Abstract
Mulberry extract from Fructus Mori contains an anthocyanin pigment and has been widely used as a food additive in China and other Eastern Asian countries. Only few research has been done on toxicological profiling of mulberry extract for its safety evaluation; however, the data is inconclusive. In the current study, mulberry extract of 4200, 1400, or 466 mg/kg were orally administrated to Sprague Dawley rats for 90 consecutive days followed by a recovery period of 28 days. No abnormalities were detected in body weights, food intake, ophthalmological, hematological, coagulation, clinical chemistry, and organ weights parameters. Discoloration of urine (red, purple, and brown) and feces (black), along with bedding material (purple) were observed in the 4200 mg/kg group. Further, microscopic examination revealed brown granules in the renal tubular cells for rats in 4200 and 1400 mg/kg groups. Since these changes were associated with excretory effect of the extract, the No Observed Adverse Effect Level was determined to be 4200 mg/kg, which was equivalent to the 1058.5 mg/kg of anthocyanin.
Keywords: mulberry extract, anthocyanin, alkaloids, sub-chronic toxicity, safety assessment
What do we already know about this topic?
Mulberry extract is widely used food additive; however, current data is insufficient for its toxicological evaluation.
How does your research contribute to the field?
Our data of sub-chronic toxicity study shows potential abnormalities in renal tubular cells and NOAEL of mulberry extract at a dose of 4200 mg/kg (or anthocyanin of 1058.5 mg/kg).
What are your research’s implications towards theory, practice, or policy?
The sub-chronic toxicity and NOAEL obtained in this investigation for mulberry extract are pivotal to its risk assessment, based on the FDA Guidance for Industry (FDA-2020-D-1936).
Introduction
Mulberry plant (Morus alba L.) grows in Eastern Asian countries like Korea and China. For centuries, this plant is regarded as a traditional medicine for treating diseases such as inflammation, cancer, and diabetes. 1 Mulberry extract or so-called mulberry red is extracted from mulberry fruit or leaves and has been widely used as a food additive to improve flavor in fruit cake, jelly, fruit wine, juice, and beverage. According to the China National Standards (GB 2760-2014: National Food Safety for Standards for Food Additives), addition of mulberry red should be less than 5.0 g/kg of total weight. The main constituents of mulberry extract pigments contains anthocyanins, 2 which are cyanidin-3-O-rutinoside (C3R) and cyanidin-3-O-glucoside (C3G), accounting 50% and 40% of total anthocyanin, respectively. 3
The pharmacological effect of mulberry extract and its components are explored in the recent years. Total mulberry extract alleviates lipid peroxides by increasing activities of superoxide dismutase and glutathione in the liver and reduces pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 4 in hypertension and type 2 diabetes mellitus. 5 Anthocyanins, which is the main active ingredients of mulberry extracts, demonstrate several biological activities, such as antioxidant, anti-inflammatory, and anti-cancer.6-8 The C3G, a major component of anthocyanins, possess in-vitro oxygen radical absorbance capacity, while demonstrating in-vivo protection effect against oxidative stress-induced hepatic ischemia-reperfusion via nuclear factor kappa B inhibition in mitogen activated protein kinase pathways.9–11 Likewise, C3R, the other most abundant component, showed protective effects against protein glycation and oxidation, 12 in methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed, 13 as well as in the intestinal lipid digestion and absorption. 14
Though mulberry extracts as a food additive have potential pharmacological effect and the various applications, its safety assessment has not been addressed sufficiently. In the year of 2018, Li et.al. have reported good safety profile in acute, subacute, and genotoxicity studies of mulberry extract. Whereas the No Observed Adverse Effect level (NOAEL) has been defined as 7.5 g/kg at the 30-day fed subacute toxicity study. 15 However, acute or subacute toxicity studies are insufficient to assess the safety profiles of mulberry extracts as food additive due to its widespread and long-term use. According to the Chinese National Standard (2014) GB2760-2014, the proportion of mulberry red should be less than 5.0 g/kg total diet weight, which is classified as Concern Levels High (III) chemical according to the Food and Drug Administration (FDA) Guidance for Industry: Summary Table of Recommended Toxicological Testing for Additives Used in Food (Docket Number: FDA-2020-D-1936, issued in June 2006). Accordingly, a package of comprehensive toxicological studies, including genetic toxicity, short-term toxicity, sub-chronic toxicity, chronic toxicity carcinogenicity, reproduction, and developmental toxicity studies, are recommended to performed in Good laboratory practice (GLP) conditions.
