Abstract
Background:
Cataract is a major cause of blindness worldwide. Researchers received much attention from the traditional systems such as Ayurveda for the solutions of cataract through antioxidant activities apart from the surgical extraction.
Aims:
A To study, the anti-cataract activity of Vasanjana (VK) prepared with Yashtimadhu (Glycyrrhyza glabra Linn) Kalka (paste) in Vasa (fat) of the domestic fowl (Gallus gallus) on glucose-induced cataract in ovine (sheep) lenses.
Materials and methods:
Artificial aqueous humor with 55 mM glucose was used to induce cataract in sheep eye lenses. Treatment was given with cow ghee (CG), plain fat, Vasanjana, and Vitamin E to the same media and lenses were incubated at the room temperature for 72 h. Biochemical parameters studied in the lens were total proteins, malondialdehyde (MDA), Na+ K+ ATPase activity and electrolytes (Na+ and K+). Photographic evaluation was also done.
Results:
The complete opacification induced by the glucose in ovine lens was observed in 72 h. Cataractous lenses showed significant increase in Na+, MDA level and significant decrease in Na+ K+ ATPase activity and total protein content. Lenses treated with Vasanjana showed non-significant increase of total protein content and decreased MDA level and prevented formation and progress of cataract by glucose, as evidenced by photographic evaluation. Glucose-induced biochemical changes were found to be reversed in statistically significant manner in CG and Vitamin E treated lenses.
Conclusion:
The anti-cataract activity of Vasanjana and CG may be because of the antioxidant and free radical scavenging activity. Further in vitro and in vivo studies in various experimental models are required to validate their anti-cataract activity.
Keywords: Antioxidant, cataract, glucose, Glycyrrhyza glabra, Vasanjana
Introduction
Cataract (the opacification of lens of the eye) is the leading cause of blindness worldwide and accounts for approximately 51% of all blindness. More than 65 million people are bilaterally blind because of cataract,[1] and 28,000 new cases are reported daily worldwide. Approximately 25% of the population over 65 years and about 50% over 80 years have serious loss of vision because of cataract. In the United Kingdom, half of the patients put on waiting lists for operation and die before getting surgery[2] often associated with old age. It is a major complication of diabetes mellitus. Higher glycosylated hemoglobin levels are significantly associated with increased risk of cataract.[3] Although many cataractogenic factors have been identified, the biochemical background of cataractogenesis is still unknown. Oxidative damage by the free radicals is also implicated in the pathology of cataractogenesis.[4] It is estimated that a delay in cataract formation of about 10 years would reduce the prevalence of visual disabling cataract by about 45%.[5] Thus, it is need to find a biochemical solutions or pharmacological intervention that will help to maintain the transparency of the lens and reverse the cataractogenous changes at least in immature stage. Although a number of agents have been tried for the prevention and therapy of cataract, none have proved useful.[6] Kador and Kinoshita have reported the important role of lens Na+ K+ ATPase activity in maintaining lens transparency. Its alteration is one of the major events leading to the cataract formation.[7] Flavonoids have been found to offer the protection from free radical damage in many experimental conditions.[8,9,10,11] The flavonoids those abundantly reported in Yashtimadhu (Glycyrhyzaglabra Linn.) may have potency to protect the cataractogenous changes in animals as well as in humans.[12,13]
Many of formulations have been mentioned in the Indian System of Medicine for the treatment of cataract. Some of them are used frequently by local healers as well as Ayurvedic practitioners. Cow ghee (CG) is described as the best agent for protection from eye diseases and for treatment of cataract as delineated in classical texts.[14] It is used as base for many formulations e.g., Triphaladi Ghrita Yoga, Mahatriphaladi Ghrita, etc.,[15] in local and systemic conditions. The Vasanjana is described in Sushruta Samhita for the treatment of Kacha/Timira, the condition that resembles to the cataract.[16] A pilot experimental study has been done on Vasanjana by Manjusha et. al. (1995) and the outcomes were exuberant.[17]
Therefore, this study was conducted to find the efficacy of an indigenous preparation Vasanjana prepared by Kalka (paste) of Yashtimadhu in fat of fowl for the prevention of experimental cataract induced by glucose.
