Abstract
Introduction:
Infections are a leading cause of mortality in patients with systemic lupus erythematosus (SLE). Among various infections, invasive fungal infections (IFIs) have a particularly high mortality rate; however, studies examining IFIs in patients with SLE are limited.
Methods:
Patients diagnosed as having SLE between 1997 and 2012 were enrolled from Taiwan’s National Health Insurance Research Database along with age- and sex-matched non-SLE controls at a ratio of 1:10. IFIs were identified based on International Classification of Diseases, Ninth Revision, Clinical Modification codes and validated by the prescriptions of systemic antifungal agents. The incidence rate (IR), incidence rate ratio (IRR), and all-cause mortality rate of IFIs and its subtypes were analyzed. A Cox multivariate regression model with time-dependent covariates was applied to analyze independent risk factors for IFIs.
Results:
A total of 24,541 patients with SLE and 245,410 non-SLE controls were included. We observed 445 IFI episodes in the SLE cohort, with an all-cause mortality rate of 26.7%. Candida spp. (52.8%) was the most common pathogen, followed by Cryptococcus spp. (18.2%) and Aspergillus spp. (18.2%). The IR of IFIs in the SLE cohort was 20.83 per 10,000 person-years, with an IRR of 11.1 [95% confidence interval (CI): 9.8–12.6] relative to the non-SLE controls. Juvenile patients with SLE aged ⩽18 years had the highest IRR of 47.2 (95% CI: 26.9–86.8). Intravenous steroid therapy administered within 60 days (hazard ratio: 29.11, 95% CI: 23.30–36.37) was the most critical risk factor for overall IFIs and each of the three major fungal pathogens. Distinct risk factors were found among different IFI subtypes.
Conclusion:
Patients with SLE had a higher risk of IFIs, especially juvenile patients. Intravenous steroid therapy is the most critical risk factor for IFIs. This study provides crucial information for the risk stratification of IFIs in SLE.
Plain Language Summaries
Patients with systemic lupus erythematosus and physicians treating this patient group should be aware of the risk of invasive fungal infections.
Invasive fungal infections (IFIs) are a severe complication with a high mortality rate among patients with systemic lupus erythematosus (SLE); however, studies on this topic are scant. We performed a nationwide population-based study in Taiwan to estimate the incidence and mortality of and risk factors for IFIs. We found an incidence rate of 20.83 per 10,000 person-years for IFIs, with a mortality rate of 26.7%. Juvenile patients aged ⩽18 years had the highest relative risk of IFIs. Although candidiasis was the most common IFI, cryptococcosis and aspergillosis should be concerned in juvenile patients as well. Intravenous steroid therapy was the most critical risk factor for all IFIs, and different immunosuppressive agents posed different risks in patients for acquiring certain fungal pathogens.
Our findings provide pivotal epidemiological information and indicate risk factors for IFIs in patients with SLE. Age and exposure to specific immunosuppressants and steroids might help predict the risk of IFIs. Because the manifestation of these infections is sometimes indistinguishable from a lupus flare, physicians should be aware of this fatal complication, especially when patients are not responsive to immunosuppressive therapy. Early recognition, implication of diagnostic tools, and empirical antimicrobial agents can be the key to treating patients with IFIs. Additional studies are required to develop a risk management program for patients with SLE.
Keywords: Systemic lupus erythematosus, invasive fungal infection, epidemiology
Introduction
Invasive fungal infections (IFIs) are severe complications with a high mortality rate among immunocompromised hosts, such as patients with hematological malignancies, solid organ transplantation, human immunodeficiency virus/acquired immunodeficiency syndrome, or critical illness.1,2 IFIs are diagnosed on the basis of the presence of fungal pathogens in deep tissue or normally sterile sites. Candida spp., Aspergillus spp., and Cryptococcus spp. are the three major pathogens that cause IFIs in humans. 3 Host defense against these fungal infections depends on both innate and adaptive immunity, particularly neutrophil and T-cell immunity.4,5
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with multisystem involvement. Infections are a leading complication and cause of death in patients with SLE.6–9 In addition to disease as an intrinsic factor, host defense mechanisms in SLE are impaired by treatment with medications such as glucocorticoids or immunosuppressants. 10 In patients with SLE, neutrophils exhibit decreased phagocytosis and hyporesponsiveness to interleukin-8, 11 and CD4+ T helper (Th) cells become dysfunctional with increased apoptosis. 12 In addition, the presence of hypocomplementemia and decreased numbers of natural killer and naïve B cells might contribute to impaired host defense in patients with SLE.
