Abstract
Coenzyme Q is a unique lipidic molecule highly conserved in evolution and essential to maintaining aerobic metabolism. It is endogenously synthesized in all cells by a very complex pathway involving a group of nuclear genes that share high homology among species. This pathway is tightly regulated at transcription and translation, but also by environment and energy requirements. Here, we review how coenzyme Q reacts within mitochondria to promote ATP synthesis and also integrates a plethora of metabolic pathways and regulates mitochondrial oxidative stress. Coenzyme Q is also located in all cellular membranes and plasma lipoproteins in which it exerts antioxidant function, and its reaction with different extramitochondrial oxidoreductases contributes to regulate the cellular redox homeostasis and cytosolic oxidative stress, providing a key factor in controlling various apoptosis mechanisms. Coenzyme Q levels can be decreased in humans by defects in the biosynthesis pathway or by mitochondrial or cytosolic dysfunctions, leading to a highly heterogeneous group of mitochondrial diseases included in the coenzyme Q deficiency syndrome. We also review the importance of coenzyme Q levels and its reactions involved in aging and age-associated metabolic disorders, and how the strategy of its supplementation has had benefits for combating these diseases and for physical performance in aging.
Keywords: coenzyme Q, ubiquinone, metabolic disease, rare disease, mitochondria
1. Introduction
Coenzyme Q (CoQ) or ubiquinone is an essential element for mitochondrial function and provides antioxidant protection of cell membranes and plasma lipids [1]. CoQ was isolated and identified by Festenstein et al. in 1955 [2], and validated by Crane et al. in 1957, who demonstrated its main function in the mitochondrial electron transport chain (ETC) [3]. CoQ also shows a potent antioxidant function in plasma membranes, endomembranes, and lipopoteins [4].
The structure of CoQ is essentially the same in all organisms, composed of a benzoquinone ring bound to a polyisoprenoid side chain that preserve the molecule inserted into a lipid bilayer. Isoprene units of the chain are species-specific: human isoform mainly encloses ten isoprene units (CoQ10). In rodents, this molecule presents mainly nine units (CoQ9), and around a 10% has ten units (CoQ10). The yeast Saccharomyces cerevisiae holds six units (CoQ6), whereas Schizosaccharomyces pombe contains ten units (CoQ10). Bacteria such as Escherichia coli contains eight isoprene units (CoQ8) [5].
Regarding its redox activity, CoQ can be found in a reduced form (CoQH2 or ubiquinol), in a completely oxidized state (CoQ or ubiquinone), or in a semi-reduced state known as a semi-ubiquinone (CoQH•). Through this redox cycle, CoQ plays an essential role in the ETC maintaining the electron flux from complexes I and II to complex III, leading a chemiosmotic gradient that is used by the ATP synthase to produce ATP by oxidative phosphorylation (OXPHOS) [6].
The CoQ-dependent oxidation/reduction cycle also confers to this molecule the property to transfer electrons in different biological processes and to act as an antioxidant at cell membranes preserving lipids, protein, and nucleic acids from oxidative damage [7,8]. By this mechanism, CoQ is the major antioxidant preventing lipid peroxidation in cell membranes and lipoproteins [9,10]. Further, CoQ also plays a key role in the maintenance of vitamin C and α-tocopherol antioxidant activities at the plasma membrane, leading the regulation of cellular redox homeostasis [11,12].
CoQ is involved in essential physiological processes in cells and tissues, and its homeostasis is maintained through highly conserved regulatory mechanisms of the biosynthesis pathway [13]. Defects in either the biosynthesis pathway or in the mitochondrial functions that affect CoQ homeostasis in tissues and organs imply an activation of metabolic diseases and aging, in which mitochondrial dysfunction is involved [1,14]. This review highlights the importance of CoQ in the maintenance of health and in the evolution of metabolic diseases and aging.
