Abstract
Background:
The prevalence of pulmonary embolism (PE) in patients of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) varies over a wide range. Early detection and treatment of PE in AECOPD is a key to improve patient outcome. The purpose of the study was to investigate the prevalence and predictors of PE in patients of AECOPD in a high burden region of North India.
Materials and Methods:
This prospective study included patients of AECOPD with no obvious cause of exacerbation on initial evaluation. Apart from routine workup, the participants underwent assessment of D-dimer, compression ultrasound and venous Doppler ultrasound of the lower limbs and pelvic veins, and a multidetector computed tomography pulmonary angiography.
Results:
A total of 100 patients of AECOPD with unknown etiology were included. PE as a possible cause of AE-COPD was observed in 14% of patients. Among the participants with PE, 63% (n = 9) had a concomitant presence of lower extremity deep venous thrombosis. Hemoptysis and chest pain were significantly higher in patients of AECOPD with PE ([35.7% vs. 7%, P = 0.002] and [92.9% vs. 38.4%, P = 0.001]). Likelihood of PE was significantly higher in patients who presented with tachycardia, tachypnea, respiratory alkalosis (PaCO2 <45 mmHg and pH >7.45), and hypotension. No difference was observed between the two groups in terms of in-hospital mortality, age, sex distribution, and risk factors for embolism except for the previous history of venous thromboembolism (35.7% vs. 12.8% P = 0.03).
Conclusion:
PE was probably responsible for AECOPD in 14% of patients with no obvious cause on initial assessment. Patients who present with chest pain, hemoptysis, tachypnea, tachycardia, and respiratory alkalosis should be particularly screened for PE.
KEY WORDS: Acute exacerbation, chronic obstructive pulmonary disease, D-dimer, pulmonary embolism
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is currently the 4th leading cause of death worldwide and 2nd in India.[1] Patients with COPD may present with acute worsening of their respiratory symptoms known as acute exacerbation of chronic obstructive pulmonary disease (AECOPD).[2] In addition to increased morbidity and health-care utilization, AE-COPD significantly reduces both in-hospital and long-term survival.[3,4,5] Majority (50%–70%) of the events of AECOPD are precipitated by respiratory tract infections, followed by exposure to environmental pollution (10%). However, in up to 30% of cases, the cause of exacerbation remains unknown.[6,7]
Various studies have reported COPD as one of the independent risk factors for venous thromboembolism (VTE).[8,9] In a systematic review and meta-analysis, the pooled prevalence of pulmonary embolism (PE) in unexplained AECOPD was reported to be 16.1%.[10] In a recent multicenter cross-sectional study (n = 740), the prevalence of PE was 5.9% (11% in patients with suspicion of PE and 4.3% in patients with no suspicion of PE on initial assessment).[11] However, higher prevalence of PE was reported in postmortem studies ranging from 28% to 51%.[12,13] In addition, patients of COPD with PE have significantly higher 3 month as well as 1 year mortality compared to patients with PE alone.[14,15] It is therefore important to identify PE as a cause of AECOPD, as delay in diagnosis and treatment is associated with poorer outcome.[16] As the symptoms of AECOPD are nonspecific, it is often difficult to differentiate PE as a cause of AECOPD based on clinical examination alone. Multidetector computed tomography (MDCT) pulmonary angiography (PA) is currently the investigation of choice in suspected PE.[17]
The reported prevalence of spirometrically defined COPD (in adults ≥ 40 years) in our part of world is 17.3% in males and 14.8% in females.[18] It is higher than that reported from other states of our country and various parts of the world.[19] We have previously reported that viral respiratory tract infections are responsible for acute exacerbation in sizable number of our COPD patients.[7] However, the cause of AECOPD remains unknown in a large number of patients. With this background, the present study was designed to know the prevalence of PE in patients with AECOPD with no identifiable etiology requiring hospitalization at a tertiary care hospital of Kashmir in North India.
MATERIALS AND METHODS
Study design and place
This study was a prospective observational study that was conducted over a period of 2 years in Sheri-Kashmir institute of medical sciences, a multidisplinary institute of north India, an 800-bedded hospital.
