Abstract
Adeno-associated virus (AAV)-based gene therapy is gaining popularity owing to its excellent safety profile and effective therapeutic outcomes in a number of diseases. Intravenous (IV) injection of AAV into the tail vein, facial vein and retro-orbital (RO) venous sinus have all been useful strategies to infuse the viral vector systemically. However, tail vein injection is technically challenging in juvenile mice, and injection at young ages (≤ postnatal day-(P)21) is essentially impossible. The temporal or facial vein is localized anterior to the ear bud and is markedly visible in the first couple of days postnatally. However, this method is age-dependent and requires a dissecting microscope. Retro-orbital injection (ROI), on the other hand, is suitable for all murine ages, including newborn and older mice, and is relatively less stressful to animals compared to tail vein injection. Although many reports have shown ROI as an effective route of AAV delivery, herein we aim to highlight and summarize the methods and benefits of ROI. To capture the full spectrum of transduction efficiency mediated by ROI, we transduced the editing-dependent reporter mice (Ai9 Cre reporter mice) with the AAV9 vector, which targets a wide range of peripheral tissues with exceptional brain tropism. We also provide a comprehensive description of the ROI technique to facilitate viral vector administration without complications.
Keywords: adeno-associated virus (AAV), retro-orbital injection, systemic
Introduction
Administration of experimental substances (e.g., viral vectors, pharmacological agents) to laboratory animals, especially mouse (Mus musculus) is often a critical component of a preclinical experimental design [1]. Intravenous (IV) delivery is usually the preferred route to maximize systemic distribution. In mice, the most common way of getting vascular access is through the tail vein, facial/temporal vein or retro-orbital (RO) vasculature [2]. Each approach has both merits and deficiencies, and preferences are primarily dependent on the age of the animal and bioavailability of the route of injection, as well as properties and fate of the test substance. To facilitate long-term AAV-mediated gene transfer, AAV vectors is administered at neonatal period [3, 4] or juvenile (at postnatal day-(P) 21) [5] rodent models in preclinical research.
Tail vein injection remains a popular choice of venipuncture in the adult mouse (at least 6 weeks of age) owing to its convenience based on their superficial location and presence in pairs (two lateral caudal veins running in parallel to the single ventral caudal artery) [6, 7]. However, this procedure is technically challenging, requires practice and accuracy, and can have a high rate of failure in inexperienced hands. This is because although the lateral veins are readily visualized, they are quite small in diameter. Hence, in preparation for tail vein venipuncture, the mouse is often placed under a heat lamp to promote peripheral vasodilation. The heat lamp poses a high risk of causing hyperthermia, heat distress and burn-related injuries to the animals. The more acceptable alternative is to dilate the veins by immersing into warm water (40 to 45°C) [8].
Tail vein injection also requires the animal to be mechanically restrained, and this can cause distress to the animals [9]. In addition, usage of a restraining device could be inappropriate depending on the animal model that is being used. For example, some genetically modified mice can phenotypically present with reduced body weight/size as side effect of a gene knockout [10,11,12]. Because of their reduced size, it could be a challenge to identify the blood vessels which are relatively smaller in size. Furthermore, while being restrained, the mouse is awake and therefore may experience a certain degree of discomfort as a direct result of the injection. A higher level of stress may be induced if the initial venipuncture is unsuccessful and repeated attempts are made. When the animal is restrained for an extended period, it tends to urinate and defecate, contaminating the restraining device with urine and fecal pellets. Consequently, this procedure can be time-consuming as the apparatus needs to be cleaned before the use of another animal. Facial vein offers another alternative route for IV access in the adult mouse, but this technique is invasive (skin flap at preauricular area), time-consuming (skin suturing) and requires anesthesia [13].
For neonatal mice, tail vein injection is not applicable as the diameter of blood vessels is not wide enough for injection. Hence, the percutaneous injection of the superficial temporal facial vein has been introduced [14] to deliver various substances, such as AAV vectors [15]. This superficial vein is easily visible (P0–P3) and accessible posterior to the eye. Unfortunately, with increased pigmentation and skin thickness in older neonates, the vein is hard to visualize, making the injection procedure technically difficult.
RO venous sinus extends between the orbital wall and the muscle cone, captures almost all the venous drainage from the eyeball and muscle cone, and communicates with the cavernous sinus, pterygoid plexus, superficial temporal vein and facial vein [16]. In mice, the RO venous sinus is a huge invaginated sac (Figs. 1A and B) occupying the retrobulbar space. Owing to its distended venous channel, retro-orbital injection (ROI) is being widely accepted as an ideal route for venous injection (Fig. 1B). ROI has been adopted in mouse models for bone marrow transplantation [17], leukemia induction [18], and administration of MRI contrast agents [19], as well as transduction by lentiviral vectors [20] and AAV vectors [5, 21,22,23]. One of the most significant advantages of ROI, over the tail and facial vein injections, is that it can be performed in mice at any age and detailed guidelines are accessible [2, 24]. Table 1 illustrates the advantages and disadvantages of tail veininjection versus ROI [25].
