Abstract
Cocaine-dependent (CD) individuals demonstrate significant anxiety and dysphoria during withdrawal, a negative emotional state that may perpetuate drug seeking and consumption. An extensive body of work has focused on characterizing reward circuit dysfunction, but relatively little is known about the pain circuit during cocaine withdrawal. In an earlier study, we highlighted how cue-elicited functional connectivity between the periaqueductal gray (PAG), a subcortical hub of the pain circuit, and ventromedial prefrontal cortex supports tonic craving in recently abstinent CD. The functional organization of the brain can be characterized by intrinsic connectivities, and it is highly likely that the resting state functional connectivity (rsFC) of the PAG may also be altered in association with cocaine use variables. Here, we examined this issue in 52 CD and 52 healthy control (HC) participants. Imaging data were processed with published routines, and the findings were evaluated with a corrected threshold. In a covariance analysis, CD as compared with HC showed higher PAG rsFC with the hypothalamus, dorsomedial prefrontal, and inferior parietal cortices. Further, these connectivities were correlated negatively with tonic cocaine craving and recent cocaine use, respectively. Higher hypothalamic and frontoparietal rsFC with the PAG may reflect a compensatory process to regulate craving and compulsive drug use. The findings provide additional evidence in humans implicating the PAG circuit and may help research of the role of negative reinforcement in sustaining habitual drug use in cocaine addiction.
Keywords: cocaine, craving, fMRI, periaqueductal gray, resting state connectivity
1 ∣. INTRODUCTION
Withdrawal from chronic drug use manifests with irritability and dysphoria.1 Individuals resort to drugs to override the negative emotional state, and the negative reinforcement perpetuates addiction.2 For instance, earlier studies showed that individual sensitivity to craving induced by negative emotions predicted relapse in cocaine-dependent (CD) individuals.3 As a key region of the pain circuit, the periaqueductal gray (PAG) is known for its role in mediating negative emotions.4,5 For instance, a meta-analysis of 162 neuroimaging studies reported consistent PAG activation during negative emotion processing.5 The PAG responded both to physical pain and to emotionally negative images, along with increases in negative affect.4 Indeed, studies have suggested the PAG as part of the cortical subcortical circuits that support avoidance behavior6 and its potential role in drug seeking and consumption during negative emotional states.
Abundant preclinical research have linked PAG circuit and negative reinforcement to drug seeking.6 For instance, hyperalgesia during alcohol withdrawal was mediated in part by amygdalar projections to the PAG in alcohol-dependent rats.7 In vivo calcium imaging showed a reduction in prefrontal cortical signaling to the PAG in mice engaged in compulsive alcohol drinking.8 Further, studies have associated PAG responses to increased rearing and other locomotor activities during morphine withdrawal.9 Following a cocaine binge, the PAG neurons were hyperresponsive to tactile stimulation in rats.10 Investigators reported increased Fos immunoreactivity in the PAG in rats during withdrawal from cocaine or after exposure to social stress.11 This body of evidence suggests a critical role of the PAG circuits in the shaping and maintenance of addiction.
On the other hand, relatively little is known about PAG circuit dysfunction in addicted individuals. In a recent study, we highlighted how cue-elicited functional connectivity between the PAG and ventromedial prefrontal cortex in response to cocaine cues supports tonic craving in recently abstinent CD.12 The functional organization of the brain can be characterized by intrinsic connectivities, and it is highly likely that the resting state functional connectivity (rsFC) of the PAG may also be altered in association with cocaine use variables. Indeed, linear association between the rsFC measures and task-induced blood oxygen level-dependent (BOLD) responses was observed in a study of 426 healthy adults.13 RsFC analysis of the PAG has been examined previously in neuro-typical populations14 as well as patients with functional dyspepsia,15 obsessive–compulsive disorder,16 generalized anxiety disorder,17 posttraumatic stress disorder,18 and pain disorder.19 These studies highlighted the feasibility of examining the rsFC of the PAG. Here, thus, we hypothesized altered rsFC of the PAG and examined how altered PAG rsFC related to clinical variables of cocaine use in CD. Further, we previously observed sex differences in cue-elicited functional connectivity of PAG in CD.12 Thus, we would also explore sex differences in the current study.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Subjects, informed consent, and assessment
Fifty-two recently abstinent subjects with CD (42 men) and 52 age- and gender-matched healthy control (HC) subjects (39 men) participated in the study (Table 1). CD met criteria for current dependence, as diagnosed by the Structured Clinical Interview for DSM-IV.20 Recent cocaine use was confirmed by urine toxicology screens. They were drug-free while staying in an inpatient unit for 7–10 days prior to the current fMRI study. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) and current or past history of psychotic disorders. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to the study.
