Abstract
This report presents a semisolid agar antifungal susceptibility (SAAS) method for the rapid susceptibility screening of yeasts and molds. The reproducibility and accuracy of the SAAS method were assessed by comparing the MICs of amphotericin B and fluconazole obtained for 10 candidate quality control (QC) American Type Culture Collection yeast strains in ≥15 replicates with those found by six independent laboratories using the National Committee for Clinical Laboratory Standards (NCCLS) M27-P broth macrodilution method (M. A. Pfaller et al., J. Clin. Microbiol. 33:1104–1107, 1995). Overall, 96% of MICs for both drugs fell within 1 log2 dilution of the modal MIC for each strain. The MICs for amphotericin B showed 99% agreement with the NCCLS proposed QC ranges within 1 log2 dilution. Likewise, the MICs for fluconazole at ≥75% growth reduction showed 99% agreement for seven strains. Three strains, Candida albicans ATCC 24333 and ATCC 76615 and Candida tropicalis ATCC 750, showed a less sharp fluconazole endpoint at ≥75% growth reduction, but at >50% growth reduction, the agreement was 98% within 1 log2 dilution of the proposed range. The MIC agreement within the proposed range for the suggested QC strains Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 was 100% for fluconazole and 100% within 1 log2 dilution of the proposed range for amphotericin B. The SAAS method demonstrated the susceptibility or resistance of 25 clinical isolates of filamentous fungi such as Aspergillus fumigatus to amphotericin B, itraconazole, and fluconazole, usually within 48 h. Although the results are preliminary, this SAAS method is promising as a rapid and cost-effective screen and is worthy of concerted investigation.
Fungal infections associated with significant morbidity and mortality are increasing in critically ill and immunocompromised patients (2, 7, 12, 14, 17). A rise in invasive disease caused by non-albicans Candida spp., such as Candida glabrata, Candida parapsilosis, and Candida tropicalis, has been documented recently and is accompanied by in vitro detection of azole resistance (1, 3, 4, 10, 12, 13). In addition, non-Candida yeast and invasive mold infections are also increasing in these patients (7, 14, 16). Accordingly, the choice of appropriate antifungal treatment is important but limited to a few licensed agents, and testing for susceptibility to these agents has only recently been standardized for yeasts and is just being developed for filamentous fungi (5, 9). Many hospital laboratories do not routinely perform antifungal susceptibility testing by the approved National Committee for Clinical Laboratory Standards (NCCLS) reference method, resulting in the delay of results while awaiting evaluation at a reference laboratory.
An optimal antifungal susceptibility screening test would be analogous to a preliminary susceptibility test for bacteria. Ideally, such a test would be a quick, easy, and cost-effective way for the hospital laboratory to report the antimicrobial susceptibility or resistance of any fungal pathogen, yeast, or mold, even before its identification. We have developed a simple antifungal susceptibility test that consists of a deep, tubed, semisolid base medium composed of heart infusion broth without dextrose and with 0.5% agar. These conditions were chosen to reduce oxygen tension and to approximate the growth conditions found in vegetations and infected tissue of patients (8).
This paper has a threefold purpose: (i) to introduce a simple, relatively quick semisolid agar antifungal susceptibility (SAAS) method that can produce results within 48 h after initial fungal isolation, (ii) to report verification studies of the new method with 10 American Type Culture Collection (ATCC) strains for which consensus regarding MICs has been obtained in multiple laboratories, and (iii) to demonstrate that the SAAS method is useful in determining antifungal susceptibility or resistance of a variety of clinical isolates of filamentous fungi.
MATERIALS AND METHODS
Study design.
Three studies were performed. In study 1, the accuracy and reproducibility of the SAAS method were ascertained by comparing the MICs obtained for the 10 candidate quality control (QC) ATCC yeast strains in a large multilaboratory study in which the NCCLS M27-P broth macrodilution method was used with the MICs obtained for the same yeasts tested 15 or more times by the SAAS method (9, 11). Study 2 evaluated the potential use of the SAAS method as an antifungal susceptibility “screen” by assessing the resistance or susceptibility of the same 10 ATCC yeast strains to two or three serum-achievable and clinically relevant concentrations of antifungal agents. Study 3 investigated whether the in vitro susceptibility or resistance of 25 isolates of filamentous fungi recovered from clinical specimens could be determined by the SAAS method.
