Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in western countries, affecting 25–30% of the general population and up to 65% in those with obesity and/or type 2 diabetes. Accumulation of visceral adipose tissue and insulin resistance (IR) contributes to NAFLD. NAFLD is not an innocent entity as it not only may cause nonalcoholic steatohepatitis and cirrhosis but also contribute to cardiovascular morbidity and mortality. More and more people with type 1 diabetes (T1D) are becoming overweight and present with features of IR, but the prevalence and impact of NAFLD in this population are still unclear. The utility of noninvasive screening tools for NAFLD in T1D is being explored. Recent data indicate that based upon ultrasonographic criteria NAFLD is present in 27% (ranging between 19% and 31%) of adults with T1D. Magnetic resonance imaging data indicate a prevalence rate of 8.6% (ranging between 2.1% and 18.6%). There are, however, multiple factors affecting these data, ranging from study design and referral bias to discrepancies in between diagnostic modalities. Individuals with T1D have a 7-fold higher risk of cardiovascular disease (CVD) and cardiovascular mortality is the most prominent cause of death in T1D. Patients with T1D and NALFD are also more prone to develop CVD, but the independent contribution of NAFLD to cardiovascular events has to be determined in this population. Furthermore, limited data in T1D also point towards a 2 to 3 times higher risk for microvascular complications in those with NAFLD. In this article, we will discuss epidemiological and diagnostic challenges of NAFLD in T1D, explore the link between IR and NAFLD and chronic complications, and examine the independent contribution of NAFLD to the presence of macro-, and microvascular complications.
Keywords: NAFLD, type 1 diabetes, cardiovascular disease, renal disease, metabolic syndrome
Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of chronic liver disorders hallmarked by intrahepatic triglyceride accumulation in the absence of excessive alcohol consumption and other causes for steatosis such as use of steatogenic drug or chronic liver disorders associated with steatosis such as viral hepatitis, Wilson’s disease or alpha-1 antitrypsin deficiency. 1 NAFLD ranges from isolated or ‘simple’ steatosis, which is relatively benign, to more harmful nonalcoholic steatohepatitis (NASH). NASH can induce progressive fibrosis which can ultimately lead to cirrhosis, with a risk of decompensation and also increasing the risk of hepatocellular carcinoma (in up to 0.5–2.6% of those with NAFLD-cirrhosis). 2 The latter can, however, also occur in noncirrhotic NAFLD. 3 NAFLD is a complex heterogeneous condition with hepatic and systemic manifestations involving multiple pathways influenced by genetic, epigenetic, and environmental factors. 4 Accumulation of visceral adipose tissue (VAT) and ensuing insulin resistance (IR) contributes to NAFLD; hence, NAFLD is regarded as the hepatic manifestation of the metabolic syndrome. The pathophysiological relationship between NAFLD and IR is bidirectional.5,6 NAFLD is present in up to 65% of individuals with obesity and/or type 2 diabetes (T2D), whereas the global prevalence is estimated around 25%.7,8 Both obesity and T2D act as accelerators of disease progression, as the proportion of people that evolve towards NASH and/or fibrosis is greater in these risk groups. 9 However, NAFLD is not always associated with IR or the metabolic syndrome, which is referred to as NAFLD in lean people.10–12 Although the strongest genetic risk alleles for NAFLD, such as the I148M allele in PNPLA3 and the E167 K allele in TM6SF2, have been associated with an increased liver fat content and progression to NASH and significant fibrosis, they are not linked to IR or the metabolic syndrome, and are unexpectedly associated with a lower risk of cardiovascular disease (CVD).13–16
At present NAFLD is the second leading cause for liver transplantation in the United States and is expected to become the primary cause of liver transplantation in Europe.17,18 Moreover, NAFLD may also contribute to cardiovascular disease (CVD). Recent meta-analyses show an increased risk for incident CVD in all NAFLD stages, which increases with histological severity in terms of activity of the disease as well as fibrosis stage, in particular with the presence of significant fibrosis.19–21 As mentioned above, to elucidate the contribution of NAFLD to CVD, independent of the intrinsic contribution of the metabolic syndrome, genetic analyses may be warranted since some of the genes most robustly associated with NAFLD have been associated with an apparent protection from CVD, possibly via their effects on lipoprotein metabolism.13–16
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by pancreatic beta-cell destruction resulting in hyperglycaemia and lifelong insulin dependency. In contrast to the classical phenotype of the person with NAFLD, patients with T1D are typically considered lean and insulin sensitive. However, having T1D does not protect against overweight. Indeed, recent evidence demonstrates that more and more people with T1D are becoming overweight and present with features of IR due to sedentary lifestyle, caloric excess, and intensive insulin therapy,22,23 but the prevalence and impact of NAFLD in this population is still unclear. Based upon ultrasonographic criteria NAFLD is reported in 27% (ranging between 19% and 31%) of adults with T1D, while magnetic resonance imaging (MRI) data indicate a prevalence rate of 8.6% (ranging between 2.1% and 18.6%). 24 There are, however, multiple factors affecting the accuracy of these data including study design and referral bias, but also discrepancies in diagnostic accuracy of the diagnostic modalities.
Individuals with T1D have a 7-fold higher risk of CVD, arising 10 years earlier in life as compared to healthy controls, and being responsible for a significant quality of life reduction and a loss of approximately 11 years of life expectancy. Furthermore, an association exists between excess VAT, IR and micro-, and macrovascular complications.22,25–30 In addition, limited data in T1D indicate that patients with T1D and NALFD are more prone to develop CVD and present a 2 to 3 times higher risk for microvascular complications as compared to those without NAFLD.29,31,32
In this article, we will discuss epidemiological and diagnostic challenges of NAFLD in T1D, explore the link between IR and NAFLD and chronic complications, and examine the potential independent contribution of NAFLD to the presence of macro-, and microvascular complications (see Figure 1).
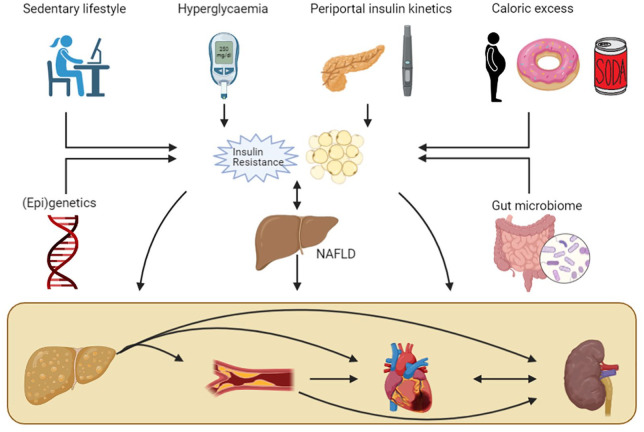
Figure 1.
The complex relationship between T1D, NAFLD, and chronic complications.
Sedentary lifestyle, hyperglycaemia and glycaemic variability, insulin kinetics in the portal circulation, caloric excess, genetic and epigenetic factors and the gut microbiome all potentially contribute to both visceral adipose tissue volume expansion and hepatic and peripheral IR in T1D. These risk factors increase both the prevalence of NAFLD and contribute to increased atherosclerosis and subsequent ischemic heart disease and nephropathy. NAFLD potentially contributes in an independent manner to these complications, increasing the total risk for cardiovascular and renal disease. Furthermore, the relationship between NAFLD and IR is bidirectional, strengthening their cumulative effect. Finally, IR increases the progression towards NASH with significant fibrosis, which in turn further increases the odds for cardiovascular or renal disease.
IR, insulin resistance; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; T1D, type 1 diabetes.
Methodology
A structured search was conducted using PubMed (MEDLINE), Web of Science and Google Scholar for studies reporting on T1D and NAFLD entering Medical Subject Headings (MeSH) terms: ‘Nonalcoholic Fatty Liver Disease’ or/and ‘type 1 diabetes mellitus’ combined with the subheadings ‘epidemiology’, ‘diagnostic imaging’, and ‘physiopathology’. We ranked articles based on their relevance and methodology. All abstracts were screened for relevance. The latest search was conducted in May 2021. Studies with a diagnosis based solely on abnormal liver enzymes were excluded. No other restrictions to type of diagnostic method were applied. All articles had to have a clear description of exclusion of patients with secondary causes of fatty liver disease to be included in the article database, in accordance with the definition of NAFLD. Language was restricted to English, articles written in other languages but with English translation were also included. Second, reference lists of relevant articles were searched for additional articles.
