Summary
Background
Phase 3 trials found mRNA-1273 was highly effective in preventing COVID-19. We conducted a prospective cohort study at Kaiser Permanente Southern California (KPSC) to determine the real-world vaccine effectiveness (VE) of mRNA-1273 in preventing COVID-19 infection and severe disease.
Methods
For this planned interim analysis, individuals aged ≥18 years receiving 2 doses of mRNA-1273 ≥24 days apart (18/12/2020-31/03/2021) were 1:1 matched to randomly selected unvaccinated individuals by age, sex, and race/ethnicity, with follow-up through 30/06/2021. Outcomes were COVID-19 infection (SARS-CoV-2 positive molecular test or COVID-19 diagnosis code) or severe disease (COVID-19 hospitalization and COVID-19 hospital death). Adjusted hazard ratios (aHR) and confidence intervals (CI) for COVID-19 outcomes comparing vaccinated and unvaccinated individuals were estimated by Cox proportional hazards models accounting for multiple comparisons. Adjusted VE was calculated as (1-aHR)x100. Whole genome sequencing was performed on SARS-CoV-2 positive specimens from the KPSC population.
Findings
This analysis included 352,878 recipients of 2 doses of mRNA-1273 matched to 352,878 unvaccinated individuals. VE (99·3% CI) against COVID-19 infection was 87·4% (84·8–89·6%). VE against COVID-19 hospitalization and hospital death was 95·8% (90·7–98·1%) and 97·9% (66·9-99·9%), respectively. VE was higher against symptomatic (88·3% [98·3% CI: 86·1–90·2%]) than asymptomatic COVID-19 (72·7% [53·4–84·0%]), but was generally similar across age, sex, and racial/ethnic subgroups. VE among individuals with history of COVID-19 ranged from 8·2–33·6%. The most prevalent variants were Alpha (41·6%), Epsilon (17·5%), Delta (11·5%), and Gamma (9·1%), with Delta increasing to 54·0% of variants by June 2021.
Interpretation
These interim results provide reassuring evidence of the VE of 2 doses of mRNA-1273 across age, sex, and racial/ethnic subgroups, and against asymptomatic and symptomatic COVID-19, and severe COVID-19 outcomes. Among individuals with history of COVID-19, mRNA-1273 vaccination may offer added protection beyond immunity acquired from prior infection. Longer follow-up is needed to fully evaluate VE of mRNA-1273 against emerging SARS-CoV-2 variants.
Funding
Moderna Inc.
Keywords: COVID-19, SARS-CoV-2, mRNA-1273, Vaccine effectiveness, Variants, Matched cohort
Research in context.
Evidence before this study
It is crucial to assess the real-world vaccine effectiveness (VE) of available COVID-19 vaccines, including mRNA-1273, to inform ongoing and future vaccination strategies. PubMed was searched for global observational studies published between December 1, 2020, and August 1, 2021, using the terms “COVID-19 AND (Moderna vaccine OR mRNA-1273) AND (effectiveness OR real-world) AND (observational OR cohort OR test-negative)”. Out of 32 articles retrieved, 7 were relevant to this topic. Overall, high VE of mRNA-1273 against SARS-CoV-2 infection (82·0-100%) and severe COVID-19 disease (86·0-95·7%) was reported. Additionally, high VE of mRNA-1273 against variants of concern B.1.1.7 (Alpha; 100%) and B.1.351 (Beta; 96·4%), was reported.
Added value of this study
This study is one of the first prospective cohort studies to assess mRNA-1273 VE among a diverse real-world population. In this planned interim analysis, VE of mRNA-1273 was shown to be >80% against COVID-19 infection, hospitalization, and death. VE was higher against symptomatic than asymptomatic COVID-19, similar across age, sex, and racial/ethnic subgroups, and lower among individuals with history of COVID-19.
Implications of all the available evidence
This study, which fulfills commitments to multiple health authorities globally, provides reassuring evidence of the VE of 2 doses of mRNA-1273 against asymptomatic infection, any COVID-19 infection, and severe outcomes. Among individuals with history of COVID-19, mRNA-1273 vaccination may offer added protection beyond immunity acquired from prior infection. Longer follow-up is needed to fully evaluate VE of mRNA-1273 against emerging SARS-CoV-2 variants.
Alt-text: Unlabelled box
Introduction
Following the sequencing of severe acquired respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccines to prevent coronavirus disease 2019 (COVID-19) were rapidly developed. These included, among others, 2-dose mRNA-based COVID-19 vaccines, mRNA-1273 (Moderna Inc, Cambridge,USA) and BNT162b2 (Pfizer Inc, New York, USA; BioNTech Manufacturing GmbH, Mainz, Germany),1,2 and a 1-dose adenoviral vector COVID-19 vaccine, Ad26.COV2.S (Johnson & Johnson, NJ, USA).3 Subsequently, the United States (US) Food and Drug Administration granted emergency use authorization in December 2020 for mRNA-1273 in individuals aged ≥18 years and BNTb162b2 in individuals aged ≥16 years,4, 5, 6 and in February 2021 for Ad26.COV2.S in individuals aged ≥18 years. 7 As of August 31, 2021, 61·3% of the vaccine-eligible US population was fully vaccinated with a COVID-19 vaccine; among fully vaccinated individuals, the majority received mRNA-based vaccines (39% received mRNA-1273 and 53% received BNTb162b2).8
In phase 3 randomized controlled trials, mRNA-1273 and BNT162b2 were 94·1% and 95·0% efficacious at preventing symptomatic, laboratory-confirmed COVID-19, respectively.1,2 As mass vaccination continues against COVID-19 in the US and globally, and as new SARS-CoV-2 variants of concern continue to emerge, there is an urgent need to continually evaluate the real-world effectiveness of COVID-19 vaccines. Real-world studies are critical as vaccine effectiveness (VE) could differ from vaccine efficacy assessed under trial conditions. Additionally, real-world studies are needed to evaluate VE in diverse populations at different time points and with longer follow-up;9,10 such data could inform vaccination strategies including selection of COVID-19 vaccine products and need for booster doses or different vaccine formulations offering broad protection against SARS-CoV-2 variants.
Several reports on real-world VE of mRNA-based vaccines have been published to date. In Qatar, VE of mRNA-1273 was 95·7% against severe, critical or fatal COVID-19 disease; high VE against infection with B.1.1.7 (Alpha) and B.1.351 (Beta) variants was also observed.11 In Israel, several large studies in early 2021 reported VE of BNTb162 of 92%-95% against COVID-19 infection and 92%-97% against severe disease.12,13 In the US, studies have focused on VE in specific populations such as health care personnel, the elderly, or military veterans, with VE estimates for early 2021 ranging from 82% to >98%.14, 15, 16, 17, 18, 19, 20, 21 With circulation of more transmissible SARS-CoV-2 variants such as Delta, however, breakthrough infections among mRNA-vaccinated individuals may be more common.22, 23, 24
Although several real-world studies have assessed VE for BNTb162b2 or mRNA-based vaccines combined, few studies have assessed VE for mRNA-1273, particularly among the general US population.25, 26 Therefore, we evaluated the VE of mRNA-1273 in a planned interim analysis of a 5-year observational study within the Kaiser Permanente Southern California (KPSC) health care system in the US.
