Abstract

Applying an asymmetric strategy to construct non-fullerene small-molecule acceptors (NFSMAs) in organic solar cells (OSCs) plays a vital role in the development of organic photovoltaic materials. In the past several years, taking advantage of the larger dipole moment and stronger intermolecular interactions, asymmetric NFSMAs have witnessed tremendous progress in OSCs with a power conversion efficiency of over 18%. From a structural point of view, besides the possible changes in the conformation effect on molecular packing, asymmetric acceptors can also achieve a balance between the solubility and the crystallinity. Herein, we systematically investigate the structure–property–performance relationships of asymmetric NFSMAs that have recently emerged and try to clarify the feasibility and practicality of an asymmetric strategy for the design of higher-performance NFSMAs. Finally, we put forward our views and a concise outlook on the asymmetric strategy.
Short abstract
An asymmetric strategy to construct non-fullerene small-molecule acceptors in organic solar cells exhibits excellent potential and plays a vital role in the development of organic photovoltaic materials.
Introduction
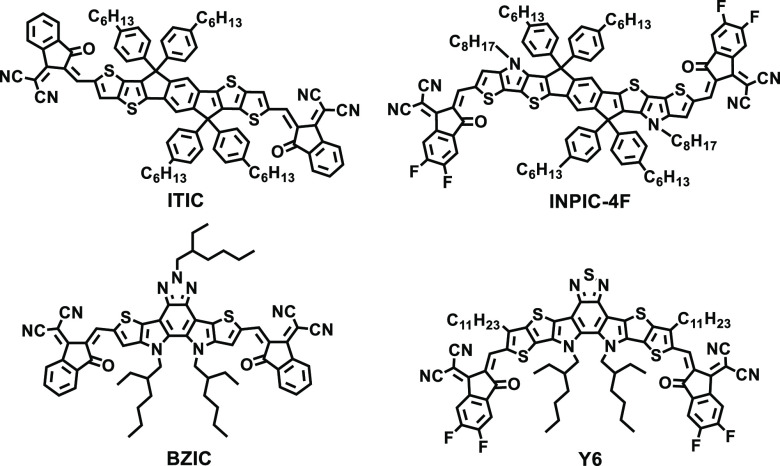
Organic solar cells (OSCs) have been developed rapidly over the past few years benefiting from chemical structural diversity, mechanical flexibility, roll-to-roll large area printing, etc.1−14 The classical active layers of single-junction OSCs usually consist of a p-type conjugated polymer as a donor and an n-type material (fullerene derivative or non-fullerene small molecule) as an acceptor.15 In the last two decades, fullerene derivatives have occupied a predominant position in electron acceptor materials due to their suitable electron affinity and isotropic electron transporting properties.16−21 However, the power conversion efficiencies (PCEs) of the most perfect fullerene-based OSCs are limited to only ∼11%.22,23 The demand for replacing fullerene in OSCs has led to the exploitation of non-fullerene small-molecule acceptors (NFSMAs). Although the efficiencies of the earlier NFSMAs remained at a relatively low status,24 their chemical structures can be varied over a fairly wide range, which affords easily tunable photoelectric properties. Facilitated by these advantages, tremendous efforts have been devoted to designing novel NFSMAs. With the emergence of A-D-A-type acceptors such as ITIC and A-DA′D-A-type acceptors such as BZIC (D: electron donor unit; A: electron acceptor unit),25−34 the PCEs of the contemporary NFSMA-based OSCs can reach over 18% (certified).35
Generally, the complementary absorption profiles, matched energy levels, and suitable phase separation morphologies of the donor and the acceptor in the active layer are the necessary factors to achieve high PCEs. All these common features are already well recognized and used to guide future molecular design.28,36−38 After the structure–property–performance relationship has been investigated sufficiently, in addition to the exploration of next-generation molecules different from the known A-D-A or A-DA′D-A-type molecular skeleton, a cost-effective strategy to further breakthroughs in PCE is subtle adjustment of the molecular structure without breaking the inherent advantages of those molecules. How is it possible to simultaneously enhance three key factors (short-circuit current density (Jsc), open circuit voltage (Voc), and fill factor (FF)) of OSCs based on NFSMAs through time-saving and productive strategies? This research topic is of great interest driven by the rapid improvement of asymmetric molecular strategies in recent years.
Asymmetric small molecules exhibit a larger dipole moment and stronger binding energy, which can intensify intermolecular interactions.39−42 By utilizing asymmetric strategies, the properties of the molecule could be subtly optimized, and the conformation effect on molecular packing, miscibility, and crystallinity will be changed. Thus, the aggregation behaviors of active layers are subtly adjusted, leading to possibly increased carrier mobility. In view of the advantages and potentials of asymmetric strategies in OSCs, two asymmetric modification projects will be concluded, followed by the display of their applications. Moreover, this outlook will focus on the structure–property–performance relationship of asymmetric NFSMAs and further rational design of high-performance asymmetric NFSMAs.
Representative Structure and Modification Strategy of NFSMAs
In 2015, Zhan’s group synthesized the classic A-D-A-type small-molecular acceptor ITIC with a PCE of 6.8% (blended with PTB7-TH). Since then, based on ITIC, significant efforts have been devoted to developing NFSMAs for OSC applications.43−46 In addition, Tang’s group drew an electron-donating unit 5,5,12,12-tetrakis(4-hexylphenyl)-indacenobis(dithieno[3,2-b:2′,3′-d])pyrrol (INP) into a conjugated backbone and synthesized an A-D-A-type acceptor INPIC-4F.47 An improved electron mobility (μe) and a relatively low energy loss (Eloss) (0.54 eV) enabled an eminent PCE up to 13.13% in INPIC-4F-based OSCs. The results demonstrated the INP unit could be a potential selection of preparing high-efficiency NFSMAs. In 2017, the first A-DA′D-A typed NFSMA BZIC was reported by Zou’s group. BZIC was endowed with a novel conjugated fused-ring molecular framework using an electron-deficient unit as the central core.25 Based on the same molecular strategy as BZIC, Y6 was then designed and synthesized, and the PM6:Y6 device achieved a record-breaking PCE of 15.7%.27 Consequently, the emergence of A-DA′D-A typed NFSMAs advanced a more rapid development of OSCs.48,49
Figure 1.
Chemical structures of typical symmetric NFSMAs.
Figure 2.

Three sections of typical NFSMAs.
By analyzing the structure of these molecules, it can be clearly seen that they mainly consist of three sections: conjugated central backbone, side chains, and terminal groups. Therefore, two corresponding modification strategies could be summarized to realize the asymmetric transformation for NFSMAs:
-
(1)
The asymmetric modification of the conjugated central backbone. The molecular conjugated length and isomerization of the conjugated backbone are critical in controlling the electronic structure and aggregation behavior of NFSMAs. Regarding the backbone modification, developing NFSMAs with an asymmetric structure by changing the sequence, species, or length of the central fused ring has shown great potential in achieving different molecular packing, energy levels, dipole moments, and device performance.
-
(2)
The introduction of diverse side chains and terminal groups. The side chains are the key to balancing the solubility, charge transport, and the aggregation behavior of blend films.38 Unlike symmetrical modification of the side chain structure, one-sided modification might be more conducive to make a subtle adjustment of the above-mentioned properties. Moreover, the modification of the asymmetric terminal group could subtly affect the energy levels as well as the intramolecular charge transfer.
Actually, these two parts complement each other and also have various emphases on affecting photoelectric properties. These strategies can effectively promote the development of asymmetric NFSMAs and give support to materials with the required properties more accurately.
Asymmetric Modification Strategy of NFSMAs
Asymmetric Backbone Strategies for NFSMAs
The conjugated central backbone is one of the most crucial components because it dictates the primary physical properties of NFSMAs, such as absorption, energy levels, and inter-/intramolecular interaction. Therefore, the molecular conjugation structure as well as the length, shape, and heteroatomic units will effectively affect the properties of NFSMAs. The asymmetric extension of NFSMAs can change the above-mentioned properties while maintaining relative π-electron delocalization.50,51
Compared with changing heteroatomic units, altering the length or shape of the conjugated backbone will significantly influence the molecular conformations. With the number of fused rings increasing, the absorption of the acceptor can be easily extended to the near-infrared that could substantially improve photocurrent generation, finally affecting the Jsc and the PCE of the OSCs (summarized in Table 1 and Figure 3). Researchers have devoted much attention to the study in the optimal fused number and shape of the conjugate backbone of asymmetric NFSMAs.