To fill the gap in the knowledge, a sub-chronic toxicity study was performed for toxicity profiling of mulberry red. The study was performed in accordance with FDA Redbook 2000: IV.C.4.a. Sub-chronic Toxicity Studies with Rodents (issued on November 2003) and Organization for Economic Co-operation and Development (OECD): Repeated Dose 90-Day Oral Toxicity Study in Rodents TG 408), a 3-months toxicity study of mulberry extract in rats has been performed in compliance with the FDA GLP regulations (FDA 21 CFR Part 58) and OECD Principles of Good Laboratory Practice. In this study, the mulberry extract was firstly characterized according to applicable requirements, then be orally administered to SD rats by gavage at dose levels of 0, 466, 1400, and 4200 mg/kg/day for 90 consecutive days, followed by 28 days of recovery period. Therefore, the study aims to determine the potential toxicity and NOAEL to understand the risk assessment of mulberry extracts.
Methods
Chemicals
The test article used in this study was extracted from mulberry fruit. The extraction was performed by Huzhou Biological Technology Co., Ltd (Huzhou, Zhejiang, China) using the method described elsewhere. 16 Briefly, the extraction solution was filtered using a filter membrane, concentrated, and then dried to obtain mulberry extract powder (Batch No.190328). The extract powder was characterized according to the Chinese Food Safety Standard for Food Additives (Standard No. GB-1886.345) and Korea Food Additives Code 2019 (INS No. 163). The overall content, their description, mesh size, ash content, loss on drying, purity (arsenic and lead), and microbial analysis (yeast and mold, E.coli, and Salmonella) were included in the certificate.
The doses were calculated based on the weekly body weight of animals. Whereas, for each dose, the test article was dissolved in ultrapure water and temporarily stored at room temperature until the administration of extract within 4 hours before dosing of preparation.
Animal and Housing Condition
Sprague Dawley (SD) rats, aged 4–5 weeks, were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. The animals were housed ad-libitum (5 rats/cage) at 20°C–26°C, relative humidity of 40%–70%, and 12 h/day of light period, with air exchange at least 15 times per hour. Detailed examination of all animals was conducted once daily during 6-day’s acclimatization period by a staff veterinarian.
Sub-Chronic Toxicity Design
The sub-chronic toxicity of mulberry extract was evaluated in compliance with US FDA 21 CFR Part 58, Good Laboratory practice for non-clinical studies, and the OECD series on principles of Good Laboratory Practice and Compliance Monitoring No.1: OECD Principle of Good laboratory Practice. The execution and interpretation of the study was done by the qualified study director quality control inspectors, pathologist, and contributed scientist.
In our laboratory, mulberry extract had a maximal solubility of 210 mg/mL in water. However, at concentration more than 210 mg/mL, it appeared as a suspension or paste. The highest orally administration volume for rats was 20 mL/kg; therefore, 4200 mg/kg was selected for the high dose group, whereas 1400 mg/kg and 466 mg/kg were set as the middle and low dose group, respectively. Totally, 104 SD rats aged 4–5 week with body weights ranging from 68–87 g were included in the study. Rats were then randomly divided into 4 groups: vehicle control group (deionized water), low dose group, middle dose group, and high dose group. All animals were orally administrated with either deionized water or the test article for 90 consecutive days, where ten male and ten female rats in each group were sacrificed at the end of dosing period. For the vehicle control and high dose group, additional 6 males and 6 females were sacrificed after 28-day’s recovery.
Animals were observed daily for altered symptoms in clinical, behavioral activities, glandular secretion, respiration, and other parameters. Whereas the body weights and food consumption were measured at least once a week. Ophthalmologic examination of conjunctiva, cornea, iris, and lens was performed at pre-treatment, end of dosing period, and end of recovery period by using YZ25C ophthalmoscopy (66 Vision Tech Co., Ltd, Suzhou, Jiangsu, China) and KJ5S1 slit lamp (66 Vision Tech Co., Ltd, Suzhou, Jiangsu, China). Tropicamide eye drop (Santen Pharmaceutical Co., Ltd Shiga Plant, Japan) was used as a mydriatics for the ophthalmological examination.