Methods
Study setting
Here, we present the first interim results of an ongoing, matched, prospective cohort study at KPSC to evaluate the VE of mRNA-1273 in preventing COVID-19 infection and severe disease. The study is a regulatory commitment to multiple health authorities globally, to continue evaluating the benefit-risk profile of mRNA-1273 post-authorization/licensure. The study protocol was submitted to regulatory agencies prior to the conduct of the study and is available in the Supplementary Material. The KPSC Institutional Review Board provided ethical approval for the study.
KPSC is an integrated health care system including more than 4.6 million members of diverse sociodemographic, racial, and ethnic backgrounds.27 Comprehensive electronic health records (EHRs) capture details of patient care from inpatient, emergency department (ED), outpatient, and virtual care settings, with care received outside of the KPSC system captured through claims.
KPSC began mRNA-1273 administration to eligible individuals on December 18, 2020. In accordance with state public health guidelines,28 individuals were prioritized for vaccination with mRNA-1273 or other available COVID-19 vaccines as follows: healthcare workers and long-term care residents (started December 2020); individuals aged ≥65 years, and workers in education and childcare, emergency services, and food and agriculture (started January 2021); individuals aged 18–64 years with underlying health conditions (started March 2021); and all individuals aged ≥18 years (started April 2021).
Molecular testing for SARS-CoV-2 at KPSC is widely available for individuals with or without symptoms who seek testing for any reason. KPSC also requires testing prior to procedures or hospital admission. The majority of samples are nasopharyngeal/oropharyngeal swabs or saliva samples, which are primarily tested by RT-PCR using the TaqPath™ COVID-19 High-Throughput Combo Kit (Thermo Fisher Scientific, Pleasanton, USA); a smaller proportion of samples are tested using the Roche cobas® SARS-CoV-2 assay or the Roche cobas® SARS-CoV-2 & Influenza A/B assay (Roche Molecular Systems, Branchburg, USA). In March 2021, KPSC began sending all positive SARS-CoV-2 specimens to a commercial laboratory (Helix, San Diego, USA) for whole genome sequencing (WGS), as detailed in Supplementary Methods.
Study objectives
The primary objectives of this planned interim analysis were to evaluate the VE of 2 doses of mRNA-1273 in preventing COVID-19 infection and severe disease. Secondary objectives evaluated at this time point included the VE of 2 doses of mRNA-1273 in preventing asymptomatic vs. symptomatic COVID-19, and COVID-19 infection stratified by age, sex, race/ethnicity, and history of COVID-19 infection. Subsequent interim and final analyses throughout the 5-year study are planned to evaluate additional secondary objectives.
Study population
Individuals aged ≥18 years who were members of KPSC for ≥12 months prior to index date (allowing a 31-day membership gap) through 14 days after the index date were eligible for inclusion in the study. The index date was defined as the date of receipt of the second dose of mRNA-1273 for vaccinated individuals and their matched unvaccinated counterparts. Individuals who received a COVID-19 vaccine other than mRNA-1273 prior to the index date, received 2 doses of mRNA-1273 <24 days apart, received any COVID-19 vaccine <14 days after the index date, had no health care utilization and no vaccination from the 2 years prior to the index date through the index date, or had a COVID-19 outcome <14 days after the index date were excluded.
For this interim analysis, eligible individuals who received 2 doses of mRNA-1273 at least 24 days apart (4-day grace period allowed prior to the recommended 28-day interval) from December 18, 2020 to March 31, 2021 were included in the vaccinated group. The unvaccinated comparison group comprised eligible individuals who had not received mRNA-1273 or any other COVID-19 vaccine as of the index date. Unvaccinated individuals were randomly selected and 1:1 matched to vaccinated individuals by age (18–44 years, 45–64 years, 65–74 years, and ≥75 years), sex, and race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, Non-Hispanic Asian, and Other/Unknown). Additional details of the study design, matching process, and analytic methods are presented in Supplementary Methods.
Exposure and outcomes
The mRNA-1273 vaccine exposure was captured from KPSC EHR vaccination records. COVID-19 vaccine providers must document COVID-19 vaccine administration data daily to the California Immunization Registry (CAIR).29 COVID-19 vaccinations received outside KPSC were regularly imported into the EHR from external sources, including CAIR, CalVax (Cal Poly Pomona mass vaccination site), Care Everywhere (system on the Epic EHR platform that allows different health care systems to exchange patient medical information), claims (e.g., retail pharmacies), and member self-report (with valid documentation).
The primary outcomes of the study were COVID-19 infection, defined as a SARS-CoV-2 positive test or a COVID-19 diagnosis code for both symptomatic and asymptomatic infections (Supplementary Table 1 and 2); and severe COVID-19 disease, including COVID-19 hospitalization (hospitalization with a SARS-CoV-2 positive test or a COVID-19 diagnosis code, or a hospitalization ≤7 days after a SARS-CoV-2 positive test, with chart review by a physician investigator [BKA] to confirm severe COVID-19 symptoms) and COVID-19 hospital death. A COVID-19 infection was considered an incident infection if there was no history of a COVID-19 diagnosis code or SARS-CoV-2 positive molecular test in the 90 days prior.30 We ascertained the first occurrence of incident COVID-19 infection or severe COVID-19 disease ≥14 days after the index date.1,31
For this interim analysis, asymptomatic COVID-19 cases were identified through positive SARS-CoV-2 tests ordered for individuals without COVID-19 symptoms (e.g., routine screening prior to procedures or hospital admission at KPSC, elective screening of KPSC employees, or testing requested for any other reason); these test orders were not used for individuals with symptoms. The remainder of the COVID-19 cases were considered symptomatic COVID-19 cases.
For individuals with history of a COVID-19 from March 1, 2020 to index date, a COVID-19 reinfection (>90 days after the most recent prior COVID-19 diagnosis code or SARS-CoV-2 positive test) during follow-up was assessed using two definitions. The first definition required a COVID-19 diagnosis code with chart-confirmed symptoms or a SARS-CoV-2 positive molecular test. The second, more specific definition required a COVID-19 diagnosis code with chart-confirmed symptoms, a SARS-CoV-2 positive molecular test with chart-confirmed symptoms, or a SARS-CoV-2 positive molecular test with an intervening SARS-CoV-2 negative molecular test.
We followed-up individuals in the EHR for occurrence of COVID-19 outcomes until the end of the interim analysis period (June 30, 2021) or censoring events (termination of KPSC membership allowing for a 31-day gap, death, or receipt of a COVID-19 vaccine). Unvaccinated individuals no longer contributed unvaccinated person-time upon receipt of a first dose of mRNA-1273 during follow-up, and contributed vaccinated person-time upon receipt of an eligible second dose of mRNA-1273.
Other variables
Baseline characteristics and potential confounders were extracted from the EHR (Supplementary Table 3). Variables assessed at index date included age, sex, race/ethnicity, socioeconomic status (Medicaid, neighborhood median household income), medical center area, pregnancy status (by trimester), and KPSC physician/employee status. Variables assessed in the two years prior to index date included smoking and body mass index (BMI). Variables assessed in the year prior to index date included Charlson comorbidity score, autoimmune conditions, health care utilization (virtual, outpatient, ED, and inpatient encounters), preventive care (other vaccinations, screenings, and well-visits), chronic diseases (kidney disease, heart disease, lung disease, liver disease, diabetes), and frailty index.32 Other variables included history of SARS-CoV-2 molecular test performed from March 1, 2020 to index date, irrespective of result, and immunocompromised status.