Table 1. Photovoltaic Properties of NFSMAs Mentioned in This Outlook.
| active layer | fused ring amount | HOMO (eV) | LUMO (eV) | Voc (V) | Jsc (mA cm–2) | FF (%) | PCE (%) | ref |
|---|---|---|---|---|---|---|---|---|
| PTB7-TH:ITIC | 7 | –5.48 | –3.83 | 0.81 | 14.21 | 59.1 | 6.8 | (26) |
| PBDB-T:INPIC-4F | 9 | –5.42 | –3.94 | 0.85 | 21.61 | 71.5 | 13.1 | (47) |
| PM6:Y6 | 7 | –5.65 | –4.10 | 0.83 | 25.30 | 74.8 | 15.7 | (27) |
| PM6:Y18 | 7 | –5.58 | –3.91 | 0.84 | 25.33 | 76.5 | 16.5 | (49) |
| PBT1-C:TPT-2F | 5 | –5.84 | –4.06 | 0.87 | 13.89 | 68.6 | 8.3 | (51) |
| PBT1-C:TPTT-2F | 6 | –5.75 | –4.04 | 0.88 | 15.82 | 73.0 | 10.2 | (51) |
| PBT1-C:TPTTT-2F | 7 | –5.69 | –4.01 | 0.92 | 17.63 | 74.5 | 12.0 | (51) |
| PBT1-C:TTPTTT-2F | 8 | –5.67 | –4.04 | 0.92 | 16.78 | 74.6 | 11.5 | (52) |
| PM6:Y26 | 5 | –5.58 | –4.03 | 0.83 | 21.63 | 74.3 | 13.3 | (59) |
| PM6:Y22 | 6 | –5.69 | –3.94 | 0.85 | 24.37 | 74.1 | 15.4 | (58) |
| PM6:BP-4F | 6 | –5.69 | –4.06 | 0.87 | 23.54 | 76.3 | 15.6 | (60) |
| PM6:BP4T-4F | 7 | –5.71 | –3.91 | 0.84 | 26.30 | 77.7 | 17.1 | (61) |
| PM6:BP5T-4F | 8 | –5.63 | –3.88 | 0.89 | 24.60 | 76.3 | 16.7 | (61) |
| PM6:ABP4T-4F | 7 | –5.65 | –3.85 | 0.92 | 22.00 | 75.1 | 15.2 | (61) |
| PM6:BDTP-4F | 7 | –5.61 | –3.90 | 0.90 | 22.54 | 75.5 | 15.2 | (62) |
| PM6:BTDTP-4F | 8 | –5.56 | –3.93 | 0.87 | 21.25 | 71.3 | 13.1 | (62) |
| PM6:IDTP-4F | 7 | –5.54 | –3.98 | 0.87 | 22.28 | 73.7 | 14.3 | (63) |
| PM6:IDTTP-4F | 8 | –5.53 | –3.97 | 0.88 | 20.21 | 71.4 | 12.6 | (63) |
| PBDB-T:IPT-2F | 7 | –5.51 | –3.96 | 0.86 | 22.40 | 72.4 | 14.0 | (64) |
| PBDB-T:IPTT-2F | 8 | –5.46 | –4.04 | 0.87 | 19.70 | 66.2 | 11.4 | (64) |
| PBDB-T:IPTTT-2T | 9 | –5.40 | –4.07 | 0.89 | 20.00 | 69.3 | 12.3 | (64) |
| PM6:S-YSS-Cl | 7 | –5.71 | –3.85 | 0.86 | 25.85 | 75.3 | 16.7 | (66) |
| PM6:A-WSSe-Cl | 7 | –5.70 | –3.86 | 0.85 | 26.58 | 77.5 | 17.5 | (66) |
| PM6:S-WSeSe-Cl | 7 | –5.66 | –3.88 | 0.83 | 26.35 | 73.4 | 16.0 | (66) |
| PM6:Y6–1O | 7 | –5.71 | –3.84 | 0.89 | 23.20 | 78.3 | 16.1 | (70) |
| PM6:Y6–2O | 7 | –5.73 | –3.76 | 0.92 | 13.30 | 53.5 | 6.6 | (70) |
| PM6:EH-HD-4F | 7 | –5.69 | –4.04 | 0.84 | 27.50 | 79.3 | 18.4 | (71) |
| PM6:Bu-OD-4F | 7 | –5.68 | –4.01 | 0.85 | 26.20 | 76.6 | 17.1 | (71) |
| PM6:BO-4F | 7 | –5.70 | –4.01 | 0.84 | 27.00 | 76.7 | 17.4 | (71) |
| PBDB-T:a-IT-2F | 7 | –5.67 | –4.07 | 0.78 | 19.06 | 68.8 | 10.2 | (72) |
| PBDB-T:a-IT-2OM | 7 | –5.61 | –3.92 | 0.93 | 18.11 | 71.5 | 12.0 | (72) |
| PBDB-TF: ITIC-2F | 7 | –5.76 | –4.07 | 0.92 | 17.30 | 65.7 | 10.3 | (73) |
| PBDB-TF: ITIC-3F | 7 | –5.73 | –4.12 | 0.90 | 19.40 | 66.5 | 11.4 | (73) |
| PM6: α-ITIC-2Cl | 7 | –5.29 | –3.77 | 0.88 | 18.91 | 73.5 | 12.2 | (77) |
| PM6:BTP-S1 | 7 | –5.55 | –4.01 | 0.93 | 22.39 | 72.6 | 15.2 | (78) |
| PM6:BTP-S2 | 7 | –5.65 | –4.01 | 0.93 | 24.07 | 72.0 | 16.3 | (78) |
| PM6:BTP-2ThCl | 7 | –5.67 | –3.95 | 0.89 | 23.46 | 69.8 | 14.5 | (79) |
| PM6:BTP-2F-ThCl | 7 | –5.70 | –3.99 | 0.87 | 25.38 | 77.4 | 17.1 | (79) |
| PM6:SY1 | 7 | –5.68 | –3.95 | 0.87 | 25.41 | 76.0 | 16.8 | (80) |
| PM6:SY2 | 7 | –5.67 | –3.99 | 0.85 | 25.29 | 74.3 | 16.0 | (80) |
| PM6:SY3 | 7 | –5.69 | –3.98 | 0.86 | 25.54 | 74.1 | 16.2 | (80) |
| PM6:BTIC-2Cl-γCF3 | 7 | –5.55 | –4.00 | 0.84 | 25.09 | 77.0 | 16.3 | (81) |
Figure 3.
(a) Energy levels and short-circuit current density of segmental acceptor molecules. (b) Chemical structures of segmental NFSMAs.
For example, the typical indaceno[1,2-b:5,6-b′]dithiophene (IDT) unit could be converted to an asymmetric backbone with increasing one-sided conjugated fused rings. Thus, three diverse membered fused ring acceptors TPT-2F, TPTT-2Fm and TPTTT-2F were designed by Sun’s group.51 As shown in Figure 3, the extended IDT backbone realized broader absorption and upshifted energy levels. Consequently, seven-membered fused ring TPTTT-2F-based devices achieved the highest efficiency of 12.03% with simultaneously increased Voc, Jsc, and FF. On the basis of the above-mentioned result, Sun and collaborators committed to exploiting an asymmetric eight-membered NFSMA named TTPTTT-2F.52 With the number of fused rings up to eight, the optical gap would be expected to be reduced. However, the TTPTTT-2F-based devices obtained a low PCE of 11.52% and a low Jsc of 16.78 mA cm–2, which is worse than seven-membered fused ring TPTTT-2F-based devices. It suggests that the PCE is determined not only by the absorption ability but also other factors such as the intermolecular packing, the intramolecular charge transfer, and the solubility.53−57 Moreover, in order to reduce the cost and complexity of synthesis, Zou et al. exploited three types fused ring acceptors Y26, Y22, and Y18, which obtained five-, six-, and seven-membered conjugated fused rings, respectively.49,58,59 As a result, the efficiency of the six-membered asymmetric acceptor Y22 is significantly higher than that of the five-membered symmetric acceptor Y26, and this improvement comes from the asymmetric expansion. Successively extending thiophene conjugation gave rise to red-shifted absorption spectra and increased intermolecular ordering with improved π–π interactions. However, their efficiencies were lower than that of devices based on the seven-membered acceptor Y18. All these examples showed that the number of fused rings had an important influence on the device performance during the asymmetric modification, and seven-membered acceptors exhibited the best intramolecular charge-transfer ability which reflects the Jsc.
