Abstract
Statins may exert protective effects against oxidative stress by upregulating specific antioxidant mechanisms. We conducted a systematic review and meta-analysis of the effect of statins on three key antioxidant enzymes: glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase. The electronic databases PubMed, Web of Science, and Scopus were searched from inception to July 2021. The risk of bias was assessed with the Joanna Briggs Institute Critical Appraisal Checklist and certainty of evidence was assessed using the GRADE framework. In 15 studies, reporting 17 treatment arms in 773 patients (mean age 53 years, 54% males), statins significantly increased the concentrations of both GPx (standardized mean difference, SMD = 0.80, 95% confidence interval, CI 0.13 to 1.46, p = 0.018; high certainty of evidence) and SOD (SMD = 1.54, 95% CI 0.71 to 2.36, p < 0.001; high certainty of evidence), but not catalase (SMD = −0.16, 95% CI −0.51 to 0.20, p = 0.394; very low certainty of evidence). The pooled SMD values were not altered in sensitivity analysis. There was no publication bias. In conclusion, statin treatment significantly increases the circulating concentrations of GPx and SOD, suggesting an antioxidant effect of these agents (PROSPERO registration number: CRD42021271589).
Keywords: statins, glutathione peroxidase, superoxide dismutase, catalase, oxidative stress, pleiotropic effects
1. Introduction
Elevations in circulating cholesterol concentrations significantly increase the risk of atherosclerosis and its clinical manifestations, particularly myocardial infarction, ischemic stroke, and peripheral arterial disease [1,2]. Statins, through the inhibition of the enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, the rate-limiting step in the mevalonate pathway through which cells synthesize cholesterol, are the most commonly prescribed drugs for the treatment of hypercholesterolaemia and the management of cardiovascular risk worldwide in view of their favourable efficacy and safety profile [3]. However, while the main action is mediated by lowering the concentrations of specific cholesterol fractions, particularly low-density lipoprotein (LDL) [4], the atheroprotective effects of statins involve other mechanisms, normally described as pleiotropic effects [5,6,7]. Such effects, generally apparent shortly after commencing statin treatment, have been shown to be mediated by specific antioxidant mechanisms [8,9,10,11].
Oxidative stress, through the generation of reactive oxygen species (ROS) and oxidized LDL, is considered to play a key pathophysiological role in the onset and the progression of atherosclerosis [12,13,14]. Specifically, oxidative stress exerts significant negative effects on cellular homeostasis by damaging lipids, thiols, DNA, and protein pools, stimulating the synthesis and release of pro-inflammatory and atherogenic cytokines, and favouring the adhesion of monocytes to the endothelium, a critical pathophysiological step in atherosclerosis and plaque formation [15,16]. The coexistence of oxidative stress and hypercholesterolemia imposes a particularly high burden on endothelial integrity, further increasing the risk of atherosclerosis and its clinical manifestations [17,18].
The effects of statin treatment, singly or in combination with other therapies, on oxidative stress have been extensively studied both in experimental models of atherosclerosis and in humans [19,20,21]. In particular, statins have been shown to inhibit key pro-oxidant enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [22,23], reduce the synthesis of the highly reactive compound malondialdehyde from lipid peroxidation of polyunsaturated fatty acids [24], as well as increase the expression, activity, and coupling of endothelial nitric oxide synthase [25], and upregulate antioxidant enzymes such as catalase [26], glutathione peroxidase (GPx) [27], and superoxide dismutase (SOD) [28,29,30]. Notably, epidemiological studies have convincingly shown that higher circulating concentrations of the antioxidant enzymes GPx, SOD, and catalase are associated with a significant reduction in the risk of coronary heart disease [31]. This suggests that pharmacological strategies that upregulate these enzymes may exert a key protective role against atherosclerosis and cardiovascular disease.
In order to investigate the complex interplay between statins and antioxidant mechanisms, we conducted a systematic review and meta-analysis of studies reporting on the effects of statin treatment on the circulating concentrations of GPx, SOD, and catalase in patients with different cardiovascular risk profiles. We hypothesised that statin treatment would significantly increase GPx, SOD, and catalase concentrations regardless of specific agents used.
2. Materials and Methods
2.1. Search Strategy and Study Selection
We searched for articles published in PubMed, Web of Science, and Scopus, from inception to 31 July 2021, using the terms “Glutathione Peroxidase” or “GPx” or “GSH-PX” or “Superoxide Dismutase” or “SOD” or “Catalase” and “Statin”. The abstracts and articles were screened by two independent investigators. The article references were also searched for additional studies. Pre-defined inclusion criteria were: (a) reporting of GPx and/or SOD and/or catalase concentrations in blood, erythrocytes, plasma, or serum at baseline and after statin treatment; (b) ≥10 participants; (c) English language; and (d) full-text availability. Data extracted included the country, type of biological matrix, age, sex distribution, GPx, SOD, and catalase concentrations before and after treatment, disease condition studied, statin and dose used, and treatment duration.
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for analytical studies was used to assess the risk of bias. Scores ≥ 5, 4, and <4 indicated low, moderate, and high risk, respectively [32]. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) working group system was used to assess the certainty of evidence. GRADE considers the study design, the risk of bias, the presence of heterogeneity, the indirectness of evidence, the imprecision of results, the effect size (small, SMD < 0.5, medium, SMD 0.5–0.8, and large, SMD > 0.8) [33], and the publication bias [34,35,36]. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement on the reporting of systematic reviews and meta-analyses (Supplementary Materials, Tables S1 and S2) [37]. The International Prospective Register of Systematic Reviews (PROSPERO) registration number was CRD42021271589.
2.2. Statistical Analysis
Because of the different units of measurement (U/mL, U/gHb, nmol/mg, or µmol/L) used to express the concentrations of GPx, SOD, and catalase, standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated to build forest plots of the differences in GPx, SOD, and catalase concentrations before and after statin treatment, with a p-value < 0.05 indicating statistical significance. When required, the means and standard deviations were derived from the corresponding medians and interquartile ranges [38], medians and ranges [39], or from graphs using the Graph Data Extractor software. Between-study heterogeneity was assessed using the Q-statistic (significance level set at p < 0.10) and the I2 statistic (I2 < 25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 > 75%, extreme heterogeneity) [40,41]. In the presence of significant heterogeneity, defined as I2 values ≥ 50%, a random-effects model was used. Sensitivity analysis was performed to assess the influence of each study on the overall risk estimate by sequentially removing individual studies [42]. Publication bias was assessed with the Begg’s test, the Egger’s test (significance level set at p < 0.05 for both), and the “trim-and-fill” procedure [43,44,45]. When possible, the effects of individual statins (e.g., lipophilic: atorvastatin, simvastatin, lovastatin, fluvastatin, cerivastatin, and pitavastatin; hydrophilic: rosuvastatin, pravastatin) were assessed and compared. Statistical analyses were performed using Stata 14 software (STATA Corp., College Station, TX, USA).