Although bacteria and viruses are the most common pathogens observed in patients with SLE, IFIs are increasingly identified because of the application of sensitive diagnostic modalities.13–15 The use of steroids and immunosuppressive agents is a well-established risk factor for IFIs among solid organ transplantation recipients; 2 however, risk factors for IFIs in patients with SLE remain unclear. A systematic review of 182 studies including 393 patients with SLE and IFIs found a high mortality rate of 50.9%; 16 however, determining the actual incidence of and risk factors for IFIs is difficult because of the limited number of cases in each study. Taiwan’s National Health Insurance (NHI) Research Database (NHIRD) provides comprehensive and uninterrupted medical information on a nationwide scale and thus can be used in epidemiological research.
We conducted this retrospective population-based cohort study by using data from the NHIRD to examine the clinical burden and outcomes of and risk factors for IFIs in patients with SLE, with a particular focus on the different patterns of risk factors for each fungal species.
Methods
Study design and participants
We conducted a population-based retrospective cohort study by using Taiwan’s NHIRD, which contains the data of beneficiaries enrolled in the NHI program, a single mandatory insurance system established in 1995 covering more than 99% of the 23 million residents of Taiwan. The NHIRD contains comprehensive medical claims data regarding demographics, diagnoses, laboratory examinations and procedures, and prescriptions in outpatient, inpatient, and emergency settings. Each of the diagnoses is defined according to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
All patients diagnosed as having SLE (ICD-9-CM code 710.0) between January 1997 and December 2012 were eligible for inclusion. The diagnosis of SLE was further validated by a catastrophic illness certification. This certification acts as a medical fee waiver for public health care services and can only be issued by legal rheumatologists and accredited by the Ministry of Health and Welfare according to the American College of Rheumatology 1982 revised criteria for the classification of SLE 17 or 1997 updated classification criteria for SLE. 18 The date of certification was considered as the date of SLE diagnosis for each patient.
Patients without SLE were selected from the 2000 Longitudinal Health Insurance Database, which contains the longitudinally linked anonymized data of 1,000,000 enrollees (approximately 5% of Taiwan’s population) randomly sampled from the NHIRD’s Registry of Beneficiaries in 2000. We matched patients with SLE with those without SLE by sex and age at a ratio of 1:10. Patients who had IFIs before enrollment were excluded. Patients who received their catastrophic illness certification before January 1997 were excluded from the analysis of time from SLE diagnosis to IFI development.
The two cohorts were followed until the development of a primary endpoint (development of IFIs), withdrawal from the NHI system (usually after death), or the end of the study period. All-cause mortality was defined as withdrawal from the NHI system.
The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB-TPEVGH No. 2017-03-010 C). The reporting of this study conforms to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement. 19
Outcomes
The primary endpoint of interest was the incidence of IFIs. The definition of IFIs used in this study was adopted from an international committee consensus for opportunistic infections in immune-mediated inflammatory disease. 20 Such infections included candidiasis (ICD-9-CM codes 112.2, 112.4, 112.5, 112.81, 112.83, 112.84, 112.85, 112.89, and 112.9), cryptococcosis (ICD-9-CM codes 117.5 and 321.0), histoplasmosis (ICD-9-CM code 115∗), blastomycosis (ICD-9-CM code 116.0), aspergillosis (ICD-9-CM codes 117.3 and 484.6), coccidioidomycosis (ICD-9-CM code 114∗), paracoccidioidomycosis (ICD-9-CM code 116.1), sporotrichosis (ICD-9-CM code 117.1), zygomycosis (ICD-9-CM code 117.7), other and unspecified mycoses (ICD-9-CM code 117.9), opportunistic mycoses (ICD-9-CM code 118), and pneumonia related to other systemic mycoses (ICD-9-CM code 484.7). Hospitalized patients with the aforementioned codes in their discharge diagnoses were defined as patients with IFIs. Only the first IFI episode for each patient was recorded.
IFI diagnoses were further validated by a record of in-hospital prescriptions of systemic antifungal agents available in Taiwan, namely fluconazole, itraconazole, posaconazole, voriconazole, amphotericin B, liposomal amphotericin B, caspofungin, micafungin, anidulafungin, and flucytosine.
Statistical analyses
Student’s t-test or the Mann–Whitney U test was used for continuous variables, and the chi-square test or Fisher’s exact test was used for categorical variables. The incidence rate [IR, per 10,000 person-years (PY)] and incidence rate ratio (IRR) of IFIs were analyzed for both SLE and non-SLE controls as well as subgroups defined by sex, age, and specific pathogens. The Poisson distribution was used to obtain the confidence interval (CI) of IRR between the groups. Kaplan–Meier curves were plotted to determine the cumulative incidence of IFIs for different age subgroups (⩽18, 19–50, and ⩾51 years), and the log-rank test and Cox proportional hazard model were employed to compare cumulative incidences.