2. Biosynthesis of CoQ
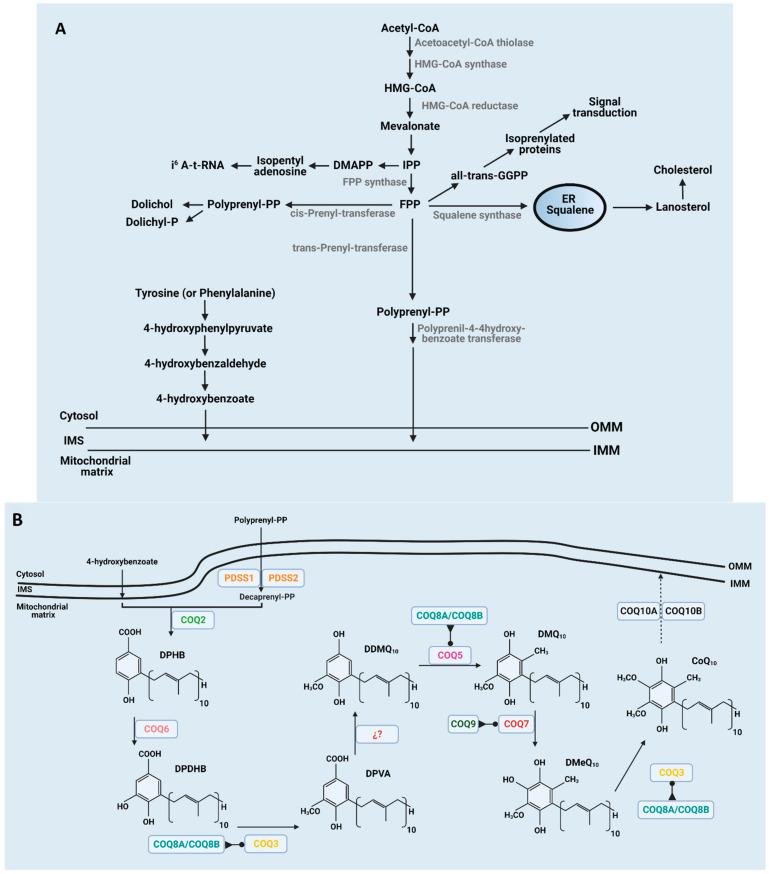
Although CoQ can be incorporated through the diet, most of the CoQ is endogenously produced inside the mitochondria of every cell and then distributed throughout the cellular membranes [15]. An overview of the CoQ biosynthesis pathway in mammals is depicted in Figure 1. The biosynthesis pathway, in which at least 13 genes are involved [16,17,18], is not fully understood, and most of the recent discoveries in humans comes from the study of CoQ deficiency syndrome [19]. Final reactions of CoQ biosynthesis take place inside mitochondria by a nuclear-encoded COQ proteins cluster, after the isoprenoid tail and benzoquinone head are bound, producing decaprenyl hydroxybenzoic acid (DPHB) at the matrix side of the inner mitochondrial membrane (IMM). A CoQ polyisoprenoid chain is produced through the mevalonate pathway in the endoplasmic reticulum shared with the synthesis of other lipids, such as cholesterol or dolichol, and heme A and protein prenylation [5]. It is initiated by the condensation of three acetyl-Coenzyme A (acetyl-CoA) molecules to form 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) due to acetoacetyl-CoA thiolase and HMG-CoA synthase activities. Mevalonate is then produced from HMG-CoA by HMG-CoA reductase. Mevalonate is phosphorylated and decarboxylated to produce isopentenyl pyrophosphate (IPP). Farnesyl pyrophosphate synthase uses IPP to make farnesyl pyrophosphate (FPP), an intermediary product for geranyl pyrophosphate (GPP) production. The next enzyme involved in CoQ biosynthesis is transprenyl-transferase that converts FPP into polyprenyl-PP, which is considered a rate-limiting step [6]. The transport of FPP into mitochondria is not fully understood yet, but it has been proposed to occur in the ER-mitochondria contacts through the ERMES complex [17,20,21]. COQ1 (PDSS1 and PDSS2) would assemble the side chain producing decaprenyl-PP (DPP) in those species containing CoQ10 [16,22]. The ring precursor para-hydroxybenzoate (pHB) seems to be synthesized in cytosol including seven proteins, such as Hfd1p and its human homolog ALDH3A1 [23,24]. Recently, it has been shown in yeast that the five aminotransferases, Aro8, Aro9, Bat2, Bna3, and Aat2, are required to produce the benzoquinone ring for CoQ synthesis, which would also be studied in undiagnosed CoQ deficiency syndrome [25].