TABLE 1.
Demographics and clinical measures of the subjects
| Subject characteristic | CD (n = 52) | HC (n = 52) | p value |
|---|---|---|---|
| Age (years) | 44.9 ± 7.2 | 44.0 ± 8.8 | 0.54a |
| Gender (M/F) | 42/10 | 39/13 | 0.48b |
| Years of drinking | 26.8 ± 10.8 | 26.1 ± 11.1 | 0.74a |
| Years of smoking | 17.5 ± 11.4 | 2.0 ± 6.5 | <0.001a |
| CCQ score | 41.8 ± 15.1 | N/A | N/A |
| CSSA score | 30.3 ± 20.1 | N/A | N/A |
| Monthly cocaine use (g, average, prior year) | 28.2 ± 26.8 | N/A | N/A |
| Cocaine amount per use (g, prior month) | 1.3 ± 1.0 | N/A | N/A |
| Days of cocaine use (prior month) | 19.6 ± 8.7 | N/A | N/A |
| Years of cocaine use | 16.9 ± 9.5 | N/A | N/A |
Note: values are mean ± S.D.
Abbreviations: CCQ: Cocaine Craving Questionnaire; CSSA: Cocaine Selective Severity Assessment; CD, cocaine dependent; HC, healthy control.
Two-tailed two-sample t test.
χ2 test.
Cocaine craving was assessed with the Cocaine Craving Questionnaire, brief version (CCQ-Brief), for all participants every 2–3 days during the inpatient stay.21 The CCQ-Brief is a 10-item questionnaire, abbreviated from the CCQ-Now.22 Each item was rated on a scale from 1 to 7, with a higher total score (ranging from 10 to 70) indicating greater craving. Here, CDs averaged 41.8 ± 15.1 in CCQ score across all assessments.
2.2 ∣. Imaging protocol
Brain images were collected using multiband imaging with a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany). Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization. Anatomical 3D MPRAGE image were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 1900 ms, TE = 2.52 ms, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional, BOLD signals were then acquired with a single-shot gradient echo planar imaging (EPI) sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap. One 10-min resting state fMRI scan was obtained for both CD and HC with eye closed but awake. Images from the first 10 TRs at the beginning of each trial were discarded to ensure only signals in steady-state equilibrium between RF pulsing and relaxation were included in the analyses.
2.3 ∣. Imaging data preprocessing
Data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were coregistered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 4 mm at full width at half maximum. The smoothed images had a voxel size of 3 mm × 3 mm × 3 mm.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity.23 The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular, and white matter signals were also included in the regression. Cordes et al. suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations.24 Thus, we applied a temporal band-pass filter (0.009 Hz < f < 0.08 Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies.23
As extensively investigated in previous study, micro-head motion (>0.1 mm) is an important source of spurious correlations in rsFC analysis.25 Therefore, we applied a “scrubbing” method proposed by Power et al.26 to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD(t) = ∣Δdx(t)∣+∣Δdy(t)∣+∣Δdz(t)∣ +r∣α(t)∣+r∣β(t)∣+r ∣ γ(t) ∣ FD(t) = ∣Δdx(t)∣+∣Δdy(t)∣+∣Δdz(t)∣+∣Δα(t)∣+∣Δβ(t)∣ + ∣ Δγ(t)∣, where (dx, dy, dz) and (α, β, γ) are the translational and rotational movements, respectively.26 The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject's correlation map, we removed every time point that exceeded the head motion limit FD(t) > 0.5 mm or DVARS(t) > 0.5%.26 On average, 1% of the time points were removed across subjects. CD and HC did not differ in FD (p = 0.72) or in DVARS (p = 0.52).