Pathogenesis of NAFLD in T1D
The pathogenesis of NAFLD is multifactorial, with multiple hits contributing sequentially and/or in parallel to the development of a fatty liver and steatohepatitis. 33 Fat accumulation in the liver occurs when the rate of hepatic lipogenesis due to increased hepatic free fatty acid (FFA) uptake and triglyceride synthesis exceeds the rate of triglyceride oxidation or efflux as very-low density lipoproteins (VLDLs). 34 Factors that may contribute to a fatty liver in T1D include ( 1 ) Acquired hepatic and peripheral IR, ( 2 ) hyperglycaemia-induced activation and upregulation of transcription factors that regulate intrahepatic lipid handling, ( 3 ) altered kinetics of insulin delivery to the liver, ( 4 ) lipoprotein abnormalities, and ( 5 ) nutritional factors5,35–43 (Figure 1). Genetic polymorphisms and alterations in gut microbial composition and functions have also been associated with NAFLD pathogenesis, but are unexplored so far in patients with T1D.
IR is often mentioned as the first hit in the pathogenesis of NAFLD, at least in the classical phenotype of metabolic syndrome-associated NAFLD, directly by increasing de novo lipogenesis and indirectly by increasing FFA flux to the liver via decreased peripheral inhibition of lipolysis. 44 Hepatic IR can be induced by several mechanisms that interfere with the insulin signalling cascade including hyperglycaemia, high concentrations of FFA, oxidative and endoplasmic reticulum stress, systemic and cellular inflammation [resulting in nuclear factor-kappa B (NF-KB) and c-Jun N-terminal kinase (JNK) pathway activation which inhibit phosphorylation of insulin receptor substrate 1 (IRS-1)], and genetic factors.
Hyperglycaemia induces IR via different pathways including the hexaminose pathway, protein kinase C pathway, sorbitol pathway, and accumulation of advanced glycation end products and oxidative stress–driven pathways.45,46 Furthermore, hyperglycaemia has been shown to increase both sterol regulatory element-binding proteins (SREBPs) and GLUT2 expression in hepatocytes. SREBPs are transcription factors that activate the expression of multiple genes dedicated to the synthesis and uptake of cholesterol, fatty acids, triglycerides, and phospholipids. 47 In the presence of high glucose levels, and independent of insulin, SREBPs such as SREBP-1c and carbohydrate-responsive element-binding protein (ChREBP) upregulate the expression of multiple lipogenic genes.37,48 Therefore, SREBPs and ChREBP may contribute to fatty liver development in T1D. 35 GLUT2 is a glucose transporter found primarily in the liver and β-cells. Several studies have demonstrated that liver GLUT2 expression is upregulated according to glycaemia and downregulated by insulin. 49 Furthermore, SREBP-1c mediates glucose-stimulated upregulation of GLUT2 gene expression. 36 Insulin decreases hepatic gluconeogenesis by suppressing gene expression of the key gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. 50 Insulin stimulates glycogenesis in the liver by activating glycogen synthetase. 51 If more glucose is taken up and/or produced by the hepatocytes than can be converted to glycogen, this additional glucose is moved towards pathways leading to synthesis of fatty acids. Thus, both hyperglycaemia and hypoinsulinaemia, which may characterize poorly-controlled T1D, may potentiate multiple pathways promoting sugar into fat conversion and lipid synthesis. 35
It is important to highlight the paradoxical increase in hepatic lipogenesis, since one would expect hepatic IR to promote hyperglycaemia, due to continuous gluconeogenesis, along with inhibition of lipogenesis. However, as outlined before, several other mechanisms can lead to increased lipogenesis in hepatocytes. 52 Furthermore, peripheral IR also needs to be taken into account, the latter leading to an increase in FFA flux from the periphery to the liver. In this regard, adipose tissue IR, mostly in relation to overweight, creating metabolically unhealthy fat depots, is increasingly considered to be relevant in T1D. 53 Adipose tissue inflammation is known to contribute to the progression of NAFLD, leading to the development of steatohepatitis and fibrosis, and its contribution to NAFLD in T1D warrants further study.54,55
Lipoprotein abnormalities are present in patients with T1D with poor metabolic control or acquired nephropathy. These abnormalities include, among others, increased serum triglycerides, increased cholesterol-to-triglyceride ratios within VLDLs, an increase in small, dense low-density lipoprotein particles and other qualitative changes in lipoproteins likely impairing their function. 43 These lipoprotein derangements are associated with hyperglycaemia and peripheral hyperinsulinaemia and could therefore contribute to hepatic fat accumulation in T1D.35,43
One potentially crucial difference between T1D and T2D is the portal concentration of insulin and its fluctuations over time. In T1D, insulin is delivered peripherally by means of subcutaneous insulin administration. This is substantially different from physiological insulin delivery directly into the portal circulation where 50–80% of insulin is subsequently cleared by the liver (first passage effect). 56 This altered exposure of hepatocytes to insulin concentrations might considerably change the rate of intrahepatic fat deposition. Wanless et al. compared liver histology of 11 patients with T1D who had received intraperitoneal insulin during peritoneal dialysis to controls not receiving intraperitoneal insulin during dialysis. Steatosis occurred in 10 out of 11 patients treated with intraperitoneal insulin compared to 0 out of 9 control patients. Mainly the subcapsular hepatocytes developed steatosis, supposedly be due to their proximity to the high concentrations of glucose and insulin in the peritoneal dialysate, stressing the steatogenic role of hyperinsulinaemia. 57 The primary cellular mechanism for hepatic uptake and degradation of insulin is receptor-mediated and is altered by IR.58,59 Interestingly, pinocytosis, which is a nonreceptor mediated uptake of insulin by hepatocytes, may be significantly upscaled in hyperinsulinaemia. 60 Hyperinsulinaemia will therefore regulate its own degradation, but not when IR is present. In normal conditions, insulin release in the portal vein is pulsatile, which is lowered in T2D, but completely absent in T1D. This loss of pulsatile action is known to alter insulin action in the liver, however, its direct effects on liver steatosis are not yet examined.38,61 The kinetics of insulin delivery may hence be a key factor leading to steatosis, but this also needs further study. This idea is strengthened by the differences seen in NAFLD prevalence between insulin-dependent and insulin-naïve T2D patients, the latter being higher compared to the first. 62
Aside from the molecular basis for NAFLD, special attention should be given to lifestyle factors. Sedentarism is an important risk factor for metabolic syndrome, and exercise is crucial for patients with T1D to ameliorate insulin sensitivity, obtain glycaemic control and keep a healthy weight.63,64 However, glycaemia remains difficult to control during exercise, and therefore many persons with T1D are reluctant to perform prolonged physical activity, mainly because of fear of hypoglycaemia. 63 A healthy diet is essential in the treatment of diabetes. Patients must try to avoid excessive intake of carbohydrates and fat to control their blood sugar, lipid profile and weight. 65
Another difference between T1D and T2D is that people with T1D are more prone to hypoglycaemia. Hypoglycaemia needs to be treated by intake of fast-acting carbohydrates such as glucose, dextrose and/or fructose. Fructose is a simple sugar that is present in fruit and honey but is also a major component of the two most commonly used sweeteners, namely sucrose (table sugar, a disaccharide of fructose and glucose), and high-fructose corn syrup (a mixture of fructose and glucose monosaccharides). Dietary fructose, sucrose, and high-fructose corn syrup have been shown to have a particular tendency to induce fatty liver.39–41 Further investigation of the independent role of dietary-induced fatty liver disease, specifically in patients with T1D patients, is therefore needed.
In addition, due to ‘defensive snacking’ in order to avoid possible impending hypoglycaemia, weight gain can occur, leading to higher insulin demands, thereby possibly increasing the risk of hypoglycaemia and thus initiating a vicious cycle. 23 In the DCCT trial, patients with intensive insulin therapy had a 33% increased risk of being overweight, and their mean weight gain was 4.6 kg more compared to people with T1D treated by conventional insulin therapy. 66
Diagnostic modalities of NAFLD and their application in T1D cohorts
The presence of liver steatosis in ⩾5% of hepatocytes is a conditio sine qua non for the diagnosis of NAFLD. 67 Liver histology remains the gold standard to assess and grade the presence of steatosis (S1 or mild steatosis = 5–33% fat content, S2 or moderate steatosis = 33–66%, S3 or severe steatosis >66%), inflammation (ballooning and influx of inflammatory cells), and/or fibrosis [ranging, according to the most widely used staging system, from no fibrosis (F0) to minimal (F1) or significant fibrosis (F2–F3) to cirrhosis (F4)], but a biopsy is invasive and not suited for epidemiological studies.1,68,69 Multiple noninvasive tools have been developed to aid in the differentiation, but to date there is no accepted method to diagnose or grade NAFLD/NASH other than biopsy.1,68,69 Nevertheless, all guidelines recommend stratification using noninvasive tools first, and biopsy when NASH or advanced fibrosis are likely present,1,68 or when co-existing liver disease cannot be ruled out. 69
In this section, we describe the most commonly used noninvasive imaging studies in NAFLD and T1D. Computed tomography (CT) can also be used to evaluate liver fat content but has not been thoroughly validated and not recommended in any guideline, also due to radiation burden and its inferiority as compared to MRI, and as no studies on patients with T1D used CT, we will not discuss CT in more detail.
