Abstract
Duchenne muscular dystrophy (DMD) is an X-linked lethal muscle disorder characterized by primary muscle degeneration. Therapeutic strategies for DMD have been extensively explored, and some are in the stage of human clinical trials. Along with the development of new therapies, sensitive outcome measures are needed to monitor the effects of new treatments. Therefore, we investigated outcome measures such as biomarkers and motor function evaluation in a dystrophic model of beagle dogs, canine X-linked muscular dystrophy in Japan (CXMDJ). Osteopontin (OPN), a myogenic inflammatory cytokine, was explored as a potential biomarker in dystrophic dogs over the disease course. The serum OPN levels of CXMDJ dystrophic dogs were elevated, even in the early disease phase, and this could be related to the presence of regenerating muscle fibers; as such, OPN would be a promising biomarker for muscle regeneration. Next, accelerometry, which is an efficient method to quantify performance in validated tasks, was used to evaluate motor function longitudinally in dystrophic dogs. We measured three-axis acceleration and angular velocity with wireless hybrid sensors during gait evaluations. Multiple parameters of acceleration and angular velocity showed notedly lower values in dystrophic dogs compared with wild-type dogs, even at the onset of muscle weakness. These parameters accordingly decreased with exacerbation of clinical manifestations along with the disease course. Multiple parameters also indicated gait abnormalities in dystrophic dogs, such as a waddling gait. These outcome measures could be applicable in clinical trials of patients with DMD or other muscle disorders.
Keywords: accelerometry, canine X-linked muscular dystrophy in Japan (CXMDJ), Duchenne muscular dystrophy, osteopontin, outcome measure
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked muscle disorder characterized by primary muscle degeneration [1]. Its prevalence in the population is estimated to be 1 in 5,000 male newborns. A mutation in the DMD gene results in the absence of dystrophin, a structural protein in muscle fibers, which leads to weakened muscle fibers following contractive force [2]. The histologic features of DMD include muscle fiber degeneration with secondary cellular inflammation and ineffective muscle fiber regeneration, with the muscle eventually being replaced by fibrous and adipose tissue [3]. Patients with DMD manifest progressive muscle weakness and contracture [4, 5]. The early symptoms of DMD, which include gait disturbance, frequent falls, and difficulty climbing stairs, usually appear by 2–5 years of age, with loss of ambulation occurring between 9 and 12 years of age. Respiratory or cardiac failure causes death in the third decade.
Steroid therapy for muscle weakness is currently applied in the clinical setting to prolong muscle strength and ambulation; however, it is not a cure for DMD [5,6,7]. Various therapeutic strategies, such as pharmaceuticals and gene and cell therapy, have been proposed and explored in human trials [8,9,10,11,12]. Among these strategies, exon skipping and stop codon read-through, which are designed to restore dystrophin expression, are the most advanced [12]. A morpholino antisense oligonucleotide, NS-065/NCNP-01 (viltolarsen), which effectively induces exon 53 skipping in the DMD gene, was recently established to be efficacious and safe in human trials [13,14,15] and was approved in Japan and the USA in 2020. In the development of new therapeutic interventions, surrogate endpoints have become increasingly important to evaluate treatment effects in detail. Therefore, sensitive outcome measures, including biomarkers and motor function evaluations, are needed.
Overview of Canine Models for DMD
Golden retriever muscular dystrophy (GRMD)
To explore the efficiency of the different preclinical treatments for DMD, there is a need for animal models that show the dystrophic phenotypes. Spontaneous genetically homologous dystrophin-deficiency has been identified in several species, including mice and dogs. The X-linked muscular dystrophy (mdx) mouse is the most widely used animal model for DMD, even though its mild phenotype does not mimic the severe symptoms of human DMD [16, 17]. By contrast, canine dystrophic models share the severe myopathy and DMD conditions [18,19,20]. Novel animal models for DMD, such as rats [21, 22], rabbits [23], pigs [24], and rhesus monkeys [25], have been generated using genome editing methods and subsequently examined for their phenotypes [26].
Canine dystrophinopathies have been reported in a number of canine breeds [27,28,29]. Canine X-linked muscular dystrophy, which is compatible with degenerative myopathy, was documented in golden retrievers in the 1980s [18, 30,31,32] and recognized as a genetic homologue of DMD due to the lack of dystrophin protein [33]. GRMD has been established in a breeding colony of golden retrievers by reproducing dystrophic dogs according to an X-linked pattern of inheritance [28, 32, 34]. Dogs with GRMD are dystrophin deficient because of a splice-site mutation resulting from a single base change in the 3’ consensus splice site of intron 6 [35]. Exon 7 is then skipped, which is predicted to result in a termination of the dystrophin reading frame within its N-terminal domain in exon 8.
In GRMD, the histologic features include widely observed segmental degeneration of muscle fibers (hyaline fibers, myophagocytosis) and regeneration (small basophilic fibers) [30, 31], following the selective involvement of muscles such as the diaphragm, tongue, and limb muscles in the neonatal phase [36, 37]. Progressive changes include the development of severe fiber size variation, endomysial and perimysial fibrosis, fat infiltration, and alterations in the fast and slow fiber-type patterns over the disease course [19, 38]. In clinical phenotypes, a severe, lethal, neonatal form of the disease has been recognized in which GRMD pups do not survive beyond 2 weeks of age [18, 28, 36, 37]; GRMD pups that survive are only mildly affected and tend to stabilize at around 2 weeks of age [28]. Clinical signs are commonly observed as an onset of generalized muscle weakness and posture and gait abnormalities with selective muscle atrophy and hypertrophy at between 6 and 9 weeks of age; these exacerbations markedly progress until 6 to 9 months of age [18, 28, 30, 39]. It has also been noted that the pelvic limbs appear stiff and are simultaneously advanced (“bunny hopping”), resulting in a stiff and shuffling (waddling-like) gait. Other clinical features include poor weight gain, kyphosis, splaying of the limbs, fatigue with exercise, neck stiffness, resistance to jaw opening, enlargement of the base of the tongue, pharyngeal and esophageal dysfunction (dysphagia and regurgitation), and occasional respiratory and cardiac distress.
Canine X-linked muscular dystrophy in Japan (CXMDJ)
CXMDJ is a dystrophic model of beagle dogs established as a breeding colony by inseminating beagles with the sperm of GRMD dystrophic dogs at the National Center of Neurology and Psychiatry [40]. CXMDJ dystrophic dogs have a mutation in the DMD gene analogue to GRMD and lack dystrophin in their muscle tissue [40,41,42]. The clinical and histologic features of CXMDJ have been found to be similar to those observed in GRMD [20]. The clinical manifestations of CXMDJ can be evaluated from clinical signs, such as gait and mobility disturbances, limb and temporal muscle atrophy, drooling, macroglossia, dysphagia, and abnormal sitting posture, by classifying the severity on a scale of 1 to 5 (from grade 1, none, to grade 5, severe) [20, 43]. The early clinical signs, including gait disturbance and distal limb and temporal muscle atrophy, are observed at around 2 months of age, corresponding to the onset of muscle weakness [20]. High mortality of CXMDJ neonates has also been observed (e.g., a mortality of 32.3% by 3 days of age in the third generation) [20], confirming our results indicating that initial pulmonary respiration causes massive diaphragm injury followed by respiratory dysfunction [44].
Pathophysiological investigations in CXMDJ
Various studies on CXMDJ have revealed the novel characteristics of dystrophic pathology. In the diaphragm of CXMDJ, the muscle fiber-type composition is found to switch from the fast type to predominantly the slow type over the disease course [45]. Matrix metalloproteinases (MMPs), which are proteolytic enzymes that degrade extracellular matrix components, are upregulated in the dystrophic muscles; MMP-9 and MMP-2 are related to degenerating muscle fibers with inflammatory cells and regenerating muscle fibers, respectively [46]. The cardiac phenotypes of CXMDJ show prominent deep Q-waves and increased Q/R ratios in leads II, III, and aVF on electrocardiography by 6 to 7 months of age [47], and they show a decreased peak radial strain rate during early diastole in the posterior segment of the left ventricle on echocardiography after 8 months of age [48]. Selective vacuolar degeneration of Purkinje fibers is found as early as 4 months of age, suggesting an association between cardiac conduction abnormality and fatal arrhythmia [49]. Myocardial fibrosis in the left ventricular posterobasal wall has been observed in several dystrophic dogs by 21 months of age [47].