2.4 ∣. Seed-based correlation
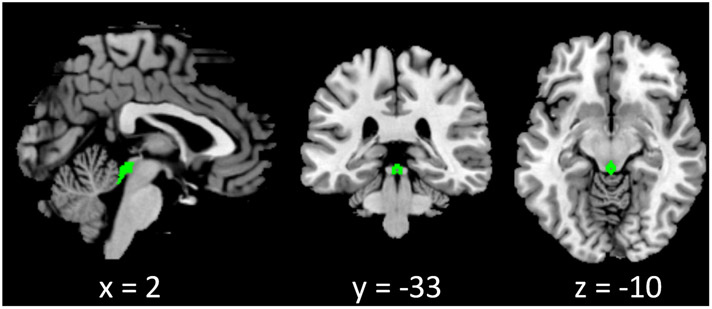
We used a PAG mask (Figure 1) from the Harvard Ascending Arousal Network (AAN) Atlas.27 The BOLD time courses were averaged spatially over the PAG mask. For individual subjects, we computed the correlation coefficient between the averaged time course of the PAG and the time courses of all other brain voxels. To assess and compare the rsFC, we converted these image maps, which were not normally distributed, to z score maps by Fisher's z transform: . The Z maps were used in group random effect analyses.
FIGURE 1.
Periaqueductal gray (PAG) mask from the Harvard Ascending Arousal Network (AAN) Atlas. The mask comprises 16 voxels
2.5 ∣. Group data analysis
We performed one-sample t test each on the Z maps of PAG mask for CD and HC and two-sample t test with age, sex, years of drinking, and years of smoking as covariates to compare the two groups. Following current reporting standards, all imaging results were evaluated with at voxel p < 0.001, uncorrected, in combination with cluster p < 0.05, FWE corrected, on the basis of Gaussian random field theory as implemented in SPM.
In region of interest (ROI) analysis, we used MarsBaR (http://marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity difference for the ROIs. Functional ROIs were defined of clusters as obtained from whole brain analysis. All voxel activations were presented in Montreal Neurological Institute (MNI) coordinates.
2.6 ∣. Mediation analysis
We performed mediation analyses,28 using the toolbox M3 (http://wagerlab.colorado.edu/tools) to examine the interrelationships between PAG–hypothalamus and PAG–dorsomedial prefrontal cortex (dmPFC) connectivities and CCQ score (see Section 3). In a mediation analysis, the relation between the independent variable X and dependent variable Y, for example, X → Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing three regression equations28:
where a represents X → M, b represents M → Y (controlling for X), c′ represents X → Y (controlling for M), and c represents X → Y. The constants i1, i2, and i3 are the intercepts, and e1, e2, and e3 are the residual errors. In the literature, a, b, c, and c′ were referred as path coefficients or simply paths,28 and we followed this notation. Variable M is said to be a mediator of the correlation X → Y if (c – c′), which is mathematically equivalent to the product of the paths a × b, is significantly different from zero.28 If the product a × b and the paths a and b are significant, one concludes that X → Y is mediated by M. In addition, if path c′ is not significant, there is no direct connection from X to Y and that X → Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
3 ∣. RESULTS
3.1 ∣. Resting state functional connectivity: CD versus HC
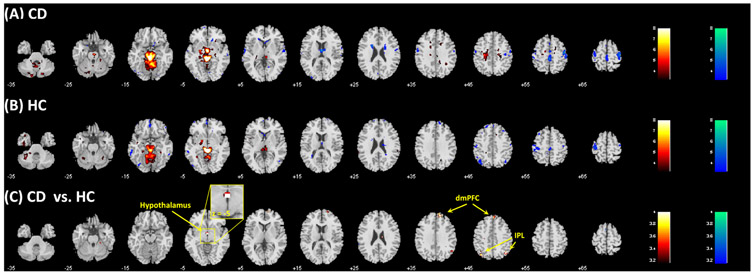
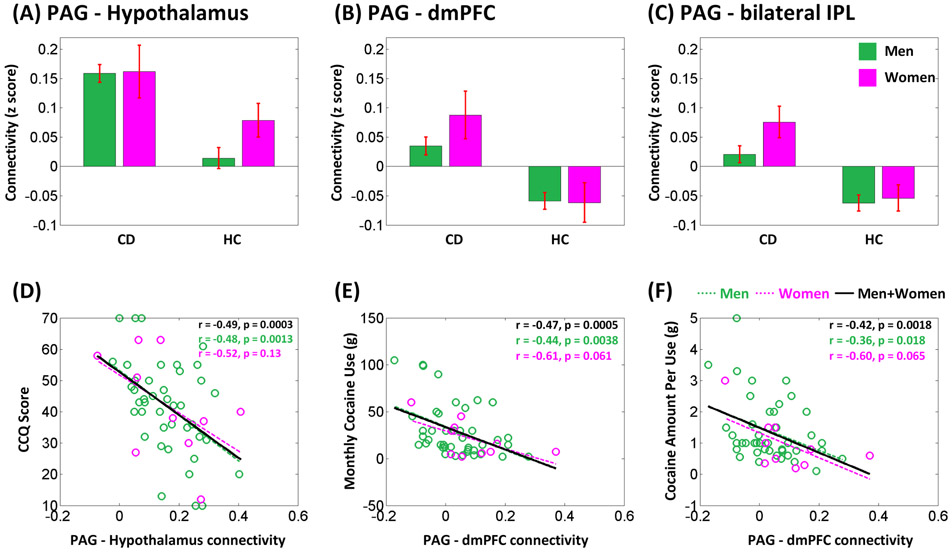
The results of a one-sample t test of the connectivity of PAG are shown in Figure 2A,B, each for CD and HC. We compared CD and HC in a two-sample t test with age, sex, years of drinking, and years of smoking as covariates (Figure 2C and Table 2). Compared with HC, CD showed increased PAG rsFC with the hypothalamus, dmPFC, and bilateral inferior parietal lobules (IPLs). No brain regions showed lower PAG rsFC in CD as compared with HC. We performed post hoc ROI analysis to examine sex differences of these rsFC's in a group (CD vs. HC) by sex (men vs. women) ANOVA with age, years of drinking, and years of smoking as covariates. There were a significant group main effect (all p's < 0.001), as expected, but no significant sex main (all p's > 0.16) or group by sex interaction effect (all p's > 0.44). We showed the mean ± S.E. of the rsFC's for CD men, CD women, HC men, and HC women in Figure 3A-C.
