Abstract
Immunosuppression from human immunodeficiency virus (HIV) may impair antibody formation, and false-negative hepatitis C virus antibody (anti-HCV) tests have been reported in individuals coinfected with HIV and HCV. It is unknown if the frequency of false-negative tests is sufficiently high to change screening recommendations in this setting. Thus, the prevalence of false-negative results for anti-HCV by third-generation tests was determined with samples from HIV-infected individuals. Sera from 559 HIV-infected and 944 HIV-negative prospectively followed injection drug users were tested for anti-HCV by a third-generation enzyme immunoassay and for HCV RNA by using a branched DNA assay and the HCV COBAS AMPLICOR system. Of 559 HIV-infected participants, 547 (97.8%) were anti-HCV positive. One of the remaining 12 anti-HCV-negative participants was HCV RNA positive, and she later developed detectable anti-HCV. Of the 944 HIV-negative participants, 825 (87.4%) were anti-HCV positive. One of the remaining 119 anti-HCV-negative participants was HCV RNA positive, and she also developed detectable anti-HCV at a later visit. These data indicate that HIV infection does not alter the approach to hepatitis C virus screening, which should be performed with third-generation assays for anti-HCV unless acute infection is suspected.
Many human immunodeficiency virus (HIV)-infected individuals are coinfected with hepatitis C virus (HCV) (10). In coinfected individuals, HCV replication is increased and progression of liver disease is accelerated presumably from HIV-induced immunosuppression (5, 9). In addition, there have been reports of HCV antibody (anti-HCV) loss in HIV-infected patients (4, 8), and there has been one report of anti-HCV return after immune system restoration with highly active antiretroviral therapy (HAART) (6). Loss of antibody to other infectious agents such as hepatitis B virus and the syphilis spirochete has also been described in HIV-infected patients (2, 7). The U.S. Public Health Service and a National Institutes of Health consensus panel recommend that tests for anti-HCV be used to screen for HCV infection (1, 3). However, it is unknown if this recommendation is sufficient for HIV-infected persons, for whom false-negative results of tests for anti-HCV have been reported for earlier generations of commercially available assays. To examine the sensitivity of the current third-generation assay for anti-HCV with samples from HIV-infected individuals, testing for both HCV antibodies and HCV RNA was performed with samples from a large cohort of injection drug users (IDUs).
MATERIALS AND METHODS
Study subjects.
The study subjects were members of the ALIVE (AIDS Link to the Intravenous Experience) cohort. ALIVE is an ongoing study that began with 2,921 IDUs who were enrolled in Baltimore, Md., from February 1988 to March 1989 and who were seen semiannually thereafter, as described previously (11). The 1,503 subjects in this analysis represent a consecutive sample of cohort participants from whom a blood sample was collected between 1 January 1995 and 31 March 1996. Serum samples were aliquoted within 2 h of collection and were stored at −20°C for less than seven days and then at −80°C.
Informed consent was obtained from all patients and was approved by the Institutional Review Board at Johns Hopkins University.
Laboratory testing.
For all HIV-infected participants and HIV-negative participants without a previous second- or third-generation test for anti-HCV, sera were tested for anti-HCV by a third-generation Ortho, version 3.0, enzyme immunoassay performed according to the manufacturer's specifications (Ortho Diagnostic Systems, Raritan, N.J.), as described previously (9). With the same sample, testing for HCV RNA was done for all subjects by a branched DNA (bDNA) assay (Quantiplex HCV RNA 2.0 Assay; Chiron Corporation, Emeryville, Calif.), which was performed according to the manufacturer's recommendations. All anti-HCV-negative, bDNA assay-negative sera were retested for HCV RNA by using the HCV COBAS AMPLICOR (COBAS) system, according to the manufacturer's specifications (COBAS AMPLICOR HCV; Roche Diagnostics, Branchburg, N.J.). The limits of viral detection for the bDNA and the COBAS assays are approximately 200,000 equivalents/ml (approximately 60,000 copies/ml) and 100 copies/ml, respectively. For participants who were anti-HCV negative and bDNA assay positive, testing by the COBAS assay was also performed with samples from the same, prior, and subsequent visits.
Statistical analysis.
Frequency data were generated by using SAS software (SAS Institute, Cary, N.C.).