Hematology, Coagulation, and Clinical Chemistry
Rats were fasted overnight but had free access to water before the necropsy. Animals were anesthetized by 30 mg/kg pentobarbital sodium, and then blood samples were collected into Ethylene Diamine Tetraacetic Acid (EDTA)-K2 anticoagulant tubes from the abdominal aorta for hematological examination using an automatic blood analyzer (ADVIA 2120i, Bayer, Germany). Additional blood sample was collected into trisodium citrate anticoagulant tubes for coagulation profile by using an automated coagulometer (CA-1500, Sysmex, Germany). Following parameters were studied: white blood cell count (WBC), red blood cell (RBC), hemoglobin concentration (HGB), hematocrit (HCT), platelet count (PLT), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet distribution width (relative number) (PDW), platelet packed volume (relative number) (PCT), red blood cell volume distribution width (RDW), mean platelet volume (MPV), red blood cell hemoglobin distribution width (absolute number, HDW), basophils% (BAS%), eosinophil (EOS%), lymphocyte% (LYMP%), monocytes% (MONO%), neutrophil% (NEU%), large unidentified cells% (LUC%), prothrombin time (PT), and activated partial thromboplastin time (APTT).
To prepare serum samples, non-heparinized blood samples were allowed to coagulate for 30 minutes and then centrifuged for 10 minutes. Thereafter, the sera were measured using an automatic biochemical analyzer (7180, Hitachi, Japan) for the following parameters: alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total cholesterol (CHO), triglyceride (TG), urea nitrogen (BUN), glucose (Glu), albumin (ALB), globulin (GLO), creatinine (CREA), albumin (ALB), creatine phosphate kinase (CPK), calcium (Ca), and total protein (TP), chloride (Cl−), sodium (Na+), and potassium (K+).
Urinalysis
At the end of dosing and recovery period, overnight urine samples were collected over approximately a 20-h period using stainless-steel metabolism cages. The urine samples were analyzed by an automated analyzer (Uritest-500B Urine Analyzer, Inc, Kyoto, Japan) or microscope (Olympus, Japan) for the following parameters: color, turbidity, urine sediment, specific gravity, pH, WBC, nitrite, vitamin C, protein, glucose, ketones, urobilinogen, and bilirubin.
Pathology Examinations
At necropsy days, animals were anesthetized by 30 mg/kg pentobarbital sodium and then sacrificed by exsanguination from the abdominal aorta. At first, gross necropsy was performed for all organs including external surfaces, orifices, thoracic, and abdominal cavities. Secondly, brain, thymus, heart, livers, spleen, kidneys, adrenal gland, uterus, ovaries, testes, and epididymides were collected for the organ weight and relative weights calculation. Lastly, the following organs and tissues were preserved in 10% neutral buffered formalin for histopathological examinations: adrenals, brain (representative regions including cerebrum, cerebellum, and medulla), colon, duodenum, epididymides, heart, jejunum, ileum, lung (with main-stem bronchi), liver, spleen, kidneys, mesenteric lymph node, ovaries, pancreas, pituitary, prostate, rectum, stomach, testes, thymus, thyroid, urinary bladder, uterus with cervix, and tissues with any gross lesions. Following at least 7 days of fixation, the organs were subjected to paraffin embedding, sectioning, hematoxylin–eosin staining, and histopathological examination.
Statistical Analysis
The statistical analyses were conducted by using SPSS statistic 21.0. The body weights, food intake, hematological, coagulation, clinical chemistry, and organ weight parameters were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett T test for pairwise comparison. P-value of less than .05 (P < .05) was considered statistically significant.
Results
Characterization of Mulberry Extracts
The final product of mulberry extract powder had purple black appearance. The anthocyanin was characterized as 25.2% of the total extracts weight (Table 1). The results of ash content, loss of drying, arsenic, and lead were within the applicable requirements of the Korea and/or Chinese Standards. Moreover, testing items that did not have either standard such as mesh size, total heavy metals, total plate counts, yeast and mold, E.coli, as well as Salmonella were tested according to the internal standards of the laboratory, all obtained results were acceptable and included in the certificate.
Table 1.