Study population
Patients of COPD who presented with AE-COPD to the emergency department of our institute severe enough to require hospitalization were assessed for the eligibility criteria. Patients of AECOPD with no obvious cause on initial assessment were included in the study. Following assessments were done to rule out the possible etiology of worsening of symptoms in patients with COPD: (a) history of fever, purulent sputum, myalgias, and running nose to rule out possibility of infective etiology; (b) chest radiography was done to look for the evidence of pneumothorax, pleural effusion, or pulmonary edema as a cause of exacerbation of symptoms. (c) Electrocardiography to look for any evidence suggestive of acute coronary syndrome and arrhythmias. Those with definite alternative cause of worsening of symptoms (like respiratory tract infection, pneumothorax, and myocardial ischemia) plus requiring mechanical ventilation or who fail to give consent were excluded from the study. Diagnosis of COPD was made based on the compatible history and postbronchodilator forced expiratory volume in 1 s to forced vital capacity ratio of <0.7. Severity of COPD as well as exacerbation of COPD was defined as per the global initiative for chronic obstructive lung disease (GOLD) criteria.[20]
Study protocol
In addition to routine investigations that included complete blood count, biochemistry, chest radiograph, and arterial blood gas (ABG) analysis, a detailed history, physical examination, the use of pretest probability scoring (simplified Geneva scoring [SGS]), and qualitative D-dimer assay were carried out in all the participants. Compression ultrasound (CUS) and venous Doppler of lower extremities were performed for the presence of deep venous thrombosis (DVT) followed by MDCT PA for PE. Reporting of CUS/Doppler study of lower extremities and MDCT-PA was done by a radiologist with more than 10-year experience in a teaching tertiary care center [Figure 1].
Figure 1.
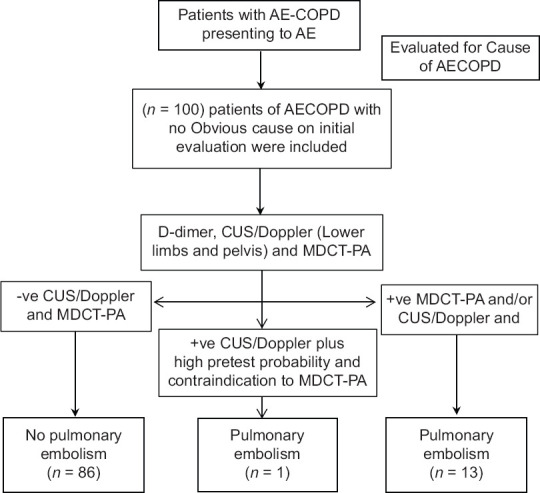
Flow chart of the study protocol
Diagnosis of deep venous thrombosis and pulmonary embolism
Noncompressibility or incomplete compressibility of deep veins of lower extremities on CUS was taken as criteria for the presence of DVT. Computed tomography pulmonary angiogram (CTPA) was considered positive for PE if there was a total occlusion of the vessel by a low attenuation material or presence of an intraluminal defect surrounded by a contrast material.[21] Patients were considered to have PE if they had positive CTPA or positive CUS/Doppler of lower extremities plus high clinical suspicion (if CTPA was not done due to any contraindication). PE was excluded in case of negative CTPA and/or lower extremity CUS/Doppler. History regarding the presence of risk factors for VTE including the presence of malignancy, immobility, recent surgery, or previous VTE was recorded. Patients who had DVT or PTE were managed with therapeutic anticoagulation and those with PE who fulfilled the criteria for thrombolysis, received thrombolysis as per the standard protocol. AECOPD was managed as per the available GOLD guidelines.[20]
Ethics and consent
The study protocol was approved by departmental ethics committee. A written consent was obtained from all patients who participated in the study.
Statistical analysis
All continuous variables were expressed as the mean ± standard deviation in case of normally distributed data and were compared using unpaired Student's t-test. The difference between the two groups was assessed using Chi-square or Fisher's exact test. Multiple logistic regression analysis was performed to identify the predictors of PE. A P < 0.05 was considered to indicate significance. Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
The 100 cases included 57% (n = 57) were males and 43% (n = 43) were females with a mean age of 66.32 ± 14.06 years. Majority of the patients (69%) belonged to age group of more than 60 years with no difference between the two groups. The demographic data of the participants are depicted in Table 1.
Table 1.