However, when the acceptors BP-4F, ABP4T-4F, and BP5T-4F were discussed, the conclusion is out of the above-mentioned expectation;60,61 the seven-membered acceptor ABP4T-4F obtains a poor PCE with the lowest Jsc among three asymmetric acceptors. Besides the different number of fused rings, it can be clearly seen that these acceptors have different shapes. These differences will also significantly affect the performance of the acceptors. The modification in shape often affects the intermolecular interaction, thereby affecting the stacking behavior of the molecules. The dipole moments for BP5T-4F (3.4 D) and ABP4T-4F (2.5 D) are significantly larger than that of BP4T-4F (0.9 D). The investigations indicate that BP4T-4F and BP5T-4F predominantly adopt a face-on orientation, and BP5T-4F has better π–π stacking than that of BP4T-4F. In contrast, ABP4T-4F exhibits an almost edge-on orientation and a stronger aggregation, which accounts for the significant shoulder peak in film absorption. The enhanced packing properties of two asymmetric NFSMAs may be caused by the strong intermolecular dipole–dipole interactions. The S-shaped conformation BP-4F, C-shaped conformation BP4T-4F, Z-shaped conformation BP5T-4F and W-shaped conformation ABP4T-4F were synthesized to conclude that the C-type conformation BP4T-4F shows the best performance among A-DA′D-A-type acceptors (Figures 3b and 4). Relative to BP4T-4F, BP5T-4F showed similarities in photoelectric properties, yet ABP4T-4F presented unfavorable characters such as blue-shifted absorption and edge-on packing in films. As a consequence, when pairing with PM6, the C-shaped BP4T-4F (seven-membered fused ring) obtained the best PCE of 17.1%, better than S-shaped BP-4F (six-membered fused ring, 15.6%), Z-shaped BP5T-4F (eight-membered fused ring, 16.7%), and W-shaped ABP4T-4F (seven-membered fused ring, 15.2%).
Figure 4.
Chemical structures of BDTP-4F, BTDTP-4F, IDTP-4F, IDTTP-4F, BP-4F, ABP4T-4F, BP4T-4F, BP5T-4F, IPT-2F, IPTT-2F, and IPTTT-2F.
For instance, Yang’s group designed two types of asymmetric NFSMAs: C-shaped BDTP-4F and S-shaped BTDTP-4F.62 It can be concluded that BTDTP-4F shows strong crystallinity and high electron mobility by grazing incidence wide-angle X-ray scattering (GIWAXS). In A-DA′D-A-type acceptors, due to the synergistic effect of the miscibility (with polymer donor) and C-shaped conformation, the PM6:BDTP-4F blend had stronger π–π stacking and a larger crystal coherence length. Moreover, the transformation of the shape could enhance intermolecular packing, which also accounts for a more balanced electron and hole mobility, eventually resulting in a higher FF (75.5%). In two traditional A-D-A-type NFSMAs (S-shaped IDTP-4F and C-shaped IDTTP-4F63), the same phenomenon also existed, but the S-shaped IDTP-4F-based OSCs obtained the best PCE (14.32%). The more balanced charge transfer and excellent morphology of PM6:BDTP-4F blend films were conducive to a much better PCE (15.24%). The C-shaped BDTP-4F (A-DA’D-A type) and S-shaped IDTP-4F (A-D-A type) showed better PCEs of OSC devices.
In addition, Tang’s group also synthesized three asymmetric A-D-A-type NFSMAs which contained the C-shaped acceptor IPTT-2F as well as S-shaped acceptors IPT-2F and IPTTT-2F.64 By GIWAXS measurements, it could be clearly seen that face-on π–π stacking of the S-shaped IPT-2F- and IPTTT-2F-based blends are obviously enhanced. In general, the more well-organized and compact packing structure could be responsible for the less trap-assisted recombination and higher FF. In IPT-2F- and IPTTT-2F-based devices, the electron mobility (μe) and hole mobility (μh) were enhanced simultaneously after extending the length of the conjugated backbone. Nevertheless, the μe/μh ratio of blends based on S-shaped IPT-2F and IPTTT-2F were more balanced than those of the C-shaped IPTT-2F blend, which contributed to high Jsc and FF. This is consistent with the BDTP-4F and IDTP-4F molecules mentioned above. Importantly, when blended with PBDB-T, S-shaped IPT-2F exhibits a perfect nanofibril morphology with homogeneous and regular phase separation, resulting in a PCE of 14% with a Jsc of 22.4 mA cm–2 and FF of 72.4%.
Previous work has shown that S-shaped asymmetric NFSMAs, which consisted of seven-membered fused ring backbone, exhibited an impressive potential for A-D-A-type acceptors materials. And for the A-DA′D-A-type acceptors, the C-shaped NFSMAs with seven-membered fused ring backbone showed the best PCE.
Compared with the modification of the length and shape of the molecular backbone, controlling the number of heteroatoms can subtly adjust the acceptor to obtain the optimal PCE while maintaining the majority of the advantages of the parent acceptor. The introduction of various heteroatoms will have different effects on the intermolecular interaction and the intramolecular charge transfer, which will be directly reflected in the changes in the Jsc and FF. For example, incorporating selenophene into the central conjugated backbone will enhance the intramolecular charge-transfer effect and improve charge mobility, as well as extend absorption.65 The asymmetric strategy provides a solution for the synthesis of high-efficiency selenophene-based acceptors. Wang and co-workers synthesized an asymmetric A-DA′D-A type NFSMA A-WSSe-Cl. A-WSSe-Cl-based OSCs yielded a remarkable PCE of 17.51%, with an improved FF of 77.50%, which outperformed the performance of symmetric S-YSS-Cl (PCE of 16.73%) and S-WSeSe-Cl (PCE of 16.01%)-based OSCs.66
Figure 5.
Chemical structures of S-YSS-Cl, A-WSSe-Cl, and S-WSeSe-Cl.
The selenophene substitution within the backbone induces a slightly higher HOMO energy level, stronger intermolecular interaction, narrower optical band gap with high absorption coefficient, and enhanced electron mobility in films. Crystallographic structures indicated the increase of selenophene substitution on the conjugated backbone induced stronger intermolecular π–π stacking besides S···N noncovalent intermolecular interactions from central benzothiadiazole. Compared with the thiophene analogue S-YSS-Cl, A-WSSe-Cl and S-WSeSe-Cl showed better ordered three-dimensional interpenetrating charge-transfer networks. The PM6:A-WSSe-Cl blend films exhibited the sharpest face-on orientation, the shortest π–π stacking distances among these three blend films. The results indicate the asymmetric molecular structure could effectively achieve a favorable morphology with the optimized molecular packing property, thus resulting in the highest Jsc and FF in a corresponding device.
Asymmetric Side Chains and Terminal Groups Strategies for NFSMAs
The great majority of non-fullerene acceptors have symmetric side chains and terminal groups, strongly affecting the molecular crystallinity, packing behavior, and thus photovoltaic performance.67 From the perspective of molecular design, it is simple and effective to modify the side chain or terminal group to optimize the device performance without changing the overall structure.
Introducing an electron-withdrawing side chain or stronger electron-donating group to replace the alkyl chain is an effective strategy to optimize the intramolecular charge transport or intermolecular stacking.68,69 In fact, asymmetric side chains strategies may achieve a balance between crystallinity and solubility; thus, it is indispensable to combine side chain engineering with asymmetric strategies for high-performance OSCs. Yan et al. synthesized two kinds of NFSMAs named Y6-1O and Y6-2O.70 Considering a conformational locking effect of the alkoxy group, Y6-2O-based cells exhibited low solubility, excessive aggregation, and poor efficiency (6.6%). However, the asymmetric Y6-1O which contained an alkyl chain and an alkoxy chain maintained fairly good solubility, leading to an outstanding efficiency of 16.1% as pairing with PM6. Surprisingly, by incorporating the third component PC71BM, the PM6:Y6–1O:PC71BM blends reached an optimized PCE of 17.6%.
