Abstract
The SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) corepressor participates in the repression of target gene expression by a variety of transcription factors, including the nuclear hormone receptors, promyelocytic leukemia zinc finger protein, and B-cell leukemia protein 6. The ability of SMRT to associate with these transcription factors and thereby to mediate repression is strongly inhibited by activation of tyrosine kinase signaling pathways, such as that represented by the epidermal growth factor receptor. We report here that SMRT function is potently inhibited by a mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK) cascade that operates downstream of this growth factor receptor. Intriguingly, the SMRT protein is a substrate for phosphorylation by protein kinases operating at multiple levels in this MAPKKK pathway, including the MAPKs, MAPK–extracellular signal-regulated kinase 1 (MEK-1), and MEK-1 kinase (MEKK-1). Phosphorylation of SMRT by MEKK-1 and, to a lesser extent, MEK-1 inhibits the ability of SMRT to physically tether to its transcription factor partners. Notably, activation of MEKK-1 or MEK-1 signaling in transfected cells also leads to a redistribution of the SMRT protein from a nuclear compartment to a more perinuclear or cytoplasmic compartment. We suggest that SMRT-mediated repression is regulated by the MAPKKK cascade and that changes both in the affinity of SMRT for its transcription factors and in the subcellular distribution of SMRT contribute to the loss of SMRT function that is observed in response to kinase signal transduction.
Eukaryotic transcription factors can exert both positive and negative effects on gene expression. A number of transcriptional regulators are, in fact, bipolar in their properties, with a given transcription factor being able to both repress and activate target gene expression. Perhaps the most extensively analyzed of these bipolar transcription factors are the nuclear hormone receptors, such as the retinoic acid receptors (RARs) and the thyroid hormone receptors (T3Rs) (2, 31, 37, 43, 64). RARs and T3Rs are ligand-regulated transcription factors that bind to specific target promoter sequences, denoted hormone response elements, in both the absence and the presence of cognate hormone. In the absence of hormone, these nuclear receptors typically repress gene transcription; conversely, binding of cognate hormone converts the nuclear receptors into strong transcriptional activators (2, 31, 37, 43, 64).
RARs and T3Rs manifest these divergent transcriptional properties through their ability to recruit auxiliary polypeptides, denoted corepressors and coactivators (12, 22, 36, 62, 76). In the absence of hormone ligand, RARs and T3Rs are able to bind to two interrelated corepressor polypeptides, denoted SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) and N-CoR (nuclear hormone receptor corepressor) (11, 21, 29, 35, 45, 46, 51, 55, 73, 79, 80); SMRT and N-CoR recruit, in turn, a larger corepressor complex including mSin3, RbAp-46, RbAp-48, SAP-18, and SAP-30, and histone deacetylases (5, 17, 47, 71). Conversely, binding of hormone ligand results in a conformational change in the nuclear hormone receptors that leads to release of the corepressor complex and recruitment of a series of coactivator complexes, many of which possess histone acetyltransferase activity (12, 22, 36, 62, 74, 76). The precise mechanisms by which corepressors and coactivators modulate transcription remain to be fully elucidated but appear to involve both modifications of the chromatin template and interactions with the general transcriptional machinery (5, 17, 28, 36, 41, 58, 59, 62, 72, 74–76).
Despite the important regulatory role of hormone ligand in T3R and RAR function, these nuclear receptors actually function as a molecular nexus at which a variety of both hormonal and nonhormonal signals converge to generate combinatorial regulation of target gene expression. Therefore, the ultimate transcriptional response mediated by nuclear hormone receptors is determined not just by the hormone status but also by the nature of the target promoter and by the actions of nonligand signal transduction pathways operative in the cell (26, 39, 43, 44, 56, 69). Particularly of note is the ability of certain protein kinases to modulate, both negatively and positively, nuclear hormone receptor function (reviewed in references 7, 8, 26, 56, and 69). The actions of these kinases can, for example, induce target gene expression by nuclear hormone receptors even in the absence of ligand or can further enhance the activation observed in the presence of hormone ligand. In some cases, these effects appear to be mediated through direct phosphorylation of the receptor itself (3, 10, 16, 23, 24, 27, 33, 63, 65). In other contexts, however, the mechanism by which these protein kinases alter the regulatory properties of the nuclear hormone receptor is not known but does not appear to involve modification of the receptor itself (6, 20, 52, 67, 69).
Might auxiliary factors, such as corepressors, serve as regulatory targets for these protein kinase signal transducers? We and others have reported that the SMRT and N-CoR corepressors are important targets of regulation by the epidermal growth factor (EGF) receptor and by cyclic AMP (20, 30, 67). EGF receptor signaling, for example, has little or no effect on T3R-mediated activation but strongly counteracts T3R-mediated repression, apparently by interfering with the ability of the SMRT or N-CoR corepressor to interact with the nuclear hormone receptor (20, 30). EGF receptor signaling similarly interferes with corepressor recruitment and transcriptional repression by RAR and by PLZF (promyelocytic leukemia zinc finger protein), a nonreceptor transcriptional repressor that also utilizes the SMRT–N-CoR corepressor complex (20). The signaling events and mechanisms by which the EGF receptor regulates corepressor function were not previously determined. Here, we report that SMRT corepressor function is regulated by components of a mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK) cascade that operates downstream of the EGF receptor. SMRT appears to be a substrate for phosphorylation by multiple components of this kinase cascade, including MAPK-extracellular signal-regulated kinase (ERK) kinase 1 (MEK-1), MEK-1 kinase (MEKK-1), p38, and possibly additional downstream protein kinases. SMRT phosphorylation in response to the actions of MEKK-1 or, to a somewhat lesser extent, MEK-1 strongly inhibits the ability of the corepressor to mediate repression by nuclear hormone receptors and by other transcription factors, such as PLZF. This MEKK-1 or MEK-1 phosphorylation of SMRT is closely paralleled by an inhibition of the ability of the corepressor to bind to nuclear receptors and by a relocalization of the SMRT corepressor from the nucleus to the cytoplasm of the cell. Our observations suggest that the MAPKKK pathway serves as a potent negative regulator of the SMRT corepressor and that this regulation appears to operate, at least in part, by altering both the subcellular location and the receptor interaction properties of the corepressor protein.
MATERIALS AND METHODS
Plasmid constructs.
The pCMV-SMRT and pCMV-SMRT-C vectors were constructed by inserting EcoRI fragments from the previously described pSG5-SMRT TRAC-2 and TRAC-1 constructs (51) into a pCR3.1 vector (Invitrogen, Carlsbad, Calif.). Mammalian two-hybrid vectors for various SMRT and T3Rα derivatives were constructed as previously described (18, 20). The pSG5-GAL4 activation domain (AD) (pSG5-GAL4AD)-RARα and retinoid X receptorα(RXRα) vectors were constructed by inserting EcoRV and XhoI fragments, generated by PCR, into the pSG5-GAL4AD vector. Construction of the glutathione S-transferase (GST) and GST-T3Rα vectors was described previously (72). The green fluorescent protein (GFP)-SMRT vector was constructed by inserting the BsrGI-EcoRI (blunt) fragment from pSG5-SMRT into the BsrGI-HindIII (blunt) sites on a CMVs-GFPTYGBH vector. Base substitution mutations, designed to abolish MAPK sites within the SMRT sequence, were created by standard PCR-mediated in vitro mutagenesis methodologies.
Expression vectors for full-length MEKK-1 and MEK-1 and for dominant negative MEK-1 expression plasmids were obtained from Chris Jamieson (University of California at San Francisco). The pMT3-HA-SAPK (p46β), pMT3-HA-p38, pEBG-SEK1, pMT3-ERK-1, pCMV5-FLAG-MEKK-1(817-1493), and pCMV5-FLAG-MEKK-1-KM(817-1493) clones were obtained from John Kyriakis (Massachusetts General Hospital). Baculovirus stocks of recombinant His6–MEKK-1α were obtained from Tom Maniatis (Harvard University).
Transient transfections.
CV-1 cell transfections were performed by a Lipofectin-mediated method using the general protocol recommended by the manufacturer (GIBCO/BRL Life Technologies, Rockville, Md.). Approximately 4 × 105 cells were transfected with 50 ng of pSG5-T3Rα or pSG5-v-ErbA plasmid, 100 ng of pCMV-lacZ or pCH110 as an internal control, and 100 ng of a ptk-luc-TRE reporter, together with expression vectors for the various signal transducers tested here (equal quantities of the equivalent empty vectors were substituted as appropriate). The cells were transferred to serum-free Dulbecco modified Eagle medium 24 h after transfection and harvested 24 h later. The luciferase and β-galactosidase assays were performed as previously described, and the relative luciferase activity was determined (19, 20).
Mammalian two-hybrid assays.
Exponentially growing CV-1 cells (7 × 104 cells per well in 12-well culture plates) were transiently transfected by Lipofectin methodology with 25 ng of the appropriate pSG5-GAL4 DNA binding domain (DBD) (pSG5-GAL4DBD) vector, 100 ng of the appropriate pSG5-GAL4AD vector, 100 ng of the pGL2-GAL4–17-mer luciferase reporter, 100 ng of the pCMV-lacZ internal control, and appropriate expression vectors for the various signal transducers tested here (or equal quantities of the equivalent empty vectors as appropriate) (20). The cells were transferred to serum-free Dulbecco modified Eagle medium 24 h after transfection and harvested an additional 24 h later. The luciferase and β-galactosidase assays were performed as previously described, and the relative luciferase activity was determined (20).
Immunoblotting.
CV-1 cells (7 × 104 per well) were transfected with the appropriate expression vectors, harvested 48 h after transfection by scraping, and lysed by being mixed with sodium dodecyl sulfate (SDS) electrophoresis sample buffer. The lysates were then sonicated to reduce viscosity, boiled for 5 min, and loaded onto an SDS–7.5% polyacrylamide–0.3% bisacrylamide gel. After electrophoresis, the proteins were electrophoretically transferred to a nitrocellulose membrane. The membrane was incubated in blocking buffer (5% nonfat dry milk in TBST [0.1% Tween 20, 150 mM NaCl, 10 mM Tris-Cl, pH 7.6]) for 1 h, and then incubated with appropriate primary antibodies (diluted in 5% bovine serum albumin in TBST) for 1 h. The membrane was next washed extensively with TBST and incubated with appropriate secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibodies [Affinity BioReagents, Golden, Colo.]; diluted 1:2,000). After extensive washing with TBST, the chemiluminescent Western detection system was used for visualization of the immunoreactive proteins as specified by the manufacturer (New England Biolabs, Beverly, Mass.).
