Abstract
The spread of resistant bacteria from livestock to the food industry promoted an increase of alternative poultry production systems, such as organic and antibiotic-free ones, based on the lack of antimicrobial use, except in cases in which welfare is compromised. We aimed to investigate the antibiotic susceptibility of commensal Escherichia coli isolated from organic, antibiotic-free, and conventional broiler farms and slaughterhouses toward several antimicrobials critically important for human health. To assess antimicrobial susceptibility, all E. coli isolates and extended spectrum beta-lactamase (ESBL) E. coli were analysed by the microdilution method. The prevalence of tigecycline, azithromycin and gentamicin E. coli-resistant strains was highest in organic samplings. Conversely, the lowest prevalence of resistant E. coli strains was observed for cefotaxime, ceftazidime and ciprofloxacin in organic systems, representing a significant protective factor compared to conventional systems. All E. coli strains were colistin-susceptible. Contamination of the external environment by drug-resistant bacteria could play a role in the presence of resistant strains detected in organic systems. Of interest is the highest prevalence of cephalosporin resistance of E. coli in conventional samplings, since they are not permitted in poultry. Our results suggest that monitoring of antibiotic resistance of the production chain may be helpful to detect “risks” inherent to different rearing systems.
Keywords: broiler chicken, antimicrobial susceptibility, microdilution test, commensal E. coli, organic system, antibiotic-free system, conventional system
1. Introduction
Over the years, the rapidly increasing demand for livestock products by the human population has led to the development of intensive production of food animals, such as cattle, poultry and pigs [1], and the increase of antimicrobial resistance, due to the uncontrolled use of antimicrobials [2,3] for the prevention and treatment of infections, promotion of growth and improvement in production [4].
Furthermore, waste from hospitals and livestock producers containing antimicrobial residues have facilitated the selection of resistomes in the environment with potential diffusion to animals and humans [5]. In this context, Escherichia coli, along with Salmonella and Campylobacter [6,7], is one of the bacteria most responsible for resistant gene co-circulation among the environment, animals and humans. In addition to resistance against classical molecules that have been overused in the zootechnical sector, there has been an increase in the prevalence of resistance toward antimicrobials prevalently used in human medicine, such as extended-spectrum cephalosporins (ESC), including the third- and fourth-generation cephalosporins [8], neomycin, apramycin and tigecycline [9,10]. Tigecycline with colistin is considered to be a last resort defence against infections due to carbapenem-resistant Enterobacteriaceae and multidrug resistant (MDR) Gram-negative bacteria [11,12]. Decreased susceptibility to colistin and tigecycline are justified by encoded intrinsic resistance and the presence of mobile colistin and tigecycline resistance genes [13,14]. A transferable plasmid-derived colistin resistance gene mcr-1 was found to be responsible for resistance occurrence worldwide [15,16]. It should be considered that colistin has been widely administered for the prevention, treatment, metaphylaxis and growth promotion in veterinary medicine for years [17,18]. Among food-producing animals, several studies have reported a notable prevalence of colistin resistance in poultry [19,20,21]. It should be stressed that there has been an alarming increase of bacteria resistant to third-generation cephalosporins, which reinforces the suspicion of possible “non-official” use in chicks at hatch [22,23]. Actually, it is known that the use of cephalosporins in poultry and other species was prohibited by the Food and Drug Administration [24], since they could be responsible for triggering resistance to these classes of antimicrobials in humans. During the last decade, a progressive increase in ESBL E. coli associated with a multi-resistance profile has also been documented on chicken farms [25]. This, in turn, has caused concern for public health, as poultry meat is widely consumed, both for its nutritional characteristics and the economic benefits related to low costs [26]. Moreover, the diffusion of ESBL could result in unsuccessful therapeutic treatment in human infections and also require the use of “last resort antibiotics” (e.g., carbapenems) causing an increased resistance to these antibiotic classes [27,28]. In this scenario, poultry producers have turned to alternative production systems, such as organic (O) and antibiotic-free (AF) farming, based on the lack of antimicrobial use, unless animal welfare is at risk [29]. In our work, we aimed to investigate the antibiotic susceptibility of commensal E. coli, especially towards antimicrobials used in human therapy, isolated in organic, antibiotic-free and conventional (C) broilers on farms and at slaughter. The diffusion of ESBL E. coli was also assayed.