Materials and methods
Test drug preparation
The test drug Vasanjana was prepared with paste of Yashtimadhu in the fat of domestic fowl (Gallus gallus).[16] The coarse powder of Yashtimadhu was procured from Alva pharmacy, Karnataka, and authenticated in Pharmacognosy Laboratory attached to the institute. Raw fat obtained from mature fowls, which were purchased from local poultry farm and dissected in Pharmacology Laboratory, of the institute for their fat. Yashtimadhu powder was mixed and triturated in to fat separately for 3 consecutive days.[18] CG was obtained from the local area.
Chemicals
D-Glucose and Vitamin E (a-tocopherol) were obtained from MERK, India. Thiobarbituric acid and other chemicals were obtained from Sisco research laboratories Pvt., Ltd., Mumbai, Maharashtra, India.
Dose selection
The standard clinical dose is described as 1 drop.[19] A micro capillary tube dipped into the drug mixture and drop instilled from the capillary was considered one drop, which weighed an average of 30.0 mg at the room temperature (25°C ± 2°C). Dose of CG was fixed by same procedure and was average of 24.6 mg, whereas plain Vasa (fat) of an average 26.0 mg at the room temperature. Vitamin E was used in 15 μg/ml (as a-tocopherol), the dose based on previous study, reported optimum anti-oxidative effect in lens culture.[20]
Animals and institutional animal ethics committee
In this study, sheep (Ovis aries) lenses were used as they were easily available. The study was approved by the Institutional Animal Ethics Committee (IAEC-05/09-10/Proj-02).
Lens culture
Fresh sheep eyeballs were procured from local abattoir immediately after killing and transported to the laboratory at 0°C–4°C in a box-containing crushed ice. The lenses were removed by extra-capsular extraction through the posterior approach and incubated in artificial aqueous humor (NaCl 140 mM, KCl 5 mM, MgCl2 2 mM, NaHCO3 0.5 mM, NaH (PO4)2 0.5 mM, CaCl2 0.4 mM and Glucose 5.5 mM), pH 7.8 at the room temperature for 72 h.[21] Penicillin 32 mg% and streptomycin 250 mg% were added to the culture media to prevent bacterial contamination. Glucose in a concentration of 55 mM was used to induce cataract.[20,21]
Study drugs and groups
A total of 36 lenses were divided into six groups (n = 06 in each group). Normal control group (NC) contains lenses with normal glucose 5.5 mM. Remaining all five groups contains lenses with high concentrated glucose 55 mM. Glucose control (GC) group remain untreated for 72 h and was referred as GC. CG group was treated by cow Ghee, VC with plain fat, VK by Vasanjana (paste of G. glabra in fat), and Vitamin E with α-tocopherol (15 μg/ml).
Homogenate preparation
After 72 h of incubation, homogenate of lenses was prepared in normal saline (10 ml) and cold distilled water (3 ml) separately. For the estimation of total proteins, malondialdehyde (MDA) level and Na+ K+ ATPase activities, homogenate was prepared in normal saline and for electrolytes (Na+ and K+) estimation, it was prepared in ice cold millipore water. The homogenates were centrifuged at 10,000 g at 4°C for 1 h and the supernatant was used for the estimation of biochemical parameters.
Biochemical estimation
Electrolytes (Na+ and K+) estimation was done by flame photometry.[22] Na+ K+ ATPase activity was assessed by the method of Bonting[23] and total protein by Lowry method.[24] The degree of oxidative stress was assessed by measuring the MDA levels by the method adopted by Ohkawa et al.[25]
Photographic evaluation
Lenses were placed on a wired net with posterior surface touching the net, and the pattern of net (number of squares visible through the lens) was observed through the lens as a measure of lens opacity.
