Abstract
Dementia with Lewy bodies (DLB) is a clinical diagnosis representing a specific presentation of a pathological α-synucleinopathy (Lewy body disease). DLB is one entity under the broader term Lewy body dementia, which also includes Parkinson’s disease dementia. Recent advances in DLB include publication of updated diagnostic criteria and recognition of prodromal DLB states, including mild cognitive impairment, delirium-onset, and psychiatric-onset forms. Research criteria for the mild cognitive impairment form of DLB were published in 2020. Increasing research shows that concomitant Alzheimer’s disease pathology in individuals with DLB is common in addition to the α-synucleinopathy pathology. This has implications for biomarker use and expected progression. Identifying biomarkers for DLB is an area of active research. Cerebrospinal fluid and skin biopsy tests are now commercially available in the United States, but their role in routine clinical care is not yet established. Additional research and biomarkers are needed. Research suggests that median survival after DLB diagnosis is 3–4 years, but there are rapidly and slowly progressive forms. Most individuals with DLB die of complications of the disease. Clinical trials for individuals with DLB have increased over the last 5 years, targeting both symptoms and underlying pathology. Effective therapies remain an unmet need, however. This review focuses on recent advances with an emphasis on literature that informs clinical care.
Keywords: dementia with Lewy bodies, Lewy body dementia, Lewy body disease [MeSH]
Introduction
Dementia with Lewy bodies (DLB) is a clinical diagnosis that reflects a specific presentation of a pathological α-synucleinopathy. Its pathology and clinical presentation is closely related to Parkinson’s disease (PD) with and without dementia, but recognition of DLB as an entity occurred much more recently than PD. The initial criteria for DLB were published in 1996. 1 Since that time, there have been advances in diagnostic criteria [e.g. inclusion of rapid eye movement (REM) sleep behavior disorder (RBD) as a core symptom], recognition of prodromal DLB states, identification of prognostic and end-of-life considerations, and an increase in clinical trials targeting both symptoms and underlying pathology. This review focuses on these advances, with an emphasis on recent literature that informs clinical care.
Vocabulary
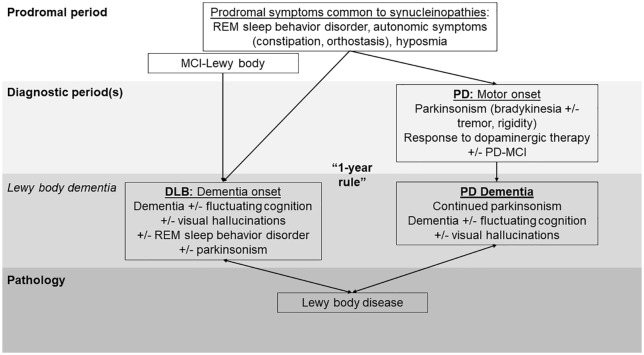
While four rounds of consensus have honed the diagnostic criteria for DLB, the vocabulary for this disorder continues to cause confusion. Dementia with Lewy bodies is distinct from Lewy body dementia, which is an umbrella term including both DLB and Parkinson’s disease dementia (PDD). Lewy body dementia is the second-most common degenerative dementia after Alzheimer’s disease (AD),1–3 but DLB is only one part of this diagnostic umbrella. DLB is also variably categorized as both an ‘atypical parkinsonism’ and an ‘Alzheimer-disease related dementia’ (ADRD). Lewy body dementia and DLB are further distinct from Lewy body disease (LBD), which is the term used to describe the pathological finding of Lewy bodies (aggregated α-synuclein inclusions in neurons) on postmortem examination (Figure 1).
Figure 1.
Lewy body dementia terminology over the clinical course. Many prodromal features are common to the synucleinopathies, including DLB and PD. Research criteria for MCI-Lewy body were published in 2020. MCI-Lewy body is thought to represent a prodromal form of DLB. If an individual has sufficient motor features at presentation to meet criteria for PD, they receive a PD diagnosis. MCI may be present at PD onset. Most individuals with PD develop dementia over time, termed PD dementia. If an individual has dementia at presentation or within 1 year of the onset of motor parkinsonism, they receive a diagnosis of DLB (assuming sufficient features are present). This ‘1-year’ rule is maintained in the most recent (2017) DLB criteria but the 1 year remains arbitrary. Together, DLB and PD dementia constitute the umbrella term Lewy body dementia. Individuals with both forms of Lewy body dementia have aggregated α-synuclein neuronal inclusions on postmortem examination, termed Lewy body disease.
DLB, dementia with Lewy bodies; MCI, mild cognitive impairment; PD, Parkinson’s disease; PD-MCI, Parkinson’s disease with mild cognitive impairment; REM, rapid eye movement.
The term Lewy body variant of Alzheimer disease was coined in the 1990s to describe individuals with clinically diagnosed and pathologically confirmed AD with concomitant Lewy bodies on pathology. 4 Because these individuals have a predominant AD dementia presentation, they are not typically grouped under the Lewy body dementia clinical umbrella. This term is no longer commonly used, but individuals with AD dementia can have co-existing Lewy bodies resulting in a dual pathological diagnosis (AD + LBD) or AD with Lewy bodies insufficient to meet formal DLB pathological criteria.5,6
Classically, DLB was distinguished from PDD by the timing of symptom onset (Figure 1). DLB was diagnosed if dementia was present at symptom onset or within 1 year of parkinsonism onset. PDD was diagnosed if an individual had a diagnosis of PD for at least 1 year prior to the onset of dementia. Dementia at onset was an exclusion criterion for the diagnosis of PD in most commonly referenced PD criteria prior to 2015, such as the United Kingdom Parkinson Disease Brain Bank Criteria. 7 The ‘one year rule’ for DLB was arbitrary but helped guide clinical practice, with the acknowledgment that this might need revision over time. 1 In 2015, the International Parkinson and Movement Disorder Society (MDS) published new criteria for the diagnosis of PD (MDS-PD Criteria). In these criteria, the task force proposed omitting the prior exclusion criterion of dementia at onset when diagnosing PD and the ‘one year rule’.8,9 In this rubric, individuals meeting criteria for PD but with dementia at onset and sufficient features to also meet DLB criteria would be diagnosed as PD or ‘PD (DLB subtype)’.8,9 The argument for this change was based on similarities in prodromal features, dementia presentations, neuropsychological findings, nonmotor profiles, imaging, genetics, and pathology.8,10 Members of the Lewy Body Dementia Association Scientific Advisory Council objected to this change, however, noting that despite important overlaps among DLB, PD, and PDD, there remained key differences. For example, some individuals with DLB never develop parkinsonism in life and those with parkinsonism might or might not have sufficient features for a PD diagnosis. Frequency of amyloid co-pathology (and related imaging findings) appears to be different in DLB versus PD and PDD. The expected clinical course is also different in DLB compared with PD/PDD. While acknowledging that the 1-year rule remains arbitrary, these authors argued that maintaining DLB and PDD as separate phenotypes has important implications for patient care (e.g. understanding expected prognosis) and research into pathophysiology, genetics, prodromal states, and potentially treatment. 11 The diagnostic criteria controversy remains unresolved, with research studies variably using the 2015 MDS-PD Criteria, the 2017 DLB criteria, or a combination. This review focuses on advances in DLB as defined by the DLB-specific criteria.