2. Results
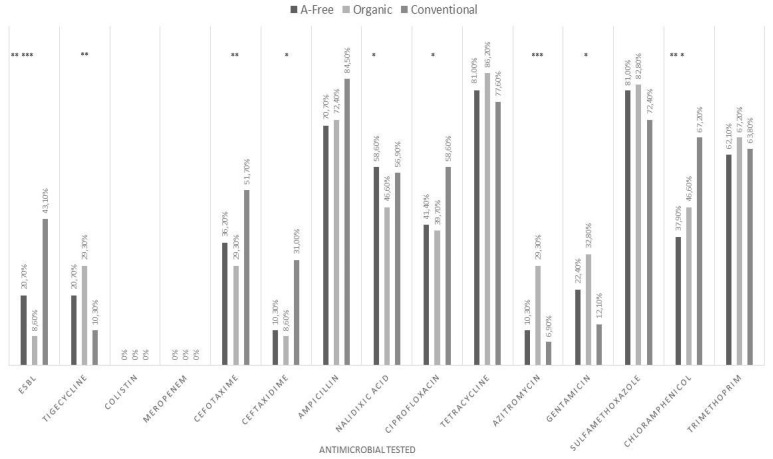
With respect to antimicrobial susceptibility, O and AF samplings showed higher prevalence of E. coli-resistant strains to tigecycline than C (29.3% in O, 20.7% in AF, and 10.3% in C) (Figure 1) with an odds ratio (OR) of 3.59 (p = 0.01) for O (Table 1). All E. coli strains isolated from O, AF and C had minimal inhibitory concentration (MIC) values for tigecycline within 1–4 μg/mL (Table S1). The O samplings showed the highest prevalence of E. coli-resistant strains for azithromycin (29.3% vs. 10.3% in AF and 6.9% in C) with OR = 4.39 (p = 0.001), and for gentamicin (32.8% vs. 22.4% in AF and 12.1% in C) with OR = 2.34 (p = 0.02) when compared to the C systems (Figure 1 and Table 1). The MIC values for the most azithromycin-resistant E. coli strains isolated from O samplings were greater than 64 μg/mL (Table S1). The O samplings exhibited the lowest prevalence of resistance of E. coli to cefotaxime and ceftazidime-resistant strains. The prevalence rates of E. coli cefotaxime-resistant strains were 29.3% in O, 36.2% in AF and 51.7% in C with OR = 0.39 (p = 0.01) and the prevalence of ceftazidime-resistant strains was 8.6% in O, 10.3% in AF and 31% in C with an OR of 0.36 (p = 0.02) (Figure 1 and Table 1).
Figure 1.
Prevalence of antimicrobial-resistant E. coli strains in conventional, organic and antibiotic-free systems. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
Table 1.
Multivariate logistic regression analysis for ESBL E. coli, MDR E. coli strain prevalence and E. coli antimicrobial susceptibility (only significant ORs, and their 95% confidence interval, are reported in the table).
| Outcome | Explanatory Variable | Level | OR * | 95 % Confidence Interval | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| ESBL strains | Type of rearing | Conventional | ref a | - | - | - | |
| Organic | 0.13 | 0.04 | 0.37 | <0.001 | |||
| AF | 0.33 | 0.14 | 0.76 | 0.009 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | 2.72 | 1.23 | 6.02 | 0.01 | |||
| MDR strains | Type of rearing | Conventional | - | - | - | ||
| Organic | - | - | - | 0.17 | |||
| AF | - | - | - | 0.76 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.85 | |||
| Tigecycline | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 3.59 | 1.3 | 9.93 | 0.01 | |||
| AF | - | - | - | 0.13 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.15 | |||
| Colistin | Type of rearing | Conventional 1 | ref | - | - | - | |
| Organic | - | - | - | 1.00 | |||
| AF | - | - | - | 1.00 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 1.00 | |||
| Meropenem | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 1.00 | |||
| AF | - | - | - | 0.98 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.61 | |||
| Cefotaxime | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.39 | 0.18 | 0.83 | 0.01 | |||
| AF | - | - | - | 0.09 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.55 | |||
| Ceftaxidime | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.36 | 0.15 | 0.83 | 0.02 | |||
| AF | - | - | - | 0.44 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.85 | |||
| Ampicillin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.14 | |||
| AF | - | - | - | 0.16 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.60 | |||
| Nalidixic acid | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | ||||
| AF | 2.32 | 1.07 | 5.07 | 0.03 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.48 | |||
| Ciprofloxacin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.46 | 0.22 | 0.97 | 0.04 | |||
| AF | - | - | - | 0.06 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.17 | |||
| Tetracycline | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.07 | |||
| AF | - | - | - | 0.23 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.45 | |||
| Azitromycin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 4.39 | 1.86 | 10.39 | 0.001 | |||
| AF | - | - | - | 0.99 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.05 | |||
| Gentamicin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 2.34 | 1.13 | 4.85 | 0.02 | |||
| AF | - | - | - | 0.13 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.89 | |||
| Sulfamethoxazole | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.2 | |||
| AF | - | - | - | 0.24 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.94 | |||
| Chloramphenicol | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.42 | 0.2 | 0.9 | 0.03 | |||
| AF | 0.3 | 0.14 | 0.64 | 0.002 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.22 | |||
| Trimethoprim | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.38 | |||
| AF | - | - | - | 0.85 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.36 | |||
* Odds ratio. Only the significant values are reported. a Reference category. p ≤ 0.05.
