Abstract
The aim of the present study was to investigate the frequency of extended-spectrum β-lactamases (ESBLs) in a consecutive collection of clinical isolates of Enterobacter spp. The abilities of various screening methods to detect ESBLs in enterobacters were simultaneously tested. Among the 68 consecutive isolates (56 Enterobacter cloacae and 12 Enterobacter aerogenes isolates) that were analyzed for β-lactamase content, 21 (25 and 58%, respectively) possessed transferable ESBLs with pIs of 8.2 and phenotypic characteristics of SHV-type enzymes, 8 (14.3%) of the E. cloacae isolates produced a previously nondescribed, clavulanate-susceptible ESBL that exhibited a pI of 6.9 and that conferred a ceftazidime resistance phenotype on Escherichia coli transconjugants, and 2 E. cloacae isolates produced both of these enzymes. Among the total of 31 isolates that were considered ESBL producers, the Vitek ESBL detection test was positive for 2 (6.5%) strains, and the conventional double-disk synergy test (DDST) with amoxicillin-clavulanate and with expanded-spectrum cephalosporins and aztreonam was positive for 5 (16%) strains. Modifications of the DDST consisting of closer application of the disks (at 20 instead of 30 mm), the use of cefepime, and the use of both modifications increased the sensitivity of this test to 71, 61, and 90%, respectively. Of the 37 isolates for which isoelectric focusing failed to determine ESBLs, the Vitek test was false positive for 1 isolate and the various forms of DDSTs were false-positive for 3 isolates.
The occurrence of extended-spectrum β-lactamases (ESBLs) in enterobacteria that possess inducible Bush group 1 chromosomal β-lactamases is increasingly reported worldwide (1, 2, 4–9, 10, 12, 15, 17, 18). In these species, the detection of ESBLs by methods based on the inhibitory effects of clavulanic acid is expected to be difficult and is dependent on the level of chromosomal enzyme production (3). From a clinical point of view, the discrimination between ESBLs and overproduced class C β-lactamases may not be critical, since the therapeutic options for infections caused by organisms that possess any of these mechanisms of resistance are similarly limited (22). Nevertheless, the detection of such “hidden” ESBLs is still of epidemiological importance for the hospital environment (3).
Derepressed production of the AmpC β-lactamase has previously been documented as a prevalent mechanism of β-lactam resistance in Enterobacter cloacae strains isolated in Greece (24). On the other hand, there are no studies on the occurrence of ESBLs in enterobacters, although high frequencies of such enzymes have repeatedly been reported in strains of Klebsiella pneumoniae, Escherichia coli, and Serratia marcescens isolated in Greek hospitals (9, 19, 23). In the present study we attempted to determine the frequency of ESBLs in a consecutive sample of clinical isolates of E. cloacae and Enterobacter aerogenes by using various screening procedures, as well as isoelectric focusing and conjugal transfer of β-lactamases.
MATERIALS AND METHODS
Bacterial strains.
A total of 68 isolates of Enterobacter spp. (56 E. cloacae and 12 E. aerogenes isolates) were studied. The isolates were nonrepetitive (one per patient) and were obtained consecutively from clinical specimens in the Hippokration General Hospital, Thessaloniki, Greece, from January 1998 through March 1999. Species identification was done by using the Vitek automated identification system (bioMérieux, Marcy l'Etoile, France) and was confirmed with the ATB-GN system (bioMérieux).
Antimicrobial susceptibility testing and screening for ESBLs.
The susceptibilities of the isolates to antibiotics were determined by the standard disk diffusion method as described in the guidelines of the National Committee for Clinical Laboratory Standards (16). The MICs of selected β-lactams were determined for isolates with representative susceptibility phenotypes and E. coli transconjugants by the Etest method (AB Biodisk, Solna, Sweden). All susceptibility results were interpreted by using the breakpoints from the National Committee for Clinical Laboratory Standards (16). The isolates were screened for the presence of ESBLs by the Vitek ESBL detection test (bioMérieux) by using cefotaxime and ceftazidime alone (at 0.5 μg/ml) and in combination with clavulanic acid (4 μg/ml). Double-disk synergy tests (DDSTs) (14) were also performed by placing disks of ceftazidime, cefotaxime, ceftriaxone, aztreonam, cefepime, and cefpirome (30 μg each) at distances of 30 and 20 mm (center to center) from a disk containing amoxicillin plus clavulanate (Amc; 20 g and 10 μg, respectively).