Figure 1.
Scheme of CoQ synthesis in mitochondria. (A) Synthesis of the mevalonate chain in the ER and quinone head processing from tyrosine in cytosol or mitochondrial matrix. (B) Assembly of benzene ring and isoprenoid tail at the inner mitochondrial membrane and further modifications of the phenol head at the inner mitochondrial membrane. Members of the CoQ-synthome are indicated in each step. In some cases, the enzyme involved in some of the steps are unknown. Regulatory enzymes such as COQ8 and COQ9 are indicated at the steps in which they are actively involved.
The next set of reactions occur through the interaction of COQ gene-encoded proteins that provide support for the existence of a CoQ biosynthesis complex or CoQ synthome [17,26]. In these reactions, a sequential series of modifications of the aromatic ring are performed. Initially, DPHB suffers from C5-hydroxylation by COQ6, resulting in decaprenyl-dihydroxybenzoate (DPDHB) formation [27]. Subsequently, DPDHB is O-methylated by COQ3 to form decaprenyl-vanillic acid (DPVA) [28,29]. Later, a C1-hydroxylation and a C1-decarboxylation are catalyzed by unidentified proteins to form demethoxy-demethyl-coenzyme Q (DDMQ). Afterwards, COQ5 carries a C2-methylation to form demethoxy-coenzyme Q (DMQ) [30]. Thereafter, DMQ is C6-hydroxylated by COQ7 to form demethyl-coenzyme Q (DMQ) [31]. Finally, COQ3 catalyzes the final step ending in CoQ from DMQ.
In recent years, other COQ proteins showing regulatory functions have been discovered. COQ8A (ADCK3) and COQ8B (ADCK4) show properties of atypical kinases that phosphorylate COQ3, COQ5, and COQ7 [32,33,34]. The function of COQ4 has not yet been elucidated, but some reports indicate that this enzyme modulates the formation and maintenance of the CoQ biosynthetic complex [35]. Moreover, COQ9 is a lipid-binding protein that modulates the activity of COQ7 [36,37]. Further, COQ10A and COQ10B possibly regulate the distribution and/or complex III binding of CoQ within the IMM where displays its functions [38].
3. CoQ as Metabolic Integrative Factor for Cellular Homeostasis
The main function of CoQ is to act as an electron carrier in the ETC that drives all electrons to complex III. Most of the redaction reactions of CoQ come from NADH-dependent complex I and FADH2-dependent complex II. However, CoQ is also reduced by diverse oxidoreductases, which represents key steps in important metabolic pathways, such as iron sulfur cluster synthesis, nucleotide synthesis, and sulfide metabolism [39,40]. These reactions and their metabolic mechanisms have been recently reviewed by [41].
As a ubiquitous component of eukaryotic lipid membranes, CoQ participates in electron transfer activities. In these redox activities, the redox state of CoQ determines where and how electrons move from the electron carrier and if leaks produce reactive oxygen species (ROS) [42]. Although free radicals have long been thought to be responsible for aging and age-related diseases, recent reports indicate that they have both positive and negative effects on longevity and health acting as integrators of cellular homeostasis [39,42,43,44]. The levels of CoQ, its redox state, and its interaction with CoQ-dependent enzymatic activities can be key in the modulation of cellular homeostasis.
