Abstract
Antimicrobial resistance (AMR) is a global crisis for human public health which threatens the effective prevention and control of ever-increasing infectious diseases. The advent of pandrug-resistant bacteria makes most, if not all, available antibiotics invalid. Meanwhile, the pipeline of novel antibiotics development stagnates, which prompts scientists and pharmacists to develop unconventional antimicrobials. Bacteriophage-derived endolysins are cell wall hydrolases which could hydrolyze the peptidoglycan layer from within and outside of bacterial pathogens. With high specificity, rapid action, high efficiency, and low risk of resistance development, endolysins are believed to be among the best alternative therapeutic agents to treat multidrug resistant (MDR) bacteria. As of now, endolysins have been applied to diverse aspects. In this review, we comprehensively introduce the structures and activities of endolysins and summarize the latest application progress of recombinant endolysins in the fields of medical treatment, pathogen diagnosis, food safety, and agriculture.
Keywords: antimicrobial resistance, endolysin, biofilm, food safety, pathogen detection
1. Introduction
Humans have used antibiotics for more than half a century to counter infectious diseases caused by pathogenic bacteria. Overuse and misuse of antibiotics have contributed to a rise in the number of antibiotic-resistant strains, including multidrug-resistant (MDR), extensively drug-resistant (XDR), and even pandrug-resistant (PDR) strains. The inherent heredity and physiology transmitted vertically within species, and the tendency of bacteria to exchange various genes horizontally between species and genera, have been recommended as possible causes of resistance to antibiotics [1,2,3]. Due to the occurrence of incurable infections, the number of medical procedure failures is expected to increase in the near future. As well, antibiotic-resistant strains are also responsible for the increased cost of livestock breeding, food industry, and agriculture. In this “post-antibiotic era”, it is imperative to search for new therapeutic approaches in the battle against bacterial infections.
Bacteriophages (phages) are the natural enemies of bacteria, which were first used as therapeutic agents in humans in 1919, just a few years after they were discovered [4]. Phages have a range of potential benefits compared to antibiotics. The major advantage of phages is their specificity for target bacteria, which significantly reduces the damage to the host’s normal flora. The phages are self-limiting, i.e., they need their hosts for continuous growth. If the specific hosts are not available, they will not last long [5]. However, the human immune system eliminates incoming phages, posing an obstacle to their use as a therapeutic agent [6,7]. Especially, the strong antibody response of in vivo phage therapy causes phages to be cleared more quickly, making long-term use of phages impossible [5]. Another disadvantage of phages is their narrow host range, making it difficult to look for suitably paired phages for a given bacterial pathogen. At last, it is very important to ensure that phage preparations are free of bacteria and bacterial toxins during the preparation of phage stocks, which increases the production cost and technical challenge. Thus, rather than administering the whole virions, one choice is to use the phage lytic enzyme, endolysin [8,9,10].
Endolysins are hydrolases produced by phages which function in vivo to lyse bacterial cell walls and release progeny phages at the end of a replication cycle [11]. These enzymes are remarkably efficient in hydrolyzing the peptidoglycan layer, resulting in a sudden drop in turgor pressure and osmotic lysis to cause bacterial cell death [12]. Endolysins are considered as a promising class of antibiotics derived from enzymes known as “enzybiotics”. The major advantage of endolysins over conventional broad-spectrum antibiotics is their high specificity. Endolysins exhibit specific bactericidal activity and do not kill the beneficial microbiota [13,14]. Through molecular engineering, the lytic spectrum of an endolysin could be changed [15]. Meanwhile, endolysins have other advantages, such as rapid bacterial cell lysis, a low risk of resistance, synergistic activity with different antibacterial agents, and the ability to effectively function in biofilms and on mucosal surfaces [16,17,18,19,20]. Due to these unique properties, endolysins are highly ranked alternatives in eradicating drug-resistant pathogens [13,21,22,23]. In the last decade, recombinant endolysins have been applied in many fields to combat MDR bacteria [13,20,24,25]. This review aims to introduce the diverse protein architectures of different endolysins and comprehensively summarize the progress of endolysin application in medical practice, pathogen diagnosis, food safety, and agriculture. Moreover, the commercialization of endolysins from lab to market is discussed.
2. Architecture of Endolysins
Phage endolysins are analogous to bacterial lysins in structure and function, which are closely linked to the small family of mammalian peptidoglycan recognition proteins [26]. The endolysin architectures are different between Gram-positive and Gram-negative phages. As for Gram-positive phages, most endolysins are composed of two domains, an N-terminal enzymatic activity domain (EAD) and a C-terminal cell wall binding domain (CBD), which are connected by a short flexible linker [27]. EAD contributes to the cleavage of different bonds in peptidoglycan, while CBD recognizes and binds specifically to the receptor of bacterial cell walls [28]. Besides this architecture, some endolysins contain unusual structures. For example, staphylococcal endolysin, λSA2, has a central CBD and two flanking EADs [29]. Recently, a novel Bacillus cereus endolysin, LysPBC2, was confirmed to have an extra spore binding domain (SBD), besides EAD and CBD, which specifically binds B. cereus spores but not to its vegetative cells [30]. Streptococcus dysgalactiae phage endolysin PlySK1249 was composed of an EAD, a central CBD, and a C-terminal CHAP domain, which is a cysteine, histidine-dependent amidohydrolases/peptidase. Interestingly, the CHAP domain of PlySK1249 was a nonbacteriolytic endopeptidase, which acted as a dechaining enzyme and exhibited a synergistic effect with the lytic amidase domain for peptidoglycan digestion and bacteriolysis [31]. Remarkably, the modular structure of Gram-positive phage endolysins facilitates protein engineering to modify bacteriolytic activity, specificity, solubility, and other physicochemical properties of endolysins [32]. For example, the full-length or EAD of LysB4, an endolysin from the B. cereus phage B4, was fused to LysSA11, an endolysin from S. aureus phage SA11, to form a hybrid endolysin and simultaneously control Staphylococcus aureus and B. cereus [33]. S. aureus phage Twort endolysin (PlyTW) was composed of three domains, including a CHAP domain, an amidase-2 domain, and a CBD. Deletion of the amidase-2 domain formed a novel shorter endolysin with stronger activity [34].
In contrast, most endolysins of Gram-negative phages are small single-domain globular proteins with molecular mass between 15 and 20 kDa, usually without a specific CBD [14]. Exceptionally, a few Gram-negative endolysins have a modular structure, such as Pseudomonas endolysin KZ144, P. fluorescens phage OBP endolysin OBPgp279, and Burkholderia phage AP3 endolysin AP3gp15 [17,35,36]. The modular endolysins with a Gram-negative background display a unique property with an N-terminal CBD and a C-terminal EAD [36]. Interestingly, CBDs from these endolysins demonstrate a broad binding spectrum, which is different from Gram-positive endolysins [28].
Most endolysins are the product of a single gene. However, one of the endolysins against Streptococcus spp., PlyC, is encoded by plyCA and plyCB. PlyC is a multimeric endolysin from the streptococcal C1 phage, comprising of two components. PlyCA is essential for enzymatic activity and PlyCB is able to direct streptococcal cell-wall-specific binding [37]. X-ray crystal diffraction revealed that the PlyC structure consists of an EAD (PlyCA) and eight copies of CBDs (PlyCB) [38]. Additionally, a few multimeric endolysins encoded by a single gene have been detected, such as CD27L from Clostridium difficile phage, CTP1L from C. tyrobutyricum phage [39,40], and Lys170 from enterococcal phage F170/08 [41]. The full-length endolysin and its CBD fragment are expressed in phage F170/08, respectively, and they interact to form the fully active endolysin. The CBD part is produced from an in-frame, alternative translation start site of the same gene [41].
3. Antimicrobial Activity of Endolysins
Depending on the mode of action, EADs are categorized into three groups: (a) glycosidases, cleaving the glycan portion of peptidoglycan (MurNAc-GlcNAc); (b) amidases, cleaving the amide bond between the glycan moiety (MurNAc) and the peptide moiety (L-alanine); and (c) endopeptidase, cleaving the peptide bond between two amino acids of the stem peptide [25]. It is worthy to note that CHAP is a special case, as this type is not classified based on which bond in peptidoglycan they cleave, but on their catalytic mechanism [42]. CHAP domains have an invariant cysteine and histidine residue in their active site for substrate cleavage [43]. The enzymatic activity of endolysins is influenced by the composition of cell walls. As for Gram-positive bacteria, the cell wall is comprised of an internal cytoplasmic cell membrane and a thick peptidoglycan layer [44]. When applied exogenously, endolysins readily access the peptidoglycan layer and hydrolyze the basic bonds of peptidoglycan, resulting in osmotic lysis and cell death [45]. However, the cell wall of Gram-negative bacteria comprises an internal cytoplasmic cell membrane, a peptidoglycan layer, and an outer membrane with a lipopolysaccharide layer [45], which inhibits access to the peptidoglycan layer [46].
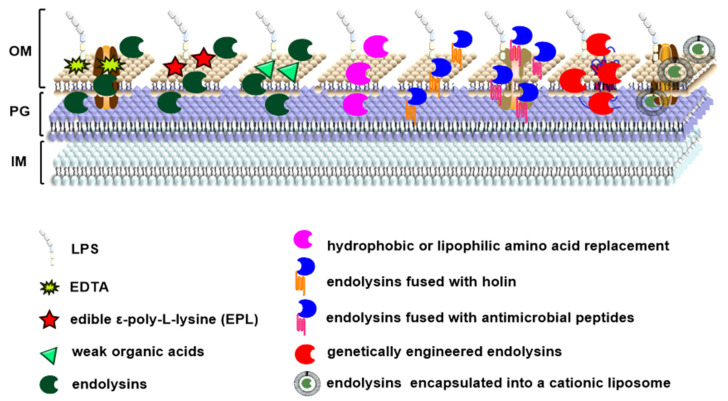
There are a few strategies to overcome the disadvantages of endolysins from Gram-negative phages (Figure 1). At first, EDTA is often used to permeabilize the outer membrane. Endolysin LysSP1 from Salmonella phage SLMP1 displayed a very broad lytic spectrum against Gram-negative and Gram-positive bacteria with the help of 5 mM EDTA [47]. Similarly, the lytic activity of the endolysin LysPN09 from P. syringae pv. actinidiae phage PN09 was improved at the presence of 1 mM EDTA [48]. Secondly, the edible ε-poly-L-lysine (EPL) can be used as an outer-membrane permeabilizer for some endolysins targeting food-borne pathogens. Salmonella endolysin LyS15S6 lysed Salmonella and three species of Enterobacteriaceae with EPL at a very low concentration [49]. Thirdly, endolysins exhibit increased and broader antibacterial activity in the presence of weak organic acids. Acinetobacter baumannii phage endolysin ABgp46 rapidly killed A. baumannii, P. aeruginosa, and Salmonella enterica serovar Typhimurium strains with the help of 3.65 mM citric acid and 4.55 mM malic acid [50]. Fourthly, amino acid replacement in endolysin improves its antimicrobial activity. After 3–12 hydrophobic amino acids were successfully added to the C-terminus of E. coli phage endolysin Lysep3, the modified Lysep3 was able to kill E. coli from outside of the cells [51]. Fifthly, genetic engineering of endolysins has been applied to make innolysins and artilysins, which can pass through the outer membrane barrier and increase the lytic activity [11,52]. Recently, to treat Helicobacter pylori infection, holin and a section of polypeptides were fused to the endolysin of H. pylori to create a novel artilysin [53]. Holin is a transmembrane protein which punches holes in the cell membrane. Usually, polycations and hydrophobic polypeptides were chosen which enable penetration of the outer membrane [53]. Sixthly, endolysins are directly fused with antimicrobial peptides. The coliphage LysECD7 was fused with the N-terminus sheep myeloid peptide (SMAP) to form LysECD7-SAMP which extended the antimicrobial spectrum and enhanced the activity. This modified endolysin can be not only used for topical treatment but also for systemic applications in the bloodstream and tissues [54]. At last, endolysins can be delivered by encapsulation into a cationic liposome. For example, Salmonella phage endolysin BSP16Lys was encapsulated by a liposome comprised of dipalmitoylphosphatidylcholine, cholesterol, and hexadecylamine, which inhibited the growth of S. Typhimurium and E. coli [55].
Figure 1.
Different strategies of overcoming the barrier of Gram-negative bacterial outer membrane in the endolysin application.
To our interest, some Gram-negative endolysins possess the ability to lyse the bacteria even in the absence of membrane permeabilizer. PD-6A3 was a novel phage of A. baumannii, which could not only inhibit A. baumannii but also E. coli and methicillin-resistant S. aureus [56]. Similarly, another A. baumannii phage endolysin, LysAB54, showed high antibacterial activity against multiple Gram-negative pathogens [57]. Ts2631, an endolysin from extremophilic Thermus scotoductus phage, was able to lyse not only its host, but also T. thermophilus, A. baumannii, P. aeruginosa, and C. freundii without membrane permeabilizer [58]. Comparative study of the structures of these Gram-negative endolysins with or without the capacity of lysing bacteria on their own is needed to reveal this interesting phenomenon.