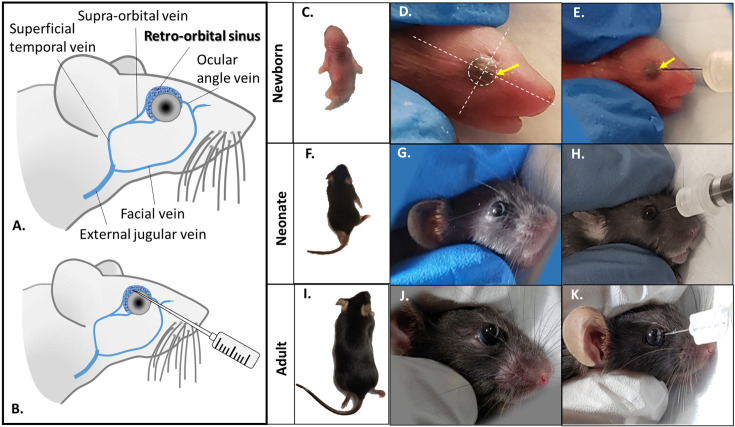
Fig. 1.
Anatomy of the retro-orbital venous (shady blue) of the mouse and route of injection (A, B). Retro-orbital injection into newborn (C–E), neonatal (F–H) and juvenile (I–K) of Ai9 mice. The eyeball of the neonate is clearly visible (outlined with dotted circle, D). Two planes (dotted lines) are used to highlight the 3 o’clock position of the eyeball, the site to insert the needle (yellow arrows, D, E).
Table 1. Comparison between tail vein and retro-orbital injection.
| Tail vein injection | Retro-orbital injection | ||
|---|---|---|---|
| 1 | Injection site | Lateral caudal vein | Retro-orbital plexus |
| 2 | Age | at least 6 weeks old | Suitable for all ages |
| 3 | Restraining device | Required | Not required |
| 4 | Anesthesia | ||
| * newborn (P0-P1) | Not applicable | cryoanesthesia | |
| * neonates (≥1 week old) | Not applicable | Inhalant anesthesia (1.5% isoflurane) | |
| * juvenile and adult | Not required | Inhalant anesthesia (2.5% isoflurane) | |
| 5 | Injection volume | ||
| * newborn (P0-P1) | Not applicable | ~ 10 μl (per eye) | |
| * neonates (P14 to P21) | Not applicable | Up to 50 μl (per eye) | |
| * juvenile and adult | Up to 200 μl | Up to 200 μl (per eye) | |
| 6 | Continuous injections | Yes | Yes |
| 7 | Stress level | High | Low |
| 8 | Vasodilation | Required (heat lamp or warm water) | Not required |
| 9 | Needle size | 28 G | 31G (for neonates) |
| 28G or smaller (for adult) | |||
| 10 | Disinfection | Swab the tail with 70% ethanol | Not required |
| 11 | Time required | Relatively longer* | Shorter |
| 12 | Visibility of the vein | Obvious in non-pigmented mouse | Not applicable |
| 13 | Other benefits | Long injection route† | - |
| 14 | Other side effects | Possible inflammation or necrosis at injection site; possible scarring or bruising of the tail if substance administered subcutaneously | Swelling adjacent to the injection site, proptosis, eye trauma |
| 15 | Other optional procedures | - | Topical ophthalmic analgesic (tetracaine, gentamicin ophthalmic) |
*Longer time required due to multiple procedures involved - to restrain animal; waiting time for vasodilation; to clean the restraint device, apply pressure on injection site to stop bleeding before returning to the home cage. †Long injection route - paired lateral vein runs the length of both lateral aspect along the tail and hence, can accommodate multiple attempts of injection, starting from distal to the proximal end of the vein.