Statistical analysis
All the results were expressed as mean ± standard error of the mean. The groups were compared using the one-way analysis of variance by post-hoc Dunnett’s test in Sigma-State version 3 Systat Software, California, US (2005) with glucose 55 mM group as control. P < 0.05 was considered statistically significant.
Results
Opacification starts at the periphery, on the posterior surface of the lens incubated in glucose 55 mM after 10 h. It was progressively increased to the center and completed by 72 h.
Biochemical changes
Glucose 55 mM treated lenses showed highly significant depletion of water soluble total proteins concentrations as well as Na+ K+ ATPase activity in the lens homogenate (P < 0.001), while MDA levels were found significantly high (P < 0.01) compared with normal lenses [Table 1].
Table 1.
Effect of Vasanjana, cow ghee and plain fat in total protein contents, malondialdehyde and Na+K + ATPase activity in cataractogenesis induced by high concentrated glucose
| Groups | Study groups | Total protein (mg/g wet tissue) | MDA (µ moles MDA released/g wet tissue) | Na+K+ ATPase activity (µ moles of phosphorus liberated/min/mg wet tissue) |
|---|---|---|---|---|
| NC | Glucose 5.5 mM | 14.533±02.051 | 08.760±08.338 | 1.802±0.345 |
| GC | Glucose 55 mM | 5.907±00.499ФФ | 37.116±03.787ФФ | 0.878±0.096Ф |
| CG | Glucose 55 mM+cow ghee 1 ng/ml | 9.515±01.723 | 16.069±03.301** | 1.544±00.149** |
| VC | Glucose 55 mM+plain fat 5 mg/ml | 7.145±00.990 | 38.276±06.269ΨΨ | 1.393±0.170 |
| VK | Glucose 55 mM+Vasaanjana 5 mg/ml | 8.268±01.107 | 32.513±14.407 | 1.706±0.597 |
| Vitamin E | Glucose 55 mM+Vitamin E 15 μg/ml | 6.585±00.464 | 27.803±01.455* | 0.993±0.022ΨΨ |
ФP<0.05, ФФP<0.01 (unpaired t-test-in comparison to 5.5 mM glucose or Normal control group), *P<0.05, **P<0.01 (unpaired t-test-in comparison to 55 mM glucose control group), ΨΨP<0.01 (unpaired t-test-in comparison to plain cow ghee treated group). NC: Normal control, GC: Glucose control, CG: Cow ghee treated, VC: Vehicle control treated with plain fat, VK: Vasaanjana prepared by Yashtimadhu Kalka, Vitamin E: Vitamin E (tocoferol) treated, MDA: Malondialdehyde
All treated groups viz.; CG, VC, VK and Vitamin E had higher concentrations of total lens proteins, compared with glucose 55 mM group. However, the difference was not found to be statistically significant between in any group.
Lenses treated with Vasanjana, Cow Ghee and Vitamin E had reduced MDA content, compared with glucose group. However, the statistically significant difference was observed only in Cow Ghee and Vitamin E treated groups. Unexpectedly, VC treated group showed aggressive increase in MDA level in comparison to both NC and GC [Table 1].
Na+ K+ ATPase activity was observed significantly low in GC group. The changes were found reversed by all treated groups except Vitamin E treatment. It was significantly higher (P < 0.01) with only Cow Ghee treatment (CG group) as compared to GC group though reversal was not found significant with Vasaanajana treated group. Vitamin E was unable to maintain the activity. [Table 1].
Glucose 55 mM treated lenses also showed significantly higher Na+ and lower K+ levels compared with control group having normal lenses [Table 2]. Cow Ghee and Vitamin E treated lenses showed significantly high K+, compared with glucose 55 mM alone group.[Table 2] CG, VK and Vitamin E treated groups showed a lower Na+ compared with glucose 55 mM group, but the difference was found to be statistically significant only with Vitamin E. However, VC treated group was again found unable to maintain electrolytes ratio and showed increase in the level of Na+, against GC and significantly less along with VK compared to CG [Table 2].
