Abstract
The immunogenicity of SARS-CoV-2 vaccines in cancer patients receiving radiotherapy is unknown. This prospective cohort study demonstrates that anti-SARS-CoV-2 spike antibody and neutralization titers are reduced in a subset of thoracic radiotherapy patients, possibly due to immunosuppressive conditions. Antibody testing may be useful to identify candidates for additional vaccine doses.
Keywords: SARS-CoV-2, mRNA vaccine, COVID-19, Lung cancer, Radiotherapy, Immunogenicity
Cancer patients are particularly vulnerable to SARS-CoV-2 infection, which is associated with increased risks of severe disease and death [1], [2]. In particular, patients with lung cancer fare poorly upon infection [3]. At the same time, accumulating evidence suggests that underlying cancer and administration of certain anti-cancer therapies can negatively affect the immune response to SARS-CoV-2 vaccines [4], [5], [6]. For example, in a recent study of 131 cancer patients who were administered SARS-CoV-2 mRNA vaccines, seroconversion as assessed by measurement of anti-spike antibody titer was reduced in patients with hematologic malignancies and in those receiving cytotoxic chemotherapy or the monoclonal antibody rituximab [5]. Nonetheless, there is currently a paucity of data on the immunogenicity of SARS-CoV-2 vaccines in specific subsets of patients with solid tumors including those with thoracic malignancies, early-stage disease, and those receiving radiotherapy.
In this rapidly evolving field, booster vaccine doses have recently been recommended by the United States Food and Drug Administration (FDA) for immunosuppressed individuals and other populations [7]. Emerging data support the efficacy of a third mRNA vaccine dose in organ transplant patients or those on hemodialysis [8], [9]. In these studies, among patients who had no detectable anti-spike antibody response to an initial mRNA vaccination schedule, approximately 40% developed an antibody response to an additional dose. In the population of patients with cancer, the immunogenicity of approved SARS-CoV-2 vaccines is not routinely assessed by measuring antibody titers or neutralization assays. Further, the immunogenicity and duration of effect of additional vaccine doses in specific subsets of patients with solid tumors remains largely unknown [10]. This constitutes an area of unmet need.
In the current prospective cohort study, we focused on determining the immunogenicity of SARS-CoV-2 vaccines approved by the FDA under Emergency Use Authorization (EUA) in a population of patients with thoracic malignancies that were treated with radiotherapy. Findings reported herein are suggestive of a subset of patients exhibiting a poor immune response to current SARS-CoV-2 vaccination schedules. The data highlight the urgent need for further investigations in this space to ultimately better protect patients with lung and other solid tumors from COVID-19 infection.
Patients and methods
Study population
The current report constitutes a subset analysis of the Cancer, Covid and Vaccination (CANVAX) prospective cohort study at Massachusetts General Hospital (MGH) reported elsewhere [10]. This study was approved by the Mass General Brigham Institutional Review Board (2021P000746). The analysis is based on CANVAX participants with completed baseline survey and post-vaccination antibody testing from April 21 through July 21, 2021. Consecutive patients with thoracic malignancies treated on the Thoracic Radiation Oncology Service between December 1, 2020 and April 30, 2021 were screened for eligibility (Supplementary Fig. S1). All participants completed SARS-CoV-2 vaccination. To be eligible for the current analysis, vaccination was to be received within three weeks of starting radiotherapy or chemotherapy (when given sequentially with radiation), during radiotherapy, or within four months post-radiotherapy completion. After informed consent was obtained, participants completed a standardized questionnaire that included questions about baseline demographics, medical history, SARS-CoV-2 exposures and infection, and vaccination information. Additional clinical information was abstracted from the electronic medical record, including cancer type, stage, medical comorbidities, medications, radiotherapy, and systemic therapy. Comparison cohorts of patients with thoracic malignancies who did not receive radiotherapy (n = 181) and healthy vaccinated controls (n = 187) were taken from reference [10].
Antibody assays
Blood was collected in serum separator tubes at least two weeks after completion of vaccination and antibody assays were performed with the Roche Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics, Indianapolis), at the CLIA-certified MGH Core Clinical laboratory. Total anti-spike (IgA/M/G) antibody concentrations >2500 U/ml triggered additional manual dilution to yield titers up to 250,000 U/ml. An antibody binding index greater than 0.8 was defined as positive [10]. Participants with a negative test result were offered confirmatory testing 7–14 days later.
