Abstract
Tetralogy of Fallot with absent pulmonary valve (TOF-APV) is a rare form of tetralogy with unique challenges due to the combination of pulmonary annular stenosis, severe pulmonary regurgitation, and airway compression secondary to aneurysmal dilatation of the pulmonary arteries. Data on the long-term outcomes of repaired TOF-APV are scarce. We used the Pediatric Cardiac Care Consortium (PCCC), a large US-based registry, to describe the postrepair transplant-free survival of patients with TOF-APV. We queried the PCCC for patients operated for TOF-APV between 1982 and 2003. Death or transplant events were ascertained from the PCCC and by linkage with the US National Death Index and the Organ Procurement Transplantation Network through December 2019. A total of 126 patients were identified with TOF-APV repair (primary n = 119, staged n = 7). The majority of them were repaired with a right ventricular to pulmonary artery conduit (n = 80, 64%) and 43 (34%) with transannular patch. In-hospital mortality occurred in 31 patients (25%); post discharge and over a median period of 19 years (IQR 0.37 to 23.7 years), 5 patients died and 2 underwent heart transplant, one of whom subsequently died. The 25-year transplant-free survival post discharge after TOF-APV repair was 92%, which was similar with the outcome of patients with simple TOF undergoing non-valve sparing procedures (94% log-rank test p = 0.455; aHR 1.37; 95% CI: 0.63 to 2.97, p = 0.432). In conclusion, early in-hospital mortality is high for TOF-APV; however, once repaired and survived to discharge, long term survival is similar to simple TOF with non-valve sparing procedures.
Tetralogy of Fallot with absent pulmonary valve (TOF-APV) accounts for approximately 3% to 6% of patients with TOF.1,2 First described by Chevers in 1847, TOF-APV, is characterized by severe regurgitation secondary to absent or rudimentary pulmonary valve, pulmonary annular stenosis and aneurysmal dilation of the main pulmonary trunk and branch pulmonary arteries in addition to the other anatomic features of TOF.3 Airway obstruction due to tracheobronchial compression results from dilation of the main pulmonary artery and branch pulmonary arteries contributing to the significant morbidity and mortality of these patients.4 To date, there are limited data on the long-term outcomes of TOF-APV from largely non-US cohorts with national health registries.5–9 Some single center reports.10–14 are limited by selection bias and institution-based treatment strategies. While valuable, the currently existing data does not adequately capture the experience in the United States due to lack of sufficient number of patients and follow up time for evaluation of long-term survival. In this study, we use the Pediatric Cardiac Care Consortium (PCCC), a large US-based registry of interventions for pediatric heart diseases to describe the in-hospital and long-term survival outcomes following TOF-APV repair on patients enrolled between 1982 and 2003.15–17 Matching of the PCCC to the National Death Index (NDI) and the Organ Procurement and Transplantation Network (OPTN) registries extends up to 2019 the prospective follow up time period, thus creating one of the largest and longest studies of this rare condition.18,19
Methods
This is a retrospective cohort study using data from 32 US centers enrolling patients in the PCCC, which were enriched with prospective data from the NDI and OPTN. The OPTN includes data on all donors, wait-listed candidates, and transplant recipients in the US. The Health Resources and Services Administration and US Department of Health and Human Services provide oversight to OPTN contractors. The study was approved by the institutional review boards of Emory University, University of Minnesota, and the NDI and OPTN registries.
The PCCC was queried for patients with the diagnosis of TOF-APV. Manual review of operative records of all patients was performed to confirm the diagnosis of TOF-APV which was absence of a functioning pulmonary valve at the first operative report. The PCCC was also queried for a comparison cohort of patients operated for simple TOF (i.e., excluding pulmonary atresia, APV or atrioventricular canal) within the same period. Surgical codes within the PCCC were used to determine the operation for surgical treatment. Inclusion criteria were: (1) surgery performed at a US center, (2) available full set of identifiers to allow reliable linkage with the NDI/OPTN registries, (3) repair before 21 years of age, (4) first surgery performed prior to 3 years of age, (5) US residency at time of enrollment in the PCCC, and (6) patients with primary repair after January 1, 1982. Patients enrolled after April 15, 2003 were excluded for long-term outcome analysis due to lacking direct identifiers after the implementation of the stricter Health Insurance Portability and Affordability Act (HIPAA).