Table 2.
Effect of Vasaanjana, Cow ghee and plain fat in Na+ and K+ percentage in cataractogenesis induced by high concentrated glucose
| Groups | Dose | Na+(mEq/L) | K+(mEq/L) |
|---|---|---|---|
| NC | Glucose 5.5 mM | 0.028±00.007 | 0.013±00.009 |
| GC | Glucose 55 mM | 0.042±00.008 | 0.004±00.001 |
| CG | Glucose 55 mM+cow ghee 1 ng/ml | 0.035±00.008 | 0.021±00.006* |
| VC | Glucose 55 mM+plain fat 5 mg/ml | 0.050±00.011 | 0.004±00.001Ψ |
| VK | Glucose 55 mM+Vasaanjana 5 mg/ml | 0.033±00.010 | 0.006±00.001Ψ |
| Vitamin E | Glucose 55 mM+Vitamin E 15 μg/ml | 0.020±00.001* | 0.029±00.004*** |
*P<0.05, ***P<0.001 (unpaired t-test-in comparison to 55 mM glucose control group), ΨP<0.05 (unpaired t-test-in comparison to plain cow ghee treated group). NC: Normal control, GC: Glucose control, CG: Cow ghee treated, VC: Vehicle control treated with plain fat, VK: Vasaanjana prepared by Yashtimadhu Kalka, Vitamin E: Vitamin E (tocoferol) treated
Photographic evaluation
After 72 h of incubation in glucose 55 mM, lens becomes completely opaque [Figure 1, group GC] as against lenses incubated in 5.5 mM glucose [Figure 2, group NC]. Incubation of lenses with Cow ghee [Figure 3, group CG], Vasaanjana [Figure 4, group VK] and tocopherol [Figure 5, group Vitamin E] at normal concentrations seems to retard the progression of lens opacification, compared with the control group (glucose 55 mM). This is because more number of squares is clearly visible in all treated groups than in GC (glucose 55 mM). Vehicle i.e., plain fat [Figure 6, group VC] group showed haziness less than GC group, but the squares were not clearly visible.
Figure 1.
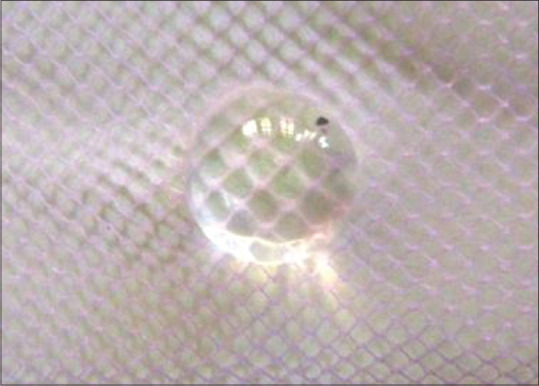
From Glucose control group – lens treated with high concentrated glucose
Figure 2.
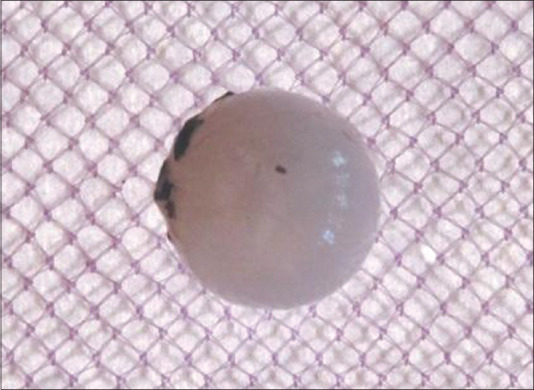
From Normal control group – normal lens, with no treatment
Figure 3.

From Cow ghee group – lens treated with high concentrated glucose and cow ghee
Figure 4.