The lowest prevalence for E. coli ciprofloxacin-resistant strains was detected in O samplings (39.7% vs. 58.6% in C and 41.4% in AF with OR = 0.46, (p = 0.04) (Figure 1 and Table 1). The highest prevalence of E. coli chloramphenicol-resistant strains was found in C samplings (67.2% in C, 46.6% in O and 37.9% in AF) (Figure 1). The prevalence of nalidixic acid-resistant E. coli in the AF, C and O systems was 58.6%, 56.9% and 46.6%, respectively (Figure 1). The majority of the resistant strains displayed MIC values ≥ 128 μg/mL for nalidixic acid (Table S1). All E. coli strains isolated from the O and C samplings were susceptible to meropenem (Table S1). We isolated 10.3% of susceptible, increased exposure [30] E. coli to meropenem from AF samplings at slaughter with 4 μg/mL MIC values. All E. coli strains were colistin-susceptible (MIC range, 0.5–1 μg/mL) (Table S1). The lowest prevalence of ESBL E. coli was found in the O and AF samplings (8.6% in O, 20.7% in AF and 43.1% in C) (Figure 1, with an OR of 0.13 (p < 0.001) and OR = 0.33 (p = 0.009), respectively (Figure 1 and Table 1). Farm, when compared to slaughterhouse, resulted in a protective factor for the presence of ESBL E. coli strains (OR = 2.72; p = 0.01) (Table 1).
No statistical differences were found in the multi-resistance among the E. coli isolates from the three systems. Most of the E. coli strains were resistant to five (AF, 22.1%; O, 30.6%; and C, 28.9%) and six antimicrobials (AF, 23.8%; O, 23.8%; and C, 17.0%) (Table S2).
3. Discussion
Escherichia coli is a normal constituent of the intestinal microbial flora of poultry, although it is also a potential pathogen associated with serious diseases, such as colibacillosis. Moreover, it can also represent a potential source of resistant genes transferable to humans, posing a public health threat [31]. In this context, we aimed to investigate if the different typology of farming can influence the antimicrobial susceptibility of E. coli isolated on farms and at slaughter. Our study revealed that the organic and antibiotic-free samplings showed the highest prevalence of E. coli-resistant strains to tigecycline. The conventional system resulted in a significant protective factor for the presence of the tigecycline-resistant strain of E. coli when compared to organic and antibiotic-free management. Tigecycline is a broad-spectrum glycylcycline synthesised to overcome tetracycline resistance [32]. Its use has indeed developed in response to increasing resistance of Staphylococcus aureus, Acinetobacter baumannii, and E. coli to a majority of antimicrobials [33]. In order to maintain tigecycline efficacy, the European Commission (EC) requested that the European Medicine Agency (EMA) provide scientific advice on the impact of its use in animals on human health and recommend the restricted use of glycylcyclines in veterinary medicine. Tigecycline has not really been used on conventional poultry farms and the high prevalence of E. coli-resistant strains found in organic systems could likely be dependent on the resistant strains present in the external environment. Recently, Sun et al. [34] demonstrated a significant presence of tigecycline-resistant E. coli strains from raw meat bearing the tet (X) gene. It should also be highlighted that in our investigation, all E. coli strains were susceptible to colistin differently than what was observed in other studies [35,36,37]. However, it should be specified that colistin, after being administrated for a long time as a prophylactic and therapeutic agent, is currently in off-label use in Italy and it was banned as a growth promoter [35]. The large use in poultry has been favoured by the lack of withdrawal time for eggs and meat [38,39]. Moreover, colistin, though considered efficient against human Enterobacteriaceae infections, was replaced because of its systemic toxicity [40]. Recently, the emergence of the MDR (multidrug resistant ) bacteria, responsible for severe infections and deaths in humans, have led to addressing the reconsideration of the colistin as a last-resort antibiotic against these “superbug” bacteria [12]. Additionally of interest are the results related to the prevalence of azithromycin and gentamicin-resistant E. coli strains, which were found to be higher in organic samples than in antibiotic-free and conventional ones. It should be considered that azithromycin has not been used in poultry and the results observed in organic samples could also be justified by external contamination likely due to wildlife [41,42]. Recently, azithromycin became the first choice drug for the treatment of enterotoxigenic E. coli (ETEC) infections in human medicine since a progressive resistance has been described for nalidixic acid, ampicillin, sulphonamides and tetracycline [43]. Gentamicin has been used against poultry diseases, such as respiratory diseases and necrotic enteritis [44,45]. At present, this medication is largely administered in industrial poultry production due to poor intestinal absorption and the lack of residues in edible chicken tissues [39,46]. The prevalence of ceftazidime and cefotaxime E. coli-resistant strains were highest in the conventional samplings. Thus, the organic system represented a significant protective factor for the presence of cefotaxime, ceftazidime and ciprofloxacin-resistant strain E. coli when compared to C management. Several E. coli strains have acquired the capability to inactivate a large number of β-lactam antibiotics, including penicillin and extended-spectrum cephalosporins, such as third- and fourth-generation cephalosporins [47]. Furthermore, in the last years ESBL/AmpC E. coli has been frequently found as a contaminant in broiler meat [48]. It should be mentioned that the increased use of amoxicillin and ampicillin in poultry therapy might have promoted the rise of ESBL E. coli. In particular, these antimicrobials have been used against enteric disorders associated with an overgrowth of Clostridium perfringens [49,50] following the ban of the use of antibiotics as feed growth promoters in industrial chickens [51]. Our results showed that antibiotic-free and organic systems, that had not used antimicrobials, were protective factors for ESBL presence if compared to conventional management.
