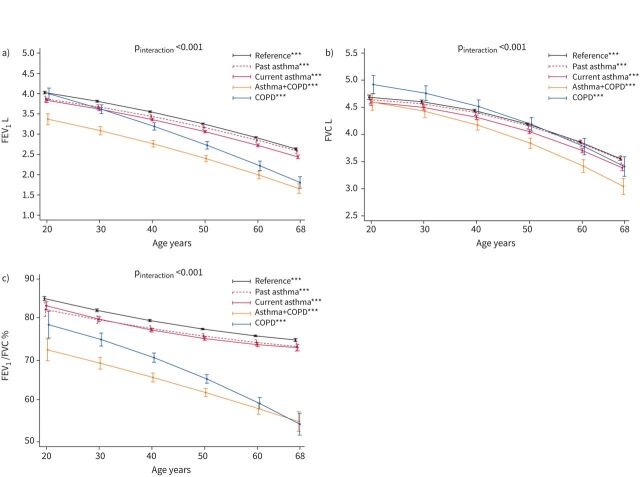
Subjects with asthma+COPD had a low lung function when they were young adults, and this condition tracked over their adult lives (supplementary table E6 and figure 3). Their raw FEV1 decline was only slightly faster than that in the current asthma alone and reference groups, whereas a steeper decline was observed for FEV1 % predicted (supplementary figure E3a); raw FVC (figure 3b), FVC % predicted (supplementary figure E3b) and FEV1/FVC (figure 3c) were also accelerated.
FIGURE 3.
Predicted trajectories for mean pre-bronchodilator a) forced expiratory volume in 1 s (FEV1), b) forced vital capacity (FVC) and c) FEV1/FVC ratio as a function of disease group and age. Number of subjects contributing data=4831 (FEV1), 4822 (FVC) and 4816 (FEV1/FVC). pinteraction obtained by Wald test (null hypothesis: true trajectories do not vary by disease group). The vertical lines represent 95% confidence intervals. Spirometer type was set to NDD EasyOne; quantitative/indicator independent variables were set equal to the mean/proportion calculated over the set of subjects included. ***: p<0.001 for the test of significance of the age-related trend within a disease group (null hypothesis: true mean is constant across ages).
We observed the steepest rate of FEV1 decline in subjects with COPD alone (figure 3a and supplementary figure E3a). Their FEV1/FVC was midway between those in the asthma+COPD group and other groups during young adulthood, but then it declined steeply and reached the low level observed in asthma+COPD during the seventh decade of life (figure 3c).
Results of the sensitivity analyses were consistent with those from the main analyses (appendix E1, supplementary figures E4–E7, supplementary tables E7, E8).
Discussion
Using data collected prospectively over 20 years from population samples, we found that subjects with both asthma and COPD at age 40–68 years were more likely to report maternal smoking and respiratory infections in childhood, but less likely to have been exposed to tobacco smoking and occupational inhalants during adult life compared to subjects with COPD alone. Estimated past trajectories of FEV1, FVC and FEV1/FVC ratio suggested that low lung function in young adulthood is more frequently observed among subjects who will be identified as having both asthma and COPD later in life, rather than COPD alone. However, a steep lung function decline is more common among adults who will subsequently be diagnosed with COPD alone. We hypothesise that early-life risk factors have a more prominent role in the development of asthma+COPD, whereas exposures during adult life could be stronger risk factors for the development of COPD that does not have features of asthma.
Disease classification and socio-demographics
At disease classification, all the participants in our study were ≥40 years, an age when the overlap between asthma and COPD generally starts to be considered as a potential diagnosis [8]. The number of subjects with COPD alone was relatively small compared to the number with asthma+COPD, possibly because the age range at ECRHS III still included a small proportion of older people, but also owing to the ECRHS design, which over-represented people with asthma [21]. The greater representation of women in the asthma+COPD group (49.7%) compared to COPD alone (42.3%) is common to several studies [15, 18, 29, 30], and consistent with the higher risk of adult asthma in women, and with the greater tobacco exposure among men in Europe [31].