We collected the following variables: gender, age group at repair (neonates: <28 days of age, infants 28 days to <1 year, children 1 year to <21 years of age), era of repair by tertile (1982 to 1993, 1994 to 1998, and 1999 to 2003), details of cardiac diagnosis, surgical staging (primary vs staged repair), surgery type [Right ventricle to Pulmonary artery (RV-PA) conduit, transannular patch (TAP), as well as performance of pulmonary artery plication or reduction], and presence of genetic syndrome. Type of surgical repair were assigned by the respective PCCC surgical codes and manual review of the operative notes were performed.
Death or transplant events were ascertained from the PCCC for events occurring during hospitalization to a PCCC center and by matching identifiers with the NDI and OPTN records through December 31, 2019. Records submitted to the NDI and OPTN included first name, middle initial (when available), surname, date of birth, gender, state of last known residence and state of birth. Underlying or contributing (multiple) causes of death (COD) were obtained from the NDI-Plus as reported on the death certificate using International Classification of Disease-9 or 10 (ICD-9 or 10) codes to assign deaths into categories as previously reported.19,20
Descriptive statistics were presented as counts with percentages for categorical variables and median with inter-quartile range (IQR) for continuous variables with skewed distributions. Comparisons between groups of interest were performed using Fisher’s exact test for categorical variables, t-test for continuous variables and Kruskal Wallis 1-way analysis of variance for comparison between 3 groups. Kaplan-Meier curves were created and log-rank tests were used to compare the long-term transplant-free survival after surgery for TOF-APV versus simple TOF. Hazard ratios (HRs) for transplant or death with 95% confidence intervals (CI) and p-values were computed using Cox’s regression models. Adjusted hazard ratios (aHR) were calculated taking into account potential risk factors such as age, surgical era, gender and presence of genetic condition. The proportionality of hazards was checked for each covariate. p-value less than 0.05 was considered statistically significant. Analyses was performed using SAS Version 9.4 (Cary, NC).
Results
The PCCC query for patients with TOF-APV revealed 143 patients that met inclusion criteria, of which, 130 met the diagnostic criteria. Among them, 22% patients had an underlying genetic condition, most commonly DiGeorge syndrome (DGS) which accounted for 20% of the whole cohort. Primary repair was pursued in 119 patients, while 11 underwent a staged approach with a shunt including 4 patients that died before having a complete repair (Figure 1). Eight of the 11 patients with shunt palliation were neonates, including the 4 patients with in-hospital death. There was a near equal gender distribution within the cohort. During the same era, a total of 2,643 patients underwent surgical repair for simple TOF with (TOF-VS) or without pulmonary valve sparing (TOF-NVS) procedures and met similar inclusion/exclusion criteria21.
Figure 1.
