Abstract
Acinetobacter baumannii strains resistant to both imipenem (IPM) and ceftazidime (CAZ) were isolated from 1994 through 1996 at Gunma University Hospital. Nine isolates from different inpatients were examined for carbapenem-hydrolyzing activity and for the carbapemase gene blaIMP by the PCR method. All nine isolates were carbapenemase-producing strains that hydrolyzed IPM and that harbored blaIMP. The blaIMP gene was transmissible by conjugation to an IPM-susceptible recipient strain of A. baumannii and conferred resistance to IPM, CAZ, cefotaxime (CTX), ampicillin (AMP), and piperacillin (PIP). Either intermediate or high-level resistance to amikacin (AMK) was transferred from two and five strains, respectively, concomitantly with blaIMP, and gentamicin (GEN) resistance was also transferred in one instance of high-level AMK resistance. Comparative examination of clinical isolates for resistance patterns to nine drugs, IPM, CAZ, CTX, aztreonam, AMP, PIP, AMK, GEN, and norfloxacin, in addition to pulsed-field gel electrophoresis patterns with NotI-digested genomic DNA, confirmed nosocomial transmission of infections involving carbapenemase-producing A. baumannii strains.
Acinetobacter baumannii is a glucose-nonfermentative gram-negative bacillus that is widely distributed in hospital environments and is one of the nosocomial pathogens that often causes serious infections, especially in immunocompromised inpatients (1, 8). Extensive use of antimicrobial chemotherapy against bacterial infections has contributed to the emergence and increase in the number of multidrug-resistant strains, especially among opportunistic pathogens such as members of the family Enterobacteriaceae and glucose-nonfermentative bacteria. Antimicrobial susceptibility testing and biological and genomic typing of Acinetobacter isolates have revealed the dissemination of drug-resistant strains in various hospitals (5, 13, 14, 20, 22, 24, 26).
We have detected A. baumannii strains resistant to imipenem (IPM) and various other β-lactam antibiotics including ceftazidime (CAZ). IPM is a potent β-lactam, partly because of its resistance to hydrolysis by most β-lactamases except carbapenemase (2). The latter enzyme, a metallo-β-lactamase belonging to molecular class B, is capable of hydrolyzing both IPM and CAZ, in addition to most β-lactam antibiotics, and confers resistance to these agents in pathogenic bacteria (11, 19). The nucleotide sequence of the gene encoding the carbapenem-hydrolyzing metallo-β-lactamase identified on a plasmid in Pseudomonas aeruginosa was completely identical to that of the Serratia marcescens gene named blaIMP (7, 16, 27). Furthermore, surveillance for the identification of blaIMP by PCR techniques revealed that the gene was disseminated among various gram-negative pathogens, especially in P. aeruginosa and S. marcescens (6, 15). Although IPM-resistant Acinetobacter species have been isolated, strains that produce the carbapenemase have not yet been reported (3, 17, 18, 21).
We detected the carbapenemase gene, blaIMP, in clinical isolates of IPM- and CAZ-resistant A. baumannii strains and investigated the strains for their dissemination mode in a single hospital.
MATERIALS AND METHODS
Bacterial strains.
IPM- and CAZ-resistant isolates of A. baumannii were obtained from different inpatients in Gunma University Hospital from 1994 through 1996.
Media.
Sheep blood agar (BBL) was used for isolation and purification of A. baumannii strains. Sensitivity testing (ST) agar and ST broth (Nissui Pharmaceutical Co., Ltd.) were used routinely for cultivation and drug susceptibility testing.
Antibacterial agents.
The abbreviations and sources of the antibacterial agents used in the study are as follows: amikacin (AMK) and IPM, Banyu Pharmaceutical Co., Ltd.; aztreonam (ATM), Bristol-Myers Squibb, Inc.; CAZ, Nippon Glaxo Co., Ltd.; cefotaxime (CTX), Hoechst Japan, Ltd.; gentamicin (GEN), Schering Corp.; norfloxacin (NOR), Kyorin Seiyaku Co., Ltd.; piperacillin (PIP), Toyama Chemical Co., Ltd.; and rifampin (RIF), Daiichi Pharmaceutical Co., Ltd.
Drug susceptibility tests.
MICs were determined by an agar dilution method with an inoculum size of 104 CFU per spot (27).
