Abstract
Simple Summary
Male fertility is often estimated by simple sperm assessment, and therefore, it is crucial to establish species-specific baselines for normal sperm parameters. In this paper, sperm physiology, function, and common abnormalities in stallions will be reviewed.
Abstract
As the use of assisted reproductive technologies (ART) and in vitro embryo production (IVP) expand in the equine industry, it has become necessary to further our understanding of semen physiology as it applies to overall fertility. This segment of our two-section review will focus on normal sperm parameters, beginning with development and extending through the basic morphology of mature spermatozoa, as well as common issues with male factor infertility in IVP. Ultimately, the relevance of sperm parameters to overall male factor fertility in equine IVP will be assessed.
Keywords: stallion, fertility, sperm, assisted reproductive techniques
1. Introduction
During natural breeding, a stallion will deposit millions of sperm within the intra-uterine environment of the mare [1]. Among this population of sperm there is a wide array of sperm “quality”, which represents the ability of the sperm to fertilize an oocyte and produce viable offspring [2]. Although some variation in sperm morphology and physiology between either individuals of the same species or within an ejaculate will not affect fertilization and embryo development outcomes, some parameters are correlated with fertilization, embryo development, and pregnancy outcomes.
This significant diversity in sperm fertility within an ejaculate becomes more pertinent when applied to in vitro embryo production (IVP), during which a smaller number of sperm are generally selected for either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Thus, it becomes necessary to understand which physiological and functional parameters are the biggest contributors to sperm fertility. This allows us to select the highest quality sperm within an ejaculate for IVP.
Studies of equine sperm fertility encompass sperm biogenesis [3,4], motility and metabolism [5,6], morphology [7], sperm ultrastructure [8], and biochemical elements of sperm function [9,10,11,12], including sperm interactions with accessory sex gland secretions [13,14,15]. The wholistic picture of sperm fertility is integral to the maximization of IVP outcomes, and, therefore, in Section I of this review we will focus on equine spermatogenesis, sperm morphology, and common sperm abnormalities leading to infertility.
2. Spermatogenesis
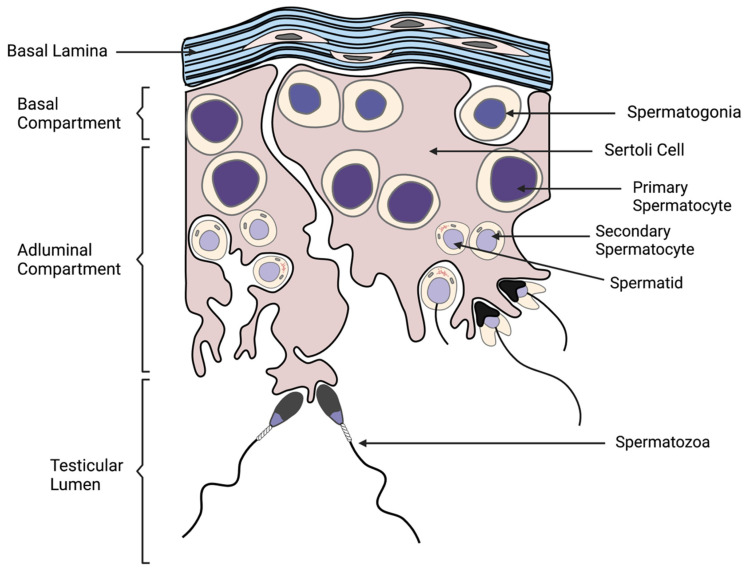
Adequate production of high-quality sperm by the male is critical to both natural and artificial reproductive processes. Therefore, it is critical to understand the pathways by which male gametes are derived. This process, known as spermatogenesis, occurs in the germinal epithelium of the seminiferous tubules of the testis, and is initiated during puberty (Figure 1) [3]. Cross sections of the seminiferous tubules reveal adjacent cellular associations that produce sperm in a cyclic manner, repeating approximately every 12 days in the stallion for the constant production of spermatozoa [3,16,17,18].
Figure 1.
Schematic presentation of spermatogenesis. Facilitated by the nurturing Sertoli cells, basal spermatogonia replicate and differentiate into primary spermatocytes, and sequentially develop into secondary spermatocytes, spermatids, and the morphologically distinct spermatozoa during spermatogenesis. Spermatozoa are released into the lumen of the seminiferous tubule of the testis during spermiation.
The seminiferous tubule is divided into a basal and an adluminal layer, which is fully surrounded by a basal lamina [3,19]. Leydig cells, which are stimulated by Luteinizing Hormone (LH) to produce sex hormones, including testosterone, are key for regulating spermatogenesis as well as being responsible for the male phenotype [20,21]. Leydig cells occupy the interstitial space of the testes between adjacent seminiferous tubules and serve as a key regulator of Sertoli cell function [21]. Within the seminiferous tubules, Sertoli cells span both the basal and adluminal layers, and their role is to host germ cells as they undergo meiosis and differentiation [22,23,24]. Specifically, Sertoli cells are stimulated by pituitary Follicle Stimulating Hormone (FSH) and secrete a variety of proteins that play a role in germ cell nourishment and development [22,24]. In the stallion, it has been shown that seasonal fertility is partially attributed to changes in the number of Sertoli cells in the testis, which is directly correlated with the numbers of spermatozoa ultimately produced [25,26].
The process by which mature spermatozoa are generated is a highly regulated process spanning across multiple domains of the testis. A non-committed store of A-spermatogonial cells exists at the basal layer and remains undifferentiated due to the expression of the Neurogenin 3 (NGN3) gene [27,28,29]. However, A-spermatogonia exist both to replenish the population of gametic stem cells and differentiate for continuation of spermatogenesis, and, therefore, a subpopulation of A-spermatogonia become committed to differentiation [27,30]. The basal store of cells begin as single cells (Asingle) and undergo either a complete mitotic division forming two single daughter cells, or several rounds of incomplete mitosis in order to create paired (Apaired) and aligned (Aaligned) cell groups connected by intercellular bridges [30]. Aaligned spermatogonia then undergo differentiation into committed A1-spermatogonia, which also reside in the basal compartment [31]. However, A1 cells do not express NGN3 and, therefore, will undergo several rounds of mitosis and differentiation while remaining connected by intercellular bridges (A1, A2, A3, B1, B2 stages) [3,4,27,32]. This period of cell replication is known as spermatocytogenesis and, ultimately, produces preleptotene primary spermatocytes [4]. These primary spermatocytes then enter the meiosis phase, where they pass into the adluminal compartment and participate in two meiotic divisions, first becoming haploid secondary spermatocytes and, ultimately, producing haploid spermatids [3,4].
Following spermatocytogenesis, the final stage of spermatogenesis is the morphological shift denoted as spermiogenesis. Here, the sperm cell acquires its characteristic shape, including a species-specific streamlined head containing penetrative enzymes, a structured midpiece, a propelling tail, and the condensation of the male genome [4]. In most cells, nuclear DNA is organized by histone proteins [33]. However, during spermiogenesis, somatic histones are replaced by protamines, the dominant nuclear proteins of mature spermatozoa [33]. The strict compaction of protamine-DNA complexes prevents transcription, provides translational control, and promotes stability in the genome until penetration of the oocyte [33]. This final form produced via spermiogenesis is known as a spermatozoon and is released into the lumen of the seminiferous tubule during the event of spermiation [4,32]. The entire process of spermatogenesis takes approximately 57 days in the stallion [3,7].
Following spermatogenesis, spermatozoa are exposed to a variety of proteinic and non-proteinic substances secreted by the accessory sex glands which aid in the acquisition of mature male fertility and sperm survival during transportation through the male tract and into the female tract [13,14,15,34]. However, the remainder of this review will be focusing on the mature ejaculated spermatozoa and its relation to IVP, a process during which seminal plasma is largely removed, and, thus, we will not be elaborating on the significance of accessory sex glands and their secretions.