Phosphorylation-dephosphorylation assays.
CV-1 cells (2.5 × 105) were transfected with the pCMV-SMRT-C, MEKK-1, MEK-1, MEKK-1 kinase mutant (MEKK1KM), or dominant negative MEK-1 expression vectors. The cells were harvested 48 h later by scraping and centrifugation in 150 μl of whole-cell extraction buffer (25 mM HEPES [pH 7.5], 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% Triton X-100, 0.1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail [Boehringer GmbH, Mannheim, Germany]). Lysates were then incubated in the presence or absence of 0.5 U of calf intestinal alkaline phosphatase (New England Biolabs) for 30 min at 30°C. The incubation reactions were terminated by mixing the samples with SDS sample buffer. The samples were boiled for 5 min, loaded onto an SDS–7.5% polyacrylamide–0.3% bisacrylamide gel, and subjected to electrophoresis and immunoblotting as described above.
Coimmunoprecipitation assays.
Approximately 7.5 × 105 COS-1 cells were transfected with various combinations of pCMV-SMRT, pCMV-FLAG-MEKK-1, or pCMV-HA-MEK-1 expression vectors by use of a Lipofectamine-Plus procedure (GIBCO/BRL Life Technologies). Whole-cell lysates were prepared by gently sonicating the cells in 600 μl of immunoprecipitation buffer (IP buffer; phosphate-buffered saline, 1 mM EDTA, 1.5 mg of iodoacetamide per ml, 100 μM Na3VO4, 0.5% Triton X-100, 20 mM β-glycerolphosphate, 0.2 mM phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail). After clarification by 5 min of centrifugation in a microcentrifuge at 4°C, the resulting supernatant was incubated for 1 h at 4°C with 2.4 μl of anti-FLAG antibody M2 (Sigma Chemical Co., St. Louis, Mo.; diluted 1:250). Fifteen microliters of protein A-Sepharose (as a 50% slurry in IP buffer; Sigma) was then added, and the samples were incubated with continuous mixing for an additional 1 h at 4°C. The protein A-Sepharose matrix was extensively washed with IP buffer, and any proteins remaining bound to it were eluted with SDS sample buffer and detected by Western analysis.
In vitro kinase assays.
GST-SMRT fusion proteins were expressed in Escherichia coli and immobilized on glutathione-agarose as previously described (19, 20). GST-SMRT proteins were then incubated with 0.4 μg of MEKK-1 (purified from recombinant E. coli; Upstate Biotechnology, Lake Placid, N.Y.), 0.5 U of activated MEK-1 (purified from recombinant E. coli; Upstate Biotechnology), or 0.5 μg of His6-tagged ΔMEKK-1 (purified from baculovirus-infected Sf9 cells) for 30 min at 30°C in 50 μl of kinase buffer (20 mM HEPES [pH 7.5], 20 mM β-glycerolphosphate, 10 mM MgCl2, 10 mM p-nitrophenyl phosphate, 100 μM Na3VO4, 2 mM DTT, 20 μM ATP) containing 5 μCi of [γ-32P]ATP. GST–SEK-1 (stress-activated protein kinase β [SAPK-β, also known as ERK kinase 1]) was used in some experiments as a positive control. The kinase reactions were terminated by adding SDS-sample buffer. The samples were boiled and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Phosphorylated proteins were visualized by autoradiography.
Immunocomplex kinase assays.
COS-1 cells were transfected with a pCMV5-MEKK1 expression vector (0.7 μg) and lysed 48 h later by gentle sonication in 600 μl of whole-cell extraction buffer. The MEKK-1 protein was immunoprecipitated by the addition of 2.4 μl of MEKK-1-directed antibodies. Immunopurified SMRT protein was obtained from pCMV-SMRT-transfected COS-1 cells in a similar manner with antibodies directed against SMRT (Affinity BioReagents). The immunopurified MEKK-1 preparation was then tested for the ability to phosphorylate the immunopurified SMRT protein or GST-SMRT protein derivatives obtained from E. coli using the in vitro kinase assay described above.
Receptor-corepressor binding assays in vitro.
GST-T3Rα proteins were expressed in E. coli and purified and immobilized by binding to a glutathione-agarose matrix (19, 20). 35S-radiolabeled SMRT-C (TRAC-1) proteins were synthesized by a coupled in vitro transcription-translation system (TnT kit; Promega, Madison, Wis.). The radiolabeled SMRT-C proteins were incubated in kinase buffer (containing 20 μM unlabeled ATP) with MEKK-1 or activated MEK-1 (each purified from recombinant E. coli) or without exogenous kinase. The SMRT-C proteins were then incubated with the immobilized GST-T3Rα polypeptides for 1 h at 4°C in 250 ml of HEMG buffer (51). The glutathione-agarose matrix was extensively washed, and proteins remaining bound to the matrix were eluted with free glutathione and analyzed by SDS-PAGE (19, 20). The electrophoretograms were visualized by autoradiography and quantified by PhosphorImager analysis (STORM; Molecular Dynamics).
Laser scanning confocal microscopy.
Approximately 105 CV-1 cells were seeded in a chambered coverslip cell culture system (Nalge-Nunc, Rochester, N.Y.). The cells were transfected with the pCMV-GFP-SMRT vector together with an appropriate expression vector for either v-ErbB, v-Ras, MEKK-1, or MEK-1 (or an equivalent empty vector as a control) using the Lipofectamine-Plus procedure. One day after transfection, the cells were transferred to serum-free Dulbecco modified Eagle medium and incubated for an additional 24 h. The subcellular location of the GFP-SMRT fusion polypeptide was visualized using a Leica TCS-SP Ar/Kr/HeNe laser scanning confocal microscope, with excitation at 488 nm and detection at 500 to 540 nm.
Subcellular fractionation assays.
CV-1 cells (2.5 × 105) were transfected with pCMV-SMRT-C together with expression vectors for the various signal transducers tested here using the Lipofectamine-Plus protocol. After 48 h, the cells were harvested in phosphate-buffered saline and incubated in hypotonic buffer (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM DTT) for 10 min at 4°C. Subsequently, the cells were lysed by 10 strokes of a Dounce microhomogenizer with a loosely fitting plunger. The cell lysates were then centrifuged at 2,000 × g for 5 min. The resulting pellets (nuclear fraction) and supernatants (cytoplasmic fraction) were subsequently solubilized in SDS sample buffer and analyzed by immunoblotting.
RESULTS
Tyrosine kinase signaling abrogates transcriptional repression and interferes with the ability of SMRT to interact with T3R in vivo.
The effects of EGF receptor signaling on T3R-mediated gene regulation were tested with a transient transfection assay using a luciferase reporter containing a thyroid hormone response element (TRE). CV-1 cells possess few or no endogenous T3Rs and display a basal level of luciferase reporter expression in either the absence or the presence of triiodothyronine (T3) hormone (Fig. 1A). As anticipated (11, 21, 51), the introduction of a T3R expression vector into these cells in the absence of T3 hormone resulted in the repression of luciferase expression to below basal levels; the addition of T3 hormone reversed this repression and led to T3R-mediated enhancement of luciferase expression to levels significantly above basal levels (Fig. 1A). Notably, cointroduction of a constitutively active form of the EGF receptor, denoted v-ErbB, into these cells reversed the repression mediated by T3R in the absence of T3, with relatively little effect on the activation mediated by T3R in the presence of T3 (Fig. 1A). The ability of EGF receptor signaling to abrogate transcriptional repression extended to repression by PLZF, a transcription factor that also utilizes the SMRT–N-CoR corepressor complex but that is not a member of the nuclear receptor family (20) (see Fig. 4C). Although v-ErbB was used in these experiments as a means of uniformly activating EGF receptor signaling in transfected cells (40, 66), analogous results were observed upon induction of wild-type EGF receptor activity (data not shown).
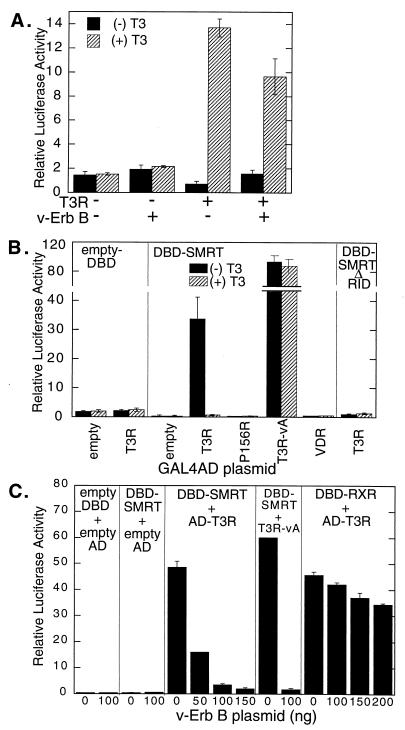
FIG. 1.