Having at least one GOLD-defined indicator of COPD was an inclusion criterion for both asthma+COPD and COPD alone [3]. However, four subjects with a post-bronchodilator FEV1/FVC<LLN did not have any of these indicators. We found that GOLD-defined key symptoms of COPD and early-life respiratory infections were not very specific for COPD alone.
A lifetime history of asthma or marked BDR (increase in FEV1>12% and >400 mL) were additional inclusion criteria for asthma+COPD, but were exclusion criteria for COPD alone [9, 32]. Nonetheless, 10.2% of subjects with COPD alone had BDR (increase in FEV1 200–400 mL), as compared to 24.3% in asthma+COPD. Almost all subjects with asthma+COPD had a history of asthma (98.9%), which implies that only a small minority of people with persistent airflow obstruction were classified in this group based on a marked BDR alone. A marked BDR is less relevant for the diagnosis of asthma+COPD because it is infrequent [33, 34], it does not reliably differentiate between COPD with and without asthma [7], and because definitions including BDR are not stable over time [19].
Clinical characteristics of asthma+COPD at disease classification
Subjects with asthma+COPD had a prevalence of asthma-like symptoms (86.4%) and chronic cough/sputum production (35.8%) that was similar to or higher than that found in subjects with current asthma alone (89.2% and 26.4%, respectively). They were also more likely to report emergency room or hospital admissions, and they were using respiratory medication more frequently. However, subjects with asthma+COPD were as physically active as individuals with current asthma alone, suggesting a marginal impairment of their daily activities. A high degree of bronchial inflammation, as indicated by FENO levels, could be linked to the excess disease burden in subjects with asthma+COPD [35, 36]. High FENO levels are a useful marker of type-2 inflammation for asthma diagnosis, phenotyping and monitoring, and could help to predict who will benefit from inhaled corticosteroid treatment [37], but their clinical relevance in COPD and asthma+COPD is still unclear.
The early-life origins of asthma+COPD
In early adulthood, subjects with asthma+COPD had airflow obstruction (low FEV1/FVC ratio and low FEV1) and small lung volumes (FVC) [38], which could be explained by several, potentially concurrent, pathogenic mechanisms, including abnormal pulmonary growth and early-life insults [39]. These subjects reported the highest frequency of maternal smoking and respiratory infections during childhood, two acknowledged risk factors for reduced lung growth and lung function decline [38, 40, 41]. Subjects with asthma+COPD had a low education level, as found by others [9, 29, 42], suggesting that socio-economic deprivation could be an additional disadvantageous factor [41]. For more than half of the subjects with asthma+COPD, asthma began in childhood, which could have contributed to the development of irreversible airflow obstruction in adulthood [40, 43]. Bui et al. [20] reported that a low lung function at age 7 years is a strong risk factor for having both current asthma and COPD at age 45 years.
Adult-life trajectories
Subjects with COPD alone had the fastest rate of FEV1 and FEV1/FVC decline, and they reported a sharp increase in medical examinations after age 50 years, which may indicate an accelerated worsening of health with ageing compared to the other groups. Subjects with asthma+COPD had a decline in FEV1 and FEV1/FVC that was intermediate between COPD and the other disease groups. Overall, these findings could be explained by the higher level of exposure to inhaled pollutants in the group with COPD alone. The age-related decrease in active and passive smoking, which we observed for all the groups as a result of the successful implementation of tobacco control policies [31, 44], occurred about one life decade later for subjects with COPD alone compared to the other groups.
Lung function decline for subjects with asthma+COPD could have been mitigated by long-term anti-inflammatory treatment. Two out of three in this group had a history of allergic sensitisation or high total IgE, and sustained inhaled corticosteroid treatment could be more effective in atopic asthma [45, 46]. Almost 90% of subjects with asthma+COPD had a history of AHR, which we previously related to low lung function in young adulthood but normal subsequent FEV1 decline in mL·year−1 [47].