Furthermore, we observed the highest prevalence of chloramphenicol-resistant E. coli strains in conventional samples. The use of chloramphenicol in livestock has been banned in Europe since 1997 [29]. In our work, the resistance to chloramphenicol described in the conventional samplings could be explained by the possible effects of repeated antibiotic treatments with amoxicillin, as described for broilers [52]. Recently, Burow et al. [53] have experimentally demonstrated an increase in resistance towards several antimicrobials (tetracycline, ciprofloxacin, nalidixic acid, gentamicin) after oral administration of amoxicillin, which is commonly used in poultry practice. Finally, ESBL E. coli strains were higher at slaughter than in the farm. A cross-contamination mechanism can occur during scalding and/or evisceration and dressing, although contamination with ESBL-producing Enterobacteriaceae may be due to workers responsible for the contamination of live animals, carcasses or organs, as occurs in pigs [54]. With respect to the detection of MDR strains, five and six patterns of multi-resistance were found, although no statistical differences in prevalence were seen among the different rearing systems. Previous works have demonstrated that meat products from conventionally raised poultry exhibited MDR bacteria more frequently than meat products from antibiotic-free or organic chickens [55,56], although some exceptions [57,58] have been reported.
4. Materials and Methods
4.1. Sampling
A total of 174 strains were collected from samplings performed on conventional (C), organic (O) and antibiotic-free (AF) chicken farms (cloacal swabs and environmental samples) and at slaughter (caecal contents and skin samples). In particular, 58 strains of E. coli were collected for each typology of farming: in C (25 and 33 on farm and in slaughterhouse, respectively), in O (31 and 27 on farm and in slaughterhouse, respectively) and in AF (25 and 33 on farm and in slaughterhouse, respectively).
4.2. Isolation and Identification of E. coli
All samples were placed in a pre-enrichment medium consisting of buffered peptone water (BPW) at a ratio of 1:10 and then incubated at 37 °C for 18–24 h in aerobiosis, after which 0.1 mL from each diluted sample was plated on MacConkey agar with an added low concentration (1 mg/L) of cefotaxime (Thermo Fisher Scientific, Milan, Italy). The plates were incubated for 24 h at 37 °C under aerobic conditions. All colonies with typical E. coli morphology were selected and confirmed by biochemical tests [59].
4.3. Antibiotic Susceptibility Testing and ESBL E. coli Detection
To assess antimicrobial susceptibility, all E. coli isolates were analysed by the broth microdilution method. Pure cultures were suspended in 4 mL of 0.90% sterile saline solution (final concentration: 5 × 107 CFU/mL CFU/mL−1), equivalent to a 0.5 McFarland turbidity level (Vitek, bioMérieux Inc., Durham, United States). Ten microlitres of bacterial suspension was transferred to 11 mL of cation-adjusted Mueller Hinton broth (Thermo Fisher Scientific, Milan, Italy) and 50 μL of bacterial suspension was dispensed into each well of Euvsec microtitre plates (Thermo Fisher Scientific, Milan, Italy) with scalar concentrations of the following antibiotics: sulfamethoxazole (SMX) (8–1024 μg/mL), trimethoprim (TMP) (0.25–32 μg/mL), ciprofloxacin (CIP) (0.03–8 μg/mL), tetracycline (TET) (2–64 μg/mL), meropenem (MERO) (0.06–16μg/mL), azithromycin (AZI) (2–64 μg/mL), nalidixic acid (NAL) (4–128 μg/mL), cefotaxime (FOT) (0.25–1 μg/mL), chloramphenicol (CHL) (8–128 μg/mL), tigecycline (TGC) (0.25–8 μg/mL), ceftazidime (TAZ) (2–8 μg/mL), colistin (COL) (1–16 μg/mL), ampicillin (AMP) (1–64 μg/mL), and gentamicin (GEN) ( 0.5–32 μg/mL). After inoculation, the plates were incubated for 24 h at 37 °C under aerobic conditions. Susceptibility to colistin was evaluated using the FRCOL Plates (0.12–128 μg/mL) (Thermo Fisher Scientific, Milan, Italy). For all E. coli, ESBL production was confirmed by the double-disc synergy test (DDST) [59] and the microdilution method using Sensititre™ ESBL plates (Thermo Fisher Scientific, Milan, Italy). E. coli ATCC 25922 was used as the quality control strain. The results were evaluated according to the breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [60], with the exception of sulfamethoxazole, tetracycline, azithromycin and nalidixic acid, for which breakpoints published by the Clinical and Laboratory Standard Institute (CLSI) were applied [61].