For the SLE group, univariate and multivariate Cox proportional hazard models were used to estimate the hazard ratio (HR) and 95% CI to determine possible risk factors for IFIs. Patient characteristics, comorbidities, and medications were used as covariates. Comorbidities, namely diabetes mellitus, end-stage renal disease, interstitial lung diseases, stroke, malignancy, cirrhosis, and congestive heart failure, were identified using the corresponding ICD-9-CM codes. Data on disease-modifying antirheumatic drugs or immunosuppressants, namely hydroxychloroquine, azathioprine, mycophenolate mofetil, cyclosporine, cyclophosphamide, methotrexate, rituximab, oral steroids, and intravenous steroids, were recorded. Because comorbidities developed and prescriptions varied over time, these factors were incorporated into Cox proportional hazard models as time-dependent covariates. For medication exposure, the follow-up period of each patient was retrospectively divided into successive 60-day blocks; the prescription status of each medication in each 60-day block and its association with the occurrence of an event at the end of each time block were analyzed.
Sensitivity analysis using 30- and 90-day blocks was performed. Daily oral steroids were categorized according to the average daily dose in a time block, with a cutoff point of 5 mg of prednisolone or its equivalent. All biologically plausible covariates were imputed into multivariate analysis with forward selection. All statistical analyses were performed using R foundation (R) version 3.2.2 and SPSS version 26.0. Stata/MP V.11 and Microsoft Excel (Office 365) were used for plotting. All tests were two sided with a 95% CI, and a p value of <0.05 indicated statistical significance.
Results
A total of 24,541 patients with SLE were identified between 1997 and 2012, and 245,410 age- and sex-matched patients were included in the non-SLE controls (Table 1). The mean age of both cohorts was 36.3 with a standard deviation (SD) of 15.8 years, and the majority of patients (88%) were women. The mean follow-up duration (years) was significantly shorter for the SLE group than the non-SLE controls (8.7 ± 5.4 versus 12.1 ± 2.6, p < 0.001) (Table 1).
Table 1.
Demographic data, prevalence, and outcomes of invasive fungal infections in patients with SLE and age- and sex-matched non-SLE controls.
| Variables | SLE n = 24,541 |
Non-SLE controls n = 245,410 |
p value |
|---|---|---|---|
| Mean age, years (SD) | 36.3 (15.8) | 36.3 (15.8) | 1.000 |
| Female, n (%) | 21,688 (88) | 216,880 (88) | 1.000 |
| Mean follow-up, years (SD) | 8.7 (5.4) | 12.1 (2.6) | <0.001 |
| Invasive fungal infections, n | 445 | 556 | |
| Prevalence (‰) | 18.1 | 2.3 | <0.001 |
| All-cause mortality (%) | 26.7 | 28.2 | 0.599 |
| Candidiasis, n | 235 | 327 | |
| Prevalence (‰) | 9.6 | 1.3 | <0.001 |
| All-cause mortality (%) | 20.4 | 32.4 | 0.002 |
| Cryptococcosis, n | 81 | 29 | |
| Prevalence (‰) | 3.3 | 0.1 | <0.001 |
| All-cause mortality (%) | 32.1 | 13.8 | 0.058 |
| Aspergillosis, n | 28 | 25 | |
| Prevalence (‰) | 1.1 | 0.1 | <0.001 |
| All-cause mortality (%) | 35.7 | 28.0 | 0.548 |
SD, standard deviation; SLE, systemic lupus erythematosus.
Overall, 445 (1.8%) of 24,541 patients with SLE were hospitalized because of IFIs during the study period (Table 1). The leading pathogen in these episodes was Candida spp. (52.8%, 235/445), followed by Cryptococcus spp. (18.2%, 81/445) and Aspergillus spp. (6.3%, 28/445). Four episodes of zygomycosis and one episode of histoplasmosis were identified in the cohort, with the remaining episodes being unspecified mycoses (ICD-9-CM codes 117.9, 118, and 484.7). A detailed analysis of zygomycosis, histoplamosis, and unspecified mycoses was not performed because of limited case numbers and clinical implications.
The IR of IFIs in the SLE group was 20.83 per 10,000 PY, which was significantly higher than that in the non-SLE controls (IRR: 11.1, 95% CI: 9.8–12.6).