3. Results
3.1. Study Selection
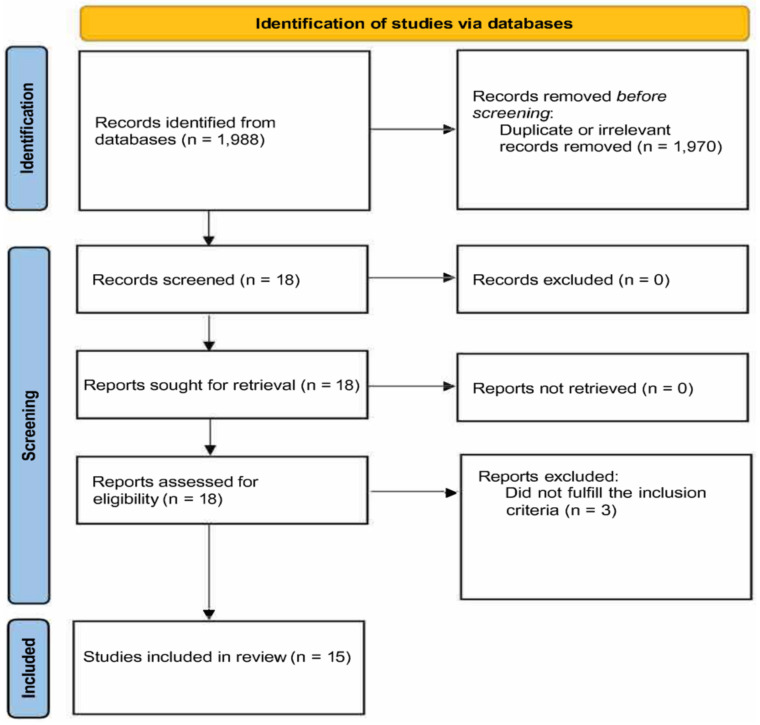
We initially identified 1988 articles. A total of 1970 were excluded (duplicates or irrelevant). After reviewing the remaining 18 articles, 3 were further excluded, leaving 15, reporting 17 treatment arms in 773 patients (mean age of 53 years, 54% males), for final analysis (Figure 1 and Table 1) [27,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
Figure 1.
PRISMA 2020 flow diagram.
Table 1.
Study characteristics.
| First Author, Year, Country [Ref] | Matrix | n | Age (yrs) |
M/F | GPx Bas Mean ± SD |
GPx Post Mean ± SD |
SOD Bas Mean ± SD |
SOD Post Mean ± SD |
Cat Bas Mean ± SD |
Cat Post Mean ± SD |
Condition | Statin and Daily Dose | Treatment (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen MF, 1997, Taiwan [46] | P | 20 | 47 | 11/9 | 0.98 ± 0.32 U/mL | 1.08 ± 0.29 U/mL | NR | NR | NR | NR | HCL | Pravastatin 5 mg | 12 |
| Yilmaz MI, 2004, Turkey [27] | E | 35 | 48 | 18/17 | 20.93 ± 10.46 U/mL | 39.13 ± 6.76 U/mL | 510 ± 190 U/mL | 589 ± 182 U/mL | NR | NR | HCL | Fluvastatin 40 mg | 12 |
| Ghayour-Mobarhan M, 1997, UK [47] | S | 11 | 52 | 7/4 | 0.36 ± 0.13 U/mL | 0.32 ± 0.13 U/mL | NR | NR | NR | NR | HCL | Simvastatin 10 mg | 16 |
| Molčányiová A, 2006, Slovakia [48] | E | 42 | 60 | 12/30 | 6.03 ± 2.97 U/mL | 9.67 ± 4.27 U/mL | NR | NR | NR | NR | HCL | Simvastatin 20 mg | 8 |
| Ruiz MC, 2006, Spain [49] | S | 21 | NR | NR | 74 ± 22 nmol/mg | 106 ± 22 nmol/mg | 170 ± 49 U/mg | 181 ± 31 U/mg | 7.48 ± 0.84 KU × 10−5/mg |
7.59 ± 1.38 KU × 10−5/mg |
Kidney Tx | Atorvastatin 10–40 mg | 24 |
| Save V, 2006, India [50] | E | 100 | 51 | 29/71 | 1.2 ± 0.2 U/mL | 1.1 ± 0.2 U/mL | 3673 ± 369 U/gHb | 6260 ± 375 U/gHb | NR | NR | T2D | Atorvastatin 10 mg | 24 |
| Su Y, 2010 (a), China [51] | E | 75 | 55 | 39/36 | 18.96 ± 1.45 µmol/L | 21.57 ± 1.63 µmol/L | 65.73 ± 17.02 mmol/L | 96.54 ± 17.34 mmol/L | NR | NR | T2D | Simvastatin 40 mg | 12 |
| Su Y, 2010 (b), China [51] | E | 76 | 56 | 43/33 | 17.31 ± 1.11 µmol/L | 21.28 ± 0.57 µmol/L | 75.15 ± 13.31 mmol/L | 100.23 ± 15.67 mmol/L | NR | NR | T2D | Atorvastatin 10 mg | 12 |
| Janic M, 2014, Slovenia [52] | WB | 25 | 44 | 25/0 | 1.10 ± 0.25 U/gHb | 1.14 ± 0.20 U/gHb | NR | NR | NR | NR | Healthy | Fluvastatin 10 mg | 4.5 |
| Sena-Evangelista KCM, 2015, Brazil [53] | WB | 38 | 63 | 23/15 | 41.33 ± 9.62 U/gHb | 44.67 ± 13.33 U/gHb | 1415 ± 340 U/gHg | 1468 ± 265 U/gHg | NR | NR | CAD | Rosuvastatin 10 mg | 16 |
| Yildiz A, 2015, Turkey [54] | E | 18 | 38 | 9/9 | 22.37 ± 7.99 U/gHb | 30.7 ± 13.4 U/gHb | 19.09 ± 4.61 U/gHg | 24.34 ± 7.99 U/gHg | NR | NR | Kidney Tx | Fluvastatin 80 mg | 4 |
| Fassett RG, 2015, Australia [55] | P | 47 | 65 | 28/19 | 32.8 ± 10.1 U/L | 31.4 ± 11.1 U/L | NR | NR | NR | NR | CKD | Atorvastatin 10 mg | 3 years |
| Hernandez-Mijares A, 2016, Spain [56] | S | 20 | 58 | 5/15 | NR | NR | 0.81 ± 0.14 U/mL | 0.92 ± 0.18 U/mL | 29.4 ± 17.9 U/mL | 28.1 ± 13.0 U/mL | HCL | Simvastatin 40 mg | 4 |
| Abdel Magid AM, 2017, Egypt [57] | S | 30 | 51 | 15/15 | 98 ± 78 U/L | 142 ± 133 U/L | NR | NR | NR | NR | HD | Simvastatin 60 mg * | 16 |
| Hadzi-Petrushev N, 2018, Macedonia [58] | S | 20 | 43 | 20/0 | 277 ± 85 U/mL | 223 ± 95 U/mL | NR | NR | 85 ± 36 U/mL | 64 ± 47 U/mL | NAFLD | Atorvastatin 20 mg | 12 |
| Mayyas F, 2018 (a), Jordan [59] | P | 122 | 51 | 81/41 | NR | NR | 28 ± 6 U/mL | 69 ± 44 U/mL | NR | NR | ASCVD | Atorvastatin 20 mg | 12 |
| Mayyas F, 2018 (b), Jordan [59] | P | 37 | 51 | 24/13 | NR | NR | 30 ± 18 U/mL | 75 ± 43 U/mL | NR | NR | ASCVD | Atorvastatin 40 mg | 12 |
Legend: P, plasma; S, serum; E, erythrocytes; WB, whole blood; GPx, glutathione peroxidase; SOD, superoxide dismutase; Cat, catalase; HCL, hypercholesterolemia; Tx, transplant; T2D, type 2 diabetes; CAD, coronary artery disease; CKD, chronic kidney disease; HD, haemodialysis; NAFLD, non-alcoholic fatty liver disease; ASCVD, atherosclerotic cardiovascular disease; NR, not reported; *, weekly.