3. Sperm Morphology
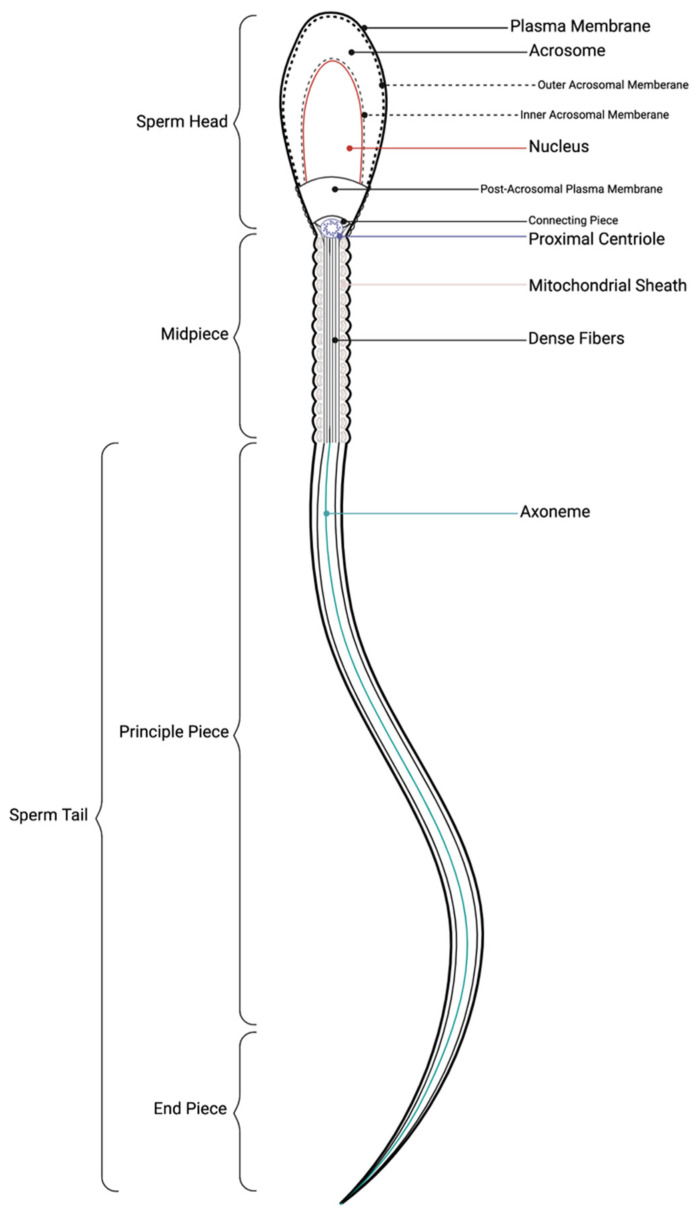
The length of the equine spermatozoa from head to tail is approximately 60 µm [35]. A spermatozoa consists of three main components: a headpiece, midpiece, and tail which are fully encapsulated by a plasma membrane (Figure 2) [7]. The sperm head is an elongated, oval shape that is also relatively flat, with some variation on an individual basis [7,36,37]. The head consists of the acrosome, the post-acrosomal lamina, and the nucleus. The acrosome covers the anterior portion of the sperm head and contains hydrolytic enzymes which are released in order for the sperm to penetrate an oocyte [35]. In addition, it is theorized that the proteases and hydrolases contained within the acrosome play a role in the penetration of the oocyte cumulus complex, in addition to the zona pellucida [38,39]. The post-acrosomal lamina covers the caudal nucleus, which contains the highly condensed male genome [7,35]. Species specific traits of the stallion sperm head include a characteristic asymmetrical head, a paraxially inserted tail, and a small acrosome relevant to other species [40].
Figure 2.
Anatomy of spermatozoa. A spermatozoon consists of three major components: head, midpiece, and tail. The sperm head is overlaid by a plasma membrane, and an acrosomal compartment containing enzymes to aid in fertilization. The nucleus, surrounded by the nuclear envelope, contains the compacted male genome. The head is connected to the midpiece by the connecting piece. The midpiece consists of the proximal centriole, the mitochondrial sheath, and an inner dense fiber structure. The tail points distally, is also covered by a plasma membrane, surrounding the structural axoneme.
The neck piece connects the sperm head to the tail and is made up of the connecting piece, the proximal centriole, and mitochondria. The neck serves as a connection point as well as orienting the tail distally [7]. The midpiece is made up of the cytoskeletal axoneme, which contains cylindrically arranged contractile microtubule doublets with attached dynein arms, which serve to facilitate tail movement. Each doublet is surrounded by dense fibers, which are, in turn, surrounded by a double spiral of mitochondria. The mitochondrial helix is critical for supplying energy to the sperm tail, allowing for the motility that is necessary in fertilization events. The end of the midpiece is defined as the caudal end of the mitochondrial sheath where the annulus, a ring of dense filaments, is located to separate the mitochondria and the sperm tail [7].
The principal piece of the propelling tail consists of the continuation of the axoneme and tapering dense fibers. The distinguishing feature of the principal piece is a protein-rich fibrous sheath that provides structure and flexibility for tail movements. The end of the fibrous sheath indicates transition from the principal piece to the end piece, which solely consists of the axoneme. All of these components are covered superficially by the sperm plasma membrane. Although parameters of a morphologically normal sperm may vary significantly on an individual basis, abnormalities in the sperm anatomy may be an indication of subfertility or a problem with spermatogenesis [7].
The outer plasma membrane can be partitioned into the acrosomal, post-acrosomal, neck, midpiece, and principal piece domains [41]. Each region of the membrane can be characterized by a phospholipid bilayer of heterogeneously expressed lipids, proteins, carbohydrates, and cholesterol that is primarily established during spermatogenesis [33,41,42,43]. The cell surface is additionally covered by a glycocalyx, a network of glycoproteins and glycolipids attached to a matrix of oligosaccharides and polysaccharides, that is known to aid in the proper function of sperm, as well as survival as it passes through the female reproductive tract [43,44]. However, spermatozoa in several species, including the ram, bull, rat, boar, buck, man, and stallion have been documented to undergo significant remodeling to the lipid and protein compositions during epididymal maturation [41,45,46,47,48,49,50].
Due to the compaction of the sperm genome and the reduction in transcription, significant changes in protein, lipid, and sugar contents are thought to be a result of the uptake of epididymal epithelial secretions [51]. Although the mechanisms of proteomic alteration are not well understood, several corresponding hypotheses exist, including (a) the reorganization of proteins into membrane specific domains [52], (b) the secretion of soluble proteins into the epididymal lumen by the epithelium and their subsequent absorption and integration into the plasma membrane [52], (c) the release of extracellular vesicles such as epididymosomes and proteasomes from the epididymis contributing micro and transfer RNAs as well as proteins [53,54,55], and (d) the potential direct anchoring of sperm heads to the epididymal epithelium for protein transfers via an unknown mechanism [56]. Specific proteomic changes to the sperm have been associated with various sperm functions including motility (flagellar, signaling cascade, and metabolic modifications) [57,58,59,60], capacitation (uptake of capacitation linked kinases) [61], acrosome reaction (modifications to the scaffolding proteins involved in acrosomal fusion and synapse) [62], and fertilization (facilitation of sperm-zona pellucida and sperm-oocyte interactions) [51,63,64,65,66].
In the stallion, remodeling of the plasma membrane has been partially described through the domain-specific patterning of filipin–sterol complexes acquired during epididymal maturation as well as changes in intermembrane proteins [40]. Changes in protein composition have been thoroughly described in several species, and a majority of studies focus on the acquisition of epididymal secretory proteins between the caput and caudal epididymis [41]. Through freeze-fracture analysis, altered quantities and distributions of various intramembrane particles were observed over the course of epididymal transit in the equine testis, which is hypothesized to play a role in the establishment of various functional domains [50,67]. It is hypothesized that functional domains assist the sperm cell in adapting to new conditions in the seminal plasma and female reproductive tract [41]. Specifically, changes in the binding affinity between sugar-binding lectins and the sperm glycocalyx indicate an altered exposure of terminal saccharide residues in the sperm membrane—thus altering the ability of the sperm to interact with its environment, such as within the uterus and oviduct, or with an oocyte [43,68].
One of the physiological outcomes of membrane protein modifications is the overall change in net surface charge. This characteristic can be estimated through the measurement of zeta potential, or electrophoretic mobility: an electrostatic potential at the slipping plane of the cell [69,70]. The slipping plane can be described as the distance from the cell at which surrounding fluid particles are no longer bonded or attached to the cell, but are completely mobile and free, and the charge at this location is proportional to surface-charge density [71,72]. Zeta potential of sperm cells has been investigated in men, rats, bulls, rabbits, golden hamsters, guinea pigs, and mongoose [69,73,74,75,76]. The source of the net negative charge is due to the addition of negatively charged sialoglycoproteins to the glycocalyx, such as the bipolar glycopeptide CD52, that appear in the sperm membrane during epididymal maturation [43,69,77,78]. These proteins, as well as the total glycoproteic population in the plasma membrane, undergo compositional changes throughout maturation, capacitation, and acrosome reaction, and are thought to play a physiological role in these processes as well as in fertilization [41,77,79]. Thus, membrane charge is both a revealing and complex trait to accurately measure and interpret.