Assay of β-lactamase activity.
Exponentially growing cells of A. baumannii in ST broth were washed with 50 mM phosphate buffer (pH 7.0) and were disrupted by sonication. The supernatant obtained after the cellular debris was removed by centrifugation (15,000 × g, 15 min, 4°C) was used as the crude enzyme extract.
β-Lactamase activity was determined spectrophotometrically by previously described methods (27). One unit of enzyme activity was defined as the amount of enzyme that hydrolyzed 1 μmol of substrate per min at 30°C.
PCR amplification.
The PCR consisted of 25 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and amplification at 72°C for 1.5 min, followed by an additional 7 min at 72°C, with the Premix Taq reagent (Takara Shuzo Co., Ltd.) used with the Program Template Control System PC-700 (ASTEC Co., Ltd.).
The nucleotide sequences of the forward and reverse primers used for detection of the carbapenemase gene, blaIMP, were those constructed by Senda et al. (23). The PCR product was 587 bp, was derived from within the 741-bp blaIMP sequence, and was detected by agarose gel electrophoresis.
Conjugation experiment.
Conjugal transmissibility of blaIMP was examined with the recipient strain A. baumannii TY44Rp, a RIF-resistant mutant of strain TY44, which was a clinical isolate that lost IPM resistance after storage in Casitone medium agar.
The membrane filter method was used for conjugation. A mixture of donor and recipient cells in broth cultures at an early stationary phase of growth was filtered through membranes (pore size, 0.45 μm), and those bacterial cells that were retained were placed on ST agar plates overnight at 30°C. The transmissibility of blaIMP was determined by transfer of CAZ resistance rather than IPM resistance, because the level of CAZ resistance expressed by blaIMP was high enough to distinguish transconjugants from the recipient. Transconjugant cells were selected on agar plates containing 8 μg of CAZ per ml and 100 μg of RIF per ml. The transfer frequency was expressed as the ratio of the number of transconjugants to the number of donor cells in the conjugation mixture.
Pulsed-field gel electrophoresis (PFGE).
Genomic DNA was prepared in agarose plugs that had been treated with lysozyme and proteinase K by using the GenePath Reagent kit (Bio-Rad) by the procedures recommended by the manufacturer. The DNA was then digested with 12.5 U of the restriction endonuclease NotI. The DNA segments generated were separated in a 1% agarose gel and were run in Tris-borate-EDTA buffer on the pulsed-field apparatus (Gene Path System; Bio-Rad) at 6.0 V/cm for 19.7 h, with pulse times ranging from 5.3 to 34.9 s.
RESULTS
Isolation of IPM-resistant A. baumannii.
A total of 251 A. baumannii strains were isolated from different inpatients during the 3 years from 1994 to 1996 in Gunma University Hospital, and 28 of these were resistant to both IPM (MICs, ≧8 μg/ml) and CAZ (MICs, ≧16 μg/ml). However, the resistance was not stable, because 19 of the 28 strains lost resistance to both IPM and CAZ after storage for a year or more in Casitone medium. The remaining nine strains that harbored IPM and CAZ resistance are listed in Table 1. The biochemical properties of these isolates were examined with the API NE20 system and were found to be the same. They were isolated from various sites from different inpatients on six wards at different times. Three of the nine isolates were obtained from patients on intensive care units (ICUs) after they were moved there from other wards.
TABLE 1.
Origins of imipenem-resistant A. baumannii strains
| Strain | Isolation
|
||
|---|---|---|---|
| Date (yr, mo) | Ward | Source | |
| TY7 | 1994, February | 1st internal medicine | Urine |
| TY8 | 1994, April | 1st internal medicine | Oral fluid |
| TY9 | 1994, July | 1st internal medicine | Sputum |
| TY10 | 1994, August | Dermatology | Bedsore |
| TY11 | 1994, September | 2nd surgery (ICU) | Blood |
| TY12 | 1994, September | 2nd surgery (ICU) | Pus |
| TY15 | 1995, February | Otolaryngology (ICU) | Drainage |
| TY40 | 1996, June | 2nd internal medicine | Sputum |
| TY42 | 1996, May | 2nd internal medicine | Sputum |
Detection of carbapenemase activity and blaIMP gene.