3.2. Glutathione Peroxidase
3.2.1. Study Characteristics
A total of 13 studies, reporting 14 treatment arms in 558 patients (mean age 54 years, 52% males), presented data on GPx concentrations [27,46,47,48,49,50,51,52,53,54,55,57,58]. Erythrocytes were assessed in five studies (six arms) [27,48,50,51,54], whole blood in two [52,53], serum in four [47,49,57,58], and plasma in the remaining two [46,55]. The statin used was atorvastatin in four studies [49,50,51,58], simvastatin in four [47,48,51,57], fluvastatin in three [27,52,54], and pravastatin [46] and rosuvastatin [53] in one, respectively. Treatment duration ranged between four weeks and three years (Table 1).
3.2.2. Risk of Bias
The risk of bias was low in 11 studies [27,46,48,49,50,51,53,54,55,57,58] and high in the remaining 2 [47,52] (Table 2).
Table 2.
The Joanna Briggs Institute Critical Appraisal Checklist.
| Study | Were the Criteria for Inclusion in the Sample Clearly Defined? | Were the Study Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Chen MF [46] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Yilmaz MI [27] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ghayour-Mobarhan M [47] | No | No | Yes | Yes | No | No | Yes | No | High |
| Molčányiová A [48] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Ruiz MC [49] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Save V [50] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Su Y [51] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Janic M [52] | No | No | Yes | Yes | No | No | Yes | No | High |
| Sena-Evangelista KCM [53] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Yildiz A [54] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Fassett RG [55] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Hernandez-Mijares A [56] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Abdel Magid AM [57] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Hadzi-Petrushev N [58] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Mayyas F [59] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
3.2.3. Results of Individual Studies and Syntheses
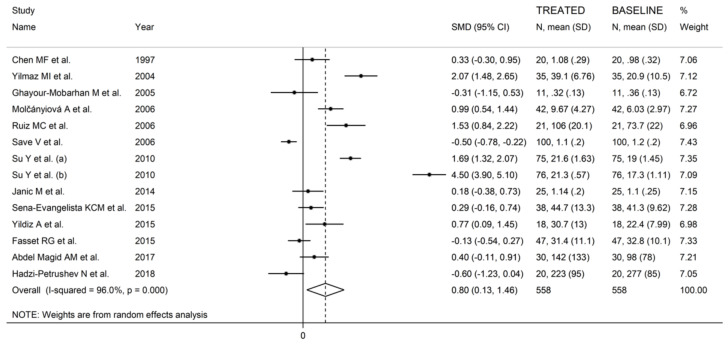
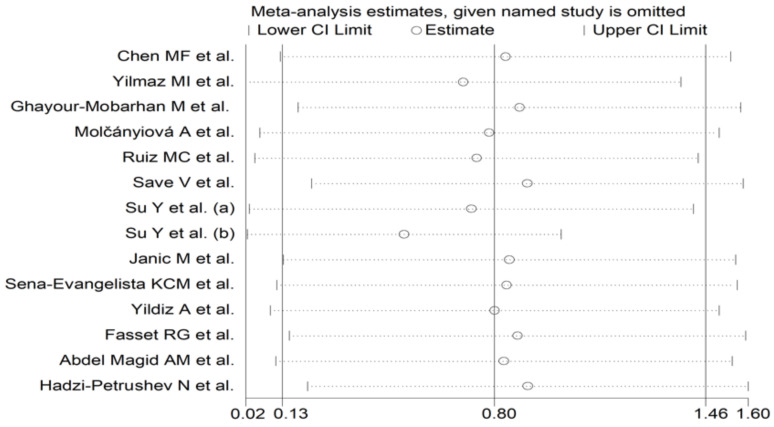
The forest plot of the GPx concentrations before and after statin treatment is shown in Figure 2. In 10 treatment arms [27,46,48,49,51,52,53,54,57], the circulating GPx concentrations were higher after statin treatment (mean difference range, 0.18 to 4.50), with a significant difference reported in six [27,48,49,51,54]. By contrast, in four arms [47,50,55,58], the GPx concentrations were lower after treatment (mean difference range, −0.13 to −0.60), with a significant difference reported in one [50]. Random-effects models were used in view of the extreme heterogeneity observed (I2 = 96.0%, p < 0.001). Pooled results showed that circulating GPx concentrations were significantly higher after statin treatment (SMD = 0.80, 95% CI 0.13 to 1.46, p = 0.018). In sensitivity analysis, the corresponding pooled SMD values were not substantially modified when individual studies were sequentially removed (effect size range between 0.52 and 0.91, Figure 3).
Figure 2.
Forest plot of studies reporting GPx concentrations before and after statin treatment.
Figure 3.
Influence of individual studies on the standardized mean difference (SMD). The hollow circles represent the SMD when the remaining study is omitted.
3.2.4. Publication Bias
There was no publication bias according to the Begg’s test (p = 0.66), the Egger’s test (p = 0.24), or the “trim-and-fill” method.