Adeno-associated virus (AAV) has been widely adopted as the gene therapy vector of choice in both preclinical studies and clinical trials, owing to its remarkable safety and efficacy [26]. Some serotypes of AAVs exhibit body wide gene transfer following single IV injection. In this study, we provide comprehensive step-to-step guidelines for ROI coupled with neuroanatomy of the mouse’s ophthalmic route (RO venous plexus). In addition, we harnessed the editing-dependent reporter mice (Ai9 [or B6.Cg-Gt (ROSA) 26Sortm9 (CAG-tdTomato) Hze/J]) [27] and transduced them with the AAV9-CBA-Cre vector, the serotype that targets multiple peripheral tissues with remarkable brain tropism [28]. Cells in Ai9 mice exhibit very low level of tdTomato fluorescence expression prior to administration of Cre recombinase. Following transduction with Cre recombinase, cells in Ai9 model will express robust and significant greater tdTomato fluorescence expression levels than those baseline levels, allowing us to capture the full spectrum of transduction efficiency mediated by ROI. ROI, once mastered, is a user friendly procedure and effective IV route to infuse AAV vector for systemic gene therapy in both neonatal and adult murine models.
Materials and Methods
Animal husbandry
The procedures described below were approved by the Institutional Animal Care and Use Committee (IACUC) for the Massachusetts General Hospital (MGH) following the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals. Breeding pairs of Ai9 (B6.Cg-Gt (ROSA) 26Sortm9 (CAG-tdTomato) Hze/J, Stock No: #007909) were purchased from Jackson Laboratories. Ai9 is a Cre reporter mouse model that uses a loxP-flanked STOP cassette to prevent tdTomato transcription driven by CAG promoter. Ai9 mice will express robust tdTomato fluorescence upon Cre recombination. The animals were bred in-house and were housed under controlled temperature with a 12-h light/dark cycle. The mice were given standard animal feed, and water was provided ad libitum.
In vivo systemic transduction using AAV9 vector
AAV9-CBA-Cre vector used in this study was as reported by Prabhakar et al., 2019 [22]. Mice at three different postnatal stages were used: newborn (P0–P1), neonate (P14) and juvenile (P21) and were injected with AAV9-CBA-Cre at the dosage of 1.8 × 1011 gc/mouse [1.5 × 1014 vg/kg for P0–P1 pups; 2.5 × 1013 vg/kg for P14 pups; 1.5 × 1013 vg/kg for P21 pups]. Mice were sacrificed two weeks post-injection with AAV9-CBA-Cre, and multiple tissues were harvested.
Retro-orbital (RO) venous sinus injection
Newborn pups (P0 to P1)
-
1.
Thaw AAV vectors and keep on ice (AAV vectors are stable at 4°C for up to 2–3 weeks)
-
2.
Prepare the injectate by diluting the viral vector to the desired dose using 31-gauge insulin syringe (BD Ultra-Fine Insulin Syringes Short Needle, 0.5 cc, 8 mm).
-
3.
Transfer the dam to a clean holding cage leaving the newborns in the home cage with the nesting materials.(Note: under the stressful condition such as restraint or during injections, the pups may emit audible and ultrasonic vocalizations that can distress the mother.)
-
4.
Cryo-anesthetize the pup (one pup at a time) by placing it on directly on ice for 3 to 4 min, until the pup’s color turns a bluish hue. (Note 1: immobilization indicates adequate anesthesia. Note 2: prolonged exposure to ice may cause deep hypothermia leading to mortality of the pup.)
-
5.
Place the pup (Fig. 1C) in left lateral recumbency, on a piece of clean paper towel, with its head facing to the right (Fig. 1D).
-
6.
Gently restrain the pup’s head with the thumb and forefinger of the non-dominant hand. (Note: excessive pressure will either compress the trachea interfering with the respiration or impede venous flow.)
-
7.
Identify the eye region on the right side (outlined with dotted circle, Fig. 1D). (Note: the darkened protruding eye with closed eyelids is easily identified.)
-
8.
Insert approximately 1/3 of the needle length (~3 mm), bevel down, at a 30–45° angle into the 3 o’clock position of the eyeball (yellow arrows, Figs. 1D and E).
-
9.
Advance the injectate into the venous sinus gently and swiftly.(Note: excess fluid outside the eye region indicates unsuccessful fluid administration, the administrator can try on the other eye.)
-
10.
Keep the needle in place for 3 to 5 s, then, gently withdraw the needle.
-
11.
Place the pup on the heating pad (37°C) until it gains mobility and turns pinkish.(Note: pups are susceptible to hypothermia.)
-
12.
Apply the gloved-hands with the nesting material (from the home cage) and gently rub it onto the pup before returning it to its mother in the home cage.(Note: during the postpartum period, the dam may be aggressive toward the pups if their odor cue is strange.)
Neonates (1 to 3 weeks old)
-
1.
Thaw AAV vectors and keep on ice.
-
2.
Prepare the injectate by diluting the viral vector to the desired volume using 31-gauge insulin syringe (BD Ultra-Fine Insulin Syringes Short Needle, 0.5 cc, 8 mm needle).