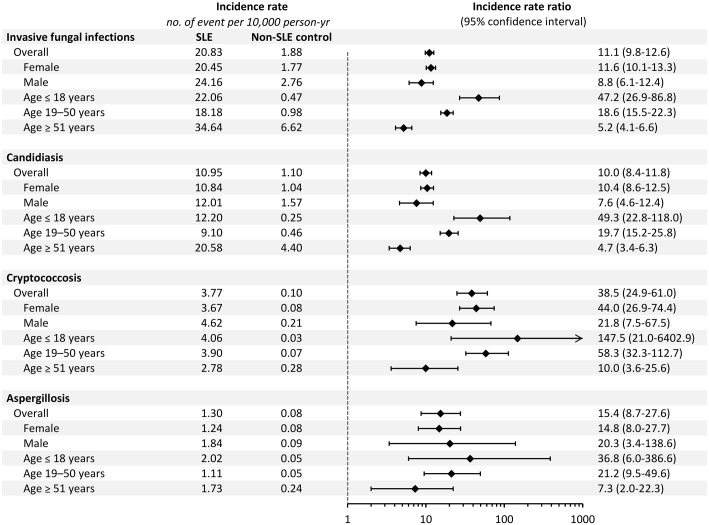
The leading IFI in the SLE cohort was candidiasis, followed by cryptococcosis and aspergillosis, with IRs of 10.95, 3.77, and 1.30 per 10,000 PY, respectively (Figure 1). In comparison with the non-SLE controls, the SLE group had the highest risk for cryptococcosis, with an IRR of 38.5 (95% CI: 24.9–61.0), followed by that of aspergillosis (IRR: 15.4, 95% CI: 8.7–27.6) and candidiasis (IRR: 10.0, 95% CI: 8.4–11.8). The same trend was observed in each subgroup analysis stratified by sex and age (Figure 1). Regarding different age strata, the IR of overall and three major IFIs rapidly increased with age in the non-SLE controls. By contrast, the juvenile subgroup in the SLE cohort had a disproportionally high IR for different IFIs, leading to the highest relative risk in this age stratum, with IRRs of 36.5, 49.3, and 147.5 for aspergillosis, candidiasis, and cryptococcosis, respectively. Notably, the IRs of cryptococcosis and aspergillosis were the highest in juvenile patients with SLE compared with other age subgroups. Figure 2 shows the cumulative incidence of IFIs in the SLE and non-SLE controls. Older patients with SLE aged ⩾51 years had the highest absolute risk of IFIs, whereas juvenile patients with SLE aged ⩽18 years had the highest relative risk of IFIs.
Figure 1.
Incidence rate and incidence rate ratio of invasive fungal infections, candidiasis, cryptococcosis, and aspergillosis in patients with systemic lupus erythematosus (SLE) and non-SLE controls.
Figure 2.
Cumulative incidence of invasive fungal infections in patients with systemic lupus erythematosus (SLE) and non-SLE controls stratified by age.
The all-cause mortality rate of IFIs was similar between the SLE cohort and non-SLE controls (26.7% versus 28.3%, p = 0.599; Table 1). However, the mortality rate of candidiasis was lower in the SLE cohort than in the non-SLE controls (20.4% versus 32.4%, p = 0.002), and the mortality rates of cryptococcosis and aspergillosis were both higher in the SLE cohort than in the non-SLE controls.
Among 18,481 patients with SLE with available information regarding the date of SLE diagnosis (Table 2), more than half of the IFIs occurred within the first 3 years after the diagnosis of SLE, with a median of 33 months [interquartile range (IQR): 8–82]. This phenomenon was consistent among age subgroups. The frequency of IFIs occurred within the first 3 years after the diagnosis of SLE was 53.5%, 50.5%, and 58.9% in patients aged ⩽18, aged 19–50, aged ⩾51 years, respectively. The median time from SLE diagnosis to the first episode of candidiasis, cryptococcosis, and aspergillosis was 38 (IQR: 10–82), 14 (IQR: 7–52), and 21 (IQR: 4.5–86) months, respectively. The difference in time from SLE diagnosis to IFIs did not reach statistical significance for these three major pathogens. Notably, cryptococcosis occurred earlier in patients aged ⩾51 years than in those aged between 19 and 50 years [median interval of 7 (IQR: 5–9) versus 27 (IQR: 8.75–67.25) months].
Table 2.
Median time from SLE diagnosis to IFI in patients with SLE [months (IQR)].
| Overall | Age ⩽18 years | Age 19–50 years | Age ⩾51 years | |
|---|---|---|---|---|
| All invasive fungal infections | 33 (8–82) | 32 (9–59) | 36 (11–86.5) | 22 (4.25–73) |
| Candidiasis | 38 (10–82) | 33.5 (12.25–54.25) | 40 (14–84) | 37 (3–84) |
| Cryptococcosis | 14 (7–54) | 10 (3.75–46) | 27 (8.75–67.25) † | 7 (5–9) † |
| Aspergillosis | 21 (4.5–86) | 50 (5–132) | 21 (5.5–86) | 5 (2.5–83) |
IFI, invasive fungal infection; IQR, interquartile range; SLE, systemic lupus erythematosus.