3.2.5. Sub-Group Analysis
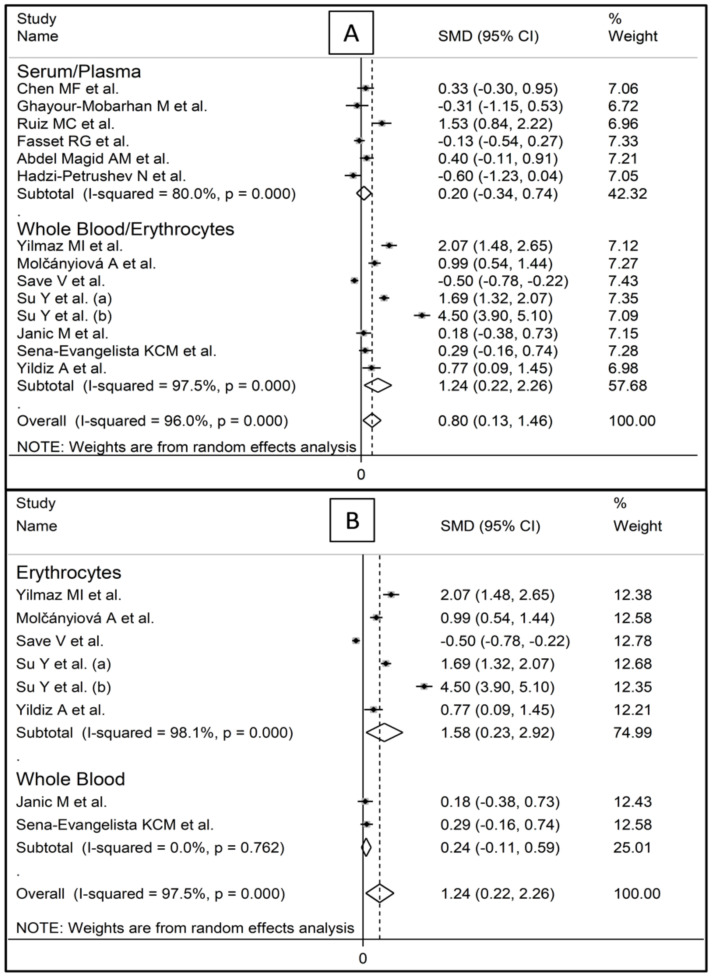
Circulating GPx concentrations were significantly higher after statin treatment in the studies assessing whole blood/erythrocytes (SMD = 1.24, 95% CI 0.22 to 2.26, p = 0.017; I2 = 97.5%, p < 0.001, Figure 4A), but not in those assessing serum/plasma (SMD = 0.20, 95% CI −0.34 to 0.74, p = 0.463; I2 = 80.0%, p < 0.001). Specifically, GPx concentrations post-treatment were significantly higher in the studies assessing erythrocytes (SMD = 1.24, 95% CI 0.22 to 2.26, p = 0.021; I2 = 98.1%, p < 0.001, Figure 4B), but not in those assessing whole blood (SMD = 0.24, 95% CI −0.11 to 0.59, p = 0.174; I2 = 97.5%, p < 0.001). In studies assessing erythrocytes, the SMD with individual statins (fluvastatin, simvastatin, atorvastatin) was similar (Figure 5).
Figure 4.
Forest plot of studies investigating GPx concentrations according to biological matrix: (A) whole blood/erythrocytes vs. plasma/serum; (B) whole blood vs. erythrocytes.
Figure 5.
Forest plot of studies of individual statins on GPx concentrations.
3.2.6. Certainty of Evidence
The initial level of certainty for GPx SMD values was moderate as the studies were interventional (rating 3, ⊕⊕⊕⊝). As 11 out of 13 studies had a low risk of bias (no rating change required), there was extreme and unexplained heterogeneity (serious limitation, downgrade one level), there was a lack of indirectness (no rating change required), the imprecision was low (narrow confidence intervals without threshold crossing, upgrade one level), the effect size was large (SMD = 0.80, upgrade one level), and there was no publication bias (no rating change required), the overall level of certainty was considered high (rating 4, ⊕⊕⊕⊕).
3.3. Superoxide Dismutase
3.3.1. Study Characteristics
A total of 8 studies, reporting 10 treatment arms in 542 patients (mean age 53 years, 52% males), presented data on SOD [27,49,50,51,53,54,56,59]. Four studies (five arms) assessed erythrocytes [27,50,51,54], two serum [49,56], and one whole blood [53] and plasma [59], respectively. The statin used was atorvastatin in four studies [49,50,51,59], simvastatin in two [51,56], fluvastatin in two [27,54], and rosuvastatin in one [53]. The treatment duration ranged between 4 and 24 weeks (Table 1).
3.3.2. Risk of Bias
The risk of bias was low in all studies [27,49,50,51,53,54,56,59] (Table 2).
3.3.3. Results of Individual Studies and Syntheses
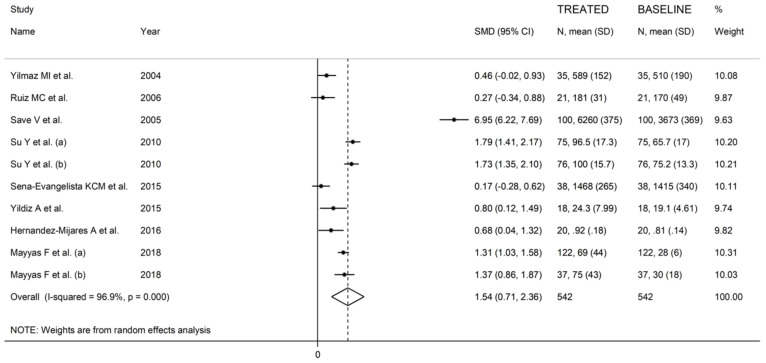
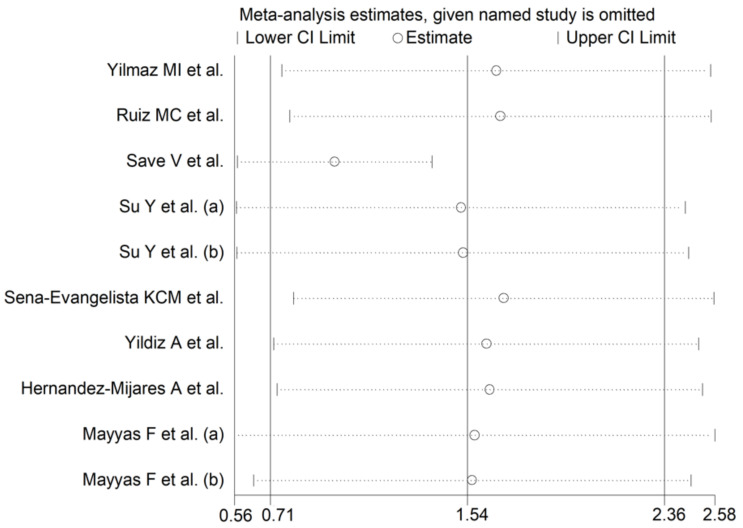
The forest plot of the circulating SOD concentrations before and after statin treatment is shown in Figure 6. In all treatment arms, SOD concentrations were higher after statin treatment (mean difference range 0.27 to 6.95), with significant differences reported in seven arms [50,51,54,56,59]. In view of the extreme heterogeneity observed (I2 = 96.9%, p < 0.001), random-effects models were used. Pooled results showed that the circulating SOD concentrations were significantly higher after statin treatment (SMD = 1.54, 95% CI 0.71 to 2.36, p < 0.001). In the sensitivity analysis, the pooled SMD values were not modified when individual studies were omitted (effect size range between 0.98 and 1.69, Figure 7).
Figure 6.
Forest plot of SOD concentrations before and after statin treatment.
Figure 7.
Sensitivity analysis describing the impact of individual studies on SOD on the standardized mean difference.
3.3.4. Publication Bias
No publication bias was observed with either the Begg’s test (p = 0.72), the Egger’s test (p = 0.61), or the “trim-and-fill” method.