The novel endolysins found in the PubMed database since 2019 are listed in Table 1 and Table 2.
Table 1.
Bacteriophage endolysins of Gram-positive bacteria since 2019.
| No. | Endolysin Name | Original Phage | Targeted Pathogens | Effective Concentration | Features of Endolysin | Reference |
|---|---|---|---|---|---|---|
| 1 | LysMR-5 | S. aureus phage | S. aureus, S. epidermidis | 500 μg/mL | encapsulation in alginate-chitosan nanoparticles | [59] |
| 2 | LysRODI | S. aureus phage | S. aureus | 20 µg/mL | encapsulation in pH-sensitive liposomes, and effective at pH 5 | [60] |
| 3 | XZ.700 | S. aureus phage | S. aureus | 250 µg/mL | chimeric endolysin and effective against S. aureus biofilms | [61] |
| 4 | LysSAP26 | S. aureus phage SAP-26 | A. baumannii, E. coli, K. pneumoniae, P. aeruginosa, S. aureus, E. faecium | 5–80 µg/mL | there was 40% protection rate in A. baumannii-infected mouse model | [62] |
| 5 | Lys84 | S. aureus phage qdsa002 | S. aureus | 10 μM | effective against biofilms | [63] |
| 6 | LysSAP33 | S. aureus phage SAP33 | S. aureus | / | higher activity against biofilms than LysK-like endolysin | [64] |
| 7 | S25-3 | S. aureus kayvirus S25-3 | S. aureus | / | genus-specific against staphylococci, particularly S. aureus | [65] |
| 8 | SAL200 | S. aureus phage | S. aureus | / | effective against severe pneumonia caused by S. aureus in a lethal murine model | [66] |
| 9 | LysCSA13 | S. aureus phage | S. aureus | 300 nM | effective against staphylococcal biofilms on various food contact surfaces | [67] |
| 10 | Lys109 | S. aureus phage | S. aureus | 100 nM | chimeric endolysin | [68] |
| 11 | LysP108 | S. aureus phage | S. aureus | / | / | [69] |
| 12 | ClyC | S. aureus phage | S. aureus | / | chimeric endolysin | [70] |
| 13 | HY-133 | S. aureus phage | S. aureus | 0.12–0.5 μg/mL | chimeric endolysin | [71] |
| 14 | LysSA11 | staphylococcal phage SA11 | S. aureus | / | expressed and surface-displayed in Saccharomyces cerevisiae | [72] |
| 15 | Ph28 | S. epidermidis phage PH15 | S. epidermidis | / | / | [73] |
| 16 | MSlys | S. pneumoniae phage MS1 | S. pneumoniae | 2 μM | / | [74] |
| 17 | LyJH307 | Streptococcus bovis phage | S. bovis, E. faecalis, S. sanguinis | 50 µg/mL | highest efficacy at pH 5.5 at 39 °C | [75] |
| 18 | LyJH307 | S. bovis | S. bovis | / | as a specific modulator for rumen | [76] |
| 19 | PlyC | streptococcal C1 phage | group A, C, and E streptococci | / | recognition of Streptococcus Group A carbohydrate backbone | [77] |
| 20 | LytSD | S. avermitilis phage phiSASD1 | S. avermitilis, B. subtilis, S. aureus, S. lutea, E. faecalis | 10 μg/mL | / | [78] |
| 21 | lys46 | B. subtilis phage | K. pneumoniae, S. Typhimurium, Proteus, E. coli | / | / | [79] |
| 22 | Ply57 | broad-host-range temperate phage, Izhevsk | B. cereus group | 1 μM | thermostability at 55 °C | [80] |
| 23 | LysPBC5 | B. cereus phage PBC5 | B. cereus | / | / | [81] |
| 24 | PlyB | B. anthracis phage vB_BanS_Bcp1 | B. cereus sensu lato group species | 16 µg/mL | potent bacteriolytic activity against all B. cereus sensu lato isolates | [82] |
| 25 | LysB4EAD-LysSA11 | B. cereus phage B4 + S. aureus phage SA11 | S. aureus, B. cereus | 3.0 µM | a hybrid endolysin | [33] |
| 26 | LysPBC2 | B. cereus phage PBC2 | Bacillus, Listeria, Clostridium | / | harboring a B. cereus spore binding domain | [30] |
| 27 | CTP1L | C. tyrobutyricum phage ΦCTP1 | C. tyrobutyricum | / | the endolysin encoding gene was introduced into the nisin producer Lactococcus lactis subsp. lactis INIA 415 | [83] |
| 28 | LysCPAS15 | C. perfringens phage CPAS-15 | C. perfringens | 45 µg/mL | C. perfringens-specific, used for pathogen detection | [84] |
| 29 | CWH | C. difficile phage phiMMP01 | C. difficile | / | cell wall binding domain prevents C. difficile spore outgrowth | [85] |
| 30 | Psa | C. perfringens phage st13 | C. perfringens | / | an amidase endolysin that specifically lyses C. perfringens | [86] |
| 31 | LysIME-EF1 | E. faecalis phage | E. faecalis | / | a novel two-component endolysin encoded by a single gene | [87] |
| 32 | ORF28 endolysin | E. faecalis phage ϕEf11 | E. faecalis | 15–31 μg/mL | multifunctional lytic enzyme, effective against E. faecalis biofilm | [88] |
| 33 | Lys08 | E. faecalis phage PHB08 | E. faecalis | 0.5–1 µg/mL | effective against E. faecalis biofilms | [89] |
| 34 | EG-LYS | E. faecalis phage | E. faecalis | 0.1 mg/mL | specific to E. faecalis | [90] |
| 35 | PBEF129 endolysin | E. faecalis phage PBEF129 | E. faecalis | 4.8 µM | effective against biofilm | [91] |
| 36 | PM-477 | Gardnerella prophage | Gardnerella | 0.13–8 µg/mL | no effect on beneficial lactobacilli or other species of vaginal bacteria | [92] |
| 37 | LysKB317 | Lactobacillus phage EcoSau | Acetobacter, Lactobacillus, Pediococcus, Streptococcus, Weissella | 0.01–1 µM | broad activity and stability from pH 4.5–7.5 up to at least 48 h; maximum activity is observed at 50 °C up to at least 72 h | [93] |
| 38 | 293 endolysin | L. monocytogenes phage vB_LmoS_293 | L. monocytogenes 473 and 3099, a serotype 4b and serogroup 1/2b-3b-7 | / | amidase | [94] |
| 39 | LysA | mycobacteriophage D29 | M. smegmatis | / | separation of M. smegmatis from a mixed culture via the cell wall binding domain | [95] |
| 40 | LysP11 | Propionibacterium phage P1.1 | E. rhusiopathiae | / | binding specifically to the E. rhusiopathiae cell wall | [96] |
| 41 | PlyPl23 | P. larvae phage phiIBB_Pl23 | P. larvae | / | first highly specific CBD targeting exclusively P. larvae cells | [97] |
Note: “/” indicates data inaccessible.
Table 2.
Bacteriophage endolysins of Gram-negative bacteria since 2019.
| No. | Endolysin Name | Original Phage | Targeted Pathogens | Effective Concentration | Features of Endolysin | Reference |
|---|---|---|---|---|---|---|
| 1 | LysSS | S. enterica serovar Enteritidis phage SS3e | Salmonella, E. coli, P. aeruginosa, A. baumannii, K. pneumoniae, S. aureus | 0.063–0.25 mg/mL | / | [98] |
| 2 | BSP16Lys | Salmonella phage | S. Typhimurium, E. coli | / | encapsulation into a cationic liposome | [55] |
| 3 | LysSE24 | Salmonella phage LPSE1 | S. enteritidis | 0.1 μM | very stable with different pH (4.0 to 10.0) at different temperatures (20 to 60 °C) | [99] |
| 4 | M4Lys | S. enterica serovar Typhimurium phage BSPM4 | S. enterica, E. coli O157:H7, P. aeruginosa | 1 mM | the lysis function was not dependent on either holin or the Sec pathway in vitro | [100] |
| 5 | LysSP1 | S. Typhimurium phage SLMP1 | S. Typhimurium | 50 μg/mL | the optimal activity was at 40 °C and was efficiently active at alkaline condition | [47] |
| 6 | LysSTG2 | Salmonella phage STG2 | Salmonella, E. coli, P. aeruginosa | 100 μg/mL | effective on S. Typhimurium biofilm | [101] |
| 7 | LyS15S6 | Salmonella-virus-FelixO1 phage BPS15S6 | 3 species of Enterobacteriaceae, Salmonella | 2 μM | edible ε-poly-L-lysine (EPL) can be used as an outer-membrane permeabilizer | [49] |
| 8 | LysECP26 | rV5-like phage | E. coli O157:H7, Salmonella spp. | 1 µg/mL | stable at 4–55 °C | [102] |
| 9 | Lysep3 | E. coli phage | E. coli | 1750 µg/mL | activity was enhanced by modification with hydrophobic amino acids | [51] |
| 10 | LysO78 | E. coli APEC O78 phage vB_EcoM_APEC | Klebsiella, Salmonella, Shigella, Burkholderia, Yersinia, Pseudomonas, C. arctica, E. coli, R. solanacearum, A. baumannii | / | the endolysin worked with the help of 50 mM EDTA as membrane permeabilizer | [103] |
| 11 | LysECD7 | coliphage | K. pneumoniae, Pseudomonas, Acinetobacter | 3000 µg/mL | effective against forming and mature biofilm | [104] |
| 12 | LysECD7-SMAP | coliphage | K. pneumoniae, Pseudomonas, Acinetobacter | 0.5 µg/mL | the endolysin was fused to either the N- or the C-terminus of membrane-destabilizing peptides | [54] |
| 13 | Ply6A3 | A. baumannii phage PD-6A3 | A. baumannii, E. coli, S. aureus | 1 mg/mL | effective in the mouse sepsis model | [56] |
| 14 | Abtn-4 | A. baumannii phage vB_AbaP_D2 | S. aureus, P. aeruginosa, K. pneumoniae, Enterococcus, Salmonella | 5 µM | / | [105] |
| 15 | LysAB54 | A. baumannii phage p54 | A. baumannii, P. aeruginosa, K. pneumoniae, E. coli | 100 μg/mL | / | [106] |
| 16 | LysPN09 | P. syringae pv. actinidiae phage PN09 | P. syringae pv. actinidiae | 12.5–400 µg/mL | only effective against the outer-membrane-permeabilized Psa strains | [48] |
| 17 | RL_Hlys | P. aeruginosa phage RL | P. aeruginosa, K. pneumoniae, Salmonella, methicillin resistant S. aureus | / | holin was fused at the N terminus of the endolysin | [107] |
| 18 | Lysqdvp001 | V. parahaemolyticus phage | V. parahaemolyticus | ≥60 U/mL | synergistic effects with ε-poly-lysine | [108] |
| 19 | artilysin | H. pylori phage KHP30 | H. pylori | 1000 µg/mL | there was a genetic linkage between an endolysin enzyme and a holin enzyme with a section of polypeptides | [53] |
| 20 | LysHP1 | H. influenzae phage HP1 | H. influenzae, E. coli | / | endolysin expression and release was regulated by signal-arrest-release (SAR) | [109] |
| 21 | Ts2631 | T. scotoductus Bacteriophage vB_Tsc2631 | the whole Enterobacteriaceae family | 1.23 µM | extremely broad antimicrobial activity, especially with EDTA | [58] |
Note: “/” indicates data inaccessible.
4. Anti-Biofilm Activity of Endolysins
Biofilms are a major concern in food and clinical setting. They form in critical areas and cause contamination to threaten the effectiveness of the existing procedures during the food process [110]. Furthermore, antibiotic-resistant bacteria housed within the biofilm network lead to treatment failure in surgeries and chronic wounds [111]. Therefore, the development of novel anti-biofilm techniques has become essential in order to provide additional control strategies.
Some anti-biofilm agents have been found, such as phages [112], metal oxide nanoparticles [113,114], and photosensitizers [115,116]. The most obvious disadvantage of phage and antibiotic therapy is that resistant bacteria are readily produced [117]. Although metal oxide nanoparticles exhibit miscellaneous functions, such as antibacterial agents, biosensors in drug and delivery formulations, and cancer therapy, some in vitro and in vivo studies have demonstrated that nanoparticles exposure can provoke oxidative stress, inflammatory responses, myocardial infarction, and thrombosis. Therefore, the cellular and molecular toxicology of nanoparticles should be investigated before use [113,118]. Photosensitizers have poor selectivity towards pathogens and also lead to the occurrence of resistant bacteria. In addition, the short excitation light wave of photosensitizers has poor penetrating power [116,119,120]. By contrast, endolysins do not produce resistant bacteria due to the importance and conservation of their targets, peptidoglycan layer, for the viability of bacteria and they also obtain access to pathogens in inner tissues and organs in vivo [112]. Therefore, endolysins are promising anti-biofilm agents that are capable of eliminating biofilms.
