Abstract
Background:
The evidence on the efficacy and safety of Ayurveda interventions as an add-on to the standard conventional care for coronavirus disease-2019 (COVID-19) is limited.
Aim and objective:
This study was planned to explore the potential of AYUSH-64 as an add-on to conventional care in improving the clinical recovery and negative reverse transcription–polymerase chain reaction (RT-PCR) conversion in asymptomatic and mild COVID-19 cases.
Materials and methods:
An open-label randomized controlled study was conducted at Government Medical College, Nagpur, Maharashtra, India, with a sample size of 60 participants. In this study, asymptomatic or mild COVID-19 patients were randomized and allocated into intervention and control groups (CG) in a 1:1 ratio. AYUSH-64 two capsules (500 mg each) were administered thrice daily, after food with water for 30 days along with standard care in the intervention group (IG), while the CG received only standard care. The primary outcome was the proportion of participants who turned RT-PCR negative for COVID-19 at 7th, 15th, 22nd and 30th days. Secondary outcomes were the proportion of participants who attained clinical recovery at 7th, 15th, 22nd and 30th days, change in laboratory parameters on the 30th day and incidence of adverse drug reactions/adverse events. The data were compared within group using paired sample t-test/Wilcoxon signed-rank test and between group using independent sample t-test/Mann–Whitney test.
Results:
Statistically significant difference was not observed in the proportion of participants who turned RT-PCR negative during each of the follow-ups (P = 0.134) and both groups demonstrated comparable efficacy. The clinical recovery in terms of complete relief in symptoms in the symptomatic participants was 60% and 37% on day 15 (P = 0.098) and 100% and 85.2% on day 30 (P = 0.112) in the intervention and CG, respectively. The improvement in the inflammatory markers such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and D-dimer was statistically significant (P < 0.05) in the IG, whereas in the CG, it was statistically significant for D-dimer only. None of the participants developed any complications nor were any significant ADR/AE observed in the groups.
Conclusions:
In patients with asymptomatic and mild COVID-19, AYUSH-64, as add-on to standard conventional care, contributed to improved clinical recovery and demonstrated potential in reducing the levels of pro-inflammatory markers such as IL-6 and TNF-α. Further, both the groups demonstrated comparable efficacy regarding negative RT-PCR for COVID-19.
Keywords: Ayurveda, AYUSH-64, coronavirus disease-2019, pandemic, SARS-CoV-2
Introduction
Coronavirus disease-2019 (COVID-19) has affected more than 181 million people around the world and around 3.9 million deaths have been reported globally as of 30th June 2021.[1] The physical, psychological, social and economic consequences of the pandemic have been very severe and have affected the world in the most unprecedented manner. Potential therapeutic and prophylactic agents should ideally have antiviral properties against SARS-CoV-2, immunomodulatory properties, and therapeutic adjuvant activity with drugs used while being safe and tolerable.[2] Although several therapeutic interventions such as hydroxychloroquine, corticosteroids, and antivirals have been suggested and tried, the outcomes have not been much promising. The current medical strategy for the prophylaxis and management of COVID-19 is broadly based on the repurposing and repositioning of existing medications and deploying them with symptomatic support. Drugs such as antiviral, antimalarial, anti-inflammatory, and monoclonal antibodies have undergone trials, based on published empirical evidence. One of the published systematic reviews reported that corticosteroids are most frequently used to treat patients with COVID-19, followed by lopinavir/ritonavir and oseltamivir.[3]
In view of the unchecked morbidity and mortality rates, the scientific community needs to also consider pluralistic traditional medicine systems used globally. Ayurveda, the Indian traditional medicine system, has a lot to offer in this ongoing COVID-19 pandemic. It may provide a much-needed effective and safe alternative or bridge the existing gaps in conventional medicine, leading to reduce disease burden.[4] Integrating Ayurveda interventions with conventional medicine could offer a novel, safe, and cost-effective strategy to effectively manage the COVID-19 pandemic. Ayurveda interventions can be repurposed for the prophylaxis and treatment of COVID-19 since their traditional use has established safety, and experimental studies have demonstrated their immunomodulating, anti-inflammatory, antioxidant properties, and antiviral activity.[5,6,7,8,9,10,11,12] In a recent development, the Government of India has also incorporated the Ayurveda interventions in the national COVID management protocol.[13]
The trial drug, AYUSH-64, was repurposed based on the report of a clinical study in which AYUSH-64 was found effective in influenza-like illness and molecular docking study which revealed that 35 phytoconstituents isolated from AYUSH-64 demonstrated antiviral activity against SARS-CoV-2.[14,15] AYUSH-64 is a polyherbal formulation developed by Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH, Government of India, through extensive pharmacological, toxicological, and clinical studies. Its efficacy and safety have already been proven in infective febrile conditions such as malaria, microfilaremia, chikungunya, and influenza as per the published clinical studies.[14,16,17,18,19] Furthermore, previous experimental studies have suggested that the constituents of AYUSH-64 might exert immunomodulating, anti-inflammatory, and antioxidant activities.[20,21,22,23,24,25] These effects could halt the intense inflammatory responses in COVID-19 that cause progression to significant morbidity.
To date, evidence on the efficacy and safety of including Ayurveda interventions as an add-on to standard care for COVID-19 is limited.[26,27,28,29] Therefore, the present open-label, randomized controlled study was planned to test the hypothesis that adding the Ayurveda intervention, AYUSH-64 to standard care was superior to standard care alone in improving the clinical status of patients with asymptomatic and mild COVID-19.