4.4. Statistical Analysis
Logistic regression was used to weigh the overall effect of the types of rearing systems (C, AF and O) or the effect of the sampling on farm or at slaughterhouse level on antibiotic resistance. These variables were examined separately for their association with antibiotic-resistant E. coli strains and the presence of EBSL E. coli. Variables scoring p ≤ 0.20 in an early univariate analysis were included in the regression model. Non-significant variables were manually removed from the model. ORs and corresponding 95% confidence intervals (CIs) were obtained by means of logistic regression. Data were analysed by commercial software R, version 2.8.1 (R, Development Core Team 2007). A value of p ≤ 0.05 was considered statistically significant for the analysis.
5. Conclusions
Our research evidenced that rearing systems can influence the antimicrobial susceptibility of E. coli, as seen in the organic system for resistance to some antibiotics, such as tigecycline and azithromycin, used in human therapy. The organic system represents a protective factor if compared to conventional system for the lowest prevalence of E. coli-resistant strains to cefotaxime and ceftazidime. Our data evidenced that the environment can represent an important source of resistant bacteria for organic farming. Wild animals [62], insects [63], wastewater, and zootechnical waste [64] used as fertilizers are described to contaminate the environment in relation to the geographical area. Based on our data it is not still possible to be conclusive in individuating the “ideal farming” in relation to antibiotic-resistance relevance. There are also numerous components influencing the choice of the system, such as the geographical area and the economic factors. Farming is generally a protective factor if compared to slaughterhouse, in relation to the prevalence of ESBL E. coli strains. Thus, particular attention must be paid to the hygiene of the slaughtering process to avoid cross-contamination that can influence the best practices adopted at the farm level to reduce the prevalence of ESBL and antibiotic resistance bacteria in poultry meat. Continuous epidemiological monitoring of the antibiotic resistance of the production chain could be helpful to notice situations of “risk” inherent to different types of farming systems in order to apply corrective factors to various categories of production (control of the breeders) and different rearing systems (i.e., control of wildlife and environment for organic farming), as well as at slaughter (carcasses and environmental contamination), in order to optimise final production and guarantee food safety for consumers.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10111321/s1, Table S1. Distribution of minimal inhibitory concentration (MIC) of E. coli strains isolated from organic, antibiotic-free and conventional systems toward the selected antimicrobials. Table S2: Resistance patterns in E. coli isolated from antibiotic-free (AF), organic (O), and conventional (C) samples.
Author Contributions
Conceptualization, M.P.F., P.C.P. and L.M.; methodology, P.C.P. and L.M.; software, M.LM.; investigation, L.M., T.S.K., V.S., F.B., C.F.M., V.T. and R.B.; resources, D.R.; data curation, M.L.M.; writing—original draft preparation, M.P.F., P.C.P., L.M. and M.L.M.; writing—review and editing, M.P.F., P.C.P. and L.M.; All authors have read and agreed to the published version of the manuscript.
Funding
L.M discloses receiving PhD scholarship funding by the Ph.D. Program of the University of Perugia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ilea R.C. Intensive Livestock Farming: Global Trends, Increased Environmental Concerns, and Ethical Solutions. J. Agric. Environ. Ethic. 2008;22:153–167. doi: 10.1007/s10806-008-9136-3. [DOI] [Google Scholar]
- 2.Alonso C.A., Zarazaga M., Ben Sallem R., Jouini A., BEN Slama K., Torres C. Antibiotic resistance inEscherichia coliin husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017;64:318–334. doi: 10.1111/lam.12724. [DOI] [PubMed] [Google Scholar]
- 3.Chimera E.T., Fosgate G.T., Etter E.M., Boulangé A., Vorster I., Neves L. A one health investigation of pathogenic trypanosomes of cattle in Malawi. Prev. Veter. Med. 2021;188:105255. doi: 10.1016/j.prevetmed.2020.105255. [DOI] [PubMed] [Google Scholar]
- 4.Castanon J. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 5.Drug-Resistant Infections: A Threat to Our Economic Future. World Bank; Washington, DC, USA: 2017. [Google Scholar]
- 6.Roila R., Ranucci D., Valiani A., Galarini R., Servili M., Branciari R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2019;18:43–52. doi: 10.17306/J.AFS.0629. [DOI] [PubMed] [Google Scholar]