Comparison of patient characteristics between acute exacerbation of chronic obstructive pulmonary disease with and without pulmonary embolism
| Patient characteristic | AECOPD with PE (n=14), n (%) | AECOPD with no PE (n=86), n (%) | P |
|---|---|---|---|
| Age in years | |||
| <60 | 3 (21.4) | 28 (32.6) | |
| ≥60 | 11 (78.6) | 58 (67.4) | 0.404 |
| Gender | |||
| Male | 7 (50) | 50 (58) | 0.750 |
| Female | 7 (50) | 36 (42) | 0.750 |
| Smoking | 12 (85.7) | 65 (75.58) | 0.403 |
| Risk factors for PE | |||
| Recent surgery | 4 (28.5) | 20 (23.2) | 0.750 |
| Recent trauma | 3 (21.4) | 16 (18.6) | 0.900 |
| Active malignancy | 3 (21.4) | 11 (12.8) | 0.500 |
| Previous history of VTE | 5 (35.7) | 11 (12.8) | 0.030 |
| Comorbid illnesses | |||
| Hypertension | 13 (92.85) | 48 (55.8) | 0.008 |
| Diabetes mellitus | 3 (21.42) | 26 (30.2) | 0.5008 |
| Obesity | 3 (21.42) | 24 (28.9) | 0.612 |
| Stage of COPD (GOLD) | |||
| Stage II | 3 (21.43) | 30 (34.9) | 0.320 |
| Stage III | 10 (71.43) | 47 (54.6) | 0.239 |
| Stage IV | 1 (7.14) | 8 (9.30) | 0.793 |
| Clinical symptoms and signs | |||
| Hemoptysis | 5 (35.7) | 6 (6.9) | 0.002 |
| Chest pain | 13 (92.8) | 33 (38.3) | 0.001 |
| Respiratory rate >30/min | 14 (100) | 36 (43.4) | 0.001 |
| Hypotension (SBP <100 mmHg) | 9 (64.3) | 1 (1.2) | 0.001 |
| Pulse >100 bpm | 13 (92.9) | 24 (28.9) | 0.001 |
| Simplified Geneva Score | 5.57±1.158 | 3.49±0.592 | <0.001 |
| Laboratory and blood gas values | |||
| Polycythemia | 8 (57.14) | 27 (31.4) | 0.06 |
| Total leucocyte count | |||
| <4000/µL | 0 (0) | 0 (0) | |
| 4000-10,000/µL | 0 (0) | 0 (0) | |
| >10,000/µL | 14 (100) | 84 (100) | ns |
| Platelet count | |||
| <150 ×103/µL | 12 (85.71) | 33 (38.37) | 0.0096 |
| >150 ×103/µL | 2 (14.28) | 53 (61.62) | |
| Positive D dimer | 13 (92.8) | 78 (90.6) | 0.694 |
| Arterial blood gas values | |||
| Oxygen saturation (mean±SD) | 73.6±8.38 | 73.84±8.12 | 0.814 |
| paO2, mmHg (mean±SD) | 50±8.6 | 49.9±8.48 | 0.548 |
| paCO2, mmHg (mean±SD) | 47.3±5.66 | 61.4±7.38 | <0.001 |
| pH (mean±SD) | 7.46±0.064 | 7.36±0.063 | <0.001 |
| pH >7.45 | 11 (78.6) | 4 (4.7) | <0.001 |
| PCO2 (<45 mmHg) | 10 (71.42) | 3 (3.48) | 0.001 |
| In hospital mortality | 2 (14.28) | 5 (5.81) | 0.249 |
PE: Pulmonary embolism, COPD: Chronic obstructive pulmonary disease, AECOPD: Acute exacerbation of COPD, SD: Standard deviation, SBP: Systolic blood pressure, VTE: Venous thromboembolism
PE was observed in 14% (n = 14) of participants. CTPA for PE was positive in 13% (n = 13) of cases, while as one patient died before CTPA was done but had DVT on CUS/Doppler. Among the participants with PE, 63% (n = 9) had the concomitant presence of lower extremity DVT. In majority of the patients (46%) with PE, thrombus was seen in main pulmonary artery, followed by segmental (38.5%) and at subsegmental (15%) level [Table 2]. None of the cases with negative CTPA had DVT on CUS/Doppler lower limbs. No significant difference was observed between severity of AECOPD and presence of PE (P = 0.521) [Table 1].
Table 2.