Materials and Methods
This study was an open-label, randomized controlled trial. The study was conducted at Government Medical College, Nagpur, Maharashtra, India, which was a designated COVID-19 referral center notified by the State Government of Maharashtra. The study was conducted from June 10, 2020, to November 2, 2020. Participants aged 18–60 years with positive reverse transcription-polymerase chain reaction (RT-PCR) assay for COVID-19, and categorized under stage I-mild (early infection) as per the Maharashtra Health Services treatment protocol for confirmed COVID-19 hospitalized patients were included in the study. As per the protocol, Stage I include asymptomatic and mild symptomatic patients with or without co-morbidities.[30]
Participants with severe COVID-19 or acute respiratory distress syndrome or severe disease as per 8-point ordinal score,[31] i.e., hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation, with chronic kidney disease, with alanine transaminase or aspartate transaminase more than two times the upper limit of normal, pregnant or lactating women, and any other clinical condition, which would jeopardize the outcome of the study, were excluded from the study.
Detailed information about the study was provided to the eligible patients and written informed consent in the participant’s language was obtained before recruiting them in the study.
In the initial phase of the study, it was planned in such a way that the enrolled participants would be kept under observation in the in-patient department of the Government Medical College, Nagpur, till discharge. However, as per the revised guidelines of the Ministry of Health and Family Welfare (MoHFW), Government of India, dated July 2, 2020, to manage asymptomatic and mild COVID-19 patients in home isolation, patients enrolled from that date were kept in home isolation and followed up on 7th, 15th, 22nd, and 30th days.[32] All the RT-PCR diagnosed eligible patients were enrolled very next day of their test. The study participants who remain RT-PCR positive at the end of the study period were discharged as per the existing MoHFW, Government of India guidelines.[33]
Study intervention
AYUSH-64 two capsules (500 mg each) were administered thrice daily after food with water to the participants for 30 days along with standard care in the intervention group (IG) and the control group (CG) received only standard conventional care. The standard care provided was as per the national as well as the state government guidelines for COVID-19 management.[30] The standard care included paracetamol, Vitamin C, zinc, hydroxychloroquine, doxycycline, azithromycin, amoxycillin with potassium clavulanate and favipiravir as per the clinical condition of the patient along with the infection prevention and control practices.
AYUSH-64 is a patent polyherbal formulation developed by the CCRAS, Ministry of AYUSH, Government of India. AYUSH-64 consists of Saptaparna (Alstonia scholaris R. Br.), Katuki (Picrorhiza kurroa Royle ex. Benth), Kiratatikta (Swertia chirata Pexbex. Karst), and Kuberaksha (Caesalpinia crista L.). The details and quality standards of the trial drug are given in Tables 1 and 2.
Table 1.
Composition of AYUSH-64 (each 500 mg capsule)
| Name of the ingredient | Botanical name | Part used | Quantity (mg) |
|---|---|---|---|
| Saptaparna (aqueous extract) | Alstonia scholaris | Bark | 100 |
| Kutaki (aqueous extract) | Picrorhiza kurroa | Rhizome | 100 |
| Kiratatikta (aqueous extract) | Swertia chirata | Whole plant | 100 |
| Latakaranja (seed powder) | Caesalpinia crista | Seed | 200 |
Table 2.
Specifications for quality control analysis of AYUSH-64 and its ingredients
| Test parameters | Ingredients (%) | Formulation (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Saptaparna (aqueous extract) | Kutaki (aqueous extract) | Kiratatikta (aqueous extract) | Latakaranja (seed powder) | ||
| Loss on drying | NMT: 9 | NMT: 6 | NMT: 8 | - | NMT: 6 |
| pH (1% Sol) | 4.5 6.5 | 4.0 7.0 | 5.0 7.0 | - | 4.0 6.5 |
| Total Ash | NMT: 12 | NMT: 5 | NMT: 15 | NMT: 5 | NMT: 25.0 |
| Acid insoluble ash | NMT: 2 | NMT: 1 | NMT: 2 | NMT: 1 | NMT: 8.0 |
| Alcohol soluble extractive | NLT: 3 | NLT: 3 | NLT: 12 | NLT: 26 | NLT: 5.0 |
| Water soluble extractive | NLT: 85 | NLT: 80 | NLT: 80 | NLT: 4 | NLT: 30.0 |
| Heavy metals | Comply with API limits | ||||
| TBC and YMC | Comply with API limits | ||||
| Specific pathogens | Comply with API limits | ||||
| Aflatoxins | Comply with API limits | ||||
| Pesticide residue# | Comply with API limits | ||||
NMT: Not more than; NLT: Not less than; API: Ayurvedic Pharmacopoeia of India; TBC: Total Bacterial Count; YMC: Yeast & Mould Count
AYUSH-64 was procured from Indian Medicines Pharmaceutical Corporation Limited, Ministry of AYUSH, Government of India. Quality control and safety parameters of the ingredients and the formulation complied with the Ayurveda Pharmacopoeia limits/in-house limits as appropriate.
Outcomes measures
Primary outcome measure
Time to negative RT-PCR conversion (from the day of randomization) was the primary outcome measure. Real-time RT-PCR test was done on the planned follow-up visits, scheduled on the 7th, 15th, 22nd and 30th day of the study.
Secondary outcome measures
The proportion of participants who attained clinical recovery at 7th, 15th, 22nd and 30th day; improvement in laboratory parameters such as total and differential leukocyte count, absolute lymphocyte count, and erythrocyte sedimentation rate, inflammatory markers such as Interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), and D-dimer; proportion of patients who progressed to severe stage of COVID-19 (with the onset of complications and requiring invasive or noninvasive oxygen therapy); and change in score of Perceived Stress Scale were the secondart outcome measures.
Safety assessment
Safety assessment involved incidence of adverse drug reaction/adverse event (ADR/AE) and change in liver function test and kidney function test at the end of the study period, i. e., 30th day.