-
3.
Transfer the dam to a clean holding cage leaving the newborns in the home cage.
-
4.
Place the animal in the sealed chamber flushed with inhalant gas (adjust the isoflurane vaporizer to 2.5% and oxygen flowmeter at 2 l/min).
-
5.
Place the pup (Fig. 1F) in left lateral recumbency, on a piece of clean paper towel, with its head facing to the right (Fig. 1G).
-
6.
Gently restrain the pup’s head with the thumb and forefinger of the non-dominant hand.
-
7.
a) For pups aged less than P13 (the eye remains closed), insert approximately 1/3 of the needle length, bevel down, at a 30–45° angle in the same area as newborn pups. b) For pup aged ≥P14 days (the eye is opened), gently apply pressure around the eye socket so that the eyeball is partially protruded from the socket, insert the needle, bevel down, at a 45° angle into the RO sinus into (Fig. 1H)
-
8.
Advance the injectate into the venous sinus gently and swiftly (excess fluid outside the eye region indicates unsuccessful fluid administration, the administrator can try on the other eye).
-
9.
Allow the needle to stay in place for 3 to 5 s, then, gently retract the needle.
-
10.
Place the pup on a heating pad (37°C) until it gains consciousness.
-
11.
Return the pup to its mother at home cage.
Juvenile and adult mouse (≥3 weeks old )
-
1.
Thaw the viral vector on ice and dilute accordingly. Using 0.5 ml 28-gauge insulin syringe (BD Lo-DoseTM U-100 Insulin Syringes, 0.5 cc, 12.7 mm needle).
-
2.
Place the animal in the sealed chamber flushed with inhalant gas (adjust the isoflurane vaporizer to 2.5% and oxygen flowmeter at 2 l/min flow rate).
-
3.
Place the mouse (Fig. 1I) in left lateral recumbency, on a piece of clean paper towel, with its head facing to the right (Fig. 1J).
-
4.
Gently restrain the animal with thumb and index finger of the non-dominant hand, pulling back the loose skin below and above the eye.
-
5.
The eye will protrude slightly from its eye socket (do not attempt to insert the needle unless the eyeball is protruded, to avoid damage to the eyeball) (Fig. 1K).
-
6.
Insert approximately 5 mm of the needle length, bevel down, at a 45° angle, into the RO sinus. (Note: the applicator can feel a mild degree of resistance indicating the needle tip has reached the bony socket.)
-
7.
Advance injectate into the retro-bulbar sinus and let the needle remain in place for 3 to 5 s.
-
8.
Withdraw the needle gently to prevent injury to the eye. (Note: no leaking of injectate and not bleeding indicate proper injection.)
-
9.
Release the grips around the eye, close the eyelid, and apply mild pressure to the injection site for a few seconds.
-
10.
Examine the injection site for swelling or other visible trauma.
-
11.
Return the mouse to its home cage.
Tissue harvesting
-
1.
Anesthetize the animal with a ketamine:xylazine mix (at the dosage of 100 mg/kg for ketamine: 10 mg/kg for xylazine) via intraperitoneal injection.
-
2.
Assess the depth of anesthesia via toe-pinch for pain reflex.
-
3.
Pin the animal on its back by the four limbs and clean the ventral skin with alcohol swap.
-
4.
Incise the skin from the thoracic inlet to the pelvic region and pull it laterally to expose the peritoneal membrane.
-
5.
Incise the peritoneal membrane just below the xiphoid process and cut laterally and caudally to expose the diaphragm and visceral organs.
-
6.
Puncture the diaphragm and cut bilaterally.
-
7.
Cut the lateral aspects of the rib cage from caudal to rostral up to the level of the second rib. Pull back the rib cage to expose the heart.
-
8.
Gently grasp the heart with a pair of forceps and insert the 25-gauge needle through the apex of the heart.
-
9.
Puncture the right atrium with the perfusion needle and then cut the right atrium with scissors, and immediately start the perfusion with 15 ml of PBS.
-
10.
Observe discoloration of the heart and liver as the perfusion progresses. (Note: blanching of the liver to a beige color indicates proper perfusion.)
-
11.
Continue to perfuse the animal with 15 ml of 4% paraformaldehyde and observe for evidence of tremors. (Note: tremors indicates good fixation as a result of the aldehyde cross-linking in the nerves and muscles.)
-
12.
Harvest multiple organs and fix them in 4% paraformaldehyde on a rotator overnight at 4°C.
-
13.
Cryoprotect the organs with 25 to 30% of sucrose at 4°C.
-
14.