Comparison was performed between different age subgroups of each IFI subtype.
Bonferroni adjusted p = 0.013.
A Cox multivariate proportional hazards model with time-dependent covariates was used to identify independent risk factors for IFIs in the SLE cohort (Table 3). Age ⩾51 years (HR: 1.40, 95% CI: 1.08–1.81); comorbidities of diabetes mellitus (HR: 1.77, 95% CI: 1.37–2.29), end-stage renal disease (HR: 1.50, 95% CI: 1.37–2.29), interstitial lung disease (HR: 1.49, 95% CI: 1.07–2.10), stroke (HR: 1.69, 95% CI: 1.32–2.18), and malignancy (HR: 2.75, 95% CI: 1.58–4.80); the use of mycophenolate mofetil (HR: 2.24, 95% CI: 1.48–3.37), cyclosporine (HR: 1.65, 95% CI: 1.10–1.75), or cyclophosphamide (HR: 1.37, 95% CI: 1.07–1.75); the daily oral steroid dose of >5 mg of prednisolone or its equivalent (HR: 1.26, 95% CI: 1.01–1.58); and intravenous steroid therapy (HR: 29.11, 95% CI: 23.30–36.37) were independent risk factors for IFI. Similar analyses were performed for different subtypes of fungal infections, and risk factors differed among the three major pathogens. Risk factors for candidiasis were similar to those for overall IFI. By contrast, only stroke (HR: 1.96, 95% CI: 1.09–3.53) and intravenous steroid therapy (HR: 63.51, 95% CI: 36.10–111.71) were identified as independent risk factors for cryptococcosis, whereas mycophenolate mofetil (HR: 4.02, 95% CI: 1.32–12.26), cyclosporine (HR: 4.94, 95% CI: 1.61–15.10), and intravenous steroid therapy (HR: 34.80, 95% CI: 15.09–80.24) were risk factors for aspergillosis. Intravenous steroid therapy was consistently the strongest predictor for IFIs overall and the three major pathogens.
Table 3.
Multivariate Cox regression analysis of risk factors for IFIs in patients with SLE.
| Invasive fungal infections | Candidiasis | Cryptococcosis | Aspergillosis | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | ||||||||
| ⩽18 | 0.93 (0.70–1.23) | 0.594 | 1.03 (0.70–1.52) | 0.889 | ||||
| 19–50 | (reference) | (reference) | ||||||
| ⩾51 | 1.40 (1.08–1.81) | 0.010 | 1.77 (1.27–2.47) | <0.001 | ||||
| Diabetes mellitus | 1.77 (1.37–2.29) | <0.001 | 1.65 (1.16–2.35) | 0.006 | ||||
| End-stage renal disease | 1.50 (1.37–2.29) | <0.001 | 1.76 (1.29–2.41) | <0.001 | ||||
| Interstitial lung disease | 1.49 (1.07–2.10) | 0.020 | ||||||
| Stroke | 1.69 (1.32–2.18) | <0.001 | 1.77 (1.26–2.47) | <0.001 | 1.96 (1.09–3.53) | 0.024 | ||
| Malignancy | 2.75 (1.58–4.80) | <0.001 | ||||||
| Mycophenolate mofetil | 2.24 (1.48–3.37) | <0.001 | 2.72 (1.60–4.61) | <0.001 | 4.02 (1.32–12.26) | 0.015 | ||
| Cyclosporine | 1.65 (1.10–1.75) | 0.016 | 4.94 (1.61–15.10) | 0.005 | ||||
| Cyclophosphamide | 1.37 (1.07–1.75) | 0.011 | 1.50 (1.07–2.10) | 0.019 | ||||
| Intravenous steroid | 29.11 (23.30–36.37) | <0.001 | 28.19 (21.17–37.52) | <0.001 | 63.51 (36.10–111.71) | <0.001 | 34.80 (15.09–80.24) | <0.001 |
| Oral steroid >5 mg/d a | 1.26 (1.01–1.58) | 0.039 | ||||||
CI, confidence interval; HR, hazard ratio; IFIs, invasive fungal infections; SLE, systemic lupus erythematosus.
All biologically plausible variables, namely including age subgroup, sex, diabetes mellitus, end-stage renal disease, interstitial lung disease, stroke, malignancy, cirrhosis, congestive heart failure, hydroxychloroquine use, azathioprine use, mycophenolate mofetil use, cyclosporine use, cyclophosphamide use, methotrexate use, rituximab use, intravenous steroid use, and oral steroid daily dose of >5 mg, were included in Cox multivariate analysis with forward selection.