Huang’s group exploited two novel asymmetric side-chain-based NFSMAs, namely, EH-HD-4F and Bu-OD-4F.71 Compared with the reference acceptor BO-4F (Figure 6), both EH-HD-4F and Bu-OD-4F showed red-shifted absorption. However, EH-HD-4F-based blend films showed better miscibility, resulting in a more favorable morphology. As a result, the device based on PM6:EH-HD-4F obtained a desirable PCE of 18.38%, which was one of the highest efficiencies in binary OSCs (significantly higher than the symmetric one).
Figure 6.
Chemical structures of asymmetric side-chain-based and corresponding symmetric NFSMAs.
In addition to the central backbone and the side chains, the property of the terminal groups (end-capping “A” moieties) is essential for determining the overall electron affinity and optical band gap. Through an easy one-pot stepwise Knoevenagel condensation, various terminal groups (Figure 7) were induced to synthesize asymmetric NFSMAs. Currently, the main superiority of asymmetric terminal group strategies is realizing a trade-off between Voc and Jsc.
Figure 7.
Chemical structures of some terminal groups.
As shown in Figure 8, based on the indacenodithieno[3,2-b]thiophene (IDTT) central backbone, Bo’s group designed two types of asymmetric acceptors a-IT-2F and a-IT-2OM.72 Compared to ITIC, a-IT-2OM realized an elevated LUMO energy level to −3.92 eV. Because of the favorable face-on orientation and more balanced charge transport behavior, the PBDB-T: a-IT-2OM devices presented a higher efficiency of 12.07%. When the active layer thickness increased to 450 nm, the PCE of 9% was still retained. In order to investigate the fluorination density on IDTT-based acceptors, Marks and co-workers synthesized a series of symmetric acceptors ITIC-0F, ITIC-4F, and ITIC-6F as well as asymmetric ones ITIC-2F and ITIC-3F.73 Along with the number of fluorine atoms increased, these NFSMAs performed contractive Egopt’s but almost constant LUMO energy levels, which exhibited an improved Jsc and an excellent Voc. Except for ITIC-6F, the asymmetric acceptors ITIC-2F- and ITIC-3F achieved a more synergistic adjustment between the Jsc and the Voc when blending with donors in OSCs. Generally, because of the larger dipole moments, chlorine substitution was regarded as an effective strategy to lower the energy levels and broaden the absorption spectra.74−76 He and co-workers synthesized two acceptors, namely, ITIC-2Cl-β and α-ITIC-2Cl (Figure 8).77 Actually, ITIC-2Cl-β possessed a linear packing structure, but α-ITIC-2Cl formed a 3D interpenetrating network with shorter π–π distances and better molecular planarity. The larger dipole moments of α-ITIC-2Cl (4.20 D) enhanced the intermolecular interaction and charge mobility, and eventually obtained a higher FF of 73.50% and a better efficiency of 12.23% when blended with PM6.
Figure 8.
Chemical structures of asymmetric terminal-group-based NFSMAs.
On the basis of the backbone of Y6, two asymmetric acceptors BTP-S1 and BTP-S2 were acquired.78 Relative to BTP-S1, BTP-S2 displayed a bit narrower Egopt and higher absorption coefficient. The decreased nonradiative loss and Eloss of PM6:BTP-S2 devices were attributed to an excellent electroluminescence quantum efficiency (2.3 × 10–2%), which was much higher than that of Y6-based devices (4.4 × 10–3%). Ultimately, the PM6:BTP-S2 demonstrated a high PCE of 16.37%, which is much higher than PM6:Y6 (15.6%). Even more impressively, the efficiency of 18.16% was achieved when fabricated as layer-by-layer processed ternary blends of PM6:BO-4Cl:BTP-S2. In an aim to finely tune energy levels, Yan’s group employed a chlorinated thiophene-fused unit as an end group to replace the end group of BTP-4F (Y6), and a new NFSMA BTP-2ThCl was reported.79 Unfortunately, compared with Y6-based devices, the PM6:BTP-2ThCl devices presented a higher Voc yet unexpectedly lower Jsc and FF, which may be an unsuitable energy level (resulting in poor charge separation). In order to minimize the HOMO offset while maintaining sufficient charge separation, they combined two different end groups (CPTCN-Cl and IC-2F), and a new asymmetric NFA BTP-2F-ThCl was synthesized successfully. BTP-2F-ThCl-based device exhibited the best PCE of 17.06%, along with a Voc of 0.869 V and a Jsc of 25.38 mA/cm2. The higher PCE is mainly ascribed to the larger value of Voc ⊆ Jsc (22.06) than those of the devices based on BTP-4F (21.18) and BTP-2ThCl (20.76). Furthermore, the shelf life and photostability of PM6:BTP-2F-ThCl blends showed a resemblance to PM6:BTP-2ThCl blends and PM6:BTP-4F blends, indicating that different end groups in Y6-series NFSMAs had less influence on device stability. By replacing the fluorine atoms with chlorine atoms on terminal groups, three asymmetric acceptors, SY1, SY2, and SY3, were synthesized.80 The SY1 with two fluorine atoms and one chlorine atom presented the highest LUMO energy level and the best molecular packing. The PM6:SY1 devices formed proper phase separation, which was conducive to charge transportation, and thus obtained a champion PCE of 16.83%.
He and co-workers reported an acceptor named BTIC-2Cl-γCF3.81 After the asymmetric strategy, it maintained both advantages of high Voc from the IC-2Cl end group and conducive stacking from a CF3 end group. The single-crystal diffraction pattern showed that there were various of intermolecular interactions in the solid state of the acceptors (as shown in Figure 9). Synergistic H- and J-aggregations as well as a 3D network packing structure are formed, which provides more electron transfer channels. The enhanced charge transportation of BTIC-2Cl-γCF3-based devices was beneficial for FF (76.99%) and PCE (16.31%). When selecting PC71ThBM as the third component, the ternary devices reached the highest efficiency (17.12%) with halogen-free solvent processing. Because of their strong absorption in the near-infrared (NIR) region, BTIC-2Cl-γCF3-based semitransparent OSCs also presented a high PCE of 13.06% with an average visible transmittance (AVT) of 24.45%.
Figure 9.

(a) 3D network packing from the c-crystallographic axis. (b) The π–π interactions in one elliptical frame. (c) The single-crystal structure of one elliptical frame. (d) The intermolecular interactions in one elliptical frame. Copyright 2020 Wiley-VCH.
Figure 10.
(a) The chemical structures and (b) energy level of the above-mentioned polymer donors.
Summary and Perspectives
As shown in this short outlook, asymmetric NFSMAs have played a vital role in the development of OSCs and exhibited no less potency than symmetrical acceptors in recent years. Benefiting from the development of A-DA′D-A-type NFSMAs, the efficiency of asymmetric acceptors based OSCs have exceeded 18%. The remarkable results have greatly inspired researchers’ confidence of asymmetric strategies in the design of NFSMAs. In view of the special advantages of asymmetric NFSMAs, we propose the following development directions:
-
(1)
The relationship between the shape of NFSMAs and the performance of OSCs is still in the exploration stage, and there is no practical theory to verify which is the best shape for NFSMAs. However, this kind of influence does exist and has important research value. Thus, finding an optimal shape to achieve high-efficiency NFSMAs through the asymmetric expansion of the conjugated central backbone provides a very important reference value.
-
(2)
The incorporation of electron-donating alkyl chains or aromatic side chains into NFSMAs has achieved reasonably good performance, as asymmetric terminal groups. These two methods can achieve a balance between the solubility and the crystallinity. With the development of synthesis technology, we can carry out the two methods at the same time to subtly adjust the performance of OSCs.
-
(3)
The formation of quasi-polymers means that two small molecules are connected to form a dimer through a single bond or π-bridge. In the previous research of PDI-based quasi-polymers, it turns out to be a feasible and superior strategy to prevent excessive aggregation.82 Combining good repeatability of the small-molecule acceptor and high stability of the polymer acceptor, quasi-polymers will have a higher commercialization potential and take its place in asymmetric NFSMAs.