Statistical analyses
We described attributes of vaccinated and unvaccinated cohorts. Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate, and continuous variables were compared using the two-sample t-test or Wilcoxon rank-sum test, as appropriate. Absolute standardized differences (ASD) were calculated to assess the balance of covariates; ASD ≤0·1 was considered a negligible difference,33 and potential confounders were determined by ASD >0·1. Matching variables (age, sex, and race/ethnicity) were considered important risk factors and kept in adjusted models. The missing indicator method was used for covariates with missing data.34
We calculated overall incidence rates of COVID-19 infection and of severe COVID-19 for vaccinated and unvaccinated cohorts (number of incident events divided by person-years). The cumulative incidences of COVID-19 infection, hospitalization, and hospital death for the 2-dose vaccinated and unvaccinated individuals were estimated by the Kaplan-Meier method and compared by the log-rank test.
Unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CIs) comparing COVID-19 infection or severe COVID-19 disease in vaccinated and unvaccinated individuals were estimated by Cox proportional hazards regression models without and with confounder adjustment. We used study time (starting from zero) in the Cox regression. We calculated confidence intervals by HR ± Z1-α/2SE, where α was significance level with or without adjustment for multiple comparisons. We used the default method (Breslow method) for continuous time in SAS Proc PHREG to deal with ties. Since multiple interim analyses are planned for this longitudinal study, the test significance level was adjusted using the Bonferroni correction to keep the overall Type I error below 0·05. As such, either 99·3% CIs (α = 0.007 = 0.05/7 tests, for VE against COVID-19 infection, hospitalization, or hospital death) or 98·3% CIs (α = 0.017 = 0.05/3 tests, for VE against COVID-19 infection by age, sex, race/ethnicity, history of COVID-19, and asymptomatic/symptomatic COVID-19 subgroups) were calculated based on the planned number of interim analyses over the course of the full study. Unadjusted VE (%) was calculated as (1-unadjusted HR) x 100. Adjusted VE (%) was calculated as (1–adjusted HR) × 100.
We conducted two post hoc sensitivity analyses. First, we repeated the main analyses using calendar time instead of study time in the Cox regression models. Second, we repeated the main analyses using the same length of follow up for matched pairs, i.e., we censored both vaccinated and unvaccinated individuals in a matched pair at same time if either individual experienced a censoring event.
Statistical power calculations are presented in Supplementary Methods. All analyses were conducted using SAS software version 9.4, Cary, USA.
Distribution of SARS-CoV-2 variants over time
To better characterize variants circulating locally during the study period, we also described the distribution of variants among successfully sequenced specimens collected from March to June 2021. These data were derived from the broader KPSC population and were not directly linked to the eligible cohort for this interim analysis.
Role of the funding source
Authors employed by Moderna, Inc. contributed to study design, interpretation of the data, the writing of the manuscript, and the decision to submit the article for publication.
Results
The study included 352,878 recipients of 2-doses of mRNA-1273 (‘vaccinated’, hereafter) and 352,878 unvaccinated individuals matched on age, sex, and race/ethnicity (Supplementary Figure 1 and Table 1). Median age was 65 years (IQR 45–73 years), thus approximately half of individuals were aged <65 years. Overall, there were more females (59·4%) than males and more non-Hispanic White (38·7%) or Hispanic (32·4%) individuals than other racial/ethnic groups. Vaccinated and unvaccinated individuals had similar baseline distributions (ASD <0·1) of BMI, smoking, Charlson comorbidity scores, frailty, chronic diseases, immunocompromised status, autoimmune conditions, pregnancy, and ED visits and hospitalizations in the year prior to index date. Compared to unvaccinated individuals, fewer vaccinated individuals had a history of COVID-19 infection, but more vaccinated individuals had received a molecular SARS-CoV-2 test. Vaccinated individuals also had more outpatient/virtual visits and preventive care visits in the year prior to index date, had a lower proportion with Medicaid, had higher median neighborhood income, and were more often KPSC physicians/employees. There were some differences in distributions of vaccinated and unvaccinated individuals across medical center areas. Approximately half of the index dates occurred in March 2021, with 44% occurring in February 2021 and 6% occurring in January 2021. Among the vaccinated group, only 88 (0·0%) individuals received mRNA-1273 concomitantly with another vaccine, as concomitant administration was not recommended during the study vaccination period. The median number of days between the first and second mRNA-1273 doses was 28 days (IQR 28–29).
Table 1.
Baseline characteristics of 2-dose mRNA-1273 vaccinated and unvaccinated cohort.
| Vaccinated | Unvaccinated | Absolute | |
|---|---|---|---|
| N=352878 | N=352878 | Standardized Difference | |
| n (%) | n (%) | ||
| Age at index date, years | N/A | ||
| 18-44 | 84052 (23.8) | 84052 (23.8) | |
| 45-64 | 85685 (24.3) | 85685 (24.3) | |
| 65-74 | 109268 (31.0) | 109268 (31.0) | |
| ≥75 | 73873 (20.9) | 73873 (20.9) | |
| Sex | N/A | ||