Sample size
The sample size for the study was calculated assuming that 85% of the participants will turn RT-PCR negative within 15 days in the IG, while this change will be observed in only 50% of the participants in the CG. With a 95% confidence level, power of 80%, and assuming the attrition rate of 20%, the number of participants to be enrolled in each group was estimated to be 30. Hence, a total of 60 participants were enrolled in the two groups of the study.
Randomization
Sixty eligible participants were randomized into two parallel groups in the ratio of 1:1. Statistical Package for Social Sciences SPSS 15.0 for Windows, 233 South Wacker Drive, 11th Floor, Chicago, Illinois, U.S.A. was used to generate the random number sequences.
Ethical consideration
The study was conducted in accordance with the principles of the Declaration of Helsinki and the ICMR’s National Ethical Guidelines for Biomedical and Health Research on Human Participants (2017). The study was reviewed, approved, and monitored by the Institutional Ethics Committee of Government Medical College, Nagpur, Maharashtra, India. The clinical trial was registered prospectively at the Clinical Trial Registry of India (CTRI/2020/05/025156). The study was monitored by Data and Safety Monitoring Board. The CONSORT guidelines were followed while reporting the study results.
Statistical analysis
The categorical variables in the study data have been summarized as numbers (percentage) and compared using the Chi-square test. The continuous data have been represented as mean (standard deviation) and median (min-max) for data not following a normal distribution. Parametric data were analyzed by paired t-test and independent sample t-test for within and between-group analysis, respectively, whereas nonparametric data were compared by Wilcoxon signed-rank test and Mann-Whitney test for within and between-group analysis, respectively. P < 0.05 has been considered as significant. The per-protocol method was used for data analysis. All the data analyses were done using the Stata/MP 16.1 for Windows, Stata Corp, 4905 Lakeway Drive, College Station, Texas, US.
Observation and Results
Patients’ enrolment
A total of 81 RT-PCR-confirmed COVID-19 patients were screened for study eligibility from June 10, 2020. Twenty-one patients were not included in the study as they were moderate or severe cases of COVID-19 (n = 13), below 18 years (n = 2), lactating women (n = 4), and not willing to participate in the study (n = 2). Sixty participants who met the inclusion criteria were included in the study. The recruitment, allocation, follow-up, and analysis of the study participants are shown as a CONSORT flow diagram in Figure 1. The clinical condition of one participant deteriorated just after the enrolment in the IG and the participant did not receive the allocated intervention. The data of 25 participants in the IG and 27 in the CG were included for final analysis as four participants in the IG and three participants in the CG did not come for the first follow-up visit on the 7th day from randomization and dropped out of the study.
Figure 1.
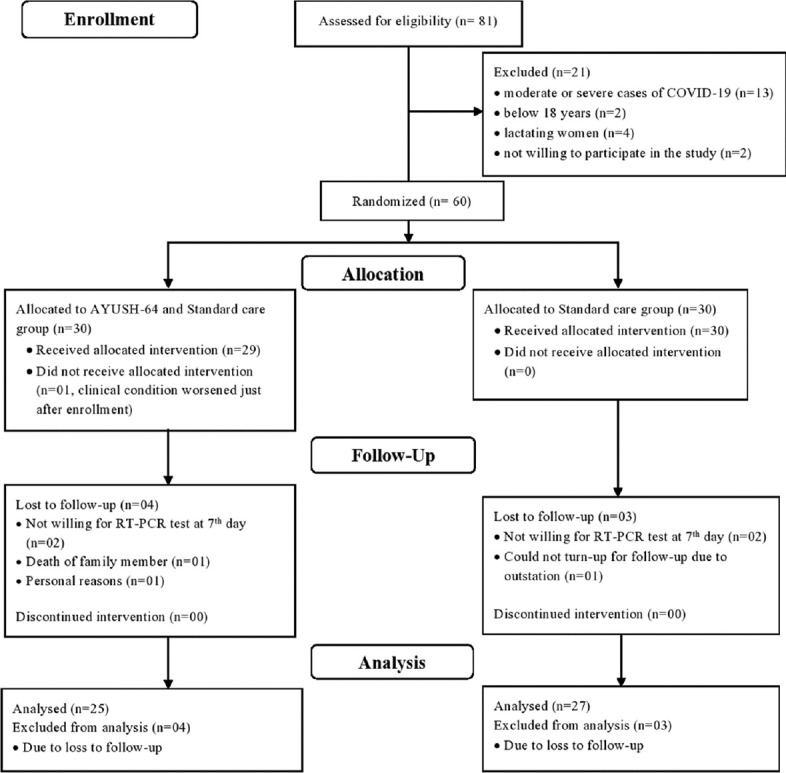
CONSORT flow diagram
Baseline clinical characteristics of study participants
The baseline characteristics of the study participants such as age, gender, symptomatic status, comorbidities, appetite, and bowel habits are shown in Table 3. There was no statistically significant difference in the baseline distribution of demographic and clinical characteristics between the two groups (P > 0.05). The majority of participants in both groups were male and the mean age of the participants in the intervention and CGs was 43.68 ± 9.97 and 35.22 ± 11.80 years, respectively. In the present study, 64% and 70.4% of patients were found symptomatic in the IG and CG, respectively. Comorbidities such as diabetes mellitus, hypertension, bronchial asthma, chronic obstructive pulmonary disease, cardiovascular disease, and thyroid dysfunction were present in the IG (n = 05) and CG (n = 10).
Table 3.