Fungal isolates.
For studies 1 and 2, overnight cultures of the 10 candidate QC strains at 35°C on Sabouraud's dextrose (SAB) agar were used; these strains were Candida albicans ATCC 90028, ATCC 24433, and ATCC 76615; C. parapsilosis ATCC 90018 and ATCC 22019; C. tropicalis ATCC 750; Candida krusei ATCC 6258; Saccharomyces cerevisiae ATCC 9763; C. glabrata ATCC 90030; and Cryptococcus neoformans ATCC 90112 (15).
Twenty-five clinical isolates of filamentous fungi and C. parapsilosis ATCC 90018 as a control were used in study 3 to assess the applicability of the SAAS method for molds: these were 17 isolates of Aspergillus; 3 of Penicillium; and 1 each of Mucor, Fusarium, Trichophyton, Paecilomyces, and Histoplasma capsulatum (6).
Description of SAAS method. (i) Media.
Five-milliliter aliquots of semisolid heart infusion broth (Difco Laboratories, Detroit, Mich.) containing 0.5% agar (Bacto Agar; Difco Laboratories) at a pH of approximately 7.4 (without dextrose, buffer, or indicator) were sterilely prepared with and without an antifungal drug in 16- by 125-mm glass tubes. Three different lots of media were used in the studies, and the QC strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used to check the suitability of each batch of medium for testing. Prepared medium was stored at 4°C and used within 1 week. SAB agar was used for subculture of all organisms.
(ii) Antifungal agents.
Amphotericin B and fluconazole were obtained from the hospital pharmacy. Itraconazole was obtained as a powder from Janssen Research Foundation (Beerse, Belgium) and was dissolved in dimethyl sulfoxide according to the manufacturer's directions. Dilutions were made in sterile distilled water. For study 1, twofold dilutions of fluconazole (0.12 to 64 μg/ml) and amphotericin B (0.12 to 2.0 μg/ml) in the semisolid agar were prepared by adding appropriate amounts of frozen drug stock to molten 0.5% heart infusion agar at 45 to 50°C. For studies 2 and 3, concentrations of antifungal agents proposed for the screening test and for use in the hospital laboratory included several clinically relevant serum-achievable concentrations (fluconazole, 2, 8, and 40 μg/ml; amphotericin B, 0.5 and 2.0 μg/ml; and itraconazole, 0.25 and 1.0 μg/ml).
(iii) Inoculum preparation and inoculation.
A suspension that was just turbid (∼0.5 McFarland standard) by visual inspection was prepared by suspending the selected yeast or mold in sterile water. Mycelial growth of filamentous fungi was preferred; if heavy particles persisted after vortexing, they were allowed to settle, and the homogeneous suspension was used for inoculation. A standard platinum loopful (∼0.001 ml) of the inoculum suspension was inserted deep into each tube of medium containing a known concentration of drug, as well as a drug-free control, by a centered down-up motion to form a two dimensional inoculum. For filamentous fungi, sterile mineral oil (∼0.5 ml) was layered on the inoculated medium to inhibit sporulation. The tubes were tightly capped. A loopful of the inoculum suspension was streaked onto SAB agar to check the purity and viability. All cultures were incubated for 48 h at 35°C or until good growth was apparent in the drug-free control.
(iv) Determination of in vitro susceptibility.