Lung function decline in COPD with and without asthma
The finding that FEV1 decline was faster for subjects with COPD alone than asthma+COPD is consistent with most literature [14–20], with two exceptions that could be linked to the heterogeneity captured by different disease definitions (supplementary table E9) [30, 34]. Lange et al. [30] found a slower FEV1 decline for subjects with early-onset asthma (age<40 years)+COPD, compared to COPD alone, but a faster decline for subjects with late-onset asthma+COPD [30]. In our study, asthma onset occurred before age 40 years for ∼90% of the subjects with asthma+COPD (table 1). At variance with our study, Lange et al. [30] did not consider the history of smoking in the definition of asthma+COPD, whereas BDR (>200 mL after terbutaline inhalation) was an exclusion criterion for COPD alone. Tkacova et al. [34] reported a faster FEV1 decline among subjects with COPD and AHR compared to COPD without AHR. AHR might be related to structural airway changes and, as the authors acknowledged, its relevance as a marker of coexisting asthma is arguable [34].
Strengths and limitations
Collecting anamnestic information can be challenging for clinicians [19]. One strength of our study was having detailed information on past medical histories and risk factor exposures, collected prospectively from individual reports up to 20 years before, which plausibly reduced recall bias. The lack of objective information on early-life factors is a limitation, because these exposures were self-reported at age 20–44 years (ECRHS I). Our sample at ECRHS III derives from self-selection of participants from previous waves, under-representing younger people, smokers and subjects with a low education level or a low lung function at baseline. We used self-reported asthma rather than a more accurate clinical diagnosis for disease classification, although higher levels of bronchial inflammation and atopy in the asthma phenotypes, compared to COPD alone, indirectly supports the validity of our definitions. Another limitation is that we did not have data on blood or sputum eosinophil counts, or repeated post-bronchodilator spirometry, which could have improved disease classification [33], nor did we have data on outdoor air pollution exposure, a factor for which evidence of association with COPD incidence is still inconclusive [48, 49].
Conclusions
The coexistence of asthma and COPD seems to be a form of severe asthma with origins early in life, as opposed to COPD alone, which is more linked to adult exposures. This was suggested in our study by a greater prevalence of respiratory infections, maternal smoking and asthma during childhood, and an impairment of lung function at younger ages among subjects with asthma+COPD. These findings imply that prevention of this condition, which is typical for older adults, should start in childhood or possibly even before [50]. Public health interventions in terms of discouraging maternal smoking, promoting immunisation in childhood and preventing young people with asthma from smoking might be most effective. By contrast, COPD without concomitant features of asthma seems predominantly linked to adult-life toxic inhalant exposures. Exposure avoidance (e.g. through smoking cessation and reduction of pollution exposure in occupational settings) may be particularly beneficial against the development of the “pure COPD” phenotype, although it is also likely to reduce the transition from asthma to asthma+COPD. In our study, COPD alone seemed to have faster progression than asthma+COPD. Nonetheless, subjects with asthma+COPD are also at increased risk of developing respiratory disability, given their marked early and progressive impairment of lung function.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04656-2020.SUPPLEMENT (1.2MB, pdf)
Shareable PDF
Acknowledgements
The ALEC study leader is Deborah Jarvis. The study was carried out under ALEC Workpackage 4 led by Judith Garcia-Aymerich. The principal investigators and team members of the original studies are reported in in appendix E2.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This article has an editorial commentary: https://doi.org/10.1183/13993003.01329-2021
Author contributions: A. Marcon and S. Accordini conceived and designed the study. A. Marcon drafted the analysis plan and the first version of the manuscript. F. Locatelli and A. Marcon performed the statistical analysis. All the authors contributed to the discussion of the analysis plan and the interpretation of results, and commented on the first manuscript draft. All the authors critically reviewed and approved the final version of the manuscript.