Preclinical investigations of therapies and outcome measures in CXMDJ
Preclinical studies of new therapies for DMD have been conducted in CXMDJ. Multi-exon skipping using morpholino antisense oligonucleotide cocktails, which induce exon 6 and 8 skipping in the DMD gene of CXMDJ, has been found to restore the dystrophin reading frame [42, 50,51,52]. The systemic injection of antisense drugs has been shown to induce therapeutic levels of dystrophin expression and improve the dystrophic phenotypes, including histology, clinical manifestations, motor dysfunction, and cardiac conduction abnormalities [42, 50, 53,54,55]. Recombinant adeno-associated virus (rAAV) vectors transduce the microdystrophin gene into muscle fibers and ameliorate the dystrophic phenotypes of CXMDJ [56,57,58,59,60]. Although host immune responses result in low and transient expression of transgene products, the efficiencies have been improved in rAAV-treated CXMDJ by co-administration of immunosuppressants [56], rAAV serotype selection [57, 60], immune tolerance [59], and injection of multipotent mesenchymal stromal cells (MSCs) [60]. The injection of MSCs, which are mesoderm-derived multipotent cells derived from bone marrow and dental pulp that have immune-modulating effects [61], results in the formation of myofiber-like tissue and downregulates severe inflammation in the dystrophic muscles of CXMDJ [61,62,63].
Novel outcome measures have been concomitantly developed in CXMDJ. Noninvasive evaluation methods, such as magnetic resonance imaging (MRI) and serum biomarkers, are used to examine the resulting temporospatial pathological changes. Dystrophic muscle lesions can be precisely assessed using conventional MRI sequences; chemical shift selective T2-weighted imaging has been shown to be sensitive for detecting muscle necrosis and inflammation by selectively canceling fat signals [64]. MicroRNAs (miRNAs), noncoding small RNAs, are considered candidate serum biomarkers for DMD. Among miRNAs, miR-1, miR-133a, miR-188, and miR-206 are elevated in serum and muscle tissues of CXMDJ, and miR-188 and miR-206 have been found to play roles in muscle regeneration [65,66,67].
Osteopontin (OPN) as a Serum Biomarker in Dystrophic Pathology
Serum biomarkers for DMD
Clinical biomarkers are necessary for the diagnosis of the disease, classification of its severity, and evaluation of therapeutic effects. Serum creatine kinase (CK) is a primary biomarker for a sensitive diagnosis of DMD that reflects muscle damage [68, 69]. However, serum CK is not sufficient for the evaluation of therapeutic effects, because its levels decrease with the progression of dystrophic disease, which corresponds to the wasting away of skeletal muscle. New candidate serum biomarkers have been explored to develop an evaluation method for dystrophic conditions [70]. MMP-9 and its endogenous inhibitor, tissue inhibitor of metalloproteinase (TIMP)-1, are strongly suggested to be DMD biomarkers [71, 72]. Serum miRNAs such as miR-1, miR-133, and miR-206 have been shown to be elevated in human DMD, as observed in CXMDJ [66, 73, 74]. The levels of various cytokines and growth factors in the blood have also been found to be elevated in association with dystrophic pathology, including tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-13, interferon-γ, transforming growth factor-β, and basic fibroblast growth factor [70, 75,76,77,78,79]. Serum levels of several proteins are correlated with the clinical severity in ambulatory and nonambulatory patients with DMD [71, 76, 80].
OPN in dystrophic pathology
OPN, a phosphorylated glycoprotein synthesized in various tissues and cells that are involved in inflammation, tissue remodeling, and tumorigenesis [81, 82], has been reported to be a potential new candidate for a DMD biomarker. As an immobilized matricellular protein and a soluble molecule with cytokine functions, the secreted form of OPN is capable of interacting with CD44 and various integrins to mediate cell–cell and cell–matrix interactions and activate intracellular signaling pathways [81, 83]. The effects of OPN binding to target cells, such as promoting cell attachment, proliferation, migration, and chemotaxis, are modulated through cleavage by thrombin, as well as MMP-3, MMP-7, and MMP-12 [84,85,86].
In injured or dystrophic muscles of rodent models, OPN is expressed in immune cells, myoblasts, and damaged or regenerating muscle fibers [87,88,89,90]. OPN deficiency in the muscles of mdx mice leads to reduced inflammatory cell infiltration and skewed M1/M2 macrophage polarization, as well as the subsequent alleviation of fibrosis via TGF-β signaling [88, 91, 92]. Therefore, OPN could be a therapeutic target as a pro-inflammatory and pro-fibrotic molecule. Meanwhile, OPN also plays important roles in aiding the fusion and differentiation of myoblasts, primarily during the early phases of myogenesis, leading to the promotion of muscle regeneration [83, 90, 93,94,95].
In patients with DMD, OPN is detected in mononuclear cell infiltrates, on some muscle fiber surfaces, in regenerating fibers, and in calcified fibers [96]. Among human OPN isoforms, including OPN-a, OPN-b, and OPN-c, OPN-a is especially abundant and functionally active in the skeletal muscle microenvironments [97]. In addition, OPN may influence the severity of disease in patients with DMD, because single-nucleotide polymorphism (SNP) in the SPP1 (OPN) promoter (rs28357094) has been shown to be correlated with DMD severity in a number of clinical trials [98,99,100] and with muscle size in healthy females [101]. The rare G allele of rs28357094 reduces its transcriptional activity compared with the ancestral T allele [102] but, conversely, enhances mRNA expression responsive to glucocorticoid drugs and estrogen [100, 101, 103].
Investigations of OPN in CXMDJ
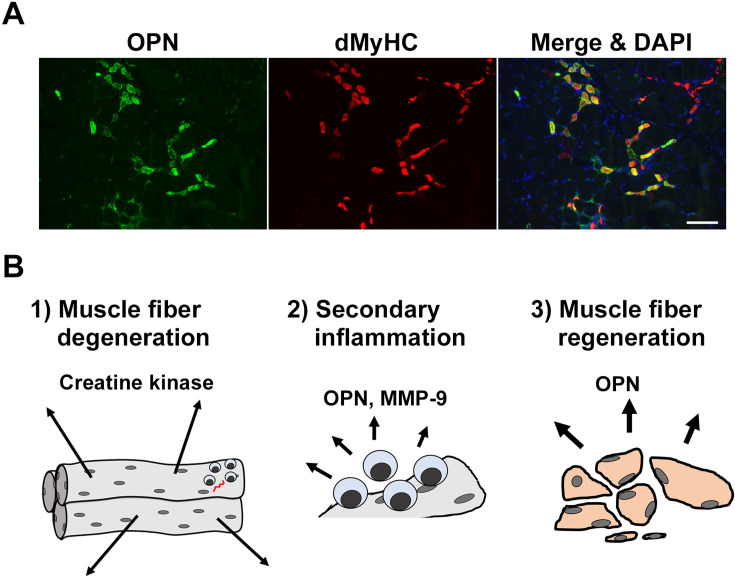
In CXMDJ dystrophic dogs, we have previously found that OPN has been upregulated in dystrophic diaphragms before initial pulmonary respiration at birth, suggesting the participation of OPN in a dystrophic muscle environment, even in the early phase [44]. It is therefore expected that OPN could potentially be a new biomarker for DMD that is expressed in a unique manner. We then monitored and compared serum OPN levels in CXMDJ dystrophic dogs over the disease course with the levels of other serum markers, including serum CK [43]. Blood samples of dystrophic and wild-type (WT) dogs were collected before birth by caesarean section, at 1 h after a birth, at 3 and 6 weeks of age, at 2, 3, 6, and 9 months of age, and at 1 and 2 years of age. Serum OPN levels in dystrophic dogs were elevated and revealed a completely different pattern compared with serum CK, an established primary biomarker for muscle injury. Serum OPN levels were significantly elevated in dystrophic dogs compared with those in WT dogs before birth, at 1 h after birth, and at 3 months of age; those at 3 weeks of age remained higher in dystrophic dogs, but not significantly higher, compared with those of the WT dogs. Meanwhile, serum CK levels were significantly higher in dystrophic than in WT dogs at all age points, and post-birth levels were substantially elevated compared with those before birth, thus confirming our observation that massive diaphragm injury is caused by initial pulmonary respiration in neonatal dystrophic dogs [44]. In dystrophic dogs, serum CK levels transiently decreased at 3 weeks of age before progressively increasing again until 3 months of age. Serum OPN levels, but not other biomarkers, were also significantly correlated with phenotypic severity in dystrophic dogs at 2 months of age, which corresponded with the onset of muscle weakness. Immunohistologically, OPN expression was observed in infiltrating CD11b- and CD18-positive macrophages or granulocytes, but not in CD3-positive T lymphocytes. OPN was also characteristically expressed in developmental myosin heavy chain (dMyHC)-positive immature regenerating muscle fibers (Fig. 1A). In particular, the number of OPN-positive regenerating muscle fibers increased with active muscle regeneration during the progressive phase but decreased with inactive muscle regeneration during the chronic phase, which was similar to the serum OPN elevation patterns over the disease course. We also noticed that OPN expression, induced by cardiotoxin injection, was detected in serum and muscle during the synergic muscle regeneration process. These observations strongly suggest that OPN is an important mediator of muscle regeneration in the early dystrophic phase. We did not identify any SNPs in CXMDJ analogue to rs28357094 in the human OPN gene promoter as a genetic modifier (unpublished).