FIGURE 2.
Resting state functional connectivity (rsFC) maps of the periaqueductal gray (PAG) in (A) cocaine dependent (CD) and (B) healthy control (HC) as revealed in one-sample t test. Voxels shown in warm/cool color represent positive/negative connectivity. (C) Differences in rsFC (CD vs. HC) shown in a covariance analysis with age, sex, and years of drinking and smoking as covariates: warm color:CD > HC; cool color: HC > CD. The results of both one-sample t tests and covariane analyses were evaluated at p < 0.001 uncorrected and cluster-level p < 0.05 corrected for familywise error of multiple comparisons. Color bars show voxel T values. dmPFC, dorsomedial prefrontal cortex; IPL, inferior parietal lobule
TABLE 2.
Regions showing differences in resting state functional connectivity of the PAG between CD and HC
| Volume |
Peak voxel |
MNI coordinates (mm) |
||||
|---|---|---|---|---|---|---|
| (mm3) | (Z) | x | y | z | Side | Identified brain region |
| CD > HC | ||||||
| 378 | 4.83 | 0 | −4 | −5 | L/R | Hypothalamus |
| 837 | 4.65 | 42 | −67 | 46 | R | Inferior parietal lobule |
| 567 | 4.58 | −36 | −73 | 46 | L | Inferior parietal lobule |
| 918 | 4.35 | 12 | 50 | 34 | L/R | Dorsomedial prefrontal cortex |
| HC > CD | ||||||
| None | ||||||
Abbreviation: CD, cocaine dependent; HC, healthy control; L, left; MNI, Montreal Neurological Institute; PAG, periaqueductal gray; R, right.
FIGURE 3.
(A–C) Histograms of the rsFC (mean ± S.E.) of (A) PAG–hypothalamus, (B) PAG–dmPFC, and (C) PAG–bilateral IPLs, shown separately for men and women and for CD and HC. (D–F) RsFC strength of (D) PAG–hypothalamus was negatively correlated with CCQ score and PAG–dmPFC was negatively correlated with (E) monthly cocaine use as well as (F) cocaine amount per use. Each data point represents one subject. CCQ, Cocaine Craving Questionnaire; CD, cocaine dependent; dmPFC, dorsomedial prefrontal cortex; IPL, inferior parietal lobule; PAG, periaqueductal gray
3.2 ∣. rsFC and cocaine use variables
In examining the relationship between the PAG rsFC's and cocaine use variables, we focused on CCQ score, CSSA score, monthly cocaine use, cocaine amount per use, days of cocaine use, and years of cocaine use and evaluated the results at a corrected p = 0.05/6 = 0.0083. Across CD, the PAG–hypothalamus connectivity strength (z score) was negatively correlated with CCQ score (r = −0.49, p = 0.0003) (Figure 3D). The PAG–dmPFC connectivity strength was negatively correlated with the monthly quantity of cocaine use (r = −0.47, p = 0.0005) (Figure 3E) and with the amount per use (r = −0.42, p = 0.0018) (Figure 3F). With men and women examined separately, the PAG–hypothalamus connectivity strength was negatively correlated with CCQ score in men (r = −0.48, p = 0.0013) but not in women (r = −0.52, p = 0.13). The PAG–dmPFC connectivity strength was negatively correlated with monthly cocaine use in men (r = −0.44, p = 0.0038) but not in women (r = −0.61, p = 0.061). The PAG–dmPFC connectivity strength was negatively correlated with cocaine amount per use in men (r = −0.36, p = 0.018) but not in women (r = −0.60, p = 0.065). However, slope test showed no differences in the correlation between men and women (all p's > 0.67).