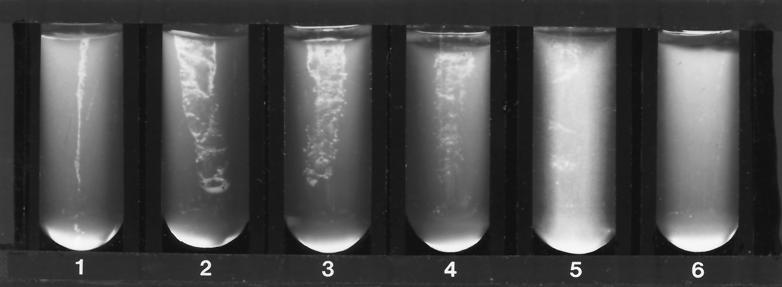
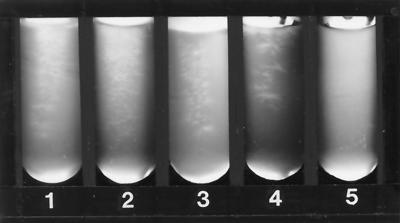
When, by visual inspection, good growth of the yeast or filamentous fungus in the drug-free medium was detected (within 48 h for yeasts and most filamentous fungi), the growth in all tubes was compared with that of the drug-free control in order to determine inhibition. For yeasts, growth was scored in the following manner: 4+, growth comparable to that of the drug-free control; 3+, growth approximately 75% that of the control; 2+, growth approximately 50% that of the control; 1+, growth 25% or less that of the control; and 0, no visible growth (Fig. 1). For filamentous fungi, the growth or inhibition of growth and the length of incubation were recorded (Fig. 2).
FIG. 1.
Growth of yeast (C. albicans) in screening test system after 48 h of incubation at 35°C. Tubes 1 and 2, side and facing views of 4+ growth (equal to that of the drug-free control); tube 3, 3+ (75% of growth control); tube 4, 2+ (50% of growth control); tube 5, 1+ (25% or less of growth control); tube 6, no growth.
FIG. 2.
Growth controls of various filamentous fungi after 48 h of incubation at 35°C (72 h for Trichophyton). Tube 1, Aspergillus fumigatus; tube 2, Mucor sp.; tube 3, Fusarium sp.; tube 4, Trichophyton sp.; tube 5, uninoculated medium.
The method for determining MIC results was modeled after the NCCLS M27-P document (now NCCLS M27-A) and established prior to our comparative study (9). The MIC results were determined by one or both of the authors independently within minutes of each other and recorded. For fluconazole, the lowest concentration at which the growth of the yeast in the semisolid medium was inhibited by 75% or more (1+) was determined to be the MIC of the drug. If the organism had a less clear endpoint at 1+ (75% or more growth reduction), the MIC was determined as the lowest concentration at which substantial growth reduction (2+; 50% or more) occurred (9). For amphotericin B, the MIC was determined to be the lowest concentration at which there was no visible growth of the organism. The few discrepant readings by the authors were reevaluated individually; if agreement was not reached, the more conservative (higher drug concentration) MIC determination was recorded.
RESULTS
Study 1.
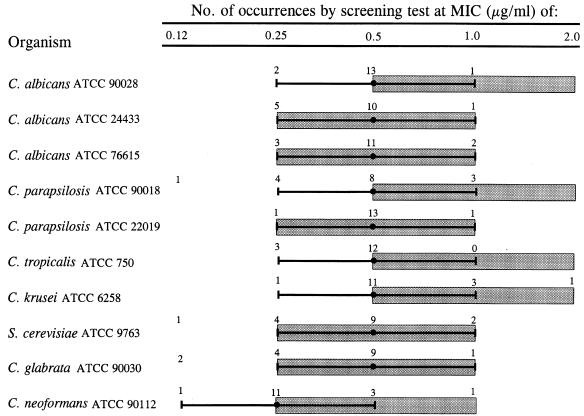
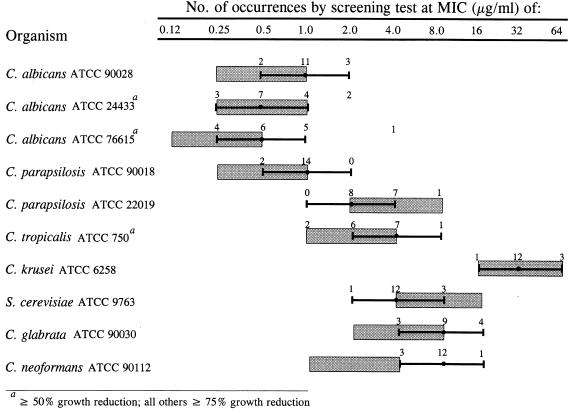
The reproducibility of the SAAS method was evaluated by determining the amphotericin B and fluconazole MICs obtained for the 10 ATCC strains in ≥15 independent experiments. Reproducibility is described by the percentage of MICs falling within 1 log2 dilution of the modal MIC. For amphotericin B, 9 of 10 strains showed ≥94% of MICs within 1 log2 dilution of the mode (Fig. 3). The C. glabrata strain ATCC 90030 had 87.5% of MICs encompass the mode ± 1 log2 dilution; 100% of the MICs fell within 4 log2 dilutions. For fluconazole, 7 of 10 strains showed ≥94% of MICs within 1 log2 dilution of the modal MIC at 75% growth reduction (Fig. 4). Three strains, C. albicans ATCC 24433 and ATCC 76615 and C. tropicalis ATCC 750, showed a less sharp endpoint at ≥75% growth reduction. However, at the ≥50% growth reduction endpoint, 87.5% of MICs fell within 1 log2 dilution of the modal MIC for C. albicans ATCC 24433 and C. tropicalis ATCC 750 and 94% of MICs fell within 1 log2 dilution of the modal MIC for C. albicans ATCC 76615. These data demonstrate that the SAAS method generated reproducible MICs for the 10 ATCC strains used in this study.