Characterization of the Mulberry Extract Powder.
| Items | Results | Chinese GB-1886.345 | Korea food additives code 2019 |
|---|---|---|---|
| Description | Purple black powder | Purple red/purple black powder | Dark red powder |
| Anthocyanidin a | 25.2% | Not applicable | Not applicable |
| Mesh size b | 100% pass 80 mesh | Not applicable | Not applicable |
| Ash content | 4.0% | ≤5.0% | Not applicable |
| Loss of drying | 4.1% | ≤8.0% | Not applicable |
| Total heavy metal b | ≤20 ppm | Not applicable | Not applicable |
| As | ≤1.0 ppm | ≤1.0 ppm | ≤4.0 ppm |
| Pb | ≤2.0 ppm | ≤2.0 ppm | ≤10.0 ppm |
| Total plate count b | ≤10 000 cfu/gm | Not applicable | Not applicable |
| Yeast and mold b | ≤1000 cfu/gm | Not applicable | Not applicable |
| E.coli b | Negative | Not applicable | Not applicable |
| Salmonella b | Negative | Not applicable | Not applicable |
Characterization was performed according to the method described in Chinese Food Safety Standard for Food Additives (Standard No. GB-1886.345) and Korea Food Additives Code 2019 (INS No. 163).
aMethod was described in the standards but without any acceptance criteria.
bInternal standard made by the laboratory but not included in either of the national standard.
Clinical Observations
Discolored urine (red, purple, and brown), feces (black), and cage bedding (purple) were observed in the high dose group. Other findings such as hair loss and occasional transient salivation was also observed after dosing but were considered as spontaneous or handling related and not related to the test article. In ophthalmological examination, except for one sporadic conjunctiva congestion noted in the right eye of a male animal of the high dose group, no test article-related adverse effect was observed.
Body Weights and Food Consumption
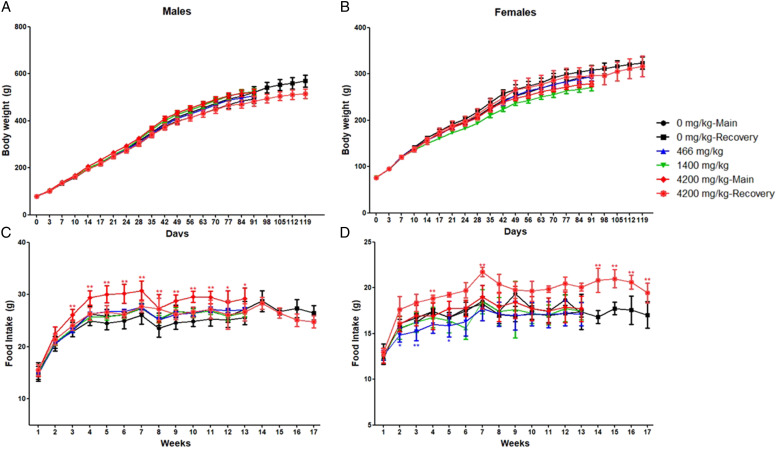
No changes in body weight were noted for the test article group when compared with the concurrent vehicle control group for both males and females (Figure 1). During the study period, food consumption in the 4200 mg/kg group was slightly higher than that in the vehicle control group (P ≤ .05 or P ≤ .01). Whereas females in the 1400 mg/kg group had a slightly lower food consumption than those in the 0 mg/kg group (Figure 1).
Figure 1.
Effect of mulberry extract on the body weights and food intakes. Body weights were measured twice weekly; food consumptions were measured. Data was presented as Meat±Standard deviations and analyzed using a one-way ANOVA followed by Dunnett T test for pairwise comparison. *P ≤ .05, ** P ≤ .01 when compared with the vehicle control group.
Clinical Pathology (Hematology, Coagulation, Clinical Chemistry, and Urinalysis)
At the end of dosing period, hematological and coagulation examination showed significant increases of NEU% and PT in male animals for the low and high dose group, as well the decreased LYM% in male for the high dose group, as compared with the vehicle control group. Meanwhile, increased BAS% was noted in the female animals of the high dose group. At the end of recovery period, significant decreases of RBC, PLT, WBC, LYM, MONO%, BAS%, and PT, but increase of MCH were noted in males of the high dose group, whereas, PDW% and PT were increased in females of the high dose group. However, these changes were regarded of no toxicological significance, as they were minimal, sporadic, no dose dependent, and within the background value of the test facility (Supplementary Table 1 and Supplementary Table 2).
