Abstract
Intracerebral hemorrhage (ICH) is the most devastating subtype of stroke with high morbidity and mortality. The previous study has confirmed the therapeutic effect of Baihui (DU20)-penetrating-Qubin (GB7) acupuncture on ICH, while the related mechanism is left to be revealed. The aim of this study was to investigate the relevant mechanisms. ICH rat models were established utilizing the autologous blood injection method and the beneficial effect was found after DU20-penetrating-GB7 acupuncture along with decreased miR-34a-5p levels in the perihemorrhagic penumbra. Inversely, upregulating miR-34a-5p expression inhibited microglia M2 polarization while accelerated M1 polarization through targeting Krüppel-like factor 4 (Klf4), and thereby diminished the protective effect of DU20-penetrating-GB7 acupuncture on ICH. The results suggested the therapeutic effect of DU20-penetrating-GB7 acupuncture on ICH might be attributed to its modulation on microglia polarization through miR-34a-5p/Klf4 signaling.
Keywords: intracerebral hemorrhage, Krüppel-like factor 4 (Klf4), microglia polarization, miR-34a-5p
Introduction
Intracerebral hemorrhage (ICH) is a life-threatening neurologic injury accounting for 10% to 20% of stroke cases globally [1, 2]. ICH is the frequent type of spontaneous intracranial haemorrhage, followed by subarachnoid haemorrhage and isolated intraventricular haemorrhage [3]. It is roughly considered that the incidence of reported ICH cases is up to 24.6 per 100,000 people annually and approximately 2 million individuals are affected by this devastating disease [4, 5]. Sex, hypertension, diabetes, alcohol intake and antiplatelet or anticoagulant medication application are the important risk factors [6]. Apart from the high incidence, ICH is the most serious and the worst functional outcomes after recovery. Poor prognosis of ICH contributes a lot to the high mortality with 1-year survival rate is probably 46% while 5-year survival rate is only 29% as stated [7]. Most ICH survivors have a high risk of recurrence and suffer varying degrees of disability, including mobility disorders and consciousness decrease [3]. Besides, there is an elevated risk of vascular events, epilepsy and dementia in ICH patients [8]. Among current therapeutics, medical and surgical interventions are the common therapy, however, there is no recognized treatment that can significantly improve the prognosis of ICH. Hence, it is urgent to investigate the pathogenesis of ICH and thereby developing efficient treatment for ICH.
Increasing evidences suggest that aggravated inflammatory response plays an essential role in facilitating ICH-related neurologic injury [9]. In addition, inflammatory cytokines released by abnormally activated microglia are the key section to aggravate the nerve injury in the central nervous system (CNS) [10]. It is well understood that microglia, one of the innate immune cells in CNS, are the major phagocytes and serve as guardians for neuronal survival and function under normal physiological conditions [11]. It is interesting to note that the effect of microglia in CNS is dual in neurological diseases, such as ICH [12, 13], spinal cord injury [14] and acute brain injury [15]. On one hand, emerging evidence suggests that the abnormally released chemokines, cytokines and other immunoregulatory molecules initiating secondary brain injury are primarily derived from the activated microglia [16]. On the other hand, the released molecules of microglia also involve in the repair processes of brain [17]. This phenomenon is due to the fact that microglia is highly plastic cells with two phenotypes, which may be harmful or beneficial to the injured site according to microenvironmental changes [18]. In respond to ICH and other brain injury, microglia is activated and developed classic proinflammatory (M1 phenotype) microglia and alternative anti-inflammatory (M2 phenotype) microglia [19, 20]. The preceding evidences suggest that microglia and macrophages tend to develop M1 phenotype than M2 phenotype, even though the markers for M1 and M2 phenotype are all increased in the acute phase of brain injury [21]. Therefore, we speculate that regulating microglia polarization may be a potentially effective strategy for ICH treatment.
Recent studies have indicated that acupuncture possesses obvious therapeutic effect on patients with ICH [22, 23]. At present, the beneficial effects of Baihui (DU20)-penetrating-Qubin (GB7) acupuncture have been confirmed in many experiments [24,25,26]. Although the neuroprotective effect of DU20-penetrating-GB7 acupuncture is basically clear, few research teams pay close attention to its mechanism on ICH. Studies have found that miR-34a-5p expression is up-regulated in the blood of stroke patients [27]. Besides, miR-34a overexpression in cerebrovascular endothelial cells disrupts blood-brain barrier permeability [28]. MiR-34a-5p overexpression can destroy blood-brain barrier and induce mitochondrial dysfunction, inversely, miR-34a-5p knockdown inhibits neuronal apoptosis, and thereby attenuating cerebral ischemia-reperfusion injury in rats [28]. However, the role of miR-34a-5p in ICH has not been left to be revealed. Since the Bioinformatics website TargetScan (www.targetscan.org) predicts the binding site of miR-34a-5p and Krüppel-like factor 4 (Klf4) mRNA, moreover, Klf4 possesses the ability to promote M2 polarization in macrophages [29, 30]. Inspired by the above background, we assume whether DU20-penetrating-GB7 acupuncture can alleviate nerve injury after ICH via promoting M2 polarization through miR-34a-5p/Klf4 signaling cascade in microglia.
Materials and Methods
Ethical statement
The animals used in this study were taken care of according to Guide for the Care and Use of Laboratory Animals (NIH, 8th). The experimental procedure was approved by ethics committee of Heilongjiang Mental Hospital Limited Company.