Embed the tissues in Tissue-Tek® optimal cutting temperature (OCT) media and stored at −80°C.
Tissue sectioning and immunohistochemistry staining
-
1.
Cryosection the PFA-fixed tissues at 12-µm thin (heart, kidney, liver lung and muscle) and 40-µm thick (brain) sections.
-
2.
Permeabilize the tissues with PBS containing 0.1% Triton X-100 (PBST) for 10 min at room temperature.
-
3.
Incubate the tissues with blocking solution (3% BSA in PBST) for 1 h at room temperature.
-
4.
Immunostain the tissue in fresh blocking solution containing the primary antibody, mouse anti-RFP (#MA5-15257, 1:1,000, Thermo Fisher Scientific, Waltham, MA, USA) at 4°C for overnight.
-
5.
The next day, wash the tissue with PBST for 3 × 5 min and incubated with the secondary antibody, Alexa Fluor 555 (1:1,000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature.
-
6.
Wash the tissue with PBST for 3 × 5 min and counterstained with DAPI prior to imaging. Fluorescence microscopy images were acquired on the Zeiss Axio Imager M2 (Carl Zeiss, Dublin, CA, USA).
Result
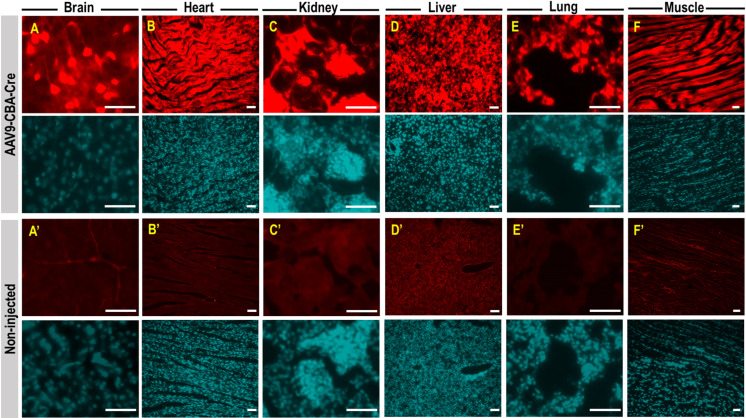
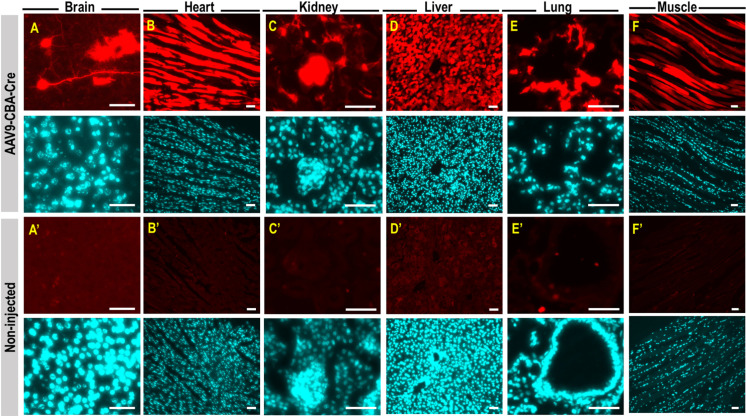
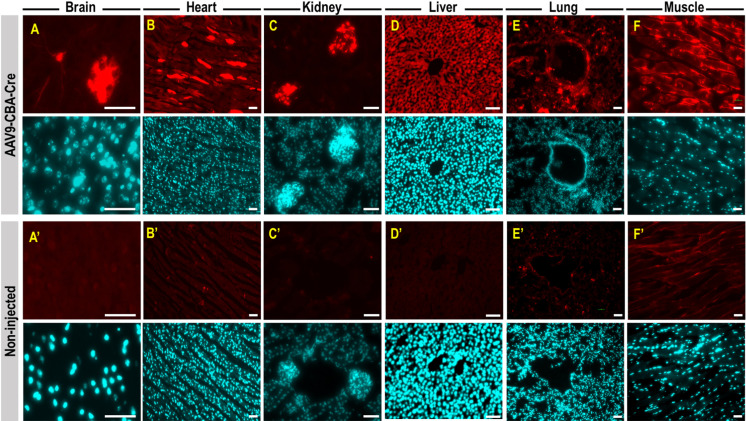
After the ROI, newborn pups were rubbed with used nesting materials prior to return to the dam and we observed 100% dam acceptance. Anatomy of the orbital venous of the mouse and route of injection was illustrated in Figs. 1A and B. Proper retro-orbital injection into newborn (Figs. 1C–E), neonatal (Figs. 1F–H) and adult mice (Figs. 1I–K) will lead to no leakage of the injectate from and around the sinus. Little or no bleeding from the eye could occur upon withdrawal of the needle from the sinus. Newborn (P0) mouse pups were intravenously injected with AAV9-CBA-Cre at dosage of 1.5 × 1014 vg/kg, via retro-orbital route. Two weeks post-injection, mice were sacrificed and perfused with 4% paraformaldehyde. Tissues were cryopreserved, sectioned, and stained for tdTomato expression and counterstained with DAPI. Fluorescence images showed intense tdTomato expression in brain cells (Fig. 2A). TdTomato immunofluorescence was detectable in other organs such as heart, kidney, liver, lung and skeletal muscle (high magnificantion in Figs. 2B–F; low magnification available in Supplementary Fig. 1). Very low tdTomato expression were detectable in the non-injected animals (Figs. 2A’–F’). Subsequent examination on the viral vector transduction following systemic delivery of AAV9-CBA-Cre via retro-orbital route at dosage of 2.5 × 1013 vg/kg in neonatal mouse, revealed tdTomato expression in both brain cells of the central nervous system (Fig. 3A) and other peripheral organs (Figs. 3B–F, high magnification; low magnification