Findings of computed tomographic pulmonary angiogram in patients with acute exacerbation of chronic obstructive pulmonary disease of unknown etiology
| Findings on CTPA | n (%) |
|---|---|
| No evidence of pulmonary embolism | 86 (86) |
| Evidence of thrombus in pulmonary arteries | 13 (13) |
| Central thrombus | 6 (46.1) |
| Segmental thrombus | 5 (38.5) |
| Subsegmental thrombus | 2 (15.4) |
CTPA: Computed tomographic pulmonary angiogram
All patients of AECOPD (irrespective of presence or absence of PE) were classified as having high risk for PE based on SGS. However, patients with PE had significantly higher values compared to patients without PE (5.57 ± 1.158 in patients with PE vs. 3.49 ± 0.592 in patients without PE, P < 0.001).
No significant difference was observed between two groups in terms of age, sex distribution, and risk factors for embolism except for previous history of VTE (35.7%, 5/14 in patients with PE vs. 12.8%, 11/86 in patients with no PE, P = 0.03) [Table 1].
Significant difference was observed in clinical presentation and vital signs among patients of AECOPD with and without PE. Hemoptysis was present in 35.7% (5 out of 14) of patients with PE and only in 7% (6 out of 84) of patients with no evidence of PE (P = 0.001). Chest pain was the presenting complaint in 92.9% (13 out of 14) of patients with PE compared to 38.4% (33 out of 84) of patients with no PE (P = 0.001). Patients with PE were more likely to have tachycardia (heart rate [HR] >100/min), tachypnea (respiratory rate [RR] >30/min), and hypotension (systolic blood pressure [SBP] <100 mmHg) on presentation as compared to patients with no evidence of PE. No significant difference was observed in terms of laboratory parameters between the two groups, except patients with PE tend to have respiratory alkalosis (pH >7.45: 78.6% vs. 4.3% P ≤ 0.001 and PCO2 ≤45: 71.4% vs. 3.4% P ≤ 0.001) and low platelet count (<1.5 l/cumm: 85.7% vs. 36.6%, P ≤ 0.001). Qualitative D-dimer assay was done in all the participants. No difference was observed in terms of percentage of positive Dimer test in both the groups (92.85% in patients with PE vs. 90.6% of patients without PE, P = 0.694) [Table 1]. The presence of previous history of VTE, hemoptysis, chest pain, simplified Geneva score of more than 5, disproportionate tachypnea (RR >30/min), tachycardia (HR >100/min), hypotension (SBP <100 mmHg), and respiratory alkalosis were significantly associated with PE in patients with AECOPD on univariate analysis [Table 3]. However, on multivariate binary logistic regression, the presence of respiratory alkalosis with PCO2 <45 mmHg and pH >7.45 predicted PE in patients with AECOPD.
Table 3.
Factors associated with high risk for pulmonary embolism in patients with acute exacerbation of chronic obstructive pulmonary disease by univariate analysis
| Patient characteristic (n) | OR | 95% CI (lower limit-upper limit) | P |
|---|---|---|---|
| Previous history of VTE | 3.79 | 1.07-13.39 | 0.050 |
| Hemoptysis | 7.40 | 1.88-29.22 | 0.005 |
| Chest pain | 20.88 | 2.61-167.10 | 0.001 |
| Tachypnea (respiratory rate >30/min) | 31.72 | 3.93-255.57 | 0.001 |
| Hypotension (SBP <100 mmHg) | 40.24 | 4.96-326.18 | 0.001 |
| Tachycardia (pulse >100 bpm) | 31.72 | 3.93-255.57 | 0.001 |
| PCO2 (<45 mmHg) | 69.17 | 13.49-354.58 | 0.001 |
| pH >7.45 | 75.16 | 14.81-381.293 | 0.001 |
| Platelet (<150,000 mm3) | 10.65 | 2.23-50.67 | 0.001 |
SBP: Systolic blood pressure, VTE: Venous thromboembolism, OR: Odds ratio, CI: Confidence interval
Management of pulmonary embolism and outcome
Out of 13 cases of AECOPD with PE, 38.46% (n = 5) fulfilled the criteria for thrombolysis. Majority of these patients (80%, n = 4) had thrombus in main pulmonary artery. Streptokinase infusion was used as an agent for systemic thrombolysis in 4 (80%) patients and t-PA in 1 (20%) patient. Four out of five (80%) patients who received thrombolysis improved clinically with hemodynamic stability. Remaining patients of PE (n = 8) who did not require thrombolysis were managed with anticoagulation alone in addition to standard of care for the management of AECOPD. Total of eight patients died during hospitalization. No significant difference was noted with regard to the in-hospital mortality between the two groups (5.81%, n = 5 in patients without PE vs. 14.28%, n = 2 in patients with PE, P = 0.25).