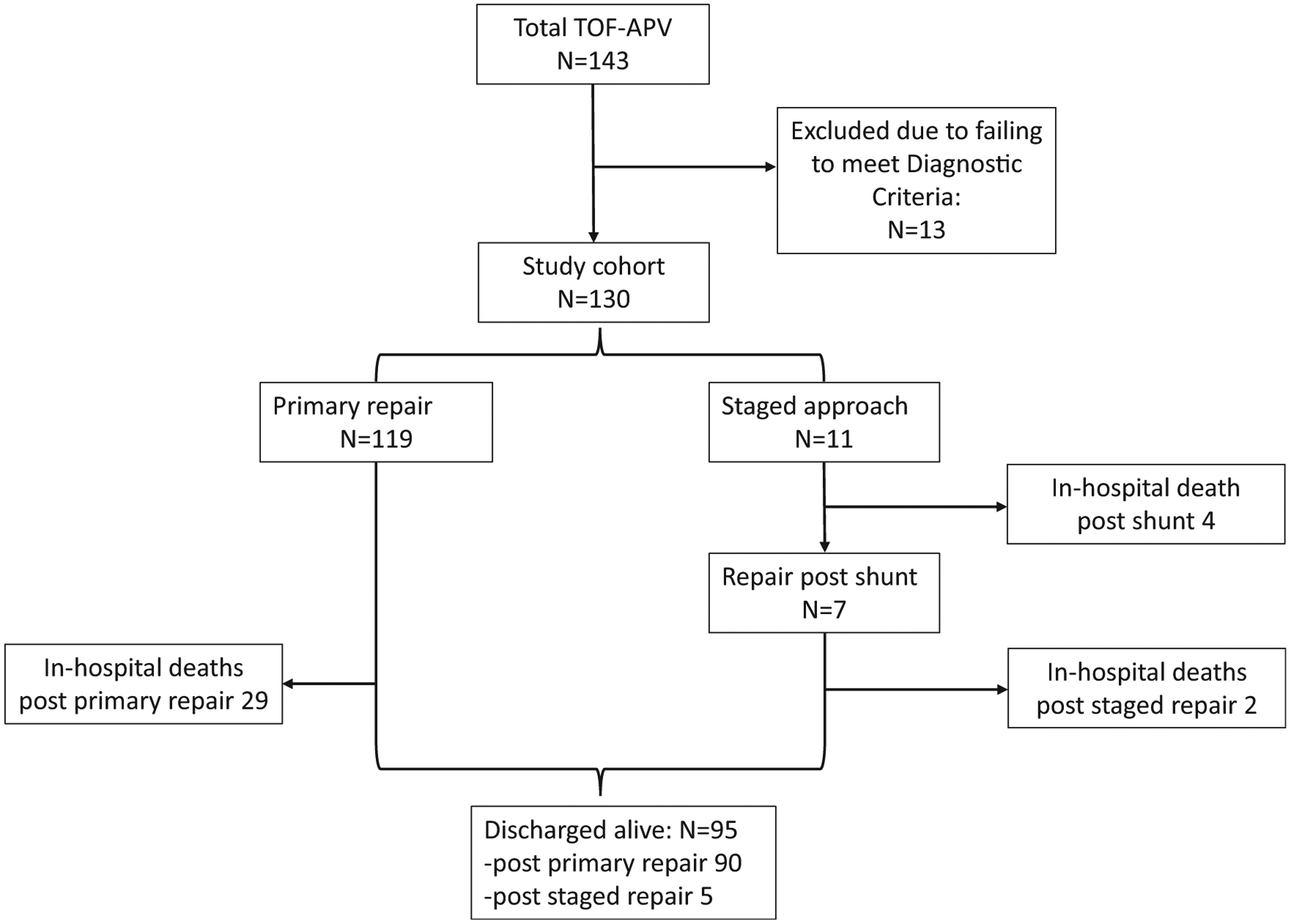
Flowchart of PCCC cohort with Tetralogy of Fallot − Absent Pulmonary Valve (TOF-APV).
Complete repair among TOF-APV in PCCC was achieved in 126 patients (97%), of which, details about the type of surgical repair were available in 123 (Table 1). Of them 43 (35%) underwent TAP, while the rest had a RV-PA conduit; 76% underwent pulmonary artery plication or reduction. Over the study period, there was no change in the age of repair (p = 0.415) or the distribution between the 2 types of surgical repair (p = 0.697). Among the patients with TOF-APV repair, in-hospital death occurred in 31 and was associated with age group (p <0.001), and surgery type (p <0.01). There were a larger percentage of neonates and those that underwent RV-PA conduit placement among the in-hospital deaths. The overall in-hospital mortality for complete repair was 25%, ranging from 50% in neonates to 15% in infants and 14% in children operated at >1 year of age. This overall hospital mortality was higher compared to patients with simple TOF repair (5%, p <0.001). In-hospital mortality for complete repair dropped from the 30% and 33% in the earlier eras to 13% after 1999.