Conjugal transfer experiments.
Strains with resistance or decreased susceptibility to expanded-spectrum cephalosporins (ESCs) as well as ESC-susceptible strains with positive indications by at least one of the ESBL screening tests used were mated with an E. coli K-12 recipient (strain 1R716; R− Strr). Transconjugants were selected on MacConkey agar containing streptomycin (500 μg/ml) and ceftazidime (10 μg/ml) or ampicillin (25 μg/ml).
IEF of β-lactamases.
Isoelectric focusing (IEF) was performed by electrophoresis of ultrasonic cell extracts on polyacrylamide gels containing ampholytes with pHs that ranged from 3.5 to 9.5 (Amersham Pharmacia Biotech AB, Uppsala, Sweden). β-Lactamases were visualized with a 0.2-mg/ml nitrocefin solution (Oxoid Ltd., Basingstoke, England) and were applied on one of two parallel focused gels immediately and on the other after it was flooded with a 1 mM solution of potassium clavulanate. This allowed the in situ distinction between class C β-lactamases that were insensitive to clavulanate and plasmid-mediated enzymes that were susceptible to clavulanate (21).
ERIC2 PCR typing.
The ESC-resistant isolates were typed by the ERIC2 PCR method (13). Extracted genomic DNA (approximately 100 ng from each isolate) was amplified in a final volume of 50 μl containing 20 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.2 mM (each) deoxynucleoside triphosphate, 2 mM MgCl2, 50 pmol of the ERIC2 primer (5′-AAGTAAGTGACTGGGGTGA GCG-3′), and 0.5 U of Taq DNA polymerase (Promega). The PCR products were separated in 1.2% agarose.
RESULTS
By disk diffusion susceptibility testing, all of the 68 Enterobacter isolates studied were resistant to cefoxitin and were classified into three main phenotypes according to their susceptibilities to the ESCs and aztreonam. Eighteen (32%) of 56 E. cloacae isolates and 3 (25%) of 12 E. aerogenes isolates were susceptible to all newer β-lactams (ESC-susceptible group). Eight (14.3%) of the E. cloacae isolates were resistant to ceftazidime and were either intermediately susceptible or susceptible to cefotaxime, ceftriaxone, and aztreonam (Caz-resistant group). The remaining 30 (53.6%) E. cloacae isolates and 9 (75%) E. aerogenes isolates were resistant to all the β-lactams listed above (ESC-resistant group). Seven of the ESC-resistant E. cloacae isolates were also resistant to cefepime and cefpirome.
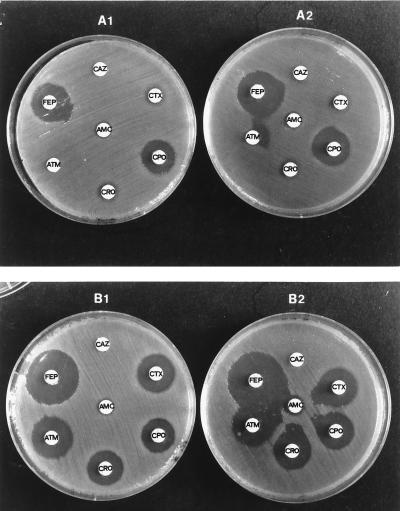
By initial screening of the isolates for ESBLs, the Vitek ESBL test was positive for two Caz-resistant E. cloacae strains and one ESC-susceptible E. cloacae strain. The conventional DDST with ESC and aztreonam disks at 30 mm from the disk of Amc was positive for six isolates, including another ESC-susceptible E. cloacae isolate. By applying the same disks at 20-mm distances, the number of isolates with positive DDST results increased to 22. Finally, the use of cefepime and cefpirome at distances 30 and 20 mm from Amc resulted in positive DDST results for 22 and 29 enterobacters, respectively. DDST results for two representative strains are shown in Fig. 1.