3.3.5. Sub-Group Analysis
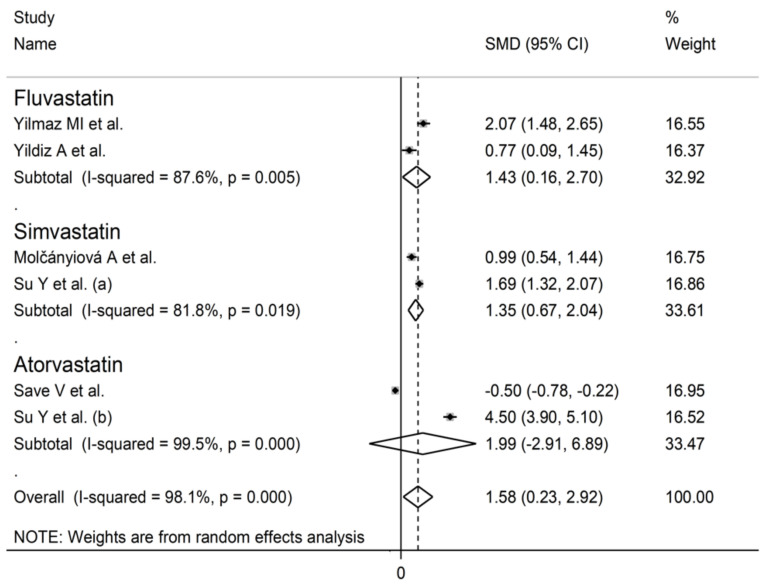
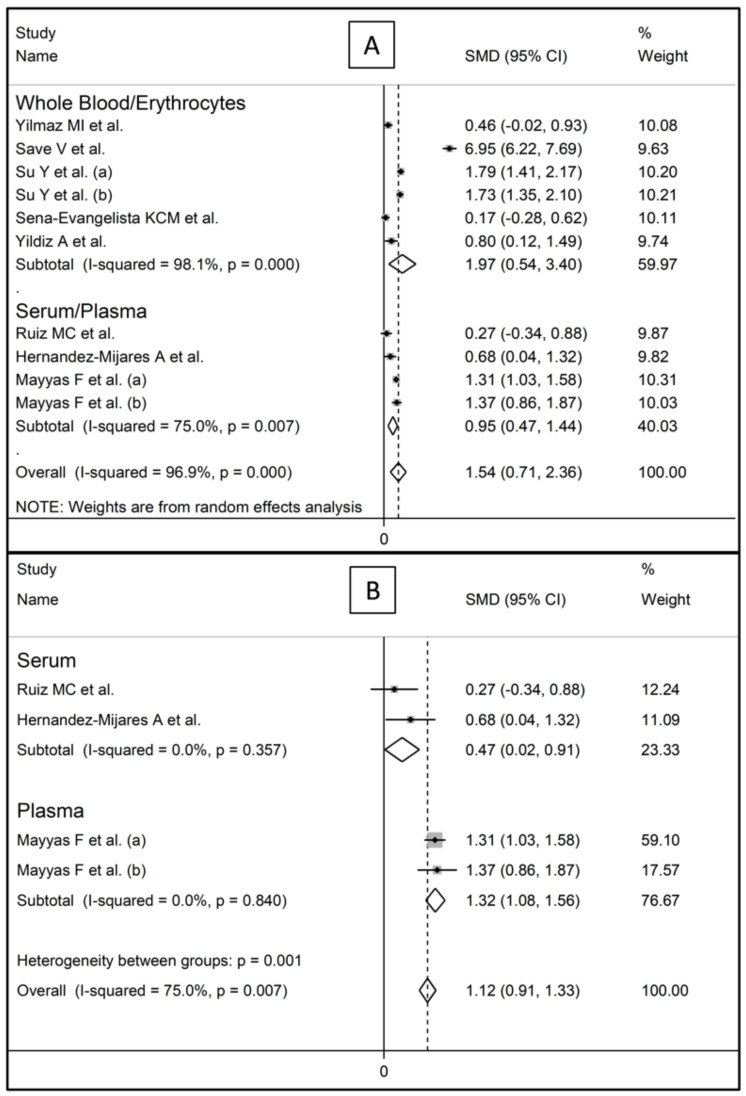
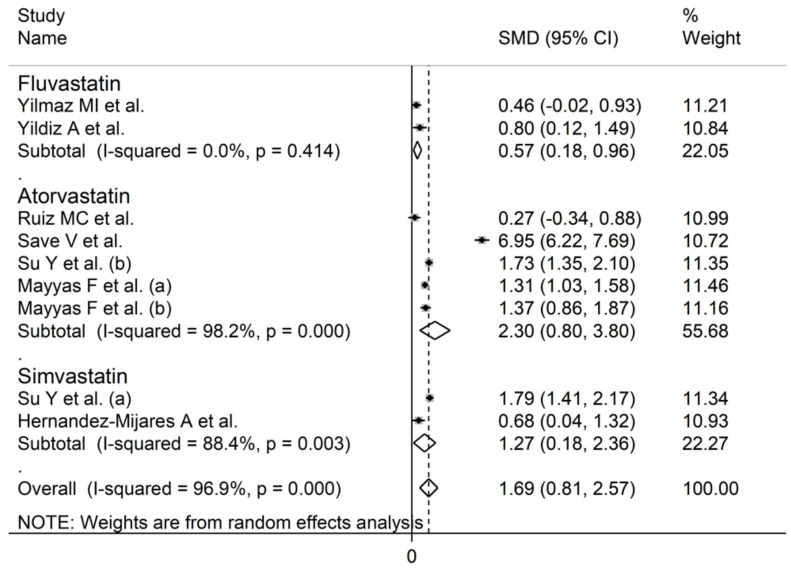
Post-treatment SOD concentrations were significantly higher both in studies assessing whole blood/erythrocytes (SMD = 1.97, 95% CI 0.54 to 3.40, p < 0.001; I2 = 98.1%, p < 0.001, Figure 8A) and in those assessing serum/plasma (SMD = 0.95, 95% CI 0.47 to 1.44, p < 0.001; I2 = 75.0%, p < 0.001). Non-significant differences in SMD (t = −3.33, p = 0.08) were observed between studies measuring SOD in plasma (SMD = 1.32, 95% CI 1.08 to 1.56, p < 0.001, Figure 8B) and those assessing serum (SMD = 0.47, 95% CI 0.02 to 0.91, p = 0.038). In both cases, however, no heterogeneity was observed (I2 = 0.0%). The SMD with individual statins (fluvastatin, simvastatin, atorvastatin) was similar (Figure 9).
Figure 8.
Forest plot of studies investigating SOD concentrations according to the biological matrix: (A) whole blood/erythrocytes vs. plasma/serum; (B) plasma vs. serum.
Figure 9.
Forest plot of studies of individual statins on SOD concentrations.