Table 1.
Summary of patient characteristics of patient undergoing repair for TOF-APV in PCCC.
| Overall (n=126) | Alive at discharge (n=95) | In-hospital mortality (n=31) | p-value | |
|---|---|---|---|---|
| Median age at repair in months (IQR) | 3.3 (0.7–6.4) | 4.3 (1.3–7.0) | 0.7 (0.1–2.6) | 0.011 |
| Age group at full repair | <0.001 | |||
| Neonate (<28 days) | 36 (29%) | 18 (19%) | 18 (58%) | |
| Infant (<1 year) | 76 (60%) | 65 (68%) | 11 (35%) | |
| ≥ 1 year | 14 (11%) | 12 (13%) | 2 (6%) | |
| Gender | 0.836 | |||
| Male | 61 (48%) | 45 (47%) | 16 (52%) | |
| Female | 65 (52%) | 50 (53%) | 15 (48%) | |
| Median weight at repair in kilograms (IQR) | 4.80 (3.30–6.70) | 5.51 (3.79–6.97) | 3.4 (2.70–4.10) | 0.001 |
| Weight <2.5 kilograms | 6 (5%) | 3 (3%) | 3 (10%) | 0.152 |
| Staged strategy | 1.000 | |||
| No | 119 (94%) | 90 (95%) | 29 (94%) | |
| Yes | 7 (6%) | 5 (5%) | 2 (6%) | |
| Pulmonary Plication/Plasty | 0.630 | |||
| Yes | 96 (76%) | 71 (75%) | 25 (81%) | |
| No | 30 (24%) | 24 (25%) | 6 (19%) | |
| Surgery Type | 0.004 | |||
| RV-PA conduit | 80 (63%) | 54 (57%) | 26 (84%) | |
| Transannular patch | 43 (34%) | 39 (41%) | 4 (13%) | |
| Othera | 3 (2%) | 2 (2%) | 1 (3%) | |
| Era | 0.052 | |||
| 1982–1993 | 33 (26%) | 23 (24%) | 10 (32%) | |
| 1994–1998 | 46 (37%) | 31 (33%) | 15 (48%) | |
| 1999–2003 | 47 (37%) | 41 (43%) | 6 (19%) | |
| Genetic condition | 0.139 | |||
| No | 98 (78%) | 77 (81%) | 21 (68%) | |
| DGS | 25 (20%) | 17 (18%) | 8 (26%) | 0.436 |
| Other genetic condition | 3 (2%) | 1 (1%) | 2 (6%) | 0.150 |
One patient underwent pulmonary artery translocation, 1 patient underwent excision of nodular tissue and for 1 patient no operative report was available.
A total of 95 TOF-APV patients were discharged alive after complete repair; among them, 5 deaths and 2 heart transplants (one of them subsequently died) occurred over 19 years of median follow up (IQR 0.37–23.7 years) for a 25-year transplant-free survival of 92% (95% CI: 83 to 96). During the study period a total of 64 reinterventions occurred in 47 unique patients, most of them for RV-PA conduit replacement (50%) or pulmonary valve placement (19%), while in the simple TOF cohort 817 reinterventions occurred in 385 unique patients (pulmonary valve replacements: 19%, RV-PA conduit replacement: 11%). The freedom from reintervention was 51% for those discharged after their complete repair, which is higher when compared to the contemporary cohort with simple TOF repair that were discharged after complete repair (385 out of 2,643 or 86%, p <0.001).
The 25-year transplant-free survival among those that underwent complete repair with TAP was not significantly different than those that underwent RV-PA conduit (90% vs 93% respectively, log-rank p = 0.954). Two of the deaths were attributed to the congenital heart disease (CHD) itself and one to cerebrovascular disease, while 2 were reported to be due to an infection or the underlying genetic syndrome. After considering the multiple causes of death information and all the events that occurred in this cohort, 5 deaths were attributed directly or indirectly to the underlying CHD, 2 deaths were attributed directly or indirectly to the presence of an infectious process (of which one of these events occurred in a patient with DGS) and 3 events (including the 2 transplants) were attributed to congestive heart failure. Deaths after complete repair occurred from 8 months of age to 22.5 years of age.