3.1. Mitochondrial Redox Reactions
3.1.1. Redox Reactions at the ETC
The CoQ-dependent redox reactions in the mitochondria are linked directly or indirectly to oxidative phosphorylation (OXPHOS), in which electrons flow through mitochondrial complexes to generate the proton gradient necessary for ATP synthesis, until they finally reach oxygen to reduce it to water [39], additionally affecting diverse metabolic pathways [41].
To access ETC, the energy of the electron flows clusters the mitochondrial redox reactions into three groups, depending on their redox potential [45]. The more energetic dehydrogenases operate at approximately −280 mV and transfer electrons from metabolites to NAD+, reducing it to NADH, and oxidizing the proper metabolite to another with lower potential energy. Within this group, enzymes, for amino acid metabolism, such as 2-oxoadipate dehydrogenase for lysine catabolism and branched-chain dehydrogenases for leucine, isoleucine and valine catabolism; hydroxyacyl-CoA-dehydrogenase for fatty-acid β-oxidation; pyruvate dehydrogenase for carbohydrate catabolism; and the three NAD-dehydrogenases integrated in the Krebs cycle: isocitrate dehydrogenase, 2-oxoglutarate dehydrogenase, and malate dehydrogenase are included. All these dehydrogenases produce NADH to feed mitochondrial complex I, which uses the energy to generate the proton gradient necessary for ATP synthesis and transfers the electrons to ubiquinone reducing it to ubiquinol, which is the substrate for complex III.
The second group of dehydrogenases operate at a lower redox potential of approximately +20 mV. They use FAD, a flavin-adenine dinucleotide that is reduced to FADH2, and transfer electrons from metabolites directly to ubiquinone, without contribute to the proton gradient generation. This group includes succinate dehydrogenase (complex II), enzymes for amino acid metabolism, such as proline dehydrogenase, dihydroorotate dehydrogenase for pyrimidine nucleotide metabolism, acyl-CoA dehydrogenases for fatty-acid beta oxidation, and glycerol-3-phosphate dehydrogenase, which shuttles the glycolytic electrons from NADH directly to the mitochondrial respiratory chain to regenerate NAD+ and avoid the blockage of the glycolysis. All these dehydrogenases use as substrate CoQ producing ubiquinol, which is reoxidized by complex III, using this energy to generate the mitochondrial proton gradient, and finally transferring the electrons to molecules of cytochrome c [41].
The third group of dehydrogenases operate at a very low redox potential of approximately +320 mV. Here, the cytochrome c linked enzymes are included, such as the sulfite dehydrogenase necessary for cysteine catabolism, which oxidizes sulfite to sulfate and reduces cytochrome c. Finally, electrons from cytochrome c go through complex IV, contributing to the proton gradient, until they reach oxygen at +600 mV.
3.1.2. CoQ Role in Reactive Oxygen Species Balance
Eleven sites in the ETC that leak electrons to oxygen producing ROS have been associated with substrate oxidation and oxidative phosphorylation in mitochondria [46]. These sites are located inside the oxoacid dehydrogenases that feed electrons to NAD+, but higher production has been recorded in proper mitochondrial complexes III, I, and II in the sites where CoQ presents its redox activity [45]. In addition, the rate of ROS production can vary depending on the tissue, showing higher production in brown adipose tissue compared with skeletal muscle, and being heart the organ with lower rates [47]. The type of substrate is also crucial, succinate is the metabolite that causes higher production of ROS, and both fatty acids and amino acids the metabolites that produces the lower levels [46]. Most of the hydrogen peroxide production takes place in complex I, while glycerol-3-phophate, fatty acids and glutamate/malate generate an equal amount in complexes I and III, and inside the proper glycerol-3-phosphate dehydrogenase. ROS is also produced by 2-oxoglutarate dehydrogenase.