DISCUSSION
The present study showed the prevalence of PE in patients with AECOPD of unknown etiology to be 14%. The prevalence of PE in COPD patients with unknown etiology varies from as low as 2% to as high as 29.1%.[11,21,22,23,24,25,26,27] Tillie-Leblond et al. reported that 15 out of 60 (25%) patients with AECOPD of unknown etiology had PE. Similarly, Gunen et al. reported 29.1% prevalence of PE in patients with AECOPD of unknown reason. However, in a study by Rutschmann et al., the prevalence of PE was only 3.3%. The low prevalence of PE might be due to selection bias excluding patients with D-dimer level <500 μg/l and with low clinical probability of PE. A study from India and Korea reported the prevalence of PE to be 2% and 5%, respectively. In a meta-analysis that included seven studies have shown that the pooled prevalence of PE in unexplained AE-COPD was 16.1% (95% confidence interval [CI]: 8.3%–25.8%).[10] These differences could be due to number of reasons that may include difference in sample size, enrollment criteria, study design, or diagnostic modalities used (V/Q scan vs. CTPA).
Various validated bedside clinical prediction scores are being used to predict the likelihood of PE and to reduce the need for imaging.[28,29] We observed that the use of clinical prediction score (simplified Geneva score) was not useful in predicting PE in patients with AECOPD. In our study, all patients with AECOPD had simplified Geneva score of >2, indicating high likelihood of PE, but only 13% of patients (13/100) had evidence of PE. However, a significant difference was noted in patients with and without PE if mean values of SGS were considered (5.57 ± 1.16 in patients with PE vs. 3.49 ± 0.59 in patients without PE, P ≤ 0.001). Therefore, likelihood of PE was significantly higher in patients of AECOPD with SGS of more than 5. Gunen et al.reported that none of the patients with low clinical prediction score and about 20% of patients with moderate score had PE. However, various studies have shown that patients of COPD with PE had lower pretest probability for PE using various PE predicting scores.[22,30,31] These observations showed that the use of clinical prediction scoring system can be misleading when used in patients of COPD in predicting PE.
We did not observe a significant association of PE in patients with AECOPD of unknown etiology with well-known risk factors except previous history of VTE. Studies have shown that, among various risk factors for PE, the presence of previous history of venous thrombosis was more likely to be associated with PE (P value 0.04, RR 0.26, 95% CI, 0.07–0.93).[23,32] Tillie-Leblond et al. reported that in addition to previous history of VTE, presence of malignancy was associated with higher risk of PE (risk ratio, 1.82 [CI, 1.13–2.92]).[21] Laurent Bet al. concluded that COPD patients with a history of venous thromboembolism, especially previous PE has increased risk of recurrence of PE and fatal PE as compared to those presenting with DVT alone.[14]
The most common symptom of PE is breathlessness at rest and with exertion, orthopnea, pleuritic chest pain, wheezing, cough, and hemoptysis, whereas the most common signs include tachypnea, tachycardia, decreased breath sounds, or accentuated second heart sound.[33,34]
Although symptoms of AECOPD due to PE are nonspecific, previous studies have reported that chest pain was more frequently reported by patients of AECOPD with PE as compared to patients with no PE.[22,23,31] Our study revealed that, among clinical features, the presence of pleuritic chest pain and hemoptysis significantly predicted PE as a cause of AECOPD compared to other symptoms (P=). However, few studies have failed to show any significant difference of symptoms in patients of AECOPD with and without PE.[21,34] The result of a recent meta-analysis that evaluated the prevalence of PE in patients of unknown etiology showed that, in patients with AE-COPD, the presence of pleuritic chest pain significantly predicted the presence of PE.[10]
Patients with peripheral pulmonary embolus usually present with chest pain and hemoptysis. In a series of 172 patients who presented with AECOPD secondary to PE, the most common symptom reported was pleuritic chest pain. About 80% of patients with PE had peripheral pulmonary embolus.[31] However, contrary to this, our study reported that 92.8% (n = 13) of patients with CTPA documented PE, presented with chest pain and 42.8%of these had centrally located thrombus. Similar findings were reported by Gunen et al. in his study.[22] These findings can be explained by the fact that massive centrally located PE can cause ischemic chest pain from right ventricular infarction.