Survival comparison of the TOF-APV cohort with the simple TOF-NVS subgroup did not reveal significant difference (94%, log-rank p = 0.455), but was lower than the survival experience in the simple TOF-VS subgroup (97%, log rank p = 0.014) (Figure 2 and Table 2). Multivariable analysis with adjustment for other covariates (age, gender, surgical era and genetic condition) potentially affecting the risk for earlier mortality or transplant did not change this conclusion. More specifically, the adjusted hazard ratio (aHR) of TOF-APV versus simple TOF-NVS for death or transplant within the study period was 1.37 (95% CI: 0.63 to 2.97, p = 0.432), while the TOF-APV versus simple TOF-VS aHR was 2.62 (95% CI: 1.14 to 6.02, p = 0.024). The number of events is too small for a meaningful analysis of those covariates as risk factors for death or transplant.
Figure 2.
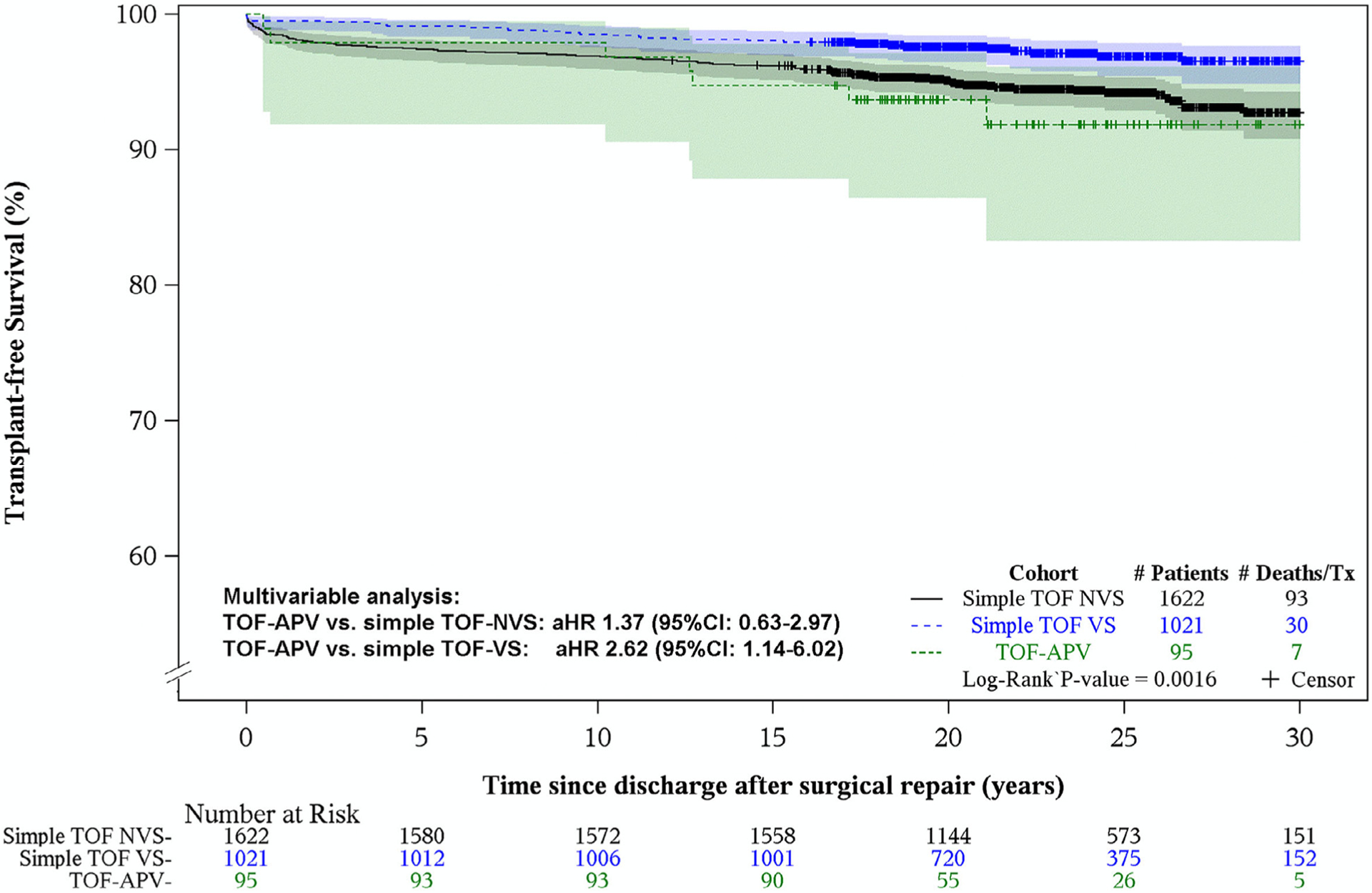
Kaplan-Meier transplant-free survival plots of PCCC patients discharged alive after repair for TOF-APV and simple TOF with valve-sparing (VS) and non-valve sparing (NVS) procedures. Shaded areas display the 95% confidence intervals. TOF-APV versus Simple TOF-NVS, Adjusted HR (95% CI): 1.37 (0.63 to 2.97), p = 0.432. TOF-APV versus Simple TOF-VS, Adjusted HR (95% CI): 2.62 (1.14 to 6.02), p = 0.024.
Table 2.
Survival table of PCCC patients discharged alive after repair for TOF-APV and simple TOF with valve-sparing (VS) and non-valve sparing (NVS) procedures
| Survival estimates (years) | Simple TOF-NVS n=1,622 | Simple TOF-VS n=1,021 | TOF-APV n=95 |
|---|---|---|---|
| 1 | 98.5 (97.7–99.0%) | 99.5 (98.8–99.8%) | 97.9 (91.8–99.5%) |
| 5 | 97.4 (96.5–98.1%) | 99.1 (98.3–99.5%) | 97.9 (91.8–99.5%) |
| 15 | 96.2 (95.1–97.0%) | 98.0 (97.0–98.7%) | 94.7 (87.8–97.8%) |
| 25 | 94.2 (92.9–95.3%) | 96.9 (95.4–97.9%) | 91.8 (83.3–96.1%) |
Discussion