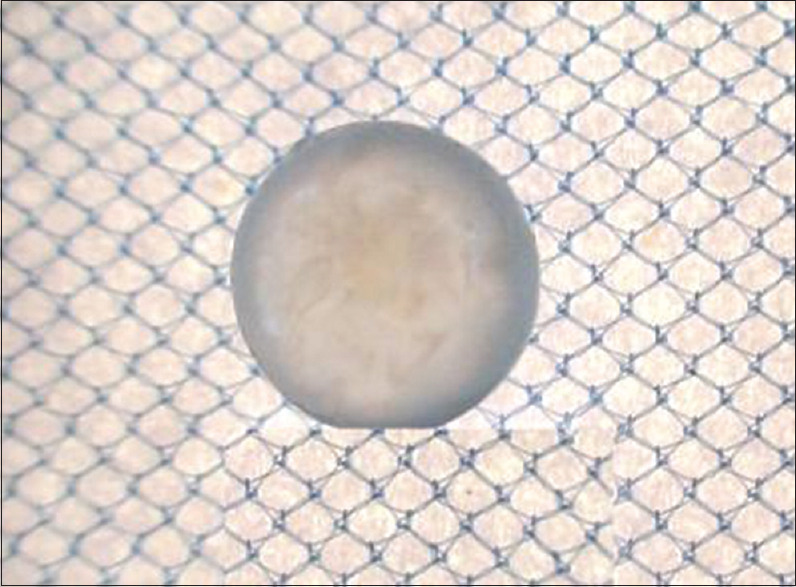
From Vasaanjana group (Vk) – lens treated with high concentrated glucose and Vasaanjana prepared by Yashtimadhu Kalka
Figure 5.
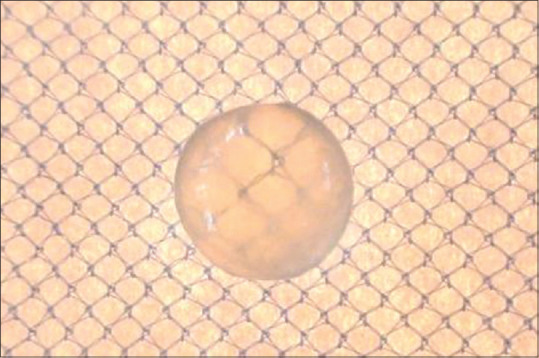
From Vitamin E group (Vitamin E) – lens treated with high concentrated glucose and Vitamin E (tocopherol)
Figure 6.
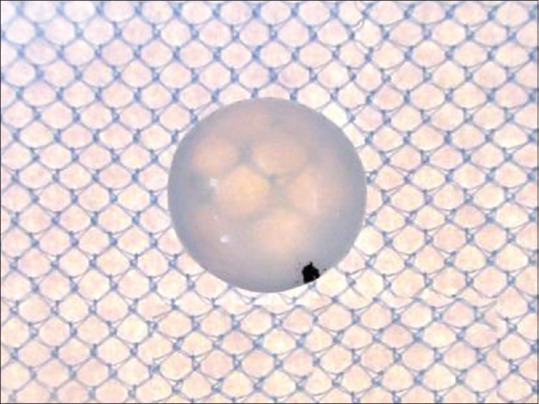
From plain fat group (VC) – lens treated with high concentrated glucose and plain fat
Discussion
Overload of glucose in the lens is metabolized through polyol pathway. During this process, sugar molecules are converted into polyols (sugar alcohols i.e., with more than two hydroxyl groups) with the help of specific enzyme, namely Aldose reductase. Accumulation of polyols causes over hydration and oxidative stress and lead to cataractogenesis.[26] The present study comprised the estimation of total proteins, MDA, Na + K+ ATPase activity and electrolytes (Na+ and K+) to evaluate the changes and oxidative stress during cataractogenesis and effect of treatment on it.