There was a significant difference in terms of physical examination findings in patients of AECOPD with and without PE. Tachycardia (HR >100/min), tachypnea (RR > 30/min), and hypotension (SBP of < 100 mmHg) was present in significantly higher percentage of AECOPD patients with PE compared to patients with no PE. Our findings were in consistent with those reported by previous studies.[26,35] Hence, the suspicion of PE should be kept higher in patients of AECOPD with disproportionate tachycardia, tachypnea, and hypotension.
Patients of COPD with acute exacerbation may present with either hypercapnic or hypoxemic respiratory failure. In the acute setting, hypercapnea may be increased secondary to increased dead space ventilation and ventilation/perfusion (V/Q) mismatch caused by increased mucus production and bronchial constriction. In patients with PE, ABG usually reveals hypoxemia, hypocapnia, and respiratory alkalosis.[36,37] We observed that patients of AECOPD with PE had significantly lower levels of paCO2 and higher pH compared to patients with no PE. These findings may be due to reflex tachypnea associated with PE secondary to increase in dead space ventilation. Our findings were in consistent with those reported by Tillie-Leblond et al., they observed that a decrease of paCO2 of at least 5 mmHg from baseline was the only ABG abnormality associated with PE.[21] Rodger et al. and Lippmann and Fein. also suggested that a decrease in paCO2 during COPD exacerbation might indicate PE.[33,38] On the other hand, no reduction in paCO2 was reported in patients of AECOPD with PE by Lesser et al. and Mispelaere et al.[34,39] It is to be noted that Shaker et al. observed that patients of AE-COPD with PE had hypercapnia rather than hypocapnia. These observations can be attributed to increased dead space ventilation and respiratory muscle fatigue present during AECOPD.
Although majority of the patients with PE have hypoxemia, no significant difference was observed between two groups in this study. Jerald et al. and Hatem et al. reported that patients of COPD with PE tend to have greater decrease in PaO2 than patients with PE alone. However, Stein et al. observed that hypoxemia can be absent or minimal in patients with PE. About 18% of patients with PE can have PaO2 between 85 mmHg and 105 mmHg and up to 6% of patients with PE have normal alveolar arterial gradient for oxygen.[40]
PE and DVT represent the spectrum of same disease. About 80% of patients with PE have evidence of DVT of lower extremities. Our study showed that 64.3% (9/13) of patients with PE had DVT of lower extremities on CUS. Absence of DVT in 30.76% of patients with PE could be due to in situ thrombus formation in pulmonary arteries or embolization of entire thrombus from lower extremities or thrombus involving iliac vessels.[41,42] Kamel et al. reported that only 26.7% of patients with PE had DVT on CUS and suggested an alternative etiology for pulmonary thrombosis such as hypoxemia and polycythemia.[25]
Limitations
Our study has some limitations. First, only qualitative D-dimer assay was performed that could be responsible for excessive use of CTPA and may explain lesser prevalence of PE as compared to previous studies. Secondly patients of AECOPD requiring invasive mechanical ventilation were excluded that could be associated with selection bias and lesser prevalence. Also it was a single-center study that was performed in a place with higher prevalence of COPD and therefore may not represent the true scenario of other places of the country. A small sample size would also call for a larger study on a country wide basis to ascertain the true burden of the disease so as to modulate the management accordingly.