Due to the topology of the mitochondria and the presence of a double membrane, these enzymes can generate superoxide either in the intermembrane space or in the matrix, but hydrogen peroxide exclusively in the matrix. The specific site of ROS production is important for redox signaling without generating catastrophic energetic effects [45]. Thus, we can distinguish intramitochondrial redox signaling, in which all targets are located inside mitochondria, and redox signaling from mitochondria to the rest of the cell (retrograde redox signaling). This ROS-dependent signaling process can even affect targets located outside cells. Among the targets affected by mitochondrial ROS are the initiation of hypoxia-inducible factor (HIF) signaling and its effect in gene expression modulation, insulin secretion stimulated by redox signaling due to the metabolism of branched-chain keto acids and fatty acids, retrograde redox signaling that modulates PGC1α during exercise in skeletal muscle, and the redox signaling that affects immune cells [48,49].
3.1.3. Mitochondrial CoQ Is Essential for Many Different Metabolic Processes
In addition to its known activity as a redox carrier among complexes I, II, and III of the ETC, CoQ also receives electrons from many different dehydrogenases [41,50,51]. CoQ receives electrons from mitochondrial glycerol-3-phosphate dehydrogenase (G3PDH) that connects glycolysis, OXPHOS, and fatty acid metabolism [52]. CoQ is also reduced by the electron-transport flavoprotein dehydrogenase (ETFDH), an essential enzyme in β-oxidation of fatty acids and in the oxidation of branched amino acids [53]. The capacity as an electron acceptor of CoQ is also essential for the activity of proline dehydrogenase (PROD), involved in glyoxylate metabolism [54], and sulphide-quinone oxidoreductase (SQR) that participates in sulphide detoxification [55], an important regulator of many cellular processes [56,57]. Further, CoQ is also reduced by choline dehydrogenase (CHDH) [58], from choline to glycine conversion, and dihydroorotate dehydrogenase (DHODH), from dihydro-orotate to orotate transformation, which is involved in pyridine nucleotide synthesis [59].
Further, CoQ also participates in the dissipation of energy from the ETC by the dissemination of the proton gradient as heat. CoQ associates to uncoupling proteins localized in the IMM playing in its regulation [60].
Moreover, it has recently been shown that the oxidation of ubiquinol by complex III of the ETC is an obligatory reaction to maintain tumor growth [61].
Another important aspect of the essential participation of CoQ in mitochondrial physiology is its role as a structural element in complexes I and III in the ETC [62,63]. In fact, CoQ is essential for the maintenance of the structure of complex III since the supplementation of yeast with CoQ restores the assembly of complex III in CoQ-deficient strains [64], and CoQ is also involved in complex I stability [65]. Interestingly, the deterioration of complexes I and III has been associated with the progression of different neurodegenerative diseases [66,67]. Further, defects of complexes downstream of the CoQ site end in the accumulation of ubiquinol in ETC that destabilizes complex I by ROS production, generating a vicious cycle [65].
CoQ is also a component of respirasome [68,69], and also participates in the assembly and the dynamics of supercomplexes [70]. Recently, it has been indicated that OPA1, a regulator of the fusion of the mitochondrial outer membrane, mediates the regulation of complex IV activity through a CoQ-dependent procedure [71]. Interestingly, a pool of CoQ is associated with complex I + III + IV supercomplexes, whereas free CoQ is dedicated to complex II-dependent respiratory chain activity [72,73,74]. This is very important as supercomplexes are associated with a balanced ETC chain activity and point to the regulatory role of CoQ in their assembly dynamics [70,75,76]. CoQ is also importantly implicated in the dynamics of the assembly and stability of these supercomplexes involved in mitochondrial efficiency that, when altered, induce mitochondrial dysfunction-mediated metabolic diseases and aging [77,78].
Recently, another key function of CoQ in mitochondria has been found in the outer membrane [79]. MitoNEET, also known as CDGS1 iron sulfur domain 1 (CISD1) protein, is a redox-active and pH-sensing protein that regulates energy metabolism, iron homeostasis, and ROS in mitochondria. MitoNEET interacts with reduced flavin mononucleotide (FMNH2) that reduces mitoNEET sulfoferric [2Fe–2S] clusters, which are oxidized back with CoQ being the most efficient electron acceptor [39,57]. As one of the proteins repaired by MitoNEET is the iron-master regulator IRP-1, which limits iron access to mitochondria to protect against ferroptosis in high ROS production, CoQ can be considered as an important redox-sensing factor in the adaptive response against oxidative injury, and a key component in the prevention of ferroptosis caused by mitochondrial dysfunction [39].