Assessment of neutralization
We measured neutralization with a SARS-CoV-2 pseudovirus neutralization assay, described elsewhere [11], [12]. Briefly, lentiviral particles encoding luciferase and ZsGreen reporter genes were pseudotyped with SARS-CoV-2 spike protein as present in the Wuhan COVID-19 strain. Particles were generated in 293T cells, titered using ZsGreen expression by flow cytometry, and utilized in an automated neutralization assay with 50–250 infectious units of pseudovirus co-incubated with serial dilutions of serum. Neutralization was then assayed on 293T-ACE2 cells. A pseudovirus neutralization titer 50 (pNT50) was calculated by taking the inverse of the serum concentration that achieved 50% neutralization of SARS-CoV-2 pseudotyped lentivirus particles entry into cells. For interpretative purposes, a value of 20% of the geometric mean titer of convalescent donors was calculated as 27.6 [10], [13].
Results and discussion
Patient and treatment characteristics
Out of 166 patients screened in the Thoracic Radiation Oncology Service, 33 patients who received thoracic radiotherapy met inclusion criteria for this study and were analyzable (Supplementary Fig. S1). The median age at time of vaccination was 68 years (range, 46–90 years) (Table 1 ). The majority of patients (70%) had localized (stage I–III) non-small lung cell carcinoma or small cell lung carcinoma (SCLC). Fourteen patients were treated with stereotactic body radiotherapy and 19 received fractionated palliative or definitive radiotherapy. Thirteen patients received chemotherapy, which can negatively affect SARS-CoV-2 vaccine immunogenicity [10], either concurrently or sequentially with radiotherapy. Nine patients, four of whom also received chemotherapy, had potentially immunosuppressive medical conditions, listed in Table 1, as defined by the Centers for Disease Control and Prevention [14]. Thus, there were a total of 18 participants with at least one immunosuppressive treatment or condition
Table 1.
Patient and treatment characteristics (n = 33).
| n (%) | Median (range) | |
|---|---|---|
| Age | ||
| Years | 68 (46–90) | |
| Gender | ||
| Female | 19 (58) | |
| Male | 14 (42) | |
| Cancer type | ||
| NSCLC | 31 (94) | |
| SCLC | 2 (6) | |
| AJCC 8th ed. stage | ||
| I | 12 (37) | |
| II | 1 (3) | |
| III | 10 (30) | |
| IV | 10 (30) | |
| Immunosuppressive condition | ||
| No | 15 (45) | |
| Yes | 18 (55) | |
| Medication* | 2 | |
| Hematologic malignancy** | 1 | |
| CKD | 6 | |
| Chemotherapy*** | 13 | |
| Thoracic radiotherapy | ||
| SBRT | 14 (42) | |
| Total Gy | 50 (24–50) | |
| Other | 19 (58) | |
| Total Gy | 60 (30–70.5) | |
| Radiotherapy target | ||
| Lung only | 20 (61) | |
| Lung/mediastinum | 7 (21) | |
| Hilum/mediastinum | 5 (15) | |
| Bone | 1 (3) |
NSCLC, non-small cell lung carcinoma; SCLC, small cell lung carcinoma; AJCC, American Joint Committee on Cancer; ed., edition; CKD, chronic kidney disease; SBRT, stereotactic body radiotherapy.
Immunosuppressive medication.
Chronic lymphocytic leukemia.
Sequential or concurrent chemotherapy, in four participants co-occurred with another immunosuppressive condition.
SARS-CoV-2 vaccination
Of the 33 participants, 10, 21, and two received two doses of mRNA-1273 (Moderna), two doses of BNT162b2 (Pfizer/BioNTech), and one dose of Ad26.COV2.S (Johnson & Johnson), respectively, during the time period of January 30 to April 30, 2021. Most participants (79%) started radiotherapy prior to vaccination, with a median time of 49 days (interquartile range (IQR), 12–77 days) from beginning of radiotherapy to the day of complete vaccination (Supplementary Fig. S2A). Blood draws for determination of antibody titers as part of the research protocol were performed at a median interval of 87 days (IQR, 60–106 days) following vaccination. The last blood draw was performed on July 21, 2021. Of note, none of the 33 participants had a documented prior SARS-CoV-2 infection and only one had a measurable anti-nucleocapsid antibody titer suggesting prior asymptomatic infection (data not shown).