Effect of EGF receptor–v-ErbB signaling on T3R transcriptional repression and interactions with SMRT. (A) Inhibition of T3R-mediated repression by v-ErbB. CV-1 cells were transfected with empty plasmid pSG5 or plasmid pSG5-T3Rα in the presence or absence of a v-ErbB expression plasmid, as indicated below the panel. Cells were then incubated in the presence (+) or absence (−) of 1 μM T3, and the expression of a T3R-responsive (TRE) luciferase reporter was determined relative to that of a constitutive pCMV-lacZ reporter used as an internal control. (B) Interaction of T3Rα with SMRT in a mammalian two-hybrid assay. A pSG5-GAL4DBD plasmid containing no insert (empty-DBD), containing the RID (amino acids 751 to 1495) of SMRT (DBD-SMRT), or containing SMRT bearing a deletion of the RID (DBD-SMRTΔRID) was cotransfected into CV-1 cells together with a GAL4–17-mer luciferase reporter and a pSG5-GAL4AD construct. The pSG5-GAL4AD construct, indicated below the panel, contained no insert (empty), the wild-type T3Rα ligand binding domain (T3R), the T3Rα ligand binding domain with a P156R mutation that disrupts SMRT association (P156), the analogous region of the v-ErbA mutant form of T3Rα (T3R-vA), or the vitamin D3 receptor (VDR). The cells were incubated in the absence or presence of 1 μM cognate hormone, and luciferase activity was determined relative to the β-galactosidase activity of the pCH110 plasmid introduced as an internal control. (C) v-ErbB inhibition of the two-hybrid interaction between T3Rα and SMRT. The same mammalian two-hybrid vectors as those described for panel B and GAL4DBD fused with the ligand binding domain of RXRα were cotransfected into CV-1 cells in various combinations, as indicated for each panel. In addition, the cells were cotransfected with the indicated amounts of the v-ErbB expression plasmid (0 to 200 ng), the GAL4–17-mer luciferase reporter, and a pCMV-lacZ internal control plasmid. The cells were incubated in the absence of T3, and the relative luciferase activity was determined. The average and standard deviation of duplicate experiments are presented.
FIG. 4.
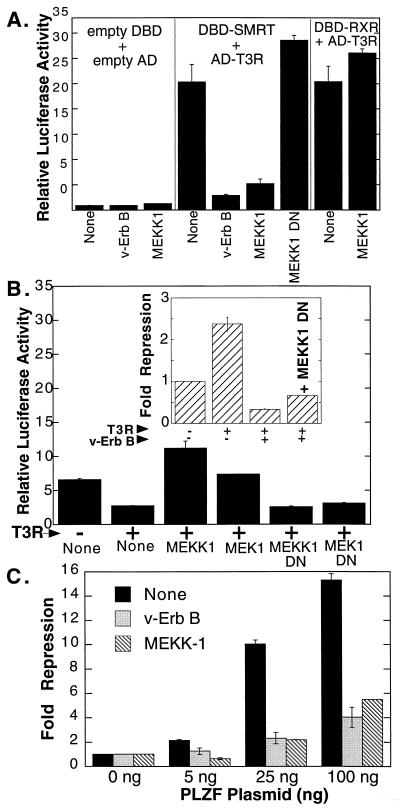
Effect of MEKK-1 signaling on transcriptional repression and T3R interactions with SMRT. (A) Inhibition by MEKK-1 of the two-hybrid interaction between SMRT and T3Rα. The effect of various signal transducers on the two-hybrid interaction between SMRT and T3Rα was tested as described in the legend to Fig. 1C by comparing an empty expression vector (None), a v-ErbB expression plasmid (v-ErbB), a full-length MEKK-1 expression plasmid (MEKK1), or a dominant negative MEKK-1 (residues 817 to 1493) expression plasmid (MEKK1 DN), as indicated below the panel. (B) Effects of MEKK-1 and MEK-1 on T3Rα-mediated repression of a TRE-luc reporter. The effect of different signal transducers on the ability of T3Rα to repress a TRE-driven promoter was tested as described in the legend to Fig. 1A. Expression plasmids for full-length MEKK-1, dominant negative MEKK-1, MEK-1, or dominant negative MEK-1 (MEK1 DN) were compared to an equivalent empty expression vector (None), as indicated below the panel. TRE reporter activity was assayed only in the absence of T3. (Inset) Effect of dominant negative MEKK-1 on the ability of v-ErbB to counteract T3R repression. Various combinations of expression plasmids for T3Rα, v-ErbB, and dominant negative MEKK-1 were transfected into CV-1 cells, as indicated below the inset panel, and the relative luciferase activity was determined. Fold repression was calculated relative to the basal levels of reporter gene expression in the absence of T3Rα. (C) Effects of EGF receptor and MEKK-1 signaling on transcriptional repression by PLZF. Various amounts of a GAL4DBD-PLZF fusion construct were introduced into CV-1 cells together with the GAL4–17-mer luciferase reporter and expression plasmids for v-ErbB or full-length MEKK-1. Relative luciferase activity was determined by normalization to an internal pCH110 β-galactosidase control; fold repression was calculated relative to the basal level of luciferase expression observed in the absence of the PLZF construct. The average and standard deviation of duplicate experiments are presented.
To examine if the abrogation of T3R- and PLZF-mediated repression by EGF receptor–v-ErbB signaling reflected changes in SMRT function, we next used a mammalian two-hybrid system as a measure of the ability of SMRT to interact with T3R in transfected cells (18, 20, 73). For this assay, a GAL4DBD-SMRT fusion construct, a GAL4AD-T3Rα fusion construct, and a luciferase reporter bearing GAL4 binding sites (GAL4–17-mer) were introduced separately or in combination into CV-1 cells. A strong activation of the GAL4–17-mer luciferase reporter was observed when all three constructs were introduced together, presumably reflecting the ability of the SMRT and T3R determinants to interact and thereby reconstitute a functional GAL4 transcription factor (Fig. 1B). Supporting this interpretation are the following: (i) each of the fusion constructs was transcriptionally inactive when introduced separately; (ii) T3 hormone abolished the T3R interaction with SMRT both in vitro and in the mammalian two-hybrid system; (iii) mutants of T3R (P156R) or other nuclear receptors, such as vitamin the D3 receptor, that do not interact significantly with SMRT in vitro (51, 73) did not interact in the two-hybrid system; (iv) irrelevant proteins or SMRT mutants (ΔRID) that fail to interact with T3R in vitro (51) did not interact with T3R in the two-hybrid system; and (v) constitutive repression mutants of T3R (T3R-vA) that bind SMRT in a hormone-independent fashion in vitro (51) exhibited a hormone-independent interaction with SMRT in the two-hybrid assay (Fig. 1B).
The ability of T3R to interact with SMRT in the mammalian two-hybrid assay was severely compromised by cointroduction of the EGF receptor–v-ErbB construct (Fig. 1C). This effect was proportional to the amount of the EGF receptor–v-ErbB construct introduced into the cells and was observed with either the wild-type T3R construct in the absence of T3 hormone or a mutant of T3R (T3R-vA) that is unable to bind T3 hormone (Fig. 1C). The inhibitory effects of EGF receptor signaling on the SMRT-T3R interaction were not limited to the v-ErbB per se and could also be mediated by the wild-type EGF receptor in response to appropriate EGF receptor ligands (data not shown).
The effects of EGF receptor signaling in the two-hybrid assay system appeared to reflect true inhibition of the SMRT-T3R interaction itself: v-ErbB had little or no effect on basal reporter expression if either (or both) of the GAL4DBD-SMRT or GAL4AD-T3R fusions was omitted from the transfection (Fig. 1C). Similarly, the introduction of v-ErbB had no effect on the expression of a β-galactosidase reporter lacking GAL4 binding sites and used as an internal control (data not shown). Therefore, the effects of v-ErbB in the two-hybrid assay are unlikely to be mediated by nonspecific inhibition of the reporter promoter itself or by a decrease in the stability or the enzymatic activity of the luciferase protein. To exclude the possibility that v-ErbB inhibited the expression or function of the GAL4DBD or GAL4AD moieties, rather than that it interfered with the SMRT-T3R interaction itself, we assayed the effects of v-ErbB on the two-hybrid interaction between T3R and RXRs. RXRs are heterodimer partners for T3Rs, and the two receptor classes can physically associate in vitro and in vivo (37). In our mammalian two-hybrid system, T3R exhibited a strong interaction with RXRs which was altered only slightly by cointroduction of v-ErbB, in clear contrast to the potent v-ErbB-mediated inhibition observed for the T3R-SMRT interaction (Fig. 1C). These results indicate that the interaction between SMRT and T3R is specifically inhibited by cointroduction of an activated EGF receptor–v-ErbB construct.
SMRT function is inhibited by a MAPKKK signaling pathway.
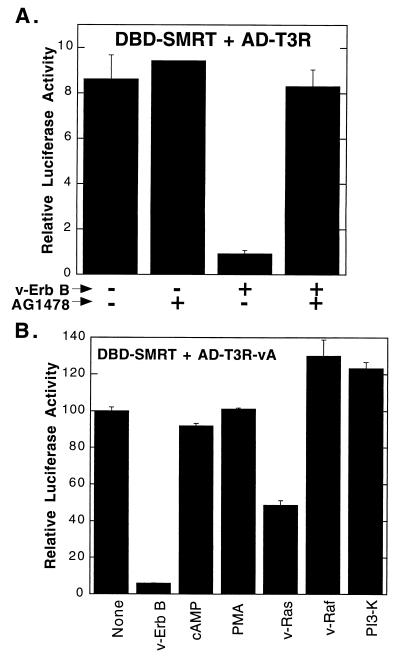
The EGF receptor can operate through a diverse array of downstream signal regulators (13, 25, 60, 61, 66, 68). To evaluate which of these downstream effectors might be responsible for the inhibition exerted by the EGF receptor on SMRT function, we tested a variety of candidate transducers and/or inhibitors of downstream signaling. A specific chemical inhibitor of the tyrosine kinase activity of the EGF receptor, AG1478 (34), abolished the effects of v-ErbB on the SMRT-T3R interaction, suggesting that the kinase activity of the EGF receptor–v-ErbB construct was essential for mediating the inhibitory phenotype (Fig. 2A). Significantly, the introduction of a v-Ras expression vector mimicked the inhibitory effects of v-ErbB on the interaction of T3R and SMRT, although not as strongly as did v-ErbB itself (Fig. 2B). In contrast, treatment of CV-1 cells with moderate levels of cyclic AMP or phorbol esters (inducers of protein kinase C) or introduction of an expression vector for the catalytic subunit of phosphatidylinositol 3-kinase failed to inhibit the T3R-SMRT interaction (Fig. 2B).
FIG. 2.