None of the rsFC strength was correlated with age (all p's > 0.14), years of drinking (all p's > 0.34), or years of smoking (all p's > 0.19). Nonetheless, we conducted additional analyses to account for age, years of drinking, and years of smoking. With these variables as covariates, the PAG–hypothalamus connectivity strength was still negatively correlated with CCQ score (r = −0.50, p = 0.0003). The PAG–dmPFC connectivity strength was still negatively correlated with monthly cocaine use (r = −0.45, p = 0.0014) and cocaine amount per use (r = −0.41, p = 0.0041). With men and women examined separately, the PAG–hypothalamus connectivity strength was negatively correlated with CCQ score in men (r = −0.52, p = 0.0008) but not in women (r = −0.52, p = 0.29). The PAG–dmPFC connectivity strength was negatively correlated with monthly cocaine use in men (r = −0.45, p = 0.0044) but not in women (r = −0.43, p = 0.39). The PAG–dmPFC connectivity strength was negatively correlated with cocaine amount per use in men (r = −0.38, p = 0.017) but not in women (r = −0.73, p = 0.097). Again, however, slope tests showed no differences in the correlation between men and women (all p's > 0.14).
3.3 ∣. Mediation analyses
The PAG–hypothalamus connectivity strength was negatively correlated with CCQ score as shown earlier. The PAG–dmPFC connectivity strength was also correlated negatively with CCQ score (r = −0.35, p = 0.011) and positively with PAG–hypothalamus connectivity strength (r = 0.33, p = 0.015). Thus, we conducted mediation analysis to examine the interrelationship between PAG–hypothalamus and PAG–dmPFC connectivities and CCQ. The results showed a significant mediation effect for the model with PAG–dmPFC connectivity contributing to CCQ and, in turn, PAG–hypothalamus connectivity (Figure 4A). Further, with the mediation of CCQ score, PAG–dmPFC connectivity was not correlated with PAG–hypothalamus connectivity (p = 0.16), suggesting a complete mediation. None of the other models were significant in the mediation effect (Figure 4).
FIGURE 4.
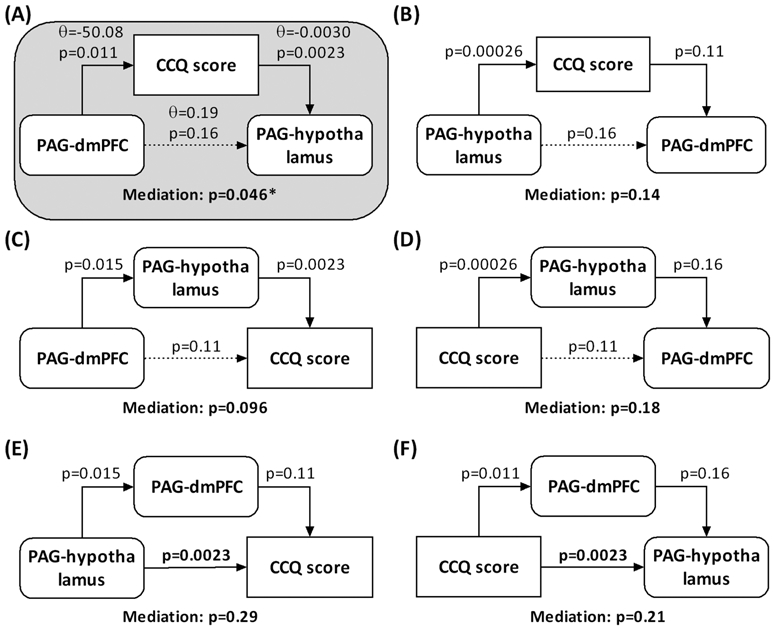
Mediation analysis of PAG–dmPFC and PAG–hypothalamus connectivities and CCQ score. The p values associated with mediation are for the path “a × b” (see Section 2). ϴ represented the path of each connection. Positive ϴ means positive connection, whereas negative ϴ means negative connection. Only Model A was significant. The model suggested that tonic cocaine craving mediated the relationship between rsFC strengths of PAG dmPFC and PAG hypothalamus. CCQ, Cocaine Craving Questionnaire; CD, cocaine dependent; dmPFC, dorsomedial prefrontal cortex; IPL, inferior parietal lobule; PAG, periaqueductal gray
4 ∣. DISCUSSION
The current study examined resting PAG connectivity in CD in contrast with HC. Compared with HC, CD showed higher PAG rsFC with the hypothalamus, dmPFC, and bilateral IPL. Notably, the strength of PAG rsFC with the hypothalamus was negatively correlated with CCQ score, and the strength of PAG rsFC with the dmPFC was negatively correlated with monthly cocaine use and cocaine amount per use. Further, CCQ score completely mediated the correlation between PAG–dmPFC and PAG–hypothalamus connectivities. These findings provide evidence in support of PAG circuit dysfunction as a neural marker of cocaine addiction.