4. Bioenergetics and Generation of Motility
As previously mentioned, the mitochondrial helix is the primary grouping of organelles responsible for active motility and metabolism in the sperm cell. The number of mitochondrial gyres in the midpiece of the equine spermatozoa varies between 40 and 50, and their organization, or more specifically a disrupted organization, has been shown to play a role in the fertility of stallions through localized ATP production for sperm flagellar movement [80,81,82]. In fact, mitochondrial function, which can be approximated by mitochondrial membrane potential and electron transport chain (ETC) activity, are known to be positively correlated with overall sperm function [82,83,84,85].
ATP production occurs on the inner mitochondrial membrane within the intermembrane space between inner and outer membranes [6,86]. In the process of oxidative phosphorylation, the primary mechanism of ATP generation in stallion sperm, a mitochondrial membrane potential is established as electrons are passaged through the respiratory enzyme complexes of the ETC of the inner membrane and energy is stored in the form of a proton gradient [82,87,88,89]. Ultimately, ATP synthase uses the proton gradient to generate ATP [6,88]. The mitochondrial membrane potential must be maintained, as reduced polarization can lead to an ATP shortage and cellular damage and hyperpolarization may produce an over-abundance of reactive oxygen species (ROS) and cause lipid peroxidation, which can be detrimental to overall cell integrity [6,90]. It is also noteworthy that oxidative phosphorylation (the primary method of ATP generation in stallion sperm) coupled with mild oxidative stress is beneficial to sperm functional pathways such as hyperactivation, capacitation, acrosome reaction, and fertilization [89]. Lesser amounts of ATP may be produced by glycolysis under oxygen depleted conditions for maintenance of high sperm velocity [91,92]. Additionally, research in stallions has shown correlations between ROS and motility, viability, and mitochondrial function [87,91,93], and, thus, it is highly beneficial to understand mitochondrial mechanisms as they relate to sperm fertility.
5. Common Abnormalities and Issues with Fertility
Sperm analysis is a significant method of infertility diagnoses and is critical in order to maximize IVP outcomes. Common issues in patients with male factor infertility can be either obvious or indiscernible to the human eye, and thus the depth of analysis by a technician depends on the technology immediately available to them. Due to the ease of analysis, sperm motility, viability, and morphology are the most common sperm assessments.
Sperm motility is essential for in vivo fertilization and in vitro fertilization (IVF), and is not necessarily required for ICSI where the sperm is manually injected [94,95,96,97]. Sperm motion can be described as either motile or hyperactivated; the latter being a result of capacitation that is required for oocyte penetration. Generally, clinics use Computer Assisted Sperm Analysis (CASA) or similar technologies to reduce subjective errors. CASA can also analyze more complicated motion parameters including the amplitude of lateral head displacement, average path velocity, straight line velocity, curvilinear velocity, linearity of the curvilinear path, and beat-cross frequency [98]. Sperm motility measures are widely considered to be indicative of fertility based on obvious biological functions, despite variable correlations with other sperm quality parameters [97,99]. In the stallion, progressive motility is used as a general estimate of fertility, with less than 50% progressively motile in raw semen or less than 10% progressively motile two hours post collection being an indicator of potential subfertility [100]. However, stallion fertility may be poor even with a highly motile population [101], and, thus, it is critical to understand other common sperm abnormalities.
Sperm viability is a generalized term that can be used to describe a number of traits, including an intact membrane, metabolic activity, and overall physiological health of the cell [102]. Generally, in sperm analysis, viability of the population is estimated by determining the percent of intact membranes using fluorescent dyes such as propidium iodide (PI) and Hoechst 33528 [103,104]. Although Hoechst is permeable with all cells, PI is only able to penetrate cells with disrupted plasma membranes. Thus, staining with two nuclear dyes is necessary for the identification of the non-viable population. Another double staining fluorescent method for viability used in the equine industry is SYBR-14 and PI for viability [103,105]. Assessment of sperm viability can also be indicative of early apoptotic changes, which could also be correlated with other severe sperm abnormalities or infertilities. Rather than, or in addition to, a viability stain with a permeable cell marker, another fluorescent dye may be added to expand upon the assessment of sperm integrity or function. Common fluorescent dyes used for equine sperm assessment include JC-1 [106,107] or rhodamine 123 [108,109] for mitochondrial membrane potential (an estimate of mitochondrial function), fluorescently conjugated Annexin-V (detection of apoptosis) [110,111], or fluorescein isothiocyanate-PNA-Lectin (FITC-PNA) (assessment of acrosomal status) [112,113]. Fluorescent dyes are a common method of sperm quality assessment and a more extensive discussion of their use in ARTs can be found in Section II of this review.
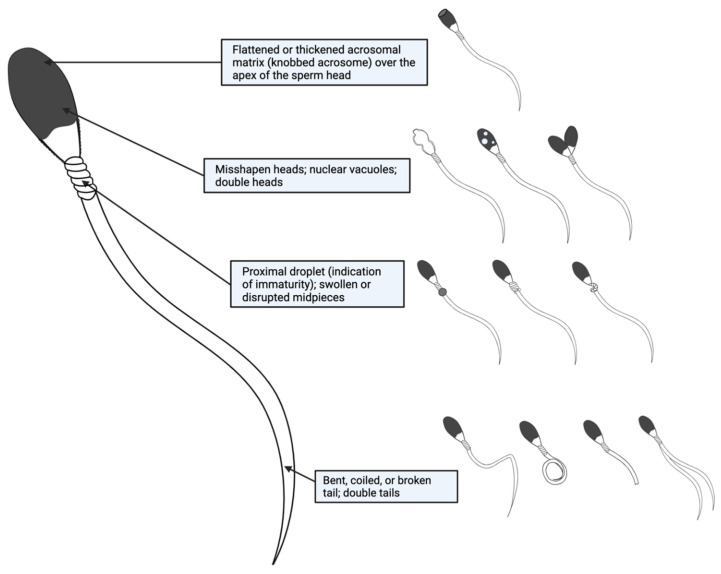
Common morphological abnormalities seen in equine spermatozoa may include bent, coiled, or broken tails, misshapen heads, flattened or thickened acrosomal matrices over the apex of the sperm head, nuclear vacuoles, the presence of proximal droplets (an indication of immaturity), swollen or disrupted midpieces, and double heads or tails (Figure 3) [7]. In humans, morphology has been identified as an indicator of quality, and worsened morphology is specifically correlated with poor motility, DNA fragmentation, chromatin immaturity, high levels of ROS, a decreased ability to bind to the oocyte zona pellucida, and an overall decreased fertilization potential [114,115,116,117,118]. Similarly, studies in stallions have identified correlations between morphologic features, motility, and pregnancy outcomes [80,119], indicating that there may be other sperm parameters associated with morphological abnormalities.
Figure 3.
Common abnormalities of equine spermatozoa. Visualizable sperm abnormalities can be broken down by anatomical region: acrosome, head, midpiece, and tail.
Prior to fertilization, the acrosome undergoes a calcium-dependent exocytotic reaction (acrosome reaction) as a result of sperm-oocyte binding that is essential for the subsequent penetration of the oocyte [120]. In equine spermatozoa, the precursor to the acrosome reaction is sperm activation, or capacitation, which occurs in the female reproductive tract as the spermatozoa approaches the oocyte. Capacitation can be generally characterized by the acquisition of both hyperactive motility and the ability to undergo the acrosome reaction through various molecular pathways and protein phosphorylation cascades [51,121,122]. Capacitation has been successfully performed in vitro in numerous species, including humans and horses [122]. The acrosome reaction has also been achieved in vitro for the horse by using various components, including calcium (Ca2+), calcium ionophore, bicarbonate (HCO3−), lysophosphatidylcholine, and progesterone leading to calcium oscillations [11,112,122,123,124]. Interestingly, sperm from stallions classified as fertile based on their breeding history are more likely to undergo the acrosome reaction in vitro when incubated with progesterone than sperm from subfertile stallions [125]. In humans, in vivo-derived inducers of calcium oscillations leading to the acrosome reaction include follicular fluid, cumulus oophorus, and the presence of granulosa cells; however, these methods are not well understood in the horse [122,126,127,128,129].