Fig. 1.
Osteopontin in the dystrophic pathology. (A) Osteopontin (OPN) expression in regenerating dystrophic muscle fibers. Images are shown of sections of tibialis cranialis muscle of a dystrophic dog immunostained for OPN (green) and developmental myosin heavy chain (dMyHC, red) at 5 months of age. A merged image co-stained with DAPI (blue; nucleus) is shown on the right. Scale bar: 100 µm. (B) Serum biomarkers related to the dystrophic pathology. 1) Muscle fiber degeneration. Muscle fibers are shown as elongated tubes containing many nuclei. Dystrophin-deficient muscle fibers are degenerated because of their fragility with respect to mechanical stress. Creatine kinase has leaked from degenerating muscle fibers, and its serum levels are drastically elevated. 2) Secondary inflammation. Inflammatory cells, including macrophages, are attracted and promote inflammation; OPN and other factors participate in these events. Matrix components are cleared by matrix metalloproteinase (MMP)-9 secreted from macrophages for tissue remodeling. 3) Muscle fiber regeneration. OPN is intensely expressed in regenerating muscle fibers, suggesting its role in muscle regeneration.
Next, we compared serum OPN levels with those of both serum MMP-9, a marker of muscle inflammation [46, 71, 104], and its inhibitor, TIMP-1, to confirm whether serum OPN is an indicator of muscle regeneration or inflammation [43]. As a result, the serum MMP-9 levels were found to be significantly elevated at 1 h after birth and 3 months of age in dystrophic dogs compared with WT dogs, and the post-birth levels were significantly increased compared with the pre-birth values. MMP-9 expression was immunohistologically observed in CD11b- and CD-18 positive macrophages or granulocytes, but not in dMyHC-positive muscle fibers. Although OPN has been shown to contribute to increased amounts of MMP-9 as an immunomodulator [105], the serum levels of OPN and MMP-9 are not similarly elevated in dystrophic dogs. MMP-9 appears to reflect a rapid inflammatory response by quickly converting the latent form (i.e., the form deposited in the extracellular matrix) to the active form through a proteolytic cascade [106] and immediately being released from storage within infiltrating granulocytes [107]. By contrast, serum levels of OPN and MMP-9 are consistently elevated at 3 months of age, which suggests the active reflection of a serial change in muscle inflammation and regeneration. The elevation pattern of serum TIMP-1 in dystrophic dogs was similar to that of serum OPN, but the levels were not significant compared with those of the WT dogs. TIMP-1 expression was also detected in CD11b- and CD-18-positive mononuclear cells and dMyHC-positive muscle fibers. Considering the similar aspects of OPN and TIMP-1, these two factors may interact with each other in an MMP-independent manner. Indeed, in addition to inhibiting MMP-9, TIMP-1 has been shown to play roles in the processes of cell growth, myogenesis, and fibrosis [72, 108, 109].
We therefore suggest that OPN is a promising biomarker for muscle regeneration in dystrophic dogs [43]. Elevated OPN levels in blood have also been reported in mdx mice and GRMD dogs, supporting our results [88, 110]. The serum biomarkers evaluated in our studies are summarized in Fig. 1B.
The perspective of OPN as a serum biomarker for DMD
In patients with DMD, OPN levels in blood are reported to be linearly correlated with muscle function [110]; however, OPN levels in blood were not found to be significantly different between healthy subjects and patients with DMD [71, 110]. A limitation of these previous studies is that they included two DMD cohorts with high mean ages (13.1 ± 6.2 and 16.1 ± 5.6 years), and at those ages, muscle regeneration is not expected to be active. Muscle regeneration in the skeletal muscles of patients with DMD becomes inactive at around 9 years of age [111]. Therefore, serum OPN levels should be analyzed in younger patients with DMD. Serum OPN is also expected to be applicable as an outcome measure in clinical trials with muscle regeneration–inducing agents [112, 113].
Motor Function Evaluations with Accelerometry
Motor function tests in patients with DMD
As patients with DMD progressively manifest abnormal gaits because of muscle weakness, sensitive outcome measures to detect even subtle motor dysfunction are concomitantly needed with the development of new therapeutic methods. Motor function tests, such as the 6-min walk test (6MWT), the timed up and go test, 10-m walk/run velocity, 4-stair climb ascent velocity, and the North Star Ambulatory Assessment, have been applied in clinical assessments of dystrophic conditions [80, 114,115,116]. The 6MWT, which measures the distance walked in 6 min, is a primary outcome measure of motor function in DMD [117, 118], but this test is not sufficiently sensitive to measure disease progression in younger DMD boys [119]. With the recent development of miniature body-worn motion sensors, accelerometry has become an efficient method to quantify performance in validated tasks and activities of daily living [120,121,122]. Acceleration parameters indicating the movement and orientation of the body and upper limbs have been measured to assess physical activities involving both ambulatory and nonambulatory conditions in patients with DMD [123,124,125,126]. Accelerometry has also been used to monitor disease progression and the effects of corticosteroid treatment for informal tasks such as walking [127, 128]. When combined with different types of information, such as angular velocity, accelerometry has been shown to be more practical for capturing motion [122, 126, 127].
Accelerometric outcomes in CXMDJ
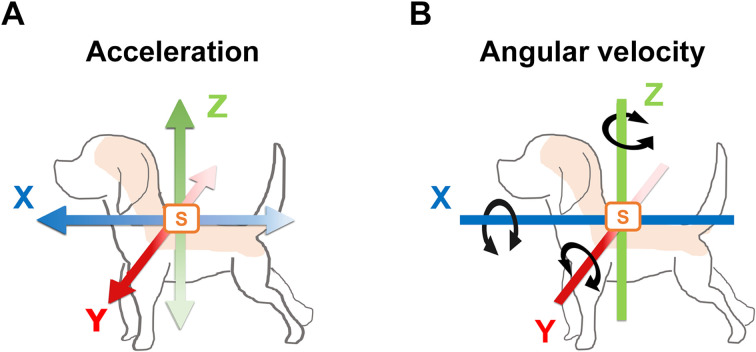
Accelerometry has been performed in GRMD dogs, and their acceleration parameters during their gaits have been found to be attenuated over the disease course [129,130,131]. In our study, gait was longitudinally evaluated in CXMDJ by measuring acceleration and angular velocity parameters that have been used to assess the relationship with clinical evaluations in dystrophic dogs [132]. We used portable wireless hybrid sensors (TSND121, ATR-Promotions, Inc., Soraku-gun, Kyoto, Japan) to measure three-axis acceleration and angular velocity values within a wide range: ± 8 G and ± 1,000 degrees per second, respectively. These miniature sensors (46 × 37 × 12 mm; weight 22 g) were affixed on the dorsal thoracic and lumbar regions of the dogs. The three axes were the X-axis (caudal–cranial), Y-axis (medial–lateral), and Z-axis (ventral–dorsal). The specific acceleration vector indicates the instantaneous inertial acceleration for each axis (Ax, Ay, and Az, respectively). The instantaneous angular velocity vector indicates the instantaneous rotation of the trunk (Gx, Gy, and Gz, respectively). The three-axis acceleration and angular velocity vectors on the dog subjects are summarized in Fig. 2. These data were recorded from trials in which the dogs ran down a 15-m hallway four times. These trials and clinical tests were performed in five CXMDJ dystrophic dogs and six WT dogs ranging from 2 to 12 months of age.