FIG. 1.
Various forms of DDST for two representative isolates of E. cloacae (TSV-287 and TSV-9) producing β-lactamases of pI 8.2 (A) and 6.9 (B), respectively. A1 and B1, tests with application of the cephalosporin disks at a standard distance of 30 mm from the clavulanate-containing disk (center to center); A2 and B2, tests with proximal application of the disks at 20 mm. AMC, amoxicillin-clavulanate; CAZ, ceftazidime; CTX, cefotaxime; CRO, ceftriaxone; FEP, cefepime; CPO, cefpirome.
To confirm these findings, we analyzed the β-lactamase contents of the isolates. On IEF gels, all of the ESC-susceptible enterobacters, as well as 12 of the ESC-resistant isolates (10 E. cloacae and 2 E. aerogenes isolates) presented single β-lactamase bands (pI range, 7.8 to 9.2) which were not inhibited by potassium clavulanate and which were thus considered class C enzymes. The remaining 35 isolates showed one or more additional β-lactamase bands which focused at pIs of 6.9, 8.2, and 5.4 and which were labile to the inhibitory action of clavulanate. In conjugation experiments in which ceftazidime was used for selection, transconjugants were obtained only from enterobacters that possessed enzymes of pI 8.2 or 6.9, and all of them showed respective β-lactamase bands on IEF gels. Transconjugant clones from four strains that produced the β-lactamase of pI 5.4 alone were selected on ampicillin. Matings of the two ESC-susceptible E. cloacae strains that were positive by ESBL screening tests showed that they were infertile. The MICs of β-lactams for representative clinical strains and E. coli transconjugants are presented in Table 1. The clones that had acquired the enzyme of pI 6.9 expressed high levels of resistance only to ceftazidime, while those that produced the β-lactamase of pI 8.2 were resistant to all ESCs and aztreonam. Both types of tranconjugant clones were more susceptible to penicillin-clavulanic acid combinations than to the respective penicillins alone, indicating efficient inhibition of the β-lactamases produced by this inhibitor. Moreover, both types of tranconjugants were positive for ESBL production by the Vitek test as well as the conventional DDST. In all cases, resistance to aminoglycosides, co-trimoxazole, and tetracycline had been cotransferred. On the basis of these results, both enzymes were considered to be ESBLs, and the number of ESBL producers among the total 68 enterobacters was confirmed to be 31 (45.6%). The β-lactamase of pI 8.2 encountered in 16 (28.6%) of the E. cloacae isolates and 7 (58.3%) of the E. aerogenes isolates and the enzyme of pI 6.9 were present in 10 (17.9%) of the E. cloacae isolates (in 2 of them together with the enzyme of pI 8.2).
TABLE 1.
MICs of selected β-lactams and β-lactamase contents for representative enterobacters and E. coli transconjugants
| Strain | β-Lactamase pI(s)a | Etest MIC (μg/ml)b
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amx | Amc | Tic | TLc | Pip | PTz | Fox | Caz | Ctx | Cro | Atm | Fep | Cpo | ||
| Caz-resistant group | ||||||||||||||