In this large multi-center cohort of patients operated between 1982 and 2003, we found high in-hospital mortality especially among neonates who needed a staged approach, which suggests that they may have a more severe form of the disease requiring palliation prior to complete repair. Conditioning survival to discharge, however, reveals a 25-year post repair survival for patients with TOF-APV of 92%, which is lower than the survival of a contemporary cohort of simple TOF (97%), but not much different when compared to the more relevant subgroup of the simple TOF with non-valve sparing procedures (94%). These findings suggest that the additional morbidity associated with TOF-APV (such as airway complications), although important for the immediate postoperative survival, does not increase the long-term risk beyond what is expected in cases of simple TOF without a functional pulmonary valve.21
Our study reflects outcomes over a 20-year period (1982 to 2003) from multiple institutions within the US, during which many changes occurred in the management of patients with CHD. In this dataset there was no significant change in the surgical approach or the age of repair over the study period. Therefore, the drop of in-hospital survival after 1999 most likely reflects improvement in immediate perioperative care and better understanding of disease pathology rather than changes in surgical approach.5,12,22,23 In terms of long term mortality, the previously published studies in the US and elsewhere8,22 report an almost 80% survival over a follow up period of 10 to 15 years. Our study extends the follow up experience with this lesion by 10 to 15 years over the longest available follow up time. Ongoing advances in medical care will continue to improve the outcome of these patients particularly during the immediate postoperative period, but the observed postrepair survival in this cohort is very reassuring for the patients with TOF-APV repair despite the challenges of the immediate postoperative care. Efforts for the timely restoration of the pulmonary valve function in this group will probably be equally beneficial for their long-term survival as in patients with simple TOF.