Conflict of interest: A. Marcon has nothing to disclose.
Conflict of interest: F. Locatelli has nothing to disclose.
Conflict of interest: S.C. Dharmage has nothing to disclose.
Conflict of interest: C. Svanes has nothing to disclose.
Conflict of interest: J. Heinrich has nothing to disclose.
Conflict of interest: B. Leynaert has nothing to disclose.
Conflict of interest: P. Burney has nothing to disclose.
Conflict of interest: A. Corsico has nothing to disclose.
Conflict of interest: G. Caliskan has nothing to disclose.
Conflict of interest: L. Calciano has nothing to disclose.
Conflict of interest: T. Gislason has nothing to disclose.
Conflict of interest: C. Janson has nothing to disclose.
Conflict of interest: D. Jarvis has nothing to disclose.
Conflict of interest: R. Jõgi received grants from the Estonian Research Council (Personal Research Grant n. 562), and personal fees for consultancy and lecturing from GSK, Boehringer and Novartis, and for travels/accommodation/meetings from GSK and Boehringer.
Conflict of interest: T. Lytras has nothing to disclose.
Conflict of interest: A. Malinovschi has nothing to disclose.
Conflict of interest: N. Probst-Hensch has nothing to disclose.
Conflict of interest: K. Toren has nothing to disclose.
Conflict of interest: L. Casas has nothing to disclose.
Conflict of interest: G. Verlato has nothing to disclose.
Conflict of interest: J. Garcia-Aymerich has nothing to disclose.
Conflict of interest: S. Accordini has nothing to disclose.
Support statement: The ALEC study was funded by the European Union's Horizon 2020 Research and Innovation programme under grant agreement number 633212. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. National funders who supported data collection in the original studies are listed in appendix E2. The funders had no role in the design of the study, in the collection, analysis and interpretation of data, in writing of the manuscript or in the decision to submit it for publication. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015; 373: 1241–1249. doi: 10.1056/NEJMra1411863 [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. 2020. www.ginasthma.org Date last accessed: July 22, 2020.
- 3.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2020. https://goldcopd.org/ Date last accessed: July 29, 2020.
- 4.American Thoracic Society . Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152: S77–S121. [PubMed] [Google Scholar]
- 5.Soriano JB, Davis KJ, Coleman B, et al. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003; 124: 474–481. doi: 10.1378/chest.124.2.474 [DOI] [PubMed] [Google Scholar]
- 6.Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax 2015; 70: 683–691. doi: 10.1136/thoraxjnl-2014-206740 [DOI] [PubMed] [Google Scholar]
- 7.Milne S, Mannino D, Sin DD. Asthma-COPD overlap and chronic airflow obstruction: definitions, management, and unanswered questions. J Allergy Clin Immunol Pract 2020; 8: 483–495. doi: 10.1016/j.jaip.2019.10.044 [DOI] [PubMed] [Google Scholar]
- 8.Bateman ED, Reddel HK, van Zyl-Smit RN, et al. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med 2015; 3: 719–728. doi: 10.1016/S2213-2600(15)00254-4 [DOI] [PubMed] [Google Scholar]
- 9.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 2017; 358: j3772. doi: 10.1136/bmj.j3772 [DOI] [PubMed] [Google Scholar]
- 10.Woodruff PG, van den Berge M, Boucher RC, et al. American Thoracic Society/National Heart, Lung, and Blood Institute asthma-chronic obstructive pulmonary disease overlap workshop report. Am J Respir Crit Care Med 2017; 196: 375–381. doi: 10.1164/rccm.201705-0973WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Marco R, Cappa V, Accordini S, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J 2012; 39: 883–892. doi: 10.1183/09031936.00061611 [DOI] [PubMed] [Google Scholar]