images available in Supplementary Fig. 2). Low level of tdTomato expression was detectable in the negative control group (Figs. 3A’–F’). Immunohistochemical staining on the juvenile mice (P21) transduced with AAV9-CBA-Cre at dosage of 1.5 × 1013 vg/kg, via retro-orbital injection showed TdTomato immunofluorescence in the brain and multiple organs (Figs. 4A–F; low magnification images available in Supplementary Fig. 3). As expected, low level of tdTomato expression were detected in non-injected animals (Figs. 4A’–F’).
Fig. 2.
TdTomato expression in central nervous system and peripheral organs following intravenous administration of AAV9-CBA-Cre via retro-orbital route of newborn pups. Newborn (P0) mouse pups were intravenously injected with AAV9-CBA-Cre at dosage of 1.5 × 1014 vg/kg, via retro-orbital route. Two weeks post injection, mice were sacrificed and perfused with 4% paraformaldehyde. Tissues were cryopreserved, sectioned, and stained for tdTomato expression (red) and counterstained with DAPI (cyan). TdTomato expression is seen in the mouse brain cells (A), heart (B), kidney (C), liver (D), lung (E) and skeletal muscle (F). Low level of tdTomato expression is observed in non-injected animals (A’ to F’). All scale bars=10 µm.
Fig. 3.
TdTomato expression in central nervous system and peripheral organs following neonatal retro-orbital injection of AAV9-CBA-Cre . Postnatal day 14 (P14) mouse pups were subjected to retro-orbital injection with AAV9-CBA-Cre at dosage of 2.5 × 1013 vg/kg. Two weeks post injection, mice were sacrificed and perfused with 4% paraformaldehyde. Tissues were cryopreserved, sectioned, and stained for tdTomato expression (red) and counterstained with DAPI (cyan). TdTomato immunofluorescence is detectable in the mouse brain cells (A), heart (B), kidney (C), liver (D), lung (E) and skeletal muscle (F). TdTomato signal is lowly expressed in the non-injected animals (A’ to F’). All scale bars=10 µm.
Fig. 4.
TdTomato immunofluorescence in central nervous system and peripheral organs of adult mouse following intravenous injection of AAV9-CBA-Cre via retro-orbital route. Postnatal day 21 (P21) mouse pups were intravenously injected with AAV9-CBA-Cre at dosage of 1.5 × 1013 vg/kg, via retro-orbital route. Two weeks post injection, mice were sacrificed and perfused with 4% paraformaldehyde. Tissues were cryopreserved, sectioned, and stained for tdTomato expression (red) and counterstained with DAPI (cyan). TdTomato immunofluorescence is detectable in the mouse brain cells (A), heart (B), kidney (C), liver (D), lung (E) and skeletal muscle (F). Low level of tdTomato expression is detectable in non-injected animals (A’ to F’). All scale bars=10 µm.