In the context of fertilization, capacitation involves calcium oscillations that trigger a complex cascade of intracellular events leading to the binding of specific zona ligands on the outer plasma membrane with the zona pellucida of the oocyte [130,131]. Subsequently, the acrosome reaction, or the fusion of the outer acrosomal membrane with the sperm plasma membrane, is marked by the exocytosis of proteolytic and hydrolytic enzymes from the acrosomal compartment [132,133]. These enzymes aid in the digestion of the zona pellucida so the sperm can penetrate the zona pellucida using hyperactivated motility acquired during capacitation. This results in the entrance of the sperm into the perivitelline space and the fusion of the inner acrosomal membrane and the equatorial region of the sperm head with the oolemma [133,134,135]. However, if a sperm cell undergoes the acrosome reaction prematurely, which can occur during cryopreservation or in vitro processing, it loses its ability to penetrate the cumulus oophorus and zona pellucida for fertilization [136,137]. In human in vitro experiments, a premature acrosome reaction precluded the binding of sperm to the oocyte, and sperm that were able to bind were less successful in penetration [131,138]. In horses, it has been demonstrated that sperm from subfertile stallions bind less frequently to the zona pellucida of the oocyte than sperm from fertile stallions, and that sperm from subfertile stallions is less likely to undergo acrosome reaction after binding [139], indicating discrepancies between fertile and subfertile sperm membrane affinities and compositions. Therefore, it is of interest to remove the prematurely acrosome reacted spermatozoa during selection procedures.
The mitochondrial helix is a sensitive structure that can be easily damaged under extreme environmental conditions, including cryopreservation [6]. Disruption of mitochondrial integrity, including the depolarization of the membrane, can disrupt ATP production and cause a sperm cell to become immotile and non-functional [6,90]. Alternatively, hyperpolarization of the mitochondrial membrane will lead to lipid peroxidation and an over-abundance of ROS, leading to cellular damage [6,90]. Although exact mechanisms of cryoinjury to equine sperm are poorly understood, potential targets include disrupted plasma and mitochondrial membranes, increased ROS production, and generation of apoptotic factors [6,93,140].
Apoptosis is also a common issue seen in sperm samples, especially those that undergo thermal, oxidative, or osmotic stressors from extending, cooling, or cryopreservation [141,142]. These stressors, as well as abnormal morphology, can initiate a variety of negative effects such as membrane and mitochondrial damage, plasma membrane restructuring (including the externalization of proteins such as phosphatidylserine), generation of ROS, and subsequent DNA damage [10,12,141,142,143].
DNA integrity assessment is one of the most valuable assessments of sperm fertilization potential due to the strong correlation with sperm reproductive competence; in fertilization as well as in subsequent embryo development and offspring phenotype [144]. Poor DNA integrity of sperm, or sperm with increased DNA fragmentation, can, thus, have detrimental effects on reproductive outcomes. DNA fragmentation is an all-encompassing term that includes both single- and double-stranded breaks, single base deletions or modifications, various non-desirable cross linkages, and mispackaging errors [145]. Causes of DNA fragmentation may include the mispackaging of chromatin during spermatogenesis [146], apoptosis [147], excessive ROS [146,148], and other environmental factors [144]. The use of spermatozoa with damaged DNA has been associated with compromised fertilization both in vivo and in vitro, as well as negative effects on embryo development, such as worsened embryo quality and blastocyst rates [144,149]. This could potentially lead to both miscarriages and altered offspring phenotypes including genetic diseases, such as Apert syndrome or achondroplasia, conditions thought to arise due to replication error mutations and cancers [144,150,151,152,153]. Thus, DNA integrity of semen can be a good indication of fertilization potential and the potential effects on embryo development and offspring characteristics.
Surface composition and the resulting membrane charge are also of interest in sperm fertility studies. A greater net negative zeta potential, a parameter determined by surface composition as described previously, is acquired during epididymal maturation through extensive membrane remodeling and has been correlated with sperm quality in men [154,155]. The acquisition of a net negative charge is primarily based on the extrusion of sialic acid (sialoglycoproteins) and other charged proteins to the outer membrane of the head region during epididymal maturation [44,69,77,156]. The charge may also change significantly as a sperm changes environments, or when it undergoes capacitation or acrosome reaction [41,79]. Specifically, membrane charge increases, or becomes less negative, when the sperm undergoes capacitation [157]. Externalization of sialoglycans by the sperm has been shown to play a role in avoidance of the uterine immune systems, as well as playing roles in capacitation and being an important component of sperm-zona pellucida binding, and, therefore, fertilization. Thus, charge is a significant factor in sperm fertility [44,77,158,159]. Extrapolating from these data, selecting sperm with a greater net negative zeta potential will theoretically select for mature, functional, and viable spermatozoa.
6. Conclusions
Thorough interpretation of sperm physiology, despite its complexity, is the best method for assessing male fertility. In particular, furthering our understanding of the relationships between sperm morphology, viability, biological composition, and metabolism for equine sperm will be extremely beneficial in understanding fertility in stallions, as well as shedding light on associated mechanisms. In addition, characterization of new biophysical properties, such as zeta potential, will not only aid in our understanding of what makes a fertile sperm, but will also allow for the development of new semen selection technologies. For a review of current and prospective sperm selection technologies, please refer to Section II of this review. In conclusion, sperm physiological assessment is an invaluable tool for the equine breeding industry and merits continued consideration in clinical and research settings.
Author Contributions
Writing—original draft preparation, M.F.O.; writing—review and editing, S.A.M. and P.D.; visualization, M.F.O.; supervision, S.A.M. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katila T. Sperm-uterine interactions. In: McKinnon A.O., Squires E.L., Vaala W.E., Varner D.D., editors. Equine Reproduction. 2nd ed. Wiley-Blackwell; Hoboken, NJ, USA: 2011. pp. 1092–1098. [Google Scholar]
- 2.Holt W.V., Van Look K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction. 2004;127:527–535. doi: 10.1530/rep.1.00134. [DOI] [PubMed] [Google Scholar]
- 3.Amann R. Spermatogenesis in the stallion: A review. J. Equine Vet. Sci. 1981;1:131–139. doi: 10.1016/S0737-0806(81)80046-9. [DOI] [Google Scholar]
- 4.Johnson L., Blanchard T., Varner D., Scrutchfield W. Factors affecting spermatogenesis in the stallion. Theriogenology. 1997;48:1199–1216. doi: 10.1016/S0093-691X(97)00353-1. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos A.B., Santana M.A., Santos A.M.C., Santoro M.M., Lagares M.A. Metabolic evaluation of cooled equine spermatozoa. Andrologia. 2010;42:106–111. doi: 10.1111/j.1439-0272.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyers S., Bulkeley E., Foutouhi A. Sperm mitochondrial regulation in motility and fertility in horses. Reprod. Domest. Anim. 2019;54:22–28. doi: 10.1111/rda.13461. [DOI] [PubMed] [Google Scholar]
- 7.Brito L.F. Evaluation of Stallion Sperm Morphology. Clin. Tech. Equine Pract. 2007;6:249–264. doi: 10.1053/j.ctep.2007.09.004. [DOI] [Google Scholar]