FIG. 3.
Amphotericin B MICs obtained by screening test method ( ) for the 10 ATCC yeast strains compared with the QC range of MICs obtained by the NCCLS M27-P broth macrodilution method (
) for the 10 ATCC yeast strains compared with the QC range of MICs obtained by the NCCLS M27-P broth macrodilution method ( ) in the multilaboratory study by Pfaller et al. (11).
) in the multilaboratory study by Pfaller et al. (11).
FIG. 4.
Fluconazole MICs obtained by screening test method ( ) for the 10 ATCC yeast strains compared with the QC range of MICs obtained by the NCCLS M27-P broth macrodilution method (
) for the 10 ATCC yeast strains compared with the QC range of MICs obtained by the NCCLS M27-P broth macrodilution method ( ) in the multilaboratory study by Pfaller et al. (11).
) in the multilaboratory study by Pfaller et al. (11).
Study 1 also assessed the accuracy of the SAAS method by comparing the range of MICs obtained by the SAAS method with that obtained by the six laboratories performing the NCCLS M27-P broth macrodilution method (11). For amphotericin B, the MICs of 9 of 10 strains showed 100% agreement within 1 log2 dilution of the proposed QC range; 94% of the MICs obtained for C. parapsilosis ATCC 90018 agreed within 1 log2 dilution of the proposed range (Fig. 3). For fluconazole, the MICs of 8 of 10 strains showed 100% agreement within 1 log2 dilution of the proposed QC range (Fig. 4). Two strains, C. albicans ATCC 76615 and C. neoformans ATCC 90112, had 94% of MICs agree within 1 log2 dilution of the proposed QC range. The fluconazole MICs obtained by the screening test for C. neoformans ATCC 90112 were consistently higher than those obtained by the NCCLS M27-P broth macrodilution method; however, all but one fell within 1 log2 dilution of the proposed QC range (1.0 to 4.0 μg/ml). For the NCCLS proposed QC strains, C. parapsilosis ATCC 22019 and C. krusei ATCC 6258, 100% of MICs for both amphotericin B and fluconazole fell within 1 log2 dilution of the NCCLS reference range. These data suggest that the MICs obtained for the 10 ATCC strains by the SAAS method are comparable to those for which a consensus was reached by six laboratories.
Study 2.
The utility of the drug concentrations chosen for rapid antifungal susceptibility screening by the SAAS method was evaluated for the 10 ATCC strains. Three clinically relevant and serum-achievable concentrations of fluconazole (2, 8, and 40 μg/ml) and two of amphotericin B (0.5 and 2.0 μg/ml) were studied. For fluconazole, five strains had modal MICs of ≤2 μg/ml and four had modal MICs of ≤8 μg/ml, indicating susceptibility. None of these nine strains demonstrated an MIC of >8 μg/ml in any of the replicated experiments. The majority of MICs for C. glabrata ATCC 90030 were ≥8 μg/ml, but none was ≥40 μg/ml. These results are suggestive of dose-dependent susceptibility for this strain, according to NCCLS guidelines for interpretive breakpoints for antifungal susceptibility testing (15). C. krusei ATCC 6258 had a fluconazole modal MIC of 32 μg/ml (Fig. 4). All of the replicates for this strain were inhibited at fluconazole concentrations of >8 and ≤40 μg/ml (data not shown). Like the results achieved by the six laboratories, none of these 10 strains demonstrated resistance to amphotericin B. Study 2 demonstrated that the drug concentrations chosen for use in the SAAS method as a rapid susceptibility screen predicted susceptibility and dose-dependent susceptibility of these 10 strains.