One special biofilm issue with extensive attention is foreign body-associated infection (FBAI), which is due to bacterial adherence to and colonization of the surfaces of foreign body materials, such as medical devices and implants [121]. Along with more and more implanted medical devices used to improve life quality, the risk of FBAI increases hugely. Additionally, FBAI is difficult to treat since these bacteria embedded in a biofilm are less susceptible to both antibiotics and host defense mechanisms [122]. To develop new anti-biofilm approaches and substances, Fursov et al. determined the efficacy of a broad-range recombinant endolysin LysECD7 against forming and mature biofilms caused by K. pneumoniae Ts 141-14 clinical isolate using the implantable diffusion chamber approach. They found that LysECD7 significantly reduced the biofilm formation and was capable of degrading the preformed biofilm in vitro [104]. Recently, a comparative study was conducted to determine the efficacies of endolysin HY-133, daptomycin, and rifampin against S. aureus biofilm on the vascular graft surface and found that daptomycin exhibited the strongest bactericidal effect, while HY-133 showed a moderate effect and rifampicin was not effective as an antimicrobial for this biofilm. If considering the risk of resistance, endolysin would be the most favorable antimicrobial agent in this setting [123].
Meanwhile, various staphylococcal endolysins and their derivative proteins are effective at removing biofilms from S. aureus and S. epidermidis. For example, the staphylococcal endolysins SAP-2 and Phi11 eliminate biofilms formed on polystyrene surfaces [124,125], while the endolysin LysH5 has staphylococcal biofilm-removal properties, with no resistant cells after treatment [126]. Similarly, safranin staining, cell reduction, and scanning electron microscopy have also revealed the effective bacterial removal activities of SAL200 endolysin [127]. PlyGRCS, a staphylococcal endolysin with a single EAD that destroys MRSA, disturbs biofilms as well [128]. The endolysin Lys84 with two catalytic domains (CHAP and amidase_2) and a CBD (SH3b) effectively removed around 90 % of the biofilms of S. aureus, and CHAP and Amidase_2 domains remained 61.20 and 59.46 % of lytic activity as well as 84.31 and 70.11 % of the anti-biofilm activity of Lys84, respectively [63]. CHAPk, a truncated LysK endolysin with only the N-terminal endopeptidase domain, can eliminate S. aureus biofilms on surfaces [129]. Meng et al. investigated the effect of LySMP, a manufactured phage lysin, on S. suis biofilms, both alone and in combination with antibiotics and phages. They reported that LySMP alone could remove >80% of the biofilm, compared to just 20% removal when the biofilm was treated with phages alone and/or antibiotics. Consistent with this, the findings showed that LySMP could treat synergistically S. suis biofilm and inactivate the released cells in a concentration-independent manner [130]. A well-known chimeric endolysin ClyR with a concentration of 50 µg/mL was found to remarkably reduce the number of viable cells in 72-h aged S. mutans and S. sobrinus biofilms after treatment for 5 min. Furthermore, continuous administration of ClyR for 40 days obviously decreased the severity of caries in the rat model infected with a single or a mixed bacteria of S. mutans and S. sobrinus [131]. The engineered phage endolysin LysRODIΔAmi prevented biofilm formation at low protein concentrations of 0.15–0.6 μM in S. aureus and had no toxicity toward human keratinocytes, even at high concentrations of 22.1 μM [132].
In addition, endolysins may be more suitable for biofilm eradication than planktonic cells. The combination of chimeric lysins Cpl-711 and PL3 showed an increased synergistic effect on the removal of biofilms compared to planktonic cells in Streptococcus pneumoniae. The synergy of Cpl-711 and PL3 was also observed in an adult zebrafish model of pneumococcal infection [133]. In other research, the amidase domain of the L. monocytogenes phage vB_LmoS_293 endolysin prevented biofilm formation on abiotic surfaces [94], while the Salmonella endolysin Lys68 in combination with malic or citric acid decreased biofilms [134]. The endolysins used for the biofilms formed by P. aeruginosa also have been developed. The endolysin LysPA26 resulted in a 1~2 log reduction in biofilm-associated P. aeruginosa on a polystyrene plate within 2 h without the use of outer membrane permeabilizers [135]. These findings indicate that endolysins are promising anti-biofilm agents. Nevertheless, the endolysin biofilm-removal abilities should be studied under more accurate conditions, such as flow cell-based models, multispecies biofilm matrixes, and surface coatings or substrates used in food processing facilities [136,137,138,139,140].
5. Endolysin Application for Pathogen Detection
The ability to identify pathogens quickly and effectively is critical for disease treatment and prevention. The majority of research has focused on foodborne bacterial detection through phage proteins. In the food industry, the most common pathogens are Gram-positive L. monocytogenes, S. aureus, and C. perfringens, and Gram-negative Salmonella and E. coli. They cause serious economic losses, foodborne diseases, and death [141,142]. Hence, the food industry requires specific and sensitive diagnostic tools to detect microbial contamination accurately and quickly. Furthermore, the approaches must be both cost-effective and convenient to use [143].
Current methods mostly rely on culturing on particular media, or PCR, antibody-based detection, and they are time-consuming and labor-intensive [144]. In addition, the failure of PCR-based methods to distinguish between live and dead cells is a significant drawback. This is important for food diagnostics as PCR will produce positive results even when pathogens have been inactivated, such as through heat treatment. Furthermore, the complex matrix of food can disturb PCR-based detection. Some advanced approaches have been developed for pathogen detection. By utilizing a host recognition protein, H-SA-BP-1, from S. aureus phage phiSLT, Idelevich et al. developed a phage-based latex agglutination to detect S. aureus, with a specificity of 92.1% and a positive predictive value of 89.6% [145]. A method for rapid detection of bacterial pathogens in blood was also achieved by engineered phages-beads and integrated real-time PCR into MicroChip [146]. Ohlsson et al. integrated acoustic separation, enrichment, and Microchip PCR for detection of bacteria in blood [147].
Interestingly, the use of CBDs for pathogen detection has obvious advantages, such as good maneuverability, lower probability of cross-reactivity, high stability and sensitivity, and rapid detection due to fast target binding [148]. Kretzer et al. proposed a process to recognize L. monocytogenes cells on magnetic beads coated with CBDs from various endolysins of Listeria phages in 2007, achieving detection rates of >90%, which is better than standard plating technique in terms of both period and sensitivity [143]. Later, Schmelcher and colleagues used this technique to collect Listeria cells from inoculated cheese and milk, and they were capable of discriminating serotypes after incubation with CBDs bound with various fluorescent proteins [149]. PlyV12 CBD-functionalized magnetic beads (CBD-MBs) were prepared and used to detect S. aureus cells with a detection limit as low as 78 CFU/mL in PBS with less than 50 min, and other bacteria associated common food-borne and nosocomial infections negligibly interfered with this detection, except for S. epidermidis [150]. The enhanced green fluorescent protein-fused CBD protein (EGFP-LysCPAS15_CBD1) is able to be used to detect C. perfringens within 5 min [84]. Endolysin-based methods for detecting B. anthracis have also been developed. Fujinami et al. established a bio-probe to identify B. anthracis based on a membrane direct blot assay using the C-terminal region of γ-phage lysin protein (PlyG), which turned out to be simpler and less costly than other genetic tools such as PCR or immunological methods using unique antibodies [151]. Later, Sainathrao et al. showed that a 10-amino-acid motif from the C-terminal region of PlyG, combined with fluorescent Qdot-nanocrystals, is enough to detect B. anthracis [152]. Another research group produced an electrochemical impedance sensor by immobilizing the CBD of the endolysin Ply500 from L. monocytogenes on a gold sensor surface. In both buffer and artificially contaminated milk, the detection of Listeria cells by electrochemical impedance spectroscopy is fast, with detection limits of 104 and 105 CFU/mL, respectively [153]. To detect S. aureus, Yu and colleagues developed a CBD-based magnetic enrichment immunoassay [154]. S. aureus was captured using immunomagnetic particles (IMPs) coated with IgG antibodies that bind staphylococcal protein A. As such the bacterial cells were concentrated and matrix interference during identification was removed. The second ‘antibody’ in the setup was the biotinylated fusion protein T-CBD of the red fluorescent protein tdTomato and a specific S. aureus phage CBD, PlyV12C. Eventually, streptavidin-linked horseradish peroxidase was used to improve the detection sensitivity. In contaminated milk, this setup resulted in a detection limit of 4x103 CFU/ml in 1.5 h [154]. Kong et al. used a surface plasmon resonance (SPR)-chip to incorporate a CBD unique for B. cereus phage LysPBC1, which resulted in a detection limit of 102 CFU/mL by using a subtractive inhibition assay [155]. A significant disadvantage of both approaches is that they need advanced and costly equipment [142]. The development of a nitrocellulose-based lateral flow assay (LFA) for the detection of B. cereus using the CBD of endolysin LysB4 from B. cereus phage B4 was able to avoid this. Briefly, the nitrocellulose membrane is first dipped in a B. cereus-containing solution. The bacterial cells adhere to the CBDs that have been immobilized at the membrane test line. In the second step, the membrane is dipped in a solution containing gold nanoparticles, which allows for visualization. The cysteine-glutathione-S-transferase-tagged CBDs (Cys-GSTCBD- AuNP) on these nanoparticles will create a red test line, enabling bacteria to be observed. To summarize, the authors created a CBD-based biosensor that is simple, sensitive, fast, and cost-effective [156]. Furthermore, an exquisite sensitivity was able to be achieved by combining CBD with qPCR, and the bacteria with a limit as low as 2 CFU/mL was detected in the approach [157].
6. Endolysin Application in Food Safety
Contamination of foodborne pathogens is a major problem in the food industry. S. aureus, Salmonella spp., E. coli, L. monocytogenes, and Clostridium spp. contamination can endanger human health, causing financial losses during food processing [46]. It is widely acknowledged that new approaches for reducing pathogenic bacteria in foods are urgently needed. Hence, endolysins have been suggested as a possible alternative biocontrol agent; further, endolysins have already been used to avoid pathogen contamination in food systems [158,159,160,161,162]. For example, endolysin PlyV12 has demonstrated a very high lytic activity against both antibiotic-resistant E. faecalis and E. faecium [163]. Surprisingly, L. monocytogenes can infect plant-based milk. Studies revealed that when LysZ5 is administered to soya milk, it has an excellent sterilization ability [164]. In addition, in the presence of hydrostatic pressure, various alternative phage endolysins such as PlyP825, PlyP40, and Ply511 have successfully treated the pathogen L. monocytogenes [165]. In human and animal medicine, S. aureus has been identified as a pathogen. Meanwhile, this bacterium is also accountable for food and milk contamination during the manufacturing process [166]. Chang et al. recently confirmed that the presence of a CBD in the staphylococcal endolysin, LysSA11, possesses a key role in specificity and antimicrobial activity as compared to endolysin LysSA97, which showed only moderate activity against S. aureus [158].
Salmonella is the most common cause of bacterial food poisoning in the United States and many other countries [167]. Salmonella disease outbreaks have been found to be related to several foods, including red meats, poultry, fruits, and vegetables [168]. Moreover, several Salmonella phage-derived recombinant endolysins have been reported [134,169]. Most of these endolysins have a broad lytic spectrum, particularly when cell membrane-permeabilizing chemicals are jointly used. Lim and colleagues expressed Salmonella phage SPN1S endolysin, and it displayed the lytic activity against both E. coli and S. Typhimurium in a buffer with EDTA to destabilize the cell membranes. Moreover, some activity was also observed for Shigella, Salmonella, Pseudomonas, Cronobacter, and Vibrio species [170]. In another study, a Salmonella phage endolysin Gp110 has a modular structure with an uncharacterized domain of unknown function (DUF3380; pfam11860) in its C terminus, which showed a remarkably high lytic activity against Salmonella and other Gram-negative bacteria [169]. Subsequent experimental data have examined the efficacy of endolysins against B. cereus as antimicrobials or preservatives for use in the food industry [171,172]. B. cereus, a Gram-positive spore-forming bacterium, is responsible for developing both an emetic and a diarrheal toxin which can cause food poisoning [171]. Loessner and colleagues isolated and characterized three different endolysins such as PlyBa, Ply12, and Ply21 from B. cereus phage Bastille, TP21, and 12826, respectively. Their effectiveness against Gram-negative and Gram-positive bacteria were measured and all three endolysins were found to be effective against 24 strains of B. cereus, as well as many other strains of B. thuringiensis [173].
Clostridial species are associated with food spoilage in addition to causing diseases in poultry. Germinated C. sporogenes and C. tyrobutyricum contribute to the development of gases and acids in the dairy industry, which alters the structural and sensory qualities of cheeses [174]. Mayer and friends isolated an N-acetylmuramoyl-L-alanine amidase, CS74, from C. sporogenes and documented that the purified protein completely lysed the C. sporogenes cells when applied exogenously. The researchers also showed that CS74L was active against C. acetobutylicum and C. tyrobutyricum using the turbidity assay and fresh bacterial cells, making it a potential cheese bio preservative [175]. Another endolysin isolated from a virulent phage CPT11 was also characterized by the same research group, but this enzyme had a more restricted host range [174]. An endolysin from C. perfringens, LysCPAS15, inhibited host cells by up to a 3-log reduction of 2 h [84].