Animals and treatment
Healthy adult male Sprague-Dawley (SD) rats at the age of eight weeks were utilized to duplicate ICH model using the autologous blood injection method according to the previous study [31]. The rats were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg), and autologous blood injection was used to induce ICH. In short, 50 µl autologous blood was collected from the tail vein. The rat was fixed on the brain stereotaxic instrument in the prone position, exposed the bregma, drilled the skull (3.5 mm on the right side and 0.2 mm on the back of the bregma). Micro-syringe was inserted into the brain tissue with a depth of 6 mm, the 50 µl autologous blood was injected at the rate of 20 µl /min. Finally, the micro-syringe was retained in the brain tissue for 5 min before removal. Rats in Sham group were drilled at the same location and injected with the same volume of normal saline. 1 h later, the ICH rats were exposed to DU20-penetrating-GB7 acupuncture for 30 min for 3 consecutive days.
For the miR-34a-5p intervention, agomiR-34a-5p or control agomiRNA (agomiR-NC) lentivirus (5 µl, 1 × 109 TU/ml) was injected into the lateral ventricle (0.8 mm after anterior fontanelle, 1.5 mm right suture and 4.5 mm deep) 7 days before autologous blood injection. The procedure of lentivirus injection was similar to that for autologous blood injection. In brief, after anesthesia with pentobarbital sodium (50 mg/kg) via intraperitoneal injection, the rat was fixed in the prone position on the brain stereotaxic instrument, exposed the bregma, drilled the skull (1.5 mm on the right side of the sagittal suture, 0.8 mm posterior to the bregma, 4.5 mm below the skull). 20 µM agomiR-34a-5p or 5 µl agomiR-NC was injected in the brain tissue at the rate of 0.5 µl /min via a 26-gauge needle of a micro-syringe.
According to the Berderson’s score [32], the Neurological function deficiency of the rats was assessed at the final day of DU20-penetrating-GB7 acupuncture. Afterwards, the rats were euthanized with intraperitoneal injection of pentobarbital sodium (200 mg/kg). The brains were carefully isolated on ice and the wet weight was recorded. Finally, the brains were kept in an oven at 100°C for 24 h to get the dry weight and the ratio of dry to wet weight represented brain water content.
Perihemorrhagic penumbra location was affirmed according to the previous literature with appropriate adjustments [33]. After the whole brain was isolated, the coronal section was made 3 to 9 mm from the anterior segment of the frontal lobe, and the remaining 6 mm thick brain tissue was collected. Afterwards, this brain tissue was cut at 2 mm along the sagittal suture, the remaining lateral brain tissuse was selected. The brain tissue was cut again at 2 mm along the sagittal suture and at an angle of 30 degrees with the sagittal suture. Finally, the remaining 2 mm thickness brain tissue was perihemorrhagic penumbra.
HE staining
Briefly, the brain were carefully isolated on ice, fixed in 4% paraformaldehyde and embedded in paraffin blocks. After sectioned into 5 µm-in thick samples by a rotary microtome, the samples were stained with hematoxylin solution (Solarbio, Beijing, China) and counterstained with eosin (Sangon, Beijing, China) according to the manufacture’s instruction. The pathologic change in the perihemorrhagic penumbra after ICH was observed under a light microscopy at 200× magnification.
Fluoro-Jade B staining
The aforementioned brain sections were used to detect neuronal apoptosis by using Fluoro-Jade B staining kits (Merckmillipore, Burlington, MA, USA) and all the procedures were in accordance with the manufacture’s instruction. Finally, the Fluoro-Jade B (FJB)-positive cells were counted under a fluorescence microscope at 400× magnification.
Immunofluorescence
In brief, the aforementioned brain sections were blocked in goat serum for 15 min, incubated in rabbit anti-CD32 (dilution: 1:50, Santa Cruz Biotechnology, Dallas, TX, USA) and mouse anti-Iba-1 (dilution: 1:300, Abcam, Cambridge, UK) or rabbit anti-CD206 (dilution: 1:200, Proteintech, Wuhan, China) and mouse anti-Iba-1 (dilution: 1:300, Abcam) over night at 4°C, and subjected to Cy3-labeled IgG antibody (dilution: 1:200, Beyotime Institute of Biotechnology, Shanghai, China) and FITC labeled IgG antibody (dilution: 1:200, Beyotime Institute of Biotechnology) for 90 min at room temperature. Finally, 4’, 6-diamidino-2-phenylindole (DAPI, Biosharp, Hefei, China) was used to visualize the nuclei and the target protein was observed under a fluorescence microscope at 400× magnification. Immunofluorescence was quantified by counting the number of co-stained cells. The number of co-stained cells was counted in three random fields.
Terminal deoxynucleotidyl transferase-mediated dUTP (2-deoxyuridine 5-triphosphate) nick-end labeling (TUNEL) assay
The brain sections were subjected to In Situ Cell Death Detection Kit (Roche, Basel, Switzerland). After all the procedure required by the manufacture’s instruction, the sections were immersed in goat serum for 30 min, incubated in mouse anti-NeuN (dilution: 1:400, abcam) over night at 4°C, Cy3-labeled IgG antibody (dilution: 1:200, Beyotime Institute of Biotechnology) for 60 min at room temperature. The co-localization of TUNEL and NeuN was observed under a fluorescence microscope at 400× magnification.
ELISA
The brain tissues in the perihemorrhagic penumbra were homogenized in ice-cold normal saline (NS) and centrifuged at 2,500 rpm at 4°C for 10 min to collect the supernatant. The protein concentration of the supernatant was determined by BCA protein assay kit (Beyotime Institute of Biotechnology). The levels of IL-4 in the perihemorrhagic penumbra were measured by commercial ELISA kits (USCN KIT INC., Wuhan, China) and all the procedures were implemented with manufacturer’s instruction.