- 12.Hosseini M, Almasi-Hashiani A, Sepidarkish M, et al. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res 2019; 20: 229. doi: 10.1186/s12931-019-1198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barczyk A, Maskey-Warzechowska M, Gorska K, et al. Asthma-COPD overlap–a discordance between patient populations defined by different diagnostic criteria. J Allergy Clin Immunol Pract 2019; 7: 2326–2336. doi: 10.1016/j.jaip.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 14.de Marco R, Marcon A, Rossi A, et al. COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J 2015; 46: 671–679. doi: 10.1183/09031936.00008615 [DOI] [PubMed] [Google Scholar]
- 15.Park HY, Lee SY, Kang D, et al. Favorable longitudinal change of lung function in patients with asthma-COPD overlap from a COPD cohort. Respir Res 2018; 19: 36. doi: 10.1186/s12931-018-0737-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu JJ, Gibson PG, Simpson JL, et al. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration 2014; 87: 63–74. doi: 10.1159/000352053 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the Hokkaido COPD cohort study. Am J Respir Crit Care Med 2016; 194: 1358–1365. doi: 10.1164/rccm.201602-0353OC [DOI] [PubMed] [Google Scholar]
- 18.Hayden LP, Hardin ME, Qiu W, et al. Asthma is a risk factor for respiratory exacerbations without increased rate of lung function decline: five-year follow-up in adult smokers from the COPDGene Study. Chest 2018; 153: 368–377. doi: 10.1016/j.chest.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrecheguren M, Pinto L, Mostafavi-Pour-Manshadi SMY, et al. Identification and definition of asthma–COPD overlap: the CanCOLD study. Respirology 2020; 25: 836–849. doi: 10.1111/resp.13780 [DOI] [PubMed] [Google Scholar]
- 20.Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med 2017; 196: 39–46. doi: 10.1164/rccm.201606-1272OC [DOI] [PubMed] [Google Scholar]
- 21.Burney PG, Luczynska C, Chinn S, et al. The European Community Respiratory Health Survey. Eur Respir J 1994; 7: 954–960. doi: 10.1183/09031936.94.07050954 [DOI] [PubMed] [Google Scholar]
- 22.ECRHS II Steering Committee . The European Community Respiratory Health Survey II. Eur Respir J 2002; 20: 1071–1079. doi: 10.1183/09031936.02.00046802 [DOI] [PubMed] [Google Scholar]
- 23.Amaral AFS, Newson RB, Abramson MJ, et al. Changes in IgE sensitization and total IgE levels over 20 years of follow-up. J Allergy Clin Immunol 2016; 137: 1788–1795. doi: 10.1016/j.jaci.2015.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 25.Chinn S, Burney P, Jarvis D, et al. Variation in bronchial responsiveness in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1997; 10: 2495–2501. doi: 10.1183/09031936.97.10112495 [DOI] [PubMed] [Google Scholar]
- 26.Nerpin E, Olivieri M, Gislason T, et al. Determinants of fractional exhaled nitric oxide in healthy men and women from the European Community Respiratory Health Survey III. Clin Exp Allergy 2019; 49: 969–979. doi: 10.1111/cea.13394 [DOI] [PubMed] [Google Scholar]
- 27.Lytras T, Kogevinas M, Kromhout H, et al. Occupational exposures and incidence of chronic bronchitis and related symptoms over two decades: the European Community Respiratory Health Survey. Occup Environ Med 2019; 76: 222–229. doi: 10.1136/OEM-2019-EPI.59 [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan BW, Grigsby MR, Siddharthan T, et al. Epidemiology and risk factors of asthma-chronic obstructive pulmonary disease overlap in low- and middle-income countries. J Allergy Clin Immunol 2019; 143: 1598–1606. doi: 10.1016/j.jaci.2018.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange P, Colak Y, Ingebrigtsen TS, et al. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med 2016; 4: 454–462. doi: 10.1016/S2213-2600(16)00098-9 [DOI] [PubMed] [Google Scholar]