The molecular structure and efficiencies of symmetrical acceptors both have been increasing rapidly in recent years. Compared with the onerous design and synthesis of novel non-fullerene acceptor systems, asymmetric modification to the structure of known acceptors appears to be a roundabout approach to get higher device performances for OSCs. With the gradual upgrade of device processing technology, the stability of devices will be enhanced with the development of packaging technology. Large-scale production will also significantly reduce the cost of the OSCs. All of these will promote the commercialization of OSCs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21875286 and 22005347) and the National Key Research and Development Program of China (2017YFA0206600).
Author Contributions
† D.L. and C.S. contributed equally to this work.
The authors declare no competing financial interest.
References
- Brabec C. J.; Heeney M.; McCulloch I.; Nelson J. Influence of Blend Microstructure on Bulk Heterojunction Organic Photovoltaic Performance. Chem. Soc. Rev. 2011, 40, 1185–1199. 10.1039/C0CS00045K. [DOI] [PubMed] [Google Scholar]
- Dai S.; Zhao F.; Zhang Q.; Lau T.-K.; Li T.; Liu K.; Ling Q.; Wang C.; Lu X.; You W.; Zhan X. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. 10.1021/jacs.6b12755. [DOI] [PubMed] [Google Scholar]
- Liu F.; Zhou Z.; Zhang C.; Zhang J.; Hu Q.; Vergote T.; Liu F.; Russell T. P.; Zhu X. Efficient Semitransparent Solar Cells with High NIR Responsiveness Enabled by a Small-Bandgap Electron Acceptor. Adv. Mater. 2017, 29, 1606574. 10.1002/adma.201606574. [DOI] [PubMed] [Google Scholar]
- Wadsworth A.; Moser M.; Marks A.; Little M. S.; Gasparini N.; Brabec C. J.; Baran D.; McCulloch I. Critical Review of the Molecular Design Progress in Non-Fullerene Electron Acceptors towards Commercially Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596–1625. 10.1039/C7CS00892A. [DOI] [PubMed] [Google Scholar]
- Gasparini N.; Paleti H. K.; Bertrandie J.; Cai G. L.; Zhang G. C.; Wadsworth A.; Lu X. H.; Yip H. L.; McCulloch I.; Baran D. Exploiting Ternary Blends for Improved Photostability in High-Efficiency Organic Solar Cells. ACS Energy Lett. 2020, 5, 1371–1379. 10.1021/acsenergylett.0c00604. [DOI] [Google Scholar]
- Du X.; Heumueller T.; Gruber W.; Classen A.; Unruh T.; Li N.; Brabec C. J. Efficient Polymer Solar Cells Based on Non-fullerene Acceptors with Potential Device Lifetime Approaching 10 Years. Joule 2019, 3, 215–226. 10.1016/j.joule.2018.09.001. [DOI] [Google Scholar]
- Duan L.; Uddin A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. 10.1002/advs.201903259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke J.; Speller E. M.; Wadsworth A.; Wyatt M. F.; Dimitrov S.; Lee H. K. H.; Li Z.; Tsoi W. C.; McCulloch I.; Bagnis D.; Durrant J. R.; Kim J.-S. Twist and Degrade—Impact of Molecular Structure on the Photostability of Nonfullerene Acceptors and Their Photovoltaic Blends. Adv. Energy Mater. 2019, 9, 1803755. 10.1002/aenm.201803755. [DOI] [Google Scholar]
- Ma Z. W.; Zhao B.; Gong Y. S.; Deng J. P.; Tan Z. A. Green-Solvent-Processable Strategies for Achieving Large-Scale Manufacture of Organic Photovoltaics. J. Mater. Chem. A 2019, 7, 22826–22847. 10.1039/C9TA09277C. [DOI] [Google Scholar]
- Zhu W.; Spencer A. P.; Mukherjee S.; Alzola J. M.; Sangwan V. K.; Amsterdam S. H.; Swick S. M.; Jones L. O.; Heiber M. C.; Herzing A. A.; Li G.; Stern C. L.; DeLongchamp D. M.; Kohlstedt K. L.; Hersam M. C.; Schatz G. C.; Wasielewski M. R.; Chen L. X.; Facchetti A.; Marks T. J. Crystallography, Morphology, Electronic Structure, and Transport in Non-Fullerene/Non-Indacenodithienothiophene Polymer:Y6 Solar Cells. J. Am. Chem. Soc. 2020, 142, 14532–14547. 10.1021/jacs.0c05560. [DOI] [PubMed] [Google Scholar]
- Sun R.; Guo J.; Wu Q.; Zhang Z.; Yang W.; Guo J.; Shi M.; Zhang Y.; Kahmann S.; Ye L.; Jiao X.; Loi M. A.; Shen Q.; Ade H.; Tang W.; Brabec C. J.; Min J. A Multi-objective Optimization-Based Layer-by-Layer Blade-Coating Approach for Organic Solar Cells: Rational Control of Vertical Stratification for High Performance. Energy Environ. Sci. 2019, 12, 3118–3132. 10.1039/C9EE02295C. [DOI] [Google Scholar]
- Sun Y.; Chang M.; Meng L.; Wan X.; Gao H.; Zhang Y.; Zhao K.; Sun Z.; Li C.; Liu S.; Wang H.; Liang J.; Chen Y. Flexible Organic Photovoltaics Based on Water-processed Silver Nanowire Electrodes. Nat. Electronics 2019, 2, 513–520. 10.1038/s41928-019-0315-1. [DOI] [Google Scholar]
- Li Y.; Sheriff H. K. M. Jr.; Liu X.; Wang C. K.; Ding K.; Han H.; Wong K. T.; Forrest S. R. Vacuum-Deposited Biternary Organic Photovoltaics. J. Am. Chem. Soc. 2019, 141, 18204–18210. 10.1021/jacs.9b09012. [DOI] [PubMed] [Google Scholar]
- Brus V. V.; Lee J.; Luginbuhl B. R.; Ko S. J.; Bazan G. C.; Nguyen T. Q. Solution-Processed Semitransparent Organic Photovoltaics: From Molecular Design to Device Performance. Adv. Mater. 2019, 31, 1900904. 10.1002/adma.201900904. [DOI] [PubMed] [Google Scholar]
- Yu G.; Gao J.; Hummelen J. C.; Wudl F.; Heeger A. J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. 10.1126/science.270.5243.1789. [DOI] [Google Scholar]
- He Y. J.; Chen H. Y.; Hou J. H.; Li Y. F. Indene-C-60 Bisadduct: A New Acceptor for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2010, 132, 1377–1382. 10.1021/ja908602j. [DOI] [PubMed] [Google Scholar]
- He Z.; Zhong C.; Su S.; Xu M.; Wu H.; Cao Y. Enhanced Power-Conversion Efficiency in Polymer Solar Cells Using an Inverted Device Structure. Nat. Photonics 2012, 6, 591–595. 10.1038/nphoton.2012.190. [DOI] [Google Scholar]
- Zhang M.; Guo X.; Zhang S.; Hou J. Synergistic Effect of Fluorination on Molecular Energy Level Modulation in Highly Efficient Photovoltaic Polymers. Adv. Mater. 2014, 26, 1118–1123. 10.1002/adma.201304427. [DOI] [PubMed] [Google Scholar]
- Ye L.; Zhang S.; Zhao W.; Yao H.; Hou J. Highly Efficient 2D-Conjugated Benzodithiophene-Based Photovoltaic Polymer with Linear Alkylthio Side. Chem. Mater. 2014, 26, 3603–3605. 10.1021/cm501513n. [DOI] [Google Scholar]