7. Endolysin Application in Agriculture
Phytopathogenic bacteria cause many food security problems worldwide [176]. Antibiotics use in agriculture is very controversial because it is unclear how much it contributes to the development of antibiotic resistance in human pathogens [177]. Preferably, an alternative strategy to control phytopathogenic bacteria would be established if its impact is minimal. As a result, endolysins have been suggested as a way to protect plants from bacterial diseases [178].
A large number of crops require treatment due to pathogenic infections. Expression of endolysins from phages Atu_ph02 and Atu_ph03 triggered lysis of C58-derived Agrobacterium tumefaciens, a Gram-negative soil-borne bacterium [21]. The endolysin PN09 (LysPN09) from P. syringae pv. actinidiae phage PN09 showed lytic activity against Psa strains. When LysPN09 was coupled with EDTA, Psa strains were effectively damaged, indicating that LysPN09 is a potential candidate for biocontrol of Psa in the kiwifruit industry [48]. The production of transgenic crops that express endolysins to provide defense against pathogenic bacteria is one proposed strategy. After bacterial pectinases break down the plant cells, endolysins accumulate in the tissue and contact with bacteria to inactive them. Transgenic tomato plants with CMP1 phage endolysins were successfully developed two decades ago to prevent infection of Clavibacter michiganensis, a bacteria that causes canker [179]. Düring et al. demonstrated the potential of this strategy by growing T4 lysozyme-expressing transgenic potatoes [180]. These genetically modified plants showed resistance to Pectobacterium carotovora (formerly Erwinia carotovora) species, which cause soft rot [181].
A previous study found that by using transgenic plants to design an endolysin-based protection mechanism, it is possible to resolve the antibiotic resistance problem. Apis (honey bees) are effective crop pollinators, but they are often infected by Paenibacillus larvae, which triggers sepsis and death [166]. Endolysin PlyV12 has high lytic activity against antibiotic-resistant E. faecalis and E. faecium, which may help to control the emergence of resistance [163]. The bacterium Xanthomonas oryzae pv. oryzae leads to leaf blight in rice [182] and several antibiotic-resistant strains have been isolated [183]. Lee et al. reported Lys411 from the phage FXo411, which had high lytic activity against Xanthomonas [184]. It also showed activity against Stenotrophomonas maltophilia, a multidrug-resistant bacterium [184], which is becoming more clinically important in the context of nosocomial infections and immunocompromised patients [185]. However, no follow-up research into Lys411 has been released, and thus the enzyme’s potential for medical or agricultural applications remains unknown. The etiologic agent of crown gall disease in a variety of orchard and vineyard crops is A. tumefaciens [186]. Because of its severity and extensive impact, it has been the subject of numerous studies [187]. At present it is generally believed that the lytic protein exhibited intriguing properties, including the ability to not only lyse the cell quickly but also prevent cell division, ensuring potent antimicrobial activity [21]. Therefore, the enzyme is a possible candidate for A. tumefaciens biocontrol. However, the mechanisms of implementation must be investigated before a feasible crop protection strategy is established.
Plants may also be used as bioreactors, not for their safety but for the low-cost, large-scale manufacturing of antimicrobial proteins for human and veterinary medicine. In the chloroplasts of tobacco plants, Oey and colleagues produced the S. pneumoniae phage endolysins Cpl-1 and Pal, as well as the group B streptococcal lysin PlyGBS, and the chloroplast-produced protein efficiently inactivated the target bacteria [188,189]. The endolysins LysP11 from Erysipelothrix rhusiopathiae produced in Nicotiana benthamiana using an Agrobacterium-mediated transient expression strategy showed strong antimicrobial activity toward E. rhusiopathiae [96]. Similarly, an endolysin-derived triple fusion protein produced in N. benthamiana showed growth inhibition against S. aureus 305 and Newman [190]. A plant-produced endolysin CP933 was found to inhibit 18% growth of Gram-positive plant pathogenic bacterium Clavibacter michiganensis [191]. The practicalities of applying these endolysins on a global scale for individual phytobacteria can be a major challenge, leading to the current lack of knowledge on the use of endolysins for plant bacterial diseases. However, given the high societal cost of plant bacterial diseases when existing treatments fail, the endolysin research should be prioritized.
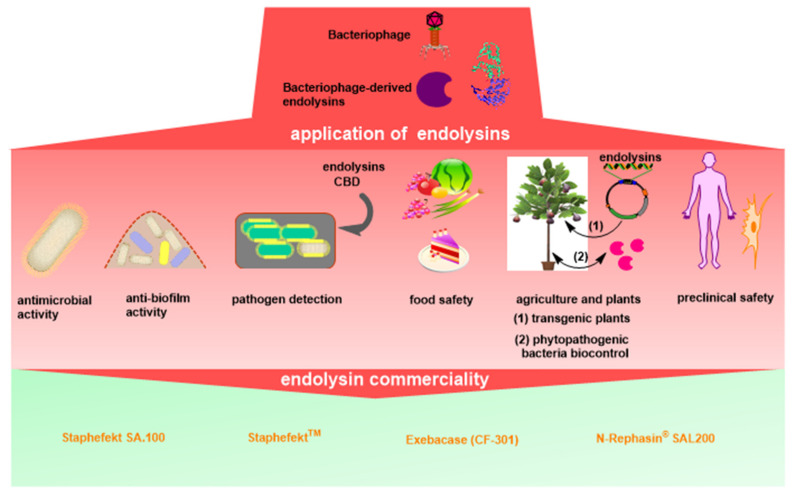
The different applications of endolysins are summarized in detail in Figure 2.
Figure 2.
Graphical summary of phage endolysin applications as promising antimicrobial agents.
8. Immunogenicity, Toxicity and Safety of Endolysins
Phages are natural components of the human microbiota; therefore, releasing phage-derived endolysins is unlikely to have a detrimental impact on human health [192]. To date, in a variety of animal model systems, the efficacy of phage endolysins has been demonstrated [18,193]. During the preclinical development of protein-based therapeutics such as endolysins, important issues such as safety, toxicity, and immunogenicity must be addressed. Immune responses to foreign proteins, such as the development of anti-drug antibodies, may alter pharmacokinetics, reduce therapeutic effectiveness, and even cause life-threatening complications including hypersensitivity reactions and anaphylaxis.
Immune responses to well-characterized endolysins such as CF-301 and SAL200 have been identified in a variety of species [166,194]. In phase 1 clinical trial, the protein SAL200 was tested in humans via intravenous infusion. SAL200 is the first MRSA therapeutic formulation based on endolysin. It emerges from the Staphylococcus phage SAP-1, which infects staphylococci such as MRSA and vancomycin-resistant S. aureus [195]. Healthy male volunteers were given single ascending intravenous doses (0.1 to 10 mg/kg) to test the pharmacokinetics, pharmacodynamics, and tolerance of SAL200 [195]. Volunteers encountered no significant side effects or infection recurrence, with the exception of fatigue, headaches, and myalgia, which were reported by more than three participants. In animal models, the effect of lytic proteins on inflammatory responses or toxicity was investigated, and it was revealed that the administration of certain lysin proteins, such as Cpl-1 and MV-L, caused an immune response that resulted in the development of antibodies against these proteins [196,197]. In another study, endolysin treatment resulted in lower levels of antibodies or cytokine formation in animals compared to untreated controls [198,199].
Despite the vast number of published animal trials, only a few endolysins have been tested in humans. Safety analyses with the pneumococcal endolysins Cpl-1 and Pal were performed and it was noticed that IgG levels in mice exposed to these enzymes elevated while IgE levels remained low, implying a low risk of hypersensitivity or allergic reactions. Consequently, no adverse health effects, increased pro-inflammatory cytokine concentrations, or complement activation were observed in mice, confirming that these endolysins have favorable safety and toxicity profiles [199].
With the increasing number of protein therapeutics on the market, studies are increasingly focusing on reducing their immunogenicity. The recognition and deletion of T cell epitopes, which can be performed using both experimental and computational methods, is one promising approach in this area. Endolysins may be amenable by similar strategies in future.
9. Commerciality of Endolysins
Staphefekt SA.100, an endolysin-based product developed by a Dutch biotech company Micreos, has been available for human use in Europe since 2017. This product is a topical chimeric endolysin that binds to the cell wall of S. aureus and cleaves the cell wall using endopeptidase and putative amidase activities [200]. StaphefektTM improved the clinical symptoms of three human subjects with chronic and recurrent S. aureus-related dermatoses in a case study, but they quickly recurred if the therapy was stopped. During chronic and repeated StaphefektTM therapy, it was also shown that long-term regular use of Staphefekt did not result in the development of bacterial resistance [201]. The application StaphefektTM on the skin, which targets only S. aureus while leaving skin commensals unharmed, improved S. aureus-related skin infections including eczema, acne, and rosacea, according to a multicenter, placebo-controlled, double-blinded, and randomized superiority trial study (ClinicalTrials.gov, NCT02840955) [202]. StaphefektTM is an over-the-counter medication available in the form of a cream or gel that is licensed as a (class 1) medical product in Europe. Several other therapies are in various stages of clinical trials, with some showing promise and paving the way for future endolysin-based therapies [203]. For example, safety and efficacy evaluation of N-Rephasin®SAL200 for a single intravenous dose (3 mg/kg) in addition to the conventional standard treatment for the treatment of persistent S. aureus bacteremia in patients has been completed in 2019 (phase IIa, NCT03089697). The safety analysis was conducted based on the data of all adverse events, physical examinations, clinical laboratory tests, and vital signs (blood pressure, pulse rate, body temperature, and respiratory rate) collected from the subjects. All subjects who enrolled in this study were defined as the safety set and included in the analysis (https://clinicaltrials.gov/ct2/show/results/NCT03089697?term=SAL-200&draw=2&rank=2) (accessed on 9 October 2021). Another study to evaluate safety, pharmacokinetics, pharmacodynamics, and immunogenicity of N-Rephasin® SAL200 in healthy male volunteers is ongoing (NCT03446053, https://clinicaltrials.gov/ct2/show/results/NCT03446053?term=SAL-200&draw=2&rank=3, accessed on 9 October 2021). Similarly, safety, efficacy, and pharmacokinetics investigation of exebacase (CF-301) for treatment of S. aureus bacteremia, including right-sided endocarditis, is ongoing (phase Ⅲ, NCT04160468). The quality control ranges of exebacase were determined as 0.25 to 2 μg/mL and 8 to 64 μg/mL against S. aureus ATCC 29213 and E. faecalis ATCC 29212, respectively, and were approved by the Clinical and Laboratory Standards Institute (CLSI) [204].
10. Conclusions and Perspectives
Since penicillin was discovered, antibiotics have profoundly changed human society and saved lives from deadly bacterial infections. However, the emergence of AMR makes these conventional chemotherapies pale and weak. Phage endolysins are promising weapons against this great challenge of AMR because they exhibit potent and rapid bactericidal and anti-biofilm activity, low induced resistance and cell toxicity, and synergy with regular antibiotics. Remarkably, their narrow antimicrobial spectrums make precise killing possible, no disturbing of the beneficial microbiota. In the meantime, phages are the most abundant and diverse biological entities on the planet. Metagenome studies reveal more and more phages and their endolysin sequences, which is a huge resource of novel endolysins. These sequences also facilitate the combination and assembly of modular domains of chimeric endolysins. In addition, the development of nanomaterial technology and membrane permeabilizer will provide a better delivery strategy for endolysins. Accordingly, endolysins are a new dawn and hope in the dark era of AMR.