CONCLUSION
PE was responsible for AECOPD in a significant number of patients (14%) with no obvious cause on initial assessment. The presence of PE should be considered in patients of COPD who present with acute worsening of their symptoms particularly when there is the presence of risk factors for DVT and clinical manifestations consistent with acute PE.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.India State-Level Disease Burden Initiative Collaborators. Nations within a nation: Variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–60. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:464–71. doi: 10.1164/rccm.201710-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Sanz MT, Cánive-Gómez JC, Senín-Rial L, Aboal-Viñas J, Barreiro-García A, López-Val E, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9:636–45. doi: 10.21037/jtd.2017.03.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koul PA, Dar HA, Jan RA, Shah S, Khan UH. Two-year mortality in survivors of acute exacerbations of chronic obstructive pulmonary disease: A North Indian study. Lung India. 2017;34:511–6. doi: 10.4103/lungindia.lungindia_41_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease.NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 7.Koul PA, Hyder M, Shabir A, Varsha P, Mandeep SC. Respiratory viruses in acute exacerbations of chronic obstructive pulmonary disease. Lung India. 2017;34:29–33. doi: 10.4103/0970-2113.197099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 9.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 10.Aleva FE, Voets LW, Simons SO, de Mast Q, van der Ven AJ, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2017;151:544–54. doi: 10.1016/j.chest.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Couturaud F, Bertoletti L, Pastre J, Roy PM, Le Mao R, Gagnadoux F, et al. Prevalence of pulmonary embolism among patients with COPD hospitalized with acutely worsening respiratory symptoms. JAMA. 2021;325:59–68. doi: 10.1001/jama.2020.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moua T, Wood K. COPD and PE: A clinical dilemma. Int J Chron Obstruct Pulmon Dis. 2008;3:277–84. doi: 10.2147/copd.s1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott SM, Richards KL, Tikoff G, Armstrong JD, Jr, Shigeoka JW. Venous thromboembolism in decompensated chronic obstructive pulmonary disease. A prospective study. Am Rev Respir Dis. 1981;123:32–6. doi: 10.1164/arrd.1981.123.1.32. [DOI] [PubMed] [Google Scholar]
- 14.Bertoletti L, Quenet S, Laporte S, Sahuquillo JC, Conget F, Pedrajas JM, et al. Pulmonary embolism and 3-month outcomes in 4036 patients with venous thromboembolism and chronic obstructive pulmonary disease: Data from the RIETE registry. Respir Res. 2013;14:75. doi: 10.1186/1465-9921-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110:1212–9. doi: 10.1378/chest.110.5.1212. [DOI] [PubMed] [Google Scholar]
- 16.Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. The importance of initial heparin treatment on long-term clinical outcomes of antithrombotic therapy.The emerging theme of delayed recurrence. Arch Intern Med. 1997;157:2317–21. [PubMed] [Google Scholar]
- 17.Rawat KS, Buxi TB, Sudarsan H, Yadav A, Ghuman SS. Current role of multi-detector computed tomography (MDCT) in diagnosis of pulmonary embolism. Curr Radiol Rep. 2014;2:68. [Google Scholar]
- 18.Koul PA, Hakim NA, Malik SA, Khan UH, Patel J, Gnatiuc L, et al. Prevalence of chronic airflow limitation in Kashmir, North India: Results from the BOLD study. Int J Tuberc Lung Dis. 2016;20:1399–404. doi: 10.5588/ijtld.15.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mejza F, Gnatiuc L, Buist AS, Vollmer WM, Lamprecht B, Obaseki DO, et al. Prevalence and burden of chronic bronchitis symptoms: Results from the BOLD study. Eur Respir J. 2017;50 doi: 10.1183/13993003.00621-2017. Eur Respir j 2017;50(5):1700621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. [Lastaccessed on 2018 Oct 10]. Available from: https://goldcoped.org/
- 21.Tillie-Leblond I, Marquette CH, Perez T, Scherpereel A, Zanetti C, Tonnel AB, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: Prevalence and risk factors. Ann Intern Med. 2006;144:390–6. doi: 10.7326/0003-4819-144-6-200603210-00005. [DOI] [PubMed] [Google Scholar]
- 22.Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35:1243–8. doi: 10.1183/09031936.00120909. [DOI] [PubMed] [Google Scholar]