Discussion
There are an increasing number of transgenic, induced mutant and naturally occurring mouse models of genetic diseases which portray the causative genes in humans, as well as facilitating cellular and molecular mechanisms of genetic diseases. Based on this information gene therapy strategies are being devised for clinical trials. Prior to pursuing clinical gene therapy for human diseases, these mouse models are essential as the translational models to facilitate proof-of-concept trials. In order to demonstrate a potential clinical efficacy achieved by AAV-mediated gene therapy, it is of the utmost importance to select an ideal route of vector delivery for the appropriate age group in the disease model [7]
ROI, as we have shown here, has successfully been used to deliver AAV vectors encoding Cre recombinase in transgenic mice (Ai9) carrying a floxed-STOP tdTomato cassette. It is a reliable way of delivering AAV to the brain as well as peripheral organs at all ages of the animals (ranging from newborn to juvenile period). Immunohistochemistry studies showed that all AAV9-CBA-Cre injected mice manifested tdTomato-positive cells in the brain and other peripheral organs such as liver (hepatocytes), lung (bronchiole and alveoli), kidney (glomerulus), limb muscle (skeletal muscle), and heart (cardiomyocytes). The ophthalmic venous sinuses are available for both eyes, hence, this procedure is equally suitable for right- and left-handed investigator, in which the position of the head of the animal can be easily determined accordingly to individual’s ergonomically convenient direction. During ROI, pups are separated from the mother and may pick up the foreign scent from the animal handler. The mother may show abandonment and rejection on the pups with unfamiliar odour. To prevent the incidence of cannibalism and aggressiveness, the handler should gently rub the pups with the nesting materials from the home cage after the procedure.
Although there are many advantages with ROI, a few limitations to the applicability of this technique should be mentioned. First of all, injection volume for newborn pups is limited to 10 µl, which is well tolerated by the animals as small as 0.8 to 1.0 g, without leakage of injectant. For the neonates, the total volume of injection should not exceed 50 µl per eye. This means a high-virus titer preparation is needed in order to deliver a high dose of the virus. In addition, anesthesia is required for ROI. We would recommend an inhalant anesthetic, such as isoflurane, as this agent has a faster induction and speedier recovery time as compared to other anesthetics, as well as minimal side effects on the animal. Additional evidence also supports the idea that isoflurane may suppress the inflammatory response [29]. Furthermore, ROI provides option for continuous intravenous administration, but eye injections should be limited to once a day (https:/www.jove.com/science-education/10214/compound-administration-iv) and precautions should be taken in order to prevent trauma to the eye. If the needle is incorrectly placed this could lead to swelling around the injection site, bleeding, loss of the viral vector and damage to the eye. Therefore, once the needle is inserted into the RO sinus, unexpected movements should be avoided (needle should be stable) in order to prevent rupturing of the vessels. Animal behavioral studies such as Morris water maze relies on visual ability to measure the learning and memory performance of the rodents. Hence, it is important to examine the gross visual impairments, if any, in the animals after the ROI. In the present study, we have not observed any swelling or infection in or around the eye after the ROI.
The detailed guidelines of the procedures of the present study are meant to serve as general starting point for researchers unfamiliar with such procedures and viral vectors. In summary, our report supports retro-orbital venous sinus injection as a rapid, hassle-free, relatively less invasive procedure for delivery of viral vector, which in turn can provide almost body wide, stable AAV-mediated transduction (in non-dividing cells) and gene editing in mouse models of at all ages.
Author Contributions
X.O.B., S.P., and C.P.S. conceived and designed the experiments; S.P., C.P.S., C.C.H and S.L. performed the experiments; X.O.B. S.P., C.P.S., C.C.H and S.L. wrote and edited the paper.
Supplementary
Acknowledgments
We thank Ms. Suzanne McDavitt for her skilled editorial assistance; Casey Maguire (The Casey Maguire Laboratory, MGH) for AAV vector packaging and production. This work was supported by DOD Army Grant W81XWH-13-1-0076 (XOB), and NIH NINDS 1R61NS108232 and BridgeBio Pharma, Inc. (XOB).