- Liang Y.; Xu Z.; Xia J.; Tsai S.-T.; Wu Y.; Li G.; Ray C.; Yu L.; et al. For the Bright Future—Bulk Heterojunction Polymer Solar Cells with Power Conversion Efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. 10.1002/adma.200903528. [DOI] [PubMed] [Google Scholar]
- Liao S. H.; Jhuo H. J.; Cheng Y. S.; Chen S. A. Fullerene Derivative-Doped Zinc Oxide Nanofilm as the Cathode of Inverted Polymer Solar Cells with Low-Bandgap Polymer (PTB7-Th) for High Performance. Adv. Mater. 2013, 25, 4766–4771. 10.1002/adma.201301476. [DOI] [PubMed] [Google Scholar]
- Scharber M. C. On the Efficiency Limit of Conjugated Polymer:Fullerene-Based Bulk Heterojunction Solar Cells. Adv. Mater. 2016, 28, 1994–2001. 10.1002/adma.201504914. [DOI] [PubMed] [Google Scholar]
- You J.; Dou L.; Yoshimura K.; Kato T.; Ohya K.; Moriarty T.; Emery K.; Chen C.-C.; Gao J.; Li G.; Yang Y. A Polymer Tandem Solar Cell with 10.6% Power Conversion Efficiency. Nat. Commun. 2013, 4, 1446. 10.1038/ncomms2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Mende L.; Fechtenkotter A.; Mullen K.; Moons E.; Friend R. H.; MacKenzie J. D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 2001, 293, 1119–1122. 10.1126/science.293.5532.1119. [DOI] [PubMed] [Google Scholar]
- Feng L.; Yuan J.; Zhang Z.; Peng H.; Zhang Z.-G.; Xu S.; Liu Y.; Li Y.; Zou Y. Thieno[3,2-b]pyrrolo-Fused Pentacyclic Benzotriazole-Based Acceptor for Efficient Organic Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 31985–31992. 10.1021/acsami.7b10995. [DOI] [PubMed] [Google Scholar]
- Lin Y. Z.; Wang J. Y.; Zhang Z. G.; Bai H. T.; Li Y. F.; Zhu D. B.; Zhan X. W. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. 10.1002/adma.201404317. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Zhang Y.; Zhou L.; Zhang G.; Yip H.-L.; Lau T.-K.; Lu X.; Zhu C.; Peng H.; Johnson P. A.; Leclerc M.; Cao Y.; Ulanski J.; Li Y.; Zou Y. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. 10.1016/j.joule.2019.01.004. [DOI] [Google Scholar]
- Cui Y.; Yao H.; Zhang J.; Xian K.; Zhang T.; Hong L.; Wang Y.; Xu Y.; Ma K.; An C.; He C.; Wei Z.; Gao F.; Hou J. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. 10.1002/adma.201908205. [DOI] [PubMed] [Google Scholar]
- Arunagiri L.; Peng Z.; Zou X.; Yu H.; Zhang G.; Wang Z.; Lin Lai J. Y.; Zhang J.; Zheng Y.; Cui C.; Huang F.; Zou Y.; Wong K. S.; Chow P. C. Y.; Ade H.; Yan H. Selective Hole and Electron Transport in Efficient Quaternary Blend Organic Solar Cells. Joule 2020, 4, 1790–1805. 10.1016/j.joule.2020.06.014. [DOI] [Google Scholar]
- Cui Y.; Yao H.; Hong L.; Zhang T.; Tang Y.; Lin B.; Xian K.; Gao B.; An C.; Bi P.; Ma W.; Hou J. Organic Photovoltaic Cell with 17% Efficiency and Superior Processability. Natl. Sci. Rev. 2020, 7, 1239–1246. 10.1093/nsr/nwz200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Jiang Y.; Jin K.; Qin J.; Xu J.; Li W.; Xiong J.; Liu J.; Xiao Z.; Sun K.; Yang S.; Zhang X.; Ding L. 18% Efficiency Organic Solar Cells. Sci. Bull. 2020, 65, 272–275. 10.1016/j.scib.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Nugraha M. I.; Firdaus Y.; Scaccabarozzi A. D.; Aniés F.; Emwas A.-H.; Yengel E.; Zheng X.; Liu J.; Wahyudi W.; Yarali E.; Faber H.; Bakr O. M.; Tsetseris L.; Heeney M.; Anthopoulos T. D. A Simple n-Dopant Derived from Diquat Boosts the Efficiency of Organic Solar Cells to 18.3%. ACS Energy Lett. 2020, 5, 3663–3671. 10.1021/acsenergylett.0c01949. [DOI] [Google Scholar]
- Zhang M.; Zhu L.; Zhou G.; Hao T.; Qiu C.; Zhao Z.; Hu Q.; Larson B. W.; Zhu H.; Ma Z.; Tang Z.; Feng W.; Zhang Y.; Russell T. P.; Liu F. Single-Layered Organic Photovoltaics with Double Cascading Charge Transport Pathways: 18% Efficiencies. Nat. Commun. 2021, 12, 309. 10.1038/s41467-020-20580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R.; Liu T.; Luo Z.; Guo Q.; Xiao Y.; Chen Y.; Li X.; Luo S.; Lu X.; Zhang M.; Li Y.; Yan H. Improving Open-Circuit Voltage by a Chlorinated Polymer Donor Endows Binary Organic Solar Cells Efficiencies over 17%. Sci. China: Chem. 2020, 63, 325–330. 10.1007/s11426-019-9669-3. [DOI] [Google Scholar]
- Li C.; Zhou J. D.; Song J. L.; Xu J. Q.; Zhang H. T.; Zhang X. N.; Guo J.; Zhu L.; Wei D. H.; Han G. C.; Min J.; Zhang Y.; Xie Z. Q.; Yi Y. P.; Yan H.; Gao F.; Liu F.; Sun Y. M. Non-Fullerene Acceptors with Branched Side Chains and Improved Molecular Packing to Exceed 18% Efficiency in Organic Solar Cells. Nat. Energy 2021, 6, 605. 10.1038/s41560-021-00820-x. [DOI] [Google Scholar]
- Mo D.; Chen H.; Zhou J.; Tang N.; Han L.; Zhu Y.; Chao P.; Lai H.; Xie Z.; He F. Alkyl Chain Engineering of Chlorinated Acceptors for Elevated Solar Conversion. J. Mater. Chem. A 2020, 8, 8903–8912. 10.1039/C9TA12558B. [DOI] [Google Scholar]
- Lai H.; Zhao Q.; Chen Z.; Chen H.; Chao P.; Zhu Y.; Lang Y.; Zhen N.; Mo D.; Zhang Y.; He F. Trifluoromethylation Enables a 3D Interpenetrated Low-Band-Gap Acceptor for Efficient Organic Solar Cells. Joule 2020, 4, 688–700. 10.1016/j.joule.2020.02.004. [DOI] [Google Scholar]
- Yuan J.; Zhang C.; Chen H.; Zhu C.; Cheung S. H.; Qiu B.; Cai F.; Wei Q.; Liu W.; Yin H.; Zhang R.; Zhang J.; Liu Y.; Zhang H.; Liu W.; Peng H.; Yang J.; Meng L.; Gao F.; So S.; Li Y.; Zou Y. Understanding Energetic Disorder in Electron-Deficient-Core-Based Non-Fullerene Solar Cells. Sci. China: Chem. 2020, 63, 1159–1168. 10.1007/s11426-020-9747-9. [DOI] [Google Scholar]
- Li C.; Fu H.; Xia T.; Sun Y. Asymmetric Nonfullerene Small Molecule Acceptors for Organic Solar Cells. Adv. Energy Mater. 2019, 9, 1900999. 10.1002/aenm.201900999. [DOI] [Google Scholar]
- Bürckstümmer H.; Kronenberg N. M.; Gsänger M.; Stolte M.; Meerholz K.; Würthner F. Tailored Merocyaninedyes for Solution-processed BHJ Solar Cells. J. Mater. Chem. 2010, 20, 240–243. 10.1039/B916181C. [DOI] [Google Scholar]
- Burckstummer H.; Tulyakova E. V.; Deppisch M.; Lenze M. R.; Kronenberg N. M.; Gsanger M.; Stolte M.; Meerholz K.; Wurthner F. Efficient Solution-processed Bulk Heterojunction Solar Cells by Antiparallel Supramolecular Arrangement of Dipolar Donor-acceptor Dyes. Angew. Chem., Int. Ed. 2011, 50, 11628–11632. 10.1002/anie.201105133. [DOI] [PubMed] [Google Scholar]