Ultrasound
Ultrasound allows to estimate the degree of fat infiltration based on echogenicity of the liver compared to the kidney parenchyma, echo beam attenuation, and loss of periportal signalling. Furthermore, it provides additional information about liver tissue integrity, biliary structures, and features of cirrhosis. It has adequate overall sensitivity to detect moderate to severe steatosis (sensitivity 84.8%, specificity 93.6%), but lacks accuracy for mild steatosis (sensitivity 61–65%) compared to histology or other imaging techniques.70–72 However, it goes without quantitative reporting. Other ultrasound-based techniques include the hepatorenal steatosis index (HSI) and Hamaguchi score, but they lack general validation. 73 Assessment of NAFLD by ultrasonography has substantial inter-observer variability and reproducibility of results is limited.74,75 Nevertheless, ultrasound is widely available, cheap, and has adequate diagnostic accuracy, and is therefore recommended (A1) as first-line diagnostic procedure in the European guideline. 1 Ultrasound is also the most commonly used method in studies in T1D cohorts. 24 However, in addition to the above-mentioned shortcomings, it is possible that ultrasound is less accurate in T1D patients than in the general population due to the presence of glycogen disturbances.76,77 More specifically, glycogenic hepatopathy, a rare condition seen in badly controlled T1D, can mimic steatosis on ultrasound since it presents with ultrasonographic hyperechogenicity resembling NAFLD.76,77 However, glycogenic hepatopathy is often accompanied by mildly to robustly elevated liver enzymes and distinct hepatomegaly, which is not commonly seen in simple steatosis. 78 Because the few data on glycogenic hepatopathy originate from case series, it is uncertain whether this condition is a mimicker of NAFLD or a confounder. Furthermore, the presence of glycogenic hepatopathy does not rule out the co-presence of NAFLD. Diagnosis is made histologically, which implies there are no reliable data available on its prevalence in T1D patients. Because of the potential for progressive liver disease and CVD in NAFLD, it is crucial to distinguish NAFLD from glycogenic hepatopathy which is generally considered a more benign and reversible condition. MRI studies could aid in the differentiation between NAFLD and glycogenic hepatopathy due to a more accurate image of steatosis on MRI (vide infra). Therefore, cross-validation of ultrasound versus MRI are needed in T1D-NAFLD studies.
Transient elastography
The most commonly used noninvasive tool to assess fibrosis is transient elastography (TE), as liver stiffness correlates with the severity of the fibrosis. Similar to magnetic resonance elastography (MRE), it utilizes ultrasound wave propagation through the liver to evaluate liver stiffness. The faster the shear wave propagates, the stiffer (and thus potentially more fibrotic) the liver is. The benefit of TE over biopsy, besides its noninvasive character, is the approximately 100 times larger sample area. 79 TE is most commonly performed using Fibroscan® (Echosens, Paris, France), which allows to simultaneously evaluate liver fat content using the controlled attenuation parameter (CAP). The Fibroscan® commonly features two probes, M and XL, to evaluate both liver stiffness and CAP, with the choice of the probe based on the skin-to-liver capsule distance (⩽25 mm for the M probe or >25 mm for the XL probe). A third probe, the S probe is used for paediatric patients, with a preferred measurement depth of 15 to 50 mm. To evaluate NAFLD in lean people studies using the S probe could be helpful, but none have been performed to date in a T1D population.
In contrast to MRE, TE is affected by body composition, inflammation, congestion, and cholestasis. 80 The diagnostic accuracies of the M and XL probes are reported to be similar. 81 TE has excellent accuracy for the detection of advanced fibrosis (sensitivity ranging 85–92% for F3 to F4), but moderate accuracy for F2 (sensitivity 79%). It is superior to prediction scores but strictu sensu inferior to MRE. However, when taking practical considerations into account, TE is the preferred first-line noninvasive tool to assess fibrosis.82–84 It is level A2 recommended in the European guideline. 1 A level B2 recommendation was also made to combine both TE and scores in the decision-making, but more studies are needed to evaluate this additional benefit. 85
A few studies have used TE in T1D cohorts. De Lédighen et al. cross-sectionally screened in a mixed diabetes cohort of 277 hospitalized patients (52% with T1D) and found a very low prevalence of hepatic fibrosis in T1D (2.1%). 86 An Egyptian study in 100 paediatric T1D patients found that liver stiffness was higher in those with abnormal liver ultrasound, but this was not always due to NAFLD. 87 A third study screened T1D patient with the FIB-4 scores and performed elastography when the score was intermediary or elevated. Of those patients (29% of patients), only 18 appeared for Fibroscan®. Of those 18 patients, only 2 had elevated liver stiffness. 88
Score systems
To allow routine screening for NAFLD several markers and risk scores have been developed. The fatty liver index (FLI) and hepatic steatosis index (HSI) are based on biochemical and anthropometrical data such as aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (gGT), triglycerides (TG), BMI, and waist circumference and have demonstrated good performance to screen for hepatic steatosis in several populations, but in patients with obesity and T2D, the clinical accuracy of HSI and FLI is variable. 1 However, little is known about the diagnostic value of these risk scores in people with T1D. In a small Latvian study examining 40 patients with T1D with MRS, the area under the receiver-operator curve for FLI (⩾60) was 0.86 and for HSI (⩾36) was 0.75. 89 Many factors may contribute to variable diagnostic efficacy of these risk scores including ethnicity, comorbidities, and mainly modality to diagnose hepatic steatosis. Important to note is that triglycerides can be high in case of metabolic dysregulation and hypoinsulinaemia in T1D, thereby influencing the score.
There is paucity of data on the use of hepatic fibrosis markers such as the FIB-4 or NAFLD Fibrosis Score (NFS) to identify liver fibrosis in a T1D population. In a retrospective chart analysis of 4,899 patients with T1D (87% Caucasian and 67% overweight or obese), Singh et al. studied the estimated prevalence of NAFLD using the HSI (and also that of advanced fibrosis using appropriate fibrosis scores such as the NAFLD Fibrosis Score (NFS), the Fibrosis-4 (FIB-4) index, AST-to-platelet ratio index (APRI) and AST/ALT ratio). NAFLD based on HSI >36 was present in 71.3% of patients and advanced fibrosis was present in 20.3% based on NFS >0.676, 6.7% based on FIB-4 >2.67, 2.1% based on APRI >1.5% and 22.1% based on AST/ALT >1.4%. 90 Despite the high prevalence of NAFLD based on HSI in this study, the mean AST and ALT were within normal limits, suggesting that normal liver enzymes do not exclude fatty liver. However, these scores were not tested/validated against ultrasound or MR. Furthermore, the wide variations in the percentages among different scores indicate the need for further validation of these scores in a T1D population. Finally, many risk scores include the absence/presence of diabetes, thereby inducing bias and risk of overestimation (see Table 1).
Table 1.
Overview of commonly used NAFLD risk scores for steatosis in T1D.
| Score system | Parameter measured | Variables included in the equation | Commentary |
|---|---|---|---|
| FLI | Steatosis | BMI, waist circumference, triglycerides, gGT | Not validated in T1D, triglycerides are highly affected by metabolic control in T1D |
| HSI | Steatosis | Gender, BMI, AST, ALT, presence of diabetes | Not validated in T1D, gives weight to the presence of diabetes, uncertain which weight to give to T1D |
| FIB-4 | Fibrosis | Age, AST, ALT, platelet count | Not validated in T1D, is used to screen for advanced fibrosis, which is not prevalent in T1D, gives less accurate information on mild fibrosis |
| NFS | Fibrosis | Age, BMI, presence of diabetes, AST, ALT, platelet count, albumin | Not validated in T1D, gives weight to the presence of diabetes, uncertain which weight to give to T1D, is used to screen for advanced fibrosis, which is not prevalent in T1D, gives less accurate information on mild fibrosis |
| AST-to-platelet ratio | Fibrosis | AST, platelet count | Not validated in T1D |
| FibroTest | Fibrosis | Alpha2-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, and gGT, adjusted for age and gender | Not validated in T1D, proprietary panel not universally available in clinical practice |
| AST/ALT ratio | Fibrosis | AST, ALT | Not validated in T1D |
ALT, alanine transferase; AST, aspartate transferase; BMI, body mass index; gGT, gamma-glutamyltransferase; FIB-4, fibrosis-4; FLI, fatty liver index; HSI, hepatic steatosis index; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD fibrosis score; T1D, type 1 diabetes.