Finally, it has been suggested that CoQ inhibits the calcium-dependent opening of the mitochondrial permeability transition pore (PTP) and modulates the mitochondrial uncoupling proteins (UCPs) [40].
3.2. Extramitochondrial Redox Reactions
Since the discovery that extramitochondrial ubiquinol possesses antioxidant functions, efforts have been made to characterize the physiological enzyme reduction systems. It has been widely described that CoQ and vitamin E inhibit lipid peroxidation by scavenging lipid peroxyl radicals, and that CoQ directly scavenges the perferryl radical, thereby preventing the initiation of lipid peroxidation [57,80].
These important functions require continuous regeneration of ubiquinol from the oxidized ubiquinone and different quinone reductases have been proposed. In this way, the cytosolic enzymes that reduce extramitochondrial ubiquinone can be grouped depending on their dependence on NADPH and NADH and if they have flavin adenine dinucleotide (FAD) as prosthetic group [80]. The highest rate of extramitochondrial ubiquinone reduction described is accomplished by flavoenzymes belonging to a unique family of pyridine nucleotide oxidoreductases, such as lipoamide dehydrogenase (LipDH), mammalian thioredoxin reductase (TrxR-1), and glutathione reductase (GR) [80]. LipDH reduces CoQ with either NADH or NADPH, works at acidic pH, and its activity depends on the presence of zinc. TrxR-1 is a selenoenzyme with very broad substrate specificity that has an optimal physiological pH of 7.5 and uses either NADPH or NADH, being the most efficient ubiquinone reductase so far tested. GR is a flavoenzyme, the activity of which is also stimulated by zinc, and has an acidic pH.
On the other hand, NAD(P)H:quinone acceptor oxidoreductase1 (NQO1) plays a central role in the processes of stress adaptation, including oxidative stress [42]. NQO1 and cytochrome b5 reductase (CYTB5R3) regulate the stress adaptation response as a central component of the transplasma membrane redox system that is responsible for preventing lipid peroxidation by reducing oxidized antioxidants, such as vitamins E and C, and the proper CoQ [81,82]. An overexpression of these enzymes in mice improved health- and life-span by modulating lipid-mediated respiration [83,84,85]. CoQ is also involved in alterations in the redox environment by receiving highly energized electrons from NAD(P)H, and possibly in the local regulation of NAD(P)H-dependent enzymes, such as sirtuins acting as metabolic switch [1,86].
CoQ in plasma membrane contributes to prevent ferroptosis, as substrate of ferroptosis suppressor protein 1 (FSP1), acting in association to the glutathione-dependent lipid hydroperoxidase glutathione peroxidase 4 (GPX4) [87,88]. The relevant extramitochondrial redox reactions that depend on CoQ, which are integrated with other pathways, are essential to regulate cellular homeostasis and metabolism [57].
CoQ also plays an key antioxidant role in the prevention of oxidative damage in plasma cholesterol lipoproteins, essentially in low-density lipoproteins (LDLs) [89]. This role has been essentially considered in the prevention of, and protection against, cardiovascular diseases and atherogenesis [4,90]. Interestingly, a NADH-dependent reductase able to reduce LDL-associated CoQ has been found in the outer side of the plasma membrane of hepatocytes [91], possibly indicating a mechanism to maintain the redox cycle of CoQ in plasma. The importance of this enzyme in the maintenance of ubiquinol in plasma in aging and metabolic diseases is under study.
4. CoQ Deficiency Syndrome
CoQ deficiency syndrome includes a subgroup of very heterogeneous mitochondrial diseases characterized by reduced levels of CoQ in cells. The first cases of CoQ deficiency were described in 1989 by Ogasahara et al. [92]. This report showed two sisters displaying a progressive muscle weakness characterized by an abnormally low activity of complex I + III (NADH-cytochrome c reductase) and II + III (succinate-cytochrome c reductase). This indicates that the activities that needed CoQ were low, while the individual complex activities were normal [92].