Baseline characteristics of the participants in both the groups
| Variables | Parameters | IG (n=25) | CG (n=27) | P $ |
|---|---|---|---|---|
| Age:Mean±SD | 43.68±9.97 | 35.22±11.80 | 0.448 | |
| Gender | Male | 18 (72.0) | 18 (66.7) | 0.677 |
| Female | 7 (28.0) | 9 (33.3) | ||
| Clinical features | Asymptomatic | 9 (36.0) | 8 (29.6) | 0.625 |
| Symptomatic | 16 (64.0) | 19 (70.4) | ||
| Stage of disease | Group A | 8 (32.0) | 8 (29.6) | 0.530 |
| Group B | 14 (56.0) | 14 (51.9) | ||
| Group C | 3 (12.0) | 5 (18.5) | ||
| Comorbidities | COPD | 1 (4.0) | 0 | - |
| Bronchial asthma | 2 (8.0) | 2 (7.4) | 0.936 | |
| Diabetes mellitus | 3 (12.0) | 2 (7.4) | 0.575 | |
| Hypertension | 2 (8.0) | 5 (18.5) | 0.267 | |
| Cardiovascular disease | 0 | 2 (7.4) | - | |
| Thyroid dysfunction | 0 | 3 (11.1) | - | |
| Bowel habits | Regular | 22 (88.0) | 22 (81.5) | 0.515 |
| Irregular | 3 (12.0) | 5 (18.5) | ||
| Appetite | Normal | 23 (92.0) | 23 (85.2) | 0.442 |
| Disturbed | 2 (8.0) | 4 (14.8) | ||
| Stool consistency | Normal | 22 (88.0) | 20 (74.1) | 0.203 |
| Constipated | 3 (12.0) | 7 (25.9) |
$Compared using Chi-square/Fisher’s exact test. Values have been expressed as n (%) for all variables except age. IG: Intervention group, CG: Control group, SD: Standard deviation, COPD: Chronic obstructive pulmonary disease
Efficacy outcomes
The efficacy outcome was evaluated through the proportion of participants who attained negative RT-PCR conversion and clinical recovery in the scheduled follow-up on the 7th, 15th, 22nd and 30th days. The proportion of participants who turned RT-PCR negative on the 7th, 15th and 30th day were 64%, 80% and 92% in the IG and 70.3%, 88.8% and 100% in the CG, respectively [Table 4]. The difference observed between the intervention and CG is statistically insignificant (P = 0.134). The remaining two RT-PCR-positive patients in the IG were also asymptomatic before the end of the study period.
Table 4.
Effect on outcome parameters in both the groups
| Outcome Parameters | IG (n=25), n (%) | CG (n=27), n (%) | P $ |
|---|---|---|---|
| Primary outcome measure | |||
| Negative RT-PCR | |||
| 7th day | 16 (64.0) | 19 (70.4) | 0.625 |
| 15th day | 20 (80.0) | 24 (88.9) | 0.375 |
| 22nd day | 23 (92.0) | 26 (96.3) | 0.507 |
| 30th day | 23 (92.0) | 27 (100.0) | 0.134 |
| Secondary outcome measures | |||
| Clinical recovery | |||
| 7th day | 9 (36.0) | 7 (25.9) | 0.432 |
| 15th day | 15 (60.0) | 10 (37.0) | 0.098 |
| 22nd day | 18 (72.0) | 15 (55.6) | 0.219 |
| 30th day | 25 (100) | 23 (85.2) | 0.112 |
| Perceived Stress Scale Score: Median (minimum-maximum) | |||
| Baseline | 21 (0-32) | 16 (0-32) | 0.205 |
| 30th day | 0 (0-18) | 0 (0-12) | 0.181 |
$Compared using Chi-square/Fisher’s exact test. IG: Intervention group, CG: Control Group, RT-PCR: Reverse transcription-polymerase chain reaction
The clinical recovery was 60% and 37% on 15th day (P = 0.098) and 100% and 85.2% at the end of the study period, i.e., 30th day (P = 0.112) in the intervention and CG, respectively [Table 4]. Clinical features such as fever, chest pain, and anorexia relieved within 7 days in all the IG participants and cough, expectoration, and breathlessness were also absent before the 15th day. Other symptoms such as sore throat, nasal discharge, bodyache, headache and nausea persisted in a very few participants till the 22nd day in the IG [Table 5]. In the CG, nasal discharge and expectoration relieved by the 15th day while nausea, bodyache and headache persisted throughout the study period. The major symptoms such as fever and breathlessness persisted in the CG till the 15th day and complete relief in cough was achieved at 22nd day in the CG.
Table 5.