High glucose (55 mM) concentration leads to a considerable drop in total protein contents and Na + K+ ATPase activity, with progression of opacity.[26,27] There is a loss of membrane permeability and leakage of free amino acids, glutathione, myoinositol, and other small molecular weight substances.[28] CG, VC, VK and Vitamin E have shown to arrest the loss of water-soluble protein contents, during the process of cataractogenesis initiated by high glucose concentration. However, none of the above changes were found to be significant compared to the GC group.
High concentration glucose-induced oxidative stress due to the formation of superoxide (O2•–) radicals and H2O and evolved in the form of cataract. This incident triggers to induce antioxidant enzymes, suggesting oxidative stress in the lens cells.[26,29] Free radicals during the oxidative stress cause the peroxidation of poly ionic lipids. Such peroxidation products have also been well correlated to the lens damage.[30] In this study, MDA levels were observed higher in high glucose (55 mM) group, compared with NC group. The MDA levels were observed reduced in the all treated groups but found to be significant only in CG treated group, whereas plain fat-treated group observed high MDA levels in comparison to GC.
The accumulation of high concentrations of polyols in the lens leads to excessive hydration, gain of sodium, and loss of potassium ions due to an increase in intracellular ionic strength.[28] The resulting hyper osmotic stress-associated oxidative insult is postulated to be the primary cause for the development of cataract.[31] This study shows concurrence with this finding. Na+ K + ATPase is very important enzyme in maintaining the ionic equilibrium in the lens, and its impairment causes marked increase of Na+ and loss of K+ with hydration and swelling of the lens fibers leading to cataractogenesis.[32] This alteration in the Na+ K+ ratio alters the protein content of the lens, leading to a decrease in water-soluble proteins content and increase in insoluble proteins. This causes lens opacification.[33] The present study showed higher Na+ K+ ATPase activity, total proteins and K+ ions whereas lower concentrations of Na+ ions with Cow Ghee, Vasanjana and Vitamin E treated groups. Therefore, these four mechanisms seem to prevent the alteration of Na+ and K+ imbalance, which may be due to a direct effect on the lens membrane by Na+ K+ ATPase or indirect effect through their free radical scavenging activity. Incubation in the presence of high glucose (55 mM) concentration simulates a state of clinical diabetes where flavonoids are commonly used in these patients to treat associated ocular complications. Stefek reviewed in detail the important role of various flavonoids in arresting cataract progression both in in vivo and in vitro studies.[34] Cazarolli et al.,[35] reviewed the mode of action of flavonoids including cellular and molecular mechanism. In their review, the authors thoroughly discussed about the various effects of the drug candidates in regulating diabetic syndromes. It has been demonstrated that flavonoid compounds act either through their capacity to prevent glucose absorption or to improve glucose tolerance.[36] The role of Vasanjana and Vitamin E exhibited in this in vitro model suggest adequate preventive role in the progression of diabetic cataracts which is also manifested by the photographic results of the lenses [Figures 3–5].
The concentrations of Vasanjana used in this study ranged about 6 mg/ml. However, higher concentrations and increased frequency of application may show better anti-cataract activity, and hence, further evaluation with higher concentrations and increased frequency of application is required.
Conclusion
Vasanjana (prepared by paste of Yashtimadhu) had in vitro preventive anti-cataract activity in the experimental cataract model induced by high glucose. Further in vitro and in vivo studies to elucidate the exact mechanism of the action of the test drug Vasanjana in the prevention of cataractogenesis are needed. This study may be followed to identify the mechanism of cataract progression and its prevention and for in approaching further clinical evaluation of Vasanjana in humans.
Financial support and sponsorship
The project was financially supported by IPGT and RA, Jamnagar, Gujarat.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The work was supported by Institute for Post Graduate Teaching and Research in Ayurveda, Gujarat Ayurved University, Jamnagar as the Departmental Research Project “Experimental study on anti-cataract effect of Vasanjana – an indigenous preparation”.
References
- 1.World Health Organization (WHO). Blindness and Vision Impairment: Key facts. World Health Organization (WHO); 8 October 2020. [Last accessed on 2020 Nov 06]. Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment .
- 2.Minassian DC, Reidy A, Desai P, Farrow S, Vafidis G, Minassian A. The deficit in cataract surgery in England and Wales and the escalating problem of visual impairment: Epidemiological modeling of the population dynamics of cataract. Bri J Ophthalmol. 2000;84:04–8. doi: 10.1136/bjo.84.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein BE, Klein R, Lee KE. Cardiovascular disease, selected cardiovascular disease risk factors, and age-related cataracts: the Beaver Dam Eye Study. Am J Ophthalmol. 1997;123:338–46. doi: 10.1016/s0002-9394(14)70129-1. [DOI] [PubMed] [Google Scholar]
- 4.Cheng HM, Fagerhoilm P, Chylack LT. Response of the lens to oxidative-osmotic stress. Exp Eye Res. 1983;37:11–21. doi: 10.1016/0014-4835(83)90145-8. [PMID: 6873202] [DOI] [PubMed] [Google Scholar]
- 5.Kupfer C. Bowman lecture. The conquest of cataract: A global challenge. Trans Ophthalmol Soc U K. 1985;104:1–10. [PMID: 3855332] [PubMed] [Google Scholar]
- 6.Gupta SK, Joshi S, Velpandian T, Awor L, Prakash J. An update on pharmacological prospectives for prevention and development of cataract. Indian J Pharmacol. 1997;29:03–10. [Google Scholar]
- 7.Kador PF, Kinoshita JH. Diabetic and galactosaemic cataracts. Ciba Found Symp. 1984;106:110–31. doi: 10.1002/9780470720875.ch7. [DOI] [PubMed] [Google Scholar]
- 8.Unakar NJ, Tsui JY. Inhibition of galactose induced alteration in ocular lens with sorbinil. Exp Eye Res. 1983;36:685–94. doi: 10.1016/0014-4835(83)90106-9. [DOI] [PubMed] [Google Scholar]
- 9.Varma SD, Mizuno A, Kinoshita JH. Diabetic cataracts and flavonoids. Science. 1977;195:205–6. doi: 10.1126/science.401544. [DOI] [PubMed] [Google Scholar]
- 10.Lim SS, Jung SH, Ji J, Shin KH, Keum SR. Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. J Pharm Pharmacol. 2001;53:653–68. doi: 10.1211/0022357011775983. [DOI] [PubMed] [Google Scholar]
- 11.Lija Y, Biju PG, Reeni A, Cibin TR, Sahasranamam V, Abraham A. Modulation of selenite cataract by the flavonoid fraction of Emilia sonchifolia in experimental animal models. Phytother Res. 2006;20:1091–5. doi: 10.1002/ptr.2005. [DOI] [PubMed] [Google Scholar]
- 12.Alqahtani A, Hamid K, Kam A, Wong KH, Abdelhak Z, Razmovski-Naumovski V, et al. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 2013;20:908–31. [PubMed] [Google Scholar]
- 13.Bahmani M, Rafieian-Kopaei M, Jeloudari M, Eftekhari Z, Delfan B, Zargaran A, et al. A review of the health effects and uses of plat licorice (Glycyrrhyza glabra L) in Iran. Asian Pac J Trop Dis. 2014;4(Suppl 2):S847–9. [Google Scholar]
- 14.Acharya YT, Ram AN, editors. 8th ed. Ch. 17, Ver. 48. Varanasi: Chaukhamba Surbharati Prakashana; 2017. Sushruta Samhita of Sushruta, Uttar Tantra; p. 630. [Google Scholar]
- 15.Mishra SN, editor. Ch. 64., Ver. 249-256. Varanasi: Chaukhamba Surbharati Prakashan; 2007. Bhaishjya Ratnavali of Govindadas Sen; p. 127. [Google Scholar]
- 16.Acharya YT, Ram AN, editors. 8th ed. Ch. 17, Ver. 35. Varanasi: Chaukhamba Surbharati Prakashan; 2017. Sushruta Samhita of Sushruta, Uttar Tantra; p. 28. [Google Scholar]
- 17.Manjusha K, Kulwant S, Ravishankar B. Jamnagar: IPGT & RA; 1995. Clinical and Experimental studies on Linganasha – Cataract with Tutthanjana and Vasaanjana, M D thesis, Summary and Conclusion. Department of Shalakya; pp. 166–8. [Google Scholar]