| Female | 209707 (59.4) | 209707 (59.4) | |
| Male | 143171 (40.6) | 143171 (40.6) | |
| Race/Ethnicity | N/A | ||
| Non-Hispanic White | 136479 (38.7) | 136479 (38.7) | |
| Non-Hispanic Black | 26235 (7.4) | 26235 (7.4) | |
| Hispanic | 114157 (32.4) | 114157 (32.4) | |
| Non-Hispanic Asian | 53843 (15.3) | 53843 (15.3) | |
| Other/Unknown | 22164 (6.3) | 22164 (6.3) | |
| Body mass indexa | 0.0750 | ||
| <18.5 | 3962 (1.1) | 6361 (1.8) | |
| 18.5 - <25 | 91604 (26.0) | 92737 (26.3) | |
| 25 - <30 | 115276 (32.7) | 109259 (31.0) | |
| 30 - <35 | 67435 (19.1) | 65857 (18.7) | |
| 35 - <40 | 29021 (8.2) | 28700 (8.1) | |
| 40 - <45 | 11403 (3.2) | 11582 (3.3) | |
| ≥45 | 6094 (1.7) | 6769 (1.9) | |
| Unknown | 28083 (8.0) | 31613 (9.0) | |
| Smokinga | 0.0470 | ||
| No | 261648 (74.1) | 254378 (72.1) | |
| Yes | 68339 (19.4) | 73230 (20.8) | |
| Unknown | 22891 (6.5) | 25270 (7.2) | |
| Charlson comorbidity scoreb | 0.0663 | ||
| 0 | 206661 (58.6) | 217936 (61.8) | |
| 1 | 57928 (16.4) | 52271 (14.8) | |
| ≥2 | 88289 (25.0) | 82671 (23.4) | |
| Frailty indexb | 0.1472 | ||
| Quartile 1 | 91442 (25.9) | 78980 (22.4) | |
| Quartile 2 | 80354 (22.8) | 102239 (29.0) | |
| Quartile 3 | 91145 (25.8) | 85158 (24.1) | |
| Quartile 4 | 89937 (25.5) | 86501 (24.5) | |
| Chronic diseasesb | |||
| Kidney disease | 35044 (9.9) | 32114 (9.1) | 0.0283 |
| Heart disease | 17187 (4.9) | 18642 (5.3) | 0.0188 |
| Lung disease | 36690 (10.4) | 33049 (9.4) | 0.0346 |
| Liver disease | 11119 (3.2) | 10757 (3.0) | 0.0059 |
| Diabetes | 68075 (19.3) | 63930 (18.1) | 0.0301 |
| Immunocompromised | 12306 (3.5) | 10070 (2.9) | 0.0362 |
| HIV/AIDS | 1428 (0.4) | 749 (0.2) | |
| Leukemia, lymphoma, congenital and other immunodeficiencies, asplenia/hyposplenia | 4794 (1.4) | 4226 (1.2) | |
| Organ transplant | 1209 (0.3) | 861 (0.2) | |
| Immunosuppressant medications | 6923 (2.0) | 5808 (1.6) | |
| Autoimmune conditionsb | 12562 (3.6) | 11277 (3.2) | 0.0202 |
| Rheumatoid arthritis | 5992 (1.7) | 5476 (1.6) | |
| Inflammatory bowel disease | 1981 (0.6) | 1692 (0.5) | |
| Psoriasis and psoriatic arthritis | 4372 (1.2) | 3775 (1.1) | |
| Multiple sclerosis | 553 (0.2) | 584 (0.2) | |
| Systemic lupus erythematosus | 846 (0.2) | 746 (0.2) | |
| Pregnant at index date | 1220 (0.3) | 2820 (0.8) | 0.0601 |
| 1st trimester | 502 (0.1) | 798 (0.2) | |
| 2nd trimester | 407 (0.1) | 984 (0.3) | |
| 3rd trimester | 311 (0.1) | 1038 (0.3) | |
| History of COVID-19 infectionc | 23152 (6.6) | 35876 (10.2) | 0.1305 |
| History of SARS-CoV-2 molecular testc | 133865 (37.9) | 116859 (33.1) | 0.1008 |
| Number of outpatient and virtual visitsa,d | 0.2560 | ||
| 0 | 24225 (6.9) | 41711 (11.8) | |
| 1-4 | 101161 (28.7) | 123530 (35.0) | |
| 5-10 | 105273 (29.8) | 94542 (26.8) | |
| ≥11 | 122219 (34.6) | 93095 (26.4) | |
| Number of emergency department visitsa,d | 0.0633 | ||
| 0 | 302454 (85.7) | 295516 (83.7) | |
| 1 | 36349 (10.3) | 39195 (11.1) | |
| ≥2 | 14075 (4.0) | 18167 (5.1) | |
| Number of hospitalizationsa,d | 0.0802 | ||
| 0 | 336442 (95.3) | 330181 (93.6) | |
| 1 | 13029 (3.7) | 17142 (4.9) | |
| ≥2 | 3407 (1.0) | 5555 (1.6) | |
| Preventive carea | 294962 (83.6) | 234773 (66.5) | 0.4021 |
| Medicaid | 16741 (4.7) | 27283 (7.7) | 0.1238 |
| Neighborhood median household income | 0.1224 | ||
| < $40,000 | 14128 (4.0) | 17262 (4.9) | |
| $40,000-$59,999 | 62153 (17.6) | 70737 (20.0) | |
| $60,000-$79,999 | 79584 (22.6) | 87153 (24.7) | |
| $80,000+ | 196775 (55.8) | 176858 (50.1) | |
| Unknown | 238 (0.1) | 868 (0.2) | |
| KPSC physician/employee | 26234 (7.4) | 6356 (1.8) | 0.2709 |
| Concomitant vaccinatione | 88 (0.0) | N/A | |
| Time between first and second doses, days | N/A | ||
| mean (sd) | 29.20 (3.25) | N/A | |
| Index date | N/A | ||
| January 2021 | 19141 (5.4) | 19141 (5.4) | |
| February 2021 | 152986 (43.4) | 152986 (43.4) | |
| March 2021 | 180751 (51.2) | 180751 (51.2) |
Medical center area not shown. There were differences in the distribution of the vaccinated and unvaccinated individuals across the 19 medical center areas.
N/A = not applicable (matching variable or pertains only to vaccinated individuals)aDefined in the two years prior to index date
Defined in the one year prior to index date
Defined based on all available medical records from March 1, 2020 to index date
Indicator of overall health care utilization / care-seeking
Among subjects with concomitant vaccines: influenza vaccine (37.5%), Tdap (19.3%), shingles vaccine (14.8%), PCV13/PPSV23 (12.5%), and other vaccine (17.0%); 68.2% concomitant with 1st dose and 33.0% concomitant with 2nd dose.
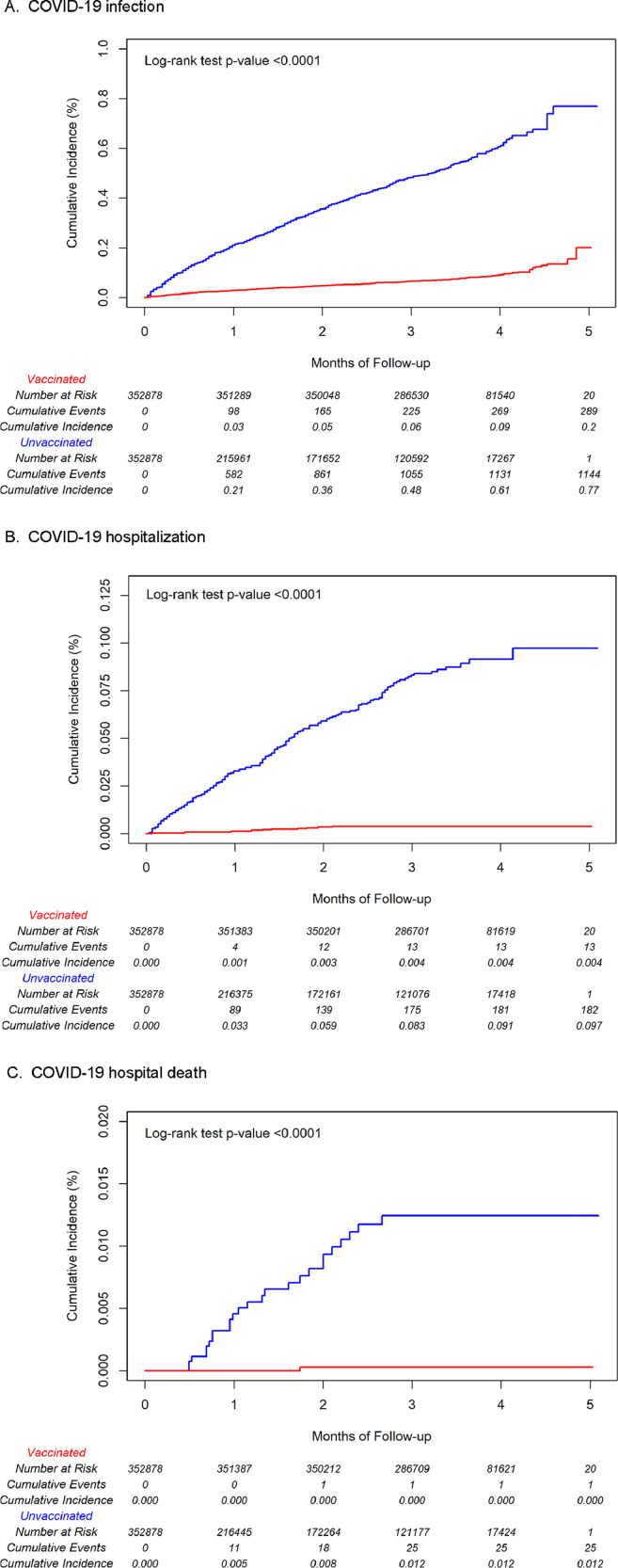
During follow-up, COVID-19 infections occurred among 289 vaccinated individuals and 1,144 unvaccinated individuals, with incidence rates per 1,000 person-years of 2.77 (95% CI: 2·47–3·11) and 20·20 (19·06–21·41), respectively (Table 2). Lower incidence rates per 1,000 person-years were observed for COVID-19 hospitalization (0·12 [0·07–0·21] for vaccinated and 3·21 [2·77–3·71] for unvaccinated individuals) and COVID-19 hospital death (0·01 [0·00–0·07] for vaccinated and 0·44 [0·30–0·65] for unvaccinated individuals). In Kaplan-Meier plots, cumulative incidence estimates of COVID-19 infection, hospitalization, and hospital death were significantly higher for unvaccinated individuals compared to vaccinated individuals (log-rank test p-values <0·0001) (Fig. 1a-c). Mean (standard deviation) follow-up time was 3·55 (0·61) months for vaccinated individuals and 1·93 (1·43) months for unvaccinated individuals.