- 8.Veeramachaneni D.R., Moeller C.L., Sawyer H.R. Sperm Morphology in Stallions: Ultrastructure as a Functional and Diagnostic Tool. Vet. Clin. N. Am. Equine Pract. 2006;22:683–692. doi: 10.1016/j.cveq.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Meyers S. Equine sperm-oocyte interaction: The role of sperm surface hyaluronidase. Anim. Reprod. Sci. 2001;68:291–303. doi: 10.1016/S0378-4320(01)00166-X. [DOI] [PubMed] [Google Scholar]
- 10.Thomas A., Meyers S., Ball B. Capacitation-like changes in equine spermatozoa following cryopreservation. Theriogenology. 2006;65:1531–1550. doi: 10.1016/j.theriogenology.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Graham J.K. Methods for Induction of Capacitation and the Acrosome Reaction of Stallion Spermatozoa. Vet. Clin. N. Am. Equine Pract. 1996;12:111–117. doi: 10.1016/S0749-0739(17)30298-5. [DOI] [PubMed] [Google Scholar]
- 12.Ball B.A., Vo A.T., Baumber J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001;62:508–515. doi: 10.2460/ajvr.2001.62.508. [DOI] [PubMed] [Google Scholar]
- 13.Moore A., Squires E., Graham J. Effect of seminal plasma on the cryopreservation of equine spermatozoa. Theriogenology. 2005;63:2372–2381. doi: 10.1016/j.theriogenology.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Guasti P., Souza F., Scott C., Papa P., Camargo L., Schmith R., Monteiro G., Hartwig F., Papa F. Equine seminal plasma and sperm membrane: Functional proteomic assessment. Theriogenology. 2020;156:70–81. doi: 10.1016/j.theriogenology.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Al-Essawe E.M., Wallgren M., Wulf M., Aurich C., Macías-García B., Sjunnesson Y., Morrell J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology. 2018;115:99–107. doi: 10.1016/j.theriogenology.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Swierstra E.E., Pickett B.W., Gebauer M.R. Spermatogenesis and duration of transit of spermatozoa through the excurrent ducts of stallions. J. Reprod. Fertil. Suppl. 1975;23:53–57. [PubMed] [Google Scholar]
- 18.Johnson L. Chapter 5—Spermatogenesis. In: Cupps P.T., editor. Reproduction in Domestic Animals. 4th ed. Academic Press; San Diego, CA, USA: 1991. pp. 173–219. [Google Scholar]
- 19.Wong C., Cheng C.Y. The Blood-Testis Barrier: Its Biology, Regulation, and Physiological Role in Spermatogenesis. Curr. Top. Dev. Biol. 2005;71:263–296. doi: 10.1016/s0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 20.Zirkin B.R., Papadopoulos V. Leydig cells: Formation, function, and regulation†. Biol. Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huhtaniemi I., Teerds K. Leydig cells. In: Skinner M.K., editor. Encyclopedia of Reproduction. 2nd ed. Academic Press; Oxford, UK: 2018. pp. 30–38. [Google Scholar]
- 22.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 23.Johnson L., Thompson D.L. Age-Related and Seasonal Variation in the Sertoli Cell Population, Daily Sperm Production and Serum Concentrations of Follicle-Stimulating Hormone, Luteinizing Hormone and Testosterone in Stallions. Biol. Reprod. 1983;29:777–789. doi: 10.1095/biolreprod29.3.777. [DOI] [PubMed] [Google Scholar]
- 24.França L.R., Hess R.A., Dufour J.M., Hofmann M.-C., Griswold M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology. 2016;4:189–212. doi: 10.1111/andr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson L., Tatum M.E. Temporal Appearance of Seasonal Changes in Numbers of Sertoli Cells, Leydig Cells, and Germ Cells in Stallions. Biol. Reprod. 1989;40:994–999. doi: 10.1095/biolreprod40.5.994. [DOI] [PubMed] [Google Scholar]
- 26.Pickett B., Voss J. Management of shuttle stallions for maximum reproductive efficiency—Part 1. J. Equine Vet. Sci. 1998;18:212–227. doi: 10.1016/S0737-0806(98)80113-5. [DOI] [Google Scholar]
- 27.Griswold M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., Suda T., Nabeshima Y.-I. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 29.Evans E., Hogarth C., Mitchell D., Griswold M. Riding the spermatogenic wave: Profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 2014;90:108. doi: 10.1095/biolreprod.114.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Rooij D.G., Russell L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 31.De Rooij D.G., Griswold M.D. Questions About Spermatogonia Posed and Answered Since 2000. J. Androl. 2012;33:1085–1095. doi: 10.2164/jandrol.112.016832. [DOI] [PubMed] [Google Scholar]
- 32.Johnson L. Seasonal Differences in Equine Spermatocytogenesis. Biol. Reprod. 1991;44:284–291. doi: 10.1095/biolreprod44.2.284. [DOI] [PubMed] [Google Scholar]
- 33.Steger K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat. Embryol. 1999;199:471–487. doi: 10.1007/s004290050245. [DOI] [PubMed] [Google Scholar]
- 34.Druart X., de Graaf S. Seminal plasma proteomes and sperm fertility. Anim. Reprod. Sci. 2018;194:33–40. doi: 10.1016/j.anireprosci.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Pesch S., Bergmann M. Structure of mammalian spermatozoa in respect to viability, fertility and cryopreservation. Micron. 2006;37:597–612. doi: 10.1016/j.micron.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Gravance C., Champion Z., Liu I., Casey P. Sperm head morphometry analysis of ejaculate and dismount stallion semen samples. Anim. Reprod. Sci. 1997;47:149–155. doi: 10.1016/S0378-4320(96)01634-X. [DOI] [PubMed] [Google Scholar]
- 37.Casey P., Gravance C., Davis R., Chabot D., Liu I. Morphometric differences in sperm head dimensions of fertile and subfertile stallions. Theriogenology. 1997;47:575–582. doi: 10.1016/S0093-691X(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 38.Bedford J. Mammalian Fertilization Misread? Sperm Penetration of the Eutherian Zona Pellucida Is Unlikely to be a Lytic Event. Biol. Reprod. 1998;59:1275–1287. doi: 10.1095/biolreprod59.6.1275. [DOI] [PubMed] [Google Scholar]
- 39.Foster J.A., Gerton G.L. The Acrosomal Matrix. Adv. Anat. Embryol. Cell. Biol. 2016;220:15–33. doi: 10.1007/978-3-319-30567-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López M.L., De Souza W. Distribution of filipin-sterol complexes in the plasma membrane of stallion spermatozoa during the epididymal maturation process. Mol. Reprod. Dev. 1991;28:158–168. doi: 10.1002/mrd.1080280209. [DOI] [PubMed] [Google Scholar]
- 41.Jones R. Plasma membrane composition and organisation during maturation of spermatozoa in the epididymis. In: Robaire B., Hinton B.T., editors. The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. Springer; Boston, MA, USA: 2002. pp. 405–416. [Google Scholar]
- 42.Flesch F., Gadella B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 2000;1469:197–235. doi: 10.1016/S0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 43.Schroter S., Osterhoff C., McArdle W., Ivell R. The glycocalyx of the sperm surface. Hum. Reprod. Updat. 1999;5:302–313. doi: 10.1093/humupd/5.4.302. [DOI] [PubMed] [Google Scholar]
- 44.Ma X., Pan Q., Feng Y., Choudhury B.P., Ma Q., Gagneux P., Ma F. Sialylation Facilitates the Maturation of Mammalian Sperm and Affects Its Survival in Female Uterus. Biol. Reprod. 2016;94:123. doi: 10.1095/biolreprod.115.137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott T.W., Voglmayr J.K., Setchell B.P. Lipid composition and metabolism in testicular and ejaculated ram spermatozoa. Biochem. J. 1967;102:456–461. doi: 10.1042/bj1020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulos A., Darin-Bennett A., White I. The phospholipid-bound fatty acids and aldehydes of mammalian spermatozoa. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973;46:541–549. doi: 10.1016/0305-0491(73)90094-1. [DOI] [PubMed] [Google Scholar]
- 47.Aveldaño M.I., Rotstein N.P., Vermouth N.T. Lipid remodelling during epididymal maturation of rat spermatozoa. Enrichment in plasmenylcholines containing long-chain polyenoic fatty acids of the n-9 series. Biochem. J. 1992;283:235–241. doi: 10.1042/bj2830235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rana A.P., Majumder G.C., Misra S., Ghosh A. Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim. Biophys. Acta (BBA)-Biomembr. 1991;1061:185–196. doi: 10.1016/0005-2736(91)90284-F. [DOI] [PubMed] [Google Scholar]