Study 3.
The application of the SAAS method to antifungal susceptibility testing of clinical isolates of filamentous fungi was evaluated. All the filamentous fungi studied grew at least as well in the drug-free heart infusion medium as on SAB agar, and the spread of hyphae from the inoculum site was easily detected by eye in indirect light. As expected, all but the yeast control grew well in the presence of 40 μg of fluconazole/ml. Table 1 summarizes the results obtained after 48 h when each of 25 clinical isolates was exposed two or more times to selected serum-achievable concentrations of amphotericin B and itraconazole. These preliminary data suggest the potential usefulness of this SAAS method as a screen for susceptibility or resistance of the filamentous fungi as well as yeasts.
TABLE 1.
Growth or inhibition of filamentous fungi in amphotericin B and itraconazole after 48 ha
| Organism (no.) | Amphotericin B
|
Itraconazole
|
||
|---|---|---|---|---|
| 0.5 μg/ml | 2.0 μg/ml | 0.25 μg/ml | 1.0 μg/ml | |
| Aspergillus fumigatus (8) | 0 | 0 | 0 | 0 |
| Aspergillus fumigatus (3) | + | 0 | 0 | 0 |
| Aspergillus flavus (1) | 0 | 0 | 0 | 0 |
| Aspergillus niger (5) | 0 | 0 | 0 | 0 |
| Fusarium sp. (1) | + | + | + | + |
| H. capsulatum (1) | 0 | 0 | 0 | 0 |
| Mucor sp. (1) | 0 | 0 | + | + |
| Paecilomyces sp. (1) | 0 | 0 | 0 | 0 |
| Penicillium spp. (2) | 0 | 0 | 0 | 0 |
| Penicillium sp. (1) | + | 0 | 0 | 0 |
| Trichophyton sp. (1) | 0 | 0 | 0 | 0 |
| C. parapsilosis ATCC 90018 (control) (1) | 0 | 0 | 0 | 0 |
Except for H. capsulatum (5 days) and Trichophyton (3 days). All strains except C. parapsilosis were resistant to fluconazole at 40 μg/ml. +, growth; 0, inhibition.
DISCUSSION
The SAAS method was developed for use in the hospital laboratory as a rapid screening test for yeasts and molds. The method was designed to predict antifungal susceptibility or resistance while awaiting formal MIC determination by the NCCLS reference method. In order to assess the reproducibility and accuracy of the SAAS method, we generated MICs for 10 ATCC candidate QC strains in ≥15 replicated experiments and compared the MICs obtained by the new method with those established for these 10 strains by the NCCLS M27-P broth macrodilution method in a multilaboratory study. We selected these 10 QC strains for study because the MIC reference ranges have been carefully validated by six independent laboratories (11). We determined the reproducibility of the method to be ≥94% for 9 of 10 strains with amphotericin and for 8 of 10 strains with fluconazole. In addition, the accuracy of the method was demonstrated by ≥98% of the MICs obtained with the SAAS method falling within the reference range proposed by the NCCLS. Concentrations of fluconazole chosen for the rapid-screening application of the SAAS method yielded consistent results for these 10 strains that would correlate with the NCCLS interpretive breakpoints of susceptibility (inhibition at ≤8 μg/ml), dose-dependent susceptibility (inhibition at >8 and ≤40 μg/ml), and resistance (inhibition at >40 μg/ml) (15). However, further study of this screening application of the SAAS method is necessary with many clinical isolates and comparing the results to results achieved by the NCCLS reference method for the same isolates.