Table 2.
Incidence rates, hazard ratios, and VE of 2 doses of mRNA-1273 vaccine in preventing COVID-19 infection, hospitalization, and hospital death
| Vaccinated (N=352878) |
Unvaccinated (N=352878) |
Hazard Ratio (95% CI) |
VE (95% CI) |
VE (99.3% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Number of cases | Incidence per 1000 person-years (95% CI) |
Number of cases | Incidence per 1000 person-years (95% CI) |
Unadjusted | Adjusteda | Unadjusted | Adjusteda | Adjusteda |
| COVID-19 infection | 289 | 2.77 (2.47–3.11) | 1144 | 20.20 (19.06–21.41) | 0.14 (0.13–0.16) | 0.13 (0.11–0.14) | 85.5% (83.5–87.3%) | 87.4% (85.6–89.1%) | 87.4% (84.8–89.6%) |
| COVID–19 hospitalization | 13 | 0.12 (0.07–0.21) | 182 | 3.21 (2.77–3.71) | 0.04 (0.02–0.07) | 0.04 (0.02–0.08) | 95.8% (92.6–97.6%) | 95.8% (92.5–97.6%) | 95.8% (90.7–98.1%) |
| COVID–19 hospital death | 1 | 0.01 (0.00–0.07) | 25 | 0.44 (0.30–0.65) | 0.02 (0.00–0.17) | 0.02 (0.00–0.16) | 97.7% (83.1–99.7%) | 97.9% (84.5–99.7%) | 97.9% (66.9–99.9%) |
Adjusted for covariates age, sex, race/ethnicity, frailty index (in quartiles), history of COVID-19 infection, history of SARS-CoV-2 molecular test, number of outpatient and virtual visits, preventive care, Medicaid, neighborhood median household income, KPSC physician/employee status, medical center area.
Fig. 1.
Cumulative incidence estimates of COVID-19 infection, COVID-19 hospitalization, and COVID-19 death by vaccination status in 2-dose mRNA-1273 vaccine cohort.
For both vaccinated and unvaccinated individuals, incidence rates varied for asymptomatic vs. symptomatic COVID-19 infection, by demographic variables, and by having a history of COVID-19 infection (Table 3). Incidence rates were lower for asymptomatic vs. symptomatic COVID-19 infection, higher among adults ages <65 years compared to those ages ≥65 years, and higher among non-Hispanic Black and Hispanic individuals compared to Non-Hispanic White or Non-Hispanic Asian individuals. When considering history of COVID-19, incidence rates were highest for unvaccinated individuals without a history of COVID-19.
Table 3.
Incidence rate, hazard ratio, and VE of 2 doses of mRNA-1273 vaccine in preventing COVID-19 infection by age, sex, race/ethnicity, history of COVID-19, and asymptomatic/symptomatic COVID-19 subgroups.
| Vaccinated (N=352878) |
Unvaccinated (N=352878) |
Hazard Ratio (95% CI) |
VE (95% CI) |
VE (98.3% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Incidence per 1000 person-years (95% CI) |
Number of cases | Incidence per 1000 person-years (95% CI) |
Unadjusted | Adjusteda | Unadjusted | Adjusteda | Adjusteda | |
| Overall | |||||||||
| Asymptomatic | 35 | 0.34 (0.24-0.47) | 66 | 1.17 (0.92-1.48) | 0.33 (0.22-0.50) | 0.27 (0.18-0.42) | 67.2% (50.3-78.3%) | 72.7% (57.6-82.4%) | 72.7% (53.4-84.0%) |
| Symptomatic | 254 | 2.44 (2.15–2.76) | 1078 | 19.04 (17.93-20.21) | 0.13 (0.12-0.15) | 0.12 (0.10-0.13) | 86.6% (84.6-88.3%) | 88.3% (86.5-89.9%) | 88.3% (86.1-90.2%) |
| Age at index date, years | |||||||||
| 18-44 | 93 | 3.58 (2.92–4.39) | 350 | 22.71 (20.45-25.22) | 0.17 (0.13-0.21) | 0.13 (0.10-0.16) | 83.3% (78.9-86.7%) | 87.2% (83.6-90.1%) | 87.2% (82.7-90.6%) |
| 45-64 | 83 | 3.18 (2.56-3.94) | 316 | 26.43 (23.67-29.51) | 0.13 (0.10-0.16) | 0.11 (0.09-0.15) | 87.3% (83.7-90.1%) | 88.7% (85.3-91.4%) | 88.7% (84.5-91.8%) |
| 65-74 | 54 | 1.77 (1.36–2.31) | 272 | 16.59 (14.73-18.68) | 0.11 (0.08-0.15) | 0.11 (0.08-0.14) | 88.6% (84.7-91.5%) | 89.4% (85.6-92.1%) | 89.4% (84.6-92.7%) |
| ≥75 | 59 | 2.72 (2.11–3.51) | 206 | 16.02 (13.97-18.36) | 0.17 (0.13-0.23) | 0.17 (0.12-0.23) | 82.5% (76.6-86.9%) | 83.0% (76.8-87.6%) | 83.0% (75.2-88.4%) |
| Sex | |||||||||
| Female | 175 | 2.81 (2.42-3.26) | 702 | 20.84 (19.35-22.44) | 0.14 (0.12-0.17) | 0.12 (0.10-0.14) | 85.9% (83.3-88.1%) | 87.9% (85.6-89.9%) | 87.9% (85.0-90.3%) |
| Male | 114 | 2.71 (2.26–3.26) | 442 | 19.27 (17.55–21.15) | 0.15 (0.12–0.18) | 0.13 (0.11–0.17) | 85.0% (81.6–87.9%) | 86.6% (83.3–89.2%) | 86.6% (82.5–89.7%) |
| Race/Ethnicity | |||||||||