4.1 ∣. Altered PAG rsFC with the hypothalamus in cocaine addiction
We observed increased PAG rsFC with the hypothalamus, dmPFC, and bilateral IPL in CD versus HC. Bidirectionally connected with the PAG anatomically,29 the hypothalamus processes motivational signals in both appetitive and aversive contexts.30,31 For instance, lateral hypothalamic neurons respond to appetitive stimuli such as food cues and food intake32,33 as well as to aversive stimuli such as tail pinch or electrical shock.32,34 In nonhuman primates too, lateral hypothalamic neurons showed consistent responses to both appetitive and aversive stimuli.35 In rodents, the transition from controlled to compulsive cocaine self-administration was associated with substantial remodeling of hypothalamic circuitry.36 In human imaging, CD relative to HC showed altered hypothalamus activation viewing erotic versus neutral pictures.37 Hypothalamus response to monetary reward versus nonreward was associated with the duration of abstinence in CD.38 We previously examined cue reactivity in 23 CDs during fMRI, and, at a corrected threshold, observed higher hypothalamic activation to cocaine versus neutral cues.39 Further, we examined cue-elicited brain activations in another cohort of 20 CDs engaged in both cocaine and food craving tasks and 24 HC matched in demographics and body mass index (BMI) in the food craving task.40 We demonstrated higher hypothalamic activation during exposure to cocaine versus food cues in CD and during exposure to food cues in CD as compared with HC, with hypothalamic response to both cocaine and food cues in correlation with daily cocaine craving and with days of cocaine use in the past month in CD. These earlier findings implicate the hypothalamus as part of the appetitive/approach circuit of addiction. Here, by demonstrating altered PAG connectivity with the hypothalamus and the correlation of PAG–hypothalamus rsFC with tonic cocaine craving, we provide evidence suggesting the role of hypothalamus in the aversive/avoidance mechanisms of cocaine misuse.
4.2 ∣. Altered PAG rsFC with the dmPFC in cocaine addiction
Rodent research indicated that the dmPFC exhibited neuronal activation concomitant with cocaine-seeking behavior, and dmPFC inactivation impaired stimulus-induced reinstatement of cocaine seeking.41 Infusion of brain-derived neurotrophic factor in the dmPFC in early withdrawal attenuated relapse to cocaine seeking.42 These findings demonstrated a key role of the dmPFC in cocaine seeking. Further, PAG-projecting neurons of the dmPFC mediated contextual fear discrimination.43 Meta-analyses showed both PAG and dmPFC within a core network that supports aversion-related behaviors.44,45 In humans, dynamic causal modeling showed that the PAG conveyed prediction errors signals to prefrontal regions, including the dmPFC, to avoid pain.46 Together, these studies suggest a role of the dmPFC in cocaine seeking and PAG–dmPFC connectivity in behavioral avoidance. Studies are needed to directly investigate how the PAG–dmPFC circuit may contribute to cocaine seeking via negative reinforcement.