- Kronenberg N. M.; Steinmann V.; Burckstummer H.; Hwang J.; Hertel D.; Wurthner F.; Meerholz K. Direct Comparison of Highly Efficient Solution- and Vacuum-Processed Organic Solar Cells Based on Merocyanine Dyes. Adv. Mater. 2010, 22, 4193–4197. 10.1002/adma.201000800. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Li S.; Yao H.; Zhang S.; Zhang Y.; Yang B.; Hou J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. 10.1021/jacs.7b02677. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang Z. G.; Bin H.; Chen S.; Gao L.; Xue L.; Yang C.; Li Y. Side-Chain Isomerization on an n-type Organic Semiconductor ITIC Acceptor Makes 11.77% High Efficiency Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. 10.1021/jacs.6b09110. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Zhao F.; He Q.; Huo L.; Wu Y.; Parker T. C.; Ma W.; Sun Y.; Wang C.; Zhu D.; Heeger A. J.; Marder S. R.; Zhan X. High-Performance Electron Acceptor with Thienyl Side Chains for Organic Photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. 10.1021/jacs.6b02004. [DOI] [PubMed] [Google Scholar]
- Hou J.; Inganäs O.; Friend R. H.; Gao F. Organic Solar Cells Based on Non-Fullerene Acceptors. Nat. Mater. 2018, 17, 119–128. 10.1038/nmat5063. [DOI] [PubMed] [Google Scholar]
- Sun J.; Ma X.; Zhang Z.; Yu J.; Zhou J.; Yin X.; Yang L.; Geng R.; Zhu R.; Zhang F.; Tang W. Dithieno[3,2-b:2′,3′-d]pyrrol Fused Nonfullerene Acceptors Enabling Over 13% Efficiency for Organic Solar Cells. Adv. Mater. 2018, 30, 1707150. 10.1002/adma.201707150. [DOI] [PubMed] [Google Scholar]
- Liu S.; Yuan J.; Deng W.; Luo M.; Xie Y.; Liang Q.; Zou Y.; He Z.; Wu H.; Cao Y. High-Efficiency Organic Solar Cells with Low Non-Radiative Recombination Loss and Low Energetic Disorder. Nat. Photonics 2020, 14, 300–305. 10.1038/s41566-019-0573-5. [DOI] [Google Scholar]
- Zhu C.; Yuan J.; Cai F.; Meng L.; Zhang H.; Chen H.; Li J.; Qiu B.; Peng H.; Chen S.; Hu Y.; Yang C.; Gao F.; Zou Y.; Li Y. Tuning the Electron-deficient Core of a Non-Fullerene Acceptor to Achieve over 17% Efficiency in a Single-junction Organic Solar Cell. Energy Environ. Sci. 2020, 13, 2459–2466. 10.1039/D0EE00862A. [DOI] [Google Scholar]
- Jia B.; Wang J.; Wu Y.; Zhang M.; Jiang Y.; Tang Z.; Russell T. P.; Zhan X. Enhancing the Performance of a Fused-Ring Electron Acceptor by Unidirectional Extension. J. Am. Chem. Soc. 2019, 141, 19023–19031. 10.1021/jacs.9b08988. [DOI] [PubMed] [Google Scholar]
- Song J. L.; Li C.; Ye L. L.; Koh C.; Cai Y. H.; Wei D. H.; Woo H. Y.; Sun Y. M. Extension of Indacenodithiophene Backbone Conjugation Enables Efficient Asymmetric A-D-A Type Non-Fullerene Acceptors. J. Mater. Chem. A 2018, 6, 18847–18852. 10.1039/C8TA07334A. [DOI] [Google Scholar]
- Li C.; Song J. L.; Ye L. L.; Koh C.; Weng K. K.; Fu H. T.; Cai Y. H.; Xie Y. P.; Wei D. H.; Woo H. Y.; Sun Y. M. High-Performance Eight-Membered Indacenodithiophene-Based Asymmetric A-D-A Type Non-Fullerene Acceptors. Sol. RRL 2019, 3, 1800246. 10.1002/solr.201800246. [DOI] [Google Scholar]
- Sun C.; Pan F.; Chen S.; Wang R.; Sun R.; Shang Z.; Qiu B.; Min J.; Lv M.; Meng L.; Zhang C.; Xiao M.; Yang C.; Li Y. Achieving Fast Charge Separation and Low Nonradiative Recombination Loss by Rational Fluorination for High-Efficiency Polymer Solar Cells. Adv. Mater. 2019, 31, 1905480. 10.1002/adma.201905480. [DOI] [PubMed] [Google Scholar]
- Pang S. T.; Zhou X.; Zhang S.; Tang H. R.; Dhakal S.; Gu X. D.; Duan C. H.; Huang F.; Cao Y. Nonfused Nonfullerene Acceptors with an A-D-A ‘-D-A Framework and a Benzothiadiazole Core for High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 16531–16540. 10.1021/acsami.0c01850. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Song X.; Liu K.-K.; Zhang M.; Qu J.; Yang C.; Yuan G.-Z.; Mahmood A.; Liu F.; He F.; Baran D.; Wang J.-L. Electron-Deficient and Quinoid Central Unit Engineering for Unfused Ring-Based A1–D–A2–D–A1-Type Acceptor Enables High Performance Nonfullerene Polymer Solar Cells with High Voc and PCE Simultaneously. Small 2020, 16, 1907681. 10.1002/smll.201907681. [DOI] [PubMed] [Google Scholar]
- He C.; Li Y.; Li S.; Yu Z.-P.; Li Y.; Lu X.; Shi M.; Li C.-Z.; Chen H. Near-Infrared Electron Acceptors with Unfused Architecture for Efficient Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 16700–16706. 10.1021/acsami.0c00837. [DOI] [PubMed] [Google Scholar]
- Chang Q.; Chen H.; Yuan J.; Hu Y.; Hai J.; Liu W.; Cai F.; Hong J.; Xiao X.; Zou Y. Diflurobenzothiadiazole Core-Based Noncovalently Fused Small Molecule Acceptor Exhibiting Over 12% Efficiency and High Fill Factor. J. Energy Chem. 2020, 51, 7–13. 10.1016/j.jechem.2020.03.036. [DOI] [Google Scholar]
- Cai F.; Peng H.; Chen H.; Yuan J.; Hai J.; Lau T.-K.; Wang J.; Hu Y.; Liu W.; Lu X.; Zou Y. An Asymmetric Small Molecule Acceptor for Organic Solar Cells with a Short Circuit Current Density over 24 mA cm-2. J. Mater. Chem. A 2020, 8, 15984–15991. 10.1039/D0TA01636E. [DOI] [Google Scholar]
- Song J.; Cai F.; Zhu C.; Chen H.; Wei Q.; Li D.; Zhang C.; Zhang R.; Yuan J.; Peng H.; So S. K.; Zou Y. Over 13%-Efficient Organic Solar Cells Based on Low-Cost Pentacyclic A-DA’D-A Type Non-Fullerene Acceptor. Solar RRL 2021, 5, 2100281. 10.1002/solr.202100281. [DOI] [Google Scholar]
- Gao W.; Ma X.; An Q.; Gao J.; Zhong C.; Zhang F.; Yang C. An Asymmetrical Fused-Ring Electron Acceptor Designed by a Cross-Conceptual Strategy Achieving 15.6% Efficiency. J. Mater. Chem. A 2020, 8, 14583–14591. 10.1039/D0TA03985C. [DOI] [Google Scholar]
- Gao W.; Fu H.; Li Y.; Lin F.; Sun R.; Wu Z.; Wu X.; Zhong C.; Min J.; Luo J.; Woo H. Y.; Zhu Z.; Jen A. K. Y. Asymmetric Acceptors Enabling Organic Solar Cells to Achieve an over 17% Efficiency: Conformation Effects on Regulating Molecular Properties and Suppressing Nonradiative Energy Loss. Adv. Energy Mater. 2021, 11, 2003177. 10.1002/aenm.202003177. [DOI] [Google Scholar]
- Luo Z.; Ma R.; Xiao Y.; Liu T.; Sun H.; Su M.; Guo Q.; Li G.; Gao W.; Chen Y.; Zou Y.; Guo X.; Zhang M.; Lu X.; Yan H.; Yang C. Conformation-Tuning Effect of Asymmetric Small Molecule Acceptors on Molecular Packing, Interaction, and Photovoltaic Performance. Small 2020, 16, 2001942. 10.1002/smll.202001942. [DOI] [PubMed] [Google Scholar]