Inhibition of the mammalian two-hybrid interaction between SMRT and T3Rα by various signal transducers. (A) Effect of tyrphostin AG1478 on the ability of v-ErbB to inhibit the SMRT–T3Rα two-hybrid interaction. The GAL4DBD-SMRT fusion, the GAL4AD-T3Rα fusion, and the GAL4–17-mer luciferase reporter were introduced into CV-1 cells in the presence (+) or absence (−) of the pSG5-v-ErbB expression plasmid, as indicated below the panel. The cells were subsequently incubated in the presence (+) or absence (−) of 30 nM tyrphostin AG1478, and the relative luciferase activity was determined. (B) Effects of different signal transducers on the two-hybrid interaction between SMRT and T3R-vA. CV-1 cells were transfected as described in panel A with GAL4DBD-SMRT and GAL4AD–T3R-vA. The cells were not treated (None), were treated with 10 μM 8-bromo-cyclic AMP (cAMP) or 5 ng of phorbol-12-myristate-13 acetate (PMA) per ml, or were cotransfected with expression plasmids for v-ErbB, v-Ras, v-Raf, or the p110 catalytic subunit of phosphatidylinositol 3-kinase (PI3-K), as indicated below the panel. The relative luciferase activity was subsequently determined. The average and standard deviation of duplicate experiments are presented.
Ras is believed to operate primarily through the ability to bind to and activate MAPKKKs, such as Raf and MEKK-1 (Fig. 3) (13, 25, 54, 60). Although Raf is a downstream effector of Ras signaling in many contexts, introduction of an activated Raf allele into our experimental system had little or no effect on the SMRT-T3R interaction (Fig. 2B). However, MEKK-1 also can serve as a downstream mediator of Ras function (13, 14, 25, 38, 48, 50, 54, 60), and introduction of either a wild-type or a constitutively activated allele of MEKK-1 into the two-hybrid assay resulted in very strong inhibition of the SMRT-T3R interaction (Fig. 4A). Similarly, induction of endogenous MEKK-1 activity with anisomycin also resulted in inhibition of the SMRT-T3R interaction (data not shown), whereas introduction of a dominant negative MEKK-1 allele into CV-1 cells actually slightly enhanced the SMRT-T3R interaction (Fig. 4A). Introduction of MEKK-1 had little or no effect on the two-hybrid interaction of T3R with RXRs, indicating that the inhibitory actions of MEKK-1 were specific for the SMRT-T3R interaction and did not represent a nonspecific or indirect action of MEKK-1 in the two-hybrid assay system itself (Fig. 4A).
FIG. 3.
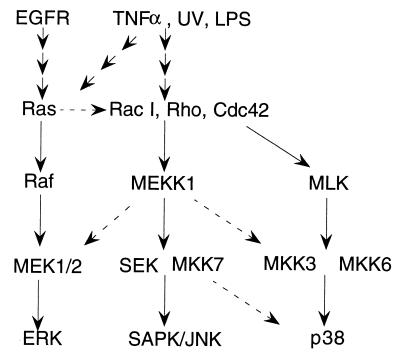
Schematic of proposed MAPKKK signal transduction pathways. The cascades include at least three major “modules,” each of which includes MAPKKKs (such as Raf, MEKK-1, or mixed-lineage kinase [MLK]), MAPKKs (such as MEK-1/2, SEK-1, MAPKK-3, MKK-6, or MKK-7), and MAPKs (such as ERK-1, SAPK-β–Jun N-terminal kinase [JNK], or p38). Upstream signals that activate these modules include the EGF receptor (EGFR), tumor necrosis factor α (TNFα), UV light, and bacterial lipopolysaccharide (LPS). The major pathways are indicated by solid lines; broken lines indicate cross talk that can occur, at least under certain circumstances, between modules.
This ability of MEKK-1 to inhibit the interaction of SMRT with T3R in a two-hybrid assay was paralleled by the ability of MEKK-1 to abrogate T3R-mediated transcriptional repression (Fig. 4B). As previously noted, T3R in the absence of hormone repressed a reporter gene bearing a TRE (Fig. 1B). Cointroduction of functional MEKK-1 reversed this repression, thereby mimicking the effects of v-ErbB (Fig. 4B). Conversely, introduction of a dominant negative MEKK-1 allele failed to reverse repression when introduced on its own and partially counteracted the inhibitory effects of v-ErbB when cointroduced together with the v-ErbB construct (Fig. 4B, inset). Taken as a whole, these results suggest that MEKK-1 is able to operate downstream of the EGF receptor to strongly inhibit both the interaction of the SMRT corepressor with T3R and the ability of T3R to function as a transcriptional repressor.
MEK-1, an MAPKK, mimics some of the actions of MEKK-1, but a variety of MAPKs do not.
We next explored which effectors might be able to operate, in turn, downstream of MEKK-1 to regulate the SMRT-T3R interaction. At least three parallel MAPKKK pathways have been identified for metazoans; although some signaling is pathway specific, there also exists detectable cross talk between the different kinase hierarchies under certain experimental conditions (Fig. 3). Intriguingly, the introduction of SEK-1, an MAPKK that in many contexts functions immediately downstream of MEKK-1, had no significant effect in our SMRT-T3R interaction assay (Fig. 5A). In contrast, the introduction of MEK-1, a second form of MAPKK that has also been reported to operate downstream of MEKK-1 (4, 14, 54, 77, 78), resulted in inhibition of both the SMRT-T3R two-hybrid interaction and T3R-mediated transcriptional repression (Fig. 4B and 5A). Consistent with these results, MEK-1 activity, but not SEK-1 activity, was enhanced in CV-1 cells in response to the introduction of MEKK-1 (data not shown). Also consistent with a role for MEK-1 in SMRT regulation, U0126 (a specific inhibitor of MEK-1 and MEK-2 [15]) counteracted the effects of MEK-1 in our transfection experiments (Fig. 5B). Notably, however, MEK-1 was less efficient at inhibiting the SMRT-T3R interaction (and T3R-mediated repression) than was MEKK-1 (Fig. 4B and 5A), and the counteracting effects of U0126 were much more pronounced for MEK-1 than for MEKK-1 (Fig. 5B). Taken as a whole, these results suggest that some, but not all, of the effects of MEKK-1 on the SMRT-T3R interaction may be mediated by the downstream kinase MEK-1.
FIG. 5.
Effects of different components of the MAPKKK cascade on the ability of SMRT to interact with T3R or PLZF. (A) Effects of different MAPKKK components on the mammalian two-hybrid interaction between SMRT and T3Rα. The two-hybrid protocol used in Fig. 2B was used but with expression vectors for v-ErbB, full-length MEKK-1, MEK-1, ERK-1, SEK-1, SAPK-β, or p38 or an empty vector, as indicated below the panel. (B) Effect of a MEK-1 inhibitor, U0126, on the SMRT-T3Rα interaction. The effects of MEKK-1 and MEK-1 on the SMRT-T3Rα two-hybrid interaction were tested as described for panel A, but in the absence (−) or presence (+) of 15 μM U0126. (C) Effects of MEKK-1 and MEK-1 on the mammalian two-hybrid interaction between SMRT and PLZF. The same protocol as that used in Fig. 2B was repeated but with either an empty GAL4AD plasmid or a GAL4AD-PLZF fusion, together with the GAL4DBD-SMRT construct previously described. The transfections were performed in the presence of 100 ng of empty vector or an expression vector for v-ErbB, MEKK-1, or MEK-1, as indicated below the panel. The data represent the average and standard deviation of duplicate experiments.
To extend these results, we examined the effects of MEKK-1 and MEK-1 on the ability of SMRT to interact with PLZF, a hormone-independent transcriptional repressor. As previously noted (20), the ability of SMRT to interact with PLZF in our two-hybrid system was strongly inhibited by cointroduction of EGF receptor–v-ErbB (Fig. 5C). Significantly, the introduction of MEKK-1 or MEK-1 also interfered with the SMRT-PLZF interaction (Fig. 5C) and with PLZF-mediated repression (Fig. 4C). We conclude that these components of the MAPKKK cascade mediate strong inhibition of the ability of SMRT to interact with a variety of its transcription factor partners.
In many regulatory pathways, the actions of MEKK-1 and MEK-1 are mediated, in turn, by MAPKs that operate still lower in the kinase cascade (13, 25, 60). However, overexpression of three different MAPKs, ERK-1, SAPK-β, and p38, separately or in combination, had little or no observable effect on the T3R-SMRT interaction in our two-hybrid system and no observable effect on T3R-mediated repression (Fig. 5A and data not shown). We conclude that the effects of MEKK-1 and MEK-1 on the T3R-SMRT interaction are not manifested through the actions of these downstream MAPKs; the same conclusion was obtained from our analysis of the sites of phosphorylation of SMRT (see below).
The SMRT protein is phosphorylated in vivo and in vitro by multiple components of the MAPKKK cascade.
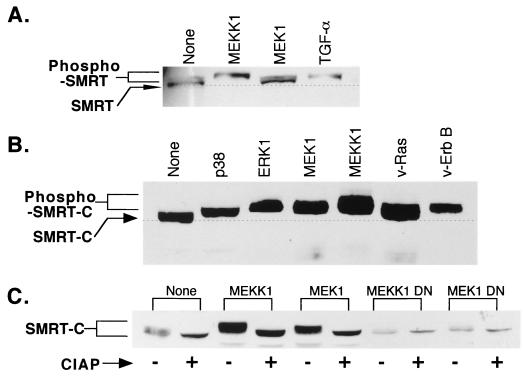
To better understand the impact of MEKK-1 signaling on the T3R-SMRT interaction, we next investigated if either T3R or SMRT was modified by components of the MAPKKK signal cascade. No indication of posttranslational modification of T3R, as evidenced by a change in the apparent molecular weight of the nuclear receptor, was observed in response to MEKK-1 or MEK-1 expression (data not shown). In contrast, the introduction of MEKK-1 into transfected cells led to a detectable change in the apparent mobility of the SMRT corepressor, as determined by Western blot analysis (Fig. 6A). A more modest shift in the mobility of SMRT was also observed in response to the introduction of MEK-1 (Fig. 6A). The changes in the mobility of the SMRT protein in response to MEKK-1 and MEK-1 were also observed upon introduction of v-ErbB or v-Ras and by treatment of the cells with EGF receptor agonists, such as transforming growth factor α (TGF-α), and were particularly evident when corepressor constructs limited to the SMRT C-terminal receptor interaction domain (RID) were used (Fig. 6A and B). The alterations in SMRT mobility were reversed by treatment of the corepressor with alkaline phosphatase and were not observed when kinase-defective dominant negative mutants of either MEK-1 or MEKK-1 were used, indicating that these mobility shifts were likely due to the phosphorylation of SMRT (Fig. 6C).