RT-PCR analysis
The total RNA in the perihemorrhagic penumbra was extracted using the high purity total RNA rapid extraction kit (BioTeke, Beijng, China) and quantified by an ultraviolet spectrophotometer. The isolated RNA was transcribed into the relevant cDNA by miR-34a-5p stem-loop RT primer or respective primer, the expression levels of miR-34a-5p and Klf4 were detected by RT-PCR utilizing Taq HS Perfect Mix (Takara, Dalian, China) and SYBR Green (Takara) in an Exicycler 96 System (Bioneer, Daejeon, Korea). 5S and β-actin were used as the internal reference. The data was analyzed by 2-ΔΔCT method.
The primers for RT-PCR were as follows:
MiR-34a-5p stem-loop RT primer: 5’-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACAACC-3’; Forward: 5’-GGACTTGGCAGTGTCTTAGCTG-3’; Reverse: 5’-GTGCAGGGTCCGAGGTATTC-3’
Klf4 Forward: 5’-GGAGCCCAAGCCAAAGAGG-3’; Reverse: 5’-CGTCCCAGTCACAGTGGTAAGGT-3’
Western blotting analysis
The total protein in the perihemorrhagic penumbra was extracted using the RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified by a BCA protein assay kit (Beyotime Institute of Biotechnology). After separation by 8%, 10% or 15% SDS-PAGE gel, the protein was transferred to an equilibrated polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific, Rockford, IL, USA) and blocked in 5% bovine serum albumin (BSA, Biosharp) for 1 h at the room temperature. Afterwards, the PVDF membranes were incubated in specific primary antibodies at the predetermined dilution over night at 4°C, anti-mouse horseradish peroxidase (HRP) labelled secondary antibody (Proteintech, category No: SA00001-1) or anti-rabbit HRP labelled secondary antibody (Proteintech, category No: SA00001-2) for 45 min at 37°C, and finally visualized using chemiluminescence (ECL, 7 Sea biotech, Shanghai, China) kits in Gel-Pro-Analyzer system. β-actin was used as the internal reference.
The primary antibodies for western blotting were as follows:
Mouse anti-CD206 (dilution: 1:1,000), CD32 (dilution: 1:500), β-actin (dilution: 1:2,000), rabbit anti-Iba1 (dilution: 1:1,000), iNOS (dilution: 1:500), Arg-1 (dilution: 1:500), IL-4 (dilution: 1:1,000) and Klf4 (dilution: 1:500).
Dual-luciferase reporter assay
TargetScan (www.targetscan.org) was used to predict the targeted binding between miR-34a-5p and Klf4 3’-UTR. HEK293T cells were co-transfected with miR-34a-5p mimics or control mimics and wild-type (WT) Klf4 3’-UTR or mutant-type (MUT) Klf4 3’-UTR reporter plasmid (Wanlei Biological Technology Co., Ltd., Shenyang, China). Afterwards, luciferase activity was etected by Dual-Luciferase assay kit (KeyGENBioTECH, Nanjing, China).
Statistical analysis
Data are reported as means ± SD and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. P value less than 0.05 was regarded statistically significant.
Results
MiR-34a-5p counteracted the therapeutical effects of Baihui-penetrating-Qubin acupuncture in ICH rats
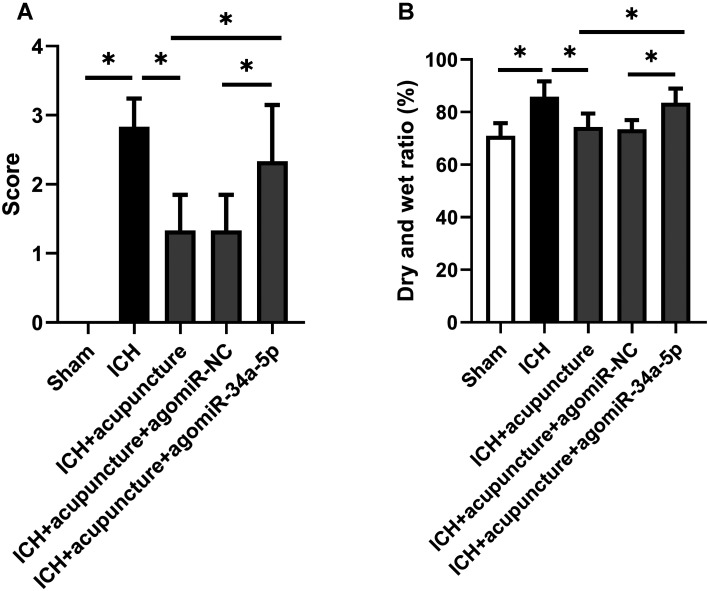
The positive effects of Baihui-penetrating-Qubin acupuncture on ICH in rats have been affirmed in the previous research, but the specific mechanism remains to be revealed. In order to confirm whether miR-34a-5p played an important role in this pathological process, we injected agomiR-34a-5p into the lateral ventricle before establishing ICH model. As is shown in Fig. 1A, neurological deficits were alleviated with Baihui-penetrating-Qubin acupuncture treatment, however, the neuroprotective effects could be counteracted by miR-34a-5p overexpression (P<0.05). In addition, the intraventricular injection of agomiR-34a-5p inhibited the decrease in cerebral edema after Baihui-penetrating-Qubin acupuncture (Fig. 1B, P<0.05). The data showed that miR-34a-5p overexpression diminished the therapeutic effects of Baihui-penetrating-Qubin acupuncture in ICH rats, indicating miR-34a-5p might be an important molecule in ICH pathological process.