3.3.6. Certainty of Evidence
The initial certainty for the SOD SMD values was moderate (interventional studies; rating 3, ⊕⊕⊕⊝). The final level of certainty was high (rating 4, ⊕⊕⊕⊕) due to the low risk of bias in all studies (no rating change), the extreme and unexplained heterogeneity (downgrade one level), the lack of indirectness (no rating change), the low imprecision (upgrade one level), the large effect size (SMD = 1.54, upgrade one level), and the absence of publication bias (no rating change).
3.4. Catalase
3.4.1. Study Characteristics
A total of 3 studies, reporting 4 treatment arms in 61 patients (mean age 51 years, 63% males), presented data on serum catalase [49,56,58]. The statin used was atorvastatin in two studies [49,58], and simvastatin in the remaining one [56]. The treatment duration ranged between 4 and 24 weeks (Table 1).
3.4.2. Risk of Bias
The risk of bias was considered low in all studies [49,56,58] (Table 2).
3.4.3. Results of Individual Studies and Syntheses
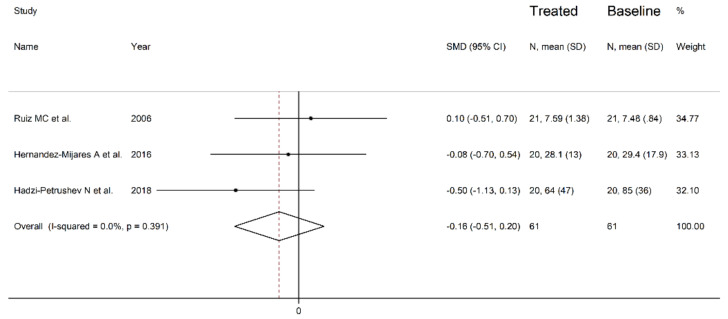
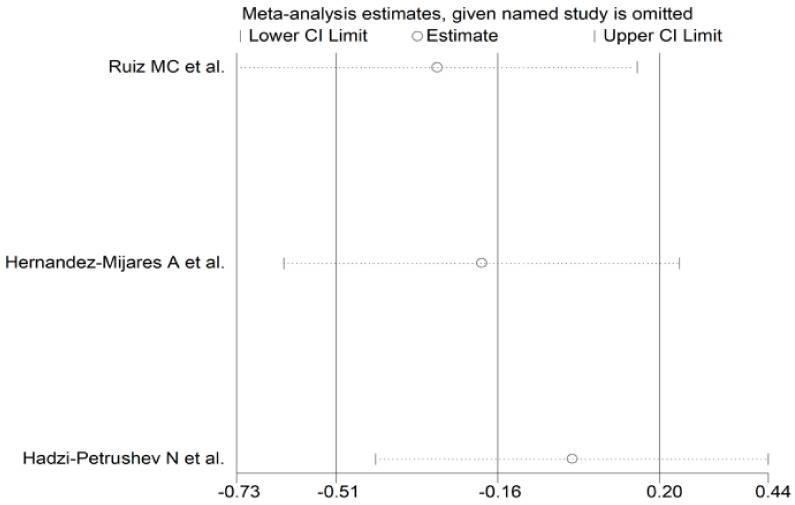
The forest plot of the circulating catalase concentrations before and after statin treatment is shown in Figure 10. The catalase concentrations increased in one study [49], and decreased in the other two [56,58]. However, in no study was a significant difference reported. Accordingly, the pooled results showed that the circulating catalase concentrations did not significantly change after statin treatment (SMD = −0.16, 95% CI −0.51 to 0.20, p = 0.391). There was a low between-study heterogeneity (I2 = 0.00%, p = 0.391). In the sensitivity analysis, the corresponding pooled SMD values were not substantially modified when individual studies were sequentially omitted (effect size range between −0.29 and 0.00, Figure 11).
Figure 10.
Forest plot of catalase concentrations before and after statin treatment.
Figure 11.
Sensitivity analysis of the influence of each study on the overall standardized mean difference.
3.4.4. Publication Bias
An assessment of publication bias was not possible due to the limited number of studies.
3.4.5. Sub-Group Analysis
A sub-group analysis was not possible due to the limited number of studies.
3.4.6. Certainty of Evidence
The initial level of certainty was moderate as the studies were interventional (rating 3, ⊕⊕⊕⊝). This was downgraded to very low (rating 0, ⊝⊝⊝⊝) after considering the low risk of bias in all studies (no change), the low heterogeneity (no change), the lack of indirectness (no change), the high imprecision (downgrade one level), the small effect size (SMD = −0.16, downgrade one level), and the lack of assessment of publication bias (downgrade one level).
4. Discussion
Statins significantly increased the circulating concentrations of the antioxidant enzymes GPx and SOD, but not catalase, in patients with various cardiovascular risk burdens. The observed SMD values for GPx (0.80) and SOD (1.54) suggest a large effect size, and therefore the presence of tangible antioxidant effects [33]. Furthermore, in sensitivity analysis, the corresponding pooled SMDs were not substantially modified when individual studies were sequentially removed. Importantly, the certainty of evidence was considered high for both GPx and SOD.
The presence of hypercholesterolemia, singly or in combination with other traditional cardiovascular risk factors, favours the production of ROS by NADPH oxidase, xanthine oxidase, the mitochondrial electron-transport chain, and uncoupled nitric oxide synthase [60,61]. This, in combination with an impaired function of key antioxidant systems that include GPx, SOD, and catalase, promotes oxidative stress and, consequently, the development of endothelial dysfunction, vascular damage, and atherosclerosis [30,62]. The main biological effects of GPx, SOD, and catalase are well established. GPx catalyses the reduction of free H2O2, a precursor of the highly reactive radical OH•, to H2O and their corresponding alcohols. While eight isoforms of GPx have been reported in humans (GPx1-8), GPx1 is the most abundant and commonly measured isoform [63]. The three isoforms of SOD (SOD1-3) catalyse the dismutation of the superoxide anion, O2−•, into O2 and H2O2 [64]. By contrast, catalase, a tetramer of four polypeptides, promotes the transformation of H2O2 into O2 and H2O [65]. The key pathophysiological role of GPx, SOD, and catalase in human atherosclerosis was highlighted in a systematic review and meta-analysis of 3 cohort and 41 case–control studies. The pooled odds ratio for coronary heart disease was significantly and inversely associated with a 1-standard deviation increase in GPx (0.51, 95% CI 0.35 to 0.75), SOD (0.48, 95% CI 0.32 to 0.72), and catalase (0.32, 95% CI 0.16 to 0.61) [31]. The associations with GPx and SOD were similar in patients with acute and chronic coronary heart disease. By contrast, the associations with catalase were stronger in patients with acute coronary heart disease [31].
Our meta-analysis supports a significant antioxidant effect of statins through the upregulation of GPx and SOD. The absence of tangible effects of statin treatment on the concentrations of catalase needs to be interpreted with caution due to the small number of eligible studies identified (n = 3). While these data are encouraging in terms of atheroprotection, the exact mechanisms involved in the statin-mediated upregulation of antioxidant enzymes require additional in vitro and in vivo studies. Furthermore, appropriately designed interventional studies are warranted to determine whether the beneficial effects of this class of drugs in terms of primary and secondary cardiovascular prevention are, at least partly, mediated by specific antioxidant effects that are independent of cholesterol lowering.