- 49.Retamal C., Urzúa J., Lorca C., López M.L., Alves E.W. Changes in the plasma membrane proteins of stallion spermatozoa during maturation in the epididymis. J. Submicrosc. Cytol. Pathol. 2000;32:229–239. [PubMed] [Google Scholar]
- 50.López M., Olea N., Retamal C. Post-testicular changes in the density and distribution of intramembrane particles of stallion sperm surface domains. Anim. Reprod. Sci. 2007;100:204–210. doi: 10.1016/j.anireprosci.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Gervasi M.G., Visconti P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology. 2017;5:204–218. doi: 10.1111/andr.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuasnicú P.S., Cohen D.J., Ellerman D.A., Busso D., Da Ros V.G., Morgenfeld M.M. Changes in specific sperm proteins during epididymal maturation. In: Robaire B., Hinton B.T., editors. The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. Springer; Boston, MA, USA: 2002. pp. 389–403. [Google Scholar]
- 53.Sullivan R., Saez F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 54.Sharma U., Conine C.C., Shea J.M., Boskovic A., Derr A.G., Bing X.Y., Belleannee C., Kucukural A., Serra R.W., Sun F., et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belleannée C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015;17:730–736. doi: 10.4103/1008-682X.155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Păunescu T.G., Shum W.W., Huynh C., Lechner L., Goetze B., Brown D., Breton S., Unescu T.G.P. High-resolution helium ion microscopy of epididymal epithelial cells and their interaction with spermatozoa. Mol. Hum. Reprod. 2014;20:929–937. doi: 10.1093/molehr/gau052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saez F., Frenette G., Sullivan R. Epididymosomes and Prostasomes: Their Roles in Posttesticular Maturation of the Sperm Cells. J. Androl. 2003;24:149–154. doi: 10.1002/j.1939-4640.2003.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 58.Eickhoff R., Baldauf C., Koyro H.-W., Wennemuth G., Suga Y., Seitz J., Henkel R., Meinhardt A. Influence of macrophage migration inhibitory factor (MIF) on the zinc content and redox state of protein-bound sulphydryl groups in rat sperm: Indications for a new role of MIF in sperm maturation. Mol. Hum. Reprod. 2004;10:605–611. doi: 10.1093/molehr/gah075. [DOI] [PubMed] [Google Scholar]
- 59.Frenette G., Lessard C., Sullivan R. Polyol pathway along the bovine epididymis. Mol. Reprod. Dev. 2004;69:448–456. doi: 10.1002/mrd.20170. [DOI] [PubMed] [Google Scholar]
- 60.Murta D.D.M., Batista M., Silva E., Trindade A., Henrique D., Duarte A., Lopes-Da-Costa L. Notch signaling in the epididymal epithelium regulates sperm motility and is transferred at a distance within epididymosomes. Andrology. 2016;4:314–327. doi: 10.1111/andr.12144. [DOI] [PubMed] [Google Scholar]
- 61.Krapf D., Ruan Y.C., Wertheimer E.V., Battistone M.A., Pawlak J., Sanjay A., Pilder S.H., Cuasnicu P., Breton S., Visconti P.E. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev. Biol. 2012;369:43–53. doi: 10.1016/j.ydbio.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshi C.S., Suryawanshi A.R., Khan S.A., Balasinor N.H., Khole V.V. Liprin α3: A putative estrogen regulated acrosomal protein. Histochem. Cell Biol. 2012;139:535–548. doi: 10.1007/s00418-012-1044-y. [DOI] [PubMed] [Google Scholar]
- 63.Oh J., Woo J.-M., Choi E., Kim T., Cho B.-N., Park Z.Y., Kim Y.C., Kim D.H., Cho C. Molecular, biochemical, and cellular characterization of epididymal ADAMs, ADAM7 and ADAM28. Biochem. Biophys. Res. Commun. 2005;331:1374–1383. doi: 10.1016/j.bbrc.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 64.Caballero J., Frenette G., D’Amours O., Belleannée C., Lacroix-Pepin N., Robert C., Sullivan R. Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida. J. Cell. Physiol. 2012;227:3876–3886. doi: 10.1002/jcp.24099. [DOI] [PubMed] [Google Scholar]
- 65.Gibbs G.M., Lo J.C.Y., Nixon B., Jamsai D., O’Connor A.E., Rijal S., Sanchez-Partida L.G., Hearn M.T.W., Bianco D.M., O’Bryan M.K. Glioma Pathogenesis-Related 1-Like 1 Is Testis Enriched, Dynamically Modified, and Redistributed during Male Germ Cell Maturation and Has a Potential Role in Sperm-Oocyte Binding. Endocrinology. 2010;151:2331–2342. doi: 10.1210/en.2009-1255. [DOI] [PubMed] [Google Scholar]
- 66.Frenette G., Sullivan R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 2001;59:115–121. doi: 10.1002/mrd.1013. [DOI] [PubMed] [Google Scholar]
- 67.Dias A.J., Maia M.S., A Retamal C., López M.L. Identification and partial characterization of α-1,4-glucosidase activity in equine epididymal fluid. Theriogenology. 2004;61:1545–1558. doi: 10.1016/j.theriogenology.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Tecle E., Gagneux P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015;82:635–650. doi: 10.1002/mrd.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishijima S.A., Okuno M., Mohri H. Zeta potential of human X- and Y-bearing sperm. Int. J. Androl. 1991;14:340–347. doi: 10.1111/j.1365-2605.1991.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 70.Esfahani M.H.N., Deemeh M.R., Tavalaee M., Sekhavati M.H., Gourabi H. Zeta Sperm Selection Improves Pregnancy Rate and Alters Sex Ratio in Male Factor Infertility Patients: A Double-Blind, Randomized Clinical Trial. Int. J. Fertil. Steril. 2016;10:253–260. doi: 10.22074/IJFS.2016.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan P.J., Jacobson J.D., Corselli J.U., Patton W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006;85:481–486. doi: 10.1016/j.fertnstert.2005.07.1302. [DOI] [PubMed] [Google Scholar]
- 72.Ohshima H., Kondo T. Electrophoresis of large colloidal particles with surface charge layers. Position of the slipping plane and surface layer thickness. Colloid Polym. Sci. 1986;264:1080–1084. doi: 10.1007/BF01410326. [DOI] [Google Scholar]
- 73.Clogston J.D., Patri A.K. Zeta potential measurement. In: McNeil S.E., editor. Characterization of Nanoparticles Intended for Drug Delivery. Humana Press; Totowa, NJ, USA: 2011. pp. 63–70. [Google Scholar]
- 74.Veres I. Negative electrical charge of the surface of bull sperm. Mikroskopie. 1968;23:166–169. [PubMed] [Google Scholar]
- 75.Yanagimachi R., Noda Y.D., Fujimoto M., Nicolson G.L. The distribution of negative surface charges on mammalian spermatozoa. Am. J. Anat. 1972;135:497–519. doi: 10.1002/aja.1001350405. [DOI] [PubMed] [Google Scholar]
- 76.Schröter S., Derr P., Conradt H.S., Nimtz M., Hale G., Kirchhoff C. Male-specific Modification of Human CD52. J. Biol. Chem. 1999;274:29862–29873. doi: 10.1074/jbc.274.42.29862. [DOI] [PubMed] [Google Scholar]
- 77.Calzada L., Salazar E.L., Pedrón N. Presence and Chemical Composition of Glycoproteic Layer on Human Spermatozoa. Arch. Androl. 1994;33:87–92. doi: 10.3109/01485019408987808. [DOI] [PubMed] [Google Scholar]
- 78.Simon L., Ge S.-Q., Carrell D.T. Sperm Selection Based on Electrostatic Charge. Methods Mol. Biol. 2012;927:269–278. doi: 10.1007/978-1-62703-038-0_25. [DOI] [PubMed] [Google Scholar]
- 79.Zeng Y., Clark E.N., Florman H.M. Sperm Membrane Potential: Hyperpolarization during Capacitation Regulates Zona Pellucida-Dependent Acrosomal Secretion. Dev. Biol. 1995;171:554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- 80.Love C.C., Varner D.D., Thompson J.A. Intra- and inter-stallion variation in sperm morphology and their relationship with fertility. J. Reprod. Fertil. Suppl. 2000;56:93–100. [PubMed] [Google Scholar]
- 81.Varner D.D., Johnson L. From a sperm’s eye view—Revisiting our perception of this intriguing cell; Proceedings of the 53rd Annual Convention of the American Association of Equine Practitioners; Orlando, FL, USA. 1–5 December 2007; pp. 104–177. [Google Scholar]