Fig. 1.
MiR-34a-5p counteracted the therapeutical effects of Baihui-penetrating-Qubin acupuncture in intracerebral hemorrhage (ICH) rats. (A) The average score of neurological function in ICH rats with Baihui-penetrating-Qubin acupuncture after miR-34a-5p treatment. (B) Ratio of dry to wet brain weight in ICH rats with Baihui-penetrating-Qubin acupuncture after miR-34a-5p treatment. Data are reported as means ± SD (n=6) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. *P<0.05 vs. the indicated group.
Effects of miR-34a-5p on Klf4 expression in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment
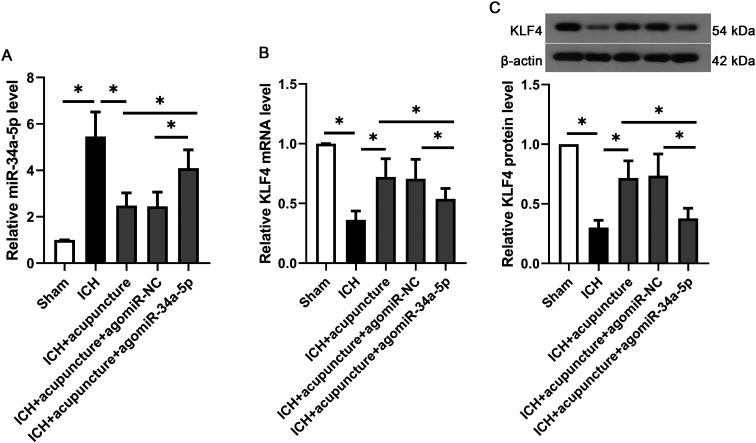
As the results shown in Fig. 2A, the levels of miR-34a-5p were increased in the perihemorrhagic penumbra after ICH injury, which could be decreased by Baihui-penetrating-Qubin acupuncture. Besides, the intraventricular injection of agomiR-34a-5p significantly increased miR-34a-5p expression in the perihemorrhagic penumbra, suggesting the in vivo transfection was effective. Contrary to the trend of miR-34a-5p expression, the mRNA and protein levels of Klf4 were decreased with miR-34a-5p overexpression (Figs. 2B and C, P<0.05), suggesting Klf4 might be one of the targets of miR-34a-5p.
Fig. 2.
Effects of miR-34a-5p on Klf4 expression in the perihemorrhagic penumbra of intracerebral hemorrhage (ICH) rats with Baihui-penetrating-Qubin acupuncture treatment. (A) The relative miR-34a-5p expression in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment. The mRNA (B) and protein (C) levels of Klf4 in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment. Data are reported as means ± SD (n=6) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. *P<0.05 vs. the indicated group.
MiR-34a-5p crippled the anti-apoptotic effects of Baihui-penetrating-Qubin acupuncture in the perihemorrhagic penumbra of ICH rats
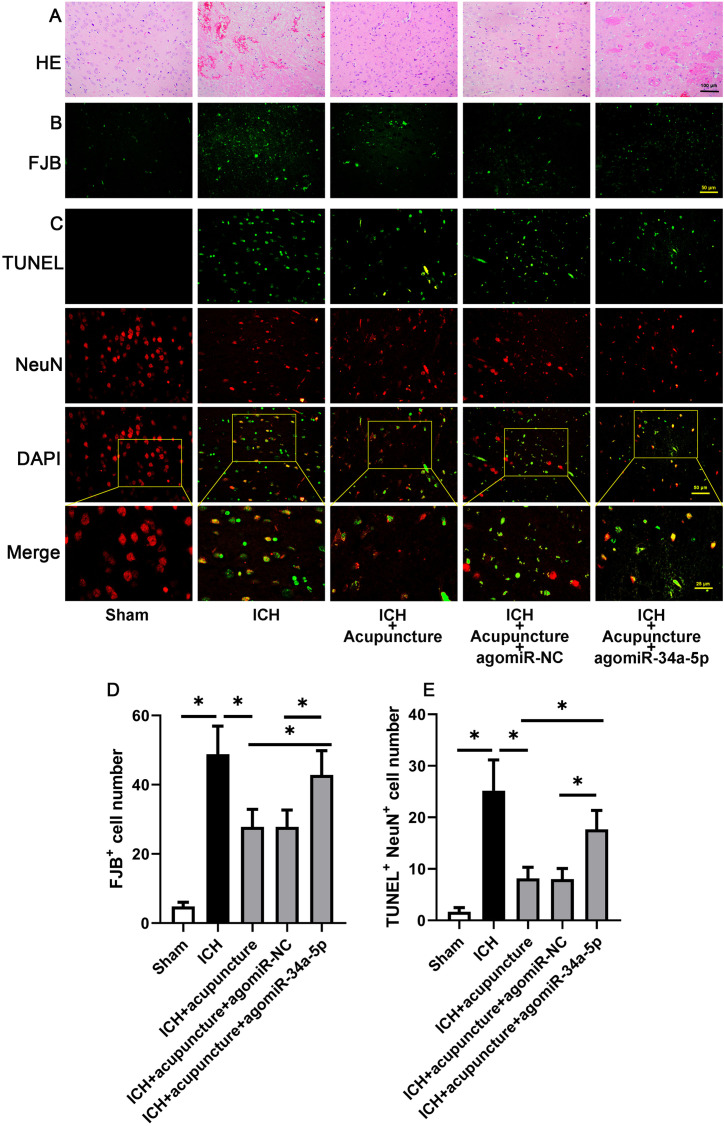
In sequence, the histopathological detection in the brain was implemented. As shown in Fig. 3A, ICH-related pathological alteration, including inflammatory infiltration and necrosis, were markedly lower after Baihui-penetrating-Qubin acupuncture in ICH rats, whereas this phenomenon could be subverted by miR-34a-5p overexpression. The results of Fluoro-Jade B staining showed that the apoptotic neurons were increased in the perihemorrhagic penumbra with agomiR-34a-5p pre-injection (Figs. 3B and D, P<0.05). As described in Figs. 3C and E, the result of immunofluorescence targeting TUNEL and NeuN was similar to Fluoro-Jade B staining (P<0.05). The result illustrated miR-34a-5p was involved in the anti-apoptotic effect of Baihui-penetrating-Qubin acupuncture in the perihemorrhagic penumbra of ICH rats.