For clinical chemistry, compared with the vehicle control group, significant decrease in ALT was noted in males of the high dose group at the end of dosing period. While female animals had significant BUN increase at the end of recovery period. However, these changes were regarded of no toxicological significance, as they were minimal, sporadic, no dose dependent, and within the background value of the test facility (Supplementary Table 3 and Supplementary Table 4).
For the urinalysis, no test article-related changes were observed either at the end of dosing or recovery period.
Pathological Examination
At necropsy, no changes were observed either at the end of dosing period or recovery period for any animals upon macroscopic examination.
Results of organ weights showed no statistical differences between the test article and the vehicle control group. However, a slight increase of liver weight in males and a decrease of ovary weight in females were noted for the high dose group (Supplementary Table 5).
Microscopically, mild to moderate renal tubular pigmentation was noted in all animals in the high dose group at the end of the dosing period. Minimal renal tubular pigmentation was also noted in four female animals of the middle dose group. As shown in the Figure 2, renal tubular cell of high dose group animals had lots of densely distributed brown granules (Figures 2(B) and 2(D)), while there were no brown granules observed in animal of the vehicle control group. Meanwhile, no other renal abnormalities, such as tubular necrosis and kidney injury, were seen in either of the groups. Further, no changes were observed for all organs at the end of recovery period.
Figure 2.
Microscopic changes in renal tubes of rats treated with mulberry extract. The renal tubules images at the end of dosing period after 90 day’s treatment of either ultrapure water or 4200 mg/kg mulberry extract. Panel A: 200× scan of animal in the control group; Panel B: 200× scan image of animal in the high dose group; Panel C: 400× scan of animal in the control group; Panel D: 400× scan of animal in the high dose group. The arrows in Panels B and D indicate pigmentation of mulberry sediment in the renal tubular cells. No pigment was noted in the vehicle control group.
Discussion
The potential toxicity of natural food additive has become an important growing issue for both regulatory and industrial agencies. Mulberry extract is widely used as natural food additive in food industry, though sufficient toxicological study results are scarce. 17 Therefore, this study investigated the toxicology profile for providing the risk assessment data of mulberry extract. The SD rats were dosed with mulberry extract for 90 consecutive days followed by 28 days’ recovery. Mortality and moribundity, clinical observations, body weights, food consumption, ophthalmic examinations, clinical pathology (hematology, coagulation, clinical chemistry, and urinalysis), organ weights, morphology, and histopathological examinations were performed. The NOAEL was also determined as 4200 mg/kg/day, which was the highest dose tested in the current investigation. Since anthocyanin account for 25.2% of the total mulberry extracts, 4200 mg/kg/day of the extract was equivalent to 1058.5 mg/kg/day of anthocyanin.
The ocular examination showed conjunctival congestion in one male animal. This anomaly is generally regarded as a common ophthalmological abnormality, arising due to hair contamination during handling procedure and not by the test article. However, sporadic but transient salivation in each of the groups might have been induced by the oral treatment procedure and cannot be considered as an adverse effect.
The test article appeared as a purple black powder, whereas the formulation was purple black solution after preparation in water. As a result, discolored urine (red, purple, and brown), feces (black), and cage bedding (purple) were observed due to the test article. Studies have reported that unchanged anthocyanins and its metabolites mainly get excreted through urine, bile, and feces.18,19 Hence, the discoloration was mainly attributed as an excretion effect and not an adverse effect. The renal tubular pigmentation (brown granules) observed in pathological examination along with urine discoloration also been considered as an excretion effect.
The other reason that the statistical changes of clinical pathological parameters been considered as non-toxicological significant was no correlated changes among the findings. For RBC-related parameters, RBC decrease (↓ 6% from the vehicle control) along with the MCH increase (↑ 5%) was noted in males of the 4200 mg/kg group at the end of recovery period, whereas no changes were observed at the end of dosing period; For WBC-related parameters, NEU% increase (↑ 41%) and LYM% decrease (↓ 6%) in males of the 466 mg/kg group, and WBC decrease (↓ 38%) and BAS% increase (↓ 48%) in males of the 4200 mg/kg group were observed, whereas no changes were observed for females. For PLT-related parameters, PLT decrease (↓ 21%) and PCT decrease (↓ 19%) in males and PDW decrease (↓ 4%) in females were noted at the end of recovery period, whereas no changes were observed at the end of dosing period. For coagulation parameters, PT increase (↑ 2%) was observed in males of the 1400 mg/kg group, whereas decrease (↓ 5% and 7%) in males and females of the 4200 mg/kg group. Similarly, the ALT decrease (↑ 19%), CHOL decrease (↓ 25%), and BUN increase (↑ 18%) were considered as spontaneous because no other changes in liver- or kidney-related biomarkers were observed.