FIG. 6.
Effects of different signal effectors and transducers on the electrophoretic mobility and phosphorylation of SMRT in transfected cells. (A) Alterations in the electrophoretic mobility of SMRT in CV-1 cells in response to MEKK-1, MEK-1, or TGF-α. CV-1 cells were transfected with pCMV-SMRT and either an empty vector (None) or an expression vector for full-length MEKK-1 or for activated MEK-1 or were treated with 100 ng of TGF-α per ml, as indicated above the panel. The cells were subsequently lysed, and the extracts were resolved by SDS-PAGE and analyzed by immunoblotting using antibody specific for the SMRT protein. The mobility of the SMRT protein in the absence of treatment is indicated by the broken line. (B) Alterations in the electrophoretic mobility of an SMRT C-terminal polypeptide in CV-1 cells in response to different components of the MAPKKK cascades. pCMV-SMRT-C (representing SMRT amino acids 751 to 1495) was introduced into CV-1 cells, together with the various expression vectors indicated above the panel. Whole-cell lysates were subsequently resolved by SDS-PAGE and visualized by immunoblotting as described for panel A. (C) Reversal by alkaline phosphatase of the alterations in SMRT electrophoretic mobility. CV-1 cells were transfected with the pCMV-SMRT-C construct and the various expression vectors indicated above the panel. Whole-cell lysates were prepared and either mock treated (−) or incubated with 0.5 U of calf intestinal alkaline phosphatase (CIAP) (+) for 30 min prior to analysis by SDS-PAGE and immunoblotting.
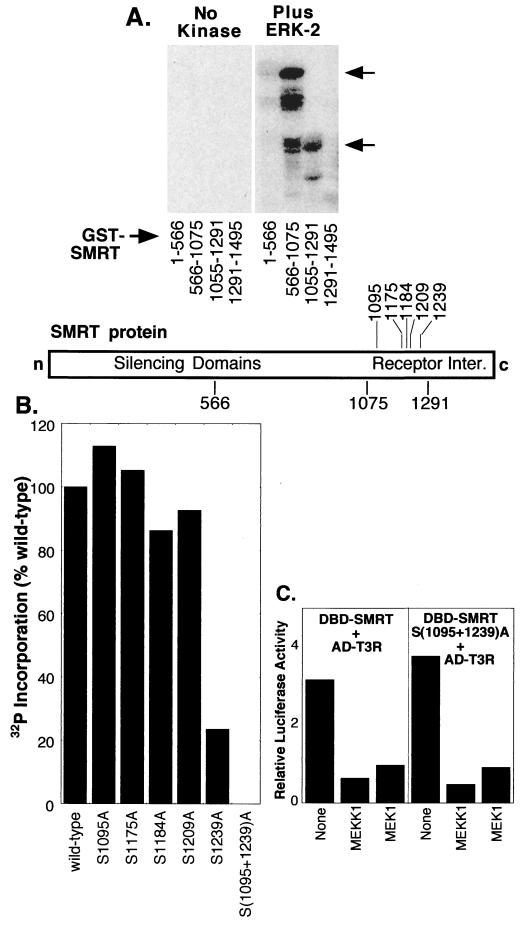
To identify the precise nature of the protein kinase(s) responsible for these modifications, we tested the ability of SMRT to be phosphorylated in vitro by purified components of the MAPKKK cascade (Fig. 7). Significantly, GST-SMRT protein fusions were phosphorylated in vitro by a variety of recombinant MEKK-1 preparations, including a MEKK-1 preparation isolated by use of a His6 tag from recombinant baculovirus-infected insect cells (Fig. 7A) and MEKK-1 purified from recombinant E. coli (Fig. 7B). Phosphorylation of SEK-1, a known substrate for MEKK-1, is shown for comparison (Fig. 7C). A GST fusion protein restricted to the C-terminal domain of SMRT (amino acids 1291 to 1495) was also phosphorylated by the recombinant MEKK-1 preparations, whereas more N-terminal SMRT domains and the GST protein itself were not (Fig. 7A and B). Apparently identical results were obtained when the kinase assay was performed with full-length MEKK-1 and full-length SMRT or SMRT C-terminal domain proteins immunopurified from suitably transfected mammalian cells (Fig. 7D). Intriguingly, activated (but not unactivated) MEK-1, either purified as a recombinant protein from bacteria or immunoenriched from transfected mammalian cells, could also phosphorylate SMRT in vitro, with the major site(s) of MEK-1 phosphorylation also mapping to a region within amino acids 1291 to 1495 of the corepressor (Fig. 7B). None of our GST-SMRT constructs was phosphorylated in the absence of an exogenous kinase (Fig. 7). We conclude that SMRT appears to be a direct substrate for phosphorylation by both MEKK-1 and MEK-1, although presumably at distinct sites, and that the principal phosphorylation sites map within the C-terminal RID of the corepressor.
FIG. 7.
Phosphorylation of SMRT in vitro and association of SMRT with MEKK-1 and MEK-1 in vivo. (A) In vitro kinase assay of SMRT using recombinant MEKK-1 purified from infected Sf9 insect cells. GST fusion proteins representing different portions of SMRT, as indicated above the panel, were synthesized in E. coli, immobilized on glutathione-agarose, and incubated with [γ-32P]ATP in the presence (right panel) or absence (left panel) of His6–MEKK-1 purified from recombinant baculovirus-infected Sf9 cells. Phosphorylated SMRT proteins are indicated by arrowheads. (B) In vitro kinase assay of SMRT protein derivatives using recombinant MEKK-1 and MEK-1 purified from E. coli. The same protocol as that used in Fig. 7A was used, except that bacterially expressed MEKK-1 or MEK-1 was used in the kinase assay. Phosphorylated SMRT proteins are indicated by arrowheads. (C) Comparison of MEKK-1 phosphorylation of SMRT and of SEK-1. GST-SMRT or GST–SEK-1 protein derivatives were incubated with [γ-32P]ATP in the presence (+) or absence (−) of recombinant murine MEKK-1 (residues 817 to 1493) purified from E. coli, as indicated below the autoradiogram. Incubations were performed with 20 mM morpholinepropanesulfonic acid (MOPS) (pH 7.2), 25 mM 2-glycerolphosphate, 5 mM EGTA, 1 mM DTT, 1 mM sodium vanadate, and 15 mM MgCl2. The locations of the phosphorylated SMRT and SEK-1 proteins are indicated by arrowheads. (D) In vitro kinase assay of SMRT proteins using full-length MEKK-1 isolated from COS-1 cells. The kinase assay was performed with SMRT proteins and full-length MEKK-1 immunopurified from transfected COS-1 cells rather than the recombinant proteins used in panel C. The positions of the phosphorylated SMRT derivatives are indicated by arrowheads, as is the position of MEKK-1, which is autophosphorylated in these assays. C-term, C terminus. (E) Physical association of SMRT with MEKK-1 and MEK-1 in mammalian cells. COS-1 cells were transfected with pCMV-SMRT and pCMV-HA-MEK-1 in the presence or absence of pCMV-FLAG-MEKK-1, as indicated below the panel. The cells were lysed, and the lysates were analyzed directly by SDS-PAGE and immunoblotting (left panel). Alternatively, the lysates were immunoprecipitated (IP) with anti-FLAG antibody M2, and the resulting immunoprecipitates were analyzed by SDS-PAGE and immunoblotting (right panel). The antisera used to visualize each immunoblot are indicated on the left.
Although overexpression of the MAPKs ERK-1, p38, and SAPK-β had no effect on the T3R-SMRT interaction in our two-hybrid assay, the SMRT protein was nonetheless capable of being phosphorylated by MAPKs both in vitro and in vivo. SMRT was shifted in mobility by coexpression of the MAPK ERK-1 or p38 in vivo, and two domains of SMRT were phosphorylated by purified MAPK in vitro: (i) amino acids 566 to 1075, overlapping the silencing domain of SMRT, and (ii) amino acids 1056 to 1291, representing the RID of SMRT (Fig. 6B and 8A and data not shown). We therefore wished to confirm that the phosphorylation of SMRT by these MAPKs was not involved in mediating the MEKK-1 inhibition phenotype. The inhibitory effects of MEKK-1 signaling on the two-hybrid interaction were observed with a SMRT construct limited to the C-terminal half of SMRT, so we focused our analysis on the MAPK sites within the corresponding SMRT region (amino acids 1055 to 1291). This region has five potential recognition sites for MAPK which we altered, individually or in combination, to alanines and tested for their effects on MAPK phosphorylation (Fig. 8B). Phosphorylation of the SMRT RID by ERK-2 was inhibited by over 75% by an S1239A substitution and virtually eliminated by an S1095A/S1239A double substitution; the remaining alanine substitution mutant proteins were phosphorylated at levels comparable to that of the wild-type SMRT protein (Fig. 8B). These results implicate serine 1095 and serine 1239 of SMRT as the primary sites of phosphorylation of the SMRT C terminus by this MAPK.
FIG. 8.