- 7.Grace D. Review of evidence on antimicrobial resistance and animal agriculture in developing countries. Evid. Demand Int. Livest. Res. Inst. 2015 doi: 10.12774/eod_cr.june2015.graced. [DOI] [Google Scholar]
- 8.Young A.L., Nicol M.P., Moodley C., Bamford C.M. The accuracy of extended-spectrum beta-lactamase detection in Escherichia coli and Klebsiella pneumoniae in South African laboratories using the Vitek 2 Gram-negative susceptibility card AST-N255. South. Afr. J. Infect. Dis. 2019;34:1–6. doi: 10.4102/sajid.v34i1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agyare C., Boamah V.E., Zumbi C.N., Osei F.B. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. Antimicrob Resist. A Glob. Threat. 2018:33–50. doi: 10.5772/intechopen.79371. [DOI] [Google Scholar]
- 10.Li J., Fu Y., Zhang J., Wang Y., Zhao Y., Fan X., Yu L., Wang Y., Zhang X., Li C. Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann. Palliat. Med. 2019;8:651–659. doi: 10.21037/apm.2019.11.06. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int. J. Antimicrob. Agents. 2016;48:614–621. doi: 10.1016/j.ijantimicag.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Poirel L., Jayol A., Nordmann P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He T., Wang R., Liu D., Walsh T., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 14.Sun J., Chen C., Cui C.-Y., Zhang Y., Liu X., Cui Z.-H., Ma X.-Y., Feng Y.-J., Fang L.-X., Lian X.-L., et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019;4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skov R.L., Monnet D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance. 2016;21:30155. doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz S., Johnson A.P. Transferable resistance to colistin: A new but old threat: Table 1. J. Antimicrob. Chemother. 2016;71:2066–2070. doi: 10.1093/jac/dkw274. [DOI] [PubMed] [Google Scholar]
- 17.Countries should Reduce Use of Colistin in Animals to Decrease the Risk of Antimicrobial Resistance|European Medicines Agency. [(accessed on 23 August 2021)]. Available online: https://www.ema.europa.eu/en/news/countries-should-reduce-use-colistin-animals-decrease-risk-antimicrobial-resistance.
- 18.Rhouma M., Beaudry F., Letellier A. Resistance to colistin: What is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents. 2016;48:119–126. doi: 10.1016/j.ijantimicag.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Kempf I., Jouy E., Chauvin C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents. 2016;48:598–606. doi: 10.1016/j.ijantimicag.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Jeannot K., Bolard A., Plésiat P. Resistance to polymyxins in Gram-negative organisms. Int. J. Antimicrob. Agents. 2017;49:526–535. doi: 10.1016/j.ijantimicag.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Webb H.E., Angulo F.J., Granier S.A., Scott H.M., Loneragan G.H. Illustrative examples of probable transfer of resistance determinants from food animals to humans: Streptothricins, glycopeptides, and colistin. F1000Research. 2017;6:1805. doi: 10.12688/f1000research.12777.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster P. The perils of poultry. Can. Med. Assoc. J. 2009;181:21–24. doi: 10.1503/cmaj.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutil L., Irwin R., Finley R., Ng L.K., Avery B., Boerlin P., Bourgault A.-M., Cole L., Daignault D., Desruisseau A., et al. Ceftiofur Resistance inSalmonella entericaSerovar Heidelberg from Chicken Meat and Humans, Canada. Emerg. Infect. Dis. 2010;16:48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antimicrobial Resistance|FDA. [(accessed on 1 September 2021)]; Available online: https://www.fda.gov/animal-veterinary/safety-health/antimicrobial-resistance.
- 25.Dierikx C.M., Van Der Goot J.A., Smith H.E., Kant A., Mevius D.J. Presence of ESBL/AmpC -Producing Escherichia coli in the Broiler Production Pyramid: A Descriptive Study. PLoS ONE. 2013;8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marangoni F., Corsello G., Cricelli C., Ferrara N., Ghiselli A., Lucchin L., Poli A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015;59:27606. doi: 10.3402/fnr.v59.27606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez F., Endimiani A., Hujer K.M., Bonomo R.A. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 2007;7:459–469. doi: 10.1016/j.coph.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black S.R., Weaver K.N., Weinstein R.A., Hayden M.K., Lin M.Y., Lavin M.A., Gerber S.I. Regional Infection Control Assessment of Antibiotic Resistance Knowledge and Practice. Infect. Control. Hosp. Epidemiol. 2015;36:381–386. doi: 10.1017/ice.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesciaroli M., Magistrali C.F., Filippini G., Epifanio E.M., Lovito C., Marchi L., Maresca C., Massacci F.R., Orsini S., Scoccia E., et al. Antibiotic-resistant commensal Escherichia coli are less frequently isolated from poultry raised using non-conventional management systems than from conventional broiler. Int. J. Food Microbiol. 2019;314:108391. doi: 10.1016/j.ijfoodmicro.2019.108391. [DOI] [PubMed] [Google Scholar]