Diagnosis
Diagnostic criteria
The most recent consensus criteria for DLB were published in 2017 (Table 1). 6 Criteria updates were based on interim research and included distinguishing between clinical features and biomarkers, removing the ‘suggestive feature’ category, elevating RBD to a core clinical feature, and demoting antipsychotic (neuroleptic) hypersensitivity to a supportive feature. 6 Clinicians now make the diagnosis of possible or probable DLB based on the presence of core clinical features and indicative biomarkers (Table 1). Additional ‘supportive’ clinical features, however, provide additional weight for a DLB diagnosis. Supportive features include severe sensitivity to antipsychotic agents, postural instability, repeated falls, syncope or other transient episodes of unresponsiveness, severe autonomic dysfunction (e.g. constipation, orthostatic hypotension, and urinary incontinence), excessive daytime sleepiness, hyposmia, hallucinations in nonvisual modalities, systematized delusions, and apathy, anxiety, and depression. These features are not sensitive or specific enough to serve as core clinical features, but their presence further supports a DLB diagnosis. Similarly, supportive biomarkers do not currently have sufficient sensitivity or specificity for formal inclusion in the DLB criteria, but they can provide additional evidence supporting a DLB diagnosis. Supportive biomarkers in the 2017 criteria were (1) relative preservation of medial temporal lobe structures on computed tomography (CT)/magnetic resonance imaging (MRI) (i.e. findings suggesting AD as less likely), (2) generalized low uptake on single-photon emission computed tomography (SPECT)/positron emission tomography (PET) perfusion/metabolism scans with reduced occipital activity and/or the cingulate island sign on FDG (fluorodeoxyglucose)-PET imaging, and (3) prominent posterior slow-wave activity on electroencephalogram (EEG) with periodic fluctuations in the pre-alpha/theta range. 6
Table 1.
Criteria for the clinical diagnosis DLB from the fourth consensus report of the DLB Consortium (2017)..
| Probable DLB: (1) Required criterion: Dementia, usually with prominent and early impairments in attention, executive function, and visuoperceptual ability (memory involvement more with progression) (2) Presence of ⩾2 core clinical features (± indicative biomarker) OR 1 core clinical feature + ⩾1 indicative biomarker(s) Possible DLB: (1) Required criterion: Dementia, usually with prominent and early impairments in attention, executive function, and visuoperceptual ability (memory involvement more with progression) (2) Presence of 1 core clinical feature (no indicative biomarker) OR ⩾1 indicative biomarker(s) (no core clinical features) | |
| Core clinical features: 1. Fluctuating cognition with pronounced variations in alertness and attention 2. Recurrent visual hallucinations 3. REM sleep behavior disorder 4. Parkinsonism (presence of one or more of: bradykinesia, rest tremor, rigidity) |
Indicative biomarkers: 1. Reduced basal ganglia dopamine transporter uptake (SPECT or PET) 2. Abnormal (low uptake) 123iodine-MIBG myocardial scintigraphy 3. Polysomnographic confirmation of REM sleep without atonia |
Source: Adapted from McKeith et al. 6
DLB, dementia with Lewy bodies; MIBG, metaiodobenzylguanidine; PET, positron emission tomography; REM, rapid eye movement; SPECT, single-photon emission computed tomography.
The criteria also include supportive clinical features and biomarkers that are helpful in diagnosing DLB but are not formally part of the criteria (see text). If parkinsonism is present, the dementia should have started first or within 1 year of motor symptom onset for a diagnosis of DLB (the ‘one year rule’). If parkinsonism is the only core clinical feature and appears only in the context of severe dementia, DLB is less likely.
Making the diagnosis
Clinicians must be alert to the possibility of DLB when evaluating individuals with cognitive impairment. It is estimated that one in three cases of DLB are missed 12 and misdiagnosis as AD is common.12,13 Diagnosis relies largely on a comprehensive history and physical examination assessing core and supportive features of DLB. Neuropsychological testing and structural imaging (typically magnetic resonance imaging) are part of many standard evaluations for dementia. 14 Other tests and biomarkers, however, are uncommonly used by specialists making DLB diagnoses. 15 Polysomnography is occasionally used by specialists to assess for REM sleep without atonia, particularly if the presence of RBD is uncertain by history. Similarly, dopamine transporter (DAT) imaging is occasionally used to strengthen suspicion of a DLB diagnosis if there is no or equivocal parkinsonism on examination. Low uptake on metaiodobenzylguanidine (MIBG) myocardial scintigraphy is an indicative biomarker in the DLB criteria, but this test is not available in many countries (including the United States) for this purpose. 15 Testing for α-synuclein aggregates in the cerebrospinal fluid (CSF) and/or via skin biopsy is now available through some commercial laboratories, but the role that these tests play in routine clinical diagnosis is yet to be established.