Controlled attenuation parameter (CAPTM)
CAP is an ultrasound-based technique available on the Fibroscan® device which allows evaluation of liver fat content. The CAP measures the attenuation of the ultrasound beam while traversing the liver. The results are given in decibels per metre and range from 100 to 400 dB/m. As mentioned above, the Fibroscan® features an M and XL probe (and an S probe in a paediatric population) to evaluate both liver stiffness and CAP, with the choice of the probe based on the skin-to-liver capsule distance. There are, however, no specific technical parameters recommended by the producers to ensure reliable measurements of CAP. As a consequence, most authors copied the quality criteria recommended for liver stiffness evaluation, which incorporates 10 valid measurements with an IQR/median < 30%. A recent study established that this quality cut-off is accurate in predicting steatosis. 91 A systematic review from 2019 reported that CAP could be a noninvasive substitute for liver biopsy with excellent sensitivity (87%) for mild steatosis but decreasing sensitivity for moderate (85%) and severe (76%) sensitivity, probably partly due to the technical difficulties to obtain valid measures in more obese patients. 92 This partially explains why the inter-observer reproducibility is good (overall concordance correlation coefficient: 0.82, >95% reliability), but lower with the use of the XL probe compared to the M probe.93,94 A meta-analysis including data of 3830 patients with different aetiologies of liver disease concluded that CAP provides an adequate measure of hepatic steatosis but that the CAP value should be corrected for the presence of NAFLD (−10 dB/m), the presence of diabetes (−10 dB/m), and body mass index (BMI; −4.4 dB/m per unit above/below 25 kg/m2 over the range of 20 to 30 kg/m2). 95 A more recent analysis of 2,979 patients with NAFLD reported a similar loss of accuracy with increasing grades of steatosis. Furthermore, this meta-analysis compared CAP with MRI and concluded that MRI was significantly more accurate to diagnose steatosis. 96 A large individual patient data meta-analysis evaluated the use of the XL probe, which is predominantly used in NAFLD patients due to the link with obesity, and concluded that CAP can help in diagnosing steatosis, but cannot adequately grade steatosis. 97 A Canadian meta-analysis compared all meta-analyses and proposed the following cut-off values: 248 dB/m, 268 dB/m, and 280 dB/m, corrected by BMI and presence of co-morbidities, to respectively diagnose S ⩾ 1, S ⩾ 2, and S3. 98 Since the XL probe is a more recent tool as compared to the M probe, the question arises whether the same cut-offs can be used for both probes. Preliminary evidence suggests small differences,35,36 and maybe universal cut-offs can be used. 99 There are no studies exploring cohort-specific cut-offs in T1D to the best of our knowledge. One study explored fibrosis in T1D with Fibroscan® but did not evaluate CAP.
MRI
MRI, either by spectroscopy (MRS) or by proton density fat fraction (MRI-PDFF) is considered as a superior noninvasive method to evaluate liver fat content, as has been demonstrated in studies using liver histology as gold standard.100–105 MR imaging is thus increasingly used in clinical trials focusing on the reduction of liver fat content.106,107 According to international guidelines, there is no indication to solely assess liver steatosis and not evaluate the presence of inflammation or fibrosis.1,68,69 Therefore, MR imaging alone is not guideline-recommended, since it does not give any information on NASH or fibrosis. 68 However, evidence is emerging that liver steatosis, even in the absence of NASH or fibrosis, can be linked to a higher risk of CVD.20,31 Therefore, the role of MRI is likely to become more prominent. A recent meta-analysis evaluated the potential use of MRI to diagnose NASH and concluded good overall diagnostic accuracy (sensitivity ranging from 76.4% to 95.3%, specificity ranging from 62.4% to 84.6%), but more studies are needed to evaluate the clinical applicability. 108 Therefore, for the time being, only histology is recommended to evaluate the presence of NASH and MRI is limited to research or trial purposes. Several studies have used MRI to assess NAFLD in T1D as will be discussed in the following.62,89,109,110 The prevalence of NAFLD is dramatically lower in these studies based on MRI compared to ultrasound studies, which can result from differences in patient characteristics and referral bias, but might also be indicative for a higher false-positive rate in case of ultrasound imaging. 24
MRE
MRE is able to determine liver stiffness through analysis of mechanical waves transmitted through the liver, similar to transient elastography (TE). 111 A pooled analysis comprising 910 patients from 12 studies concluded that MRE has a high accuracy to diagnose and stage fibrosis in patients with NAFLD with the lowest sensitivity for mild fibrosis (0.77%, 9% CI: 0.69–0.83%), and increasing accuracy as fibrosis stage inclines. MRE has an important advantage over TE because it is not influenced by BMI nor inflammation and allows for mapping of the entire liver (so accounting for regional variations in fibrosis severity and hence sampling variability). On the other hand, MRE has a long processing time, a high cost and limited availability of the required software and tools.111,112 To the best of our knowledge, there are no studies assessing liver stiffness in T1D based on MRE so far.
Epidemiology of NAFLD in T1D
The prevalence of NAFLD has been described in one meta-analysis including 20 studies conducted between 2009 and 2019 involving 3,901 patients, both children and adults with T1D. 24 Thirteen studies used ultrasound including 3054 patients, four used MRI including 394 patients, two used a combination of risk scores and transient elastography including 396 patients, and only one study used histology to diagnose NAFLD in 57 individuals with T1D. Most studies included in the meta-analysis defined NAFLD by the presence of steatosis, while only two studies looked at fibrosis. The pooled prevalence was 19.3% (95% CI: 12.3–27.5%) but there was a very high level of heterogeneity, depending mostly on the age of the patients and the diagnostic modality. The prevalence rate was highest in ultrasound studies (27.1%, 95% CI: 18.7–36.3%), lower in MRI studies (8.6%, 95% CI: 2.1–18.6%) and lowest in the two studies combining risk scores with transient elastography (2.3%, 95% CI: 0.6–4.8%). The biopsy study reported a prevalence of 19.4% (95% CI: 10.0–30.7%) in 57 individuals with T1D, but this number needs to be interpreted with caution because of potential selection bias.
Since no age- and/or BMI-matched group exists, and differences in ethnicities also play a role, no direct comparisons can be made to other populations. In a meta-analysis of 45 studies including 55,936 lean/nonobese patients, the pooled prevalence of NALFD was 10.2% (95% CI: 7.6–13.6%) and 15.7% (95% CI: 12.5–19.6%) in lean or nonobese individuals, respectively. 113 Compared with Western studies, the NAFLD prevalence in the lean or nonobese population was lower in Eastern studies. Furthermore, the prevalence rates reported differ greatly depending on the diagnostic modality used. Only one study compared the MRI-based prevalence of NALFD between patients with T1D and age- and BMI-matched individuals without diabetes and concluded that T1D is not associated with an increased prevalence of NALFD (4.7% in T1D versus 13.4% in matched controls). 109 Although most studies included in the meta-analysis adjusted for BMI or obesity or waist circumference as well as for age, it is possible that other unidentified factors might explain the different prevalence rates of steatosis. It is also important to note that the haemoglobin A1c (HbA1c) values reported in all studies ranged from 60 to 115 mmol/mol (or 7.6% to 12.7%), which exceeds target HbA1c and is on average above the mean HbA1c observed in recent T1D epidemiological reports.114,115 A higher HbA1c has been observed in individuals with compared to those without NAFLD (mean difference of 2.7 mmol/mol (95% CI: 1.4–4.0 mmol/mol). 24 Metabolic control is highly dependent on patient education, motivation, socioeconomic status, and access to healthcare services.116,117 Therefore, it is important to collect data on NAFLD in people with T1D with different levels of metabolic control.
When it comes to NASH, evidence is scarce. Only Harman et al. assessed the presence of NASH in 49 patients by retrospectively assessing liver biopsies and yielded a prevalence of 20.4%. 118 However, liver biopsy studies are prone to selection bias including only patients with a high suspicion (e.g. substantially elevated liver tests), thereby most likely inducing overestimation. Histologically proven significant fibrosis was never reported in studies of T1D patients. Unpublished results from our own study group obtained in 407 consecutively screened T1D patients without preselection showed indeed a low prevalence of significant fibrosis on TE (3.7%).
Risk scores for fibrosis are highly discrepant compared to each other in T1D, as demonstrated by Singh et al. 90 Since the NFS gives additional weight to the presence of diabetes, it is confounded in T1D patients and therefore the results are generally higher compared to the FIB-4. Indeed, the FIB-4 score estimates the presence of significant fibrosis based on clinico-biochemical variables that are not substantially influenced by the sole presence of T1D. In our own cohort, no patients had a FIB-4 score >3.25, but 2% of patients had a high risk according to the NFS >0.765 (unpublished data). Finally, there are no data regarding cirrhosis or liver transplantation due to NAFLD in patients with T1D.