Effect on chief complaints in both the groups
| Chief complaints | Baseline, n (%) | 7th day, n (%) | 15th day, n (%) | 22nd day, n (%) | 30th day, n (%) |
|---|---|---|---|---|---|
| Fever | |||||
| IG | 11 (44.0) | 0 | 0 | 0 | 0 |
| CG | 15 (55.6) | 3 (11.1) | 1 (3.7) | 0 | 0 |
| P$ | 0.405 | - | - | - | - |
| Cough | |||||
| IG | 13 (52.0) | 6 (24.0) | 0 | 0 | 0 |
| CG | 15 (55.6) | 6 (22.2) | 4 (14.8) | 1 (3.7) | 0 |
| P$ | 0.797 | 0.879 | - | - | - |
| Breathlessness | |||||
| IG | 10 (40.0) | 3 (12.0) | 0 | 0 | 0 |
| CG | 8 (29.6) | 3 (11.1) | 2 (7.4) | 0 | 0 |
| P$ | 0.432 | 0.920 | - | - | - |
| Sore throat | |||||
| IG | 13 (52.0) | 6 (24.0) | 2 (8.0) | 1 (4.0) | 0 |
| CG | 14 (51.9) | 7 (25.9) | 3 (11.1) | 2 (7.4) | 0 |
| P$ | 0.991 | 0.873 | 0.704 | 0.599 | - |
| Expectoration of sputum | |||||
| IG | 2 (8.0) | 2 (8.0) | 0 | 0 | 0 |
| CG | 4 (14.8) | 2 (7.4) | 0 | 0 | 0 |
| P$ | 0.442 | 0.936 | - | - | - |
| Nausea | |||||
| IG | 4 (16) | 6 (24) | 1 (4) | 0 | 0 |
| CG | 5 (18.5) | 3 (11.1) | 1 (3.7) | 3 (11.1) | 1 (3.7) |
| P$ | 0.810 | 0.220 | 0.956 | - | - |
| Bodyache/myalgia | |||||
| IG | 11 (44.0) | 8 (32.0) | 4 (16.0) | 5 (20.0) | 0 |
| CG | 10 (37.0) | 6 (22.2) | 9 (33.3) | 6 (22.2) | 2 (7.4) |
| P$ | 0.609 | 0.427 | 0.149 | 0.845 | - |
| Abdominal pain | |||||
| IG | 2 (4.8) | 0 | 0 | 0 | 0 |
| CG | 2 (7.4) | 1 (3.7) | 1 (3.7) | 0 | 0 |
| P$ | 0.936 | - | - | - | - |
| Nasal discharge/nasal congestion | |||||
| IG | 3 (12.0) | 0 | 3 (12.0) | 0 | 0 |
| CG | 1 (3.7) | 1 (3.7) | 0 | 0 | 0 |
| P$ | 0.262 | - | - | - | - |
| Chest pain | |||||
| IG | 2 (4.8) | 0 | 0 | 0 | 0 |
| CG | 2 (7.4) | 1 (3.7) | 1 (3.7) | 0 | 0 |
| P$ | 0.936 | - | - | - | - |
| Anorexia | |||||
| IG | 1 (4.0) | 0 | 0 | 0 | 0 |
| CG | 2 (7.4) | 2 (7.4) | 1 (3.7) | 2 (7.4) | 0 |
| P$ | 0.599 | - | - | - | - |
| Headache | |||||
| IG | 15 (60.0) | 9 (36.0) | 4 (16.0) | 1 (4.0) | 0 |
| CG | 15 (55.6) | 9 (33.3) | 6 (22.2) | 7 (25.9) | 4 (14.8) |
| P$ | 0.746 | 0.273 | 0.569 | 0.051 | - |
$Compared using Chi-square/Fisher’s exact test. Values have been represented as n (%). IG: Intervention group (n=25), CG: Control group (n=27)
The levels of inflammatory markers/cytokines such as IL-6, TNF-a, and D-dimer are shown in Table 6. The reduction in the levels of IL-6 and TNF-a at the end of the study period was statistically significant in the IG (P < 0.05), whereas it was statistically insignificant in the CG. The D-dimer levels significantly reduced after the treatment in both the groups (P < 0.05). The Perceived Stress Scale score also improved at the end of the study period in both the groups [Table 4].
Table 6.
Effect on laboratory parameters in both the groups
| Laboratoryparameters | IG (n=25) | CG (n=27) | P # |
|---|---|---|---|
| Total leucocyte count (103/µL) | |||
| Baseline | 6.04±1.78 | 5.89±1.42 | 0.736 |
| 30th day | 6.56±1.57 | 6.93±1.98 | 0.459 |
| P$ | 0.091 | 0.008* | |
| Neutrophils(%) | |||
| Baseline | 57.4±11.16 | 58.7±8.83 | 0.641 |
| 30th day | 60.28±8.76 | 58.44±6.60 | 0.396 |
| P$ | 0.194 | 0.871 | |
| Lymphocytes(%) | |||
| Baseline | 34.04±11.54 | 32.85±8.67 | 0.675 |
| 30th day | 31.32±8.71 | 33.51±6.73 | 0.311 |
| P$ | 0.203 | 0.681 | |
| Eosinophils(%) | |||
| Baseline | 2.84±1.06 | 3.29±1.97 | 0.311 |
| 30th day | 3.6±2.23 | 3.37±1.64 | 0.673 |
| P$ | 0.103 | 0.854 | |
| Absolute lymphocyte count (per mm3) | |||
| Baseline | 1992.88±669.35 | 1897.03±540.39 | 0.571 |
| 30th day | 2099.4±673.12 | 2300.5±693.93 | 0.295 |
| P$ | 0.451 | <0.001* | |
| ESR (mm/h) | |||
| Baseline | 17.6±9.97 | 20.55±11.90 | 0.338 |
| 30th day | 21.44±9.62 | 18.51±10.68 | 0.307 |
| P$ | 0.070 | 0.348 | |
| D-dimer (µg/mL)a | |||
| Baseline | 233.8 (173.9 628.3) | 250.0 (157.8 293.7) | 0.782 |
| 30th day | 185.0 (129.4 263.7) | 132.0 (84.7 213.5) | 0.055 |
| P$ | 0.015* | 0.005* | |
| IL-6 (pg/mL)a | |||
| Baseline | 5.6 (2.3 13.8) | 3.6 (1.7 4.8) | 0.067 |
| 30th day | 1.9 (0.15 5.3) | 0.4 (0.2 6.6) | 0.905 |
| P$ | 0.037* | 0.361 | |
| TNF-α (pg/mL) | |||
| Baseline | 5.70±2.15 | 5.58±1.32 | 0.809 |
| 30th day | 4.13±1.85 | 6.48±4.14 | 0.012* |
| P$ | 0.014* | 0.318 |
*P<0.05 has been considered as significant, aData have been reported as median (Q1-Q3), #Between group P value, compared using independent sample t-test/Mann-Whitney test, $Within group P value, compared using paired sample t-test/Wilcoxon signed-rank test. Values have been represented as mean±SD. IG: Intervention group, CG: Control Group, ESR: Erythrocyte sedimentation rate, IL-6: Interleukin-6, TNF-α: Tumor necrosis factor-α, SD: Standard deviation
Vital parameters such as SpO2, pulse rate, respiratory rate, and blood pressure were within normal limits in both groups, during the study period. None of the participants required invasive or noninvasive oxygen therapy or developed complications such as pneumonia, acute respiratory distress syndrome, sepsis, arrhythmia, etc. during the study period in both groups.