Limitations of the study include its retrospective and registry-based nature, which limits the information available for analysis. Even though, to our knowledge this is the largest reported cohort of TOF-APV and with the longest period of follow up, the number of events is too small for meaningful analysis of potential risk factors affecting long-term outcomes.
In conclusion, patients with TOF-APV who survive initial surgical procedure have good long-term outcomes that are similar to simple TOF with non-sparing pulmonary valve. Ongoing monitoring will be required to assess the effects of aging superimposed on a background of operated TOF-APV.
Acknowledgment
The authors thank the program directors and data collection coordinators from the participating PCCC centers.
This study was supported in part by grants from the National Institute of Health’s National Heart, Lung, Blood Institute Bethesda, Maryland (R01 HL122392) and National Center for Advancing Translational Sciences Bethesda, Maryland (UL1TR002494) as well as the Department of Defense Detrick, Maryland (PR180683).
Footnotes
Disclosure
The authors have no financial relationships relevant to this article to disclose.
References
- 1.Nagao GI, Daoud GI, McAdams AJ, Schwartz DC, Kaplan S. Cardio-vascular anomalies associated with tetralogy of fallot. Am J Cardiol 1967;20:206–215. 10.1016/0002-9149(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 2.Lev M, Eckner FA. The pathologic anatomy of tetralogy of fallot and its variations. Dis Chest 1964;45:251–261. 10.1378/chest.45.3.251. [DOI] [PubMed] [Google Scholar]
- 3.Chevers N Recherches sur les maladies del’ art[notdef]ere pulmonaire. Arch Gen Med 1847;15:488–508. [Google Scholar]
- 4.Rabinovitch M, Rabinovitch S, David I, Van Praagh R, Sauer U, Buhlmeyer K, Castaneda AR, Reid L. Compression of intrapulmonary bronchi by abnormally branching pulmonary arteries associated with absent pulmonary valves. Am J Cardiol 1982;50:804–813. 10.1016/0002-9149(82)91238-3. [DOI] [PubMed] [Google Scholar]
- 5.Yong MS, Yim D, Brizard CP, Robertson T, Bullock A, d’Udekem Y, Konstantinov IE. Long-term outcomes of patients with absent pulmonary valve syndrome: 38 years of experience. Ann Thorac Surg 2014;97:1671–1677. 10.1016/j.athoracsur.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Dorobantu DM, Stoicescu C, Tulloh RM, Stoica SC. Surgical repair of tetralogy of fallot with absent pulmonary valve: favorable long-term results. Semin Thorac Cardiovasc Surg 2019;31:847–849. 10.1053/j.semtcvs.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Hu R, Zhang H, Xu Z, Liu J, Su Z, Ding W. Late outcomes for the surgical management of absent pulmonary valve syndrome in infants. Interact Cardiovasc Thorac Surg 2013;16:792–796. 10.1093/icvts/ivt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nørgaard MA, Alphonso N, Newcomb AE, Brizard CP, Cochrane AD. Absent pulmonary valve syndrome: surgical and clinical outcome with long-term follow-up. Eur J Cardio-thoracic Surg 2006;29:682–687. 10.1016/j.ejcts.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Talwar S, Divya A, Choudhary SK, Gupta SK, Ramakriahnan S, Kothari SS, Juneja R, Saxena A, and Airan B. Mid-term results of correction of tetralogy of fallot with absent pulmonary valve. Indian Heart J 2017;69:767–771. 10.1016/j.ihj.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. Surgical treatment of absent pulmonary valve syndrome associated with bronchial obstruction. Ann Thorac Surg 2006;82:2221–2226. 10.1016/j.athoracsur.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Godart F, Houyel L, Lacour-Gayet F, Serraf A, Sousa-Uva M, Bruniaux J, Petit J, Piot JD, Binet JP, Conte S, Planche C. Absent pulmonary valve syndrome: surgical treatment and considerations. Ann Thorac Surg 1996;62:136–142. 10.1016/0003-4975(96)00276-7. [DOI] [PubMed] [Google Scholar]