Fig. 3.
MiR-34a-5p crippled the anti-apoptotic effects of Baihui-penetrating-Qubin acupuncture in the perihemorrhagic penumbra of intracerebral hemorrhage (ICH) rats. (A) Representative images of H&E staining in the perihemorrhagic penumbra of ICH rats at 200× magnification. (B) Representative Fluoro-Jade B staining in the perihemorrhagic penumbra of ICH rats at a 400× magnification. (C) Immunofluorescence targeting TUNEL and NeuN (neuron marker) in the perihemorrhagic penumbra of ICH rats at 400× magnification. (D) Quantification of Fluoro-Jade B-positive cells in the perihemorrhagic penumbra of ICH rats. (E) Quantification of TUNEL and NeuN-positive cells in the perihemorrhagic penumbra of ICH rats. Data are reported as means ± SD (n=6) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. *P<0.05 vs. the indicated group.
Effects of miR-34a-5p on microglia polarization in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment
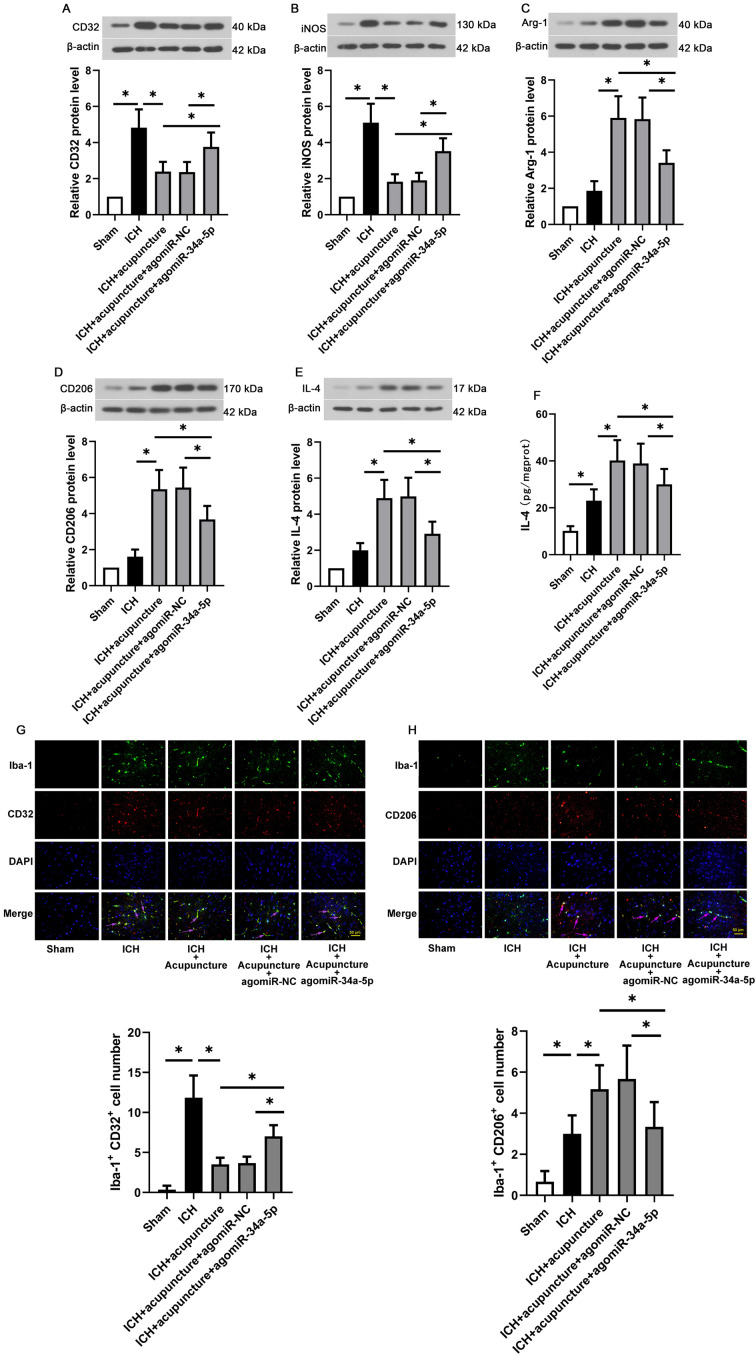
Since microglia is the sources of inflammatory cytokine in central nervous system, microglia polarization was evaluated in the present experiments. As shown in Figs. 4A and B, the markers of M1 microglia, such as CD32 and iNOS (P<0.05), were enhanced with agomiR-34a-5p administration in IHC rat treated with Baihui-penetrating-Qubin acupuncture. On the contrary, the M2 microglia markers (Arg-1, CD206 and IL-4) in the perihemorrhagic penumbra were decreased with miR-34a-5p overexpression (P<0.05). Moreover, as exhibited in Fig. 4G, the expressions of CD32 in Iba-1-positive cells were decreased after Baihui-penetrating-Qubin acupuncture treatment compared with ICH rats, whereas the inhibiting effects of acupuncture on M1 microglia polarization were diminished by miR-34a-5p overexpression. Similarly, the expression of CD206, marker of M2 microglia polarization, showed the opposite trend (Fig. 4H). The results of this section suggested that miR-34a-5p reduced the therapeutic effects of Baihui-penetrating-Qubin acupuncture on IHC via regulating microglia polarization in the perihemorrhagic penumbra.