Pathology and co-pathology
Lewy body disease has multiple types, including brainstem-predominant, transitional (limbic), and diffuse (neocortical) forms associated with increasing degrees of pathological burden. Using the updated DLB criteria, assessments of the likelihood that pathologic findings are associated with a typical DLB clinical syndrome involve evaluating both the degree of Lewy-related pathology (diffuse neocortical, limbic/transitional, brainstem-predominant, amygdala-predominant, or olfactory bulb only) and the amount of AD neuropathological change. In this rubric, the degree of AD neuropathologic change is classified in one of three categories: National Institute on Aging–Alzheimer’s Association (NIA-AA) category of none/low (Braak stage 0–II), NIA-AA intermediate (Braak stage III–IV), or NIA-AA high (Braak stage V–VI). Likelihood of parkinsonism is subclassified based on whether substantia nigra neuronal loss is none, mild, moderate, or severe. 6 Other staging systems for Lewy body pathology also exist, and a recent multicenter consortium published Lewy pathology consensus criteria in attempts to improve detection of Lewy pathology and interrater reliability. 16 This method uses a dichotomous approach for the scoring of Lewy pathology (present versus absent) applied to pathological categories used in the DLB consortium diagnostic criteria. 16
As suggested by inclusion of both Lewy pathology and AD pathology in the DLB criteria, AD neuropathological change is common in individuals diagnosed clinically with DLB. It is estimated that half of all individuals with Lewy body disease have sufficient pathology for a secondary neuropathological diagnosis of AD at autopsy. 17 Different distributions of α-synuclein and tau pathology associate with the phenotypic expression of DLB. When the distribution of α-synuclein pathology is greater than tau pathology, a clinical diagnosis of DLB is highly likely. A clinical DLB diagnosis is less likely when the distribution of tau pathology is greater than α-synuclein, with some caveats. 18 The distribution of α-synuclein, tau, and amyloid pathology also contributes to disease duration in DLB. Individuals with diffuse Lewy body disease have shorter survival than individuals with transitional Lewy body disease, with α-synuclein pathology predicting disease duration, both independently and synergistically with tau and amyloid pathology. 19 The presence of AD pathology may also influence the presence of depression/dysphoria in DLB. 20
Prodromal DLB
Prodromal features of synucleinopathies are well established, including RBD/REM sleep without atonia,21,22 olfactory dysfunction, dysautonomia, and psychiatric disturbance (e.g. anxiety and depression). 22 Mild cognitive impairment (MCI) can also precede the dementia of DLB (Figure 1). Studies consistently show that nonamnestic MCI (usually affecting attention, visuospatial/visuoperceptual function), particularly in the context of associated RBD, fluctuations, and/or subtle parkinsonism, can herald subsequent DLB.23–25 This research culminated in the publication of research criteria for prodromal DLB in 2020. These criteria describe three prodromal phenotypes of DLB: MCI-onset, delirium-onset, and psychiatric-onset. Evidence was only sufficient to propose diagnostic criteria for MCI-onset DLB, termed MCI with Lewy bodies (MCI-LB). 26
Research criteria for MCI-LB (Table 2) overlap heavily with DLB criteria (Table 1). Similar to DLB criteria, ‘supportive’ clinical features for MCI-LB include severe sensitivity to antipsychotic agents, postural instability, repeated falls, syncope or other transient episodes of unresponsiveness, severe autonomic dysfunction (e.g. constipation, orthostatic hypotension, and urinary incontinence), excessive daytime sleepiness, hyposmia, hallucinations in nonvisual modalities, systematized delusions, and apathy, anxiety, and depression, with the addition of sense of presence phenomena (in the psychosis spectrum) and prolonged or recurrent delirium as supportive features. Also similarly, relative preservation of medial temporal lobe structures on CT/MRI, low occipital uptake on SPECT/PET perfusion/metabolism scans, and quantitative EEG showing slowing and dominant frequency variability are ‘potential’ biomarkers of MCI-LB, with the addition of insular thinning and gray matter volume loss on MRI. 26
Table 2.
Research criteria for the diagnosis of mild cognitive impairment with Lewy bodies (2020)..
| Probable MCI-LB: (1) Required criterion: Mild cognitive impairment as defined by the presence of: (1) subjective cognitive complaint (from patient, informant, or clinician), (2) impairment in 1 or more domains (typically attention-executive or visual processing), AND (3) preserved or minimally affected independence in functional abilities (2) Presence of ⩾2 core clinical features (± indicative biomarker) OR 1 core clinical feature + ⩾1 indicative biomarker(s) Possible MCI-LB: (1) Required criterion: Mild cognitive impairment as defined by the presence of: (1) subjective cognitive complaint (from patient, informant, or clinician), (2) impairment in 1 or more domains (typically attention-executive or visual processing), AND (3) preserved or minimally affected independence in functional abilities (2) Presence of 1 core clinical feature (no indicative biomarker) OR ⩾ 1 indicative biomarkers (no core clinical features) | |
| Core clinical features: 1. Fluctuating cognition with variations in alertness and attention 2. Recurrent visual hallucinations 3. REM sleep behavior disorder 4. Parkinsonism (presence of one or more of: bradykinesia, rest tremor, rigidity) |
Proposed biomarkers: 1. Reduced basal ganglia dopamine transporter uptake (SPECT or PET) 2. Abnormal (low uptake) 123iodine-MIBG myocardial scintigraphy 3. Polysomnographic confirmation of REM sleep without atonia |
Source: Adapted from McKeith et al. 26
MCI-LB, mild cognitive impairment with Lewy bodies; MIBG, metaiodobenzylguanidine; PET, positron emission tomography; REM, rapid eye movement; SPECT, single-photon emission computed tomography.
The criteria also include supportive clinical features and potential biomarkers that may be helpful in diagnosing MCI-LB but are not formally part of the criteria (see text).