RESULTS
A total of 1,503 subjects were tested; of these subjects, 559 (37.2%) were HIV infected and 944 (62.8%) were HIV negative. The mean age, race, gender, and current drug use were similar between the HIV-positive and HIV-negative participants (Table 1). At the visit when anti-HCV testing was performed, 33% of the HIV-infected subjects had CD4+ cell counts of <200 cells/mm3, and 9% had counts of <50 cells/mm3. Prior to the visit when testing for anti-HCV was performed, the median time that participants had <200 CD4+ cells/mm3 was 17.5 months (mean, 20.7 months), and the CD4+ cell count at the visit tested was the nadir for all but three participants.
TABLE 1.
Characteristics of study participantsa
| Characteristic | HIV positive (n = 559) | HIV negative (n = 944) |
|---|---|---|
| Age, <40 years (no. [%]) | 303 (54.2) | 447 (47.3) |
| Race (no. [%]) | ||
| African American | 535 (95.7) | 887 (94) |
| Other | 24 (4.3) | 56 (6) |
| Gender (no. [%]) | ||
| Male | 422 (75.5) | 694 (73.5) |
| Female | 137 (24.5) | 250 (26.5) |
| Current drug use (no. [%]) | 322 (57.6) | 620 (65.7) |
| Anti-HCV status (no. [%]) | ||
| Positive | 547 (97.8) | 825 (87.4) |
| Negative | 12 (2.2) | 119 (12.6) |
| HCV RNA status for anti-HCV-negative subjects (no. [%]) | ||
| Positive | 1 (8.3)b | 1 (0.9)b |
| Negative | 11 (91.7) | 118 (99.1) |
| CD4+ T-cell count (cells/mm3) (no. [%]) | ||
| 0–49 | 52 (9.3) | NAc |
| 50–199 | 134 (24.0) | NA |
| 200–499 | 249 (44.5) | NA |
| >500 | 110 (19.6) | NA |
| Unknown | 14 (2.5) | NA |
Age and CD4+ count were defined at the time of testing. Drug use status was defined in the 6 months prior to testing.
Both individuals became anti-HCV positive on their next visit.
NA, not applicable.
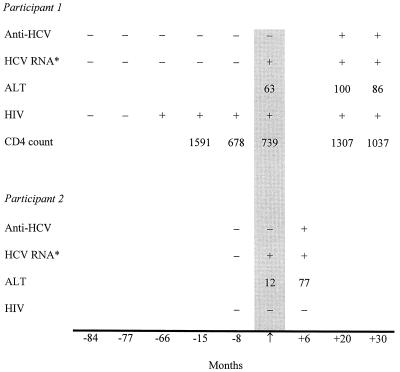
Of the 559 HIV-infected participants, 547 (97.8%) were anti-HCV positive. Of the remaining 12 anti-HCV-negative subjects, HCV RNA was detected in only 1 subject, a 32-year-old woman who maintained a high CD4+ cell count without antiretroviral therapy. Anti-HCV and HCV RNA were detected in sera collected 20 months later, at her next study visit, but not in several samples that had been collected 8 to 80 months earlier, indicating that she had recently acquired HCV infection (Fig. 1). In addition, serum alanine aminotransferase levels were elevated at the first visit when she was found to be HCV RNA positive and at subsequent visits.
FIG. 1.
Testing profiles for two anti-HCV-negative, HCV RNA-positive participants. These 2 participants represent the only subjects for whom false-negative anti-HCV tests were found among 559 HIV-positive and 944 HIV-negative persons. The arrow and shaded area represent the time of testing for anti-HCV and HCV RNA in this study. ∗, results obtained with the COBAS system; ALT, alanine aminotransferase.
Of the 944 HIV-negative participants, 825 (87.4%) were anti-HCV positive. Of the remaining 119 (12.6%) anti-HCV-negative participants, 1, a 29-year-old female, had detectable HCV RNA. Anti-HCV and HCV RNA were detected in sera collected 6 months later but not 8 months before this visit, indicating that she, too, had recently acquired HCV infection. Thus, the sensitivity of the third-generation anti-HCV assay was >99% for both HIV-infected and HIV-negative individuals, and both false-negative anti-HCV tests occurred in the seroconversion window.