Anti-SARS-CoV-2 spike antibody and neutralization titers
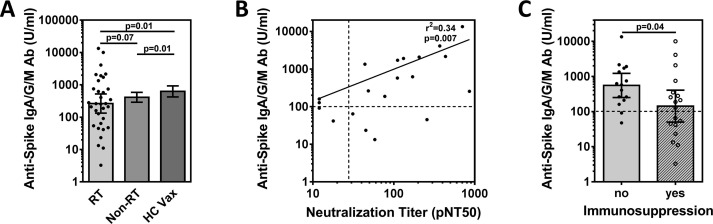
Antibody responses to the three SARS-CoV-2 vaccines under study are directed against the viral spike protein. We analyzed combined anti-spike IgA/G and M antibody concentrations (Fig. 1 A), and neutralization titers (Supplementary Fig. S2B). Interestingly, the geometric mean spike antibody concentration in log10 U/ml (GMC) was 2.42 (95% CI, 2.13–2.72), which trended lower than in patients with thoracic malignancies from our institution who did not receive radiotherapy (GMC = 2.62; 2.46–2.77; p = 0.07) (Fig. 1A) and appeared also lower than in previously reported cancer cohorts [4], [5]. In contrast, vaccinated healthy controls had higher spike antibody concentrations (GMC = 2.80; 2.63–2.97; p = 0.01).
Fig. 1.
Measures of SARS-Cov-2 vaccine immunogenicity in cancer patients who received thoracic radiotherapy. (A) Left bar, Quantitative SARS-CoV-2 spike IgG/A/M antibody concentrations (Roche Elecsys Anti-SARS-CoV-2 assay) in U/ml of serum at a median interval of 12 weeks after complete vaccination in participants who received thoracic radiotherapy (RT). Dots indicate individual concentrations (n = 33). Middle bar, geometric mean antibody concentration in patients with thoracic malignancies who did not receive radiotherapy (n = 181). Right bar, healthy controls vaccinated (HC Vax) for COVID (n = 187). Error bars indicate 95% CI around the geometric mean. (B) Correlation of spike antibody concentrations with pseudovirus neutralization titer 50 (pNT50), which was defined as the titer at which the serum achieves 50% neutralization of SARS-CoV-2 wild-type pseudovirus entry into ACE2 expressing 293T cells (in 20/33 thoracic radiotherapy patients for whom neutralization titers were available). Solid line represents linear regression. Dotted vertical line corresponds to a pNT50 titer of 27.6 equivalent to 20% of the convalescent titer that is predicted to be associated with 50% protection. (C) Data from thoracic radiotherapy patients in Panel (A) grouped according to immunosuppressive condition listed in Table 1. Statistical comparisons by Mann Whitney Test on log transformed values, two-sided.
There was a weak negative correlation of spike antibody concentrations with age (Supplementary Fig. S2C). Neutralization titers were available in 20 participants, with a geometric mean neutralization titer in log10 units (GMT) of 1.94. Neutralization and spike antibody titers were significantly correlated with each other (p = 0.007) (Fig. 1B). While there is no established threshold indicating protection against SARS-CoV-2, a neutralization titer greater than 20% of the GMT in convalescent individuals corresponds to a 50% reduction in infection risk in modeling studies [13]. Four participants (25%) had a titer <20% of GMT and all of them had spike antibody titers of only approximately 100 U/ml or less.
Vaccine immunogenicity as a function of immune status
Of the four participants with a neutralization titer <20% GMT, one had chronic lymphocytic leukemia and one underwent concurrent chemotherapy – known risk factors for a poor immune response to SARS-CoV-2 vaccines [4], [15]. The other two had no identifiable immunosuppressive conditions though one received the Ad26.COV2.S vaccine which may be associated with lower immunogenicity [10].
The patient with the second highest neutralization titer also had the highest spike antibody titer among all participants. Remarkably, she experienced an abscopal effect from radiotherapy which overlapped with her vaccination schedule, suggesting the presence of a uniquely active host immune response.
We next grouped all 33 participants according to the presence (n = 18) or absence (n = 15) of a co-existing, potentially immunosuppressive condition, including the receipt of sequential or concurrent chemotherapy with radiotherapy (Table 1). For this comparison, we analyzed anti-spike antibody concentrations as we were not able to acquire neutralization titers from all participants (Fig. 1C). Participants in the immunosuppressed group had a significantly lower antibody GMC than the non-immunosuppressed group (p = 0.04). There was also a higher percentage of participants with immunosuppressive conditions who had antibody levels <100 U/ml compared to participants without (44% vs 13%). The GMC of patients in the non-immunosuppressed group was not statistically different from the GMC of healthy controls (p = 0.3), suggesting that radiotherapy in of itself does not affect antibody levels. However, further study with larger sample sizes will be needed to answer this question definitively.