While existing evidence is only sufficient to propose diagnostic criteria for MCI-LB, clinicians should be alert to other presentations that may signify an increased risk of subsequent development of DLB. Numerous case reports and series describe provoked or unprovoked delirium as a presenting feature of DLB, sometimes occurring in individuals without cognitive impairment and years before the DLB diagnosis. It remains unclear whether this reflects mistaking cognitive fluctuations for delirium or a greater vulnerability to delirium in individuals with prodromal DLB. The occurrence of delirium should prompt an assessment for other features that would suggest DLB and caution in antipsychotic use for the treatment of delirium, as antipsychotic medications can provoke hypersensitivity reactions in individuals with DLB. 26 Late-onset major depressive disorder and late-onset psychosis are potentially types of psychiatric-onset DLB. It can be difficult to differentiate prodromal DLB from late-onset psychiatric disturbance unrelated to Lewy pathology, however, and it remains unclear how to best differentiate these individuals. 26
Biomarkers
A biomarker is defined as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’. 27 Biomarkers can be used in clinical care to help with differential diagnosis and prognosis and in research to help with participant selection and assessing therapeutic response. Biomarker development for DLB is a priority re-emphasized by the NIH 2019 ADRD Summit 28 and a research need identified by individuals living with DLB and their caregivers, particularly for improved diagnosis. 29
Neuroimaging
Structural imaging with MRI is used in DLB to evaluate for findings suggestive of AD co-pathology and as part of a standard clinical dementia evaluation.6,15 Volumetric MRI assesses patterns of neuronal loss which can be a clue in differential diagnosis. Individuals with Lewy body spectrum disorders tend to have gray matter atrophy in the posterior parietal cortices with relative sparing of medial temporal lobe structures (commonly affected in AD). 30 Additional MRI techniques under investigation for use in DLB include susceptibility-weighted imaging, functional MRI, and MRI diffusion-tensor imaging. 30
DAT imaging can be performed with PET and SPECT techniques. Reduced DAT binding suggests nigrostriatal degeneration but is not specific to an exact parkinsonian diagnosis. Because reduced DAT binding would not be expected in AD, a positive DAT scan can be helpful to help diagnose DLB, 6 particularly if parkinsonism is equivocal on examination. 15 Research with a pathology cohort confirmed that DAT imaging accurately distinguishes individuals with Lewy body versus AD pathology, although 10% of individuals in the study met pathological criteria for Lewy body disease while having normal DAT imaging. 31
Generalized low uptake on SPECT/PET perfusion/metabolism scans with reduced occipital activity and/or the cingulate island sign on FDG-PET imaging is a supportive biomarker in the 2017 DLB criteria. 6 Research performed subsequent to criteria publication further support a role for FDG-PET in DLB diagnosis, with temporo-parietal and occipital hypometabolism and preservation of medial temporal lobe metabolism distinguishing DLB from AD. 30 The ‘cingulate island sign’, where individuals with LBD have a high ratio of glucose metabolism in the posterior cingulate region compared with the precuneus and cuneus, is also highly suggestive of DLB. 30
Amyloid and tau PET imaging are used in DLB – primarily on a research basis – to help with differential diagnosis or assess the degree of suspected AD co-pathology in individuals with the DLB clinical phenotype. 30 Specific binding patterns may distinguish between Lewy body disease and AD pathology. 30 A recent autopsy study found that that lower 11 C-Pittsburgh compound B (PiB) uptake on PET in individuals with probable DLB in life or Lewy body disease on autopsy accurately distinguished these cases from cases with AD or mixed pathology. 32 This and other studies suggest that over half of individuals with probable DLB have elevated amyloid deposition on PET scans. 32 As demonstrated in pathology studies, amyloid and tau findings (as assessed by imaging or CSF) associate with specific clinical presentations in individuals with probable DLB (e.g. evidence of tau pathology is associated with a lower frequency of DLB clinical features). 33 PET imaging for α-synuclein deposition is highly desired, but α-synuclein radiotracers are still in development and testing. Existing tracers are not adequately specific for synuclein. 30
Fluid biomarkers
To date, specialists have used CSF testing in DLB primarily on a research basis and primarily to assess for evidence of AD co-pathology using Aβ42, total tau (t-tau), and phosphorylated tau (p-tau) levels. 15 In individuals with Lewy body disease, higher t-tau/Aβ1-42 and lower Aβ1-42 levels predict increasing cerebral AD pathology at autopsy. 34 As noted earlier, abnormal amyloid and tau biomarkers (on imaging or CSF) associate with the clinical presentation of individuals with probable DLB 33 and may predict the rate of cognitive decline. 35
Multiple recent studies investigate the role of CSF α-synuclein real-time quaking-induced conversion (RT-QuIC) assays in individuals with DLB. In a study using both neuropathologically verified cases (n = 77) and clinical cases (n = 36), the α-synuclein RT-QuIC CSF assay had 93% sensitivity and 96% specificity for α-synucleinopathies versus non-α-synucleinopathies and 65% sensitivity and 100% specificity for a clinical diagnosis of possible or probable DLB versus probable AD dementia. 36 Another research group also showed high sensitivity and specificity of α-synuclein RT-QuIC assays in individuals with DLB versus normal controls. 37 In prodromal populations, α-synuclein RT-QuIC approaches separated individuals with MCI-LB from cognitively unimpaired controls and, to a lesser extent, individuals with MCI due to AD or unspecified MCI. 38 Numerous additional studies investigate these techniques in other synucleinopathies such as PD. In the United States, CSF testing for evidence of a synucleinopathy is available through a Food and Drug Administration ‘breakthrough device designation’, but this test is not currently covered by insurance providers and its role in routine clinical diagnosis is not yet established.
Plasma and serum α-synuclein levels are also under study as potential biomarkers in synucleinopathies. A 2011 study found that serum α-synuclein levels might distinguish individuals with DLB versus individuals with AD and normal controls, 39 but subsequent research (largely in PD) had varied results. Recently, researchers presented data comparing α-synuclein levels measured by enzyme-linked immunosorbent assay in individuals with DLB and PDD (n = 54) versus individuals with AD (n = 31) and normal controls (n = 28). Individuals with DLB and PDD had lower plasma α-synuclein levels. After eliminating outliers, the sensitivity and specificity of the test for identifying individuals with Lewy body dementia were 58% and 85%, respectively. 40 Blood biomarkers remain research-only at the present time.
Skin biopsy
In 2017, researchers published findings showing that skin biopsies from proximal (cervical) and distal (thigh and distal leg) sites demonstrated phosphorylated α-synuclein in the cutaneous nerves of individuals with DLB (n = 18) versus individuals with other dementias (n = 23) and healthy controls (n = 25). 41 Using skin biopsies to identify pathological α-synuclein continues to be an area of active research in DLB, PD, and other synucleinopathies,42–45 including prodromal states (RBD). 44 Immunofluorescence and RT-QuIC approaches to detecting α-synuclein in skin biopsies show comparable efficacy 46 and one study showed similar diagnostic accuracy when comparing RT-QuIC assays using CSF versus skin samples. 45 Commercial testing for synucleinopathies using skin punch biopsies is now available in the United States. As with CSF α-synuclein testing, the role that skin biopsy plays in routine clinical diagnosis of DLB is yet to be established.