Especially encouraging was the fact that highly reproducible susceptibility data were obtained with three different lots of chemically undefined heart infusion medium. Similarly, although the lack of available amphotericin B reagent grade powder required us to use pharmacy stock antifungal agents, susceptibility results varied little despite the fact that different lots of the antifungal agents were used. These observations support the potential use of the SAAS method in the hospital laboratory setting. Clinical yeast isolates (>50) studied so far have grown well in the heart infusion agar medium, and their susceptibility to varying concentrations of antifungal agents has been demonstrated by the SAAS method (S. Hadley and H. Provine, unpublished data). These results notwithstanding, the SAAS method, like the disk diffusion susceptibility test for bacteria, cannot be substituted for more rigorous MIC studies.
The proposed SAAS method shows promise for screening the antifungal susceptibility of filamentous fungi. The test can be set up as soon as the mold is isolated because only mycelial growth, the invasive form, is required for the inoculum (unlike the proposed NCCLS method, in which a calibrated conidial suspension is necessary) (5). No special expertise or expensive equipment is needed, because the procedure is simple and the same for all fungi. In addition, the preliminary susceptibility test and identification of the organism (which often requires several days) can be carried out simultaneously. The test may prove useful for fungi with varied susceptibilities to amphotericin B, such as Trichosporon, Fusarium, and Pseudallescheria spp. To our knowledge, this is the first example of an antifungal susceptibility screening method that can be used to test susceptibilities of both yeasts and filamentous fungi without special adaptations for specific organisms or antifungal agents.
The SAAS method is simple, accurate, and highly reproducible in our hands. It is inexpensive, may be performed on a single isolate, and can be completed before final identification of the organism. A major advantage of the new method is its potential for screening both pathogenic yeasts and molds. Validation of the SAAS method as a clinical antifungal susceptibility screen requires the correlation of results with the NCCLS reference method and patients' outcomes, confirmation of its accuracy for a greater variety of drugs and clinical isolates, and demonstration of interlaboratory reproducibility.
ACKNOWLEDGMENTS
We thank Kenneth Lawrence, Pharm D and the BIDMC Hospital Pharmacy, and Janssen Research Foundation, Beerse, Belgium, for providing drugs. We also thank George M. Eliopoulos for his thoughtful review of the manuscript.
This study was supported in part by a grant from Pfizer, Inc.
REFERENCES
- 1.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–1128. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sague C M, Jarvis W R National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 3.Berrouane Y F, Herwaldt L A, Pfaller M A. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J Clin Microbiol. 1999;37:531–537. doi: 10.1128/jcm.37.3.531-537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boschman C R, Bodnar U R, Tornatore M A, Obias A A, Noskin G A, Englund K, Postelnick M A, Suriano T, Peterson L R. Thirteen-year evolution of azole resistance in yeast isolates and prevalence of resistant strains carried by cancer patients at a large medical center. Antimicrob Agents Chemother. 1998;42:734–738. doi: 10.1128/aac.42.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridkin S K, Jarvis W R. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hitchens A P. Advantages of culture mediums containing small percentages of agar. J Infect Dis. 1921;29:390–407. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: Emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller M A, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G, Cooper C R, McGinnis M R. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J Clin Microbiol. 1995;33:1104–1107. doi: 10.1128/jcm.33.5.1104-1107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller M A, Jones R N, Doern G V, Sader H S, Hollis R J, Messer S A and for the Sentry Participant Group. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. J Clin Microbiol. 1998;36:1886–1889. doi: 10.1128/jcm.36.7.1886-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P The SCOPE Participant Group. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;30:121–129. doi: 10.1016/s0732-8893(97)00192-2. [DOI] [PubMed] [Google Scholar]
- 14.Rees J R, Pinner R W, Hajjeh R A, Brandt M E, Reingold A L. The epidemiological features of invasive mycotic infections in the San Francisco bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–1147. [PubMed] [Google Scholar]
- 15.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 16.Vartivarian S E, Anaissie E J, Bodey G P. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin Infect Dis. 1993;17(Suppl. 2):S487–S491. doi: 10.1093/clinids/17.supplement_2.s487. [DOI] [PubMed] [Google Scholar]
- 17.Wey S B, More M, Pfaller M A, Wollson R F, Wenzel R P. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]