NAFLD and cardiovascular risk
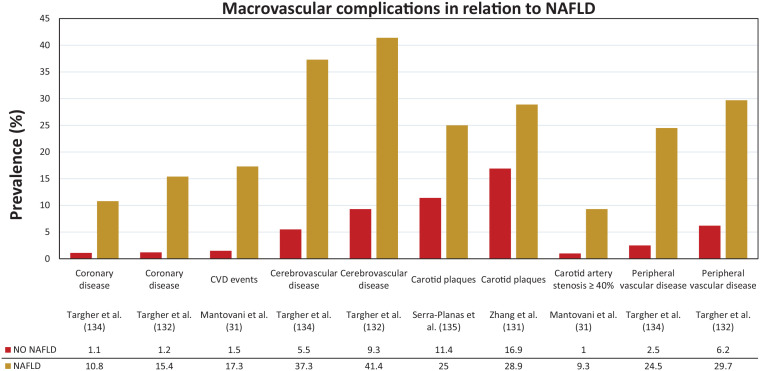
Several cross-sectional studies support a link between NAFLD and subclinical manifestations of atherosclerosis in nondiabetic and in T2D patients,119–128 although not all studies confirmed these observations.129,130 Data in T1D are scarce (see Figure 2). In a cross-sectional study of 722 patients with T1D, Zhang et al. observed that individuals with ultrasound-diagnosed NAFLD had a markedly greater carotid intima media thickness and higher frequency of carotid plaques (28.9% versus 16.9%) than those without liver steatosis. Regression analysis revealed that age, NAFLD, and high-sensitive C-reactive protein were independently associated with intima media thickness in all patients. 131
Figure 2.
Macrovascular complications in relation to NAFLD in T1D.
NAFLD, nonalcoholic fatty liver disease; T1D, type 1 diabetes.
Accumulating evidence suggests that NAFLD, especially in its necro-inflammatory form (NASH), is not only a marker of CVD, but also might contribute to its pathogenesis through the systemic release of several pro-inflammatory (e.g. C-reactive protein, interleukin-6, tumour necrosis factor-alpha) and pro-coagulant factors (e.g. plasminogen activator inhibitor-1, fibrinogen, factor VII, factor VIII) from the fatty and/or inflamed liver or through the contribution of NAFLD itself to hepatic and systemic IR, atherogenic dyslipidaemia, and post-prandial lipaemia. 132 The presence of NAFLD has indeed been linked to an increased prevalence of cardiovascular morbidity and mortality in both nondiabetic and T2D individuals. The proportion of deaths due to CVD in patients with NAFLD ranges between 12.7% and 38%. 42 In a recent meta-analysis of 498,501 patients, the presence of NAFLD was associated with an elevated risk of all-cause mortality, but not with an increased risk of death by CVD. 133 Another review by Wu et al. encompassing 164,494 patients concluded that NAFLD was associated with an increased risk of incident and prevalent CVD, but not with overall mortality nor with CVD mortality. 21 A meta-analysis by Targher et al. corroborated this conclusion, but in their analysis of 16 observational studies, the risk of fatal CVD was elevated, and higher with increasing NAFLD severity. 19 The association between the histological severity and the likelihood of CVD was confirmed in the meta-analysis of Wu et al. 21 Within the NAFLD spectrum, the fibrosis stage is the strongest predictor not only of hepatic but also cardiovascular morbidity and mortality. 20 Several factors may confound the results, namely the great heterogeneity between included studies, the presence of different cardiovascular risk factors among patients, the use of different diagnostic criteria to grade NAFLD and CVD, and the study design of several included studies. In summary, the association with CVD is dependent on the number of metabolic comorbidities, the diagnostic modality, and stage of severity of NAFLD. 20
The group of Targher and colleagues examined the association between CVD and ultrasound-diagnosed NAFLD in a cross-sectional study of 202 patients with T1D. 134 CVD was assessed by patient history, chart review, electrocardiogram, and echo-Doppler scanning of carotid and lower limb arteries. When compared to those without steatosis, individuals with NAFLD (n = 111) had a remarkably higher age- and sex-adjusted prevalence of coronary (10.8% versus 1.1%), cerebrovascular (37.3% versus 5.5%), and peripheral arterial (24.5% versus 2.5%) diseases. In logistic regression analysis, NAFLD was associated with prevalent CVD (as composite endpoint) independently of age, sex, diabetes duration, HbA1c, smoking status, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and medication use (adjusted odds ratio 7.36, 95% CI: 1.60–34.3). In another study of the group of Targher, examining 343 patients with T1D, individuals with NAFLD (n = 182) had a higher age- and sex-adjusted prevalence of coronary (15.4% versus 1.2%), cerebrovascular (41.4% versus 9.3%) and peripheral arterial (29.7 versus 6.2%) diseases as compared to those without steatosis. 132 NAFLD was associated with a 7.6-fold higher odds ratio of composite CVD independent of age, sex, BMI, family history of CVD, smoking status, physical activity, alcohol consumption, diabetes duration, HbA1c, systolic blood pressure, plasma lipids, estimated glomerular filtration rate, albuminuria, and use of anti-hypertensive, lipid-lowering and anti-platelet drugs. It is important to underline that there was no overlap between the two studies because the patients attended two different diabetes clinics. Targher et al. concluded that because NAFLD was associated with CVD independently of the above-mentioned risk factors, it is possible that NAFLD itself could, at least in part, contribute to accelerated atherogenesis. However, cross-sectional studies cannot prove causality. In a retrospective cohort study of 286 T1D patients, NAFLD was suggested to be an independent predictor of CVD. 31 In comparison with patients without NAFLD, during a mean follow-up period of 5.3 ± 2.1 years, the incidence of composite CVD was 8.16 times higher (95% CI: 1.9–35.1) in patients with NAFLD (17.3% versus 1.5%). The hazard ratio adjusted for age, sex, BMI, smoking, diabetes duration, HbA1c, dyslipidaemia, hypertension, chronic kidney disease, prior ischaemic heart disease and serum gGT levels did not appreciably attenuate the association between NAFLD and incident CVD (HR: 6.73, 95% CI: 1.2–38.1). This study was limited by several factors including the diagnostic modality not being the gold standard, a small number of events, selection bias due to retrospective design and it being a single-centre study, but nonetheless provides a suggestion that NAFLD itself acts as a predictor of incident CVD in T1D. In a Spanish study evaluating 100 T1D individuals for the presence of subclinical atherosclerosis, all patients were also evaluated by liver ultrasound. NAFLD was present in 12% and patients with NAFLD had a greater carotid intima media thickness (CIMT), but no differences were observed in coronary artery calcification score or presence of carotid plaques. Linear regression analysis identified age and BMI as factors independently with CIMT, but not NAFLD. 135
It is important to highlight that all reports of a positive association come from one group of researchers, while the only study that indicates otherwise is from another research group. This emphasizes the need for additional research. The above-mentioned findings nevertheless suggest that the identification of NAFLD in individuals with T1D might help in CVD risk stratification with relevant implications for clinical management. Whether NAFLD is related to cardiovascular mortality in T1D, and treatment of NAFLD would result in a reduction in mortality, has to the best of our knowledge not yet been examined.
NAFLD and chronic kidney disease
Patients with diabetes have an increased risk to develop chronic kidney disease (CKD) mainly caused by the negative effects of hyperglycaemia and arterial hypertension on the glomerulus. Other contributing factors are age and metabolic risk factors but there is no information on the role of NAFLD in the development of CKD in people with T1D. Growing evidence suggests that NAFLD and CKD share common pathogenetic mechanisms (such as increased caloric intake, abdominal obesity, and insulin resistance) and potential therapeutic targets, as reviewed by Musso et al. 136 Special interest must be taken into dietary fructose because it not only increases hepatic de novo lipogenesis but it also increases serum uric acid levels and in case of hyperuricemia the increased urinary excretion of uric acid can lead to CKD. 137 Furthermore, NAFLD per se may affect CKD through hypertension, altered lipoprotein metabolism, and hepatokine secretion, which have pro-inflammatory and procoagulant characteristics. 138 Targeting the renal tubule by sodium–glucose cotransporter 2 inhibitors may hold the potential to improve both CKD and NAFLD. 139 Other drug pipelines for both nephropathy and liver steatosis are summarized in a recent article by Sumida and Yoneda. 140
Cross-sectional studies have documented that NAFLD is associated with an increased prevalence of CKD both in nondiabetic and T2D patients.141–144 In a meta-analysis of nine observational studies with nearly 100,000 individuals of predominantly Asian descent who were followed over a median period of 5.2 years, individuals with NAFLD had a higher risk of incident CKD compared to controls (HR: 1.37, 95% CI: 1.20–1.53). The risk also seemed higher with progressive fibrosis. NAFLD was diagnosed using biochemistry, FLI, or ultrasound. 145 An older meta-analysis, which includes biopsy-proven NAFLD, also provided evidence for the link between NASH or advanced fibrosis and the risk of incident CKD. 136 It is important to highlight that CKD patients have an increased risk of CVD. In light of this knowledge, a study in 1148 CKD patients showed that those with CKD and ultrasound-proven NAFLD had an additional twofold risk of CVD (HR: 2.00, 95% CI: 1.10–3.66). 146
There is paucity of data on the link between NAFLD and CKD in T1D (Figure 3). One retrospective cohort study of 261 T1D patients with preserved kidney function and no macroalbuminuria at baseline evaluated the link between ultrasound-diagnosed NAFLD and incident CKD, defined as estimated glomerular filtration rate (eGFR < 60 mL/min/1.72 m2 and/or macroalbuminuria) after a follow-up period of 5.2 years. 32 During follow-up, 61 patients developed incident CKD. The frequency of a renal functional decline (arbitrarily defined as ⩾25% loss of baseline eGFR) was greater among those with than among those without NAFLD (26% versus 11%, p = 0.005). NAFLD was associated with an increased risk of incident CKD (HR: 2.85, 95% CI: 1.59–5.10). Adjustments for age, sex, duration of diabetes, HbA1c, hypertension, and baseline eGFR did not appreciably attenuate this association (adjusted HR: 2.03, 95% CI: 1.10–3.77). Previously, the same authors observed in a cross-sectional study of 202 patients with T1D that the prevalence of CKD was higher in those with NAFLD (OR: 3.90, 95% CI: 1.5–10.1). 142 These studies have some limitations; NAFLD was diagnosed using ultrasound, number of people developing CKD was small, and selection bias due to retrospective design and single-centre study. Nevertheless, the possible clinical implication of these findings is that T1D patients with NAFLD may benefit from more intensive surveillance or early treatment interventions to decrease the risk for CKD.