Electroencephalography
Findings on EEG serve as a supportive biomarker in the DLB criteria 6 and a potential biomarker in the MCI-LB criteria. 26 A recent systematic review of the role of EEG in DLB identified 43 studies, most of which used quantitative EEG. 47 Slowing of the dominant EEG rhythm (<8 Hz) was identified in approximately 90% of individuals with DLB and only approximately 10% of individuals with AD dementia. Other results were heterogeneous, likely secondary to differences in EEG techniques and study design. 47 EEG is uncommonly used in clinical practice by DLB specialists in the United States, who cite a need for more research on the role of this technique, but practices may be different in Europe where much of the EEG research occurs. 15
Progression
No staging system for DLB currently exists. In a meta-analysis of studies comparing survival time postdiagnosis in individuals with DLB versus AD dementia, mean survival of individuals with DLB was 4.11 ± 4.10 years, shorter than in individuals with AD dementia (5.66 ± 5.32 years). 48 This estimate is similar to the median 3- to 4-year survival postdiagnosis reported in studies of individuals with DLB using different study designs.49,50 Experiences, however, are diverse, with both rapid and slowly progressive DLB forms. In one study, over 10% of individuals with DLB died less than 1 year after diagnosis, while another 10% lived more than 7 years after diagnosis, and a small percent lived more than 10 years after diagnosis. 49 Most individuals with DLB die of complications of the disease. Failure to thrive is the most commonly reported cause of death (65%), followed by swallowing difficulties associated with aspiration and pneumonia (23%; multiple causes of death allowed by study). 49 Suicide was the reported cause of death for 1% of individuals with DLB. 49 This should prompt clinicians to routinely assess for suicide risk factors such as psychiatric disorders, social disconnectedness, and access to lethal means (e.g. firearms) in people with DLB and treat possible contributors (e.g. psychosis and depression) when identified. 51
There is currently no DLB-specific guidance for predicting end of life. Clinicians are left using local regulations for hospice use in dementia (e.g. Medicare criteria in the United States), although these may not be sensitive. 52 In one study, only 40% of families reported that clinicians discussed what to expect at the end of life in DLB, and only 22% said it was discussed to a helpful degree. 49 Almost 80% of individuals with DLB used hospice prior to death in that study, although often this was only for the last few weeks. 49 Research is underway to better understand advanced stages of DLB and predictors of end of life. 53
Management
There are no disease-modifying therapies for DLB. Research attempting to slow the progression of neurodegeneration in synucleinopathies focuses primarily on individuals with PD. All attempts to develop effective disease-modifying agents for PD have failed to date, 54 however, with the possible exception of high-intensity exercise, which needs further study. 55
Treatment for DLB has a modest evidence base and relies on studies in PD and AD and expert consensus. 56 A key principle in DLB management is addressing the diverse symptoms that can be problematic in the disease, for example, cognitive, neurobehavioral, motor, and autonomic (Table 3). Based on 2020 guidance for treatment of Lewy body dementia generally (not just DLB), the cholinesterase inhibitors donepezil and rivastigmine have the most evidence for use for cognitive impairment, with mixed evidence for memantine. 56
Table 3.
Pharmacological therapies for dementia with Lewy bodies a .
| Symptom | Pharmacological options |
|---|---|
| Cognitive impairment | Cholinesterase inhibitors (best evidence for donepezil, rivastigmine) Memantine (evidence mixed) |
| Neuropsychiatric symptoms | Psychosis: Quetiapine, pimavanserin, clozapine Other neuropsychiatric symptoms: SSRIs, SNRIs, memantine |
| Parkinsonism | Levodopa preparations (e.g. carbidopa/levodopa) Zonisamide (adjunctive) |
| Autonomic dysfunction | Orthostatic hypotension: midodrine, fludrocortisone, droxidopa Constipation: Stool softeners, laxatives Sialorrhea: Botulinum toxin injections, glycopyrrolate Urinary dysfunction: Mirabegron |
| REM sleep behavior disorder | Melatonin, clonazepam; potentially memantine |
DLB, dementia with Lewy bodies; REM, rapid eye movement; SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors.
Nonpharmacological therapies and research-only approaches not included. Therapies may be ‘off label’ for use depending on location and local regulatory determinations. Level of evidence for use of each agent varies in DLB and some of these are pragmatic approaches rather than evidence-based. Treatment should be individualized.
Behavioral/neuropsychiatric symptoms in DLB can include psychosis (visual hallucinations, hallucinations in other modalities, systematized delusions), depression, anxiety, apathy, and aggression. 6 While the evidence specifically in DLB is lacking, nonpharmacological interventions (e.g. environmental modifications and music therapy) are recommended as the first-line approach for treating psychosis based on the low associated risk and evidence from other dementias. 56 Pharmacological therapy for psychosis is recommended only if symptoms are severe or distressing, and if triggers (e.g. infection) are excluded. When treatment is needed, systematic reviews suggest that cholinesterase inhibitors may have some benefit for psychiatric symptoms including psychosis, in addition to their indication for treating cognition. 56 If problematic psychosis persists despite cholinesterase inhibitor treatment, clinicians must weigh potential benefits of antipsychotic treatment with the risk of severe sensitivity reactions in individuals with DLB. There is minimal evidence regarding the efficacy of antipsychotic agents in Lewy body dementia, but quetiapine, clozapine, and pimavanserin are the three medications felt to be safest. 56 Clozapine requires frequent blood monitoring given agranulocytosis risks. Pimavanserin is currently approved only in the United States and only for the indication of PD psychosis. The HARMONY trial, published in 2021, was a phase III, double-blind, randomized, placebo-controlled pimavanserin discontinuation trial enrolling individuals with dementia-related psychosis from AD, PDD, DLB, frontotemporal dementia, or vascular dementia. Individuals in the trial received open-label pimavanserin for 12 weeks and those with study-defined improvement were randomized to then receive pimavanserin or placebo for up to 26 weeks. A relapse of psychosis occurred in 13% (12/195) of individuals in the pimavanserin group and 28% (28/99) of the placebo group [hazard ratio (HR) = 0.35; 95% confidence interval (CI) = 0.17–0.73; p = 0.005], prompting early termination of the study due to demonstration of efficacy at the interim analysis. 57 Seven percent of enrolled participants had DLB and 15% had PDD. The study was not designed to assess efficacy in individual subgroups but individuals with PDD showed efficacy (HR = 0.054, 95% CI = 0.017–0.175). There were insufficient participants with DLB for subanalysis. 57 Though depression, anxiety, and apathy are common in DLB, studies investigating optimal treatment are lacking. 56 Based on approaches to treating psychiatric symptoms in dementia more generally, options include mirtazapine, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, with care guided by individual symptoms, tolerability, and response. 6
Parkinsonism in DLB is generally treated with levodopa monotherapy, 56 as levodopa has the highest chance of efficacy in improving parkinsonism and the lowest risk of side effects that overlap with symptoms in DLB (e.g. psychosis and orthostasis). Levodopa is combined with dopa-decarboxylase inhibitors (e.g. carbidopa and benserazide) to limit peripheral side effects of levodopa and enhance levodopa levels in the plasma and brain. Zonisamide, commonly used as an anti-epileptic medication, has been approved for use for the treatment of parkinsonism in Japan since 2009. 58 The mechanism for this benefit is uncertain, but may relate to inhibition of monoamine oxidase-B. In a study published in 2020, individuals with DLB with parkinsonism had improved motor function when treated with zonisamide 25 or 50 mg/day as an adjunct to levodopa therapy, without associated exacerbation of psychiatric symptoms. 58 There are no formal studies evaluating the efficacy of therapy in individuals with DLB, but physical therapy, occupational therapy, speech therapy, and swallow evaluations are likely beneficial clinically for helping mobility, addressing fall risk and fall prevention, identifying helpful resources to assist function (e.g. shower bars, commodes, and bed rails), and addressing dysphagia.