| E. cloacae TSV-9 | (8.0) + 6.9 | >256 | 96 | >256 | 128 | 128 | 16 | 256 | >256 | 8 | 12 | 8 | 0.75 | 8 |
| trcc TSV-9 | (8.5) + 6.9 | >256 | 8 | >256 | 32 | 32 | 1.5 | 3 | >256 | 2 | 4 | 4 | 0.38 | 4 |
| E. cloacae TSV-213d | (8.0) + 6.9 | >256 | 128 | >256 | 256 | 48 | 1.5 | 256 | 192 | 4 | 6 | 3 | 0.38 | 6 |
| ESC-resistant group | ||||||||||||||
| E. cloacae TSV-43 | (8.0) + 6.9 + 5.4 | >256 | 192 | >256 | 192 | 128 | 16 | 192 | >256 | 12 | 32 | 16 | 1.5 | 16 |
| trc TSV-43 | (8.5) + 6.9 | >256 | 12 | >256 | 64 | 64 | 1.5 | 2 | >256 | 2 | 3 | 4 | 0.25 | 3 |
| E. cloacae TSV-287 | (8.0) + 8.2 | >256 | 192 | >256 | 128 | >256 | 64 | 192 | >256 | 128 | 192 | >256 | 6 | 16 |
| trc TSV-287 | (8.5) + 8.2 | >256 | 6 | >256 | 4 | 128 | 12 | 2 | 48 | 4 | 1.5 | 24 | 0.25 | 0.75 |
| E. aerogenes TSV-89 | (8.3) + 8.2 | >256 | 12 | >256 | >256 | >256 | 32 | >256 | >256 | 24 | 64 | >256 | 2 | 8 |
| trc TSV-89 | (8.5) + 8.2 | >256 | 8 | >256 | 6 | 128 | 16 | 3 | 64 | 6 | 2 | 32 | 0.5 | 1.5 |
| E. cloacae TSV-144e | (8.0) + 5.4 | >256 | 192 | >256 | 128 | 192 | 64 | >256 | 32 | 32 | 32 | 16 | 0.38 | 2 |
| trc TSV-144 | (8.5) + 5.4 | >256 | 8 | >256 | 6 | 128 | 8 | 2 | 0.19 | 0.047 | 0.064 | 0.094 | 0.047 | 0.5 |
| E. cloacae TSV-68 | (9.0) | >256 | 192 | >256 | >256 | >256 | >256 | >256 | 128 | >256 | >256 | 96 | 8 | 24 |
| E. aerogenes TSV-272 | (8.3) | 128 | 16 | 256 | 192 | 128 | 64 | >256 | 64 | 24 | >32 | 8 | 0.75 | 4 |
| ESC-susceptible group | ||||||||||||||
| E. cloacae TSV-323f | (8.0) | 256 | 32 | 6 | 4 | 2 | 2 | 96 | 0.5 | 0.125 | 0.125 | 0.064 | 0.032 | 0.094 |
| E. cloacae TSV-123e | (9.2) | 96 | 12 | 4 | 3 | 4 | 3 | >256 | 0.38 | 0.125 | 0.125 | 0.094 | 0.032 | 0.094 |
| E. aerogenes TSV-92 | (8.3) | 128 | 12 | 2 | 3 | 4 | 3 | >256 | 0.5 | 0.19 | 0.19 | 0.125 | 0.064 | 0.064 |
| E. coli 1R716 (recipient) | (8.5) | 3 | 3 | 1 | 1.5 | 1 | 1 | 4 | 0.25 | 0.064 | 0.064 | 0.125 | 0.032 | 0.047 |
pIs in parentheses refer to those of β-lactamases considered chromosomal. Boldface numbers indicate pIs of ESBLs.
Abbreviations: Amx, amoxicillin; Amc, amoxicillin-clavulanate; Tic, ticarcillin; TLc, ticarcillin-clavulanate; Pip, piperacillin; PTz, piperacillin-tazobactam; Fox, cefoxitin; Caz, ceftazidime; Ctx, cefotaxime; Cro, ceftriaxone; Atm, aztreonam; Fep, cefepime; Cpo, cefpirome.
trc, transconjugated.
Strains with true-positive Vitek ESBL detection test results.
Two of three strains had false-positive DDST results.
Strains with false-positive Vitek ESBL detection test results.
By ERIC2 PCR typing, all eight isolates with the Caz resistance phenotype and the β-lactamase of pI 6.9 exhibited the same pattern. Similarly, the majority (11 of 16) of the E. cloacae isolates that possessed the enzyme of pI 8.2 exhibited a different pattern, which was also present in four of the ESC-resistant, non-ESBL-producing E. cloacae isolates. The remaining 15 ESC-resistant isolates were distributed into three unrelated ERIC2 PCR patterns. Of the nine ESC-resistant E. aerogenes isolates, eight, including all those that were confirmed to be ESBL producers, showed the same ERIC2 PCR pattern (data not shown).