Author Contributions
Writing—original draft preparation, M.u.R. and W.W.; reviewing and editing, Q.S., J.A.S., Y.S., Y.L., C.L. and B.Z.; supervision, W.C. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (31770152, 81701973, and 32170114), the General Program of Jiangsu Provincial Health Commission (M2020019), and National Key R&D Program of China (2021YFC1808902).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hadizadeh M., Norouzi A., Taghadosi R., Mohebi S., Mohammadi M., Hasanzade A., Moghadam M.T. Prevalence of qnr, intI, and intII genes in extendedspectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from clinical samples in Iran. Trop. J. Pharm. Res. 2017;16:141–147. doi: 10.4314/tjpr.v16i1.18. [DOI] [Google Scholar]
- 2.Yang Y.-S., Wei W., Hu X.-X., Tang S., Pang J., You X.-F., Fan T.-Y., Wang Y.-X., Song D.-Q. Evolution and antibacterial evaluation of 8-hydroxy-cycloberberine derivatives as a novel family of antibacterial agents against MRSA. Molecules. 2019;24:984. doi: 10.3390/molecules24050984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers W. Felix d1Herelle and the Origins of Molecular Biology. Yale University Press; New Haven, CT, USA: 1999. Bacteriophage discovered; pp. 47–59. [Google Scholar]
- 5.Clark J.R., March J.B. Bacteriophages and biotechnology: Vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006;24:212–218. doi: 10.1016/j.tibtech.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Dabrowska K., Switała-Jelen K., Opolski A., Weber-Dabrowska B., Gorski A. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 2005;98:7–13. doi: 10.1111/j.1365-2672.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark J.R., March J.B. Bacterial viruses as human vaccines? Expert Rev. Vaccines. 2004;3:463–476. doi: 10.1586/14760584.3.4.463. [DOI] [PubMed] [Google Scholar]
- 8.López R., García E., García P. Enzymes for anti-infective therapy: Phage lysins. Drug Discov. Today Ther. Strateg. 2004;1:469–474. doi: 10.1016/j.ddstr.2004.09.002. [DOI] [Google Scholar]
- 9.Fischetti V.A. Bacteriophage lytic enzymes: Novel anti-infectives. Trends Microbiol. 2005;13:491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Borysowski J., Weber-Dąbrowska B., Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 11.Briers Y., Walmagh M., Van Puyenbroeck V., Cornelissen A., Cenens W., Aertsen A., Oliveira H., Azeredo J., Verween G., Pirnay J. Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio. 2014;5:e01379-14. doi: 10.1128/mBio.01379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollmer W., Bertsche U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta Biomembr. 2008;1778:1714–1734. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Nelson D., Loomis L., Fischetti V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA. 2001;98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelcher M., Donovan D.M., Loessner M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani H.H., Schmelcher M., Sabzalipoor H., Hosseini E.S., Moniri R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018;31:e00071-17. doi: 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López R., García E., García P., García J.L. The pneumococcal cell wall degrading enzymes: A modular design to create new lysins? Microb. Drug Resist. 1997;3:199–211. doi: 10.1089/mdr.1997.3.199. [DOI] [PubMed] [Google Scholar]
- 17.Briers Y., Schmelcher M., Loessner M.J., Hendrix J., Engelborghs Y., Volckaert G., Lavigne R. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem. Biophys. Res. Commun. 2009;383:187–191. doi: 10.1016/j.bbrc.2009.03.161. [DOI] [PubMed] [Google Scholar]
- 18.Yang H., Linden S.B., Wang J., Yu J., Nelson D.C., Wei H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domenech M., García E., Moscoso M. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob. Agents Chemother. 2011;55:4144–4148. doi: 10.1128/AAC.00492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuch R., Nelson D., Fischetti V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 21.Attai H., Rimbey J., Smith G.P., Brown P.J. Expression of a peptidoglycan hydrolase from lytic bacteriophages Atu_ph02 and Atu_ph03 triggers lysis of Agrobacterium tumefaciens. Appl. Environ. Microbiol. 2017;83:e01498-17. doi: 10.1128/AEM.01498-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santamaría R.I., Bustos P., Sepúlveda-Robles O., Lozano L., Rodríguez C., Fernández J.L., Juárez S., Kameyama L., Guarneros G., Dávila G., et al. Narrow-host-range bacteriophages that infect Rhizobium etli associate with distinct genomic types. App. Environ. Microbiol. 2014;80:446–454. doi: 10.1128/AEM.02256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’flaherty S., Coffey A., Meaney W., Fitzgerald G., Ross R.P. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005;187:7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelcher M., Powell A.M., Becker S.C., Camp M.J., Donovan D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012;78:2297–2305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelrahman F., Easwaran M., Daramola O.I., Ragab S., Lynch S., Oduselu T.J., Khan F.M., Ayobami A., Adnan F., Torrents E., et al. Phage-Encoded Endolysins. Antibiotics. 2021;10:124. doi: 10.3390/antibiotics10020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low L.Y., Yang C., Perego M., Osterman A., Liddington R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011;286:34391–34403. doi: 10.1074/jbc.M111.244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira H., Melo L.D., Santos S.B., Nóbrega F.L., Ferreira E.C., Cerca N., Azeredo J., Kluskens L.D. Molecular aspects and comparative genomics of bacteriophage endolysins. J. Virol. 2013;87:4558–4570. doi: 10.1128/JVI.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira H., Azeredo J., Lavigne R., Kluskens L.D. Bacteriophage endolysins as a response to emerging foodborne pathogens. Trends Food Sci. Technol. 2012;28:103–115. doi: 10.1016/j.tifs.2012.06.016. [DOI] [Google Scholar]
- 29.García P., García J., García E., Sánchez-Puelles J., López R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- 30.Kong M., Na H., Ha N.C., Ryu S. LysPBC2, a Novel Endolysin Harboring a Bacillus cereus Spore Binding Domain. Appl. Environ. Microbiol. 2019;85:e02462-18. doi: 10.1128/AEM.02462-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oechslin F., Menzi C., Moreillon P., Resch G. The multidomain architecture of a bacteriophage endolysin enables intramolecular synergism and regulation of bacterial lysis. J. Biol. Chem. 2021;296:100639. doi: 10.1016/j.jbc.2021.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.São-José C. Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials. Antibiotics. 2018;7:29. doi: 10.3390/antibiotics7020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son B., Kong M., Cha Y., Bai J., Ryu S. Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics. 2020;9:906. doi: 10.3390/antibiotics9120906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker S.C., Swift S., Korobova O., Schischkova N., Kopylov P., Donovan D.M., Abaev I. Lytic activity of the staphylolytic Twort phage endolysin CHAP domain is enhanced by the SH3b cell wall binding domain. FEMS Microbiol. Lett. 2015;362:1–8. doi: 10.1093/femsle/fnu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maciejewska B., Źrubek K., Espaillat A., Wiśniewska M., Rembacz K.P., Cava F., Dubin G., Drulis-Kawa Z. Modular endolysin of Burkholderia AP3 phage has the largest lysozyme-like catalytic subunit discovered to date and no catalytic aspartate residue. Sci. Rep. 2017;7:14501. doi: 10.1038/s41598-017-14797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walmagh M., Briers Y., dos Santos S.B., Azeredo J., Lavigne R. Characterization of modular bacteriophage endolysins from Myoviridae phages OBP, 201φ2-1 and PVP-SE1. PLoS ONE. 2012;7:e36991. doi: 10.1371/journal.pone.0036991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson D., Schuch R., Chahales P., Zhu S., Fischetti V.A. PlyC: A multimeric bacteriophage lysin. Proc. Natl. Acad. Sci. USA. 2006;103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGowan S., Buckle A.M., Mitchell M.S., Hoopes J.T., Gallagher D.T., Heselpoth R.D., Shen Y., Reboul C.F., Law R.H., Fischetti V.A. X-ray crystal structure of the streptococcal specific phage lysin PlyC. Proc. Natl. Acad. Sci. USA. 2012;109:12752–12757. doi: 10.1073/pnas.1208424109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunne M., Mertens H.D., Garefalaki V., Jeffries C.M., Thompson A., Lemke E.A., Svergun D.I., Mayer M.J., Narbad A., Meijers R. The CD27L and CTP1L endolysins targeting Clostridia contain a built-in trigger and release factor. PLoS Pathog. 2014;10:e1004228. doi: 10.1371/journal.ppat.1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunne M., Leicht S., Krichel B., Mertens H.D., Thompson A., Krijgsveld J., Svergun D.I., Gómez-Torres N., Garde S., Uetrecht C. Crystal structure of the CTP1L endolysin reveals how its activity is regulated by a secondary translation product. J. Biol. Chem. 2016;291:4882–4893. doi: 10.1074/jbc.M115.671172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proença D., Velours C., Leandro C., Garcia M., Pimentel M., São-José C. A two-component, multimeric endolysin encoded by a single gene. Mol. Microbiol. 2015;95:739–753. doi: 10.1111/mmi.12857. [DOI] [PubMed] [Google Scholar]
- 42.Sanz-Gaitero M., Keary R., Garcia-Doval C., Coffey A., van Raaij M.J. Crystallization of the CHAP domain of the endolysin from Staphylococcus aureus bacteriophage K. Acta Crystallogr. Sect. F. 2013;69:1393–1396. doi: 10.1107/S1744309113030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broendum S.S., Buckle A.M., McGowan S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018;110:879–896. doi: 10.1111/mmi.14134. [DOI] [PubMed] [Google Scholar]
- 44.Sutcliffe I.C. A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol. 2010;18:464–470. doi: 10.1016/j.tim.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Navarre W.W., Ton-That H., Faull K.F., Schneewind O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage φ11: Identification of a D-alanyl-glycine endopeptidase activity. J. Biol. Chem. 1999;274:15847–15856. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 46.Loessner M.J. Bacteriophage endolysins—Current state of research and applications. Curr. Opin. Microbiol. 2005;8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y., Xu D., Wang L., Qu M., Li F., Tan Z., Yao L. Characterization of a broad-spectrum endolysin LysSP1 encoded by a Salmonella bacteriophage. Appl. Microbiol. Biotechnol. 2021;105:5461–5470. doi: 10.1007/s00253-021-11366-z. [DOI] [PubMed] [Google Scholar]
- 48.Ni P., Wang L., Deng B., Jiu S., Ma C., Zhang C., Almeida A., Wang D., Xu W., Wang S. Characterization of a Lytic Bacteriophage against Pseudomonas syringae pv. actinidiae and Its Endolysin. Viruses. 2021;13:631. doi: 10.3390/v13040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han H., Li X., Zhang T., Wang X., Zou J., Zhang C., Tang H., Zou Y., Cheng B., Wang R. Bioinformatic analyses of a potential Salmonella-virus-FelixO1 biocontrol phage BPS15S6 and the characterisation and anti-Enterobacteriaceae-pathogen activity of its endolysin LyS15S6. Antonie Van Leeuwenhoek. 2019;112:1577–1592. doi: 10.1007/s10482-019-01283-7. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira H., Vilas Boas D., Mesnage S., Kluskens L.D., Lavigne R., Sillankorva S., Secundo F., Azeredo J. Structural and Enzymatic Characterization of ABgp46, a Novel Phage Endolysin with Broad Anti-Gram-Negative Bacterial Activity. Front. Microbiol. 2016;7:208. doi: 10.3389/fmicb.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan G., Yang R., Fan K., Dong H., Gao C., Wang S., Yu L., Cheng Z., Lei L. External lysis of Escherichia coli by a bacteriophage endolysin modified with hydrophobic amino acids. AMB Express. 2019;9:106. doi: 10.1186/s13568-019-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zampara A., Sørensen M.C.H., Grimon D., Antenucci F., Briers Y., Brøndsted L. Innolysins: A novel approach to engineer endolysins to kill Gram-negative bacteria. BioRxiv. 2018:408948. doi: 10.1101/408948. [DOI] [Google Scholar]