| Non–Hispanic White | 86 | 2.15 (1.74–2.66) | 399 | 17.46 (15.83–19.26) | 0.13 (0.10–0.16) | 0.11 (0.09–0.14) | 87.1% (83.7–89.8%) | 88.7% (85.5–91.1%) | 88.7% (84.7–91.6%) |
| Non–Hispanic Black | 23 | 3.04 (2.02–4.58) | 107 | 25.60 (21.19–30.94) | 0.11 (0.07–0.18) | 0.11 (0.07–0.17) | 88.6% (82.1–92.8%) | 89.2% (82.6–93.4%) | 89.2% (80.6–94.0%) |
| Hispanic | 142 | 4.24 (3.60–5.00) | 462 | 25.11 (22.92–27.51) | 0.18 (0.15–0.22) | 0.16 (0.13–0.19) | 81.7% (77.8–84.9%) | 84.4% (80.9–87.2%) | 84.4% (80.0–87.8%) |
| Non–Hispanic Asian | 22 | 1.32 (0.87–2.01) | 116 | 15.83 (13.20–18.99) | 0.10 (0.06–0.15) | 0.08 (0.05–0.13) | 90.5% (84.8–94.0%) | 91.8% (86.6–94.9%) | 91.8% (85.1–95.5%) |
| History of COVID–19 infection | |||||||||
| No | 245 | 2.51 (2.22–2.85) | 1104 | 21.66 (20.42–22.98) | 0.12 (0.11–0.14) | 0.11 (0.09–0.12) | 87.8% (85.9–89.4%) | 89.3% (87.6–90.8%) | 89.3% (87.2–91.1%) |
| Yesb | 44 | 6.50 (4.84–8.73) | 40 | 7.07 (5.19–9.64) | 0.91 (0.59–1.40) | 0.92 (0.58–1.45) | 9.1% (0.0–41.0%) | 8.2% (0.0–41.8%) | 8.2% (0.0–47.3%) |
| Yesc | 27 | 3.99 (2.73–5.81) | 31 | 5.48 (3.85–7.79) | 0.69 (0.41–1.16) | 0.66 (0.38–1.15) | 31.1% (0.0–59.0%) | 33.6% (0.0–61.5%) | 33.6% (0.0–65.8%) |
Adjusted for covariates: age (not adjusted in the age subcohort analysis), sex (not adjusted in the sex subcohort analysis), race/ethnicity (not adjusted in the race/ethnicity subcohort analysis), frailty index (in quartiles), history of COVID-19 infection (not adjusted in the history of COVID-19 infection subcohort analysis), history of SARS-CoV-2 molecular test, number of outpatient and virtual visits, preventive care, Medicaid, neighborhood median household income, KPSC physician/employee status, medical center area.
Cases in this category were defined as having a COVID-19 diagnosis code with chart-confirmed symptoms or a SARS-CoV-2 positive molecular test, with no history of a COVID-19 diagnosis code or SARS-CoV-2 positive molecular test in the 90 days prior.
Cases in this category were defined as having a COVID-19 diagnosis code with chart-confirmed symptoms, a SARS-CoV-2 positive molecular test with chart-confirmed symptoms, or a SARS-CoV-2 positive molecular test with an intervening SARS-CoV-2 negative molecular test; cases also had no history of a COVID-19 diagnosis code or SARS-CoV-2 positive molecular test in the 90 days prior.
The adjusted HR (95% CI) of COVID-19 infection comparing vaccinated and unvaccinated individuals was 0·13 (0·11–0·14), yielding an adjusted VE of 87·4% (99·3% CI: 84·8–89·6%) (Table 2). VE against COVID-19 hospitalization was 95·8% (90·7–98·1%) and VE against COVID-19 hospital death was 97·9% (66·9–99·9%). VE was higher against symptomatic COVID-19 (88·3% [98·3% CI: 86·1–90·2%]) than asymptomatic COVID-19 (72·7% [53·4–84·0%]), but was generally similar in subgroups by age, sex, and race/ethnicity, with point estimates ranging from 83·0% to 91·8%. VE among individuals with a history of COVID-19 ranged from 8·2% (0·0–47·3%) to 33·6% (0·0–65·8%), depending on reinfection definition (Table 3).
VE estimates were robust using different analytic approaches. In the sensitivity analyses using calendar time instead of study time in the Cox regression models, VE estimates were nearly the same (Supplementary Table 4). In the sensitivity analyses censoring both vaccinated and unvaccinated individuals within a pair if either individual experienced a censoring event, VE estimates were also nearly the same (Supplementary Table 5).
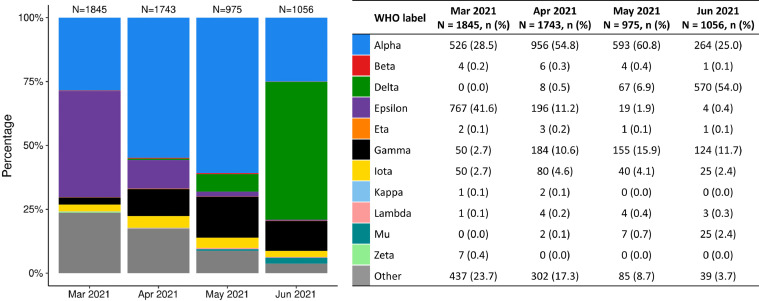
From March–June 2021, 5,619 SARS-CoV-2 positive specimens were successfully sequenced (Fig. 2). Over the study period, the most prevalent variants in the broader KPSC population were 41·6% Alpha (B.1.1.7, Q.3), 17·5% Epsilon (B.1.427, B.1.429), 11·5% Delta (B.1.617.2, AY.*), and 9·1% Gamma (P.1, P.1.1, P.1.10). While Epsilon was the most prevalent variant in March 2021 (41·6% of variants), the proportion of specimens attributed to Alpha increased from 28·5% in March 2021 to 60·8% in May 2021, while the proportion attributed to Delta increased from 0% in March 2021 to 54·0% in June 2021.
Fig. 2.