Fig. 4.
Effects of miR-34a-5p on microglia polarization in the perihemorrhagic penumbra of intracerebral hemorrhage (ICH) rats with Baihui-penetrating-Qubin acupuncture treatment. Representative western blot for (A) CD32, (B) iNOS, (C) Arg-1, (D) CD206 and (E) IL-4 in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment. (F) IL-4 release in the perihemorrhagic penumbra of ICH rats with Baihui-penetrating-Qubin acupuncture treatment. (G) Immunofluorescence targeting CD32 and Iba-1 (microglia marker) in the perihemorrhagic penumbra of ICH rats at 400× and 800× magnification. (H) Immunofluorescence targeting CD206 and Iba-1 (microglia marker) in the perihemorrhagic penumbra of ICH rats at 400× and 800× magnification. Data are reported as means ± SD (n=6) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. *P<0.05 vs. the indicated group.
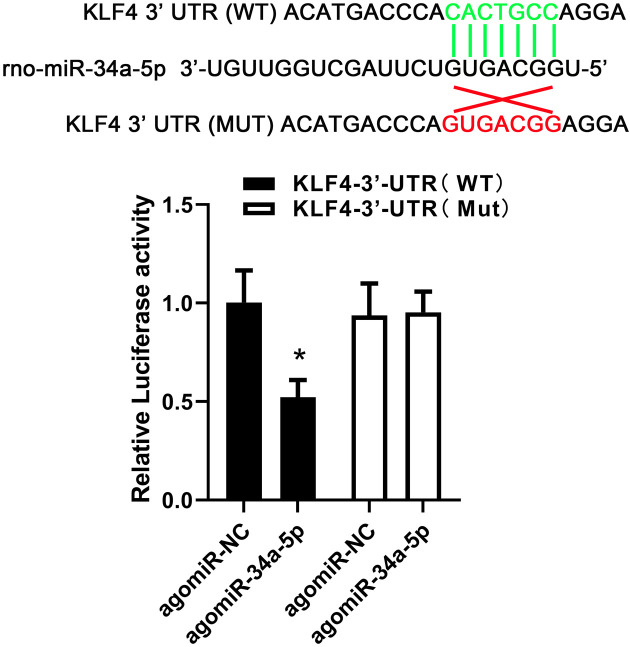
Targeted binding between miR-34a-5p and Klf4 3’-UTR
To study the underlying molecular mechanism between miR-34a-5p and Klf4, Dual-Luciferase reporter assay was carried out. As shown in Fig. 5A, relative firefly luciferase activity was obviously reduced in co-expressed miR-34a-5p and wild-type Klf4 3’-UTR (P<0.05). The results confirmed the hypothesis that miR-34a-5p negatively regulated Klf4 level via targeted binding Klf4 3’-UTR.
Fig. 5.
Targeted binding between miR-34a-5p and Klf4 3’-UTR. Dual-Luciferase reporter assay was performed between miR-34a-5p and Klf4 3’-UTR in HEK293T cells. Data are reported as means ± SD (n=3) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. *P<0.05 vs. the indicated group.
Discussion
It is well accepted that acupuncture as a bright pearl of Chinese traditional medicine plays a significant role in the treatment of multifarious diseases, including stroke, chronic pain and even depression. Acupuncture has been applied in the treatment and rehabilitation of stroke for thousands of years and its therapeutic effect has been confirmed in clinic, but the accurate mechanism remains to be explored. In the present study, we explored the effect of miR-34a-5p overexpression on therapeutic outcome of DU20-penetrating-GB7 acupuncture after ICH in rat and evaluated the potential molecular mechanisms of miR-34a-5p/Klf4 signaling on microglia polarization. Our present results indicated that DU20-penetrating-GB7 acupuncture mitigated ICH injury, which was accompanied by miR-34a-5p down-regulation, Klf4 up-regulation and promoted microglia M2 polarization. Surprisingly, the therapeutic effects of DU20-penetrating-GB7 acupuncture on ICH could be diminished by miR-34a-5p overexpression, suggesting the pivotal role of miR-34a-5p and its target mRNA in ICH treatment. Therefore, our work revealed that DU20-penetrating-GB7 acupuncture mitigated ICH injury by promoting microglia M2 polarization via regulating miR-34a-5p/Klf4 system in rat models.
miRNA pertains to the small non-coding RNA with 18–24 nucleotides and possesses the function of facilitating degradation or inhibiting translation of the target genes [34]. Due to the continuously deepening research on miRNA function, MicroRNA is found to be related to a variety of physiological and pathophysiological processes, such as focal cerebral ischemia [35], comorbid pain and depression [36]. The preceding study exhibited that miR-34a-5p deficiency attenuated cognitive deficits in Alzheimer’s disease (AD) mice [37], indicating miR-34a-5p might serve as a regulator of central nervous system disease. Conversely, the expressions of miR-34a-5p were found to be elevated in blood of acute ischemic stroke patients when compared with the controls [38]. Our results supported that miR-34a-5p levels were up-regulated in the perihemorrhagic penumbra of ICH rats with DU20-penetrating-GB7 acupuncture. Additionally, the therapeutical effect of DU20-penetrating-GB7 acupuncture on ICH was diminished by miR-34a-5p overexpression accompanied by increased neurological function deficiency and edema degree. With the aggravating neuronal apoptosis and morphological changes in the perihemorrhagic penumbra after miR-34a-5p overexpression, we preliminarily inferred that miR-34a-5p might be the key factor regulating the therapeutic effect of DU20-penetrating-GB7 acupuncture.