- 53.Xu D., Zhao S., Dou J., Xu X., Zhi Y., Wen L. Engineered endolysin-based "artilysins" for controlling the gram-negative pathogen Helicobacter pylori. AMB Express. 2021;11:63. doi: 10.1186/s13568-021-01222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonova N.P., Vasina D.V., Rubalsky E.O., Fursov M.V., Savinova A.S., Grigoriev I.V., Usachev E.V., Shevlyagina N.V., Zhukhovitsky V.G., Balabanyan V.U., et al. Modulation of Endolysin LysECD7 Bactericidal Activity by Different Peptide Tag Fusion. Biomolecules. 2020;10:440. doi: 10.3390/biom10030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai J., Yang E., Chang P.-S., Ryu S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzym. Microb. Technol. 2019;128:40–48. doi: 10.1016/j.enzmictec.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Wu M., Hu K., Xie Y., Liu Y., Mu D., Guo H., Zhang Z., Zhang Y., Chang D., Shi Y. A Novel Phage PD-6A3, and Its Endolysin Ply6A3, with Extended Lytic Activity Against Acinetobacter baumannii. Front. Microbiol. 2018;9:3302. doi: 10.3389/fmicb.2018.03302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan F.M., Gondil V.S., Li C., Jiang M., Li J., Yu J., Wei H., Yang H. A Novel Acinetobacter baumannii Bacteriophage Endolysin LysAB54 With High Antibacterial Activity Against Multiple Gram-Negative Microbes. Front. Cell. Infect. Microbiol. 2021;11:70. doi: 10.3389/fcimb.2021.637313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plotka M., Kapusta M., Dorawa S., Kaczorowska A.K., Kaczorowski T. Ts2631 Endolysin from the Extremophilic Thermus scotoductus Bacteriophage vB_Tsc2631 as an Antimicrobial Agent against Gram-Negative Multidrug-Resistant Bacteria. Viruses. 2019;11:657. doi: 10.3390/v11070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur J., Kour A., Panda J.J., Harjai K., Chhibber S. Exploring Endolysin-Loaded Alginate-Chitosan Nanoparticles as Future Remedy for Staphylococcal Infections. AAPS PharmSciTech. 2020;21:233. doi: 10.1208/s12249-020-01763-4. [DOI] [PubMed] [Google Scholar]
- 60.Portilla S., Fernández L., Gutiérrez D., Rodríguez A., García P. Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics. 2020;9:242. doi: 10.3390/antibiotics9050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuiper J.W.P., Hogervorst J.M.A., Herpers B.L., Bakker A.D., Klein-Nulend J., Nolte P.A., Krom B.P. The novel endolysin XZ.700 effectively treats MRSA biofilms in two biofilm models without showing toxicity on human bone cells in vitro. Biofouling. 2021;37:184–193. doi: 10.1080/08927014.2021.1887151. [DOI] [PubMed] [Google Scholar]
- 62.Kim S., Jin J.S., Choi Y.J., Kim J. LysSAP26, a New Recombinant Phage Endolysin with a Broad Spectrum Antibacterial Activity. Viruses. 2020;12:1340. doi: 10.3390/v12111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ning H., Lin H., Wang J., He X., Lv X., Ju L. Characterizations of the endolysin Lys84 and its domains from phage qdsa002 with high activities against Staphylococcus aureus and its biofilms. Enzym. Microb. Technol. 2021;148:109809. doi: 10.1016/j.enzmictec.2021.109809. [DOI] [PubMed] [Google Scholar]
- 64.Yu J.H., Park D.W., Lim J.A., Park J.H. Characterization of staphylococcal endolysin LysSAP33 possessing untypical domain composition. J. Microbiol. 2021;59:840–847. doi: 10.1007/s12275-021-1242-1. [DOI] [PubMed] [Google Scholar]
- 65.Imanishi I., Uchiyama J., Tsukui T., Hisatsune J., Ide K., Matsuzaki S., Sugai M., Nishifuji K. Therapeutic Potential of an Endolysin Derived from Kayvirus S25-3 for Staphylococcal impetigo. Viruses. 2019;11:769. doi: 10.3390/v11090769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae J.Y., Jun K.I., Kang C.K., Song K.H., Choe P.G., Bang J.H., Kim E.S., Park S.W., Kim H.B., Kim N.J., et al. Efficacy of Intranasal Administration of the Recombinant Endolysin SAL200 in a Lethal Murine Staphylococcus aureus Pneumonia Model. Antimicrob. Agents Chemother. 2019;63:e02009-18. doi: 10.1128/AAC.02009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cha Y., Son B., Ryu S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol. 2019;84:103245. doi: 10.1016/j.fm.2019.103245. [DOI] [PubMed] [Google Scholar]
- 68.Son B., Kong M., Lee Y., Ryu S. Development of a Novel Chimeric Endolysin, Lys109 With Enhanced Lytic Activity Against Staphylococcus aureus. Front. Microbiol. 2020;11:615887. doi: 10.3389/fmicb.2020.615887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu Y., Wang Y., Wang J., Zhao Y., Zhong Q., Li G., Fu Z., Lu S. Phage Endolysin LysP108 Showed Promising Antibacterial Potential Against Methicillin-resistant Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021;11:668430. doi: 10.3389/fcimb.2021.668430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee C., Kim J., Son B., Ryu S. Development of Advanced Chimeric Endolysin to Control Multidrug-Resistant Staphylococcus aureus through Domain Shuffling. ACS Infect. Dis. 2021;7:2081–2092. doi: 10.1021/acsinfecdis.0c00812. [DOI] [PubMed] [Google Scholar]
- 71.Knaack D., Idelevich E.A., Schleimer N., Molinaro S., Kriegeskorte A., Peters G., Becker K. Bactericidal activity of bacteriophage endolysin HY-133 against Staphylococcus aureus in comparison to other antibiotics as determined by minimum bactericidal concentrations and time-kill analysis. Diagn. Microbiol. Infect. Dis. 2019;93:362–368. doi: 10.1016/j.diagmicrobio.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Chun J., Bai J., Ryu S. Yeast Surface Display System for Facilitated Production and Application of Phage Endolysin. ACS Synth. Biol. 2020;9:508–516. doi: 10.1021/acssynbio.9b00360. [DOI] [PubMed] [Google Scholar]
- 73.Muharram M.M., Abulhamd A.T., Aldawsari M.F., Alqarni M.H., Labrou N.E. Development of Staphylococcus Enzybiotics: The Ph28 Gene of Staphylococcus epidermidis Phage PH15 Is a Two-Domain Endolysin. Antibiotics. 2020;9:148. doi: 10.3390/antibiotics9040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva M.D., Oliveira H., Faustino A., Sillankorva S. Characterization of MSlys, the endolysin of Streptococcus pneumoniae phage MS1. Biotechnol. Rep. 2020;28:e00547. doi: 10.1016/j.btre.2020.e00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim H., Lee H.G., Kwon I., Seo J. Characterization of Endolysin LyJH307 with Antimicrobial Activity Against Streptococcus bovis. Animals. 2020;10:963. doi: 10.3390/ani10060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H., Park T., Kwon I., Seo J. Specific inhibition of Streptococcus bovis by endolysin LyJH307 supplementation shifts the rumen microbiota and metabolic pathways related to carbohydrate metabolism. J. Anim. Sci. Biotechnol. 2021;12:93. doi: 10.1186/s40104-021-00614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broendum S.S., Williams D.E., Hayes B.K., Kraus F., Fodor J., Clifton B.E., Geert Volbeda A., Codee J.D.C., Riley B.T., Drinkwater N., et al. High avidity drives the interaction between the streptococcal C1 phage endolysin, PlyC, with the cell surface carbohydrates of Group A Streptococcus. Mol. Microbiol. 2021;116:397–415. doi: 10.1111/mmi.14719. [DOI] [PubMed] [Google Scholar]
- 78.Lu N., Sun Y., Wang Q., Qiu Y., Chen Z., Wen Y., Wang S., Song Y. Cloning and characterization of endolysin and holin from Streptomyces avermitilis bacteriophage phiSASD1 as potential novel antibiotic candidates. Int. J. Biol. Macromol. 2020;147:980–989. doi: 10.1016/j.ijbiomac.2019.10.065. [DOI] [PubMed] [Google Scholar]
- 79.Sarjoughian M.R., Rahmani F., Abolmaali S., Astaneh S.D.A. Bacillus phage endolysin, lys46, bactericidal properties against Gram-negative bacteria. Iran. J. Microbiol. 2020;12:607–615. doi: 10.18502/ijm.v12i6.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skorynina A.V., Piligrimova E.G., Kazantseva O.A., Kulyabin V.A., Baicher S.D., Ryabova N.A., Shadrin A.M. Bacillus-infecting bacteriophage Izhevsk harbors thermostable endolysin with broad range specificity. PLoS ONE. 2020;15:e0242657. doi: 10.1371/journal.pone.0242657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K.O., Kong M., Kim I., Bai J., Cha S., Kim B., Ryu K.S., Ryu S., Suh J.Y. Structural Basis for Cell-Wall Recognition by Bacteriophage PBC5 Endolysin. Structure. 2019;27:1355–1365.e1354. doi: 10.1016/j.str.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Schuch R., Pelzek A.J., Nelson D.C., Fischetti V.A. The PlyB Endolysin of Bacteriophage vB_BanS_Bcp1 Exhibits Broad-Spectrum Bactericidal Activity against Bacillus cereus Sensu Lato Isolates. Appl. Environ. Microbiol. 2019;85:e00003-19. doi: 10.1128/AEM.00003-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garde S., Calzada J., Sánchez C., Gaya P., Narbad A., Meijers R., Mayer M.J., Ávila M. Effect of Lactococcus lactis expressing phage endolysin on the late blowing defect of cheese caused by Clostridium tyrobutyricum. Int. J. Food Microbiol. 2020;329:108686. doi: 10.1016/j.ijfoodmicro.2020.108686. [DOI] [PubMed] [Google Scholar]
- 84.Cho J.H., Kwon J.G., O’Sullivan D.J., Ryu S., Lee J.H. Development of an endolysin enzyme and its cell wall-binding domain protein and their applications for biocontrol and rapid detection of Clostridium perfringens in food. Food Chem. 2021;345:128562. doi: 10.1016/j.foodchem.2020.128562. [DOI] [PubMed] [Google Scholar]
- 85.Mondal S.I., Akter A., Draper L.A., Ross R.P., Hill C. Characterization of an Endolysin Targeting Clostridioides difficile That Affects Spore Outgrowth. Int. J. Mol. Sci. 2021;22:5690. doi: 10.3390/ijms22115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sekiya H., Okada M., Tamai E., Shimamoto T., Shimamoto T., Nariya H. A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm. Antibiotics. 2021;10:245. doi: 10.3390/antibiotics10030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou B., Zhen X., Zhou H., Zhao F., Fan C., Perčulija V., Tong Y., Mi Z., Ouyang S. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 2020;16:e1008394. doi: 10.1371/journal.ppat.1008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H., Buttaro B.A., Fouts D.E., Sanjari S., Evans B.S., Stevens R.H. Bacteriophage φEf11 ORF28 Endolysin, a Multifunctional Lytic Enzyme with Properties Distinct from All Other Identified Enterococcus faecalis Phage Endolysins. Appl. Environ. Microbiol. 2019;85:e00555-19. doi: 10.1128/AEM.00555-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang D., Chen Y., Sun E., Hua L., Peng Z., Wu B. Characterization of a Lytic Bacteriophage vB_EfaS_PHB08 Harboring Endolysin Lys08 Against Enterococcus faecalis Biofilms. Microorganisms. 2020;8:1332. doi: 10.3390/microorganisms8091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsui H., Uchiyama J., Ogata M., Nasukawa T., Takemura-Uchiyama I., Kato S.I., Murakami H., Higashide M., Hanaki H. Use of Recombinant Endolysin to Improve Accuracy of Group B Streptococcus Tests. Microbiol. Spectr. 2021;9:e0007721. doi: 10.1128/Spectrum.00077-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh H.K., Hwang Y.J., Hong H.W., Myung H. Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime. Viruses. 2021;13:426. doi: 10.3390/v13030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Landlinger C., Tisakova L., Oberbauer V., Schwebs T., Muhammad A., Latka A., Van Simaey L., Vaneechoutte M., Guschin A., Resch G., et al. Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo. Pathogens. 2021;10:54. doi: 10.3390/pathogens10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu S.Y., Bischoff K.M., Rich J.O., Liu S., Skory C.D. Recombinant bacteriophage LysKB317 endolysin mitigates Lactobacillus infection of corn mash fermentations. Biotechnol. Biofuels. 2020;13:157. doi: 10.1186/s13068-020-01795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pennone V., Sanz-Gaitero M., O’Connor P., Coffey A., Jordan K., van Raaij M.J., McAuliffe O. Inhibition of L. monocytogenes Biofilm Formation by the Amidase Domain of the Phage vB_LmoS_293 Endolysin. Viruses. 2019;11:722. doi: 10.3390/v11080722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nair G., Jain V. Separation of Mycobacterium smegmatis From a Mixed Culture Using the Cell Wall Binding Domain of D29 Mycobacteriophage Endolysin. Front. Microbiol. 2020;11:1119. doi: 10.3389/fmicb.2020.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Islam M.R., Son N., Lee J., Lee D.W., Sohn E.J., Hwang I. Production of bacteriophage-encoded endolysin, LysP11, in Nicotiana benthamiana and its activity as a potent antimicrobial agent against Erysipelothrix rhusiopathiae. Plant Cell Rep. 2019;38:1485–1499. doi: 10.1007/s00299-019-02459-1. [DOI] [PubMed] [Google Scholar]
- 97.Santos S.B., Oliveira A., Melo L.D.R., Azeredo J. Identification of the first endolysin Cell Binding Domain (CBD) targeting Paenibacillus larvae. Sci. Rep. 2019;9:2568. doi: 10.1038/s41598-019-39097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim S., Lee D.W., Jin J.S., Kim J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2020;22:32–39. doi: 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Ding Y., Zhang Y., Huang C., Wang J., Wang X. An Endolysin LysSE24 by Bacteriophage LPSE1 Confers Specific Bactericidal Activity against Multidrug-Resistant Salmonella Strains. Microorganisms. 2020;8:737. doi: 10.3390/microorganisms8050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bai J., Lee S., Ryu S. Identification and in vitro Characterization of a Novel Phage Endolysin that Targets Gram-Negative Bacteria. Microorganisms. 2020;8:447. doi: 10.3390/microorganisms8030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y., Huang H.H., Duc H.M., Masuda Y., Honjoh K.I., Miyamoto T. Endolysin LysSTG2: Characterization and application to control Salmonella Typhimurium biofilm alone and in combination with slightly acidic hypochlorous water. Food Microbiol. 2021;98:103791. doi: 10.1016/j.fm.2021.103791. [DOI] [PubMed] [Google Scholar]