- 23.Rutschmann OT, Cornuz J, Poletti PA, Bridevaux PO, Hugli OW, Qanadli SD, et al. Should pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease? Thorax. 2007;62:121–5. doi: 10.1136/thx.2006.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KJ, Cha SI, Shin KM, Lee J, Hwangbo Y, Yoo SS, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration. 2013;85:203–9. doi: 10.1159/000335904. [DOI] [PubMed] [Google Scholar]
- 25.Kamel MM, Moussa H, Ismail A. Prevalence of venous thrombo-embolism in acute exacerbations of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2013;62:557–66. [Google Scholar]
- 26.Jindal A, Singh RY, Joshi V, Jain S, Khippal N. A cross-sectional study for the evaluation of pulmonary embolism in unexplained dyspnea in acute exacerbation of chronic obstructive pulmonary disease. Indian J Respir Care. 2020;9:191–5. [Google Scholar]
- 27.Dutt TS, Udwadia ZF. Prevalence of venous thromboembolism in acute exacerbations of chronic obstructive pulmonary disease: An Indian perspective. Indian J Chest Dis Allied Sci. 2011;53:207–10. [PubMed] [Google Scholar]
- 28.Klok FA, Mos IC, Nijkeuter M, Righini M, Perrier A, Le Gal G, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–6. doi: 10.1001/archinte.168.19.2131. [DOI] [PubMed] [Google Scholar]
- 29.Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295:199–207. doi: 10.1001/jama.295.2.199. [DOI] [PubMed] [Google Scholar]
- 30.Fernández C, Jiménez D, De Miguel J, Martí D, Díaz G, Sueiro A. [Chronic obstructive pulmonary disease in patients with acute symptomatic pulmonary embolism] Arch Bronconeumol. 2009;45:286–90. doi: 10.1016/j.arbres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Akpinar EE, Hoşgün D, Akpýnar S, Ataç GK, Doğanay B, Gülhan M. Incidence of pulmonary embolism during COPD exacerbation. J Bras Pneumol. 2014;40:38–45. doi: 10.1590/S1806-37132014000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann IJ, Hagen PJ, Melissant CF, Postmus PE, Prins MH. Diagnosing acute pulmonary embolism: Effect of chronic obstructive pulmonary disease on the performance of D-dimer testing, ventilation/ perfusion scintigraphy, spiral computed tomographic angiography, and conventional angiography. ANTELOPE Study Group. Advances in New Technologies Evaluating the Localization of Pulmonary Embolism. Am J Respir Crit Care Med. 2000;162:2232–7. doi: 10.1164/ajrccm.162.6.2006030. [DOI] [PubMed] [Google Scholar]
- 33.Lippmann M, Fein A. Pulmonary embolism in the patient with chronic obstructive pulmonary disease.A diagnostic dilemma. Chest. 1981;79:39–42. doi: 10.1378/chest.79.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Lesser BA, Leeper KV, Stein PD, Saltzman HA, Chen J, Thompson BT, et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992;102:17–22. doi: 10.1378/chest.102.1.17. [DOI] [PubMed] [Google Scholar]
- 35.Suzan S, Olfat ES, Raafat T, Elham Abd ES, Ali H. Predictors for pulmonary embolism in patients with acute exacerbation of COPD. Egypt J Bronchol. 2008;2:280–91. [Google Scholar]
- 36.Prediletto R, Miniati M, Tonelli L, Formichi B, Di Ricco G, Marini C, et al. Diagnostic value of gas exchange tests in patients with clinical suspicion of pulmonary embolism. Crit Care. 1999;3:111–6. doi: 10.1186/cc352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruno CM, Valenti M. Acid-base disorders in patients with chronic obstructive pulmonary disease: A pathophysiological review. J Biomed Biotechnol. 2012;2012:915150. doi: 10.1155/2012/915150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodger MA, Jones G, Rasuli P, Raymond F, Djunaedi H, Bredeson CN, et al. Steady-state end-tidal alveolar dead space fraction and D-dimer: Bedside tests to exclude pulmonary embolism. Chest. 2001;120:115–9. doi: 10.1378/chest.120.1.115. [DOI] [PubMed] [Google Scholar]
- 39.Mispelaere D, Glerant JC, Audebert M, Remond A, Sevestre-Pietri MA, Jounieaux V. Pulmonary embolism and sibilant types of chronic obstructive pulmonary disease decompensations. Rev Mal Respir. 2002;19:415–23. [PubMed] [Google Scholar]
- 40.Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, Hales CA, et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am J Med. 2007;120:871–9. doi: 10.1016/j.amjmed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo A, De Luca M, Vigna C, De Rito V, Pacilli M, Lombardo A, et al. Central pulmonary artery lesions in chronic obstructive pulmonary disease: A transesophageal echocardiography study. Circulation. 1999;100:1808–15. doi: 10.1161/01.cir.100.17.1808. [DOI] [PubMed] [Google Scholar]
- 42.Goldhaber SZ. Pulmonary embolism. N Engl J Med. 1998;339:93–104. doi: 10.1056/NEJM199807093390207. [DOI] [PubMed] [Google Scholar]