Apart from renal cells pigmentation, no other abnormal changes were observed in either kidney or other organs. Besides, organ weight and/or organ coefficients, hematological, clinical chemistry examinations, and urinalysis were unremarkable. In addition, reports showed the pigmentation was considered as an excretory effect of the anthocyanins and its metabolites.18,19 Thus, the pigmentation did not have any adverse impact on the structure and function of kidney or other organs, whereas at the end of recovery period, no discoloration or pigmentation was noted in renal cells.
Slightly higher food consumption in males and females of the 4200 mg/kg, whereas the lower food consumption in females of the 1400 mg/kg were noted in this study. As shown in Figure 1, the differences were more likely to be caused by the biological deviations within the 4200 mg/kg than changes between groups. As previous reports showed no effect of mulberry extract on the appetite and food consumption,15,20,21 the causal relationship between food consumption and the test article need further investigation. Besides, the slight increase or decrease in food consumption had no impact on the body weight in both male and female rats, thus the changes were not considered as the adverse effect.
Conclusion
In conclusion, oral administration of mulberry extract once daily for 90 consecutive days at dose levels of 4200, 1400, and 466 mg/kg followed by a 28-day recovery period did not have any significant adverse effect. Although, renal tubular pigmentation and discharge coloration were seen in fed animals, it was an excretory effect of the test article rather than an adverse effect. Overall, for mulberry extract, the NOAEL was determined to be 4200 mg/kg/day, which is equivalent to the 1058.5 mg/kg/day of anthocyanin.
Supplemental Material
Supplemental Material, sj-pdf-1-inq-10.1177_00469580211056044 for A 90-day Sub-chronic Oral Toxicity Assessment of Mulberry Extract in Sprague Dawley Rats by Min Hong, Min Lu, Yimin Qian, Liping Wei, Yaqun Zhang, Xueying Pan, Hua Li, Huaying Chen and Naping Tang in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Acknowledgments
The authors thank Yimei Wang, Xin Wang, Yan Chen, and Caiyun Li from the facility for the study design, animal husbandry, dosing, necropsy, sample collection, and data analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Key R&D Program of China (Grant Number: 2017YFC1600200).
Ethical Approval Statement: The study was in compliance with all applicable local, national, and international guidelines of animal welfare. Study protocol and procedure were approved (approval number: 2019-086a) and monitored by the IACUC of the facility, which is an Association for Assessment and Accreditation of Laboratory Animal Care international (AAALAC)-accredited laboratory.
Supplementary Material: Supplementary material for this article is available online.
ORCID iD
Huaying Chen https://orcid.org/0000-0003-4084-733X
References
- 1.Park Y, An M, Kim J, Lim YH. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J Ethnopharmacol. 2020;251:112542. [DOI] [PubMed] [Google Scholar]
- 2.Du QZ, Zheng Jf, Xu Y. Composition of anthocyanins in mulberry and their antioxidant activity. J Food Compos Anal. 2008;21(5):390-395. [Google Scholar]
- 3.Zhou XJ, Zhu CT, Zhang LY, You S, Wu FA, Wang J. Enrichment and purification of red pigments from defective mulberry fruits using biotransformation in a liquid-liquid-solid three-phase system. Environ Sci Pollut Res Int. 2021;28(19):24432-24440. [DOI] [PubMed] [Google Scholar]
- 4.Yang HJ, Kim MJ, Kang ES, Kim DS, Park S. Red mulberry fruit aqueous extract and silk proteins accelerate acute ethanol metabolism and promote the anti-oxidant enzyme systems in rats. Mol Med Rep. 2018;18(1):1197-1205. [DOI] [PubMed] [Google Scholar]