- 102.Park D.W., Park J.H. Characterization of Endolysin LysECP26 Derived from rV5-like Phage vB_EcoM-ECP26 for Inactivation of Escherichia coli O157:H7. J. Microbiol. Biotechnol. 2020;30:1552–1558. doi: 10.4014/jmb.2005.05030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng S., Xu Q., Fu Y., Liang L., Wu Y., Peng F., Gao M. Genomic Analysis of a Novel Phage Infecting the Turkey Pathogen Escherichia coli APEC O78 and Its Endolysin Activity. Viruses. 2021;13:1034. doi: 10.3390/v13061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fursov M.V., Abdrakhmanova R.O., Antonova N.P., Vasina D.V., Kolchanova A.D., Bashkina O.A., Rubalsky O.V., Samotrueva M.A., Potapov V.D., Makarov V.V., et al. Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In vitro and in vivo Study. Viruses. 2020;12:545. doi: 10.3390/v12050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan Y., Li X., Wang L., Li G., Cong C., Li R., Cui H., Murtaza B., Xu Y. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb. Biotechnol. 2021;14:403–418. doi: 10.1111/1751-7915.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hammond R.W., Swift S.M., Foster-Frey J.A., Kovalskaya N.Y., Donovan D.M. Optimized production of a biologically active Clostridium perfringens glycosyl hydrolase phage endolysin PlyCP41 in plants using virus-based systemic expression. BMC Biotechnol. 2019;19:101. doi: 10.1186/s12896-019-0594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Basit A., Qadir S., Qureshi S., Rehman S.U. Cloning and expression analysis of fused holin-endolysin from RL bacteriophage; Exhibits broad activity against multi drug resistant pathogens. Enzym. Microb. Technol. 2021;149:109846. doi: 10.1016/j.enzmictec.2021.109846. [DOI] [PubMed] [Google Scholar]
- 108.Ning H.Q., Lin H., Wang J.X. Synergistic effects of endolysin Lysqdvp001 and ε-poly-lysine in controlling Vibrio parahaemolyticus and its biofilms. Int. J. Food Microbiol. 2021;343:109112. doi: 10.1016/j.ijfoodmicro.2021.109112. [DOI] [PubMed] [Google Scholar]
- 109.Adamczyk-Popławska M., Tracz-Gaszewska Z., Lasota P., Kwiatek A., Piekarowicz A. Haemophilus influenzae HP1 Bacteriophage Encodes a Lytic Cassette with a Pinholin and a Signal-Arrest-Release Endolysin. Int. J. Mol. Sci. 2020;21:4013. doi: 10.3390/ijms21114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donlan R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 111.Sharma U., Vipra A., Channabasappa S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today. 2018;23:848–856. doi: 10.1016/j.drudis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 112.Vukotic G., Obradovic M., Novovic K., Di Luca M., Jovcic B., Fira D., Neve H., Kojic M., McAuliffe O. Characterization, Antibiofilm, and Depolymerizing Activity of Two Phages Active on Carbapenem-Resistant Acinetobacter baumannii. Front. Med. 2020;7:426. doi: 10.3389/fmed.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shkodenko L., Kassirov I., Koshel E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms. 2020;8:1545. doi: 10.3390/microorganisms8101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okamoto I., Miyaji H., Miyata S., Shitomi K., Sugaya T., Ushijima N., Akasaka T., Enya S., Saita S., Kawasaki H. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega. 2021;6:9279–9290. doi: 10.1021/acsomega.1c00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zagami R., Franco D., Pipkin J.D., Antle V., De Plano L., Patanè S., Guglielmino S., Monsù Scolaro L., Mazzaglia A. Sulfobutylether-β-cyclodextrin/5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphine nanoassemblies with sustained antimicrobial phototherapeutic action. Int. J. Pharm. 2020;585:119487. doi: 10.1016/j.ijpharm.2020.119487. [DOI] [PubMed] [Google Scholar]
- 116.Tonin M.H., Brites F.C., Mariano J.R., Freitas K.M.S., Ortiz M.A.L., Salmeron S. Low-Level Laser and Antimicrobial Photodynamic Therapy Reduce Peri-implantitis-related Microorganisms Grown In Vitro. Eur. J. Dent. 2021 doi: 10.1055/s-0041-1731926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kortright K.E., Doss-Gollin S., Chan B.K., Turner P.E. Evolution of Bacterial Cross-Resistance to Lytic Phages and Albicidin Antibiotic. Front. Microbiol. 2021;12:658374. doi: 10.3389/fmicb.2021.658374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Girigoswami K. Toxicity of Metal Oxide Nanoparticles. In: Saquib Q., Faisal M., Al-Khedhairy A.A., Alatar A.A., editors. Cellular and Molecular Toxicology of Nanoparticles. Springer International Publishing; Cham, Switzerland: 2018. pp. 99–122. [DOI] [Google Scholar]
- 119.Dehghan Esmatabadi M.J., Bozorgmehr A., Hajjari S.N., Sadat Sombolestani A., Malekshahi Z.V., Sadeghizadeh M. Review of new insights into antimicrobial agents. Cell. Mol. Biol. 2017;63:40–48. doi: 10.14715/cmb/2017.63.2.6. [DOI] [PubMed] [Google Scholar]
- 120.Xiang M., Zhou Q., Shi Z., Wang X., Li M., Jia Y., Li S., Yang F., Wang W., Chen T., et al. A Review of Light Sources and Enhanced Targeting for Photodynamic Therapy. Curr. Med. Chem. 2021;28:6437–6457. doi: 10.2174/0929867328666210121122106. [DOI] [PubMed] [Google Scholar]
- 121.Jansen B., Peters G. Foreign body associated infection. J. Antimicrob. Chemother. 1993;32((Suppl. A)):69–75. doi: 10.1093/jac/32.suppl_A.69. [DOI] [PubMed] [Google Scholar]
- 122.Nowakowska J., Landmann R., Khanna N. Foreign Body Infection Models to Study Host-Pathogen Response and Antimicrobial Tolerance of Bacterial Biofilm. Antibiotics. 2014;3:378–397. doi: 10.3390/antibiotics3030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Idelevich E.A., Knaack D., Nugroho N.T., Peters G., Bisdas T., Molinaro S., Torsello G.B., Becker K., Herten M. Comparative in vitro activity of bacteriophage endolysin HY-133 against Staphylococcus aureus attached to vascular graft surface. Med. Microbiol. Immunol. 2020;209:51–57. doi: 10.1007/s00430-019-00638-1. [DOI] [PubMed] [Google Scholar]
- 124.Sass P., Bierbaum G. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007;73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Son J.-S., Lee S.-J., Jun S.Y., Yoon S.J., Kang S.H., Paik H.R., Kang J.O., Choi Y.-J. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010;86:1439–1449. doi: 10.1007/s00253-009-2386-9. [DOI] [PubMed] [Google Scholar]
- 126.Gutierrez D., Ruas-Madiedo P., Martínez B., Rodríguez A., García P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE. 2014;9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jun S.Y., Jung G.M., Yoon S.J., Oh M.-D., Choi Y.-J., Lee W.J., Kong J.-C., Seol J.G., Kang S.H. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int. J. Antimicrob. Agents. 2013;41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Linden S.B., Zhang H., Heselpoth R.D., Shen Y., Schmelcher M., Eichenseher F., Nelson D.C. Biochemical and biophysical characterization of PlyGRCS, a bacteriophage endolysin active against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2015;99:741–752. doi: 10.1007/s00253-014-5930-1. [DOI] [PubMed] [Google Scholar]
- 129.Fenton M., Keary R., McAuliffe O., Ross R.P., O’Mahony J., Coffey A. Bacteriophage-derived peptidase eliminates and prevents Staphylococcal biofilms. Int. J. Microbiol. 2013;2013:625341. doi: 10.1155/2013/625341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meng X., Shi Y., Ji W., Meng X., Zhang J., Wang H., Lu C., Sun J., Yan Y. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011;77:8272–8279. doi: 10.1128/AEM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu J., Yang H., Bi Y., Li W., Wei H., Li Y. Activity of the Chimeric Lysin ClyR against Common Gram-Positive Oral Microbes and Its Anticaries Efficacy in Rat Models. Viruses. 2018;10:380. doi: 10.3390/v10070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gutiérrez D., Rodríguez-Rubio L., Ruas-Madiedo P., Fernández L., Campelo A.B., Briers Y., Nielsen M.W., Pedersen K., Lavigne R., García P., et al. Design and Selection of Engineered Lytic Proteins with Staphylococcus aureus Decolonizing Activity. Front. Microbiol. 2021;12:723834. doi: 10.3389/fmicb.2021.723834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vázquez R., García P. Synergy Between Two Chimeric Lysins to Kill Streptococcus pneumoniae. Front. Microbiol. 2019;10:1251. doi: 10.3389/fmicb.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oliveira H., Thiagarajan V., Walmagh M., Sillankorva S., Lavigne R., Neves-Petersen M.T., Kluskens L.D., Azeredo J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE. 2014;9:e108376. doi: 10.1371/journal.pone.0108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo M., Feng C., Ren J., Zhuang X., Zhang Y., Zhu Y., Dong K., He P., Guo X., Qin J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017;8:293. doi: 10.3389/fmicb.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Purevdorj-Gage B., Orr M.E., Stoodley P., Sheehan K.B., Hyman L.E. The role of FLO11 in Saccharomyces cerevisiae biofilm development in a laboratory based flow-cell system. FEMS Yeast Res. 2007;7:372–379. doi: 10.1111/j.1567-1364.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 137.Snel G., Malvisi M., Pilla R., Piccinini R. Evaluation of biofilm formation using milk in a flow cell model and microarray characterization of Staphylococcus aureus strains from bovine mastitis. Vet. Microbiol. 2014;174:489–495. doi: 10.1016/j.vetmic.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 138.Elias S., Banin E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012;36:990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 139.Rickard A.H., Gilbert P., High N.J., Kolenbrander P.E., Handley P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100. doi: 10.1016/S0966-842X(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 140.Chorianopoulos N., Tsoukleris D., Panagou E., Falaras P., Nychas G.-J. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 2011;28:164–170. doi: 10.1016/j.fm.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 141.Bai J., Kim Y.-T., Ryu S., Lee J.-H. Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins. Front. Microbiol. 2016;7:474. doi: 10.3389/fmicb.2016.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmelcher M., Loessner M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2016;37:76–87. doi: 10.1016/j.copbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 143.Kretzer J.W., Lehmann R., Schmelcher M., Banz M., Kim K.-P., Korn C., Loessner M.J. Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl. Environ. Microbiol. 2007;73:1992–2000. doi: 10.1128/AEM.02402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hagens S., Loessner M.J. Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 2007;76:513–519. doi: 10.1007/s00253-007-1031-8. [DOI] [PubMed] [Google Scholar]
- 145.Idelevich E.A., Walther T., Molinaro S., Li X., Xia G., Wieser A., Peters G., Peschel A., Becker K. Bacteriophage-based latex agglutination test for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 2014;52:3394–3398. doi: 10.1128/JCM.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rizzo M.G., Carnazza S., De Plano L., Franco D., Nicolo M., Zammuto V., Petralia S., Calabrese G., Gugliandolo C., Conoci S., et al. Rapid detection of bacterial pathogens in blood through engineered phages-beads and integrated Real-Time PCR into MicroChip. Sens. Actuators B Chem. 2021;329:129227. doi: 10.1016/j.snb.2020.129227. [DOI] [Google Scholar]
- 147.Ohlsson P., Evander M., Petersson K., Mellhammar L., Lehmusvuori A., Karhunen U., Soikkeli M., Seppä T., Tuunainen E., Spangar A., et al. Integrated Acoustic Separation, Enrichment, and Microchip Polymerase Chain Reaction Detection of Bacteria from Blood for Rapid Sepsis Diagnostics. Anal. Chem. 2016;88:9403–9411. doi: 10.1021/acs.analchem.6b00323. [DOI] [PubMed] [Google Scholar]
- 148.Jones H.J., Shield C.G., Swift B.M. The Application of Bacteriophage Diagnostics for Bacterial Pathogens in the Agricultural Supply Chain: From Farm-to-Fork. PHAGE. 2020;1:176–188. doi: 10.1089/phage.2020.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schmelcher M., Shabarova T., Eugster M.R., Eichenseher F., Tchang V.S., Banz M., Loessner M.J. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl. Environ. Microbiol. 2010;76:5745–5756. doi: 10.1128/AEM.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yi Z., Wang S., Meng X., Wu A., Li Q., Song Y., Zhao R., Qiao J. Lysin cell-binding domain-functionalized magnetic beads for detection of Staphylococcus aureus via inhibition of fluorescence of Amplex Red/hydrogen peroxide assay by intracellular catalase. Anal. Bioanal. Chem. 2019;411:7177–7185. doi: 10.1007/s00216-019-02099-0. [DOI] [PubMed] [Google Scholar]
- 151.Fujinami Y., Hirai Y., Sakai I., Yoshino M., Yasuda J. Sensitive detection of Bacillus anthracis using a binding protein originating from gamma-phage. Microbiol. Immunol. 2007;51:163–169. doi: 10.1111/j.1348-0421.2007.tb03894.x. [DOI] [PubMed] [Google Scholar]