Fig. 2.
Illustrations of the three axes of acceleration and angular velocity in a dog subject. Wireless sensors (indicated by S in orange) are affixed to the dorsal region of a dog to detect three-axis acceleration and angular velocity values. The three axes are indicated as the X-axis (blue; caudal–cranial), Y-axis (red; medial–lateral), and Z-axis (green; ventral–dorsal). (A) Three axes of acceleration are shown with colored bidirectional arrows, including the inertial instantaneous acceleration for each axis. Dense colored arrows for the X-, Y-, and Z-axes indicate the fore, left, and upper directions, respectively, as vectors with positive values, while pale colored arrows indicate the opposite directions as vectors with negative values. (B) Three axes of angular velocity are shown with bidirectional arrows and indicate the instantaneous rotation of the colored bars for each axis. Clockwise rotation indicates vectors with positive values, while the opposite rotation indicates vectors with negative values.
The dystrophic dogs showed a bunny hop at the stance and swing phases during gallop and changed their gait pattern to a trot or walk according to the severity. The instantaneous vectors of acceleration (Ax, Ay, Az) were calculated as the average of the absolute values for each axis. The results showed that all three-axis vectors were lower in dystrophic dogs than in WT dogs in both the dorsal thoracic and lumbar regions and were progressively attenuated over the disease course. The acceleration magnitude (AM) was then calculated from the three acceleration vectors (Ax, Ay, Az) as the square root of the sum of the three-axis values (AM = √Ax2 + Ay2 + Az2) and averaged for each trial. The results showed that the AMs, especially those in the thoracic region, were already notably lower in dystrophic dogs than in WT dogs at 2 months of age, which is when the onset of muscle weakness occurs in dystrophic dogs. AMs in the lumbar region were drastically attenuated over the disease course in dystrophic dogs. These results demonstrate differences in AMs between the dorsal thoracic and lumbar regions. It has been reported that forelimb and hindlimb gait movements strongly influence thoracic and lumbar motion, respectively [133]. In the standing position, the load in the vertical direction is more strongly applied to the forelimb than to the hindlimb, with a ratio of at least 6:4 [133,134,135,136,137], which suggests that the forelimbs are mainly loaded from the early stage at the initiation of walking. Owing to the strut load, dystrophic dogs might experience muscle involvement, especially in the forelimb, before the onset of muscle weakness, which could lead to reduced acceleration in the thoracic region. Thus, the drastic decline in AM in the lumbar region is likely related to the progressive involvement of the hindlimb muscles during the disease course.
We also analyzed the acceleration ratios (Ax ratio, Ay ratio, Az ratio), which are the relative components of the AM along the three axes (%), to detect three-axis biases for acceleration as a whole. The acceleration ratios were calculated by dividing the absolute values of each axis by the AM and then averaged in each trial. The Ax ratios in the dorsal thoracic and lumbar regions were lower in dystrophic dogs than in WT dogs. In dystrophic dogs, the Ay ratio progressively increased in the thoracic region over the disease course, whereas that in the lumbar region slightly increased at 10 and 11 months of age. By contrast, the Az ratio was slightly higher in the lumbar region in dystrophic dogs than in WT dogs at 8 months of age. The attenuation of the Ax ratio in dystrophic dogs reflects a progressive decay in the forward propulsive force, whereas the increase in the Ay and Az ratios might be indicative of a heightening motion potentially related to a waddling-like gait and a bunny hop, respectively.
The instantaneous vectors of angular velocity (Gx, Gy, Gz) were calculated as the average of the absolute values of each axis. Three-axis vectors were lower in dystrophic dogs than in WT dogs in both the dorsal thoracic and lumbar regions and, except for Gz in the lumbar region, were found to be attenuated over the disease course. Gy in the thoracic region was substantially increased in WT dogs and drastically different from that in dystrophic dogs. Limb behavior during the gait of dogs causes a wider sweeping motion of the thorax because of forelimb movement in the horizontal and vertical axes [133]. In WT dogs, an increase in Gy in the thoracic region reflected dynamic forelimb motion during a gallop; therefore, greater power was applied to that leg. By providing a strong load in the early phase, the intense motion of the thorax in dystrophic dogs resulted in forelimb muscle involvement, which, in turn, led to gait dysfunction and a reduced Gy in the thoracic region. Gz in the lumbar region was lower in dystrophic dogs compared with WT dogs, but it showed an increase to that in WT dogs at 8 months of age. This observation could be the result of waddling at the girdle, which suggests the importance of motion evaluation in the lumbar region.
Multiple parameters of acceleration and angular velocity were correlated with the clinical manifestations, as determined using a grading scale of clinical signs in CXMDJ, which was described earlier. The coefficients of the total grading scores for multiple parameters were less than 0, except for Gz in the lumbar region. These findings revealed that a number of parameters mostly decreased with exacerbated severity, in contrast to Gz in the lumbar region, which increased. Among the acceleration ratios, the coefficient for the Ay ratio in the thoracic region was greater than 0, indicating that acceleration in the medial–lateral direction in the thoracic region increased with exacerbated severity. These findings suggest that an increased Gz in the lumbar region and a higher Ay ratio in the thoracic region are associated with a waddling-like gait in the severe phenotype of CXMDJ.
From these results, multiple parameters of acceleration and angular velocity can be considered remarkably sensitive for evaluating pathological conditions. We found that these parameters also increased in association with high spontaneous locomotor activity in dystrophic dogs [132], implying a relationship between activities of daily living and motor dysfunction similar to that in patients with DMD [124, 125, 128]. Cell therapies such as MSC treatment in dystrophic dogs have been shown to ameliorate the acceleration parameters [62]. Accelerometry has been found to provide quantitative, multifaceted kinematic indices in combination with conventional motor function tests in clinical trials.
Conclusion
We investigated serum OPN expression and accelerometry in CXMDJ to develop outcome measures for the clinical evaluation of DMD. The results in CXMDJ suggested that serum OPN is a promising biomarker for muscle regeneration and that multiple acceleration and angular velocity parameters are also efficient outcome measures for the quantification of motor function according to the disease course and severity. It is necessary to carefully examine these outcome measures in patients with DMD, which shows different pathological conditions depending on symptom onset and disease phase from those of canine dystrophic models [28, 138]. It is also necessary to define the accelerometric characteristics of dystrophic quadrupedal gaits and to cautiously extrapolate this information to the bipedal gaits of patients. These outcome measures would be potentially applicable to patients with hereditary neuromuscular disorders, including DMD.
Acknowledgments
We are grateful to En Kimura, Tetsuya Nagata, Takashi Okada, Michihiro Imamura, Hisateru Tachimori, Hirofumi Komaki, Naohiro Yonemoto, Yuko Nitahara-Kasahara, Yumi Hayashita-Kinoh, Naohiro Kato, Takashi Saito, Janek Hyzewicz, Ryoko Nakagawa, Naoko Yugeta, Masanori Kobayashi, Hironori Okada, Satoru Masuda, Chiaki Masuda, Tomoko Chiyo, Naoki Ito, Hiroyuki Shibasaki, Akihiko Shin, Yasuyuki Iwata, Hiroyuki Yajima, Eri Takeshita, and Yuko Shimizu-Motohashi. We would like to thank Hideki Kita, Shin-ichi Ichikawa, Yumiko Yahata-Kobayashi, Aya Kuriyama, Takayoshi Hikage, Akane Hanaoka-Hayashi, Namiko Ogawa, and other staff members of JAC Co. for their care of the dogs.