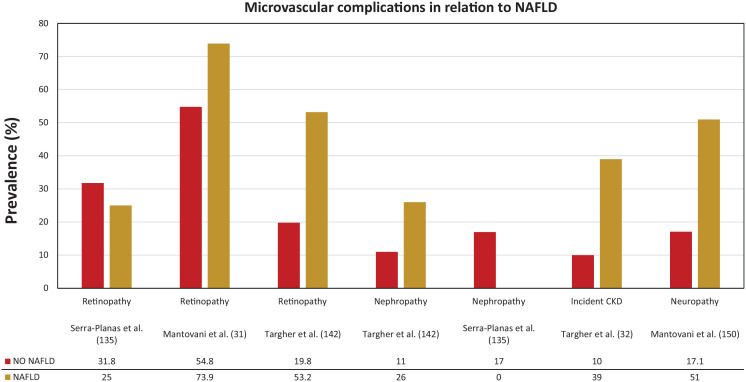
Figure 3.
Microvascular complications in relation to NAFLD in T1D.
NAFLD, nonalcoholic fatty liver disease; T1D, type 1 diabetes.
NAFLD and other microvascular complications
The group of Targher and colleagues also published cross-sectional data on the association between ultrasound-diagnosed NAFLD and diabetic retinopathy (Figure 3). The age- and sex-adjusted prevalence of diabetic retinopathy (53.2% versus 19.8%) was markedly higher in patients with compared to those without NAFLD. In multivariate logistic regression analysis, NAFLD was associated with prevalent retinopathy (adjusted OR: 3.31, 95% CI: 1.4–7.6). These associations were independent of age, sex, diabetes duration, HbA1c, medication use, and presence of the metabolic syndrome. 142
Data on the link between NAFLD and distal symmetric polyneuropathy in people with diabetes are scarce and conflicting. At least one-third of patients with diabetes have diabetic polyneuropathy.147–149 Poor glycaemic control is the strongest risk factor for the development of diabetic peripheral neuropathy, but other factors, namely dyslipidaemia, hypertension, presence of diabetic retinopathy, presence of nephropathy and smoking, may also contribute to the increased risk estimation. Whether NAFLD might also contribute to the presence of diabetic polyneuropathy is not well studied. In a cohort of 286 patients with T1D, the group of Mantovani observed an association between ultrasound-diagnosed NAFLD and the presence of distal symmetric polyneuropathy (detected using the Michigan Neuropathy Screening Instrument and the biothesiometer Vibrotest). 150 Individuals with NAFLD had a higher prevalence of distal symmetric polyneuropathy compared to their counterparts without NAFLD (51.0% versus 17.1%; Figure 3). After adjustment for age, sex, diabetes duration, HbA1c, diabetic retinopathy, smoking, metabolic syndrome, chronic kidney disease, and carotid artery stenoses ⩾40%, this association remained significant (adjusted OR: 2.23, 95% CI: 1.1–4.8).
Conclusion
NAFLD appears to be rather common in T1D but available studies show great heterogeneity, mostly due to differences in diagnostic accuracy of the used noninvasive diagnostics, selection bias due to retrospective study designs, but also other factors such as metabolic control and patient education or additional (yet unidentified) parameters. Further studies are needed that include cross-validation of noninvasive diagnostics in T1D patients and identification of possible additional risk factors for steatosis. T1D is a very heterogeneous disease with some individuals being more prone to develop overweight, obesity, and IR. Longitudinal data from new-onset T1D are essential to clarify the natural history and importance of NAFLD in the cardiometabolic profile of T1D patients. Data from mainly one group of researchers suggest an independent association between NALFD and microvascular (retino-, neuro-, and nephropathy) and macrovascular disease. Evidence is growing that features of IR are increasingly prevalent among patients with T1D, making them vulnerable for NAFLD and NAFLD-associated comorbidities.
Acknowledgments
We would like to thank Kristof Van Dessel for the design of the figures.
Footnotes
Author contributions: J.M. contributed to data curation, formal analysis, methodology, and wrote the manuscript. L.V.G. contributed to conceptualization, provided supervision, and edited the manuscript. S.F. contributed to conceptualization, methodology, provided supervision, and reviewed the manuscript. C.D.B. provided the initial idea, contributed to conceptualization, data curation, formal analysis, methodology, project administration, and edited and reviewed the manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the specific research, authorship, and/or publication of this article.
JM has no conflicts of interest in general. LVG declares to be a member of the advisory board J Mertens, LF Van Gaal et al. journals.sagepub.com/home/tae 15 and/or speakers bureau of Astrazeneca, Boehringer Ingelheim, Eli Lilly, MSD, and Novo Nordisk. SF has a senior clinical research mandate from the Fund for Scientific Research (FWO) Flanders (1802154N) and has acted as adviser and/or lecturer for Roche, Gilead, Abbvie, Bayer, BMS, MSD, Janssen, Actelion, Astellas, Genfit, Inventiva, Intercept, Genentech, Galmed, Promethera, Coherus and NGM Bio. CDB reports consulting fees and honoraria for speaking for Abbott, AstraZeneca, Boehringer Ingelheim, Menarini Diagnostics, Eli Lilly, Medtronic, Novo Nordisk and Roche.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Prior publications: This work was presented in part at the 14th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD), 2–5 June 2021 and the preliminary data discussed in this paper were presented in part at the 56th annual meeting of the European Association for the Study of Diabetes (EASD), 21–25 September 2020.
ORCID iDs: Jonathan Mertens  https://orcid.org/0000-0002-4822-4113
https://orcid.org/0000-0002-4822-4113
Christophe De Block  https://orcid.org/0000-0002-0679-3203
https://orcid.org/0000-0002-0679-3203
Contributor Information
Jonathan Mertens, Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Drie Eikenstraat 655, 2650 Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Department of Gastroenterology & Hepatology, Antwerp University Hospital, Edegem, Belgium.