Treating autonomic function in individuals with DLB is critical, but an evidence base for DLB-specific care is lacking. 56 For orthostatic hypotension, nonpharmacological therapies are first-line therapy, including rising slowly, increasing fluid intake, increasing salt intake (if no medical contraindication), and potentially using compression stockings or elevation of the head of the bed. If pharmacological therapy is needed, midodrine, fludrocortisone, and droxidopa are the most commonly used agents (where droxidopa is available) (Table 3). All three can contribute to supine hypertension, and fludrocortisone is also associated with electrolyte disturbances and edema. 56
When treating constipation in DLB, a first step is to identify and stop potentially contributing medications (e.g. anticholinergics). Dietary changes, hydration, and exercise can also be helpful. Over-the-counter stool softeners and laxatives are used when pharmacological intervention is needed. Management of gastroparesis includes drinking during meals, avoiding high fat foods, and walking after meals. If the risk-benefit analysis favors treatment, domperidone is available in some countries (but not the United States) for treating gastroparesis. Based on evidence in PDD, strategies for treating dysphagia in DLB include chin tucks during swallowing and honey-thickened fluids. 56 Treating sialorrhea is part of dysphagia treatment but can also improve quality of life in individuals with DLB generally. Approaches are based on studies in PD and where the highest degree of evidence supports botulinum toxin injections and glycopyrrolate.56,59 Urinary urgency, frequency, and incontinence are common symptoms in DLB, but research on optimal treatment strategies is lacking. Many pharmacological agents used to treat these symptoms have a high risk of cognitive side effects due to anticholinergic properties, precluding or limiting their use. Mirabegron is a β3-adrenoceptor agonist lacking typical anticholinergic side effects. A retrospective study of its use in PD suggested it was effective for symptoms relating to overactive bladder and well tolerated.56,60
Evidence for treatment of sleep disorders and excessive daytime sleepiness in DLB is lacking. Based on limited evidence, melatonin is the first-line therapy for symptoms of RBD. Clonazepam may be helpful but must be used cautiously given side effect risks. One study in DLB and PDD suggested memantine might be helpful. 56 Similarly, treating insomnia in DLB must balance benefits of improved sleep for the person with DLB and caregiver versus potential cognitive side effects. Managing excessive daytime sleepiness is difficult and largely relies on identifying potential contributors and approaches for good sleep hygiene. An open-label pilot study in individuals with DLB reported improvements with armodafinil.56,61
Both disease-modifying and symptomatic treatments are identified needs in DLB. 28 While there was a dearth of DLB studies for years, investigator-led and pharma-funded phase II clinical trials in DLB have increased over the last 5 years (Table 4). Areas of interest for potential disease modification include α-1-selective adrenergic blockers, based on a large cohort study suggesting a decreased risk of PD in individuals taking these medications. 62 Multiple tyrosine kinase inhibitors are under investigation (Table 4), although recent results in PD populations were discouraging.63,64 Multiple studies are investigating ambroxol (in DLB and PD) to increase glucocerebrosidase activity and protein levels. 65 Clenbuterol, a β2 adrenergic agonist, is under study in DLB and PD given preclinical studies suggesting that β2 adrenergic agonists might reduce synuclein production. 66
Table 4.
Phase II clinical trials for dementia with Lewy bodies since 2016.
| Goal a | Drug (mechanism of action) | ClinicalTrials.gov Identifier | Phase | Status a |
|---|---|---|---|---|
| Disease modification | Terazosin (α-1-selective adrenergic blocker) | NCT04760860 | Phase I/II | Not yet recruiting |
| K0706 (tyrosine kinase inhibitor) | NCT03996460 | Phase II | Recruiting | |
| Nilotinib (tyrosine kinase inhibitor) | NCT04002674 | Phase II | Recruiting | |
| Bosutinib (tyrosine kinase inhibitor) | NCT03888222 | Phase II | Active, not recruiting | |
| Ambroxol [molecular chaperone for the lysosomal enzyme glucocerebrosidase (GCase)] | NCT04405596 | Phase I/II | Not yet recruiting | |
| Ambroxol [molecular chaperone for the lysosomal enzyme glucocerebrosidase (GCase)] | NCT04588285 | Phase II | Recruiting | |
| CST-103/clenbuterol + CST-107/nadolol (clenbuterol is a β2 adrenergic agonist; combined with nadolol to reduce side effects) | NCT04739423 | Phase II | Recruiting | |
| Symptomatic (parkinsonism, gait, cognition, psychosis) | RVT-101/intepirdine (5-HT-6 receptor antagonist) | NCT02669433 NCT02928445 NCT02910102 |
Phase II | Completed 67 |
| Symptomatic (cognition) | E2027 (selective inhibitor of phosphodiesterase 9; goal to increase cyclic GMP levels) | NCT03467152 NCT04764669 |
Phase II | Completed (NCT03467152); recruiting (NCT04764669) |
| Symptomatic (cognition) | LY3154207/mevidalen (D1 receptor positive allosteric modulator) | NCT03305809 | Phase II | Completed |
| Symptomatic (cognition) | Neflamapimod [p38 MAP kinase alpha (p38α) inhibitor] | NCT04001517 | Phase II | Active, not recruiting |
| Symptomatic (cognition) | NYX-458 (NMDA receptor modulator) | NCT04148391 | Phase II | Recruiting |
| Symptomatic (psychosis, REM sleep behavior disorder) | Nelotanserin (selective antagonist at 5-HT2A serotonin receptor) | NCT02640729 NCT02708186 NCT02871427 |
Phase II | Completed (NCT02871427 terminated due to changes in development program) |
| Symptomatic (cognition, psychosis) | HTL0018318 (selective activation of the muscarinic acetylcholine receptor M1) | NCT03592862 | Phase II | Withdrawn (pending investigation of an unexpected animal toxicology finding) |
REM, rapid eye movement.