References
- 1.Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg. 2002; 10: 138–151. doi: 10.5435/00124635-200203000-00009 [DOI] [PubMed] [Google Scholar]
- 2.Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994; 17: 2–15. doi: 10.1002/mus.880170103 [DOI] [PubMed] [Google Scholar]
- 3.Cullen MJ, Mastaglia FL. Morphological changes in dystrophic muscle. Br Med Bull. 1980; 36: 145–152. doi: 10.1093/oxfordjournals.bmb.a071630 [DOI] [PubMed] [Google Scholar]
- 4.McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995; 74:(5 Suppl): S70–S92. doi: 10.1097/00002060-199509001-00003 [DOI] [PubMed] [Google Scholar]
- 5.Goto M, Komaki H, Takeshita E, Abe Y, Ishiyama A, Sugai K, et al. Long-term outcomes of steroid therapy for Duchenne muscular dystrophy in Japan. Brain Dev. 2016; 38: 785–791. doi: 10.1016/j.braindev.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008; CD003725. [DOI] [PubMed] [Google Scholar]
- 7.Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen GJ, Kunkel LM. The Pathogenesis and Therapy of Muscular Dystrophies. Annu Rev Genomics Hum Genet. 2015; 16: 281–308. doi: 10.1146/annurev-genom-090314-025003 [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Vlcek V, Balabanov P, Salmonson T, Bakchine S, Markey G, et al. European Medicines Agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord. 2015; 25: 5–13. doi: 10.1016/j.nmd.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 9.Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, Shao J, et al. Eteplirsen Study Group and Telethon Foundation DMD Italian Network. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016; 79: 257–271. doi: 10.1002/ana.24555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan JM, Zimmer M, Offman E, Grant T, Jirousek M. A Novel NF-κB Inhibitor, Edasalonexent (CAT-1004), in Development as a Disease-Modifying Treatment for Patients With Duchenne Muscular Dystrophy: Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics in Adult Subjects. J Clin Pharmacol. 2017; 57: 627–639. doi: 10.1002/jcph.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafner P, Bonati U, Rubino D, Gocheva V, Zumbrunn T, Gueven N, et al. Treatment with L-citrulline and metformin in Duchenne muscular dystrophy: study protocol for a single-centre, randomised, placebo-controlled trial. Trials. 2016; 17: 389. doi: 10.1186/s13063-016-1503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu-Motohashi Y, Komaki H, Motohashi N, Takeda S, Yokota T, Aoki Y. Restoring Dystrophin Expression in Duchenne Muscular Dystrophy: Current Status of Therapeutic Approaches. J Pers Med. 2019; 9: 1. doi: 10.3390/jpm9010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komaki H, Nagata T, Saito T, Masuda S, Takeshita E, Sasaki M, et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci Transl Med. 2018; 10: eaan0713. doi: 10.1126/scitranslmed.aan0713 [DOI] [PubMed] [Google Scholar]
- 14.Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, Smith EC, et al. CINRG DNHS Investigators. Safety, Tolerability, and Efficacy of Viltolarsen in Boys With Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2020; 77: 982–991. doi: 10.1001/jamaneurol.2020.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komaki H, Takeshima Y, Matsumura T, Ozasa S, Funato M, Takeshita E, et al. Viltolarsen in Japanese Duchenne muscular dystrophy patients: A phase 1/2 study. Ann Clin Transl Neurol. 2020; 7: 2393–2408. doi: 10.1002/acn3.51235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984; 81: 1189–1192. doi: 10.1073/pnas.81.4.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulton GR, Morgan JE, Partridge TA, Sloper JC. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol. 1988; 14: 53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x [DOI] [PubMed] [Google Scholar]
- 18.Valentine BA, Cooper BJ, de Lahunta A, O’Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988; 88: 69–81. doi: 10.1016/0022-510X(88)90206-7 [DOI] [PubMed] [Google Scholar]
- 19.Valentine BA, Cooper BJ, Cummings JF, de Lahunta A. Canine X-linked muscular dystrophy: morphologic lesions. J Neurol Sci. 1990; 97: 1–23. doi: 10.1016/0022-510X(90)90095-5 [DOI] [PubMed] [Google Scholar]
- 20.Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M, et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol. 2005; 24: 145–154. [PubMed] [Google Scholar]
- 21.Nakamura K, Fujii W, Tsuboi M, Tanihata J, Teramoto N, Takeuchi S, et al. Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci Rep. 2014; 4: 5635. doi: 10.1038/srep05635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larcher T, Lafoux A, Tesson L, Remy S, Thepenier V, François V, et al. Characterization of dystrophin deficient rats: a new model for Duchenne muscular dystrophy. PLoS One. 2014; 9: e110371. doi: 10.1371/journal.pone.0110371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui T, Lau YS, Liu D, Liu T, Xu L, Gao Y, et al. A novel rabbit model of Duchenne muscular dystrophy generated by CRISPR/Cas9. Dis Model Mech. 2018; 11: dmm032201. doi: 10.1242/dmm.032201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu HH, Zhao H, Qing YB, Pan WR, Jia BY, Zhao HY, et al. Porcine Zygote Injection with Cas9/sgRNA Results in DMD-Modified Pig with Muscle Dystrophy. Int J Mol Sci. 2016; 17: 1668. doi: 10.3390/ijms17101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Zheng Y, Kang Y, Yang W, Niu Y, Guo X, et al. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum Mol Genet. 2015; 24: 3764–3774. doi: 10.1093/hmg/ddv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim KRQ, Nguyen Q, Dzierlega K, Huang Y, Yokota T. CRISPR-Generated Animal Models of Duchenne Muscular Dystrophy. Genes (Basel). 2020; 11: 342. doi: 10.3390/genes11030342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkmeyer-Langford C, Kornegay JN. Comparative Genomics of X-linked Muscular Dystrophies: The Golden Retriever Model. Curr Genomics. 2013; 14: 330–342. doi: 10.2174/13892029113149990004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornegay JN. The golden retriever model of Duchenne muscular dystrophy. Skelet Muscle. 2017; 7: 9. doi: 10.1186/s13395-017-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nghiem PP, Bello L, Balog-Alvarez C, López SM, Bettis A, Barnett H, et al. Whole genome sequencing reveals a 7 base-pair deletion in DMD exon 42 in a dog with muscular dystrophy. Mamm Genome. 2017; 28: 106–113. doi: 10.1007/s00335-016-9675-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentine BA, Cooper BJ, Cummings JF, deLahunta A. Progressive muscular dystrophy in a golden retriever dog: light microscope and ultrastructural features at 4 and 8 months. Acta Neuropathol. 1986; 71: 301–310. doi: 10.1007/BF00688053 [DOI] [PubMed] [Google Scholar]
- 31.Kornegay JN, Tuler SM, Miller DM, Levesque DC. Muscular dystrophy in a litter of golden retriever dogs. Muscle Nerve. 1988; 11: 1056–1064. doi: 10.1002/mus.880111008 [DOI] [PubMed] [Google Scholar]
- 32.Cooper BJ, Valentine BA, Wilson S, Patterson DF, Concannon PW. Canine muscular dystrophy: confirmation of X-linked inheritance. J Hered. 1988; 79: 405–408. doi: 10.1093/oxfordjournals.jhered.a110543 [DOI] [PubMed] [Google Scholar]
- 33.Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988; 334: 154–156. doi: 10.1038/334154a0 [DOI] [PubMed] [Google Scholar]
- 34.Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Grange RW. Golden retriever muscular dystrophy (GRMD): Developing and maintaining a colony and physiological functional measurements. Methods Mol Biol. 2011; 709: 105–123. doi: 10.1007/978-1-61737-982-6_7 [DOI] [PubMed] [Google Scholar]
- 35.Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992; 13: 115–121. doi: 10.1016/0888-7543(92)90210-J [DOI] [PubMed] [Google Scholar]
- 36.Valentine BA, Cooper BJ. Canine X-linked muscular dystrophy: selective involvement of muscles in neonatal dogs. Neuromuscul Disord. 1991; 1: 31–38. doi: 10.1016/0960-8966(91)90040-Y [DOI] [PubMed] [Google Scholar]
- 37.Nguyen F, Cherel Y, Guigand L, Goubault-Leroux I, Wyers M. Muscle lesions associated with dystrophin deficiency in neonatal golden retriever puppies. J Comp Pathol. 2002; 126: 100–108. doi: 10.1053/jcpa.2001.0526 [DOI] [PubMed] [Google Scholar]