Phosphorylation of SMRT in vitro by MAPK. (A) Phosphorylation of SMRT by ERK-2. GST fusions representing different regions of SMRT were incubated with [γ-32P]ATP and either no kinase (left panel) or purified ERK-2 (right panel). The proteins were resolved by SDS-PAGE and visualized by PhosphorImager analysis. Arrows indicate phosphorylated GST-SMRT derivatives. A schematic below the gel depicts the regions of SMRT represented by the different GST fusions. Inter., interaction domains. (B) Effects of alanine substitutions on the phosphorylation of SMRT by ERK-2. Different serine codons were converted into alanines by site-directed mutagenesis, as indicated below the panel. The different mutants were expressed as GST-SMRT (residues 1291 to 1495) fusion proteins and were tested for the ability to be phosphorylated by ERK-2 in vitro as described for panel A. The amount of 32P incorporated into each mutant or wild-type protein was quantified by PhosphorImager analysis. (C) Response of an MAPK-deficient mutant of SMRT to MEKK-1 and MEK-1 signaling. The mammalian two-hybrid experiment shown in Fig. 5A was repeated with pSG5-GAL4AD-T3Rα and either pSG5-GAL4DBD-SMRT (wild type) (left panel) or pSG5-GAL4DBD-SMRT bearing a double substitution (S1095A/S1239A) (right panel).
Although unable to be phosphorylated by MAPK, the 1095A/1239A double mutant of SMRT was fully susceptible to inhibition by MEKK-1 and MEK-1 in the two-hybrid T3R interaction assay (Fig. 8C and data not shown). In addition, the mobility shift observed for SMRT in transfected cells in response to MEKK-1 and MEK-1 signaling was not eliminated by introduction of the 1095A/1239A double mutation, indicating that this mutant remains a substrate for MEKK-1 and MEK-1 (data not shown). We conclude that although MAPKs are able to phosphorylate SMRT, they do not appear to be the principal effectors by which the inhibitory effects of MEKK-1 or MEK-1 on the SMRT-T3R interaction were manifested in our two-hybrid assay. Instead, inhibition of SMRT function was closely correlated with phosphorylation of the corepressor by MEKK-1 and, to a lesser extent, MEK-1.
SMRT can be isolated from cells in the form of a complex with MEKK-1 and MEK-1.
The individual kinases within certain MAPKKK cascades appear able to associate together to form a physical complex that can, in turn, bind to and phosphorylate substrate polypeptides (reviewed in references 53 and 70). To test if a similar physical complex might be involved with the phosphorylation of SMRT, we cotransfected cells with expression vectors for SMRT and for a FLAG-tagged MEKK-1 or a hemagglutinin (HA)-tagged MEK-1 construct. Immunoprecipitates of FLAG-tagged MEKK-1 or HA-tagged MEK-1 contained high levels of associated SMRT protein in Western analysis, whereas little or no SMRT was immunoprecipitated by anti-FLAG antibodies in the absence of a tagged MEKK-1 construct (Fig. 7E). Identical results were observed in immunoprecipitations of full-length, rather than epitope-tagged, MEKK-1 (data not shown). Notably, FLAG-tagged MEK-1 was also detected in immunoprecipitates of FLAG-tagged MEKK-1 and visa versa (e.g., Fig. 7E). Taken as a whole, our results indicate that MEKK-1 and MEK-1 can be found in a physical complex in cells and that this complex itself appears able to bind to and phosphorylate the SMRT corepressor.
Phosphorylation by MEKK-1 or by MEK-1 interferes with the ability of SMRT to bind to nuclear hormone receptors in vitro.
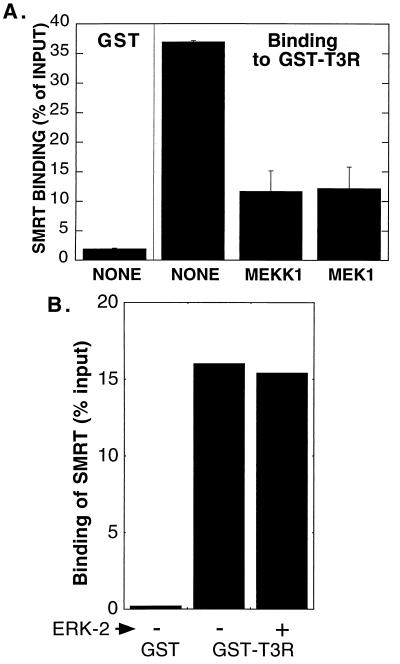
We next determined if the inhibitory effects of MEKK-1 and MEK-1 on the interaction between SMRT and T3R in our two-hybrid system were mimicked in vitro. We tested the ability of radiolabeled SMRT, synthesized by in vitro translation, to bind to a GST-T3R fusion protein synthesized in bacteria. A strong interaction was observed between SMRT and the GST-T3R polypeptide in the absence of exogenous kinases (Fig. 9A). Preincubation of the SMRT protein with either purified MEKK-1 or purified MEK-1 led to more than 66% inhibition of the ability of the corepressor to bind to the GST-T3R construct (Fig. 9A). The inhibitory effects of SMRT phosphorylation in vitro may be even greater than this 66% value suggests: the SMRT protein population that bound to the GST-T3R matrix after kinase treatment migrated with a mobility characteristic of unphosphorylated SMRT, suggesting that it may have escaped being phosphorylated by the kinase or was dephosphorylated during subsequent incubations (data not shown). Incubation of SMRT with purified MAPKs, such as ERK-2, had no effect on the ability of the corepressor to bind to T3R (Fig. 9B). We conclude that phosphorylation of SMRT by MEKK-1 or MEK-1 specifically interferes with the ability of the corepressor to bind to nuclear receptors, such as T3R, in vitro as well as in vivo.
FIG. 9.
Inhibition of the SMRT-T3Rα interaction in vitro by phosphorylation by MEKK-1 and MEK-1. (A) Inhibition by MEKK-1 or MEK-1 of the ability of SMRT to bind to GST-T3Rα in vitro. Nonrecombinant GST or a GST-T3Rα fusion protein was synthesized in E. coli and immobilized on glutathione-agarose. The immobilized GST proteins were then incubated with 35S-labeled SMRT protein that had been previously incubated without kinase, with bacterially produced MEKK-1, or with bacterially produced, activated MEK-1, as indicated below the panel. Radiolabeled SMRT remaining bound to the glutathione-agarose after extensive washing was eluted with free glutathione and was resolved by SDS-PAGE. The amount of bound SMRT was quantified by PhosphorImager analysis and is presented as a percentage of the total input for each binding reaction. The data represent the average and range of duplicate experiments. (B) Lack of an effect of ERK-2 phosphorylation on the ability of SMRT to bind to GST-T3Rα in vitro. An experiment similar to that shown in panel A was performed but with purified ERK-2 instead of MEKK-1 or MEK-1.
MEKK-1 signaling alters the subcellular localization of the SMRT protein.
We next examined the effects of the MEKK-1 cascade on the subcellular distribution of SMRT. N-CoR and SMRT are nuclear proteins. In agreement with prior observations on native SMRT (57), a GFP fusion of SMRT was located virtually exclusively in the nucleus of unstimulated transfected cells, forming a punctate pattern of bright fluorescent spots superimposed on a more diffuse fluorescent nucleoplasm (Fig. 10A). Cointroduction of a v-ErbB, MEKK-1, or MEK-1 expression vector into these cells led to a change in the GFP-SMRT pattern, manifested as coalescence of the punctate spots into a smaller number of larger dots per nucleus. In many cells, the GFP-SMRT signal also shifted out of the nucleus into a perinuclear or cytoplasmic fluorescence pattern; this effect was most evident in response to MEKK-1 and was not observed with a kinase-defective MEKK-1 mutant (Fig. 10A and data not shown). No change in the morphology or integrity of the nuclei themselves was observed. The introduction of MAPKs, such as ERK-1 or p38, had no observable effect on the subcellular distribution of SMRT. The 1095A/1239A mutant construct of SMRT, which is defective for MAPK phosphorylation in vitro, underwent the same change in subcellular distribution in response to MEKK-1 signaling as did the wild-type SMRT protein (data not shown).
FIG. 10.
Effect of transient MEKK-1 expression on subcellular localization of SMRT proteins. (A) Alteration of subcellular localization of GFP-SMRT protein by MEKK-1 and MEK-1 signaling. CV-1 cells were transfected with pCMV-GFP-SMRT together with an empty expression plasmid or were cotransfected with expression vectors for v-ErbB, MEKK-1, or MEK-1. The GFP signal was subsequently visualized by confocal microscopy. Representative cell fields are shown at different magnifications. Arrows indicate the nuclear envelope. (B) Biochemical subcellular fractionation of SMRT proteins. pCMV-SMRT-C was introduced into CV-1 cells with either an empty vector (None) or v-ErbB, full-length MEKK-1, MEK-1, or v-Ras expression vectors, as indicated above the immunoblot. The cells were harvested and separated into nuclear and cytoplasmic fractions as described in Materials and Methods. SMRT proteins were not detected in the cytoplasmic fraction when coexpressed with p38 or SEK-1 (data not shown).
We confirmed these results using a biochemical subcellular fractionation procedure (Fig. 10B). Consistent with the GFP fusion data, in the absence of MEKK-1 signaling all of the SMRT protein detected by Western analysis was found in the nuclear fraction, whereas the introduction of MEKK-1 or, to a somewhat lesser extent, MEK-1 resulted in a significant redistribution of the SMRT protein from the nuclear to the cytoplasmic fraction (Fig. 10B). V-ErbB and v-Ras overexpression also led to a redistribution of SMRT into the cytoplasmic fraction (Fig. 10B). We conclude that MEKK-1 signaling can result in a change in the subcellular distribution of the SMRT protein from an exclusively nuclear compartment to a more perinuclear and cytoplasmic distribution.
DISCUSSION
A MAPKKK cascade acts to inhibit SMRT function.
The ability of nuclear hormone receptors and of nonreceptor transcription factors, such as PLZF, to repress transcription is strongly counteracted by the EGF receptor signal transduction pathway (20). This inhibition of repression by EGF receptor signaling appears to be due to an inhibition of the ability of the SMRT corepressor to physically interact with the transcription factor partner, as observed both in a mammalian two-hybrid assay and by coprecipitation analyses (20, 30). Given that EGF receptor signaling had comparable inhibitory effects on the ability of SMRT to interact with T3R, RAR, and PLZF, we had previously proposed that the corepressor itself was likely to represent the common target through which these EGF receptor-initiated events manifest their inhibitory effects (20).