An average dose is defined as the accumulated equivalent prednisolone dose (milligrams) in a 60-day block divided by 60 (days).
Discussion
To our knowledge, this is the first population-based cohort study to investigate detailed epidemiological data including the incidence and outcomes of and risk factors for overall and different IFIs in an SLE cohort on a national scale. We found a higher risk of IFIs in the SLE cohort than in the non-SLE controls. Whereas candidiasis was the most common IFI, the patients with SLE had a much higher relative risk of cryptococcosis than did those without SLE. The increased risk was the most prominent in the juvenile SLE subgroup. The all-cause mortality rate of IFIs in hospitalized patients with SLE was as high as 26.7% and could reach up to 32.1% and 35.7% for cryptococcosis and aspergillosis, respectively. Intravenous steroid therapy was the most critical risk factor for all IFIs, and different immunosuppressive agents increased the risk of patients acquiring certain fungal pathogens.
The prevalence rate of IFIs in the SLE cohort observed in the present study (1.8%) is consistent with that reported in the largest relevant systematic review (0.6% to 3.2%), which was conducted by Wang et al. 16 The comprehensiveness of the NHIRD enabled us to estimate an IR of 20.83 per 10,000 PY for IFIs in the SLE cohort. Because the study conducted by Wang et al. included reports from as early as the 1960s, when diagnostic tools and options for antifungal agents were limited, the pooled all-cause mortality rate of IFIs in the SLE cohort was higher than that in our study (50.9% versus 26.7%). Similar to the present investigation, another single-center cohort study investigating 140 patients with SLE and IFIs consisting of 140 IFIs in SLE 14 found Candida spp. as the leading fungal pathogen, whereas Wang et al. 16 suggested that Cryptococcus spp. and Aspergillus spp. were the two leading pathogenic fungi. The incidence of invasive candidiasis declined over time in patients with hematological malignancy because of the use of prophylactic antifungal agents in high-risk populations. However, this prophylactic strategy is rarely applied in patients with SLE. The high prevalence of Cryptococcus spp. and Aspergillus spp. in the systematic review 16 might be attributable to publication bias caused by a high fatality rate associated with these two pathogens. We found that more than half of the IFIs occurred within the first 3 years after SLE diagnosis; this finding is consistent with those of previous studies.15,16
The human defensive mechanism against fungal infections is a complex network involving phagocytes, plasmacytoid dendritic cells, subcellular inflammasomes, T cells, and, to a lesser extent, B cells. In addition to intrinsic immune defects associated with SLE, immunosuppressive treatments further compromise immunity. Corticosteroids and various immunosuppressants modulate immune cells differently. Among these cells, neutrophils and CD4+ T helper cells are considerably critical for the control of fungal pathogens; however, the clearance of different fungal pathogens depends on certain mechanisms to varying degrees. For example, Th1 responses are crucial for the control of Candida spp., Cryptococcus spp., and Aspergillus spp;5,21–23 however, Th17 responses are exclusively essential for fighting Candida spp. infection, as reported by the previous clinical trial on the IL-17 inhibitor, which raised concerns regarding candidiasis but not other pathogens. 24 Whereas neutrophil extracellular trap (NET) formation or NETosis aids in the clearance of Candida spp., neutrophils rely more on reactive oxygen species for the elimination of Aspergillus spp.5,22,23,25 Corticosteroids, which universally suppress both innate and adaptive immunities, are a well-known dose-dependent risk factor for IFIs.26,27 We found a 30- to 60-fold higher risk of IFIs, namely candidiasis, cryptococcosis, and aspergillosis, after the administration of intravenous steroid therapy. By contrast, an oral dose of >5 mg/day of prednisolone or an equivalent dose resulted in a negligible risk of IFIs in patients with SLE. Our findings are consistent with those of previous studies suggesting a high dose of steroids as a potent risk factor.14,16 Cyclosporine, mycophenolate mofetil, and cyclophosphamide are immunosuppressants that inhibit lymphocyte function with variable specificity and reversibility. Except for intravenous steroid therapy, these immunosuppressants pose diverse risks of individual fungal species infections. This implies that humans fight against infection from different fungal species by using different mechanisms. Additional studies are required to elucidate these mechanisms for improving risk stratification and treatment outcomes for these patients.