Luc F. Van Gaal, Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Edegem, Belgium Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Sven M. Francque, Department of Gastroenterology & Hepatology, Antwerp University Hospital, Drie Eikenstraat 655, 2650 Edegem, Belgium Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Christophe De Block, Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Drie Eikenstraat 655, 2650 Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
References
- 1. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 2. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. Epub ahead of print 28 May 2021. DOI: 10.1016/j.cgh.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 4. Rosato V, Masarone M, Dallio M, et al. NAFLD and extra-hepatic comorbidities: current evidence on a multi-organ metabolic syndrome. Int J Environ Res Public Health 2019; 16: 3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep 2019; 1: 312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2: 901–910. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 8. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 9. Younossi ZM, Golabi P, De Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019; 71: 793–801. [DOI] [PubMed] [Google Scholar]
- 10. VanWagner LB, Armstrong MJ. Lean NAFLD: a not so benign condition? Hepatol Commun 2018; 2: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren TY, Fan JG. What are the clinical settings and outcomes of lean NAFLD? Nat Rev Gastroenterol Hepatol 2021; 18: 289–290. [DOI] [PubMed] [Google Scholar]
- 12. Francque S, Wong VW. NAFLD in lean individuals: not a benign disease. Gut. Epub ahead of print 12 March 2021. DOI: 10.1136/gutjnl-2021-324162. [DOI] [PubMed] [Google Scholar]
- 13. Chandrasekharan K, Alazawi W. Genetics of non-alcoholic fatty liver and cardiovascular disease: implications for therapy. Front Pharmacol 2019; 10: 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324. [DOI] [PubMed] [Google Scholar]
- 15. Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology 2015; 62: 1742–1756. [DOI] [PubMed] [Google Scholar]
- 16. Lauridsen BK, Stender S, Kristensen TS, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J 2018; 39: 385–393. [DOI] [PubMed] [Google Scholar]
- 17. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148: 547–555. [DOI] [PubMed] [Google Scholar]
- 18. Adam R, Karam V, Cailliez V, et al. 2018 Annual report of the European liver transplant registry (ELTR) – 50-year evolution of liver transplantation. Transpl Int 2018; 31: 1293–1317. [DOI] [PubMed] [Google Scholar]
- 19. Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016; 65: 589–600. [DOI] [PubMed] [Google Scholar]
- 20. Kasper P, Martin A, Lang S, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol 2021; 110: 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep 2016; 6: 33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Block CE, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care 2005; 28: 1649–1655. [DOI] [PubMed] [Google Scholar]
- 23. Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin. Diabetes Ther 2018; 9: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vries M, Westerink J, Kaasjager K, et al. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. J Clin Endocrinol Metab 2020; 105: 3842–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Šimonienė D, Platu¯kiene A, Prakapienė E, et al. Insulin resistance in type 1 diabetes mellitus and its association with patient’s micro- and macrovascular complications, sex hormones, and other clinical data. Diabetes Ther 2020; 11: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chillarón JJ, Goday A, Flores-Le-Roux JA, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab 2009; 94: 3530–3534. [DOI] [PubMed] [Google Scholar]
- 27. Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: ‘double diabetes’ in the Diabetes Control and Complications Trial. Diabetes Care 2007; 30: 707–712. [DOI] [PubMed] [Google Scholar]
- 28. Pambianco G, Costacou T, Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care 2007; 30: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 29. Popa SG, Simion AM, Soare M, et al. Insulin resistance and hepatic steatosis in type 1 diabetes mellitus and their association with diabetic chronic complications. Minerva Endocrinol. Epub ahead of print 2 October 2020. DOI: 10.23736/S0391-1977.20.03290-3. [DOI] [PubMed] [Google Scholar]
- 30. De Block CEM, Shivalkar B, Goovaerts W, et al. Coronary artery calcifications and diastolic dysfunction versus visceral fat area in type 1 diabetes: VISCERA study. J Diabetes Complications 2018; 32: 271–278. [DOI] [PubMed] [Google Scholar]
- 31. Mantovani A, Mingolla L, Rigolon R, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol 2016; 225: 387–391. [DOI] [PubMed] [Google Scholar]
- 32. Targher G, Mantovani A, Pichiri I, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014; 37: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 33. Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology 2021; 73: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 2018; 75: 3313–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Regnell SE, Lernmark Å. Hepatic steatosis in type 1 diabetes. Rev Diabet Stud 2011; 8: 454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Im SS, Kang SY, Kim SY, et al. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes 2005; 54: 1684–1691. [DOI] [PubMed] [Google Scholar]
- 37. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergsten P. Pathophysiology of impaired pulsatile insulin release. Diabetes Metab Res Rev 2000; 16: 179–191. [DOI] [PubMed] [Google Scholar]
- 39. Alwahsh SM, Gebhardt R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch Toxicol 2017; 91: 1545–1563. [DOI] [PubMed] [Google Scholar]
- 40. Basaranoglu M, Basaranoglu G, Bugianesi E. Carbohydrate intake and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr 2015; 4: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013; 57: 2525–2531. [DOI] [PubMed] [Google Scholar]
- 42. Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis 2012; 32: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vergès B. Lipid disorders in type 1 diabetes. Diabetes Metab 2009; 35: 353–360. [DOI] [PubMed] [Google Scholar]
- 44. Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017; 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 46. Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism 2015; 64: 1629–1639. [DOI] [PubMed] [Google Scholar]
- 47. Xu X, So JS, Park JG, et al. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis 2013; 33: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Azzout-Marniche D, Bécard D, Guichard C, et al. Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochem J 2000; 350: 389–393. [PMC free article] [PubMed] [Google Scholar]
- 49. Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch 2020; 472: 1273–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hatting M, Tavares CDJ, Sharabi K, et al. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci 2018; 1411: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han HS, Kang G, Kim JS, et al. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med 2016; 48: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Santoleri D, Titchenell PM. Resolving the paradox of hepatic insulin resistance. Cell Mol Gastroenterol Hepatol 2019; 7: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol 2020; 8: 616–627. [DOI] [PubMed] [Google Scholar]
- 54. Cordeiro A, Costa R, Andrade N, et al. Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity. Clin Res Hepatol Gastroenterol 2020; 44: 394–402. [DOI] [PubMed] [Google Scholar]
- 55. Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun 2020; 4: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Field JB. Extraction of insulin by liver. Annu Rev Med 1973; 24: 309–314. [DOI] [PubMed] [Google Scholar]
- 57. Wanless IR, Bargman JM, Oreopoulos DG, et al. Subcapsular steatonecrosis in response to peritoneal insulin delivery: a clue to the pathogenesis of steatonecrosis in obesity. Mod Pathol 1989; 2: 69–74. [PubMed] [Google Scholar]
- 58. Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab 2019; 104: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bojsen-Møller KN, Lundsgaard AM, Madsbad S, et al. Hepatic insulin clearance in regulation of systemic insulin concentrations-role of carbohydrate and energy availability. Diabetes 2018; 67: 2129–2136. [DOI] [PubMed] [Google Scholar]
- 60. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 1998; 19: 608–624. [DOI] [PubMed] [Google Scholar]
- 61. Matveyenko AV, Liuwantara D, Gurlo T, et al. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 2012; 61: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017; 19: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 63. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016; 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Colberg SR, Laan R, Dassau E, et al. Physical activity and type 1 diabetes: time for a rewire? J Diabetes Sci Technol 2015; 9: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019; 42: 731–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 2013; 127: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Makri E, Goulas A, Polyzos SA. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res 2021; 52: 25–37. [DOI] [PubMed] [Google Scholar]
- 68. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 69. Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018; 33: 70–85. [DOI] [PubMed] [Google Scholar]
- 70. Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009; 51: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 2010; 256: 159–168. [DOI] [PubMed] [Google Scholar]
- 72. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol 2019; 25: 6053–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cengiz M, Sentürk S, Cetin B, et al. Sonographic assessment of fatty liver: intraobserver and interobserver variability. Int J Clin Exp Med 2014; 7: 5453–5460. [PMC free article] [PubMed] [Google Scholar]
- 75. Strauss S, Gavish E, Gottlieb P, et al. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol 2007; 189: W320–W323. [DOI] [PubMed] [Google Scholar]
- 76. Sumida Y, Yoneda M. Glycogen hepatopathy: an under-recognized hepatic complication of uncontrolled type 1 diabetes mellitus. Intern Med 2018; 57: 1063–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Torbenson M, Chen YY, Brunt E, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol 2006; 30: 508–513. [DOI] [PubMed] [Google Scholar]
- 78. Sherigar JM, Castro J, Yin YM, et al. Glycogenic hepatopathy: a narrative review. World J Hepatol 2018; 10: 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mikolasevic I, Orlic L, Franjic N, et al. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease – where do we stand? World J Gastroenterol 2016; 22: 7236–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oeda S, Tanaka K, Oshima A, et al. Diagnostic accuracy of FibroScan and factors affecting measurements. Diagnostics 2020; 10: 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oeda S, Takahashi H, Imajo K, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan ® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol 2020; 55: 428–440. [DOI] [PubMed] [Google Scholar]