Distribution of SARS-CoV-2 variants from March to June 2021 at KPSC. Alpha: B.1.1.7, Q.3 Beta: B.1.351, B.1.351.3 Delta: AY.2, AY.3, AY.4, AY.10, AY.12, AY.13, AY.15, AY.24, AY.25, B.1.617.2 Epsilon: B.1.427, B.1.429 Eta: B.1.525 Gamma: P.1, P.1.1, P.1.10 Iota: B.1.526, B.1.526.1, B.1.526.2 Kappa: B.1.617.1 Lambda: C.37 Mu: B.1.621 Zeta: P.2 Other variants: A, A.2.5, A.2.5.1, A.5, AZ.3, B, B.1, B.1.1, B.1.1.28, B.1.1.174, B.1.1.222, B.1.1.304, B.1.1.316, B.1.1.318, B.1.1.328, B.1.1.335, B.1.1.368, B.1.1.413, B.1.1.416, B.1.1.432, B.1.1.519, B.1.126, B.1.153, B.1.2, B.1.232, B.1.234, B.1.241, B.1.243, B.1.280, B.1.289, B.1.311, B.1.324, B.1.349, B.1.377, B.1.384, B.1.393, B.1.396, B.1.36.21, B.1.400, B.1.404, B.1.438.1, B.1.523, B.1.530, B.1.550, B.1.551, B.1.555, B.1.561, B.1.575, B.1.577, B.1.595, B.1.596, B.1.599, B.1.609, B.1.612, B.1.619, B.1.623, B.1.627, B.1.628, B.1.637, C.36, D.2, R.1
Discussion
These interim results from a prospective cohort study conducted confirm high effectiveness of 2 doses of mRNA-1273, with VE of 87·4% against COVID-19 infection, 95·8% against COVID-19 hospitalization, and 97·9% against COVID-19 hospital death. The study included a large cohort of individuals eligible to receive mRNA-1273 for diverse reasons (health care workers, long term care residents, individuals aged ≥65 years, workers in education, childcare, emergency services, food and agriculture, and individuals aged 18-64 with underlying conditions) who were followed until June 2021, a period that overlapped with the emergence of Delta in the US.
Our results add to limited other reports of real-world VE estimates specific to mRNA-1273 among the general population, although several studies were primarily conducted before Delta was predominant. A test-negative study conducted in California found 2-dose VE of mRNA-1273 of 86·2% against SARS-CoV-2 infection (87·0% for BNT162b2), consistent with our results.25 In addition, a test-negative study in Ontario, Canada found 2-dose mRNA-1273 VE of 94% against a positive SARS-CoV-2 test (91% for BNT162b2) and 96% against severe COVID-19 outcomes (96% for BNT162b2).35
We found higher VE for mRNA-1273 against COVID-19 hospitalization and COVID-19 hospital death than against COVID-19 infection. This is aligned with other studies suggesting that protection of mRNA-based vaccines is higher against more severe disease.1,15 Few previous studies have reported VE of 2 doses of mRNA-1273 against asymptomatic infections. One study found mRNA-1273 VE against asymptomatic infection of 92·5% and against symptomatic infection of 98·6%,11 while another study reported mRNA-based VE against asymptomatic infection of 68·3%, 25 and against symptomatic infection of 91·3%, similar to the results of our study.
The US Centers for Disease Control and Prevention (CDC) recommends COVID-19 vaccine for individuals with history of COVID-19.36 Vaccination can further boost antibody levels in those with past infection and has the potential to improve durability and breadth of protection.37 Several small studies showed that a single mRNA vaccine (BNT162b2) dose among individuals with evidence of prior SARS-CoV-2 infection boosted binding and neutralizing antibody and cell-mediated immune responses compared with individuals without a history of infection.38, 39, 40 Our study suggests that among individuals with history of COVID-19, mRNA-1273 vaccination may provide additional benefit (8·2-33·6%) beyond protection from natural infection, supporting current guidance that individuals with history of COVID-19 should be vaccinated. As immunologic data suggest that vaccination after COVID-19 infection produces more durable and broader protection compared to COVID-19 infection without vaccination, 35 further study is needed to evaluate long-term protection of mRNA-1273 and protection against variants in those with history of COVID-19.
Results of WGS conducted for SARS-CoV-2 specimens positive by RT-PCR from the broader KPSC population from March to June 2021 demonstrate an increase in the prevalence of Delta during the study period. In our cohort, the emergence of Delta may correspond to an observed spike in cumulative incidence of COVID-19 infection between 4 to 5 months of follow-up. Further studies of mRNA-1273 are needed to assess variant-specific VE and waning protection over time, in order to inform strategies for booster doses in different populations.
Our study has several other strengths and limitations. As one of the first population-based real-world studies reporting VE estimates specific to mRNA-1273, our study was conducted in a large integrated health care system with a diverse and stable population. EHRs enabled comprehensive capture of COVID-19 vaccine exposures, COVID-19 outcomes, and extensive demographic, care-seeking, and clinical covariates. The matched cohort design allows generalizability to the general population eligible for mRNA-1273, in contrast to test-negative designs in which generalizability is limited to those who are tested. Nevertheless, observational studies may be susceptible to residual confounding from factors that affect risk of COVID-19 exposure and are also associated with vaccination. Individual risk avoidance behaviors vary and are difficult to predict and measure between the vaccinated and unvaccinated populations. Individuals prioritized for vaccination during the study period may have been at higher risk of COVID-19 than their unvaccinated matches in ways we could not account for through matching and adjustment; this could have led to underestimation of VE. Alternatively, individuals who remained unvaccinated during the study period could have taken fewer COVID-19 precautions, thereby increasing their risk; this could potentially overestimate VE.
Although misclassification of vaccine exposure is unlikely due to comprehensive capture of vaccinations within and outside of KSPC, misclassification of COVID-19 infection may have occurred. This may have been due to false positive test results or erroneous entry of diagnosis codes from claims, or for those with history of COVID-19, if viral shedding persisted >90 days; such misclassification is likely to be non-differential, underestimating VE. We observed a lower incidence of asymptomatic as compared to symptomatic infections, which may reflect incomplete capture of asymptomatic infections, as most individuals are not regularly tested. Vaccinated individuals may be less likely to present for SARS-CoV-2 testing for mild or no symptoms if they are confident in the protection afforded by vaccination. Alternatively, unvaccinated individuals may be less concerned about COVID-19 and less likely to seek testing.
In addition, several methodologic limitations should be considered. First, vaccinated individuals were matched upon receipt of the second dose of mRNA-1273, instead of upon receipt of the first dose, as in some prior studies.13 Although this approach can lead to selection bias, this is likely minimal in our study, as 94% of first dose recipients received the second dose on time. Second, we identified potential confounders a priori and used ASD>0·1 to select variables to adjust for in analyses, which may be less optimal than other approaches for determining confounders.41 Third, few COVID-19 hospital deaths were observed, resulting in wide confidence intervals for this outcome. Confidence intervals were also wide for analyses of VE among individuals with history of COVID-19.
Additional interim and final analyses are planned over the course of the 5-year study to examine VE in additional subgroups (adolescents once authorized, individuals with chronic diseases, individuals who are immunocompromised, individuals with autoimmune conditions, frail individuals, pregnant women, and recipients of concomitant vaccinations); VE of 1 dose of mRNA-1273 against COVID-19 infection and severe COVID-19 disease; VE against SARS-CoV-2 variants; and durability of 2 doses of mRNA-1273 in preventing COVID-19 infection and severe COVID-19 disease.
In conclusion, interim results of this matched cohort study with follow-up through June 2021 provide reassuring evidence of the effectiveness of 2 doses of mRNA-1273 against asymptomatic infection, any COVID-19 infection (including across age, sex, and race/ethnicity) and severe outcomes (COVID-19 hospitalization and death). Among those with history of COVID-19, mRNA-1273 vaccination may offer some additional protection beyond that afforded by natural infection. Long-term follow-up is needed to fully evaluate effectiveness of mRNA-1273 against emerging SARS-CoV-2 variants.