- 30.EUCAST: New S, I and R definitions. [(accessed on 23 August 2021)]. Available online: https://www.eucast.org/newsiandr/
- 31.Shang Y., Kumar S., Oakley B., Kim W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Veter. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhanel G.G., Homenuik K., Nichol K., Noreddin A., Vercaigne L., Embil J., Gin A., Karlowsky J.A., Hoban D.J. The Glycylcyclines. Drugs. 2004;64:63–88. doi: 10.2165/00003495-200464010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Rose W.E., Rybak M.J. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006;26:1099–1110. doi: 10.1592/phco.26.8.1099. [DOI] [PubMed] [Google Scholar]
- 34.Sun H., Wan Y., Du P., Liu D., Li R., Zhang P., Wu Y., Fanning S., Wang Y., Bai L. Investigation of tigecycline resistant Escherichia coli from raw meat reveals potential transmission among food-producing animals. Food Control. 2020;121:107633. doi: 10.1016/j.foodcont.2020.107633. [DOI] [Google Scholar]
- 35.Huang X., Yu L., Chen X., Zhi C., Yao X., Liu Y., Wu S., Guo Z., Yi L., Zeng Z., et al. High Prevalence of Colistin Resistance and mcr-1 Gene in Escherichia coli Isolated from Food Animals in China. Front. Microbiol. 2017;8:562. doi: 10.3389/fmicb.2017.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed S., Das T., Islam Z., Herrero-Fresno A., Biswas P.K., Olsen J.E. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-75608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majewski M., Łukomska A., Wilczyński J., Wystalska D., Racewicz P., Nowacka-Woszuk J., Pszczola M., Anusz K. Colistin resistance of non-pathogenic strains of Escherichia coli occurring as natural intestinal flora in broiler chickens treated and not treated with colistin sulphate. J. Veter. Res. 2020;64:399–405. doi: 10.2478/jvetres-2020-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botsoglou N. Drug Residues in Foods: Pharmacology, Food Safety, and Analysis. Marcel Dekker; New York , NY, USA: 2001. [Google Scholar]
- 39.Goetting V., Lee K.A., Tell L.A. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: A review of the literature. J. Veter. Pharmacol. Ther. 2011;34:521–556. doi: 10.1111/j.1365-2885.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 40.Lim L.M., Ly N., Anderson D., Yang J.C., Macander L., Jarkowski A., Forrest A., Bulitta J., Tsuji B.T. Resurgence of Colistin: A Review of Resistance, Toxicity, Pharmacodynamics, and Dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010;30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaza-Rodríguez C., Alt K., Grobbel M., Hammerl J.A., Irrgang A., Szabo I., Stingl K., Schuh E., Wiehle L., Pfefferkorn B., et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Veter. Sci. 2021;7 doi: 10.3389/fvets.2020.627821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swift B.M., Bennett M., Waller K., Dodd C., Murray A., Gomes R.L., Humphreys B., Hobman J.L., Jones M.A., Whitlock S.E., et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total. Environ. 2018;649:12–20. doi: 10.1016/j.scitotenv.2018.08.180. [DOI] [PubMed] [Google Scholar]
- 43.Ibekwe A.M., Murinda S.E., Debroy C., Reddy G.B. Potential pathogens, antimicrobial patterns and genotypic diversity ofEscherichia coliisolates in constructed wetlands treating swine wastewater. FEMS Microbiol. Ecol. 2016;92:fiw006. doi: 10.1093/femsec/fiw006. [DOI] [PubMed] [Google Scholar]
- 44.Alhendi A.B., Musa Homeida A.-A., Gaili E.-S., Alhendi A.B., Homeida A.-A.M. Drug residues in broiler chickens fed with antibiotics in ration GAILI: Drug residues in broiler chickens fed with antibiotics in ration. Vet. Arh. 2000;70:199–205. [Google Scholar]
- 45.Zakeri B., Wright G.D. Chemical biology of tetracycline antibioticsThis paper is one of a selection of papers published in this Special Issue, entitled CSBMCB—Systems and Chemical Biology, and has undergone the Journal's usual peer review process. Biochem. Cell Biol. 2008;86:124–136. doi: 10.1139/O08-002. [DOI] [PubMed] [Google Scholar]
- 46.Kabir J., Umoh V., Audu-Okoh E., Umoh J., Kwaga J. Veterinary drug use in poultry farms and determination of antimicrobial drug residues in commercial eggs and slaughtered chicken in Kaduna State, Nigeria. Food Control. 2004;15:99–105. doi: 10.1016/S0956-7135(03)00020-3. [DOI] [Google Scholar]