The absolute incidences of all IFIs, candidiasis, cryptococcosis, and aspergillosis all increased with age in the non-SLE controls; the same finding was noted in patients with hematological malignancy.1,26 By contrast, patients with SLE aged ⩽18 years had the highest absolute and relative risk of cryptococcosis and aspergillosis. This finding highlights the need for the clinical awareness of IFIs in this vulnerable population, particularly for those whose condition continues to deteriorate while they receive intravenous steroid therapy, to facilitate early access to diagnostic modalities and antifungal treatment.
The findings of this study are believed to be robust for the following reasons. First, the SLE population was verified by a catastrophic illness certification, which is issued only after strict peer review in the NHI system. Patients with IFIs were generally ill; thus, only those who were hospitalized and discharged with prespecified IFI-related ICD-9-CM codes were included in the analysis. Furthermore, the diagnosis of IFIs was additionally validated based on the prescription of systemic antifungal agents. These agents can only be claimed in the reimbursement system after the confirmation of diagnosis in hospital settings with clinically relevant laboratory or radiological evidence. Therefore, the populations of patients with both SLE and IFIs are considered representative. Because the present study was conducted using a nationwide population-based database, unbiased epidemiological data were extracted. Moreover, we conducted subgroup analysis based on pathogens, age, and sex, which provided insights into risk stratification. Finally, we analyzed certain risk factors and immunosuppressants as time-dependent covariates with 60-day blocks, and the results were stable and consistent in sensitivity analysis using 30- and 90-day blocks.
Although we used reliable data in the present study, it has several potential limitations. The NHIRD is a claims-based data set; thus, it contains no information regarding clinical manifestations, infectious focus, detailed species of fungi, diagnostic laboratory data, or the chronological relationship among invasive procedures. Therefore, the severity of organ involvement could not be accurately examined. The modest association between IFIs and stroke might have originated from unadjusted confounding factors, such as disease activity, which is suggested to be correlated with the intensity of immunosuppressants. Because of the lack of identification and the susceptibility of microbiological isolates, we were unable to define the appropriateness of antifungal treatment or the existence of coinfections that precluded the analysis of risk factors for mortality. Belimumab is not covered by NHI reimbursement for SLE in Taiwan; thus, it was not included in the analysis.
In conclusion, this nationwide population-based cohort study revealed the high risk and mortality from IFIs in patients with SLE, including a particularly high risk in juvenile patients with SLE. Intravenous steroid therapy within 60 days is the most critical predictor of IFIs. Different immunosuppressants may modify the risk of acquiring certain fungal pathogens. The present investigation provides pivotal epidemiological information regarding IFIs in SLE and highlights the need for further research to establish a risk management program.
Acknowledgments
The authors would like to thank Fang-Yi Wu for her valuable statistical consultation.
Footnotes
Author contributions: Chin-Fang Su and Yu-Sheng Chang conceived and designed the study. Chin-Fang Su performed the literature search and drafted the manuscript. Yu-Sheng Chang and Chien-Chih Lai conducted data extraction, methodological quality assessments and performed the analysis. Tzu-Hao Li and Yu-Fang Chang assisted in critical interpretation of data. Yi-Tsung Lin, Wei-Sheng Chen, Yen-Po Tsao, Wen-Hsiu Wang performed critical revision of the manuscript for important intellectual content. Yu-Sheng Chang and Chang-Youh Tsai supervised the whole process of the study. All authors read and approved the final version of submitted manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Taipei Veterans General Hospital (V109A-006 and V110A-002).
ORCID iDs: Chin-Fang Su  https://orcid.org/0000-0003-1979-6056
https://orcid.org/0000-0003-1979-6056
Chien-Chih Lai  https://orcid.org/0000-0002-0546-8695
https://orcid.org/0000-0002-0546-8695
Yu-Sheng Chang  https://orcid.org/0000-0002-2622-7524
https://orcid.org/0000-0002-2622-7524
Contributor Information
Chin-Fang Su, Division of Allergy, Immunology and Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei; Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, Taipei Medical University –Shuang Ho Hospital, New Taipei City.
Chien-Chih Lai, Division of Allergy, Immunology and Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; Institute of Clinical Medicine, National Yang-Ming University, Taipei.
Tzu-Hao Li, Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; Institute of Clinical Medicine, National Yang-Ming University, Taipei; Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, Shin Kong Wu Ho-Su Memorial Hospital, Taipei.
Yu-Fan Chang, Institute of Clinical Medicine, National Yang-Ming University, Taipei; Department of Ophthalmology, Taipei Veterans General Hospital, Taipei.
Yi-Tsung Lin, Division of Infectious Diseases, Department of Medicine, Taipei Veterans General Hospital, Taipei; Institute of Emergency and Critical Care Medicine, National Yang-Ming University, Taipei.