- 38.Lanfossi M, Cozzi F, Bugini D, Colombo S, Scarpa P, Morandi L, et al. Development of muscle pathology in canine X-linked muscular dystrophy. I. Delayed postnatal maturation of affected and normal muscle as revealed by myosin isoform analysis and utrophin expression. Acta Neuropathol. 1999; 97: 127–138. doi: 10.1007/s004010050965 [DOI] [PubMed] [Google Scholar]
- 39.Kornegay JN, Cundiff DD, Bogan DJ, Bogan JR, Okamura CS. The cranial sartorius muscle undergoes true hypertrophy in dogs with golden retriever muscular dystrophy. Neuromuscul Disord. 2003; 13: 493–500. doi: 10.1016/S0960-8966(03)00025-7 [DOI] [PubMed] [Google Scholar]
- 40.Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, et al. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp Anim. 2003; 52: 93–97. doi: 10.1538/expanim.52.93 [DOI] [PubMed] [Google Scholar]
- 41.Honeyman K, Carville KS, Howell JM, Fletcher S, Wilton SD. Development of a snapback method of single-strand conformation polymorphism analysis for genotyping Golden Retrievers for the X-linked muscular dystrophy allele. Am J Vet Res. 1999; 60: 734–737. [PubMed] [Google Scholar]
- 42.Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009; 65: 667–676. doi: 10.1002/ana.21627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuraoka M, Kimura E, Nagata T, Okada T, Aoki Y, Tachimori H, et al. Serum Osteopontin as a Novel Biomarker for Muscle Regeneration in Duchenne Muscular Dystrophy. Am J Pathol. 2016; 186: 1302–1312. doi: 10.1016/j.ajpath.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 44.Nakamura A, Kobayashi M, Kuraoka M, Yuasa K, Yugeta N, Okada T, et al. Initial pulmonary respiration causes massive diaphragm damage and hyper-CKemia in Duchenne muscular dystrophy dog. Sci Rep. 2013; 3: 2183. doi: 10.1038/srep02183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuasa K, Nakamura A, Hijikata T, Takeda S. Dystrophin deficiency in canine X-linked muscular dystrophy in Japan (CXMDJ) alters myosin heavy chain expression profiles in the diaphragm more markedly than in the tibialis cranialis muscle. BMC Musculoskelet Disord. 2008; 9: 1. doi: 10.1186/1471-2474-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda S, et al. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord. 2007; 8: 54. doi: 10.1186/1471-2474-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yugeta N, Urasawa N, Fujii Y, Yoshimura M, Yuasa K, Wada MR, et al. Cardiac involvement in Beagle-based canine X-linked muscular dystrophy in Japan (CXMDJ): electrocardiographic, echocardiographic, and morphologic studies. BMC Cardiovasc Disord. 2006; 6: 47. doi: 10.1186/1471-2261-6-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takano H, Fujii Y, Yugeta N, Takeda S, Wakao Y. Assessment of left ventricular regional function in affected and carrier dogs with Duchenne muscular dystrophy using speckle tracking echocardiography. BMC Cardiovasc Disord. 2011; 11: 23. doi: 10.1186/1471-2261-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urasawa N, Wada MR, Machida N, Yuasa K, Shimatsu Y, Wakao Y, et al. Selective vacuolar degeneration in dystrophin-deficient canine Purkinje fibers despite preservation of dystrophin-associated proteins with overexpression of Dp71. Circulation. 2008; 117: 2437–2448. doi: 10.1161/CIRCULATIONAHA.107.739326 [DOI] [PubMed] [Google Scholar]
- 50.Yokota T, Nakamura A, Nagata T, Saito T, Kobayashi M, Aoki Y, et al. Extensive and prolonged restoration of dystrophin expression with vivo-morpholino-mediated multiple exon skipping in dystrophic dogs. Nucleic Acid Ther. 2012; 22: 306–315. doi: 10.1089/nat.2012.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miskew Nichols B, Aoki Y, Kuraoka M, Lee JJ, Takeda S, Yokota T. Multi-exon Skipping Using Cocktail Antisense Oligonucleotides in the Canine X-linked Muscular Dystrophy. J Vis Exp. 2016; 24: 53776. doi: 10.3791/53776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maruyama R, Echigoya Y, Caluseriu O, Aoki Y, Takeda S, Yokota T. Systemic Delivery of Morpholinos to Skip Multiple Exons in a Dog Model of Duchenne Muscular Dystrophy. Methods Mol Biol. 2017; 1565: 201–213. doi: 10.1007/978-1-4939-6817-6_17 [DOI] [PubMed] [Google Scholar]
- 53.Echigoya Y, Nakamura A, Nagata T, Urasawa N, Lim KRQ, Trieu N, et al. Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2017; 114: 4213–4218. doi: 10.1073/pnas.1613203114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maruyama R, Aoki Y, Takeda S, Yokota T. In Vivo Evaluation of Multiple Exon Skipping with Peptide-PMOs in Cardiac and Skeletal Muscles in Dystrophic Dogs. Methods Mol Biol. 2018; 1828: 365–379. doi: 10.1007/978-1-4939-8651-4_23 [DOI] [PubMed] [Google Scholar]
- 55.Lim KRQ, Echigoya Y, Nagata T, Kuraoka M, Kobayashi M, Aoki Y, et al. Efficacy of Multi-exon Skipping Treatment in Duchenne Muscular Dystrophy Dog Model Neonates. Mol Ther. 2019; 27: 76–86. doi: 10.1016/j.ymthe.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007; 14: 1249–1260. doi: 10.1038/sj.gt.3302984 [DOI] [PubMed] [Google Scholar]
- 57.Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009; 17: 73–80. doi: 10.1038/mt.2008.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koo T, Okada T, Athanasopoulos T, Foster H, Takeda S, Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med. 2011; 13: 497–506. doi: 10.1002/jgm.1602 [DOI] [PubMed] [Google Scholar]
- 59.Hayashita-Kinoh H, Yugeta N, Okada H, Nitahara-Kasahara Y, Chiyo T, Okada T, et al. Intra-amniotic rAAV-mediated microdystrophin gene transfer improves canine X-linked muscular dystrophy and may induce immune tolerance. Mol Ther. 2015; 23: 627–637. doi: 10.1038/mt.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashita-Kinoh H, Guillermo PH, Nitahara-Kasahara Y, Kuraoka M, Okada H, Chiyo T, et al. Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment. Mol Ther Methods Clin Dev. 2020; 20: 133–141. doi: 10.1016/j.omtm.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Okada H, Wada-Maeda M, Nakamura A, et al. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol Ther. 2012; 20: 168–177. doi: 10.1038/mt.2011.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nitahara-Kasahara Y, Kuraoka M, Guillermo PH, Hayashita-Kinoh H, Maruoka Y, Nakamura-Takahasi A, et al. Dental pulp stem cells can improve muscle dysfunction in animal models of Duchenne muscular dystrophy. Stem Cell Res Ther. 2021; 12: 78. doi: 10.1186/s13287-020-02099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nitahara-Kasahara Y, Kuraoka M, Oda Y, Hayashita-Kinoh H, Takeda S, Okada T. Enhanced cell survival and therapeutic benefits of IL-10-expressing multipotent mesenchymal stromal cells for muscular dystrophy. Stem Cell Res Ther. 2021; 12: 105. doi: 10.1186/s13287-021-02168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi M, Nakamura A, Hasegawa D, Fujita M, Orima H, Takeda S. Evaluation of dystrophic dog pathology by fat-suppressed T2-weighted imaging. Muscle Nerve. 2009; 40: 815–826. doi: 10.1002/mus.21384 [DOI] [PubMed] [Google Scholar]
- 65.Yuasa K, Hagiwara Y, Ando M, Nakamura A, Takeda S, Hijikata T. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct. 2008; 33: 163–169. doi: 10.1247/csf.08022 [DOI] [PubMed] [Google Scholar]
- 66.Mizuno H, Nakamura A, Aoki Y, Ito N, Kishi S, Yamamoto K, et al. Identification of muscle-specific microRNAs in serum of muscular dystrophy animal models: promising novel blood-based markers for muscular dystrophy. PLoS One. 2011; 6: e18388. doi: 10.1371/journal.pone.0018388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibasaki H, Imamura M, Arima S, Tanihata J, Kuraoka M, Matsuzaka Y, et al. Characterization of a novel microRNA, miR-188, elevated in serum of muscular dystrophy dog model. PLoS One. 2019; 14: e0211597. doi: 10.1371/journal.pone.0211597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emery AEH, Muntoni F. Duchenne Muscular Dystrophy. Great Clarendon Street, Oxford OX2 6DP, UK: Oxford University Press; 2003. [Google Scholar]