Safety outcomes
ADRs or serious AEs were not observed/reported by any of the study participants in both groups. Liver function test and kidney function test were found to be within the normal limits throughout the study period in both groups [Table 7].
Table 7.
Effect on liver and kidney function in both the groups
| Laboratory parameters | IG (n=25) | CG (n=27) | P # |
|---|---|---|---|
| Blood urea (mg/dl) | |||
| Baseline | 16.87±7.67 | 20.14±13.38 | 0.290 |
| 30th day | 15.34±6.08 | 16.40±4.18 | 0.462 |
| P$ | 0.147 | 0.118 | |
| Serum uric acid (mg/dl) | |||
| Baseline | 4.94±1.43 | 4.99±1.70 | 0.920 |
| 30th day | 4.63±1.20 | 5.11±1.30 | 0.170 |
| P$ | 0.205 | 0.625 | |
| Serum creatinine (mg/dl)a | |||
| Baseline | 0.7 (0.55-0.85) | 0.6 (0.7-0.9) | 0.317 |
| 30th day | 0.7 (0.6-0.9) | 0.6 (0.7-0.8) | 0.679 |
| P$ | 0.119 | 0.085 | |
| SGOT (U/L) | |||
| Baseline | 24.36±15.08 | 22.18±8.19 | 0.517 |
| 30th day | 21.36±8.59 | 23.48±11.01 | 0.445 |
| P$ | 0.316 | 0.552 | |
| SGPT (U/L) | |||
| Baseline | 23.40±15.08 | 18.62±13.36 | 0.184 |
| 30th day | 20.68±11.08 | 23.18±25.12 | 0.648 |
| P$ | 0.168 | 0.283 | |
| Serum alkaline phosphatase (IU/L) | |||
| Baseline | 82.68±29.03 | 79.88±15.44 | 0.664 |
| 30th day | 77.28±25.67 | 80.96±22.19 | 0.582 |
| P$ | 0.029* | 0.682 | |
| Total protein (g/dl) | |||
| Baseline | 6.99±0.39 | 7.10±0.40 | 0.323 |
| 30th day | 7.06±0.41 | 7.16±0.43 | 0.425 |
| P$ | 0.521 | 0.497 | |
| Serum albumin (g/dl) | |||
| Baseline | 4.40±0.28 | 4.44±0.41 | 0.717 |
| 30th day | 4.53±0.28 | 4.56±0.34 | 0.694 |
| P$ | 0.172 | 0.126 | |
| Serum globulin (g/dl) | |||
| Baseline | 2.58±0.31 | 2.63±0.38 | 0.620 |
| 30th day | 2.54±0.39 | 2.59±0.31 | 0.627 |
| P$ | 0.675 | 0.442 | |
| Serum bilirubin conjugated (mg/dl) | |||
| Baseline | 0.18±0.07 | 0.17±0.08 | 0.565 |
| 30th day | 0.19±0.13 | 0.19±0.08 | 0.869 |
| P$ | 0.671 | 0.176 | |
| Serum bilirubin unconjugated (mg/dl) | |||
| Baseline | 0.41±0.47 | 0.24±0.15 | 0.085 |
| 30th day | 0.32±0.24 | 0.26±0.17 | 0.294 |
| P$ | 0.282 | 0.538 |
*P<0.05 has been considered as significant, aData have been reported as median (Q1-Q3), $Within group P value, compared using paired sample t-test/Wilcoxon signed-rank test, #Between group P value, compared using independent sample t-test/Mann-Whitney test. Values have been represented as mean±SD. IG: Intervention group, CG: Control group, SD: Standard deviation, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase
Discussion
In the present open-label randomized controlled study, the proportion of participants who attained negative RT-PCR conversion on the scheduled follow-up visits was the primary outcome measure to evaluate the efficacy of the study intervention. The difference observed in the proportion of participants who turned RT-PCR negative for COVID-19 during each of the follow-ups is statistically insignificant between the intervention and CG. Incidentally, the status of RT-PCR in patients who have clinically recovered from COVID-19 bears little relevance as it is evident that SARS-CoV-2 virus can rarely be cultured in respiratory samples after 9 days of symptom onset, especially in patients with mild disease.[34]
The clinical endpoint of the study was the time to attain clinical recovery within 30 days after randomization and was observed to be not significantly different between groups, but there was trend of good symptomatic response in the IG than the CG. Further, only mild cases of COVID-19 were selected, so the number of participants having symptoms at baseline was low and not sufficient for statistically significant difference. Furthermore, the enrolled participants were kept in isolation at home after baseline evaluation. Hence, evaluation of clinical recovery was done only when the patients attended the follow-ups and due to recall bias, patient-reported duration of symptomatic relief could not be recorded in all the participants. Hence, the exact proportion of participants who attained clinical recovery on each day following the enrolment could not be elicited. Hence, the absence of statistical difference could not be used for limiting the possible role of the study intervention on clinical outcomes.