DISCUSSION
U.S. Public Health Service guidelines recommend HCV screening for all HIV-infected persons (3a). Case reports based on prior versions of commercially available anti-HCV assays have suggested that false-negative results by tests for anti-HCV detection may occur for HIV-infected individuals. However, our data indicate that a third-generation assay for anti-HCV detection has high sensitivity, even with samples from HIV-infected persons. This investigation also underscores the importance of either testing for HCV RNA or serial testing for anti-HCV for the diagnosis of acute HCV infection. Our serial tests for HCV RNA and anti-HCV indicated that the only two anti-HCV-negative, HCV RNA-positive participants were undergoing HCV seroconversion. Thus, even this infrequent occurrence of false-negative results by tests for anti-HCV was attributed not to HIV infection but to the well-known window of seronegativity following acute hepatitis C.
A high sensitivity of anti-HCV testing was found in this investigation, despite the inclusion of over 150 subjects with CD4+ counts below 200 cells/mm3. This high sensitivity cannot be attributed to the use of HAART in any members of this cohort since sera were collected prior to the initiation of HAART. The sensitivity of testing for anti-HCV could not have been substantially exaggerated by a low sensitivity of testing for HCV RNA since 97.8% of HIV-infected participants had anti-HCV and the most sensitive commercially available test for HCV RNA was used.
It is possible that a lower sensitivity for detection of anti-HCV would be found in other settings. In the IDU cohort described here, HCV infection almost always precedes HIV infection (10). The durability of the HCV antibody response might be lower if HCV infection followed HIV infection since HIV-related immunosuppression decreases the antibody response to vaccination and some infections. Both false-negative results of tests for anti-HCV in this study were for persons with acute HCV infection, which is now relatively uncommon in this cohort of long-term IDUs. Thus, the sensitivities of tests for anti-HCV could also be lower in settings in which new HCV infections are common, irrespective of the HIV infection status.
We conclude that a third-generation assay for anti-HCV can be used to screen for HCV in persons who acquire HIV in the context of intravenous drug use. Further research is warranted to reassess the sensitivities of assays for anti-HCV in other settings where it has been questioned, including persons on hemodialysis and those infected with HIV through nonparenteral routes. Testing for HCV RNA is indicated for anti-HCV-negative persons when acute HCV infection is suspected or when there is other evidence of liver disease, such as unexplained elevated liver enzyme levels.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DA-04334, DA-08004, DA-023201, DA-05887, and AI-40035 from the National Institute on Drug Abuse.
REFERENCES
- 1.Anonymous. National Institutes of Health consensus development conference panel statement: management of hepatitis C. Hepatology. 1997;26:2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 2.Biggar R J, Goedert J J, Hoofnagle J. Accelerated loss of antibody to hepatitis B surface antigen among immunodeficient homosexual men infected with HIV. N Engl J Med. 1987;316:630–631. doi: 10.1056/NEJM198703053161015. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Morbid Mortal Weekly Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 3a.Centers for Disease Control and Prevention. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with Human Immunodeficiency Virus: disease-specific recommendations. Morbid Mortal Weekly Rep. 1999;48:1–82. [PubMed] [Google Scholar]
- 4.Chamot E, Hirschel B, Wintsch J, Robert C F, Gabriel V, Deglon J J, Yerly S, Perrin L. Loss of antibodies against hepatitis C virus in HIV-seropositive intravenous drug users. AIDS. 1990;4:1275–1277. doi: 10.1097/00002030-199012000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Eyster M E, Diamondstone L S, Lien J M, Ehmann W C, Quan S, Goedert J J. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. The Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1993;6:602–610. [PubMed] [Google Scholar]
- 6.John M, Flexman J, French M A H. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Johnson P D R, Graves S R, Stewart L, Warren R, Dwyer B, Lucas C R. Specific syphilis serological tests may become negative in HIV infection. AIDS. 1991;5:419–423. doi: 10.1097/00002030-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Sorbi D, Shen D, Lake-Bakaar G. Influence of HIV disease on serum anti-HCV antibody titers: a study of intravenous drug users. J Acquir Immune Defic Syndr. 1996;13:295–296. doi: 10.1097/00042560-199611010-00014. [DOI] [PubMed] [Google Scholar]
- 9.Thomas D L, Shih J W, Alter H J, Vlahov D, Cohn S, Hoover D R, Cheung L, Nelson K E. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 10.Villano S A, Vlahov D, Nelson K E, Lyles C M, Cohn S, Thomas D L. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlahov D, Anthony J C, Muñoz A, Margolik J, Celentano D D, Solomon L, Polk B F. The ALIVE Study: a longitudinal study of HIV-1 infection in intravenous drug users: description of methods. J Drug Issues. 1991;21:759–776. [PubMed] [Google Scholar]