- 69.Ebashi S, Toyokura Y, Momoi H, Sugita H. High Creatine Phosphokinase Activity of Sera with Progressive Muscular Dystrophy. J Biol Chem. 1959; 46: 103–104. [Google Scholar]
- 70.Grounds MD, Terrill JR, Al-Mshhdani BA, Duong MN, Radley-Crabb HG, Arthur PG. Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress. Dis Model Mech. 2020; 13: dmm043638. doi: 10.1242/dmm.043638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadarajah VD, van Putten M, Chaouch A, Garrood P, Straub V, Lochmüller H, et al. Serum matrix metalloproteinase-9 (MMP-9) as a biomarker for monitoring disease progression in Duchenne muscular dystrophy (DMD). Neuromuscul Disord. 2011; 21: 569–578. doi: 10.1016/j.nmd.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 72.Sun G, Haginoya K, Chiba Y, Uematsu M, Hino-Fukuyo N, Tanaka S, et al. Elevated plasma levels of tissue inhibitors of metalloproteinase-1 and their overexpression in muscle in human and mouse muscular dystrophy. J Neurol Sci. 2010; 297: 19–28. doi: 10.1016/j.jns.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 73.Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009; 23: 3335–3346. doi: 10.1096/fj.08-128579 [DOI] [PubMed] [Google Scholar]
- 74.Li X, Li Y, Zhao L, Zhang D, Yao X, Zhang H, et al. Circulating muscle-specific miRNAs in Duchenne muscular dystrophy patients. Mol Ther Nucleic Acids. 2014; 3: e177. doi: 10.1038/mtna.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Salam E, Abdel-Meguid I, Korraa SS. Markers of degeneration and regeneration in Duchenne muscular dystrophy. Acta Myol. 2009; 28: 94–100. [PMC free article] [PubMed] [Google Scholar]
- 76.Cruz-Guzmán OR, Rodríguez-Cruz M, Escobar Cedillo RE. Systemic inflammation in Duchenne muscular dystrophy: association with muscle function and nutritional status. BioMed Res Int. 2015; 2015: 891972. doi: 10.1155/2015/891972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terrill JRP, Grounds MD, Arthur PG. Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016; 8: ecurrents.md.77be6ec30e8caf19529a00417614a072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005; 65: 826–834. doi: 10.1212/01.wnl.0000173836.09176.c4 [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, Lu H. Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol. 2010; 69: 771–776. doi: 10.1097/NEN.0b013e3181e9a34b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dang UJ, Ziemba M, Clemens PR, Hathout Y, Conklin LS, Hoffman EP. CINRG Vamorolone 002/003 Investigators. Serum biomarkers associated with baseline clinical severity in young steroid-naïve Duchenne muscular dystrophy boys. Hum Mol Genet. 2020; 29: 2481–2495. doi: 10.1093/hmg/ddaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008; 19: 333–345. doi: 10.1016/j.cytogfr.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 82.Morimoto J, Kon S, Matsui Y, Uede T. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets. 2010; 11: 494–505. doi: 10.2174/138945010790980321 [DOI] [PubMed] [Google Scholar]
- 83.Pagel CN, Wasgewatte Wijesinghe DK, Taghavi Esfandouni N, Mackie EJ. Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J Cell Commun Signal. 2014; 8: 95–103. doi: 10.1007/s12079-013-0217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem. 2001; 276: 28261–28267. doi: 10.1074/jbc.M103608200 [DOI] [PubMed] [Google Scholar]
- 85.Goncalves DaSilva A, Liaw L, Yong VW. Cleavage of osteopontin by matrix metalloproteinase-12 modulates experimental autoimmune encephalomyelitis disease in C57BL/6 mice. Am J Pathol. 2010; 177: 1448–1458. doi: 10.2353/ajpath.2010.091081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamaguchi Y, Shao Z, Sharif S, Du XY, Myles T, Merchant M, et al. Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage. J Biol Chem. 2013; 288: 3097–3111. doi: 10.1074/jbc.M112.362954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, et al. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003; 163: 203–215. doi: 10.1016/S0002-9440(10)63644-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, et al. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009; 119: 1583–1594. doi: 10.1172/JCI37662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbosa-Souza V, Contin DK, Filho WB, de Araújo AL, Irazusta SP, da Cruz-Höfling MA. Osteopontin, a chemotactic protein with cytokine-like properties, is up-regulated in muscle injury caused by Bothrops lanceolatus (fer-de-lance) snake venom. Toxicon. 2011; 58: 398–409. doi: 10.1016/j.toxicon.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 90.Uaesoontrachoon K, Wasgewatte Wijesinghe DK, Mackie EJ, Pagel CN. Osteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Dis Model Mech. 2013; 6: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kramerova I, Kumagai-Cresse C, Ermolova N, Mokhonova E, Marinov M, Capote J, et al. Spp1 (osteopontin) promotes TGFβ processing in fibroblasts of dystrophin-deficient muscles through matrix metalloproteinases. Hum Mol Genet. 2019; 28: 3431–3442. doi: 10.1093/hmg/ddz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, et al. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J Cell Biol. 2016; 213: 275–288. doi: 10.1083/jcb.201510086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008; 40: 2303–2314. doi: 10.1016/j.biocel.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 94.Pereira RO, Carvalho SN, Stumbo AC, Rodrigues CA, Porto LC, Moura AS, et al. Osteopontin expression in coculture of differentiating rat fetal skeletal fibroblasts and myoblasts. In Vitro Cell Dev Biol Anim. 2006; 42: 4–7. doi: 10.1290/0509058.1 [DOI] [PubMed] [Google Scholar]
- 95.Maeda Y, Yonemochi Y, Nakajyo Y, Hidaka H, Ikeda T, Ando Y. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci Rep. 2017; 7: 3305. doi: 10.1038/s41598-017-02928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zanotti S, Gibertini S, Di Blasi C, Cappelletti C, Bernasconi P, Mantegazza R, et al. Osteopontin is highly expressed in severely dystrophic muscle and seems to play a role in muscle regeneration and fibrosis. Histopathology. 2011; 59: 1215–1228. doi: 10.1111/j.1365-2559.2011.04051.x [DOI] [PubMed] [Google Scholar]
- 97.Many GM, Yokosaki Y, Uaesoontrachoon K, Nghiem PP, Bello L, Dadgar S, et al. OPN-a induces muscle inflammation by increasing recruitment and activation of pro-inflammatory macrophages. Exp Physiol. 2016; 101: 1285–1300. doi: 10.1113/EP085768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bello L, Piva L, Barp A, Taglia A, Picillo E, Vasco G, et al. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology. 2012; 79: 159–162. doi: 10.1212/WNL.0b013e31825f04ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, et al. Cooperative International Neuromuscular Research Group. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011; 76: 219–226. doi: 10.1212/WNL.0b013e318207afeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vianello S, Pantic B, Fusto A, Bello L, Galletta E, Borgia D, et al. SPP1 genotype and glucocorticoid treatment modify osteopontin expression in Duchenne muscular dystrophy cells. Hum Mol Genet. 2017; 26: 3342–3351. doi: 10.1093/hmg/ddx218 [DOI] [PubMed] [Google Scholar]
- 101.Hoffman EP, Gordish-Dressman H, McLane VD, Devaney JM, Thompson PD, Visich P, et al. Alterations in osteopontin modify muscle size in females in both humans and mice. Med Sci Sports Exerc. 2013; 45: 1060–1068. doi: 10.1249/MSS.0b013e31828093c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giacopelli F, Marciano R, Pistorio A, Catarsi P, Canini S, Karsenty G, et al. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol Genomics. 2004; 20: 87–96. doi: 10.1152/physiolgenomics.00138.2004 [DOI] [PubMed] [Google Scholar]
- 103.Barfield WL, Uaesoontrachoon K, Wu CS, Lin S, Chen Y, Wang PC, et al. Eccentric muscle challenge shows osteopontin polymorphism modulation of muscle damage. Hum Mol Genet. 2014; 23: 4043–4050. doi: 10.1093/hmg/ddu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdière-Sahuqué M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999; 205: 158–170. doi: 10.1006/dbio.1998.9107 [DOI] [PubMed] [Google Scholar]