Taking into consideration the essential cellular functions carried out by CoQ in mitochondria [57], a depletion of these molecules mainly leads to the impairment of OXPHOS, causing lower rates of ATP synthesis, and rising ROS generation through the disruption of its participation in supercomplex formation [65,69]. CoQ deficiency leads to the development of mitochondrial diseases affecting organs or tissues with higher CoQ levels and bioenergetic requirements. Although these processes are the main features of CoQ deficiency, other processes where CoQ is involved contribute significantly to the development of the diseases [57]. Generally, CoQ deficiency has been associated with many age-related diseases [39], neurological disorders [93], kidney and liver diseases [94,95], and heart failure [96], among others.
Several causes, including reduced dietary CoQ intake, excessive CoQ cell metabolism, and biosynthesis defects, have been proposed as being important to the development of CoQ deficiency syndrome [97,98]. To date, although the number of cases reported is increasing, the pathophysiology of this syndrome is still poorly understood. It has been suggested that several mechanisms could contribute to development of the disease, such as the pleiotropic functions of CoQ, genotypic variability, and the unknown functions of CoQ intermediates in the biosynthesis pathway [14].
4.1. Primary CoQ Deficiency
4.1.1. Characteristics of Primary CoQ Deficiencies
Primary CoQ deficiencies are autosomal recessive conditions caused by a biallelic mutation in gene-encoding proteins implicated in the CoQ biosynthesis pathway [19]. These diseases in humans are highly heterogeneous and with a broad spectrum of clinical phenotypes. The estimated overall prevalence of this primary form is less than 1:100,000 [99]. Generally, patients manifest a combination of symptoms ranging from isolated affects on organs or tissues, such as the kidney, skeletal muscle, the heart, or the brain, to complex multisystem disorders with different severity levels and age of onset [19,97,100]. To date, defects in PDSS1, PDSS2, COQ2, COQ4, COQ5, COQ6, COQ7, COQ8A, COQ8B, and COQ9 genes have been described in CoQ deficiency-related diseases. However, there is no relationship between mutation and a specific phenotype. In this regard, mutations in COQ8A/ADCK3 cause ataxic cerebellar syndrome, and COQ8B/ADCK4 and COQ6 genes cause steroid-resistant nephrotic syndrome, while mutations in other COQ genes are more pleiotropic [19]. For this reason, the most ambitious clinical challenge is to be able to establish the correlation between genotype and phenotype to accelerate diagnosis and improve therapies [19].
According to the clinical symptoms, CoQ deficiency has been classified in five major groups of diseases:
-
(1)
Encephalomyopathy with recurrent myoglobinuria, myopathy, and brain involvement [101];
-
(2)
Severe infantile multisystemic disease characterized by encephalopathy and nephropathy [102,103];
- (3)
- (4)
-
(5)
Nephropathy [100].
However, this classification can cause confusion in the difference between primary and secondary CoQ deficiencies, at least until the genetic diagnostic is completed. In fact, this classification needs to be updated due to the emerging number of patients who show overlapping clinical manifestations [108]. However, in the last few years, the use of next-generation sequencing (NGS) technologies, such as whole exome sequencing (WES) and whole genome sequencing (WGS), has helped to progress rapid diagnosis [109]. This means that clinical and biochemical techniques are useful for the rapid identification of CoQ deficiency, but only molecular diagnosis may elucidate the etiology of this syndrome.