- 47.Carattoli A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008;14:117–123. doi: 10.1111/j.1469-0691.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 48.Musa L., Proietti P.C., Branciari R., Menchetti L., Bellucci S., Ranucci D., Marenzoni M.L., Franciosini M.P. Antimicrobial Susceptibility of Escherichia coli and ESBL-Producing Escherichia coli Diffusion in Conventional, Organic and Antibiotic-Free Meat Chickens at Slaughter. Animals. 2020;10:1215. doi: 10.3390/ani10071215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hafez H.M. Enteric Diseases of Poultry with Special Attention to Clostridium perfringens. Pak. Vet. J. 2011;31:175–184. [Google Scholar]
- 50.Cooper K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Veter. Diagn. Investig. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- 51.The European Union . Proceedings of the European Commission. Regulation. European Commission; Brussels, Belgium: 2006. Ban on antibiotics as growth promoters in animal feed enters into effect. [Google Scholar]
- 52.Jiménez-Belenguer A., Doménech E., Villagrá A., Fenollar A., Ferrús M.A. Antimicrobial resistance of Escherichia coli isolated in newly-hatched chickens and effect of amoxicillin treatment during their growth. Avian Pathol. 2016;45:501–507. doi: 10.1080/03079457.2016.1168515. [DOI] [PubMed] [Google Scholar]
- 53.Burow E., Grobbel M., Tenhagen B.-A., Simoneit C., Szabó I., Wendt D., Kürbis C., Ladwig-Wiegard M., Banneke S., Käsbohrer A. Antibiotic Resistance in Escherichia coli from Broiler Chickens After Amoxicillin Treatment in an Experimental Environment. Microb. Drug Resist. 2020;26:1098–1107. doi: 10.1089/mdr.2019.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dohmen W., VAN Gompel L., Schmitt H., Liakopoulos A., Heres L., Urlings B.A., Mevius D., Bonten M.J.M., Heederik D.J.J. ESBL carriage in pig slaughterhouse workers is associated with occupational exposure. Epidemiol. Infect. 2017;145:2003–2010. doi: 10.1017/S0950268817000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui S., Ge B., Zheng J., Meng J. Prevalence and Antimicrobial Resistance of Campylobacter spp. and Salmonella Serovars in Organic Chickens from Maryland Retail Stores. Appl. Environ. Microbiol. 2005;71:4108–4111. doi: 10.1128/AEM.71.7.4108-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda J.M., Vázquez B.I., Fente C.A., Calo-Mata P., Cepeda A., Franco C.M. Comparison of Antimicrobial Resistance in Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes Strains Isolated from Organic and Conventional Poultry Meat. J. Food Prot. 2008;71:2537–2542. doi: 10.4315/0362-028X-71.12.2537. [DOI] [PubMed] [Google Scholar]
- 57.Millman J.M., Waits K., Grande H., Marks A.R., Marks J.C., Price L.B., Hungate B.A. Prevalence of antibiotic-resistant E. coli in retail chicken: Comparing conventional, organic, kosher, and raised without antibiotics. F1000Research. 2013;2:155. doi: 10.12688/f1000research.2-155.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mollenkopf D.F., Cenera J.K., Bryant E.M., King C.A., Kashoma I., Kumar A., Funk J.A., Rajashekara G., Wittum T.E. Organic or Antibiotic-Free Labeling Does Not Impact the Recovery of Enteric Pathogens and Antimicrobial-ResistantEscherichia colifrom Fresh Retail Chicken. Foodborne Pathog. Dis. 2014;11:920–929. doi: 10.1089/fpd.2014.1808. [DOI] [PubMed] [Google Scholar]
- 59.ISO ISO 16649-1:2018—Microbiology of the food chain—Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—Part 1: Colony-count technique at 44 degrees C using membranes and 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. [(accessed on 1 September 2021)]. Available online: https://www.iso.org/standard/64951.html.
- 60.European Committe on Antimicrobial Susceptability Testing Clinical Breakpoints and Dosing of Antibiotics. [(accessed on 1 August 2020)]. Available online: https://eucast.org/clinical_breakpoints/
- 61.Weinstein M.P., Patel J.B., Bobenchik A.M., Campeau S., Cullen S.K., Galas M.F., Gold H., Humphries R.M., Kirn T.J., Lewis Ii J.S., et al. Performance Standards for Antimicrobial Susceptibility Testing Performance Standards for Antimicrobial Susceptibility Testing. CLSI; Wayne, PA, USA: 2020. M100 Performance Standards for Antimicrobial Susceptibility Testing A CLSI supplement for global application. [Google Scholar]
- 62.Bonnedahl J., Järhult J.D. Antibiotic resistance in wild birds. Upsala J. Med. Sci. 2014;119:113–116. doi: 10.3109/03009734.2014.905663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boiocchi F., Davies M.P., Hilton A.C. An Examination of Flying Insects in Seven Hospitals in the United Kingdom and Carriage of Bacteria by True Flies (Diptera: Calliphoridae, Dolichopodidae, Fanniidae, Muscidae, Phoridae, Psychodidae, Sphaeroceridae) J. Med. Èntomol. 2019;56:1684–1697. doi: 10.1093/jme/tjz086. [DOI] [PubMed] [Google Scholar]
- 64.Bouki C., Venieri D., Diamadopoulos E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013;91:1–9. doi: 10.1016/j.ecoenv.2013.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.