- 5.Amin AR, Kassab RB, Abdel Moneim AE, Amin HK. Comparison among Garlic, Berberine, resveratrol, Hibiscus sabdariffa, Genus Zizyphus, Hesperidin, Red Beetroot, Catha edulis, Portulaca oleracea, and Mulberry Leaves in the Treatment of Hypertension and Type 2 DM. Nat Prod Commu 2020;15(4):1-24. doi: 10.1177/1934578x20921623. [DOI] [Google Scholar]
- 6.Chen B, Stephen Inbaraj BJN. Nanoemulsion and Nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients. 2019;11(5):1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlowska A, Oleszek W, Braca AJJoa, chemistry f. Quali-quantitative analyses of Flavonoids of Morus nigra L. and Morus alba L. (Moraceae) fruits. J Agric Food Chem. 2008;56(9):3377-3380. [DOI] [PubMed] [Google Scholar]
- 8.Adaku C, Skaar I, Byamukama R, Jordheim M, Andersen ØM. Anthocyanin profile and antioxidant property of anti-asthma flowers of Cordyline terminalis (L.) Kunth (Agavaceae). Nat Prod Commun 2020;15(5):1-7. doi: 10.1177/1934578X20922637. [DOI] [Google Scholar]
- 9.Tsuda T, Horio F, Osawa T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors. 2000;13(1-4):133-139. [DOI] [PubMed] [Google Scholar]
- 10.Vendrame S, Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr Rev. 2015;73(6):348-358. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Yoon Y, Yoon H, Park H, Song S, Yeum KJN. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9(10):1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thilavech T, Ngamukote S, Abeywardena M, Adisakwattana S. Protective effects of cyanidin-3-rutinoside against monosaccharides-induced protein glycation and oxidation. Int J Biol Macromol. 2015;75:515-520. [DOI] [PubMed] [Google Scholar]
- 13.Thilavech T, Abeywardena MY, Adams M, Dallimore J, Adisakwattana S. Naturally occurring anthocyanin cyanidin-3-rutinoside possesses inherent vasorelaxant actions and prevents methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed. Biomed Pharmacother. 2017;95:1251-1259. [DOI] [PubMed] [Google Scholar]
- 14.Thilavech T, Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Compl Alternative Med. 2019;19(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zhang X, Liang C, Hu J, Yu Z. Safety evaluation of mulberry leaf extract: acute, subacute toxicity and genotoxicity studies. Regul Toxicol Pharmacol. 2018;95:220-226. [DOI] [PubMed] [Google Scholar]
- 16.Jeong JC, Jang SW, Kim TH, Kwon CH, Kim YK. Mulberry fruit (Moris fructus) extracts induce human glioma cell death in vitro through ROS-dependent mitochondrial pathway and inhibits glioma tumor growth in vivo. Nutr Cancer. 2010;62(3):402-412. [DOI] [PubMed] [Google Scholar]
- 17.Oplatowska-Stachowiak M, Elliott CT. Food colors: existing and emerging food safety concerns. Crit Rev Food Sci Nutr. 2017;57(3):524-548. [DOI] [PubMed] [Google Scholar]
- 18.Borges G, Roowi S, Rouanet JM, Duthie GG, Lean ME, Crozier A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol Nutr Food Res. 2007;51(6):714-725. [DOI] [PubMed] [Google Scholar]
- 19.Czank C, Cassidy A, Zhang Q, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97(5):995-1003. [DOI] [PubMed] [Google Scholar]
- 20.Wu CT, Chiu CY, Huang CF, Peng FC, Liu SH. Genotoxicity and 28-day oral toxicity studies of a functional food mixture containing maltodextrin, white kidney bean extract, mulberry leaf extract, and niacin-bound chromium complex. Regul Toxicol Pharmacol. 2018;92:67-74. [DOI] [PubMed] [Google Scholar]
- 21.Yimam M, Jiao P, Hong M, et al. Repeated dose 28-day oral toxicity study of a botanical composition composed of Morus alba and Acacia catechu in rats. Regul Toxicol Pharmacol. 2018;94:115-123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-inq-10.1177_00469580211056044 for A 90-day Sub-chronic Oral Toxicity Assessment of Mulberry Extract in Sprague Dawley Rats by Min Hong, Min Lu, Yimin Qian, Liping Wei, Yaqun Zhang, Xueying Pan, Hua Li, Huaying Chen and Naping Tang in INQUIRY: The Journal of Health Care Organization, Provision, and Financing