The scores obtained by the various ESBL screening tests are presented in Table 2. The more efficient tests were the DDSTs with cefepime or aztreonam at 20 mm, which were found to be true positive for 28 (90.3%) and 21 (67.7%) of 31 ESBL producers, respectively, and false positive for none of the 37 isolates for which IEF and conjugal transfer experiments failed to confirm the presence of ESBLs. DDSTs with cefpirome were generally less sensitive than those with cefepime. The conventional DDST, as well as the Vitek ESBL detection test, were inadequate. The Vitek test was false positive for one strain, and the DDSTs with ceftazidime, aztreonam, and cefpirome at 30 mm were false positive for another E. cloacae strain in the ESC-susceptible group. Two more (ESC-resistant) strains for which the DDSTs of cefepime and cefpirome indicated the presence of ESBLs were considered false positive, since they produced only their chromosomal β-lactamase plus an enzyme of pI 5.4 that conferred a penicillinase resistance phenotype on the respective E. coli transconjugants (Table 1).
TABLE 2.
Comparative evaluation of the Vitek ESBL detection test and the various forms of DDSTs applied for screening of ESBLs among 56 E. cloacae and 12 E. aerogenes isolates
| ESBL screening method | No (%) of positive tests among confirmed ESBL producers
|
No (%) of positive tests among non-ESBL producers
|
||||
|---|---|---|---|---|---|---|
| E. cloacae (n = 24) | E. aerogenes (n = 7) | All isolates (n = 31) | E. cloacae (n = 32) | E. aerogenes (n = 5) | All isolates (n = 37) | |
| Vitek ESBL test | 2 (8.3) | 0 | 2 (6.4) | 1 (3.1) | 0 | 1 (2.7) |
| DDSTs | ||||||
| Amc, 30 mm from | ||||||
| Caz | 0 | 0 | 0 | 1 | 0 | 1 (2.7) |
| Ctx | 0 | 2 | 2 | 0 | 0 | 0 |
| Cro | 2 | 1 | 1 | 0 | 0 | 0 |
| Atm | 2 | 0 | 2 | 1 | 0 | 1 |
| At least one combination | 3 (12.5) | 2 (28.6) | 5 (16.1) | 1 (3.1) | 0 | 1 (2.7) |
| Amc, 20 mm from | ||||||
| Caz | 7 | 2 | 9 | 0 | 0 | 0 |
| Ctx | 8 | 4 | 12 | 0 | 0 | 0 |
| Cro | 8 | 4 | 12 | 0 | 0 | 0 |
| Atm | 17 | 4 | 21 | 0 | 0 | 0 |
| At least one combination | 17 (70.8) | 5 (71.4) | 22 (71) | 0 | 0 | 0 |
| Amc, 30 mm from | ||||||
| Cpo | 7 | 4 | 11 | 3 | 0 | 3 |
| Fep | 13 | 6 | 19 | 2 | 0 | 2 |
| At least one combination | 13 (54.2) | 6 (85.7) | 19 (61.3) | 3 (9.4) | 0 | 3 (8.1) |
| Amc, 20 mm from | ||||||
| Cpo | 18 | 6 | 24 | 1 | 0 | 1 |
| Fep | 22 | 6 | 28 | 0 | 0 | 0 |
| At least one combination | 22 (91.7) | 6 (85.7) | 28 (90.3) | 1 (3.1) | 0 | 1 (2.7) |
| At least one DDST | 24 (100) | 6 (85.7) | 30 (96.8) | 3 (9.4) | 0 | 3 (8.1) |
DISCUSSION
The results of this study showed a high frequency of ESBLs in the sample of E. aerogenes and E. cloacae isolates examined. The most frequently encountered ESBL of pI 8.2 is most likely the SHV-5-type β-lactamase, which is widely disseminated among clinical strains of K. pneumoniae, E. coli, and S. marcescens isolated in Greek hospitals (9, 19, 23). The available data do not allow the assignment of the pI 6.9 enzyme to any of the known ESBL groups. However, the characterization of this probably novel ESBL is beyond of the scope of this study. On the basis of ERIC2 PCR typing, the ESBL-producing isolates seemed to represent a limited number of types; nevertheless, the presence of the same β-lactamase in strains of different types and species is indicative of the fact that horizontal gene spread may also be responsible for the high frequency of detection of ESBLs among enterobacters in this setting.