In the current study, we found evidence that the SMRT corepressor is phosphorylated in response to EGF receptor signaling, at least in part in response to a MAPKKK pathway operating downstream of the EGF receptor, whereas T3R is not; this corepressor phosphorylation closely correlates with and may mediate the inhibition of SMRT function reported previously. Intriguingly, the SMRT corepressor is subject to phosphorylation by kinases operating at a variety of levels of the MAPKKK regulatory cascade, including MEKK-1, MEK-1, and MAPKs, such as ERK-1, ERK-2, and p38. The strongest inhibitory effects on SMRT function are associated with the actions of MEKK-1, with overexpression of MEKK-1 resulting in both a dramatic decrease in the ability of SMRT to interact with its transcription factor partners in a two-hybrid interaction assay and a parallel inhibition of transcriptional repression by these transcription factors. MEK-1, an MAPKK that operates at a level below that of MEKK-1, mimicked some, but not all, of the actions of MEKK-1. In contrast, phosphorylation of SMRT by several different MAPKs had no detectable effect on corepressor function in the assays described here (see below).
MEKK-1 signaling is coupled to that of growth factor receptors, such as the EGF receptor, by the actions of Ras (reviewed in references 13, 25, 54, and 60). Consistent with the proposal that MEKK-1 can operate downstream of the EGF receptor to inhibit SMRT, Ras expression also inhibits SMRT function, although less efficiently than does the expression of MEKK-1. MEKK-1 also plays an important role in responding to signals of cell stress (13, 54, 60), and induction of cell stress with anisomycin leads to strong inhibition of both the SMRT-T3R interaction and SMRT-mediated repression, consistent with our proposed role of MEKK-1 as a negative modulator of SMRT function. MEKK-1 appears to be relatively specific in its ability to inhibit SMRT; Raf, a second MAPKKK that also operates downstream of Ras, had no detectable effect on SMRT function in our experiments.
SMRT appears to be a direct substrate for MEKK-1-mediated phosphorylation.
The ability of MEKK-1-mediated signaling to inhibit SMRT function was closely paralleled by an increase in the overall level of phosphorylation of the SMRT protein, as manifested by changes in the mobility of SMRT on Western blots that could be reversed with phosphatase treatment. SMRT could also be phosphorylated in vitro using a variety of preparations of purified or enriched MEKK-1. These results indicate that SMRT is phosphorylated either by MEKK-1 itself or possibly by a tightly associated kinase that copurifies with MEKK-1. We favor the former hypothesis for several reasons. (i) MEKK-1 could phosphorylate SMRT in vitro when both the kinase and the substrate were purified as recombinant proteins from E. coli, eliminating the possibility that these preparations were contaminated with other eukaryotic kinases. (ii) Kinase-defective mutants of MEKK-1 were impaired in the ability to phosphorylate SMRT (unpublished observations). (iii) In common with many previously identified substrates of MEKK-1, SMRT could be isolated in the form of a physical complex with MEKK-1. We have mapped a major site of MEKK-1-mediated phosphorylation of SMRT in vitro to within the SMRT RID; however, given that the substrate sequence specificity of MEKK-1 remains poorly understood, we have not yet defined the location of this phosphorylation to a specific amino acid within this corepressor domain.
It might appear paradoxical that MEKK-1, thought to be largely a cytoplasmic or an inner plasma membrane protein, is able to phosphorylate SMRT, a transcriptional modulator that functions in the nucleus. MEKK-1 may be able to access the nuclear compartment, perhaps transiently, during different stages of the cell cycle (14). Alternatively, MEKK-1 may phosphorylate nascent SMRT after its synthesis on cytoplasmic ribosomes but before translocation of the corepressor into the nucleus. It may be relevant that the actions of MEKK-1 lead to an increased cytoplasmic localization of SMRT, perhaps further increasing its availability for modification by this kinase. It is intriguing in this regard that another target of MEKK-1 regulation is the NF-κB–IκB transcription factor complex, which resides in the cytoplasm in unstimulated cells. MEKK-1 can phosphorylate the IκB kinase, leading to phosphorylation of IκB and release of NF-κB (32). MEKK-1 leads to nuclear translocation and activation of NF-κB, whereas we propose that MEKK-1 phosphorylation of SMRT leads to cytoplasmic retention and inactivation of the corepressor.
SMRT is also a target for phosphorylation by a diverse array of additional protein kinases.
As detailed above, SMRT phosphorylation is increased in cells by the introduction of active MEKK-1, and at least one component of this enhanced phosphorylation appears to be due to direct phosphorylation by MEKK-1. However, SMRT is also phosphorylated at distinct sites by MEK-1 and by MAPKs. Phosphorylation of SMRT by MEK-1 can mimic some of the effects of MEKK-1, although more weakly, whereas phosphorylation of SMRT by MAPKs does not have any observable effect on the ability of SMRT either to associate with nuclear receptors in vivo or in vitro or to mediate transcriptional repression under the conditions tested. In addition to the phosphorylation of SMRT by known components of the MAPKKK cascade, we have also observed that SMRT is phosphorylated in vitro and in vivo by casein kinase II and apparently by as-yet-unidentified kinases found physically associated with SMRT in CV-1 cell lysates (unpublished observations).
It is intriguing that SMRT serves as a substrate for so many kinases operating both within and without the known MAPKKK cascades. Although typically portrayed as acting in a linear and hierarchical fashion, MAPKKK signal transduction cascades often operate at multiple levels. For example, as shown here for SMRT and as noted elsewhere for IκB kinase and Smad2 (9, 32), MEKK-1 can function not only by transducing signals to kinases lower in the hierarchy, such as MEK-1 and MAPKs, but also by itself phosphorylating important substrates directly. In addition, the different MAPKKK cascades in eukaryotic cells can exhibit considerable cross talk between one another, depending on the cell type and experimental conditions. Thus, MEKK-1 is an important transducer of the cell stress signal but also appears to respond to growth factor signals mediated through Ras. Similarly, although SEK-1 is believed to be the principal MAPKK operating downstream of MEKK-1, MEK-1 appears able to serve this role in the CV-1 cell experiments described here.
Mechanism of the inhibition of SMRT function by MEKK-1.
How does MEKK-1 and MEK-1 signaling operate at the molecular level to inhibit the interaction between SMRT and its transcription factor partners? Notably, the ability of SMRT to physically associate with T3R in vitro is inhibited by incubation with either purified MEKK-1 or purified MEK-1, suggesting that the phosphorylation of the corepressor by these kinases can decrease the affinity of the corepressor for transcription factors and may account, at least in part, for the corresponding inhibition of the two-hybrid interaction observed in cells. This hypothesis is consistent with the sites of MEKK-1 and MEK-1 phosphorylation within SMRT, which both map within the RID of the corepressor. In contrast to this inhibition mediated by MEKK-1 and MEK-1, the phosphorylation of SMRT in vitro by MAPKs did not detectably affect the interaction between the corepressor and T3R.
However, the direct inhibitory effects of MEKK-1 and MEK-1 phosphorylation observed in vitro may not represent the only inhibitory effect of the MAPKKK pathway on SMRT function. Significantly, we observed that overexpression of MEKK-1 or, to a lesser extent, of MEK-1 led to relocalization of SMRT from an almost exclusively nuclear compartment to a perinuclear or cytoplasmic compartment in transfected cells. This relocalization of SMRT was observed using a GFP-SMRT immunofluorescence technique and, independently, in biochemical subcellular fractionation experiments. We propose that in addition to the direct inhibitory effect of MEKK-1 phosphorylation on the interaction of SMRT with its transcription factor partners in vitro, MAPKKK signaling also leads to a redistribution of SMRT from the nucleus to the cytoplasm of the cell. This change in the subcellular localization of SMRT may contribute, at least in part, to the loss of interaction of the corepressor and nuclear transcription factors observed in two-hybrid assays and the loss of SMRT-mediated repression seen in transcription assays.
We do not yet understand the precise mechanisms by which MEKK-1 and MEK-1 signaling leads to the alteration in subcellular localization observed for SMRT. This change in SMRT localization may occur directly in response to the phosphorylation of the corepressor itself or may be mediated by a more indirect mechanism, such as an MAPKKK-induced change in the nuclear export or import machinery of the cell. We also do not yet know if the change in the subcellular localization of SMRT represents a direct effect of the actions of the MAPKKK pathway or if the SMRT redistribution is a secondary effect occurring as a consequence of the loss of tethering of the corepressor to its transcription factor partners. We are currently investigating these issues in more detail.
Protein kinases and nuclear hormone receptors—a convergence of cell signaling pathways.
Many tantalizing links have been established between the actions of protein kinases and those of nuclear hormone receptors. For example, many nuclear hormone receptors are themselves targets of phosphorylation by a variety of kinases, such as protein kinase A, the cyclin-dependent kinases, DNA-dependent protein kinases, and casein kinases; phosphorylation by these kinases can either enhance or impair nuclear receptor function (reviewed in references 6 to 8, 26, 56, and 69). Intriguingly, there appears to be a particularly intimate series of interconnections between the functions of the nuclear hormone receptors and MAPKKK regulatory cascades (10, 16, 23, 24, 26, 27, 49, 56). Here we have shown that the corepressor SMRT is also an important target of modification by a diverse array of protein kinases, including components of the MAPKKK cascades, and that at least some of these phosphorylation events can disrupt SMRT function. It appears likely that the actions of coactivators and corepressors are subject to similar forms of posttranslational regulation (1, 42). Taken as a whole, our results suggest that multiple interactions take place between ligand-dependent and ligand-independent signal transduction pathways and that these interactions operate at multiple levels to generate both convergence and integration of different signals and thus to generate the correct combinatorial regulation of target gene expression.
ACKNOWLEDGMENTS
We thank J. M. Bishop, R. M. Evans, L. Freedman, J. Kyriakis, M. Lazar, and T. Maniatis for generously providing molecular clones used in this research.
This work was supported by Public Health Service grants R37 CA-53394 and R01 DK-53528 from NIH.