Both the PAG and hypothalamus support the physiological responses to stress and defensive behavior through the hypothalamus–pituitary–adrenal axis and autonomic nervous system. A meta-analysis of 162 human studies reported consistent activation of the PAG, dmPFC, and hypothalamus during negative emotional processing.5 Importantly, the coactivation of dmPFC and hypothalamus was mediated by the PAG. In current study, we observed that the relationship between PAG–dmPFC and PAG–hypothalamus rsFCs was mediated by tonic cocaine craving, as assessed by the CCQ, in CD. Many preclinical studies have supported a hypothalamic orexinergic mechanism in modulation PAG responses to pain.47,48 For instance, intra-PAG administration of a selective orexin-1 receptor antagonist dose dependently prevented the development of analgesia induced by chemical stimulation of the lateral hypothalamus in rats.47 Pharmacological inactivation of the dorsal premammillary nucleus of the hypothalamic defensive circuit impaired the acquisition of olfactory fear conditioning promoted by stimulation of the N-methyl-D-aspartate receptor in the PAG.48 Optogenetic activation of the ventromedial hypothalamus induces defensive behaviors, including inflexible immobility and active avoidance, via distinct pathways to the PAG.49 Together, the findings suggested that the PAG as well as the PAG connectivity with the dmPFC and hypothalamus plays an important role in the response to negative emotional state during cocaine withdrawal and craving.
Using the fMRI data collected of a cue reactivity paradigm from the same 52 CD, we previously observed sex differences in task-related PAG connectivity during exposure to cocaine versus neutral cues.12 Specifically, we observed that the PAG–vmPFC connectivity in response to viewing cocaine versus neutral cues was positively correlated with the CCQ score for men but negatively for women, and the sex difference was confirmed by a slope test. Further, the time series of PAG Granger caused the time series of the vmPFC in men, but the reverse was true in women, suggesting sex differences in directional interactions of the PAG and vmPFC. In contrast, we did not observe sex difference in PAG rsFC in the current study. The findings suggest that the sex differences in PAG circuit activity we observed previously may be specific to cocaine cue exposure and cue-induced craving. It remains to be seen whether sex differences also manifest in avoidance learning and other psychological processes that implicate the PAG and relate to the pathophysiology of cocaine addiction.
4.3 ∣. Limitations of the study, conclusions, and future research
Several limitations of the study need to be considered. First, participants stayed eyes-closed for the resting state scan. A recent study reported higher reliability of rsFC for eyes-opened as compared with eyes-closed state.50 Although the authors also pointed out that the difference was very small, the current findings would need to be replicated using eyes-opened resting state data. Second, the sample size was moderate. In particular, men and women were unevenly represented. The findings of lack of sex differences would need to be revisited with a larger sample. Third, CDs and HCs showed significant difference in years of smoking. Although we included years of smoking and other clinical variables as covariates in data analyses, we could not entirely rule out the effects of cigarette smoking on the current findings. Fourth, other than clinical measures of tonic cocaine craving and cocaine use, we did not have behavioral quantifiers of how the negative emotional state may impact decision making or cocaine use. Studies are warranted to address this important issue. Finally, we did not formulate specific hypotheses as to how PAG rsFC may differentiate CD and HC. Thus, the study should be considered as exploratory in nature.
In summary, we showed increased PAG rsFC with the hypothalamus and dmPFC in CD as compared with HC. Importantly, the strength of the PAG rsFC was associated cocaine use severity. The findings provide additional evidence in support of PAG circuit dysfunction as a neural marker of cocaine addiction.
ACKNOWLEDGEMENTS
Supported by NIH grants DA040032, DA045743, DA044749, and DA023248, as well as the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. The funding agencies otherwise have no roles in the conceptualization of the study, data collection and analysis, or the decision to publish these results. We thank the medical and nursing staff at the Clinical Neuroscience Research Unit, Connecticut Mental Health Center for medical care of the participants. We declare no financial interests in the current work.