- 82.Ramalho-Santos J., Amaral S. Mitochondria and mammalian reproduction. Mol. Cell. Endocrinol. 2013;379:74–84. doi: 10.1016/j.mce.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Sousa A.P., Amaral A., Baptista M., Tavares R., Campo P.C., Peregrín P.C., Freitas A., Paiva A., Almeida-Santos T., Ramalho-Santos J. Not All Sperm Are Equal: Functional Mitochondria Characterize a Subpopulation of Human Sperm with Better Fertilization Potential. PLoS ONE. 2011;6:e18112. doi: 10.1371/journal.pone.0018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallon F., Marchetti C., Jouy N., Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006;86:1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti P., Ballot C., Jouy N., Thomas P., Marchetti C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia. 2011;44:136–141. doi: 10.1111/j.1439-0272.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 86.Mannella C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta BBA Mol. Cell Res. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Gibb Z., Lambourne S.R., Aitken R.J. The Paradoxical Relationship Between Stallion Fertility and Oxidative Stress. Biol. Reprod. 2014;91:77. doi: 10.1095/biolreprod.114.118539. [DOI] [PubMed] [Google Scholar]
- 88.Saraste M. Oxidative Phosphorylation at the fin de siècle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 89.Moraes C.R., Meyers S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018;194:71–80. doi: 10.1016/j.anireprosci.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Wolken G.G., Arriaga E.A. Simultaneous Measurement of Individual Mitochondrial Membrane Potential and Electrophoretic Mobility by Capillary Electrophoresis. Anal. Chem. 2014;86:4217–4226. doi: 10.1021/ac403849x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Darr C.R., Varner D.D., Teague S., Cortopassi G.A., Datta S., Meyers S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016;95:34. doi: 10.1095/biolreprod.116.140707. [DOI] [PubMed] [Google Scholar]
- 92.Davila M.P., Muñoz P.M., Tapia J.A., Ortega-Ferrusola C., Balao da Silva C.C., Peña F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE. 2015;10:e0138777. doi: 10.1371/journal.pone.0138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Darr C.R., Cortopassi G.A., Datta S., Varner D.D., Meyers S.A. Mitochondrial oxygen consumption is a unique indicator of stallion spermatozoal health and varies with cryopreservation media. Theriogenology. 2016;86:1382–1392. doi: 10.1016/j.theriogenology.2016.04.082. [DOI] [PubMed] [Google Scholar]
- 94.Nijs M., Vanderzwalmen P., Vandamme B., Segal-Bertin G., Lejeune B., Segal L., van Roosendaal E., Schoysman R. Andrology: Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum. Reprod. 1996;11:2180–2185. doi: 10.1093/oxfordjournals.humrep.a019073. [DOI] [PubMed] [Google Scholar]
- 95.Gaddum-Rosse P. Some observations on sperm transport through the uterotubal junction of the rat. Am. J. Anat. 1981;160:333–341. doi: 10.1002/aja.1001600309. [DOI] [PubMed] [Google Scholar]
- 96.Fraser L.R., Quinn P.J. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. Reproduction. 1981;61:25–35. doi: 10.1530/jrf.0.0610025. [DOI] [PubMed] [Google Scholar]
- 97.Turner R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003;24:790–803. doi: 10.1002/j.1939-4640.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 98.Amann R.P., Waberski D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology. 2014;81:5–17.e3. doi: 10.1016/j.theriogenology.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Jasko D.J., Little T.V., Lein D.H., Foote R.H. Comparison of spermatozoal movement and semen characteristics with fertility in stallions: 64 cases (1987–1988) J. Am. Vet. Med. Assoc. 1992;200:979–985. [PubMed] [Google Scholar]
- 100.Hurtgen J.P. Evaluation of the Stallion for Breeding Soundness. Vet. Clin. N. Am. Equine Pract. 1992;8:149–165. doi: 10.1016/S0749-0739(17)30472-8. [DOI] [PubMed] [Google Scholar]
- 101.Voss J.L., Pickett B.W., Squires E.L. Stallion spermatozoal morphology and motility and their relationships to fertility. J. Am. Vet. Med. Assoc. 1981;178:287–289. [PubMed] [Google Scholar]
- 102.Johnson S., Nguyen V., Coder D. Assessment of Cell Viability. Curr. Protoc. Cytom. 2013;64:9.2.1–9.2.26. doi: 10.1002/0471142956.cy0902s64. [DOI] [PubMed] [Google Scholar]
- 103.Garner D.L., Johnson L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide. Biol. Reprod. 1995;53:276–284. doi: 10.1095/biolreprod53.2.276. [DOI] [PubMed] [Google Scholar]
- 104.Pintado B., de la Fuente J., Roldan E.R. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: Accuracy in the assessment of cell viability. J. Reprod. Fertil. 2000;118:145–152. doi: 10.1530/jrf.0.1180145. [DOI] [PubMed] [Google Scholar]
- 105.Glazar A.I. Assessment of Sperm Plasma Membrane Integrity and Viability: Propidium Iodide/SYBR-14. Equine Reprod. Proced. 2014:476–477. doi: 10.1002/9781118904398.ch145. [DOI] [Google Scholar]
- 106.Baumber J., Ball B.A., Gravance C.G., Medina V., Davies-Morel M.C.G. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000;21:895–902. [PubMed] [Google Scholar]
- 107.Gravance C., Garner D., Baumber J., Ball B. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology. 2000;53:1691–1703. doi: 10.1016/S0093-691X(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 108.O’Connell M., McClure N., Lewis S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002;17:704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 109.Glazar A.I., McCue P.M. Assessment of Sperm Mitochondrial Function. Equine Reprod. Proced. 2021:637–638. doi: 10.1002/9781119556015.ch173. [DOI] [Google Scholar]
- 110.Baumber J. Changes in Membrane Lipid Order with Capacitation in Rhesus Macaque (Macaca mulatta) Spermatozoa. J. Androl. 2006;27:578–587. doi: 10.2164/jandrol.05135. [DOI] [PubMed] [Google Scholar]
- 111.Brum A., Sabeur K., Ball B. Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology. 2008;69:1041–1055. doi: 10.1016/j.theriogenology.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Cheng F.-P., Gadella B.M., Voorhout W.F., Fazeli A., Bevers M.M., Colenbrander B. Progesterone-Induced Acrosome Reaction in Stallion Spermatozoa Is Mediated by a Plasma Membrane Progesterone Receptor. Biol. Reprod. 1998;59:733–742. doi: 10.1095/biolreprod59.4.733. [DOI] [PubMed] [Google Scholar]
- 113.Cheng F.P., Fazeli A., Voorhout W.F., Marks A., Bevers M.M., Colenbrander B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996;17:674–682. [PubMed] [Google Scholar]
- 114.Franken D.R. How accurate is sperm morphology as an indicator of sperm function? Andrologia. 2014;47:720–723. doi: 10.1111/and.12324. [DOI] [PubMed] [Google Scholar]
- 115.Dariš B., Goropevšek A., Hojnik N., Vlaisavljević V. Sperm morphological abnormalities as indicators of DNA fragmentation and fertilization in ICSI. Arch. Gynecol. Obstet. 2009;281:363–367. doi: 10.1007/s00404-009-1140-y. [DOI] [PubMed] [Google Scholar]
- 116.Oumaima A., Tesnim A., Zohra H., Amira S., Ines Z., Sana C., Intissar G., Lobna E., Ali J., Meriem M. Investigation on the origin of sperm morphological defects: Oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ. Sci. Pollut. Res. 2018;25:13775–13786. doi: 10.1007/s11356-018-1417-4. [DOI] [PubMed] [Google Scholar]
- 117.Kruger T.F., Acosta A.A., Simmons K.F., Swanson R.J., Matta J.F., Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil. Steril. 1988;49:112–117. doi: 10.1016/S0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 118.Oehninger S., Mahony M., Özgür K., Kolm P., Kruger T., Franken D. Clinical significance of human sperm-zona pellucida binding. Fertil. Steril. 1997;67:1121–1127. doi: 10.1016/S0015-0282(97)81449-5. [DOI] [PubMed] [Google Scholar]
- 119.Love C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology. 2011;76:547–557. doi: 10.1016/j.theriogenology.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Hirohashi N., Yanagimachi R. Sperm acrosome reaction: Its site and role in fertilization†. Biol. Reprod. 2018;99:127–133. doi: 10.1093/biolre/ioy045. [DOI] [PubMed] [Google Scholar]
- 121.Yanagimachi R., Hamana K., Hafez E.S.E. The movement of golden hamster spermatozoa before and after capacitation. Reproduction. 1970;23:193–196. doi: 10.1530/jrf.0.0230193. [DOI] [PubMed] [Google Scholar]
- 122.Leemans B., Stout T.A.E., De Schauwer C., Heras S., Nelis H., Hoogewijs M., Van Soom A., Gadella B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction. 2019;157:R181–R197. doi: 10.1530/REP-18-0541. [DOI] [PubMed] [Google Scholar]