REFERENCES
- 1.Ait-Si-Ali S, Carlisi D, Ramirez S, Upegui-Gonzalez L C, Duquet A, Robin P, Rudkin B, Harel-Bellan A, Trouche D. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999;262:157–162. doi: 10.1006/bbrc.1999.1132. [DOI] [PubMed] [Google Scholar]
- 2.Apriletti J W, Ribeiro R C, Wagner R L, Feng W, Webb P, Kushner P J, West B L, Nilsson S, Scanlan T S, Fletterick R J, Baxter J D. Molecular and structural biology of thyroid hormone receptors. Clin Exp Pharmacol Physiol Suppl. 1998;25:S2–S11. doi: 10.1111/j.1440-1681.1998.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnold S F, Vorojeikina D P, Notides A C. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. J Biol Chem. 1995;270:30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]
- 4.Avdi N J, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Mapping pathways for mitogen-activated protein kinase activation. J Biol Chem. 1996;271:33598–33606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 5.Ayer D E. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. doi: 10.1016/s0962-8924(99)01536-6. [DOI] [PubMed] [Google Scholar]
- 6.Bai W, Rowan B G, Allgood V E, O'Malley B W, Weigel N L. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J Biol Chem. 1997;272:10457–10463. doi: 10.1074/jbc.272.16.10457. [DOI] [PubMed] [Google Scholar]
- 7.Blok L J, de Ruiter P E, Brinkmann A O. Androgen receptor phosphorylation. Endocr Res. 1996;22:197–219. doi: 10.3109/07435809609030508. [DOI] [PubMed] [Google Scholar]
- 8.Bodwell J E, Webster J C, Jewell C M, Cidlowski J A, Hu J M, Munck A. Glucocorticoid receptor phosphorylation: overview, function and cell cycle-dependence. J Steroid Biochem Mol Biol. 1998;65:91–99. doi: 10.1016/s0960-0760(97)00185-4. [DOI] [PubMed] [Google Scholar]
- 9.Brown J D, DiChiara M R, Anderson K R, Gimbrone M A, Jr, Topper J N. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J Biol Chem. 1999;274:8797–8805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- 10.Bunone G, Briand P A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen J D, Li H. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit Rev Eukaryot Gene Expr. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 13.Denhardt D T. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318:729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanger G R, Johnson N L, Johnson G L. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4672. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 16.Hammer G D, Krylova I, Zhang Y, Darimont B D, Simpson K, Weigel N L, Ingraham H A. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 17.Hassig C A, Schreiber S L. Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol. 1997;1:300–308. doi: 10.1016/s1367-5931(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 18.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARalpha) and PLZF-RARalpha oncoproteins associated with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S H, Privalsky M L. Retinoid isomers differ in the ability to induce release of SMRT corepressor from retinoic acid receptor-alpha. J Biol Chem. 1999;274:2885–2892. doi: 10.1074/jbc.274.5.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S H, Wong C W, Privalsky M L. Signaling by tyrosine kinases negatively regulates the interaction between transcription factors and SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) corepressor. Mol Endocrinol. 1998;12:1161–1171. doi: 10.1210/mend.12.8.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hörlein A J, Näär A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass C K, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 23.Joel P B, Smith J, Sturgill T W, Fisher T L, Blenis J, Lannigan D A. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juge-Aubry C E, Hammar E, Siegrist-Kaiser C, Pernin A, Takeshita A, Chin W W, Burger A G, Meier C A. Regulation of the transcriptional activity of the peroxisome proliferator-activated receptor alpha by phosphorylation of a ligand-independent trans-activating domain. J Biol Chem. 1999;274:10505–10510. doi: 10.1074/jbc.274.15.10505. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 26.Katzenellenbogen B S. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod. 1996;54:287–293. doi: 10.1095/biolreprod54.2.287. [DOI] [PubMed] [Google Scholar]
- 27.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo M H, Allis C D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa R, Söderström M, Hörlein A, Halachmi S, Brown M, Rosenfeld M G, Glass C K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 30.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Del-Rio A L, Ricote M, Ngo S, Gemsch J, Hilsenbeck S G, Osborne C K, Glass C K, Rosenfeld M G, Rose D W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazar M A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 32.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 33.Leitman D C, Costa C H, Graf H, Baxter J D, Ribeiro R C. Thyroid hormone activation of transcription is potentiated by activators of cAMP-dependent protein kinase. J Biol Chem. 1996;271:21950–21955. doi: 10.1074/jbc.271.36.21950. [DOI] [PubMed] [Google Scholar]
- 34.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Leo C, Schroen D J, Chen J D. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]
- 36.Lin R J, Kao H Y, Ordentlich P, Evans R M. The transcriptional basis of steroid physiology. Cold Spring Harbor Symp Quant Biol. 1998;63:577–585. doi: 10.1101/sqb.1998.63.577. [DOI] [PubMed] [Google Scholar]
- 37.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 39.Meier C A. Regulation of gene expression by nuclear hormone receptors. J Recept Signal Transduct Res. 1997;17:319–335. doi: 10.3109/10799899709036612. [DOI] [PubMed] [Google Scholar]
- 40.Meyer S, LaBudda L, McGlade J, Hayman M J. Analysis of the role of the Shc and Grb2 proteins in signal transduction by the v-ErbB protein. Mol Cell Biol. 1994;14:3253–3262. doi: 10.1128/mcb.14.5.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muscat G E, Burke L J, Downes M. The corepressor N-CoR and its variants RIP13a and RIP13Delta1 directly interact with the basal transcription factors TFIIB, TAFII32 and TAFII70. Nucleic Acids Res. 1998;26:2899–2907. doi: 10.1093/nar/26.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M R. The signal-dependent coactivator CBP is a nuclear target for pp90RSK. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 43.Niles R M. Control of retinoid nuclear receptor function and expression. Subcell Biochem. 1998;30:3–28. doi: 10.1007/978-1-4899-1789-8_1. [DOI] [PubMed] [Google Scholar]
- 44.O'Malley B W, Schrader W T, Mani S, Smith C, Weigel N L, Conneely O M, Clark J H. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 45.Ordentlich P, Downes M, Xie W, Genin A, Spinner N B, Evans R M. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc Natl Acad Sci USA. 1999;96:2639–2644. doi: 10.1073/pnas.96.6.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park E J, Schroen D J, Yang M, Li H, Li L, Chen J D. SMRTe, a silencing mediator for retinoid and thyroid hormone receptors—extended isoform that is more related to the nuclear receptor corepressor. Proc Natl Acad Sci USA. 1999;96:3519–3524. doi: 10.1073/pnas.96.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pazin M J, Kadonaga J T. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez M T, Sah V P, Zhao X L, Hunter J J, Chien K R, Brown J H. The MEKK-JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 49.Rogatsky I, Logan S K, Garabedian M J. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci USA. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell M, Lange-Carter C A, Johnson G L. Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1) J Biol Chem. 1995;270:11757–11760. doi: 10.1074/jbc.270.20.11757. [DOI] [PubMed] [Google Scholar]
- 51.Sande S, Privalsky M L. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 52.Sartorius C A, Tung L, Takimoto G S, Horwitz K B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993;268:9262–9266. [PubMed] [Google Scholar]
- 53.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlesinger T K, Fanger G R, Yujiri T, Johnson G L. The TAO of MEKK. Front Biosci. 1998;3:D1181–D1186. doi: 10.2741/a354. [DOI] [PubMed] [Google Scholar]
- 55.Seol W, Mahon M J, Lee Y K, Moore D D. Two receptor interacting domains in the nuclear hormone receptor corepressor RIP13/ N-CoR. Mol Endocrinol. 1996;10:1646–1055. doi: 10.1210/mend.10.12.8961273. [DOI] [PubMed] [Google Scholar]
- 56.Smith C L. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58:627–632. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- 57.Söderström M, Vo A, Heinzel T, Lavinsky R M, Yang W M, Seto E, Peterson D A, Rosenfeld M G, Glass C K. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- 58.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 59.Suka N, Carmen A A, Rundlett S E, Grunstein M. The regulation of gene activity by histones and the histone deacetylase RPD3. Cold Spring Harbor Symp Quant Biol. 1998;63:391–399. doi: 10.1101/sqb.1998.63.391. [DOI] [PubMed] [Google Scholar]
- 60.Tan P B, Kim S K. Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 1999;15:145–149. doi: 10.1016/s0168-9525(99)01694-7. [DOI] [PubMed] [Google Scholar]
- 61.Tonks N K, Neel B G. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 62.Torchia J, Glass C, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay A, Tremblay G B, Labrie F, Giguère V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 64.Tsai M J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 65.Tzagarakis-Foster C, Privalsky M L. Phosphorylation of thyroid hormone receptors by protein kinase A regulates DNA recognition by specific inhibition of receptor monomer binding. J Biol Chem. 1998;273:10926–10932. doi: 10.1074/jbc.273.18.10926. [DOI] [PubMed] [Google Scholar]
- 66.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 67.Wagner B L, Norris J D, Knotts T A, Weigel N L, McDonnell D P. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol. 1998;18:1369–1378. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waskiewicz A J, Cooper J A. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 69.Weigel N L. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitmarsh A J, Davis R J. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 71.Wolffe A P. Transcriptional control. Sinful repression. Nature. 1997;387:16–17. doi: 10.1038/387016a0. [DOI] [PubMed] [Google Scholar]
- 72.Wong C W, Privalsky M L. Transcriptional repression by the SMRT-mSin3 corepressor: multiple interactions, multiple mechanisms, and a potential role for TFIIB. Mol Cell Biol. 1998;18:5500–5510. doi: 10.1128/mcb.18.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong C W, Privalsky M L. Transcriptional silencing is defined by isoform- and heterodimer-specific interactions between nuclear hormone receptors and corepressors. Mol Cell Biol. 1998;18:5724–5733. doi: 10.1128/mcb.18.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Workman J L, Kingston R E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 75.Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 76.Xu L, Glass C K, Rosenfeld M G. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 77.Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb M H. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1995;92:6808–6812. doi: 10.1073/pnas.92.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1913. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 79.Zamir I, Harding H P, Atkins G B, Hörlein A, Glass C K, Rosenfeld M G, Lazar M A. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16:5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zamir I, Zhang J, Lazar M A. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]