Due to multiple pathways have been confirmed to be associated with the function of miR-34a-5p, we only study related molecules that have potential target binding to miR-34a-5p. As a zinc-finger-containing protein, Klf4 plays an important role in a variety of physiological processes [39], including embryonic stem cell self-renewal and differentiation [40]. Recently, emerging evidence suggested that Klf4 was involved in the protective effect of microvascular endothelial cells from ischemic stroke injury by inhibiting apoptosis and inflammation [41]. In line with these, Klf4 downregulation was observed in the perihemorrhagic penumbra of ICH rats, which could be upregulated by DU20-penetrating-GB7 acupuncture. Moreover, miR-34a-5p overexpression inhibited acupuncture-induced Klf4 elevation in the perihemorrhagic penumbra. Inspired by the negative correlation between miR-34a-5p and Klf4 expression, we wondered whether Klf4 was the potential target genes of miR-34a-5p. In order to verify this hypothesis, Dual-Luciferase reporter assay was performed and the results confirmed the targeting binding between miR-34a-5p and Klf4 3’-UTR. These data indicated that miR-34a-5p/Klf4 axis activation was related to the therapeutic effect of DU20-penetrating-GB7 acupuncture in ICH rats.
Given that microglia is responsible for the course destruction, restoration and regeneration in CNS [42]. It has been reported that the pro-inflammatory microglia (M1 phenotype) can aggravate the inflammatory response, and thereby leading to secondary damage, while the anti-inflammatory phenotype promotes restoration and regeneration in the CNS [42]. As mentioned in previous study, suppressing M1 polarization of microglia and alleviating inflammatory reaction could be an underlying intervention strategy in ICH-related brain injuries [43]. In our study, M1 phenotype polarization was inhibited while M2 phenotype was promoted after DU20-penetrating-GB7 acupuncture, which was reflected by polarization marker CD32, iNOS, Arg-1, CD206 and IL-4. Besides, miR-34a-5p overexpression could reverse the therapeutic effect of DU20-penetrating-GB7 acupuncture, indicating the protective role of DU20-penetrating-GB7 acupuncture might be mediated by regulating microglia polarization.
In conclusion, our work revealed that DU20-penetrating-GB7 acupuncture alleviated ICH related neurological function deficiency, which could be attributed to the effects of down-regulating microglia M1 polarization while up-regulating microglia M2 polarization in the perihemorrhagic penumbra via miR-34a-5p/Klf4 signaling.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
This work was supported by Heilongjiang Province Postdoctoral Foundation (LBH-Z19034) and China Postdoctoral Science Foundation (2020M670940).
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009; 8: 355–369. doi: 10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- 2.Van Matre ET, Sherman DS, Kiser TH. Management of intracerebral hemorrhage--use of statins. Vasc Health Risk Manag. 2016; 12: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018; 392: 1257–1268. doi: 10.1016/S0140-6736(18)31878-6 [DOI] [PubMed] [Google Scholar]
- 4.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010; 9: 167–176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010)GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013; 1: e259–e281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariesen MJ, Claus SP, Rinkel GJE, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003; 34: 2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D [DOI] [PubMed] [Google Scholar]
- 7.Poon MTC, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014; 85: 660–667. doi: 10.1136/jnnp-2013-306476 [DOI] [PubMed] [Google Scholar]
- 8.Thabet AM, Kottapally M, Hemphill JC., 3rd. Management of intracerebral hemorrhage. Handb Clin Neurol. 2017; 140: 177–194. doi: 10.1016/B978-0-444-63600-3.00011-8 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol Neurobiol. 2017; 54: 1874–1886. doi: 10.1007/s12035-016-9785-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1. Transl Stroke Res. 2016; 7: 478–487. doi: 10.1007/s12975-016-0472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017; 13: 420–433. doi: 10.1038/nrneurol.2017.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010; 92: 463–477. doi: 10.1016/j.pneurobio.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016; 142: 23–44. doi: 10.1016/j.pneurobio.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009; 111: 1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x [DOI] [PubMed] [Google Scholar]
- 15.Lan X, Liu R, Sun L, Zhang T, Du G. Methyl salicylate 2-O-β-D-lactoside, a novel salicylic acid analogue, acts as an anti-inflammatory agent on microglia and astrocytes. J Neuroinflammation. 2011; 8: 98. doi: 10.1186/1742-2094-8-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014; 115: 25–44. doi: 10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Zheng K, Su Z, Su H, Zhong M, He X, et al. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J Neuroimmunol. 2016; 299: 28–34. doi: 10.1016/j.jneuroim.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 18.Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain. 2015; 138: 1138–1159. doi: 10.1093/brain/awv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012; 43: 3063–3070. doi: 10.1161/STROKEAHA.112.659656 [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013; 33: 1864–1874. doi: 10.1038/jcbfm.