- 105.Dahiya S, Givvimani S, Bhatnagar S, Qipshidze N, Tyagi SC, Kumar A. Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy. J Immunol. 2011; 187: 2723–2731. doi: 10.4049/jimmunol.1101342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Michaluk P, Kaczmarek L. Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ. 2007; 14: 1255–1258. doi: 10.1038/sj.cdd.4402141 [DOI] [PubMed] [Google Scholar]
- 107.Delclaux C, Delacourt C, D’Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996; 14: 288–295. doi: 10.1165/ajrcmb.14.3.8845180 [DOI] [PubMed] [Google Scholar]
- 108.Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012; 180: 12–16. doi: 10.1016/j.ajpath.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 109.Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil. 2000; 21: 223–233. doi: 10.1023/A:1005670507906 [DOI] [PubMed] [Google Scholar]
- 110.Galindo CL, Soslow JH, Brinkmeyer-Langford CL, Gupte M, Smith HM, Sengsayadeth S, et al. Translating golden retriever muscular dystrophy microarray findings to novel biomarkers for cardiac/skeletal muscle function in Duchenne muscular dystrophy. Pediatr Res. 2016; 79: 629–636. doi: 10.1038/pr.2015.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, et al. Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta Myol. 2011; 30: 16–23. [PMC free article] [PubMed] [Google Scholar]
- 112.Hayashiji N, Yuasa S, Miyagoe-Suzuki Y, Hara M, Ito N, Hashimoto H, et al. G-CSF supports long-term muscle regeneration in mouse models of muscular dystrophy. Nat Commun. 2015; 6: 6745. doi: 10.1038/ncomms7745 [DOI] [PubMed] [Google Scholar]
- 113.Kim AR, Kim KM, Byun MR, Hwang JH, Park JI, Oh HT, et al. Catechins activate muscle stem cells by Myf5 induction and stimulate muscle regeneration. Biochem Biophys Res Commun. 2017; 489: 142–148. doi: 10.1016/j.bbrc.2017.05.114 [DOI] [PubMed] [Google Scholar]
- 114.Goemans N, Mercuri E, Belousova E, Komaki H, Dubrovsky A, McDonald CM, et al. DEMAND III study group. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul Disord. 2018; 28: 4–15. doi: 10.1016/j.nmd.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 115.Miller NF, Alfano LN, Iammarino MA, Connolly AM, Moore-Clingenpeel M, Powers BR, et al. Natural History of Steroid-Treated Young Boys With Duchenne Muscular Dystrophy Using the NSAA, 100m, and Timed Functional Tests. Pediatr Neurol. 2020; 113: 15–20. doi: 10.1016/j.pediatrneurol.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 116.Beenakker EA, Maurits NM, Fock JM, Brouwer OF, van der Hoeven JH. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2005; 9: 387–393. doi: 10.1016/j.ejpn.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 117.McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, Gappmaier E, et al. PTC124-GD-007-DMD Study Group. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013; 48: 357–368. doi: 10.1002/mus.23905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDonald CM, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, et al. PTC124-GD-007-DMD Study Group. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013; 48: 343–356. doi: 10.1002/mus.23902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu QL, Cirak S, Partridge T. What Can We Learn From Clinical Trials of Exon Skipping for DMD? Mol Ther Nucleic Acids. 2014; 3: e152. doi: 10.1038/mtna.2014.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neugebauer JM, Hawkins DA, Beckett L. Estimating youth locomotion ground reaction forces using an accelerometer-based activity monitor. PLoS One. 2012; 7: e48182. doi: 10.1371/journal.pone.0048182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rowlands AV, Fraysse F, Catt M, Stiles VH, Stanley RM, Eston RG, et al. Comparability of measured acceleration from accelerometry-based activity monitors. Med Sci Sports Exerc. 2015; 47: 201–210. doi: 10.1249/MSS.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 122.Salarian A, Russmann H, Vingerhoets FJ, Burkhard PR, Aminian K. Ambulatory monitoring of physical activities in patients with Parkinson’s disease. IEEE Trans Biomed Eng. 2007; 54: 2296–2299. doi: 10.1109/TBME.2007.896591 [DOI] [PubMed] [Google Scholar]
- 123.Ganea R, Jeannet PY, Paraschiv-Ionescu A, Goemans NM, Piot C, Van den Hauwe M, et al. Gait assessment in children with duchenne muscular dystrophy during long-distance walking. J Child Neurol. 2012; 27: 30–38. doi: 10.1177/0883073811413581 [DOI] [PubMed] [Google Scholar]
- 124.Kimura S, Ozasa S, Nomura K, Yoshioka K, Endo F. Estimation of muscle strength from actigraph data in Duchenne muscular dystrophy. Pediatr Int. 2014; 56: 748–752. doi: 10.1111/ped.12348 [DOI] [PubMed] [Google Scholar]
- 125.Davidson ZE, Ryan MM, Kornberg AJ, Walker KZ, Truby H. Strong correlation between the 6-minute walk test and accelerometry functional outcomes in boys with Duchenne muscular dystrophy. J Child Neurol. 2015; 30: 357–363. doi: 10.1177/0883073814530502 [DOI] [PubMed] [Google Scholar]
- 126.Le Moing AG, Seferian AM, Moraux A, Annoussamy M, Dorveaux E, Gasnier E, et al. A Movement Monitor Based on Magneto-Inertial Sensors for Non-Ambulant Patients with Duchenne Muscular Dystrophy: A Pilot Study in Controlled Environment. PLoS One. 2016; 11: e0156696. doi: 10.1371/journal.pone.0156696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeannet PY, Aminian K, Bloetzer C, Najafi B, Paraschiv-Ionescu A. Continuous monitoring and quantification of multiple parameters of daily physical activity in ambulatory Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2011; 15: 40–47. doi: 10.1016/j.ejpn.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 128.Nishizawa H, Shiba N, Nakamura A. Usefulness of continuous actigraph monitoring in the assessment of the effect of corticosteroid treatment for Duchenne muscular dystrophy: a case report. J Phys Ther Sci. 2016; 28: 3249–3251. doi: 10.1589/jpts.28.3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barthélémy I, Barrey E, Thibaud JL, Uriarte A, Voit T, Blot S, et al. Gait analysis using accelerometry in dystrophin-deficient dogs. Neuromuscul Disord. 2009; 19: 788–796. doi: 10.1016/j.nmd.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 130.Barthélémy I, Barrey E, Aguilar P, Uriarte A, Le Chevoir M, Thibaud JL, et al. Longitudinal ambulatory measurements of gait abnormality in dystrophin-deficient dogs. BMC Musculoskelet Disord. 2011; 12: 75. doi: 10.1186/1471-2474-12-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barthélémy I, Pinto-Mariz F, Yada E, Desquilbet L, Savino W, Silva-Barbosa SD, et al. Predictive markers of clinical outcome in the GRMD dog model of Duchenne muscular dystrophy. Dis Model Mech. 2014; 7: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuraoka M, Nitahara-Kasahara Y, Tachimori H, Kato N, Shibasaki H, Shin A, et al. Accelerometric outcomes of motor function related to clinical evaluations and muscle involvement in dystrophic dogs. PLoS One. 2018; 13: e0208415. doi: 10.1371/journal.pone.0208415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Catavitello G, Ivanenko YP, Lacquaniti F. Planar Covariation of Hindlimb and Forelimb Elevation Angles during Terrestrial and Aquatic Locomotion of Dogs. PLoS One. 2015; 10: e0133936. doi: 10.1371/journal.pone.0133936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goslow GE, Jr, Seeherman HJ, Taylor CR, McCutchin MN, Heglund NC. Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol. 1981; 94: 15–42. doi: 10.1242/jeb.94.1.15 [DOI] [PubMed] [Google Scholar]
- 135.Gregersen CS, Silverton NA, Carrier DR. External work and potential for elastic storage at the limb joints of running dogs. J Exp Biol. 1998; 201: 3197–3210. doi: 10.1242/jeb.201.23.3197 [DOI] [PubMed] [Google Scholar]
- 136.Deban SM, Schilling N, Carrier DR. Activity of extrinsic limb muscles in dogs at walk, trot and gallop. J Exp Biol. 2012; 215: 287–300. doi: 10.1242/jeb.063230 [DOI] [PubMed] [Google Scholar]
- 137.Walter RM, Carrier DR. Effects of fore-aft body mass distribution on acceleration in dogs. J Exp Biol. 2011; 214: 1763–1772. doi: 10.1242/jeb.054791 [DOI] [PubMed] [Google Scholar]
- 138.McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech. 2015; 8: 195–213. doi: 10.1242/dmm.018424 [DOI] [PMC free article] [PubMed] [Google Scholar]