- 123.Cheng F.P., Fazeli A., Voorhout W.F., Tremoleda J.L., Bevers M.M., Colenbrander B. Progesterone in mare follicular fluid induces the acrosome reaction in stallion spermatozoa and enhances In Vitro binding to the zona pellucida. Int. J. Androl. 2002;21:57–66. doi: 10.1046/j.1365-2605.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 124.McPartlin L., Littell J., Mark E., Nelson J., Travis A., Bedford-Guaus S. A defined medium supports changes consistent with capacitation in stallion sperm, as evidenced by increases in protein tyrosine phosphorylation and high rates of acrosomal exocytosis. Theriogenology. 2008;69:639–650. doi: 10.1016/j.theriogenology.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 125.Meyers S.A., Overstreet J.W., Liu I.K., Drobnis E.Z. Capacitation in vitro of stallion spermatozoa: Comparison of progesterone-induced acrosome reactions in fertile and subfertile males. J. Androl. 1995;16:47–54. [PubMed] [Google Scholar]
- 126.Mortimer D., Camenzind A. The role of follicular fluid in inducing the acrosome reaction of human spermatozoa incubated in vitro. Hum. Reprod. 1989;4:169–174. doi: 10.1093/oxfordjournals.humrep.a136866. [DOI] [PubMed] [Google Scholar]
- 127.Sun T.T., Chung C.M., Chan H.C. Acrosome reaction in the cumulus oophorus revisited: Involvement of a novel sperm-released factor NYD-SP8. Protein Cell. 2011;2:92–98. doi: 10.1007/s13238-011-1022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siiteri J.E., Dandekar P., Meizel S. Human sperm acrosome reaction-initiating activity associated with the human cumulus oophorus and mural granulosa cells. J. Exp. Zool. 1988;246:71–80. doi: 10.1002/jez.1402460110. [DOI] [PubMed] [Google Scholar]
- 129.Leemans B., Gadella B.M., Stout T.A.E., De Schauwer C., Nelis H., Hoogewijs M., Van Soom A. Why doesn’t conventional IVF work in horses. Reproduction. 2016;152:R233–R245. doi: 10.1530/REP-16-0420. [DOI] [PubMed] [Google Scholar]
- 130.Brucker C. The human sperm acrosome reaction: Physiology and regulatory mechanisms. An update. Hum. Reprod. Updat. 1995;1:51–62. doi: 10.1093/humupd/1.1.51. [DOI] [PubMed] [Google Scholar]
- 131.Esteves S.C. Relationship of in Vitro Acrosome Reaction to Sperm Function: An Update. Open Reprod. Sci. J. 2011;3:72–84. doi: 10.2174/1874255601103010072. [DOI] [Google Scholar]
- 132.Avella M.A., Dean J. Fertilization with acrosome-reacted mouse sperm: Implications for the site of exocytosis. Proc. Natl. Acad. Sci. USA. 2011;108:19843–19844. doi: 10.1073/pnas.1118234109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gahlay G.K., Rajput N. The enigmatic sperm proteins in mammalian fertilization: An overview†. Biol. Reprod. 2020;103:1171–1185. doi: 10.1093/biolre/ioaa140. [DOI] [PubMed] [Google Scholar]
- 134.Katz D.F., Yanagimachi R., Dresdner R.D. Movement characteristics and power output of guinea-pig and hamster spermatozoa in relation to activation. Reproduction. 1978;52:167–172. doi: 10.1530/jrf.0.0520167. [DOI] [PubMed] [Google Scholar]
- 135.Fraser L.R. Sperm capacitation and the acrosome reaction. Hum. Reprod. 1998;13:9–19. doi: 10.1093/humrep/13.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 136.Cummins J.M., Yanagimachi R. Development of ability to penetrate the cumulus oophorus by hamster spermatozoa capacitated in vitro, in relation to the timing of the acrosome reaction. Gamete Res. 1986;15:187–212. doi: 10.1002/mrd.1120150302. [DOI] [Google Scholar]
- 137.Florman H.M., Jungnickel M.K., Sutton K.A. Regulating the acrosome reaction. Int. J. Dev. Biol. 2008;52:503–510. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- 138.Tesarik J., Mendoza C. Alleviation of acrosome reaction prematurity by sperm treatment with egg yolk. Fertil. Steril. 1995;63:153–157. doi: 10.1016/S0015-0282(16)57311-7. [DOI] [PubMed] [Google Scholar]
- 139.Meyers S.A., Liu I.K., Overstreet J.W., Vadas S., Drobnis E.Z. Zona pellucida binding and zona-induced acrosome reactions in horse spermatozoa: Comparisons between fertile and subfertile stallions. Theriogenology. 1996;46:1277–1288. doi: 10.1016/S0093-691X(96)00299-3. [DOI] [PubMed] [Google Scholar]
- 140.Peña F., Martínez H.R., Tapia J., Ferrusola C.O., Fernández L.G., García B.M. Mitochondria in Mammalian Sperm Physiology and Pathology: A Review. Reprod. Domest. Anim. 2009;44:345–349. doi: 10.1111/j.1439-0531.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 141.Ball B.A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 2008;107:257–267. doi: 10.1016/j.anireprosci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 142.Aitken R., Koppers A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sabeur K., Ball B.A. Detection of superoxide anion generation by equine spermatozoa. Am. J. Vet. Res. 2006;67:701–706. doi: 10.2460/ajvr.67.4.701. [DOI] [PubMed] [Google Scholar]
- 144.Shamsi M.B., Imam S.N., Dada R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J. Assist. Reprod. Genet. 2011;28:1073–1085. doi: 10.1007/s10815-011-9631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Esteves S.C., Sharma R.K., Gosálvez J., Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int. Urol. Nephrol. 2014;46:1037–1052. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 146.Shamsi M.B., Kumar R., Dada R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J. Med. Res. 2008;127:115–123. [PubMed] [Google Scholar]
- 147.Sakkas D., Moffatt O., Manicardi G.C., Mariethoz E., Tarozzi N., Bizzaro D. Nature of DNA Damage in Ejaculated Human Spermatozoa and the Possible Involvement of Apoptosis. Biol. Reprod. 2002;66:1061–1067. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 148.Henkel R., Kierspel E., Stalf T., Mehnert C., Menkveld R., Tinneberg H.-R., Schill W.-B., Kruger T.F. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil. Steril. 2005;83:635–642. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 149.Seli E., Gardner D., Schoolcraft W.B., Moffatt O., Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 150.Baker M.A., Aitken R.J. Reactive oxygen species in spermatozoa: Methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 2005;3:67–69. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 152.Goriely A., McVean G.A.T., Röjmyr M., Ingemarsson B., Wilkie A.O.M. Evidence for Selective Advantage of Pathogenic FGFR2 Mutations in the Male Germ Line. Science. 2003;301:643–646. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- 153.Tiemann-Boege I., Navidi W., Grewal R., Cohn D., Eskenazi B., Wyrobek A., Arnheim N. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc. Natl. Acad. Sci. USA. 2002;99:14952–14957. doi: 10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Giuliani V., Pandolfi C., Santucci R., Pelliccione F., Macerola B., Focarelli R., Rosati F., Della Giovampaola C., Francavilla F. Expression of gp20, a human sperm antigen of epididymal origin, is reduced in spermatozoa from subfertile men. Mol. Reprod. Dev. 2004;69:235–240. doi: 10.1002/mrd.20166. [DOI] [PubMed] [Google Scholar]
- 155.Ionov M., Gontarek W., Bryszewska M. Zeta potential technique for analyzing semen quality. MethodsX. 2020;7:100895. doi: 10.1016/j.mex.2020.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kaneko S., Oshio S., Kobayashi T., Iizuka R., Mohri H. Human X- and Y-bearing sperm differ in cell surface sialic acid content. Biochem. Biophys. Res. Commun. 1984;124:950–955. doi: 10.1016/0006-291X(84)91050-7. [DOI] [PubMed] [Google Scholar]
- 157.Focarelli R., Rosati F., Terrana B. Sialylglycoconjugates Release During In Vitro Capacitation of Human Spermatozoa. J. Androl. 1990;11:97–104. doi: 10.1002/j.1939-4640.1990.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 158.Levinsky H., Singer R., Malik Z., Sagiv M., Cohen A.M., Servadio C., Allalouf D. Distribution of Sialic Acid in Human Sperm Membranes. Arch. Androl. 1983;10:209–212. doi: 10.3109/01485018308987566. [DOI] [PubMed] [Google Scholar]
- 159.Velásquez J.G., Canovas S., Barajas P., Marcos J., Jimenez-Movilla M., Gallego R.G., Ballesta J., Avilés M., Coy P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Repeod. Dev. 2006;74:617–628. doi: 10.1002/mrd.20619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.