The levels of IL-6 demonstrated within-group statistically significant findings when compared with the baseline, while TNF-a demonstrated within-group and between-group significant findings. The levels of these inflammatory cytokines were within the accepted reference range during the study period in all the recruited participants. Pro-inflammatory cytokines such as IL-6, secreted by the monocytes are implicated in triggering signalling multiple cytokine cascades with higher chances for tissue damage and organ failure, which in COVID-19 implies elevated chances of mortality and morbidity and is associated with disease severity and chances of respiratory failure necessitating mechanical ventilation.[35,36,37] TNF-a more or less acts as an amplifier of inflammation and TNF-a blockade has been clinically used in the management of many inflammatory diseases. Clinical evidence in this area suggests that modulators of elevated cytokines/chemokines may provide precision management and the findings in the present study show that the interventions have a potential to limit the persistent elevation of plasma cytokines which are often seen, even after attaining negative viral titers. The possible role of the intervention in these highly dynamic inflammatory cytokines would need further exploration in COVID-19 patients with significantly elevated cytokines. D-dimer is a biomarker that reflects fibrin formation and degradation and higher levels have been linked with higher mortality in COVID-19 patients with increased risk of clinically diagnosed thrombotic events, critical illness, and death.[38] In the present study, the D-dimer levels were within the preferred upper reference range throughout the study. However, the depletion in the D-dimer levels within group and between the groups at 30th day was statistically significant.
Ayurveda consider diseases characterized by pyrexia as the cardinal symptom under the spectrum of Jwara, the pathogenesis of which is characterized by Amavastha (circulating endogenous or exogenous substances including infectious agents, inflammatory products) in the initial stages wherein the disease activity will be profound, followed by a stage where the disease activity limits itself or undergo resolution either as a host response or due to medical intervention. AYUSH-64 is an intervention that was developed for Vishamajwara (fever characterized by the onset of symptoms in a paroxysms or cyclical manifestation) and is expected to neutralize the Ama at the level of tissues. The baseline disease assessment in the present study shows that the asymptomatic participants do not fit in the category of classical Jwara and the laboratory parameters also do not support a profound Amavastha or Dhatugata Avastha (localization of disease at the level of various tissues).
None of the participants had study intervention prematurely stopped by the investigators because of AEs including gastrointestinal symptoms (anorexia, nausea, and vomiting) and impaired liver function and renal function. It would be prudent to reflect that the Ayurveda intervention was safe to be used with contemporary medicine and less likely to cause any drug-drug interactions when taken together.
Strategies to enhance the potency of AYUSH-64 such as multiple divided doses during the 24-hour period, combination with other Ayurvedic interventions with Jwarahara (drugs which alleviate disease conditions with pyrexia) potential, administered with suitable Anupana (adjunct administered either along with or just after the principal medicine to enhance its therapeutic action) based on individual constitution or disease state to mitigate immune-pathological host responses shall be adopted focusing on the individualized treatment regimen of Ayurveda. Furthermore, multicentric study with more number of participants should be conducted to further investigate the role of combinational therapy and explore viral dynamics.
Limitations of the study
Despite the carefully designed protocol for the present study, some limitations merit mention. We included only patients with asymptomatic and mild COVID-19, so the study findings cannot be extrapolated to patients with severe disease. Further, the study was designed as open-label and single-center study. Furthermore, the participants were in home isolation, so the exact duration for attaining clinical recovery cannot be assessed, which is a major outcome of interest in the study.
Conclusions
In asymptomatic and mild COVID-19 patients, AYUSH-64, as an add-on to standard conventional care, contributed to improvement in clinical recovery and also demonstrated the potential in reducing the levels of pro-inflammatory markers such as IL-6 and TNF-a. However, the difference observed in the proportion of participants who turned RT-PCR negative for COVID-19 is statistically insignificant between both groups.
Financial support and sponsorship
This study was financially supported by Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, Government of India.
Conflicts of interest
The authors declare that they have no financial conflict of interest related to this study. The authors Govind Reddy, Manisha Talekar, Arunabh Tripathi, Babita Yadav, Amit K Rai, Sophia Jameela, Rakesh Rana, Shruti Khanduri, Bhagwan S Sharma, Bhogavalli Chandrasekhararao and Narayanam Srikanth work in Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH, Government of India, New Delhi.
Acknowledgment
The authors are thankful to the administration of Government Medical College, Nagpur, Maharashtra, India, for their support for this study. The authors are also thankful to Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH, Government of India, for the financial support for this study. The authors are also thankful to Dr. Ravindra Ghooi, Director, Scientia Clinical Services, Pune, for providing intellectual insights while finalizing the manuscript.
References
- 1.World Health Organization. World Health Organization Coronavirus Disease (COVID-19) Dashboard. [Last accessed on 2021 Mar 05]. Available from: https://covid19.who.int/
- 2.Borse S, Joshi M, Saggam A, Bhat V, Walia S, Sagar S, et al. Ayurveda botanicals in COVID-19 management: An in silico-multitarget approach. PLoS ONE. 2021;16(6):e0248479. doi: 10.1371/journal.pone.0248479. [doi.org/10.1371/journal.pone.0248479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarentino AL, Maley F. A comparison of the substrate specificities of endo-beta-N-acetylglucosaminidases from Streptomyces griseus and Diplococcus Pneumoniae. Biochem Biophys Res Commun. 1975;67:455–62. doi: 10.1016/0006-291x(75)90337-x. [DOI] [PubMed] [Google Scholar]
- 4.Tillu G, Salvi S, Patwardhan B. AYUSH for COVID-19 management. J Ayurveda Integr Med. 2020;11:95–6. doi: 10.1016/j.jaim.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern Med Rev. 2006;11:128–50. [PubMed] [Google Scholar]
- 6.Burns JJ, Zhao L, Taylor EW, Spelman K. The influence of traditional herbal formulas on cytokine activity. Toxicology. 2010;278:140–59. doi: 10.1016/j.tox.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Roy A, Patwardhan B, Chaguturu R. Reigniting pharmaceutical innovation through holistic drug targeting. Drug Discov World. 2016;17:45–55. [Google Scholar]
- 8.Rege AA, Chowdhary AS. Evaluation of some medicinal plants as putative HIV-protease inhibitors. Indian Drugs. 2013;50:24–8. [Google Scholar]
- 9.Sharma U, Bala M, Kumar N, Singh B, Munshi RK, Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol. 2012;141:918–26. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinosporacordifolia (Willd.) Hook. f. and Thoms. (Guduchi) – validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112–21. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurya VK, Kumar S, Prasad AK, Bhatt ML, Saxena SK. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31:179–93. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi AJ, Rupareliya JD, Shukla VJ, Donga SB, Acharya R. An ayurvedic perspective along with in silico study of the drugs for the management of SARS-CoV-2. J Ayurveda Integr Med. 2020 Jul 21; doi: 10.1016/j.jaim.2020.07.002. doi: 10.1016/j.jaim.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of AYUSH, Govt. of India. National Clinical Management Protocol based on Ayurveda and Yoga for management of Covid-19. 2020. [Last accessed on 2020 Nov 12]. Available from: https://www.ayush.gov.in/docs/ayush-Protocol-covid-19.pdf .