References
- 1.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011; 50: 600–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Gessler DJ, Tai PWL, Li J, Gao G. Intravenous Infusion of AAV for widespread gene delivery to the nervous system. Methods Mol Biol. 2019; 1950: 143–163. doi: 10.1007/978-1-4939-9139-6_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerebtsova M, Ye X, Ray PE. A simple technique to establish a long-term adenovirus mediated gene transfer to the heart of newborn mice. Cardiovasc Hematol Disord Drug Targets. 2009; 9: 136–140. doi: 10.2174/187152909788488645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerebtsova M, Liu XH, Ye X, Ray PE. Adenovirus-mediated gene transfer to glomerular cells in newborn mice. Pediatr Nephrol. 2005; 20: 1395–1400. doi: 10.1007/s00467-005-1882-0 [DOI] [PubMed] [Google Scholar]
- 5.Cheah PS, Prabhakar S, Yellen D, Beauchamp RL, Zhang X, Kasamatsu S, et al. Gene therapy for tuberous sclerosis complex type 2 in a mouse model by delivery of AAV9 encoding a condensed form of tuberin. Sci Adv. 2021; 7: eabb1703. doi: 10.1126/sciadv.abb1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatakeyama S, Yamamoto H, Ohyama C. Tumor formation assays. Methods Enzymol. 2010; 479: 397–411. [DOI] [PubMed] [Google Scholar]
- 7.Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY). 2008; 37: 26–32. doi: 10.1038/laban0108-26 [DOI] [PubMed] [Google Scholar]
- 8.Ellenberger MA. The use of animal models in behavioural pharmacology. In: F. van Haaren, editor. Techniques in the behavioural and neurological sciences. Vol. 10: Methods in behavioural pharmacology. Amsterdam: Elsevier B.V.; 1993. pp. 1–22. [Google Scholar]
- 9.Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY). 2008; 37: 26–32. [DOI] [PubMed] [Google Scholar]
- 10.Cheah PS, Ramshaw HS, Thomas PQ, Toyo-oka K, Xu X, Martin S, et al. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. 2012; 17: 451–466. [DOI] [PubMed] [Google Scholar]
- 11.Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genet. 2008; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991; 7: 265–275. doi: 10.1016/0896-6273(91)90265-2 [DOI] [PubMed] [Google Scholar]
- 13.Levene HB, Zhang M, Erb CJ, Jallo JI, Loftus CM, Tuma RF. Method to perform IV injections on mice using the facial vein. J Neurosci Methods. 2007; 164: 304–307. doi: 10.1016/j.jneumeth.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 14.Gombash Lampe SE, Kaspar BK, Foust KD. Intravenous injections in neonatal mice. J Vis Exp. 2014; e52037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011; 19: 1440–1448. doi: 10.1038/mt.2011.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita T, Takahashi A, Honjin R. Spatial aspect of the mouse orbital venous sinus. Okajimas Folia Anat Jpn. 1980; 56: 329–336. [DOI] [PubMed] [Google Scholar]
- 17.Leon-Rico D, Fernández-García M, Aldea M, Sánchez R, Peces-Barba M, Martinez-Palacio J, et al. Comparison of haematopoietic stem cell engraftment through the retro-orbital venous sinus and the lateral vein: alternative routes for bone marrow transplantation in mice. Lab Anim. 2015; 49: 132–141. doi: 10.1177/0023677214567915 [DOI] [PubMed] [Google Scholar]
- 18.Somervaille TCP, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006; 10: 257–268. doi: 10.1016/j.ccr.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Nojima M, Inoue Y, Ohtomo K, Kiryu S. Assessment of MRI contrast agent kinetics via retro-orbital injection in mice: Comparison with tail vein injection. PLoS One. 2015; 10: e0129326. doi: 10.1371/journal.pone.0129326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Brand BT, Vermeij EA, Waterborg CEJ, Arntz OJ, Kracht M, Bennink MB, et al. Intravenous delivery of HIV-based lentiviral vectors preferentially transduces F4/80+ and Ly-6C+ cells in spleen, important target cells in autoimmune arthritis. PLoS One. 2013; 8: e55356. doi: 10.1371/journal.pone.0055356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C, et al. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther. 2014; 22: 1299–1309. doi: 10.1038/mt.2014.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhakar S, Cheah PS, Zhang X, Zinter M, Gianatasio M, Hudry E, et al. Long-Term Therapeutic Efficacy of Intravenous AAV-Mediated Hamartin Replacement in Mouse Model of Tuberous Sclerosis Type 1. Mol Ther Methods Clin Dev. 2019; 15: 18–26. doi: 10.1016/j.omtm.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruntman AM, Su L, Flotte TR. Retro-Orbital Venous Sinus Delivery of rAAV9 Mediates High-Level Transduction of Brain and Retina Compared with Temporal Vein Delivery in Neonatal Mouse Pups. Hum Gene Ther. 2017; 28: 228–230. doi: 10.1089/hum.2017.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY). 2011; 40: 155–160. doi: 10.1038/laban0511-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. Guide for the care and use of laboratory animals, 8th edition, Washington, DC: National Academies Press; 2011. [Google Scholar]
- 26.He X, Xie H, Liu X, Gu F. Basic and clinical application of adeno-associated virus-mediated genome editing. Hum Gene Ther. 2019; 30: 673–681. doi: 10.1089/hum.2018.190 [DOI] [PubMed] [Google Scholar]
- 27.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010; 13: 133–140. doi: 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manfredsson FP, Rising AC, Mandel RJ. AAV9: a potential blood-brain barrier buster. Mol Ther. 2009; 17: 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol. 2006; 13: 281–288. doi: 10.1128/CVI.13.2.281-288.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.