Goal other than safety/tolerability; there is some overlap as some agents are hoped to be disease-modifying but are studied initially for evidence of symptomatic effects. Status as of 13 September 2021.
In addition to these active disease-modifying trials, numerous trials of symptomatic therapies with diverse mechanisms of action are completed or underway (Table 4). Unfortunately, many of these studies, including the recently published HEADWAY-DLB trial, 67 showed no clear efficacy, although peer-reviewed publications for several studies remain lacking. While disappointing, these trials demonstrate that clinical trials in DLB are feasible, including in international settings. 67 Recently, the AscenD-LB study (NCT04001517) met its primary objective of improving cognition in individuals with DLB and a phase III study is planned. 68
Conclusions
Recent advances in DLB include updated diagnostic criteria; recognition of prodromal DLB states, including MCI-LB; identification of prognostic and end-of-life considerations; and an increase in disease-modifying and symptomatic therapy clinical trials. DLB remains underrecognized, however, and biomarkers to assist diagnosis, prognosis, and identification of co-pathology are needed. Therapies for slowing the disease and effectively treating its various symptoms also remain an unmet need.
Footnotes
Author contributions: MJA conceptualized the review, drafted and revised the article, and approved the final version for publication.
Conflict of interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MJA is supported by grants from the NIA (R01AG068128, P30AG047266) and the Florida Department of Health (grant 20A08). Lewy body dementia research at the University of Florida is also supported by the Dorothy Mangurian Lewy Body Dementia Research Fund and the Raymond E. Kassar Research Fund for Lewy Body Dementia. MJA serves as an investigator for a Lewy Body Dementia Association Research Center of Excellence. She serves as a DSMB member for ATRI (Alzheimer’s Therapeutic Research Institute)/ACTC (Alzheimer’s Clinical Trials Consortium) and Alzheimer’s Disease Cooperative Study (ADCS). She receives publishing royalties for Parkinson’s Disease: Improving Patient Care (Oxford University Press, 2014).
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Melissa J. Armstrong  https://orcid.org/0000-0002-2163-1907
https://orcid.org/0000-0002-2163-1907
References
- 1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996; 47: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 2. Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the state of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002; 16: 203–212. [DOI] [PubMed] [Google Scholar]
- 3. Goodman RA, Lochner KA, Thambisetty M, et al. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement 2017; 13: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 1990; 40: 1–8. [DOI] [PubMed] [Google Scholar]
- 5. Savica R, Beach TG, Hentz JG, et al. Lewy body pathology in Alzheimer’s disease: a clinicopathological prospective study. Acta Neurol Scand 2019; 139: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berg D, Postuma RB, Bloem B, et al. Time to refine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 2014; 29: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–1601. [DOI] [PubMed] [Google Scholar]
- 10. Postuma RB, Berg D, Stern M, et al. Abolishing the 1-year rule: how much evidence will be enough. Mov Disord 2016; 31: 1623–1627. [DOI] [PubMed] [Google Scholar]
- 11. Boeve BF, Dickson DW, Duda JE, et al. Arguing against the proposed definition changes of PD. Mov Disord 2016; 31: 1619–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas AJ, Taylor JP, McKeith I, et al. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry 2017; 32: 1280–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord 2010; 16: 388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ngo J, Holroyd-Leduc JM. Systematic review of recent dementia practice guidelines. Age Ageing 2015; 44: 25–33. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong MJ, Irwin DJ, Leverenz JB, et al. Biomarker use for dementia with Lewy body diagnosis: survey of US experts. Alzheimer Dis Assoc Disord 2021; 35: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol 2021; 141: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Irwin DJ, Hurtig HI. The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism 2018; 8: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferman TJ, Aoki N, Boeve BF, et al. Subtypes of dementia with Lewy bodies are associated with alpha-synuclein and tau distribution. Neurology 2020; 95: e155–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018; 14: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Oliveira FF, Miraldo MC, de Castro-Neto EF, et al. Associations of neuropsychiatric features with cerebrospinal fluid biomarkers of amyloidogenesis and neurodegeneration in dementia with Lewy bodies compared with Alzheimer’s disease and cognitively healthy people. J Alzheimers Dis 2021; 81: 1295–1309. [DOI] [PubMed] [Google Scholar]
- 21. Galbiati A, Verga L, Giora E, et al. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev 2019; 43: 37–46. [DOI] [PubMed] [Google Scholar]
- 22. Savica R, Boeve BF, Mielke MM. When do alpha-synucleinopathies start? An epidemiological timeline: a review. JAMA Neurol 2018; 75: 503–509. [DOI] [PubMed] [Google Scholar]
- 23. Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013; 81: 2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadiq D, Whitfield T, Lee L, et al. Prodromal dementia with Lewy bodies and prodromal Alzheimer’s disease: a comparison of the cognitive and clinical profiles. J Alzheimers Dis 2017; 58: 463–470. [DOI] [PubMed] [Google Scholar]
- 25. Cagnin A, Bussè C, Gardini S, et al. Clinical and cognitive phenotype of mild cognitive impairment evolving to dementia with Lewy bodies. Dement Geriatr Cogn Dis Extra 2015; 5: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020; 94: 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 28. Schneider J, Jeon S, Gladman JT, et al. ADRD summit 2019 report to the National Advisory Neurological Disorders and Stroke Council. In: Alzheimer’s disease-related dementias summit, 14–15 March 2019. Bethesda, MD: National Institutes of Health, https://www.ninds.nih.gov/sites/default/files/2019_adrd_summit_recommendations_508c.pdf (accessed 13 September 2021). [Google Scholar]
- 29. Armstrong MJ, Gamez N, Alliance S, et al. Research priorities of caregivers and individuals with dementia with Lewy bodies: an interview study. PLoS ONE 2020; 15: e0239279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scamarcia PG, Agosta F, Caso F, et al. Update on neuroimaging in non-Alzheimer’s disease dementia: a focus on the Lewy body disease spectrum. Curr Opin Neurol 2021; 34: 532–538. [DOI] [PubMed] [Google Scholar]