Nine IPM- and CAZ-resistant isolates and one susceptible strain (TY44; MICs of IPM and CAZ, <0.5 μg/ml), whose resistance had been lost after storage, were examined for IPM-hydrolyzing activity with crude enzyme extracts and for the blaIMP gene by the PCR method. All of the strains except for TY44 were capable of hydrolyzing IPM and were PCR positive, indicating that they harbored blaIMP (Table 2).
TABLE 2.
Properties of IPM- and CAZ-resistant A. baumannii strains
| Strain | Hydrolysis of IPM (sp act)a | Detection of blaIMP gene | Conjugal transferability of IPM and CAZ resistanceb |
|---|---|---|---|
| TY7 | 0.51 | + | 10−3 |
| TY8 | 0.36 | + | 10−3 |
| TY9 | 0.28 | + | 10−4 |
| TY10 | 0.38 | + | 10−3 |
| TY11 | 0.39 | + | 10−2 |
| TY12 | 0.59 | + | 10−3 |
| TY15 | 0.63 | + | 10−3 |
| TY40 | 1.43 | + | 10−2 |
| TY42 | 0.58 | + | 10−3 |
| TY44c | <0.01 | − | Not done |
See Materials and Methods for description of specific activity. One unit of enzyme activity is the amount of enzyme that hydrolyzed 1 μmol of substrate per min at 30°C.
Transfer frequency was expressed as the ratio of the number of transconjugant cells to the number of recipient cells.
Susceptible strain.
Transfer of carbapenemase gene by conjugation.
The IPM- and CAZ-resistant strains were mated with TY44Rp on membrane filters, and transconjugants were selected on ST agar containing both CAZ and RIF. Transconjugants were obtained at frequencies of 10−2 to 10−4 per donor cell from all nine strains (Table 2). For all transconjugants, the MICs of IPM were higher than those for the recipient strain and the gene blaIMP was detectable by PCR (data not shown).
Susceptibilities of IPM-resistant A. baumannii strains to various chemotherapeutic agents.
The drug susceptibilities of the clinical isolates and their transconjugants were determined and are shown in Table 3. The IPM MICs for nine clinical isolates were 8 to 16 μg/ml, and IPM resistance was accompanied by resistance to CAZ, CTX, and PIP but not always by resistance to ATM. Seven strains were highly resistant (MICs, >128 μg/ml) or intermediately resistant (MICs, 32 to 64 μg/ml) to AMK, and six strains were resistant to GEN (MICs, 8 to 16 μg/ml). Four strains were obviously resistant to NOR (MICs, 8 to 16 μg/ml). Identical drug resistance patterns were observed between TY11 and TY12 and between TY40 and TY42. The latter two strains, which were isolated relatively late in the study, showed characteristic resistance to multiple potent chemotherapeutic agents such as β-lactams, aminoglycosides, and quinolones.
TABLE 3.
Susceptibilities of A. baumannii strains to various drugs
| Strain | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IPM | CAZ | CTX | ATM | AMP | PIP | AMK | GEN | NOR | |
| Clinical isolate | |||||||||
| TY11 | 8 | >128 | 128 | 16 | 128 | 64 | 2 | 16 | 8 |
| TY12 | 8 | >128 | 128 | 16 | 128 | 64 | 2 | 16 | 8 |
| TY40 | 8 | >128 | >128 | 32 | >128 | 128 | 32 | 16 | 16 |
| TY42 | 8 | >128 | >128 | 32 | >128 | >128 | 32 | 16 | 16 |
| TY8 | 8 | 128 | 128 | 8 | 64 | 32 | 64 | 8 | 2 |
| TY9 | 8 | >128 | 128 | 4 | 128 | 128 | >128 | 16 | <1 |
| TY7 | 8 | >128 | 128 | 16 | 128 | 32 | 32 | 4 | 4 |
| TY10 | 16 | >128 | 128 | 16 | 32 | 32 | >128 | 16 | 2 |
| TY15 | 16 | >128 | 128 | 8 | 64 | 64 | 32 | 8 | 2 |
| Transconjugant from: | |||||||||
| TY11 | 2 | 16 | 32 | <1 | 32 | 16 | 1 | 1 | <1 |
| TY12 | 4 | 32 | 64 | <1 | 64 | 16 | 1 | 1 | <1 |