- 18.Manjusha R, Ravishankar B, Ashok BK, Gupta Varun B. Gujarat Ayurved University, Jamnagar: IPGT & RA; 2013. Experimental study on anti-cataract effect of Vasaanjana – An indigenous preparation (Project-Department of Shalakya). Summary and Conclusion; pp. 98–100. [Google Scholar]
- 19.Rajasekaran A, Kumaran KS, Preetha JP, Karthika K. A comparative review on conventional and advanced ocular drug delivery formulations. Int J Pharm Tech Res. 2010;2:668–74. [Google Scholar]
- 20.Shetty LJ, Harikiran H, Sharma A. In vitro prophylactic cataract prevention study on glucose induced cataract by quercetin and alpha-tocopherol. IJPSR. 2010;1:41–5. [Google Scholar]
- 21.Chandorkar AG, Albal MV, Bulakh PM, Muley MP. Lens organ culture. Indian J Opthalmol. 1981;29:151–2. [PubMed] [Google Scholar]
- 22.Raghuramulu N, Nair KM, Kalyanasundaram S. Hyderabad, India: National Institute of Nutrition (NIN); 2003. A Manual of Laboratory Techniques; pp. 187–8. [Google Scholar]
- 23.Bonting SL. London: Wiley Inter Science; 1970. Presence of Enzyme System in Mammalian Tissues, Membrane and Ion Transport; pp. 257–63. [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [PMID: 36810] [DOI] [PubMed] [Google Scholar]
- 26.Langade DG, Rao G, Grime RC, Patki PS, Bulakh PM. In vitro prevention by ACE inhibitors of cataract induced by glucose. Indian J Pharmacol. 2006;38:107–10. [Google Scholar]
- 27.Unakar NJ, Tsui JY. Measurement of Na+ K+ ATPase activity. Invest Ophthalmol Vis Sci. 1980;19:378–85. [PMID: 6247293] [PubMed] [Google Scholar]
- 28.Kinoshita JH. A thirty year journey in the polyol pathway. Exp Eye Res. 1990;50:567–73. doi: 10.1016/0014-4835(90)90096-d. [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45:471–7. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- 30.Murata T, Nisbida T, Eto S, Mukar N. Lipid peroxidation in diabetic rat retina. Metabol Pediatr Ophthalmol. 1981;5:83. [PMID: 6247293] [PubMed] [Google Scholar]
- 31.Williamson J, Kilo C, Tilton RG. In: Hyperglycemia, Diabetes and Vascular Disease. Ruderman N, Williamson JR, Brownlee M, editors. New York: Oxford University Press; 1992. pp. 691–714. [Google Scholar]
- 32.Chylack LT, Jr, Kinoshita JH. A biochemical evaluation of a cataract induced in a high-glucose medium. Invest Ophthalmol. 1969;8:401–12. [PMID: 5796271] [PubMed] [Google Scholar]
- 33.Shinohara T, Piatigorsky J. Regulation of protein synthesis, intracellular electrolytes and cataract formation in vitro. Nature. 1977;270:406–11. doi: 10.1038/270406a0. [DOI] [PubMed] [Google Scholar]
- 34.Stefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip Toxicol. 2011;4:69–77. doi: 10.2478/v10102-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, et al. Flavonoids: Prospective drug candidates. Mini Rev Med Chem. 2008;8:1429–40. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 36.Brahmachari G. Bio-Flavonoids with Promising Anti-Diabetic Potentials: A Critical Survey. Book: Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Research Signpost. (1st edition) 2011;(Ch. 6):187–212. [Google Scholar]