- 31. Thomas AJ, Attems J, Colloby SJ, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 2017; 88: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kantarci K, Lowe VJ, Chen Q, et al. Beta-amyloid PET and neuropathology in dementia with Lewy bodies. Neurology 2020; 94: e282–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreira D, Przybelski SA, Lesnick TG, et al. Beta-amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 2020; 95: e3257–e3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irwin DJ, Xie SX, Coughlin D, et al. CSF tau and beta-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018; 90: e1038–e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdelnour C, van Steenoven I, Londos E, et al. Alzheimer’s disease cerebrospinal fluid biomarkers predict cognitive decline in Lewy body dementia. Mov Disord 2016; 31: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 36. Bongianni M, Ladogana A, Capaldi S, et al. Alpha-synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol 2019; 6: 2120–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bargar C, Wang W, Gunzler SA, et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol Commun 2021; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossi M, Baiardi S, Teunissen CE, et al. Diagnostic value of the CSF alpha-synuclein real-time quaking-induced conversion assay at the prodromal MCI stage of dementia with Lewy bodies. Neurology 2021; 9: e930–e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laske C, Fallgatter AJ, Stransky E, et al. Decreased alpha-synuclein serum levels in patients with Lewy body dementia compared to Alzheimer’s disease patients and control subjects. Dement Geriatr Cogn Disord 2011; 31: 413–416. [DOI] [PubMed] [Google Scholar]
- 40. Senanarong V, Wachirutmangur L, Rattanabunnakit C, et al. Plasma alpha synuclein (a-syn) as a potential biomarker of diseases with synucleinopathy. Alzheimers Dement 2020; 16: e044409. [Google Scholar]
- 41. Donadio V, Incensi A, Rizzo G, et al. A new potential biomarker for dementia with Lewy bodies: skin nerve alpha-synuclein deposits. Neurology 2017; 89: 318–326. [DOI] [PubMed] [Google Scholar]
- 42. Manne S, Kondru N, Jin H, et al. Blinded RT-QuIC analysis of alpha-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 2020; 35: 2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Z, Becker K, Donadio V, et al. Skin alpha-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 2021; 78: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al-Qassabi A, Tsao TS, Racolta A, et al. Immunohistochemical detection of synuclein pathology in skin in idiopathic rapid eye movement sleep behavior disorder and parkinsonism. Mov Disord 2021; 36: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mammana A, Baiardi S, Quadalti C, et al. RT-QuIC detection of pathological alpha-synuclein in skin punches of patients with Lewy body disease. Mov Disord 2021; 36: 2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donadio V, Wang Z, Incensi A, et al. In vivo diagnosis of synucleinopathies: a comparative study of skin biopsy and RT-QuIC. Neurology 2021; 96: e2513–e2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Law ZK, Todd C, Mehraram R, et al. The role of EEG in the diagnosis, prognosis and clinical correlations of dementia with Lewy bodies – a systematic review. Diagnostics 2020; 10: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller C, Soysal P, Rongve A, et al. Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: a meta-analysis of longitudinal studies. Ageing Res Rev 2019; 50: 72–80. [DOI] [PubMed] [Google Scholar]
- 49. Armstrong MJ, Alliance S, Corsentino P, et al. Cause of death and end-of-life experiences in dementia with Lewy bodies. J Am Geriatr Soc 2019; 67: 67–73. [DOI] [PubMed] [Google Scholar]
- 50. Larsson V, Torisson G, Londos E. Relative survival in patients with dementia with Lewy bodies and Parkinson’s disease dementia. PLoS ONE 2018; 13: e0202044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Armstrong MJ, Sullivan JL, Amodeo K, et al. Suicide and Lewy body dementia: report of a Lewy body dementia association working group. Int J Geriatr Psychiatry 2021; 36: 373–382. [DOI] [PubMed] [Google Scholar]
- 52. Mitchell SL. Advanced dementia. N Engl J Med 2015; 372: 2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Armstrong MJ, Paulson HL, Maixner SM, et al. Protocol for an observational cohort study identifying factors predicting accurately end of life in dementia with Lewy bodies and promoting quality end-of-life experiences: the PACE-DLB study. BMJ Open 2021; 11: e047554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lang AE, Espay AJ. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov Disord 2018; 33: 660–677. [DOI] [PubMed] [Google Scholar]
- 55. Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol 2018; 75: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taylor JP, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol 2020; 19: 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tariot PN, Cummings JL, Soto-Martin ME, et al. Trial of pimavanserin in dementia-related psychosis. N Engl J Med 2021; 385: 309–319. [DOI] [PubMed] [Google Scholar]
- 58. Murata M, Odawara T, Hasegawa K, et al. Effect of zonisamide on parkinsonism in patients with dementia with Lewy bodies: a phase 3 randomized clinical trial. Parkinsonism Relat Disord 2020; 76: 91–97. [DOI] [PubMed] [Google Scholar]
- 59. Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 2019; 34: 180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peyronnet B, Vurture G, Palma JA, et al. Mirabegron in patients with Parkinson disease and overactive bladder symptoms: a retrospective cohort. Parkinsonism Relat Disord 2018; 57: 22–26. [DOI] [PubMed] [Google Scholar]
- 61. Lapid MI, Kuntz KM, Mason SS, et al. Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with Lewy bodies: a pilot study. Dement Geriatr Cogn Disord 2017; 43: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simmering JE, Welsh MJ, Liu L, et al. Association of glycolysis-enhancing alpha-1 blockers with risk of developing Parkinson disease. JAMA Neurol 2021; 78: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pagan FL, Hebron ML, Wilmarth B, et al. Nilotinib effects on safety, tolerability, and potential biomarkers in Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol 2020; 77: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simuni T, Fiske B, Merchant K, et al. Efficacy of nilotinib in patients with moderately advanced Parkinson disease: a randomized clinical trial. JAMA Neurol 2021; 78: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rongve A, Holst-Larsen E, Steingildra S, et al. The ANeED study: ambroxol in new and early dementia with Lewy bodies. Alzheimers Dement 2020; 16: e042589. [Google Scholar]
- 66. Mittal S, Bjørnevik K, Im DS, et al. Beta2-adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science 2017; 357: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lang FM, Kwon DY, Aarsland D, et al. An international, randomized, placebo-controlled, phase 2b clinical trial of intepirdine for dementia with Lewy bodies (HEADWAY-DLB). Alzheimers Dement 2021; 7: e12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. EIP Pharma. EIP Pharma announces positive phase 2 results for neflamapimod in mild-to-moderate dementia with Lewy bodies, https://www.eippharma.com/news/eip-pharma-announces-positive-phase-2-results-for-neflamapimod-in-mild-to-moderate-dementia-with-lewy-bodies-dlb/ (2020, accessed 13 September 2021).