| TY40 | 4 | 16 | 64 | <1 | >128 | 128 | 8 | 2 | <1 |
| TY42 | 4 | 16 | 64 | <1 | >128 | 128 | 4 | 1 | <1 |
| TY8 | 8 | 32 | 128 | <1 | 32 | 8 | 32 | <1 | <1 |
| TY9 | 8 | 32 | 128 | <1 | 32 | 16 | 64 | <1 | <1 |
| TY7 | 4 | 64 | 64 | <1 | 64 | 16 | 64 | <1 | <1 |
| TY10 | 8 | 16 | 128 | <1 | 32 | 8 | 64 | 8 | <1 |
| TY15 | 4 | 32 | 32 | <1 | 32 | 8 | 64 | <1 | <1 |
| Recipient, TY44Rp | <1 | <1 | 4 | <1 | 8 | 1 | 1 | <1 | <1 |
In all cases, resistance to the β-lactam antibiotics IPM, CAZ, CTX, ampicillin (AMP), and PIP was transferred concomitantly, whereas neither ATM nor NOR resistance was transmissible. Transconjugants obtained from strains TY11 and TY12 or from strains TY40 and TY42 had almost identical drug resistance patterns. High-level resistance to AMP and PIP was transferred from the latter two strains. Transfer of high-level or intermediate resistance to AMK was observed from five strains, strains TY7, TY8, TY9, TY10, and TY15. Resistance to GEN was transferred from TY10.
PFGE patterns of A. baumannii strains.
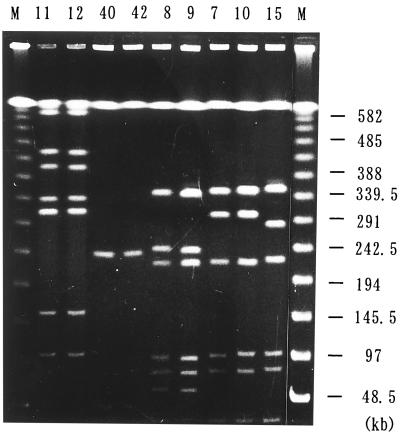
The PFGE patterns after digestion with restriction endonuclease NotI are shown in Fig. 1, in which strains with identical patterns were placed in adjacent lanes. The following five different patterns were obtained for nine TY strains: pattern 1, strains TY11 and TY12; pattern 2, strains TY40 and TY42; pattern 3, strains TY8 and TY9; pattern 4, strains TY7 and TY10; and pattern 5, strain TY15. The two strains with pattern 1 (TY11 and TY12) and those with pattern 2 (TY40 and TY42) also had identical drug resistance patterns and transmissible drug resistance patterns (Table 3) and were isolated from the same ward and in the same month of 1994 and within a 3-month period in 1996, respectively (Table 1). The two strains with pattern 3 (TY8 and TY9) were isolated from the same ward in 1994 within 3 months of each other (Table 1), but the levels of resistance to PIP and AMK were higher for the latter strain. The drug resistance patterns of the transconjugants of these strains were identical (Table 3). The two strains with pattern 4 (TY7 and TY10) were isolated from different wards in 1994, and the AMP, AMK, and GEN MICs for the strains were different. Resistance to GEN was transferred from TY10 along with the gene blaIMP.
FIG. 1.
PFGE patterns of genomic DNAs from A. baumannii isolates. The TY numbers of 9 A. baumannii isolates are indicated above the lanes. The size markers of the DNA segments are indicated in lanes M, with sizes shown on the right.
The presence of strains with identical PFGE and drug resistance patterns or with identical PFGE patterns and similar drug resistance patterns strongly suggested that different inpatients in the same or different wards were infected with the same strain or with derivatives of the same strain.
DISCUSSION
Acinetobacter spp. are important causes of nosocomial infections, and it has been reported that they acquire resistance to chemotherapeutic agents in various hospitals (1, 13, 20, 22, 26). Although β-lactam antibiotics are potent chemotherapeutic agents, Acinetobacter strains resistant to multiple β-lactam antibiotics, mainly due to hydrolysis by β-lactamases, have been increasing (14, 25). Some β-lactamases in A. baumannii were reported to be involved in resistance to IPM, but their catalytic activities for IPM were very low or were barely detectable (4, 17).