Wei-Sheng Chen, Division of Allergy, Immunology and Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; Institute of Clinical Medicine, National Yang-Ming University, Taipei.
Yen-Po Tsao, Division of Allergy, Immunology and Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei; Institute of Clinical Medicine, National Yang-Ming University, Taipei.
Wen-Hsiu Wang, Department of Medicine, Mackay Medical College, New Taipei City; Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei.
Yu-Sheng Chang, Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, Taipei Medical University–Shuang Ho Hospital, No. 291, Zhongzheng Road, Zhonghe District, New Taipei City 23561; Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei.
Chang-Youh Tsai, Division of Allergy, Immunology and Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei.
References
- 1. Colombo AL, de Almeida Júnior JN, Slavin MA, et al. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis 2017; 17: e344–e356. [DOI] [PubMed] [Google Scholar]
- 2. Kabir V, Maertens J, Kuypers D. Fungal infections in solid organ transplantation: an update on diagnosis and treatment. Transplant Rev 2019; 33: 77–86. [DOI] [PubMed] [Google Scholar]
- 3. Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly 2016; 146: w14281. [DOI] [PubMed] [Google Scholar]
- 4. Levitz SM. Overview of host defenses in fungal infections. Clin Infect Dis 1992; 14(Suppl. 1): S37–S42. [DOI] [PubMed] [Google Scholar]
- 5. Lord AK, Vyas JM. Host defenses to fungal pathogens. In: Rich RR, Fleisher TA, Shearer WT, et al. (eds) Clinical immunology: principles and practice. 5th ed. London: Elsevier, 2019, pp. 413–424. [Google Scholar]
- 6. Tselios K, Gladman DD, Sheane BJ, et al. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971-2013). Ann Rheum Dis 2019; 78: 802–806. [DOI] [PubMed] [Google Scholar]
- 7. Tektonidou MG, Wang Z, Dasgupta A, et al. Burden of serious infections in adults with systemic lupus erythematosus: a national population-based study, 1996-2011. Arthritis Care Res 2015; 67: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol 2018; 14: 1043–1053. [DOI] [PubMed] [Google Scholar]
- 9. Kedves M, Kósa F, Kunovszki P, et al. Large-scale mortality gap between SLE and control population is associated with increased infection-related mortality in lupus. Rheumatology 2020; 59: 3443–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azoulay E, Russell L, Van de, Louw A, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med 2020; 46: 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai CY, Li KJ, Hsieh SC, et al. What’s wrong with neutrophils in lupus? Clin Exp Rheumatol 2019; 37: 684–693. [PubMed] [Google Scholar]
- 12. Grammatikos AP, Tsokos GC. Immunodeficiency and autoimmunity: lessons from systemic lupus erythematosus. Trends Mol Med 2012; 18: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen HS, Tsai WP, Leu HS, et al. Invasive fungal infection in systemic lupus erythematosus: an analysis of 15 cases and a literature review. Rheumatology 2007; 46: 539–544. [DOI] [PubMed] [Google Scholar]
- 14. Chen D, Xie J, Chen H, et al. Infection in Southern Chinese Patients with systemic lupus erythematosus: spectrum, drug resistance, outcomes, and risk factors. J Rheumatol 2016; 43: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 15. Santamaría-Alza Y, Sánchez-Bautista J, Fajardo-Rivero JF, et al. Invasive fungal infections in Colombian patients with systemic lupus erythematosus. Lupus 2018; 27: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 16. Wang LR, Barber CE, Johnson AS, et al. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum 2014; 44: 325–330. [DOI] [PubMed] [Google Scholar]
- 17. Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 18. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 19. Vandenbroucke JP, von Elm E, Altman DG, et al. STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winthrop KL, Novosad SA, Baddley JW, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015; 74: 2107–2116. [DOI] [PubMed] [Google Scholar]
- 21. Vonk AG, Netea MG, van der Meer JW, et al. Host defence against disseminated Candida albicans infection and implications for antifungal immunotherapy. Expert Opin Biol Ther 2006; 6: 891–903. [DOI] [PubMed] [Google Scholar]
- 22. Segal BH. Aspergillosis. N Engl J Med 2009; 360: 1870–1884. [DOI] [PubMed] [Google Scholar]
- 23. Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 2015; 43: 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol 2017; 177: 47–62. [DOI] [PubMed] [Google Scholar]
- 25. Urban CF, Nett JE. Neutrophil extracellular traps in fungal infection. Semin Cell Dev Biol 2019; 89: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marr KA, Carter RA, Boeckh M, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100: 4358–4366. [DOI] [PubMed] [Google Scholar]
- 27. Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet 2003; 362: 1828–1838. [DOI] [PubMed] [Google Scholar]