Contributors
Concept and design: KJB, LSS, LQ, CAT, HFT
Acquisition, analysis, or interpretation of data: KJB, LSS, LQ, BKA, AF, CAT, HFT
Drafting of the manuscript: KJB, LSS, AF
Critical revision of the manuscript for important intellectual content: LQ, BKA, YL, GSL, YT, HST, JET, CAT, HFT
Statistical analysis: LQ, YL, YT, JET
Obtained funding: CAT, HFT
Administrative, technical, or material support: CAT, GSL, HST, LSS
Supervision: CAT, HFT
Declaration of interests
KJB was employed by Kaiser Permanente Southern California during the conduct of the study and is now an adjunct investigator at Kaiser Permanente Southern California. LSS, LQ, BKA, YL, GSL, YT, AF, HST, JET, HFT are employees of Kaiser Permanente Southern California, which has been contracted by Moderna for the conduct of this present study. CAT is an employee of and a shareholder in Moderna Inc. KJB received funding from GlaxoSmithKline, Dynavax, Pfizer, Gilead, and Seqirus unrelated to this manuscript. LSS received funding from GlaxoSmithKline, Dynavax, and Seqirus unrelated to this manuscript. LQ received funding from GlaxoSmithKline and Dynavax unrelated to this manuscript. BKA received funding from GlaxoSmithKline, Dynavax, Seqirus and Pfizer unrelated to this manuscript. YL received funding from GlaxoSmithKline, Seqirus and Pfizer unrelated to this manuscript. GSL received funding from GlaxoSmithKline unrelated to this manuscript. YT received funding from GlaxoSmithKline unrelated to this manuscript. AF received funding from Pfizer, GlaxoSmithKline, and Gilead unrelated to this manuscript. HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript. JET received funding from Pfizer unrelated to this manuscript. HFT received funding from GlaxoSmithKline and Seqirus unrelated to this manuscript; HFT also served in advisory boards for Janssen and Pfizer.
Acknowledgments
Acknowledgments
This study was funded by Moderna Inc. Medical writing and editorial assistance was provided by Srividya Ramachandran, PhD, and Jared Mackenzie, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna Inc, and under the direction of the authors. The authors would like to acknowledge the following Kaiser Permanente Southern California staff: Donald Kaplan, PharmD, Daniel Ehrlich, PharmD, Dale Timothy, PharmD, Patrick Kerrigan, PharmD, David Cheng, PharmD, Kevin Ohara, Pharm D, Joel Christian, PharmD, Danny Byun, PharmD, Erin Matsushita, PharmD, Dennis Curtis, PharmD, Victoria Hong, PharmD, and Arthur Librea, PharmD, for their role in COVID-19 vaccine logistics and coordination; Michael Aragones, MD, Soon Kyu Choi, Jennifer Charter, Joy Gelfond, Radha Bathala, and Lee Childs for their coordination in processing SARS-CoV-2 specimens; and Raul Calderon, Kourtney Kottman, Ana Acevedo, Elmer Ayala,and Jonathan Arguello for their technical and laboratory support in processing SARS-CoV-2 specimens. The authors would like to acknowledge Helix OpCo, LLC, for their whole genome sequencing of SARS-CoV-2 specimens. The authors would also like to acknowledge the contributions by Moderna staff: Groves Dixon, PhD, Yamuna Paila, PhD, Stephanie Zafonte, DNP, RN, CCRP, and Julie Vanas. The authors thank the patients of Kaiser Permanente for their partnership with us to improve their health. Their information, collected through our electronic health record systems, leads to findings that help us improve care for our members and can be shared with the larger community.
Data sharing
Individual-level data reported in this study are not publicly shared. Upon request, and subject to review, KPSC may provide the deidentified aggregate-level data that support the findings of this study. Deidentified data (including participant data as applicable) may be shared upon approval of an analysis proposal and a signed data access agreement.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100134.
Appendix. Supplementary materials
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration. Moderna COVID-19 Vaccine. December 18 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine Accessed July 19 2021.
- 5.United States Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. December 11 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. Accessed July 19 2021.
- 6.United States Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic. May 10 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use. Accessed July 22 2021.
- 7.United States Food and Drug Administration. FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine. February 27 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine. Accessed August 4 2021.
- 8.Centers for Disease Control and Prevention. COVID Data Tracker. 19 July 2021. https://covid.cdc.gov/covid-data-tracker. Accessed 31 August 2021.
- 9.Goodman JL, Grabenstein JD, Braun MM. Answering key questions about COVID-19 vaccines. Jama. 2020;324(20):2027–2028. doi: 10.1001/jama.2020.20590. [DOI] [PubMed] [Google Scholar]
- 10.Patel MK, Bergeri I, Bresee JS, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the world health organization. Vaccine. 2021;39(30):4013–4024. doi: 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 12.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. Eng J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilishvili T, Fleming-Dutra KE, Farrar JL, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel - 33 U.S. Sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swift MD, Breeher LE, Tande AJ, et al. Effectiveness of messenger RNA coronavirus disease 2019 (COVID-19) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a cohort of healthcare personnel. Clin Infect Dis. 2021;73(6):e1376–e1379. doi: 10.1093/cid/ciab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann Intern Med. 2021:M21–1577. doi: 10.7326/M21-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults Aged ≥65 Years - COVID-NET, 13 States, February-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1088–1093. doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paris C, Perrin S, Hamonic S, et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.06.043. S1198-743X(21) 00379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen AE, Cohen S, Bryson-Cahn C, et al. Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State. Clin Infect Dis. 2021:ciab581. doi: 10.1093/cid/ciab581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowlkes A GM, Groover K, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanduri S PT, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrejko KL, Pry J, Myers JF, et al. Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clinical Infectious Diseases. 2021:ciab640. doi: 10.1093/cid/ciab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med. 2021;385:1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDPH. Updated COVID-19 Vaccine Eligibility Guidelines. California Department of Public Health 2021. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/VaccineAllocationGuidelines.aspx. Accessed 27 Aug 2021.
- 29.CDPH. California COVID-19 Vaccination Program. https://eziz.org/assets/docs/COVID19/IMM-1329.pdf. Accessed 27 Aug 2021.
- 30.Common investigation protocol for investigating suspected SARS-CoV-2 reinfection. Centers Dis Control Prevent. 2020 https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html Accessed 27 Aug 2021. [Google Scholar]
- 31.FDA . FDA; 2021. Fact sheet for healthcare providers administering vaccine (Vaccination Providers) emergency use authorization (EUA) of the Moderna Covid-19 vaccine to prevent coronavirus disease 2019 (COVID-19)https://www.fda.gov/media/144637/download Accessed 27 Aug 2021. [Google Scholar]
- 32.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miettinen OS. John Wiley & Sons; New York (NY): 1985. Theoretical epidemiology: principles of occurrence research. [Google Scholar]
- 35.Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. Centers for Disease Control and Prevention. 2021 https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html Accessed 27 Aug 2021. [Google Scholar]
- 37.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos L, Czartoski J, Wan Y-H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372(6549):1418. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Ann Am Thorac Soc. 2021;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.