We isolated A. baumannii strains resistant to multiple β-lactam antibiotics, including IPM and CAZ, and in those strains we detected IPM-hydrolyzing activity and the carbapenemase gene blaIMP.
The blaIMP-bearing strains of A. baumannii isolated during a 3-year period in our hospital were typed according to their patterns of resistance to nine drugs and by PFGE analysis of the genome. PFGE pattern analysis has been reported to be the most discriminatory method for the genomic typing of nosocomial Acinetobacter spp. (1, 12). The restriction enzyme NotI was chosen for digestion of DNA, because the number of cutting sites associated with NotI is fewer than that associated with ApaI or SmaI, simplifying discrimination of digestion patterns.
Among nine isolates, two types each consisted of two strains from the same ward, and the strains had identical resistance and PFGE patterns. They were all isolated from different inpatients, indicating nosocomial transmission. Strains of one type, strains TY40 and TY42, were resistant to six β-lactams, IPM, CAZ, CTX, ATM, AMP, and PIP, as well as two aminoglycosides, AMK and GEN, and a quinolone. The resistance of these strains to all β-lactams except ATM was transmissible, resulting from the transfer of blaIMP, and intermediate resistance to AMK and GEN was transferred concomitantly. Transfer of high-level AMK resistance accompanied by transfer of blaIMP was observed in five other isolates, two of which, TY8 and TY9, were from the same ward and had identical PFGE patterns, although the MICs of PIP and AMK were slightly different. The drug resistance patterns that were transferred from these two strains were identical. They were probably derived from the same strain with some additional changes in the MICs for strains from different hosts.
IPM resistance encoded by blaIMP appeared to be readily lost after prolonged storage in Casitone medium. Furthermore, this resistance was transmissible by conjugation to susceptible strains. These findings strongly suggest that blaIMP is a foreign gene introduced from another species of bacteria and is retained only in environments supplied with IPM.
The frequencies of conjugal transfer of blaIMP were rather high in our study. This was mainly because of the availability of the proper recipient. We used a RIF-resistant mutant of a blaIMP-negative strain, strain TY44, which was a clinical isolate that lost the gene after storage in Casitone medium agar. It was suggested that such a strain like TY44 would be a good recipient for the blaIMP gene.
We attempted to detect plasmid DNA in our isolates, but the physical identification was unsuccessful.
In Acinetobacter spp. from various geographic areas in France, dissemination of an AMK resistance gene was reported. The gene was conjugally transmissible in some strains, although the plasmids that carried the AMK resistance gene were not revealed (9).
On the other hand, one transmissible plasmid of 63 kb and another of ca. 45 kb were reported to confer resistance to AMK and IPM, respectively, in A. baumannii strains (10, 21). The resistance to IPM was due to a β-lactamase but not a carbapenemase, since hydrolysis of IPM was not demonstrated by enzyme assay but was demonstrated only by microbiological methods.
Acquisition of blaIMP and nosocomial transmission of A. baumannii are important considerations for chemotherapy, especially in instances of multidrug resistance to aminoglycosides and quinolones as well as to β-lactams.