Interestingly, one participant who did not have neutralization titers performed was treated with sequential chemoradiotherapy for stage II SCLC and had co-existing rheumatoid arthritis for which he was taking adalimumab, a tumor necrosis factor inhibitor. His initial spike antibody titer taken eight weeks after the second shot of mRNA-1273 was only 11.1 U/ml and upon repeat draw was 8.24 U/ml. Outside of this study, he was offered a third dose of mRNA-1273 after which his titer transiently peaked at 1061 U/ml (Supplementary Fig. S3A). Three more patients received additional vaccine doses, with one patient also experiencing a similar spike antibody increase (Supplementary Fig. S3B). These observations illustrate the promise of additional vaccine doses in selected patients while the durability of the effect requires further study.
Limitations
The number of participants in this cohort study is small which limits both comparisons with other studies and subset analysis of the data. For example, it would be interesting to determine if the size/location of radiotherapy target volumes would affect vaccine immunogenicity through lymphopenia [16]; however, larger cohorts will be needed. Measures of immune response analyzed here served as a surrogate measure of protection against infection while we could not assess the incidence of breakthrough SARS-CoV-2 infections and their severity during the short study time. We only assessed neutralization of the original Wuhan strain of SARS-CoV-2 but other variants may show much lower neutralization [12].
Conclusions
Despite the small sample size, the observed variations in spike antibody and neutralization titers support the notion of heterogeneity in the immunogenicity of SARS-CoV-2 vaccines in the thoracic radiotherapy population, which is characterized by advanced age and medical co-morbidities. Larger studies are warranted to define the impact of specific immunosuppressive conditions, and of radiotherapy itself, on the vaccine response in patients with localized solid tumors. The observed increase in spike antibody titer in an immunosuppressed patient who received a third vaccine dose upon discovering low initial spike antibody titers illustrates the potential utility of antibody testing in this vulnerable population. Further research is urgently needed to better understand the immunogenicity of additional vaccine doses and duration of effect.
Funding
Funded in part by Lambertus Family Foundation and Donald Glazer Fund.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Gainor has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech, Ariad/Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Moderna, AstraZeneca, Pfizer, Novartis, Merck, and GlydeBio; research support from Novartis, Genentech/Roche, and Ariad/Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. None of the other authors has declared a competing interest.
Acknowledgements
The authors wish to thank Claire Pernat for her kind assistance in organizing the study, Dr. Shruthi Mahalingaiah for a critical review of the manuscript, and our patients for their willingness to participate.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2021.11.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bakouny Z., Hawley J.E., Choueiri T.K., Peters S., Rini B.I., Warner J.L., et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper-Vallillo A.J., Mooradian M.J., Meador C.B., Yeap B.Y., Peterson J., Sakhi M., et al. Coronavirus disease 2019 infection in a patient population with lung cancer: incidence, presentation, and alternative diagnostic considerations. JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barriere J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addeo A., Shah P.K., Bordry N., Hudson R.D., Albracht B., Di Marco M., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098 e1092. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monin L., Laing A.G., Munoz-Ruiz M., McKenzie D.R., Del Molino Del I., Barrio T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.USFDA, FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations, 2021.
- 8.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longlune N., Nogier M.B., Miedouge M., Gabilan C., Cartou C., Seigneuric B., et al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naranbhai V., Pernat C.A., Gavralidis A., St Denis K.J., Lam E.C., Spring L.M., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX Cohort Study. J Clin Oncol. 2021:2101891. doi: 10.1200/JCO.21.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488 e411. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383 e2379. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 14.Oliver S. ACIP Meeting, Centers for Disease Control and Prevention; 2021. Data and clinical considerations for additional doses in immunocompromised people. [Google Scholar]
- 15.Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyay R., Venkatesulu B.P., Giridhar P., Kim B.K., Sharma A., Elghazawy H., et al. Risk and impact of radiation related lymphopenia in lung cancer: A systematic review and meta-analysis. Radiother Oncol. 2021;157:225–233. doi: 10.1016/j.radonc.2021.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.