- 152.Sainathrao S., Mohan K.V.K., Atreya C. Gamma-phage lysin PlyG sequence-based synthetic peptides coupled with Qdot-nanocrystals are useful for developing detection methods for Bacillus anthracis by using its surrogates, B. anthracis-Sterne and B. cereus-4342. BMC Biotechnol. 2009;9:1–7. doi: 10.1186/1472-6750-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tolba M., Ahmed M.U., Tlili C., Eichenseher F., Loessner M.J., Zourob M. A bacteriophage endolysin-based electrochemical impedance biosensor for the rapid detection of Listeria cells. Analyst. 2012;137:5749–5756. doi: 10.1039/c2an35988j. [DOI] [PubMed] [Google Scholar]
- 154.Yu J., Zhang Y., Li H., Yang H., Wei H. Sensitive and rapid detection of Staphylococcus aureus in milk via cell binding domain of lysin. Biosens. Bioelectron. 2016;77:366–371. doi: 10.1016/j.bios.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 155.Kong M., Sim J., Kang T., Nguyen H.H., Park H.K., Chung B.H., Ryu S. A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur. Biophys. J. 2015;44:437–446. doi: 10.1007/s00249-015-1044-7. [DOI] [PubMed] [Google Scholar]
- 156.Kong M., Shin J.H., Heu S., Park J.-K., Ryu S. Lateral flow assay-based bacterial detection using engineered cell wall binding domains of a phage endolysin. Biosens. Bioelectron. 2017;96:173–177. doi: 10.1016/j.bios.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 157.Kwon S.J., Kim D., Lee I., Nam J., Kim J., Dordick J.S. Sensitive multiplex detection of whole bacteria using self-assembled cell binding domain complexes. Anal. Chim. Acta. 2018;1030:156–165. doi: 10.1016/j.aca.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 158.Chang Y., Kim M., Ryu S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017;68:112–120. doi: 10.1016/j.fm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 159.Chang Y., Yoon H., Kang D.-H., Chang P.-S., Ryu S. Endolysin LysSA97 is synergistic with carvacrol in controlling Staphylococcus aureus in foods. Int. J. Food Microbiol. 2017;244:19–26. doi: 10.1016/j.ijfoodmicro.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 160.Misiou O., van Nassau T.J., Lenz C.A., Vogel R.F. The preservation of Listeria-critical foods by a combination of endolysin and high hydrostatic pressure. Int. J. Food Microbiol. 2018;266:355–362. doi: 10.1016/j.ijfoodmicro.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 161.Ibarra-Sánchez L.A., Van Tassell M.L., Miller M.J. Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018;72:128–134. doi: 10.1016/j.fm.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 162.Chang Y. Bacteriophage-Derived Endolysins Applied as Potent Biocontrol Agents to Enhance Food Safety. Microorganisms. 2020;8:724. doi: 10.3390/microorganisms8050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yoong P., Schuch R., Nelson D., Fischetti V.A. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004;186:4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang H., Bao H., Billington C., Hudson J.A., Wang R. Isolation and lytic activity of the Listeria bacteriophage endolysin LysZ5 against Listeria monocytogenes in soya milk. Food Microbiol. 2012;31:133–136. doi: 10.1016/j.fm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 165.Van Nassau T.J., Lenz C.A., Scherzinger A.S., Vogel R.F. Combination of endolysins and high pressure to inactivate Listeria monocytogenes. Food Microbiol. 2017;68:81–88. doi: 10.1016/j.fm.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 166.Love M.J., Bhandari D., Dobson R.C., Billington C. Potential for bacteriophage endolysins to supplement or replace antibiotics in food production and clinical care. Antibiotics. 2018;7:17. doi: 10.3390/antibiotics7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.World Health Organization . WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 168.Gómez-Aldapa C.A., Torres-Vitela M.d.R., Villarruel-López A., Castro-Rosas J. The role of foods in Salmonella infections. In: Mahmoud B., editor. Salmonella—A Dangerous Foodborne Pathogen. IntechOpen; Rijeka, Croatia: 2012. pp. 21–46. [DOI] [Google Scholar]
- 169.Rodríguez-Rubio L., Gerstmans H., Thorpe S., Mesnage S., Lavigne R., Briers Y. DUF3380 domain from a Salmonella phage endolysin shows potent N-acetylmuramidase activity. Appl. Environ. Microbiol. 2016;82:4975–4981. doi: 10.1128/AEM.00446-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lim J.-A., Shin H., Kang D.-H., Ryu S. Characterization of endolysin from a Salmonella Typhimurium-infecting bacteriophage SPN1S. Res. Microbiol. 2012;163:233–241. doi: 10.1016/j.resmic.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 171.Son B., Yun J., Lim J.-A., Shin H., Heu S., Ryu S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012;12:1–9. doi: 10.1186/1471-2180-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park J., Yun J., Lim J.-A., Kang D.-H., Ryu S. Characterization of an endolysin, LysBPS13, from a Bacillus cereus bacteriophage. FEMS Microbiol. Lett. 2012;332:76–83. doi: 10.1111/j.1574-6968.2012.02578.x. [DOI] [PubMed] [Google Scholar]
- 173.Loessner M.J., Maier S.K., Daubek-Puza H., Wendlinger G., Scherer S. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J. Bacteriol. 1997;179:2845–2851. doi: 10.1128/jb.179.9.2845-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mayer M.J., Payne J., Gasson M.J., Narbad A. Genomic sequence and characterization of the virulent bacteriophage ΦCTP1 from Clostridium tyrobutyricum and heterologous expression of its endolysin. Appl. Environ. Microbiol. 2010;76:5415–5422. doi: 10.1128/AEM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mayer M.J., Gasson M.J., Narbad A. Genomic sequence of bacteriophage ATCC 8074-B1 and activity of its endolysin and engineered variants against Clostridium sporogenes. Appl. Environ. Microbiol. 2012;78:3685–3692. doi: 10.1128/AEM.07884-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Strange R.N., Scott P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005;43:83–116. doi: 10.1146/annurev.phyto.43.113004.133839. [DOI] [PubMed] [Google Scholar]
- 177.McManus P.S., Stockwell V.O., Sundin G.W., Jones A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002;40:443–465. doi: 10.1146/annurev.phyto.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- 178.Gaeng S., Scherer S., Neve H., Loessner M.J. Gene Cloning and Expression and Secretion of Listeria monocytogenes Bacteriophage-Lytic Enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 2000;66:2951–2958. doi: 10.1128/AEM.66.7.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hausbeck M., Bell J., Medina-Mora C., Podolsky R., Fulbright D. Effect of bactericides on population sizes and spread of Clavibacter michiganensis subsp. michiganensis on tomatoes in the greenhouse and on disease development and crop yield in the field. Phytopathology. 2000;90:38–44. doi: 10.1094/PHYTO.2000.90.1.38. [DOI] [PubMed] [Google Scholar]
- 180.Düring K., Porsch P., Fladung M., Lörz H. Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. Plant J. 1993;3:587–598. doi: 10.1046/j.1365-313X.1993.03040587.x. [DOI] [Google Scholar]
- 181.De Vries J., Harms K., Broer I., Kriete G., Mahn A., Düring K., Wackernagel W. The bacteriolytic activity in transgenic potatoes expressing a chimeric T4 lysozyme gene and the effect of T4 lysozyme on soil-and phytopathogenic bacteria. Syst. Appl. Microbiol. 1999;22:280–286. doi: 10.1016/S0723-2020(99)80075-7. [DOI] [Google Scholar]
- 182.Dow J.M., Ma G.-S., Daniels M.J. Cloning and characterization of the rpfC gene of Xanthomonas oryzae pv. oryzae: Involvement in exopolysaccharide production and virulence to rice. Mol. Plant Microbe Interact. 1996;9:664–666. doi: 10.1094/mpmi-9-0664. [DOI] [PubMed] [Google Scholar]
- 183.Xu Y., Luo Q.-Q., Zhou M.-G. Identification and characterization of integron-mediated antibiotic resistance in the phytopathogen Xanthomonas oryzae pv. oryzae. PLoS ONE. 2013;8:e55962. doi: 10.1371/journal.pone.0055962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee C.-N., Lin J.-W., Chow T.-Y., Tseng Y.-H., Weng S.-F. A novel lysozyme from Xanthomonas oryzae phage ϕXo411 active against Xanthomonas and Stenotrophomonas. Protein Expr. Purif. 2006;50:229–237. doi: 10.1016/j.pep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 185.Brooke J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pulawska J. Crown gall of stone fruits and nuts, economic significance and diversity of its causal agents: Tumorigenic agrobacterium spp. J. Plant Pathol. 2010;92:S87–S98. [Google Scholar]
- 187.Mansfield J., Genin S., Magori S., Citovsky V., Sriariyanum M., Ronald P., Dow M., Verdier V., Beer S.V., Machado M.A. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oey M., Lohse M., Scharff L.B., Kreikemeyer B., Bock R. Plastid production of protein antibiotics against pneumonia via a new strategy for high-level expression of antimicrobial proteins. Proc. Natl. Acad. Sci. USA. 2009;106:6579–6584. doi: 10.1073/pnas.0813146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oey M., Lohse M., Kreikemeyer B., Bock R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009;57:436–445. doi: 10.1111/j.1365-313X.2008.03702.x. [DOI] [PubMed] [Google Scholar]
- 190.Kovalskaya N.Y., Herndon E.E., Foster-Frey J.A., Donovan D.M., Hammond R.W. Antimicrobial activity of bacteriophage derived triple fusion protein against Staphylococcus aureus. AIMS Microbiol. 2019;5:158–175. doi: 10.3934/microbiol.2019.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kovalskaya N., Foster-Frey J., Donovan D.M., Bauchan G., Hammond R.W. Antimicrobial Activity of Bacteriophage Endolysin Produced in Nicotiana benthamiana Plants. J. Microbiol. Biotechnol. 2016;26:160–170. doi: 10.4014/jmb.1505.05060. [DOI] [PubMed] [Google Scholar]
- 192.Navarro F., Muniesa M. Phages in the human body. Front. Microbiol. 2017;8:566. doi: 10.3389/fmicb.2017.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Peng Z., Wang S., Gide M., Zhu D., Lamabadu Warnakulasuriya Patabendige H.M., Li C., Cai J., Sun X. A novel bacteriophage lysin-human defensin fusion protein is effective in treatment of Clostridioides difficile infection in mice. Front. Microbiol. 2019;9:3234. doi: 10.3389/fmicb.2018.03234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dams D., Briers Y. Enzybiotics: Enzyme-based antibacterials as therapeutics. Ther. Enzym. Funct. Clin. Implic. 2019:233–253. doi: 10.1007/978-981-13-7709-9_11. [DOI] [PubMed] [Google Scholar]
- 195.Jun S.Y., Jang I.J., Yoon S., Jang K., Yu K.-S., Cho J.Y., Seong M.-W., Jung G.M., Yoon S.J., Kang S.H. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 2017;61:e02629-16. doi: 10.1128/AAC.02629-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rashel M., Uchiyama J., Ujihara T., Uehara Y., Kuramoto S., Sugihara S., Yagyu K.-I., Muraoka A., Sugai M., Hiramatsu K. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ϕMR11. J. Infect. Dis. 2007;196:1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 197.Entenza J., Loeffler J., Grandgirard D., Fischetti V., Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 2005;49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Daniel A., Euler C., Collin M., Chahales P., Gorelick K.J., Fischetti V.A. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010;54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Witzenrath M., Schmeck B., Doehn J.M., Tschernig T., Zahlten J., Loeffler J.M., Zemlin M., Müller H., Gutbier B., Schütte H. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit. Care Med. 2009;37:642–649. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 200.Herpers B., Badoux P., Pietersma F., Eichenseher F., Loessner M. Specific lysis of methicillin susceptible and resistant Staphylococcus aureus by the endolysin Staphefekt SA. 100 TM; Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); Barcelona, Spain. 10–13 May 2014. [Google Scholar]
- 201.Totté J.E., van Doorn M.B., Pasmans S.G. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin Staphefekt SA. 100: A report of 3 cases. Case Rep. Dermatol. 2017;9:19–25. doi: 10.1159/000473872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Totté J., de Wit J., Pardo L., Schuren F., van Doorn M., Pasmans S. Targeted anti-staphylococcal therapy with endolysins in atopic dermatitis and the effect on steroid use, disease severity and the microbiome: Study protocol for a randomized controlled trial (MAAS trial) Trials. 2017;18:1–8. doi: 10.1186/s13063-017-2118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Abdelkader K., Gerstmans H., Saafan A., Dishisha T., Briers Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses. 2019;11:96. doi: 10.3390/v11020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Traczewski M.M., Ambler J.E., Schuch R. Determination of MIC Quality Control Parameters for Exebacase, a Novel Lysin with Antistaphylococcal Activity. J. Clin. Microbiol. 2021;59:e0311720. doi: 10.1128/JCM.03117-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.