The relatively small number of studies identified for analysis and the extreme between-study heterogeneity when reporting GPx and SOD concentrations represent the significant limitations of our study. However, virtually no heterogeneity was observed in a subgroup of studies investigating SOD concentrations specifically in serum of plasma. Additional limitations include the different biological matrices used for the assessment of GPx and SOD, and the lack of serial assessment of these enzymes throughout the treatment period. Significant strengths include the lack of publication bias and the high certainty of evidence with GPx and SOD, suggesting that the effect of statins on these enzymes is both genuine and biologically plausible.
5. Conclusions
Statin treatment significantly increases the circulating concentrations of the antioxidant enzymes GPx and SOD using a range of biological matrices, suggesting the protective effects of these agents against oxidative stress. Intervention studies are warranted to investigate the antioxidant effects of statins as important mediators of their beneficial effects for primary and secondary cardiovascular prevention, to determine the most suitable biological matrix for GPx and SOD assessment, and to identify specific patient groups that are more likely to benefit from a combined antioxidant and the lipid-lowering effect of this class of drugs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antiox10111841/s1: Table S1: PRISMA 2020 abstract checklist; and Table S2: PRISMA 2020 checklist and search strategy.
Author Contributions
A.Z. and A.A.M. designed the study, screened the articles, assessed the risk of bias, extracted the data, and analysed and interpreted the data. A.A.M. wrote the first draft of the manuscript. A.A.M. and A.Z. reviewed the subsequent versions and the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu H., Daugherty A. Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015;35:485–491. doi: 10.1161/ATVBAHA.115.305380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michos E.D., McEvoy J.W., Blumenthal R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019;381:1557–1567. doi: 10.1056/NEJMra1806939. [DOI] [PubMed] [Google Scholar]
- 3.Cai T., Abel L., Langford O., Monaghan G., Aronson J.K., Stevens R.J., Lay-Flurrie S., Koshiaris C., McManus R.J., Hobbs F.R., et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021;374:n1537. doi: 10.1136/bmj.n1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirtori C.R. The pharmacology of statins. Pharmacol. Res. 2014;88:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Oesterle A., Laufs U., Liao J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouhpeikar H., Delbari Z., Sathyapalan T., Simental-Mendia L.E., Jamialahmadi T., Sahebkar A. The Effect of Statins through Mast Cells in the Pathophysiology of Atherosclerosis: A Review. Curr. Atheroscler. Rep. 2020;22:19. doi: 10.1007/s11883-020-00837-9. [DOI] [PubMed] [Google Scholar]
- 7.Bahrami A., Parsamanesh N., Atkin S.L., Banach M., Sahebkar A. Effect of statins on toll-like receptors: A new insight to pleiotropic effects. Pharmacol. Res. 2018;135:230–238. doi: 10.1016/j.phrs.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 8.John S., Schneider M.P., Delles C., Jacobi J., Schmieder R.E. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am. Heart J. 2005;149:473. doi: 10.1016/j.ahj.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Tsiara S., Elisaf M., Mikhailidis D.P. Early vascular benefits of statin therapy. Curr. Med. Res. Opin. 2003;19:540–556. doi: 10.1185/030079903125002225. [DOI] [PubMed] [Google Scholar]
- 10.Ray K.K., Cannon C.P. Early time to benefit with intensive statin treatment: Could it be the pleiotropic effects? Am. J. Cardiol. 2005;96:54F–60F. doi: 10.1016/j.amjcard.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Walter D.H. Insights into early and rapid effects of statin therapy after coronary interventions. Curr. Pharm. Des. 2004;10:369–373. doi: 10.2174/1381612043453360. [DOI] [PubMed] [Google Scholar]
- 12.Marchio P., Guerra-Ojeda S., Vila J.M., Aldasoro M., Victor V.M., Mauricio M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019;2019:8563845. doi: 10.1155/2019/8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahotupa M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic. Res. 2017;51:439–447. doi: 10.1080/10715762.2017.1319944. [DOI] [PubMed] [Google Scholar]
- 14.Khatana C., Saini N.K., Chakrabarti S., Saini V., Sharma A., Saini R.V., Saini A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020;2020:5245308. doi: 10.1155/2020/5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forstermann U., Xia N., Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 16.Khosravi M., Poursaleh A., Ghasempour G., Farhad S., Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019;400:711–732. doi: 10.1515/hsz-2018-0397. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Li Y., Li Y., Ren X., Zhang X., Hu D., Gao Y., Xing Y., Shang H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017;8:600. doi: 10.3389/fphys.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli C., Lerman L.O. Involvement of oxidation-sensitive mechanisms in the cardiovascular effects of hypercholesterolemia. Mayo Clin. Proc. 2001;76:619–631. doi: 10.1016/S0025-6196(11)62413-0. [DOI] [PubMed] [Google Scholar]
- 19.Rosenson R.S. Statins in atherosclerosis: Lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 20.Hermida N., Balligand J.L. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: The role of statins. Antioxid. Redox Signal. 2014;20:1216–1237. doi: 10.1089/ars.2013.5537. [DOI] [PubMed] [Google Scholar]
- 21.Margaritis M., Channon K.M., Antoniades C. Statins as regulators of redox state in the vascular endothelium: Beyond lipid lowering. Antioxid. Redox Signal. 2014;20:1198–1215. doi: 10.1089/ars.2013.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colucci R., Fornai M., Duranti E., Antonioli L., Rugani I., Aydinoglu F., Ippolito C., Segnani C., Bernardini N., Taddei S., et al. Rosuvastatin prevents angiotensin II-induced vascular changes by inhibition of NAD(P)H oxidase and COX-1. Br. J. Pharmacol. 2013;169:554–566. doi: 10.1111/j.1476-5381.2012.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rueckschloss U., Galle J., Holtz J., Zerkowski H.R., Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: Antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 24.Zinellu A., Paliogiannis P., Usai M.F., Carru C., Mangoni A.A. Effect of statin treatment on circulating malondialdehyde concentrations: A systematic review and meta-analysis. Ther. Adv. Chronic. Dis. 2019;10:2040622319862714. doi: 10.1177/2040622319862714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margaritis M., Sanna F., Antoniades C. Statins and oxidative stress in the cardiovascular system. Curr. Pharm. Des. 2017;23:7040–7047. doi: 10.2174/1381612823666170926130338. [DOI] [PubMed] [Google Scholar]