Our findings support previous suggestions that the currently used ESBL detection methods, which are based on the inhibitory effect of clavulanic acid on the activities of ESBLs against ESCs, are inadequate in cases of overproduction of Bush group 1 β-lactamases (3, 25). The Vitek ESBL detection test and the conventional DDST were the least sensitive methods in our setting. In a previous study, the Vitek test was found to be efficient with E. coli and Klebsiella spp., but its reliability with Enterobacter spp. and Serratia spp. remained questionable (20). In a recent report, the Vitek ESBL detection test, as well as the conventional DDST, were unable to detect the SHV-5 β-lactamase in a K. pneumoniae strain that produced a plasmid-borne AmpC-type β-lactamase; the ESBL present in that strain had been successfully detected by a DDST that combined Amc with cefepime (25). In accordance with this observation, the use of cefepime increased the sensitivity of the DDST with ESCs for the detection of ESBLs in enterobacters from 16 to 61% when the disks were applied at the standard distance of 30 mm from clavulanate and from 71 to 90% with closer application of the disks. The greater efficacy of cefepime and cefpirome instead of ESCs by ESBL detection methods on the basis of the inhibitory effect of clavulanic acid is expected, since the former, but not the latter, retain activity against derepressed variants of enterobacters (11). “Proximal” DDSTs with cephalosporin disks placed 20 mm from the disk containing clavulanate were more successful than those with disks placed the standard distance of 30 mm.
The presence of ESBLs in enterobacters has been documented previously, but the reported numbers of ESBL producers are generally low, especially in E. cloacae (5, 6, 8, 10, 20). In most of these studies, as well as in studies that have evaluated various ESBL detection methods, the DDST with ESC has been used for the initial screening. The findings of this study indicate that the frequency of ESBLs can easily be underestimated in populations of enterobacters characterized by a high prevalence of derepressed variants. For such situations, the application of DDSTs that combine Amc with cefepime may increase the possibility of ESBL detection.
ACKNOWLEDGMENTS
This work was partly supported by bioMérieux.
We thank L. S. Tzouvelekis for helpful suggestions.
REFERENCES
- 1.Arpin C, Coze C, Rogues A M, Gachie J P, Bebear C, Quentin C. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J Clin Microbiol. 1996;34:2163–2169. doi: 10.1128/jcm.34.9.2163-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barroso H, Freitas-Vieira A, Duarte A. Molecular characterization of a ceftazidime-resistant Morganella morganii isolate producing a TEM-10 β-lactamase. Antimicrob Agents Chemother. 1999;43:434–435. doi: 10.1128/aac.43.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K. Is it important to identify extended-spectrum beta-lactamase-producing isolates? Eur J Clin Microbiol Infect Dis. 1996;15:361–364. doi: 10.1007/BF01690090. [DOI] [PubMed] [Google Scholar]
- 4.Chanal C, Sirot D, Romaszko J P, Bret L, Sirot J. Survey of extended-spectrum β-lactamases among Enterobacteriaceae. J Antimicrob Chemother. 1996;38:127–132. doi: 10.1093/jac/38.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Coudron P E, Moland E S, Sanders C C. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J Clin Microbiol. 1997;35:2593–2597. doi: 10.1128/jcm.35.10.2593-2597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Champs C, Sirot D, Chanal C, Poupart M-C, Dumas M-P, Sirot J. Concomitant dissemination of three extended-spectrum β-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991;27:441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- 7.El Harrif-Heraud Z, Arpin C, Benliman S, Quentin C. Molecular epidemiology of a nosocomial outbreak due to SHV-4-producing strains of Citrobacter diversus. J Clin Microbiol. 1997;35:2561–2567. doi: 10.1128/jcm.35.10.2561-2567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery C L, Weymouth L A. Detection and clinical significance of extended-spectrum β-lactamases in a tertiary-care medical unit. J Clin Microbiol. 