- Guo Q.; Ma R.; Hu J.; Wang Z.; Sun H.; Dong X.; Luo Z.; Liu T.; Guo X.; Guo X.; Yan H.; Liu F.; Zhang M. Over 15% Efficiency Polymer Solar Cells Enabled by Conformation Tuning of Newly Designed Asymmetric Small-Molecule Acceptors. Adv. Funct. Mater. 2020, 30, 2000383. 10.1002/adfm.202000383. [DOI] [Google Scholar]
- Yang L.; Song X.; Yu J.; Wang H.; Zhang Z.; Geng R.; Cao J.; Baran D.; Tang W. Tuning of the Conformation of Asymmetric Nonfullerene Acceptors for Efficient Organic Solar Cells. J. Mater. Chem. A 2019, 7, 22279–22286. 10.1039/C9TA07634D. [DOI] [Google Scholar]
- Fei Z.; Han Y.; Gann E.; Hodsden T.; Chesman A. S. R.; McNeill C. R.; Anthopoulos T. D.; Heeney M. Alkylated Selenophene-Based Ladder-Type Monomers via a Facile Route for High-Performance Thin-Film Transistor Applications. J. Am. Chem. Soc. 2017, 139, 8552–8561. 10.1021/jacs.7b03099. [DOI] [PubMed] [Google Scholar]
- Yang C.; An Q.; Bai H.-R.; Zhi H.-F.; Ryu H. S.; Mahmood A.; Zhao X.; Zhang S.; Woo H. Y.; Wang J.-L. Synergistic Strategy of Manipulating the Number of Selenophene Units and Asymmetric Central Core of Small Molecular Acceptors Enables Polymer Solar Cells with 17.5% Efficiency. Angew. Chem., Int. Ed. 2021, 60, 19241. 10.1002/anie.202104766. [DOI] [PubMed] [Google Scholar]
- Chang M. J.; Wang Y. C.; Yi Y. Q. Q.; Ke X.; Wan X. J.; Li C. X.; Chen Y. S. Fine-tuning the Side-chains of Non-fullerene Small Molecule Acceptors to Match with Appropriate Polymer Donors. J. Mater. Chem. A 2018, 6, 8586–8594. 10.1039/C8TA00764K. [DOI] [Google Scholar]
- Zhu J.; Xiao Y.; Wang J.; Liu K.; Jiang H.; Lin Y.; Lu X.; Zhan X. Alkoxy-Induced Near-Infrared Sensitive Electron Acceptor for High-Performance Organic Solar Cells. Chem. Mater. 2018, 30, 4150–4156. 10.1021/acs.chemmater.8b01677. [DOI] [Google Scholar]
- Li S.; Ye L.; Zhao W.; Zhang S.; Ade H.; Hou J. Significant Influence of the Methoxyl Substitution Position on Optoelectronic Properties and Molecular Packing of Small-Molecule Electron Acceptors for Photovoltaic Cells. Adv. Energy Mater. 2017, 7, 1700183. 10.1002/aenm.201700183. [DOI] [Google Scholar]
- Chen Y.; Bai F.; Peng Z.; Zhu L.; Zhang J.; Zou X.; Qin Y.; Kim H. K.; Yuan J.; Ma L. K.; Zhang J.; Yu H.; Chow P. C. Y.; Huang F.; Zou Y.; Ade H.; Liu F.; Yan H. Asymmetric Alkoxy and Alkyl Substitution on Nonfullerene Acceptors Enabling High□Performance Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003141. 10.1002/aenm.202003141. [DOI] [Google Scholar]
- Chen S.; Feng L.; Jia T.; Jing J.; Hu Z.; Zhang K.; Huang F. High-Performance Polymer Solar Cells with Efficiency over 18% Enabled by Asymmetric Side Chain Engineering of Non-fullerene Acceptors. Sci. China: Chem. 2021, 64, 1192. 10.1007/s11426-021-1013-0. [DOI] [Google Scholar]
- Li M.; Zhou Y.; Zhang J.; Song J.; Bo Z. Tuning the Dipole Moments of Nonfullerene Acceptors with an Asymmetric Terminal Strategy for Highly Efficient Organic Solar Cells. J. Mater. Chem. A 2019, 7, 8889–8896. 10.1039/C8TA12530A. [DOI] [Google Scholar]
- Aldrich T. J.; Matta M.; Zhu W.; Swick S. M.; Stern C. L.; Schatz G. C.; Facchetti A.; Melkonyan F. S.; Marks T. J. Fluorination Effects on Indacenodithienothiophene Acceptor Packing and Electronic Structure, End-Group Redistribution, and Solar Cell Photovoltaic Response. J. Am. Chem. Soc. 2019, 141, 3274–3287. 10.1021/jacs.8b13653. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Yao H.; Zhang J.; Zhang T.; Wang Y.; Hong L.; Xian K.; Xu B.; Zhang S.; Peng J.; Wei Z.; Gao F.; Hou J. Over 16% Efficiency Organic Photovoltaic Cells Enabled by a Chlorinated Acceptor with Increased Open-circuit Voltages. Nat. Commun. 2019, 10, 2515. 10.1038/s41467-019-10351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Yao H.; Zhang S.; Qin Y.; Zhang J.; Yang L.; Li W.; Wei Z.; Gao F.; Hou J. Fluorination vs. Chlorination: A Case Study on High Performance Organic Photovoltaic Materials. Sci. China: Chem. 2018, 61, 1328–1337. 10.1007/s11426-018-9260-2. [DOI] [Google Scholar]
- Zhang H.; Yao H.; Hou J.; Zhu J.; Zhang J.; Li W.; Yu R.; Gao B.; Zhang S.; Hou J. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated Nonfullerene Small-Molecule Acceptors. Adv. Mater. 2018, 30, 1800613. 10.1002/adma.201800613. [DOI] [PubMed] [Google Scholar]
- Lai H.; Chen H.; Zhou J.; Qu J.; Wang M.; Xie W.; Xie Z.; He F. 3D Interpenetrating Network for High-Performance Nonfullerene Acceptors via Asymmetric Chlorine Substitution. J. Phys. Chem. Lett. 2019, 10, 4737–4743. 10.1021/acs.jpclett.9b01931. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhan L.; Jin Y.; Zhou G.; Lau T. K.; Qin R.; Shi M.; Li C. Z.; Zhu H.; Lu X.; Zhang F.; Chen H. Asymmetric Electron Acceptors for High-Efficiency and Low-Energy-Loss Organic Photovoltaics. Adv. Mater. 2020, 32, 2001160. 10.1002/adma.202001160. [DOI] [PubMed] [Google Scholar]
- Luo Z. H.; Ma R. J.; Liu T.; Yu J. W.; Xiao Y. Q.; Sun R.; Xie G. S.; Yuan J.; Chen Y. Z.; Chen K.; Chai G. D.; Sun H. L.; Min J.; Zhang J.; Zou Y. P.; Yang C. L.; Lu X. H.; Gao F.; Yan H. Fine-Tuning Energy Levels via Asymmetric End Groups Enables Polymer Solar Cells with Efficiencies over 17%. Joule 2020, 4, 1236–1247. 10.1016/j.joule.2020.03.023. [DOI] [Google Scholar]
- Liu T.; Zhang Y.; Shao Y.; Ma R.; Luo Z.; Xiao Y.; Yang T.; Lu X.; Yuan Z.; Yan H.; Chen Y.; Li Y. Asymmetric Acceptors with Fluorine and Chlorine Substitution for Organic Solar Cells toward 16.83% Efficiency. Adv. Funct. Mater. 2020, 30, 2000456. 10.1002/adfm.202000456. [DOI] [Google Scholar]
- Chen H.; Lai H.; Chen Z.; Zhu Y.; Wang H.; Han L.; Zhang Y.; He F. 17.1%-Efficient Eco-Compatible Organic Solar Cells from a Dissymmetric 3D Network Acceptor. Angew. Chem. 2021, 133, 3275–3283. 10.1002/ange.202013053. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Lu Z.; Ye L.; Zhan C.; Hou J.; Zhang S.; Jiang B.; Zhao Y.; Huang J.; Zhang S.; Liu Y.; Shi Q.; Liu Y.; Yao J.; et al. A Potential Perylene Diimide Dimer-Based Acceptor Material for Highly Efficient Solution-Processed Non-Fullerene Organic Solar Cells with 4.03% Efficiency. Adv. Mater. 2013, 25, 5791–5797. 10.1002/adma.201300897. [DOI] [PubMed] [Google Scholar]