- 31.Marcon A, Pesce G, Calciano L, et al. Trends in smoking initiation in Europe over 40 years: a retrospective cohort study. PLoS One 2018; 13: e0201881. doi: 10.1371/journal.pone.0201881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012; 48: 331–337. doi: 10.1016/j.arbr.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 33.Miravitlles M. Diagnosis of asthma-COPD overlap: the five commandments. Eur Respir J 2017; 49: 1700506. doi: 10.1183/13993003.00506-2017 [DOI] [PubMed] [Google Scholar]
- 34.Tkacova R, Dai DLY, Vonk JM, et al. Airway hyperresponsiveness in chronic obstructive pulmonary disease: a marker of asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol 2016; 138: 1571–1579. doi: 10.1016/j.jaci.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 35.Goto T, Camargo CA Jr, Hasegawa K. Fractional exhaled nitric oxide levels in asthma-COPD overlap syndrome: analysis of the National Health and Nutrition Examination Survey, 2007–2012. Int J Chron Obstruct Pulmon Dis 2016; 11: 2149–2155. doi: 10.2147/COPD.S110879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannino DM, Gan WO, Wurst K, et al. Asthma and chronic obstructive pulmonary disease overlap: the effect of definitions on measures of burden. Chronic Obstr Pulm Dis 2017; 4: 87–96. doi: 10.15326/jcopdf.4.2.2016.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price DB, Buhl R, Chan A, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med 2018; 6: 29–39. doi: 10.1016/S2213-2600(17)30424-1 [DOI] [PubMed] [Google Scholar]
- 38.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019; 7: 358–364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 39.Lange P, Celli B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 111–122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 40.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 41.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010; 65: 14–20. doi: 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 42.de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One 2013; 8: e62985. doi: 10.1371/journal.pone.0062985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai A, Tran H, Roberts M, et al. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014; 69: 805–810. doi: 10.1136/thoraxjnl-2013-204815 [DOI] [PubMed] [Google Scholar]
- 44.Olivieri M, Murgia N, Carsin AE, et al. Effects of smoking bans on passive smoking exposure at work and at home. The European Community Respiratory Health Survey. Indoor Air 2019; 29: 670–679. doi: 10.1111/ina.12556 [DOI] [PubMed] [Google Scholar]
- 45.de Marco R, Marcon A, Jarvis D, et al. Inhaled steroids are associated with reduced lung function decline in subjects with asthma with elevated total IgE. J Allergy Clin Immunol 2007; 119: 611–617. doi: 10.1016/j.jaci.2006.11.696 [DOI] [PubMed] [Google Scholar]
- 46.Marcon A, Marchetti P, Anto JM, et al. Atopy modifies the association between inhaled corticosteroid use and lung function decline in patients with asthma. J Allergy Clin Immunol Pract 2020; 8: 980–988. doi: 10.1016/j.jaip.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 47.Marcon A, Locatelli F, Keidel D, et al. Airway responsiveness to methacholine and incidence of COPD: an international prospective cohort study. Thorax 2018; 73: 825–832. doi: 10.1136/thoraxjnl-2017-211289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schikowski T, Mills IC, Anderson HR, et al. Ambient air pollution: a cause of COPD? Eur Respir J 2014; 43: 250–263. doi: 10.1183/09031936.00100112 [DOI] [PubMed] [Google Scholar]
- 49.Park J, Kim HJ, Lee CH, et al. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ Res 2021; 194: 110703. doi: 10.1016/j.envres.2020.110703 [DOI] [PubMed] [Google Scholar]
- 50.Accordini S, Calciano L, Johannessen A, et al. A three-generation study on the association of tobacco smoking with asthma. Int J Epidemiol 2018; 47: 1106–1117. doi: 10.1093/ije/dyy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04656-2020.SUPPLEMENT (1.2MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04656-2020.Shareable (737.2KB, pdf)