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014; 83: 1098–1116. doi: 10.1016/j.neuron.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 22.Wang HQ, Bao CL, Jiao ZH, Dong GR. Efficacy and safety of penetration acupuncture on head for acute intracerebral hemorrhage: A randomized controlled study. Medicine (Baltimore). 2016; 95: e5562. doi: 10.1097/MD.0000000000005562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HQ, Dong GR, Bao CL, Jiao ZH. Immediate effect of scalp acupuncture on the gait of patients with subacute intracerebral haemorrhage analysed by three-dimensional motion: secondary analysis of a randomised controlled trial. Acupunct Med. 2018; 36: 71–79. doi: 10.1136/acupmed-2016-011272 [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Dai XH, Yu XP, Zou W, Teng W, Sun XW, et al. Baihui (DU20)-penetrating-Qubin (GB7) acupuncture inhibits apoptosis in the perihemorrhagic penumbra. Neural Regen Res. 2018; 13: 1602–1608. doi: 10.4103/1673-5374.237123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XY, Dai XH, Zou W, Yu XP, Teng W, Wang Y, et al. Acupuncture through Baihui (DU20) to Qubin (GB7) mitigates neurological impairment after intracerebral hemorrhage. Neural Regen Res. 2018; 13: 1425–1432. doi: 10.4103/1673-5374.235298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou W, Chen QX, Sun XW, Chi QB, Kuang HY, Yu XP, et al. Acupuncture inhibits Notch1 and Hes1 protein expression in the basal ganglia of rats with cerebral hemorrhage. Neural Regen Res. 2015; 10: 457–462. doi: 10.4103/1673-5374.153696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren X, Engler-Chiurazzi EB, Russell AE, Sarkar SN, Rellick SL, Lewis S, et al. MiR-34a and stroke: Assessment of non-modifiable biological risk factors in cerebral ischemia. Neurochem Int. 2019; 127: 73–79. doi: 10.1016/j.neuint.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X. MiR-34a regulates blood-brain barrier permeability and mitochondrial function by targeting cytochrome c. J Cereb Blood Flow Metab. 2016; 36: 387–392. doi: 10.1177/0271678X15606147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SP, Wang D, Li HX, Liu L, Duan XH. Influence of miR-34a on cerebral neuronal apoptosis in rats with cerebral ischemia reperfusion through the Notch1 signaling pathway. Eur Rev Med Pharmacol Sci. 2019; 23: 8049–8057. [DOI] [PubMed] [Google Scholar]
- 30.Han YH, Kim HJ, Na H, Nam MW, Kim JY, Kim JS, et al. RORα Induces KLF4-Mediated M2 Polarization in the Liver Macrophages that Protect against Nonalcoholic Steatohepatitis. Cell Rep. 2017; 20: 124–135. doi: 10.1016/j.celrep.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Xiao H, Gan H, Zhang L, Wang L, Li S, et al. Hypoxia-inducible factor 2α exerts neuroprotective effects by promoting angiogenesis via the VEGF/Notch pathway after intracerebral hemorrhage injury in rats. Neuroscience. 2020; 448: 206–218. doi: 10.1016/j.neuroscience.2020.07.010 [DOI] [PubMed] [Google Scholar]
- 32.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986; 17: 472–476. doi: 10.1161/01.STR.17.3.472 [DOI] [PubMed] [Google Scholar]
- 33.Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998; 29: 1037–1046, discussion 1047. doi: 10.1161/01.STR.29.5.1037 [DOI] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136: 215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang G, Liu Z, Wang L, Chen X, Wang X, Dong Q, et al. MicroRNA-195 protection against focal cerebral ischemia by targeting CX3CR1. J Neurosurg. 2018; 131: 1–10. [DOI] [PubMed] [Google Scholar]
- 36.Satyanarayanan SK, Shih YH, Wen YR, Palani M, Lin YW, Su H, et al. miR-200a-3p modulates gene expression in comorbid pain and depression: Molecular implication for central sensitization. Brain Behav Immun. 2019; 82: 230–238. doi: 10.1016/j.bbi.2019.08.190 [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Chen P, Wang X, Yao J, Zhuang S. miR-34a deficiency in APP/PS1 mice promotes cognitive function by increasing synaptic plasticity via AMPA and NMDA receptors. Neurosci Lett. 2018; 670: 94–104. doi: 10.1016/j.neulet.2018.01.045 [DOI] [PubMed] [Google Scholar]
- 38.Liang TY, Lou JY. Increased expression of mir-34a-5p and clinical association in acute ischemic stroke patients and in a rat model. Med Sci Monit. 2016; 22: 2950–2955. doi: 10.12659/MSM.900237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon BS, Bai J, Cai M, Liu C, Shi J, Lu W. Kruppel-like factor 4-dependent Staufen1-mediated mRNA decay regulates cortical neurogenesis. Nat Commun. 2018; 9: 401. doi: 10.1038/s41467-017-02720-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010; 90: 1337–1381. doi: 10.1152/physrev.00058.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Xi X, Zhao B, Su Z, Wang Z. KLF4 protects brain microvascular endothelial cells from ischemic stroke induced apoptosis by transcriptionally activating MALAT1. Biochem Biophys Res Commun. 2018; 495: 2376–2382. doi: 10.1016/j.bbrc.2017.11.205 [DOI] [PubMed] [Google Scholar]
- 42.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007; 10: 1387–1394. doi: 10.1038/nn1997 [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Cheng L, Chen ZL, Mungur R, Xu SH, Wu J, et al. Hyperbaric oxygen preconditioning attenuates brain injury after intracerebral hemorrhage by regulating microglia polarization in rats. CNS Neurosci Ther. 2019; 25: 1126–1133. doi: 10.1111/cns.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]