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid for Scientific Research [grant (C) 08670300] from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Bergogne-Berezin E, Towner K J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush K, Jacoby G A, Medeiros A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehrlein M, Leying H, Cullmann W, Wendt S, Opferkuch W. Imipenem resistance in Acinetobacter baumannii is due to altered penicillin-binding proteins. Chemotherapy (Basel) 1991;37:405–412. doi: 10.1159/000238887. [DOI] [PubMed] [Google Scholar]
- 4.Hornstein M, Sautjeau-Rostoker C, Peduzzi J, Vessieres A, Hong L T H, Barthelemy M, Scavizzi M, Labia R. Oxacillin-hydrolyzing β-lactamase involved in resistance to imipenem in Acinetobacter baumannii. FEMS Microbiol Lett. 1997;153:333–339. doi: 10.1111/j.1574-6968.1997.tb12593.x. [DOI] [PubMed] [Google Scholar]
- 5.Horrevorts A, ten Hagen G, Hekster Y, Tjernberg I, Dijkshoorn L. Development of resistance to ciprofloxacin in Acinetobacter baumannii strains isolated during a 20-month outbreak. J Antimicrob Chemother. 1997;40:460–461. doi: 10.1093/jac/40.3.460. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyobe S, Minami S, Yamada H. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 8.Jawad A, Seifert H, Snelling A M, Heritage J, Hawkey P M. Survival of Acinetobacter baumannii on dry surface: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert T, Gerbaud G, Bouvet P, Vieu J F, Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990;34:1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert T, Gerbaud G, Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3′-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988;32:15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore D M. Carbapenemases. J Antimicrob Chemother. 1992;29:609–616. doi: 10.1093/jac/29.6.609. [DOI] [PubMed] [Google Scholar]
- 12.Marcos M A, Jimenez de Anta M T, Vila J. Correlation of six methods for typing nosocomial isolates of Acinetobacter baumannii. J Med Microbiol. 1995;42:328–335. doi: 10.1099/00222615-42-5-328. [DOI] [PubMed] [Google Scholar]
- 13.Marques M B, Waites K B, Mangino J E, Hines B B, Moser S A. Genotypic investigation of multidrug-resistant Acinetobacter baumannii infections in a medical intensive care unit. J Hosp Infect. 1997;37:125–135. doi: 10.1016/s0195-6701(97)90182-1. [DOI] [PubMed] [Google Scholar]
- 14.Melissa A, Jacobs M R, Moore T D, Renzi F A, Appelbaum P C. Activities of β-lactams against Acinetobacter genospecies as determined by agar dilution and E-test methods. Antimicrob Agents Chemother. 1997;41:767–770. doi: 10.1128/aac.41.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami S, Akama M, Araki H, Watanabe Y, Narita H, Iyobe S, Mitsuhashi S. Imipenem and cephem resistant Pseudomonas aeruginosa carrying plasmids for class B β-lactamases. J Antimicrob Chemother. 1996;37:433–444. doi: 10.1093/jac/37.3.433. [DOI] [PubMed] [Google Scholar]
- 16.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paton R, Miles R S, Hood J, Amyes S G B. ARI 1: β-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int J Antimicrob Agents. 1993;2:81–88. doi: 10.1016/0924-8579(93)90045-7. [DOI] [PubMed] [Google Scholar]
- 18.Perilli M, Felici A, Oratore A, Bonfiglio G, Rossolini G M, Amicosante G. Characterization of the chromosomal cephalosporinases produced by Acinetobacter lwoffii and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother. 1996;40:715–719. doi: 10.1128/aac.40.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley T V, Webb S A, Cadwallader H, Briggs B D, Christiansen L, Bowman R A. Outbreak of gentamicin-resistant Acinetobacter baumannii in an intensive care unit: clinical, epidemiological and microbiological features. Pathology. 1996;28:359–363. doi: 10.1080/00313029600169354. [DOI] [PubMed] [Google Scholar]
- 21.Scaife W, Young H K, Paton R H, Amyes S G B. Transferable imipenem-resistance in Acinetobacter species from a clinical source. J Antimicrob Chemother. 1995;36:585–586. doi: 10.1093/jac/36.3.585. [DOI] [PubMed] [Google Scholar]
- 22.Seifert H, Baginski R, Schulze A, Pulverer G. Antimicrobial susceptibility of Acinetobacter species. Antimicrob Agents Chemother. 1993;37:750–753. doi: 10.1128/aac.37.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traub W H, Spohr M. Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. baumannii, A. haemolyticus, genospecies 3, and genospecies 6) Antimicrob Agents Chemother. 1989;33:1617–1619. doi: 10.1128/aac.33.9.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahaboglu H, Ozturk R, Aygun G, Coskunkan F, Yaman A, Kaygusuz A, Leblebiciglu H, Balik I, Aydin K, Otkun M. Widespread detection of PER-type extended-spectrum β-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob Agents Chemother. 1997;41:2265–2269. doi: 10.1128/aac.41.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vila J, Ruiz J, Navia M, Becerril B, Garcia I, Perea S, Lopez-Hernandez I, Alamo I, Ballester F, Planes A M, Martinez-Beltran J, Jimenez de Anta T. Spread of amikacin resistance in Acinetobacter baumannii strains in Spain due to an epidemic strain. J Clin Microbiol. 1999;37:758–761. doi: 10.1128/jcm.37.3.758-761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]