- 26.Ota H., Eto M., Kano M.R., Kahyo T., Setou M., Ogawa S., Iijima K., Akishita M., Ouchi Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler. Thromb. Vasc. Biol. 2010;30:2205–2211. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz M.I., Baykal Y., Kilic M., Sonmez A., Bulucu F., Aydin A., Sayal A., Kocar I.H. Effects of statins on oxidative stress. Biol. Trace Elem. Res. 2004;98:119–127. doi: 10.1385/BTER:98:2:119. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser U., Bahlmann F., Mueller M., Spiekermann S., Kirchhoff N., Schulz S., Manes C., Fischer D., de Groot K., Fliser D., et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 29.Carrepeiro M.M., Rogero M.M., Bertolami M.C., Botelho P.B., Castro N., Castro I.A. Effect of n-3 fatty acids and statins on oxidative stress in statin-treated hypercholestorelemic and normocholesterolemic women. Atherosclerosis. 2011;217:171–178. doi: 10.1016/j.atherosclerosis.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Horke S., Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Flores-Mateo G., Carrillo-Santisteve P., Elosua R., Guallar E., Marrugat J., Bleys J., Covas M.I. Antioxidant enzyme activity and coronary heart disease: Meta-analyses of observational studies. Am. J. Epidemiol. 2009;170:135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 32.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Lisy K., Qureshi R., Mattis P., et al. Systematic reviews of etiology and risk. In: Aromataris E., Munn Z., editors. Joanna Briggs Institute Reviewer’s Manual. Johanna Briggs Institute; Adelaide, Australia: 2017. [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 34.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Hultcrantz M., Rind D., Akl E.A., Treweek S., Mustafa R.A., Iorio A., Alper B.S., Meerpohl J.J., Murad M.H., Ansari M.T., et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017;87:4–13. doi: 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Coello P.A., Guyatt G.H., Yepes-Nuñez J.J., Akl E.A., Hazlewood G., Pardo-Hernandez H., Etxeandia-Ikobaltzeta I., Qaseem A., Williams J.W., Jr., et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J. Clin. Epidemiol. 2019;111:83–93. doi: 10.1016/j.jclinepi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 42.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999;47:15–17. [Google Scholar]
- 43.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 44.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 45.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen M.F., Hsu H.C., Lee Y.T. Short-term treatment with low-dose pravastatin attenuates oxidative susceptibility of low-density lipoprotein in hypercholesterolemic patients. Cardiovasc. Drugs Ther. 1997;11:787–793. doi: 10.1023/A:1007722426016. [DOI] [PubMed] [Google Scholar]
- 47.Ghayour-Mobarhan M., Lamb D.J., Taylor A., Vaidya N., Livingstone C., Wang T., Ferns G.A. Effect of statin therapy on serum trace element status in dyslipidaemic subjects. J. Trace Elem. Med. Biol. 2005;19:61–67. doi: 10.1016/j.jtemb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Molcanyiova A., Stancakova A., Javorsky M., Tkac I. Beneficial effect of simvastatin treatment on LDL oxidation and antioxidant protection is more pronounced in combined hyperlipidemia than in hypercholesterolemia. Pharmacol. Res. 2006;54:203–207. doi: 10.1016/j.phrs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz M.C., Moreno J.M., Ruiz N., Vargas F., Asensio C., Osuna A. Effect of statin treatment on oxidative stress and renal function in renal transplantation. Transplant. Proc. 2006;38:2431–2433. doi: 10.1016/j.transproceed.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 50.Save V., Patil N., Moulik N., Rajadhyaksha G. Effect of atorvastatin on type 2 diabetic dyslipidemia. J. Cardiovasc. Pharmacol. Ther. 2006;11:262–270. doi: 10.1177/1074248406295523. [DOI] [PubMed] [Google Scholar]
- 51.Su Y., Xu Y., Sun Y.M., Li J., Liu X.M., Li Y.B., Liu G.D., Bi S. Comparison of the effects of simvastatin versus atorvastatin on oxidative stress in patients with type 2 diabetes mellitus. J. Cardiovasc. Pharmacol. 2010;55:21–25. doi: 10.1097/FJC.0b013e3181bfb1df. [DOI] [PubMed] [Google Scholar]
- 52.Janic M., Lunder M., Prezelj M., Sabovic M. A combination of low-dose fluvastatin and valsartan decreases inflammation and oxidative stress in apparently healthy middle-aged males. J. Cardiopulm. Rehabil. Prev. 2014;34:208–212. doi: 10.1097/HCR.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 53.Sena-Evangelista K.C.M., Pedrosa L.F.C., Paiva M.S.M.O., Dias P.C.S., Ferreira D.Q.C., Cozzolino S.M.F., Faulin T.E.S., Abdalla D.S.P. The hypolipidemic and pleiotropic effects of rosuvastatin are not enhanced by its association with zinc and selenium supplementation in coronary artery disease patients: A double blind randomized controlled study. PLoS ONE. 2015;10:e0119830. doi: 10.1371/journal.pone.0119830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yildiz A., Gul C.B., Ocak N., Ersoy A.L.P.A.R.S.L.A.N., Sag S., Oruc A., Ayar Y., Dagel T., Dirican M., Gullulu M. Fluvastatin Decreases Oxidative Stress in Kidney Transplant Patients. Transplant. Proc. 2015;47:2870–2874. doi: 10.1016/j.transproceed.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Fassett R.G., Robertson I.K., Ball M.J., Geraghty D.P., Coombes J.S. Effects of atorvastatin on oxidative stress in chronic kidney disease. Nephrology. 2015;20:697–705. doi: 10.1111/nep.12502. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez-Mijares A., Bañuls C., Rovira-Llopis S., Diaz-Morales N., Escribano-Lopez I., de Pablo C., Alvarez A., Veses S., Rocha M., Victor V.M. Effects of simvastatin, ezetimibe and simvastatin/ezetimibe on mitochondrial function and leukocyte/endothelial cell interactions in patients with hypercholesterolemia. Atherosclerosis. 2016;247:40–47. doi: 10.1016/j.atherosclerosis.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 57.Abdel Magid A.M., Abbassi M.M., Iskander E.E.M., Mohamady O., Farid S.F. Randomized comparative efficacy and safety study of intermittent simvastatin versus fenofibrate in hemodialysis. J. Comp. Eff. Res. 2017;6:413–424. doi: 10.2217/cer-2016-0076. [DOI] [PubMed] [Google Scholar]
- 58.Hadzi-Petrushev N., Dimovska K., Jankulovski N., Mitrov D., Mladenov M. Supplementation with Alpha-Tocopherol and Ascorbic Acid to Nonalcoholic Fatty Liver Disease’s Statin Therapy in Men. Adv. Pharmacol. Sci. 2018;2018:4673061. doi: 10.1155/2018/4673061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayyas F., Baydoun D., Ibdah R., Ibrahim K. Atorvastatin Reduces Plasma Inflammatory and Oxidant Biomarkers in Patients With Risk of Atherosclerotic Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2018;23:216–225. doi: 10.1177/1074248417753677. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira H.C.F., Vercesi A.E. Mitochondrial bioenergetics and redox dysfunctions in hypercholesterolemia and atherosclerosis. Mol. Asp. Med. 2020;71:100840. doi: 10.1016/j.mam.2019.100840. [DOI] [PubMed] [Google Scholar]
- 61.Sozen E., Ozer N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas S.R., Witting P.K., Drummond G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 63.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A.F., Teixeira M., Valentine J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandi A., Yan L.J., Jana C.K., Das N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019;2019:9613090. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.