- 82. Campos-Murguía A, Ruiz-Margáin A, González-Regueiro JA, et al. Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease. World J Gastroenterol 2020; 26: 5919–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017; 66: 1486–1501. [DOI] [PubMed] [Google Scholar]
- 84. Cassinotto C, Boursier J, De Lédinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016; 63: 1817–1827. [DOI] [PubMed] [Google Scholar]
- 85. Petta S, Wong VW, Cammà C, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther 2017; 46: 617–627. [DOI] [PubMed] [Google Scholar]
- 86. De Lédinghen V, Vergniol J, Gonzalez C, et al. Screening for liver fibrosis by using FibroScan ® and FibroTest in patients with diabetes. Dig Liver Dis 2012; 44: 413–418. [DOI] [PubMed] [Google Scholar]
- 87. Elkabbany ZA, Elbarbary NS, Ismail EA, et al. Transient elastography as a noninvasive assessment tool for hepatopathies of different etiology in pediatric type 1 diabetes mellitus. J Diabetes Complications 2017; 31: 186–194. [DOI] [PubMed] [Google Scholar]
- 88. Marjot T, Sbardella E, Moolla A, et al. Prevalence and severity of non-alcoholic fatty liver disease are underestimated in clinical practice: impact of a dedicated screening approach at a large university teaching hospital. Diabet Med 2018; 35: 89–98. [DOI] [PubMed] [Google Scholar]
- 89. Svikla¯ne L, Olmane E, Dze¯rve Z, et al. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol 2018; 33: 270–276. [DOI] [PubMed] [Google Scholar]
- 90. Singh A, Le P, Lopez R, et al. The utility of noninvasive scores in assessing the prevalence of nonalcoholic fatty liver disease and advanced fibrosis in type 1 diabetic patients. Hepatol Int 2018; 12: 37–43. [DOI] [PubMed] [Google Scholar]
- 91. Semmler G, Wöran K, Scheiner B, et al. Novel reliability criteria for controlled attenuation parameter assessments for non-invasive evaluation of hepatic steatosis. United European Gastroenterol J 2020; 8: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pu K, Wang Y, Bai S, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol 2019; 19: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferraioli G, Tinelli C, Lissandrin R, et al. Interobserver reproducibility of the controlled attenuation parameter (CAP) for quantifying liver steatosis. Hepatol Int 2014; 8: 576–581. [DOI] [PubMed] [Google Scholar]
- 94. Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018; 67: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 96. Gu Q, Cen L, Lai J, et al. A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease. Eur J Clin Invest 2021; 51: e13446. [DOI] [PubMed] [Google Scholar]
- 97. Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2021; 6: 185–198. [DOI] [PubMed] [Google Scholar]
- 98. Sirli R, Sporea I. Controlled attenuation parameter for quantification of steatosis: which cut-offs to use. Can J Gastroenterol Hepatol 2021; 2021: 6662760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chan WK, Nik Mustapha NR, Mahadeva S, et al. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis. J Gastroenterol Hepatol 2018; 33: 1787–1794. [DOI] [PubMed] [Google Scholar]
- 100. Wang XM, Zhang XJ, Ma L. Diagnostic performance of magnetic resonance technology in detecting steatosis or fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Medicine 2018; 97: e10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Boudinaud C, Abergel A, Joubert-Zakeyh J, et al. Quantification of steatosis in alcoholic and nonalcoholic fatty liver disease: evaluation of four MR techniques versus biopsy. Eur J Radiol 2019; 118: 169–174. [DOI] [PubMed] [Google Scholar]
- 102. Zhou JH, Cai JJ, She ZG, et al. Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol 2019; 25: 1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019; 156: 1264–1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Idilman IS, Keskin O, Celik A, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol 2016; 57: 271–278. [DOI] [PubMed] [Google Scholar]
- 105. Yokoo T, Serai SD, Pirasteh A, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology 2018; 286: 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016; 65: 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013; 58: 1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim TH, Jeong CW, Jun HY, et al. Accuracy of proton magnetic resonance for diagnosing non-alcoholic steatohepatitis: a meta-analysis. Sci Rep 2019; 9: 15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Petit JM, Pedro L, Guiu B, et al. Type 1 diabetes is not associated with an increased prevalence of hepatic steatosis. Diabet Med 2015; 32: 1648–1651. [DOI] [PubMed] [Google Scholar]
- 110. Regnell SE, Peterson P, Trinh L, et al. Magnetic resonance imaging reveals altered distribution of hepatic fat in children with type 1 diabetes compared to controls. Metabolism 2015; 64: 872–878. [DOI] [PubMed] [Google Scholar]
- 111. Piazzolla VA, Mangia A. Noninvasive diagnosis of NAFLD and NASH. Cells 2020; 9: 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Loomba R, Cui J, Wolfson T, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol 2016; 111: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shi Y, Wang Q, Sun Y, et al. The prevalence of lean/nonobese nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol 2020; 54: 378–387. [DOI] [PubMed] [Google Scholar]
- 114. Lavens A, Nobels F, De Block C, et al. Effect of an integrated, multidisciplinary nationwide approach to type 1 diabetes care on metabolic outcomes: an observational real-world study. Diabetes Technol Ther 2021; 23: 565–576. [DOI] [PubMed] [Google Scholar]
- 115. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019; 21: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gesuita R, Skrami E, Bonfanti R, et al. The role of socio-economic and clinical factors on HbA1c in children and adolescents with type 1 diabetes: an Italian multicentre survey. Pediatr Diabetes 2017; 18(3): 241–248. [DOI] [PubMed] [Google Scholar]
- 117. Andrade CS, Ribeiro GS, Santos C, et al. Factors associated with high levels of glycated haemoglobin in patients with type 1 diabetes: a multicentre study in Brazil. BMJ Open 2017; 7: e018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Harman DJ, Kaye PV, Harris R, et al. Prevalence and natural history of histologically proven chronic liver disease in a longitudinal cohort of patients with type 1 diabetes. Hepatology 2014; 60: 158–168. [DOI] [PubMed] [Google Scholar]
- 119. Bonci E, Chiesa C, Versacci P, et al. Association of nonalcoholic fatty liver disease with subclinical cardiovascular changes: a systematic review and meta-analysis. Biomed Res Int 2015; 2015: 213737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ampuero J, Gallego-Durán R, Romero-Gómez M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: meta-analysis. Rev Esp Enferm Dig 2015; 107: 10–16. [PubMed] [Google Scholar]
- 121. Brea A, Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. Int J Cardiol 2013; 167: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 122. VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 2014; 235: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012; 56: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Targher G, Bertolini L, Padovani R, et al. Non-alcoholic fatty liver disease is associated with carotid artery wall thickness in diet-controlled type 2 diabetic patients. J Endocrinol Invest 2006; 29: 55–60. [DOI] [PubMed] [Google Scholar]
- 125. Sung KC, Wild SH, Kwag HJ, et al. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care 2012; 35: 2359–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Puig J, Blasco G, Daunis-I-Estadella J, et al. Nonalcoholic fatty liver disease and age are strong indicators for atherosclerosis in morbid obesity. Clin Endocrinol 2015; 83: 180–186. [DOI] [PubMed] [Google Scholar]
- 127. Kim KS, Oh HJ, Kim DJ, et al. The association between non-alcoholic fatty liver disease and carotid atherosclerosis in subjects with within-reference range alanine aminotransferase levels. Endocr J 2013; 60: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 128. McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008; 103: 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Petit JM, Guiu B, Terriat B, et al. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab 2009; 94: 4103–4106. [DOI] [PubMed] [Google Scholar]
- 130. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care. Atherosclerosis 2013; 230: 258–267. [DOI] [PubMed] [Google Scholar]
- 131. Zhang L, Guo K, Lu J, et al. Nonalcoholic Fatty Liver Disease is Associated with Increased Carotid Intima-Media Thickness in Type 1 Diabetic Patients. Sci Rep 2016; 6: 26805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Targher G, Pichiri I, Zoppini G, et al. Increased prevalence of cardiovascular disease in Type 1 diabetic patients with non-alcoholic fatty liver disease. J Endocrinol Invest 2012; 35: 535–540. [DOI] [PubMed] [Google Scholar]
- 133. Liu Y, Zhong G-C, Tan H-Y, et al. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Scientific Reports 2019; 9: 11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Targher G, Bertolini L, Padovani R, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol 2010; 53: 713–718. [DOI] [PubMed] [Google Scholar]
- 135. Serra-Planas E, Aguilera E, Castro L, et al. Low prevalence of non-alcoholic fatty liver disease in patients with type 1 diabetes is associated with decreased subclinical cardiovascular disease. J Diabetes 2017; 9: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 136. Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mosca A, Nobili V, De Vito R, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol 2017; 66: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 138. Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol 2020; 72: 785–801. [DOI] [PubMed] [Google Scholar]
- 139. Gastaldelli A, Stefan N, Häring HU. Liver-targeting drugs and their effect on blood glucose and hepatic lipids. Diabetologia 2021; 64: 1461–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 2018; 53: 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yilmaz Y, Alahdab YO, Yonal O, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism 2010; 59: 1327–1330. [DOI] [PubMed] [Google Scholar]
- 142. Targher G, Bertolini L, Chonchol M, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia 2010; 53: 1341–1348. [DOI] [PubMed] [Google Scholar]
- 143. Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010; 5: 2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yasui K, Sumida Y, Mori Y, et al. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism 2011; 60: 735–739. [DOI] [PubMed] [Google Scholar]
- 145. Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018; 79: 64–76. [DOI] [PubMed] [Google Scholar]
- 146. Chinnadurai R, Ritchie J, Green D, et al. Non-alcoholic fatty liver disease and clinical outcomes in chronic kidney disease. Nephrol Dial Transplant 2019; 34: 449–457. [DOI] [PubMed] [Google Scholar]
- 147. American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care 2014; 37(Suppl. 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 148. Vinik AI. CLINICAL PRACTICE. Diabetic sensory and motor neuropathy. N Engl J Med 2016; 374: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 149. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mantovani A, Rigolon R, Mingolla L, et al. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J Diabetes Complications 2017; 31: 1021–1026. [DOI] [PubMed] [Google Scholar]