- 14.Gundeti MS, Bhurke LW, Mundada PS, Murudkar S, Surve A, Sharma R, et al. AYUSH 64, a polyherbalAyurvedic formulation in influenza like illness: Results of a pilot study. J Ayurveda Integr Med. 2020 May 14:S0975-9476(20)30025-5. doi: 10.1016/j.jaim.2020.05.010. doi: 10.1016/j.jaim.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ram TS, Munikumar M, Raju VN, Devaraj P, Boiroju NK, Hemalatha R, et al. In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease. J Ayurveda Integr Med. 2021 Feb 25; doi: 10.1016/j.jaim.2021.02.004. doi: 10.1016/j.jaim.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia D. Role of AYUSH-64 in malaria epidemic. J Res Ay Sid. 1997;18:71–6. [Google Scholar]
- 17.Chari MV, Venkataraghavan S, Seshadri C, Shetty BR, Gowri N. A double blind clinical trial with ayush-64 an ayurvedic drug in P. vivax malaria. J Res Ay Sid. 1982;6:105–16. [Google Scholar]
- 18.Pandey PN, Kishore P. Effect of Ayush-64 and saptaparnaghanavati on microfilaraemia. J Res Ay Sid. 1989;12:145–50. [Google Scholar]
- 19.Anonymous. Management of Chikungunya Through Ayurveda and Siddha – A Technical Report. Central Council for Research in AyurvedicSciences, Department of AYUSH, Ministry of Health & Family Welfare, Govt. of India. 2009 [Google Scholar]
- 20.Zhao YL, Shang JH, Pu SB, Wang HS, Wang B, Liu L, et al. Effect of total alkaloids from Alstoniascholaris on airway inflammation in rats. J Ethnopharmacol. 2016;178:258–65. doi: 10.1016/j.jep.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Hu TY, Ju JM, Mo LH, Ma L, Hu WH, You RR, et al. Anti-inflammation action of xanthones from swertiachirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine. 2019;55:214–21. doi: 10.1016/j.phymed.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Shukla S, Mehta A, Mehta P, Vyas SP, Shivaprasad HN. In vivo immunomodulatory activities of the aqueous extract of bonduc nut Caesalpinia bonducella seeds. Pharm Biol. 2010;48:227–30. doi: 10.3109/13880200903085474. [DOI] [PubMed] [Google Scholar]
- 23.Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem Toxicol. 2010;48:61–4. doi: 10.1016/j.fct.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, et al. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from picrorhizakurroa. Intimmunopharmacol. 2006;6:1543–9. doi: 10.1016/j.intimp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Gupta YK, Singh S, Raj A. Anti-inflammatory effect of picrorhizakurroa in experimental models of inflammation. Planta Med. 2016;82:1403–9. doi: 10.1055/s-0042-106304. [DOI] [PubMed] [Google Scholar]
- 26.Wanjarkhedkar P, Sarade G, Purandare B, Kelkar D. A prospective clinical study of an Ayurveda regimen in COVID 19 patients. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.10.008. Oct 19:S0975-9476(20)30098-X. doi: 10.1016/j.jaim.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi JA, Puthiyedath R. Outcomes of ayurvedic care in a COVID-19 patient with hypoxia-A case report. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.10.006. Oct 13. doi: 10.1016/j.jaim.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girija PL, Sivan N. Ayurvedic treatment of COVID-19/SARS-CoV-2: A casereport. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.06.001. Jun 19:S0975-9476(20)30042-5. doi: 10.1016/j.jaim.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Prasad G, Srivastav S, Gautam VK, Sharma N. A retrospective study on efficacy and safety of guduchighanvati for COVID-19 asymptomatic patients. medRxiv. 2020.07.23.20160424. [doi.org/10.11 01/2020.07.23.20160424] [Google Scholar]
- 30.Directorate of Health Services, Government of Maharashtra, India 2020. Revised Treatment Protocol for COVID-19 dated April 15, 2020 [Google Scholar]
- 31.World Health Organization. COVID-19 Therapeutic Trial Synopsis. [Last assessed on 2020 Nov 25]. Available from: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf .
- 32.Ministry of Health and Family Welfare, Govt. of India. Revised Guidelines for Home Isolation of Very mild/Pre-Symptomatic/Asymptomatic COVID-19 Cases. [Last accessed on 2020 Jul 02]. Available from: https://www.mohfw.gov.in/pdf/RevisedHomeIsolationGuidelines.pdf .
- 33.Ministry of Health and Family Welfare, Govt. of India. Revised Discharge Policy for COVID-19. 2020. [Last assessed on 2020 Nov 25]. Available from: https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf .
- 34.World Health Organization. Criteria for releasing COVID19 patients from isolation. [Last accessed on 2020 Nov 12]. Available from: https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-fromisolation .
- 35.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, et al. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2021;93:35–7. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475–82. doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