Funding information
National Institute on Drug Abuse, Grant/Award Numbers: DA023248, DA040032, DA044749, DA045743; Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol. 2015;753:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl). 2000; 152(2):140–148. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63 (3):324–331. [DOI] [PubMed] [Google Scholar]
- 4.Buhle JT, Kober H, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci. 2013;8(6):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George DT, Ameli R, Koob GF. Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci. 2019;42(5):349–360. [DOI] [PubMed] [Google Scholar]
- 7.Avegno EM, Lobell TD, Itoga CA, et al. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J Neurosci. 2018;38 (36):7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siciliano CA, Noamany H, Chang CJ, et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science. 2019;366 (6468):1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261(2):669–677. [PubMed] [Google Scholar]
- 10.Mutschler NH, Miczek KA, Hammer RP. Reduction of zif268 messenger RNA expression during prolonged withdrawal following "binge" cocaine self-administration in rats. Neuroscience. 2000;100(3):531–538. [DOI] [PubMed] [Google Scholar]
- 11.Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology (Berl). 1999;141(3):225–234. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Zhornitsky S, Wang W, Dhingra I, Le TM, Li CR. Cue-elicited functional connectivity of the periaqueductal gray and tonic cocaine craving. Drug Alcohol Depend. 2020;216:108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasi D, Volkow ND. Association between brain activation and functional connectivity. Cereb Cortex. 2019;29(5):1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010; 211(2):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Wang G, Liu Y, et al. Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: a resting-state fMRI study. Neurogastroenterol Motil. 2017;29(8):e13060. [DOI] [PubMed] [Google Scholar]
- 16.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66(11):1189–1200. [DOI] [PubMed] [Google Scholar]
- 17.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harricharan S, Rabellino D, Frewen PA, et al. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 2016;6(12):e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper DE, Ichesco E, Schrepf A, et al. Resting functional connectivity of the periaqueductal gray is associated with normal inhibition and pathological facilitation in conditioned pain modulation. J Pain. 2018; 19(6):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID). Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- 21.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–237. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a Cocaine Craving Questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. [DOI] [PubMed] [Google Scholar]
- 23.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 24.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71(6):531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vianna DML, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36(5):557–566. [DOI] [PubMed] [Google Scholar]
- 30.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–577. [DOI] [PubMed] [Google Scholar]
- 31.Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res. 2012;226(2):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono T, Nakamura K, Nishijo H, Fukuda M. Hypothalamic neuron involvement in integration of reward, aversion, and cue signals. J Neurophysiol. 1986;56(1):63–79. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda M, Ono T, Nishino H, Sasaki K. Visual responses related to food discrimination in monkey lateral hypothalamus during operant feeding-behavior. Brain Res. 1986;374(2):249–259. [DOI] [PubMed] [Google Scholar]
- 34.Sikdar SK, Oomura Y. Selective-inhibition of glucose-sensitive neurons in rat lateral hypothalamus by noxious stimuli and morphine. J Neurophysiol. 1985;53(1):17–31. [DOI] [PubMed] [Google Scholar]
- 35.Noritake A, Nakamura K. Encoding prediction signals during appetitive and aversive Pavlovian conditioning in the primate lateral hypothalamus. J Neurophysiol. 2019;121(2):396–417. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed SH, Lutjens R, van der Stap LD, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S a. 2005;102(32):11533–11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asensio S, Romero MJ, Palau C, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI study. Addict Biol. 2010;15(4):504–516. [DOI] [PubMed] [Google Scholar]
- 38.Bustamante JC, Barros-Loscertales A, Costumero V, et al. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol. 2014;19(5):885–894. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Zhornitsky S, Angarita GA, Li CR. Hypothalamic response to cocaine cues and cocaine addiction severity. Addict Biol. 2020;25(1):e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Zhornitsky S, Le TM, Li CR. Hypothalamic responses to cocaine and food cues in individuals with cocaine dependence. Int J Neuropsychopharmacol. 2019;22(12):754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs RA, Evans KA, Ledford CC, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. [DOI] [PubMed] [Google Scholar]
- 42.McGinty JF, Zelek-Molik A, Sun WL. Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal. Brain Res. 2015;1628(Pt A):82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozeske RR, Jercog D, Karalis N, et al. Prefrontal-periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron. 2018;97(4):898–910. [DOI] [PubMed] [Google Scholar]
- 44.Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci. 2012;13(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes DJ, Northoff G. Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front Integr Neurosci. 2011;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17(11):1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esmaeili MH, Reisi Z, Ezzatpanah S, Haghparast A. Functional interaction between orexin-1 and CB1 receptors in the periaqueductal gray matter during antinociception induced by chemical stimulation of the lateral hypothalamus in rats. Eur J Pain. 2016;20 (10):1753–1762. [DOI] [PubMed] [Google Scholar]
- 48.Kincheski GC, Mota-Ortiz SR, Pavesi E, Canteras NS, Carobrez AP. The dorsolateral periaqueductal gray and its role in mediating fear learning to life threatening events. PLoS ONE. 2012;7(11):e50361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Chen IZ, Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron. 2015;85(6):1344–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou QH, Miao XY, Liu DP, Wang DJJ, Zhuo Y, Gao JH. Reliability comparison of spontaneous brain activities between BOLD and CBF contrasts in eyes-open and eyes-closed resting states. Neuroimage. 2015;121:91–105. [DOI] [PubMed] [Google Scholar]