1997;35:2061–2067. doi: 10.1128/jcm.35.8.2061-2067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianneli D, Tzelepi T, Tzouvelekis L S, Mentis A F, Nikolopoulou C. Dissemination of cephalosporin-resistant Serratia marcescens strains producing a plasmidic SHV type beta-lactamase in Greek hospitals. Eur J Clin Microbiol Infect Dis. 1994;13:764–767. doi: 10.1007/BF02276063. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein F W, Pean Y, Rosato A, Gertner J, Gutmann L the Vigil'Roc Study Group. Characterization of ceftriaxone-resistant Enterobacteriaceae: a multicenter study in 26 French hospitals. J Antimicrob Chemother. 1993;32:595–603. doi: 10.1093/jac/32.4.595. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E W, Bellido F. Factors involved in the enhanced efficacy against gram-negative bacteria of fourth-generation cephalosporins. J Antimicrob Chemother. 1992;29(Suppl. A):1–6. doi: 10.1093/jac/29.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 12.Hibbert-Rodgers L C F, Heritage J, Gascoyne-Binzi D M, Hawkey P M, Todd N, Lewis I J, Bailey C. Molecular epidemiology of ceftazidime resistant Enterobacteriaceae from patients on a pediatric oncology ward. J Antimicrob Chemother. 1995;36:65–82. doi: 10.1093/jac/36.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Hulton C S, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 14.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 15.Naas T, Philippon L, Poirel L, Ronco E, Nordman P. An SHV-derived β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1281–1284. doi: 10.1128/aac.43.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2-A6 (M100-S7). Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Neuwirth C, Siebor E, Lopez J, Pechinot A, Kazmierczak A. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum β-lactamase to other members of the family Enterobacteriaceae. J Clin Microbiol. 1996;34:76–79. doi: 10.1128/jcm.34.1.76-79.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitout J D D, Thomson K S, Hanson N D, Ehrhardt A F, Coudron P, Sanders C C. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob Agents Chemother. 1998;42:596–600. doi: 10.1128/aac.42.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinarakis E E, Tzelepi E, Gazouli M, Mentis A F, Tzouvelekis L S. Characterization of a novel SHV β-lactamase variant that resembles the SHV-5 enzyme. FEMS Microbiol Lett. 1996;139:229–234. doi: 10.1111/j.1574-6968.1996.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanders C C, Barry A L, Washington J A, Shubert C, Moland E S, Traczewski M M, Knapp C, Mulder R. Detection of extended-spectrum β-lactamase-producing members of the family Enterobacteriaceae with the Vitek ESBL test. J Clin Microbiol. 1996;34:2997–3001. doi: 10.1128/jcm.34.12.2997-3001.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders C C, Sanders W E, Molland E S. Characterization of β-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986;30:951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith Moland E, Sanders C C, Thomson K S. Can results obtained with commercially available MicroScan microdilution panels serve as an indicator of β-lactamase production among Escherichia coli and Klebsiella isolates with hidden resistance to expanded-spectrum cephalosporins and aztreonam? J Clin Microbiol. 1998;36:2575–2579. doi: 10.1128/jcm.36.9.2575-2579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsakris A, Tzouvelekis L S, Douboyas J, Panagea T, Tzelepi E. Diversity of β-lactamases among Klebsiella pneumoniae strains isolated in a university hospital in Greece. Eur J Epidemiol. 1997;13:103–107. doi: 10.1023/a:1007350016055. [DOI] [PubMed] [Google Scholar]
- 24.Tzelepi E, Tzouvelekis L S, Vatopoulos A C, Mentis A F, Tsakris A, Legakis N J. High prevalence of stably derepressed class-I β-lactamase expression in multiresistant clinical isolates of Enterobacter cloacae from Greek hospitals. J Med Microbiol. 1992;37:91–95. doi: 10.1099/00222615-37-2-91. [DOI] [PubMed] [Google Scholar]
- 25.Tzouvelekis L S, Vatopoulos A C, Katsanis G, Tzelepi E. Rare case of failure by an automated system to detect extended-spectrum β-lactamase in a cephalosporin-resistant Klebsiella pneumoniae isolate. J Clin Microbiol. 1999;37:2388. doi: 10.1128/jcm.37.7.2388-2388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]