- 12.McDonnell BE, Raff GW, Gaynor JW, Rychick J, Godinez RI, DeCampli WM, Spray TL. Outcome after repair of Tetralogy of Fallot with absent pulmonary valve. Ann Thorac Surg 1999;67:1391–1395. 10.1016/S0003-4975(99)00250-7. [DOI] [PubMed] [Google Scholar]
- 13.Watterson KG, Malm TK, Karl TR, Mee RBB. Absent pulmonary valve syndrome: operation in infants with airway obstruction. Ann Thorac Surg 1992;54:1116–1119. 10.1016/0003-4975(92)90078-I. [DOI] [PubMed] [Google Scholar]
- 14.Snir E, de Leval MR, Elliott MJ, Stark J. Current surgical technique to repair fallot’s tetralogy with absent pulmonary valve syndrome. Ann Thorac Surg 1991;51:979–982. 10.1016/0003-4975(91)91023-O. [DOI] [PubMed] [Google Scholar]
- 15.Moller JH, Borbas C. The pediatric cardiac care consortium: a physician-managed clinical review program. Qual Rev Bull 1990;16:310–316. [DOI] [PubMed] [Google Scholar]
- 16.Moller JH, Hills CB, Pyles LA. A multi-center cardiac registry: a method to assess outcome of catheterization intervention or surgery. Prog Pediatr Cardiol 2005;20:7–12. [Google Scholar]
- 17.Vinocur JM, Moller JH, Kochilas LK. Putting the pediatric cardiac care consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes 2012;5:577–579. 10.1161/CIRCOUTCOMES.111.964841. [DOI] [PubMed] [Google Scholar]
- 18.Spector LG, Menk JS, Vinocur JM, Oster ME, Harvey BA, Louis JS, Moller JH, Kochilas LK. In-hospital vital status and heart transplants after intervention for congenital heart disease in the pediatric cardiac care consortium: completeness of ascertainment using the national death index and united network for organ sharing datasets. J Am Heart Assoc 2016;5:1–12. 10.1161/JAHA.116.003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spector LG, Menk JS, Knight JH, MCCracken C, Thomas AS, Vincour JM, Oster ME, Louis JS, Moller JH, Kochilas LK. Trends in long-term mortality after congenital heart surgery. J Am Coll Cardiol 2018;71:2434–2446. 10.1016/j.jacc.2018.03.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCracken C, Spector LG, Menk JS, Knight JH, Vincour JM, Thomas AS, Oster ME, Louis JS, Moller JH, Kochilas LK. Mortality following pediatric congenital heart surgery: an analysis of the causes of death derived from the national death index. J Am Heart Assoc 2018;7. 10.1161/JAHA.118.010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CA, McCracken C, Thomas AS, Spector LG, Louis JS, Oster ME, Moller JH, Kochilas LK. Long-term outcomes of Tetralogy of Fallot: a study from the pediatric cardiac care consortium. JAMA Cardiol 2019;4:34–41. 10.1001/jamacardio.2018.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hew CC, Daebritz SH, Zurakowski D, del Nido PI, Mayer JE, Jonas RA. Valved homograft replacement of aneurysmal pulmonary arteries for severely symptomatic absent pulmonary valve syndrome. Ann Thorac Surg 2002;73:1778–1785. 10.1016/S0003-4975(02)03511-7. [DOI] [PubMed] [Google Scholar]
- 23.Alsoufi B, Williams WG, Hua Z, Cai S, Karamlou T, Chan CC, Coles JG, Van Arsdell GS, Caldarone CA. Surgical outcomes in the treatment of patients with Tetralogy of Fallot and absent pulmonary valve. Eur J Cardio-thoracic Surg 2007;31:354–359. 10.1016/j.ejcts.2006.12.001. [DOI] [PubMed] [Google Scholar]