4.1.2. Relationship of Primary CoQ Deficiency and Pathological Phenotype
In order to clarify the relationship between COQ mutations and phenotypes, recent revisions have been published [19,50]. Many of the mutations directly affecting members of the CoQ-synthome produce effects on the central nervous system, being COQ4 [110], COQ5 [111], COQ7 [112] and COQ9 [102], the mutated genes that generate stronger developmental delays including motor, cognitive, speech, emotional, and social and communication skill dysfunctions [113]. Further, many of the mutations found in the members of the COQ-synthome have been associated with intellectual disability characterized by impaired intellectual and adaptive functioning. Further, many mutated COQ proteins have also been involved in epilepsy, hypotonia, and the peripheral nervous system, as well as sensory organ deficiencies such as hearing loss [19].
Many of the mutations in COQ genes also affect kidney function. In general, renal dysfunction accompanied by altered morphological and physiological instability has been found especially in PDSS2 [114], COQ2 [115], COQ6 [116], and COQ8B [117] patients. All these patients evolve to end-stage renal disease within childhood if not treated, indicating the severity of the mutations.
Surprisingly, cardiac diseases are infrequent in primary CoQ10-deficient patients, despite the high importance of mitochondria in cardiac physiology. To date, the most commonly reported heart defect is hypertrophic cardiomyopathy that has been found more frequently in some COQ4 [118] and COQ7 [112] patients, and in a few PDSS2 [114], COQ2 [103], COQ9 [102], and COQ8B [119] patients. Other functional heart defects include valvulopathies, cardiomegaly, septal defects, and heart hypoplasia, but no clear relationship between phenotype and mutation has been found [19,50].
Despite the importance of mitochondria in muscle physiology, muscle is relatively unaffected in these patients. In primary CoQ deficiency, isolated myopathy has been not found, although it is more common in secondary deficiency patients [100,108]. However, myopathy in COQ patients has been reported but in association with a multisystemic phenotype and not always affecting all the patients with a specific mutation [19,50]. The clearest muscle physiological defects are exercise intolerance, muscle weakness, and muscle fatigue, which are found more frequently in COQ8 patients [120].
Other clinical pathologies associated with these mutations include respiratory distress and apnea [118], and respiratory failure [121] but without a clear adscription to a set of COQ mutations. Liver dysfunction has also been found in some COQ2 patients as well as in some COQ8 patients, but again without showing a clear pathological phenotype [19,50].
In general, it seems clear that neurological and renal dysfunctions in addition to hearing loss are the most common manifestations of COQ gene mutations. Other pathological manifestations can be a consequence of a different degree of sensibility to mitochondrial dysfunction depending on other factors or the equilibrium with other genes. Further research is needed, although the low number of patients with a specific mutation has made it impossible to establish a clear relationship between mutation and direct phenotypic characteristics to date.
4.2. Secondary CoQ Deficiency Due to Mitochondrial Dysfunction
Secondary CoQ deficiencies were found in affected OXPHOS or non-OXPHOS processes and also in non-mitochondrial process [14,122], and are much more frequent and diverse than primary CoQ deficiency [122].
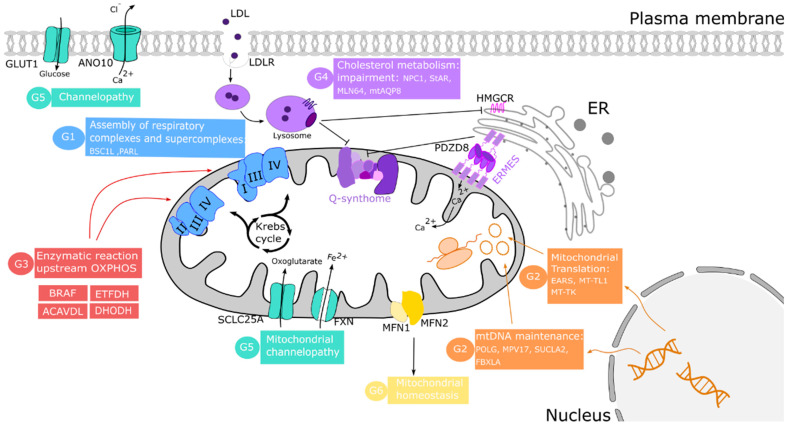
Table 1 includes those published cases of secondary CoQ deficiency due to mitochondrial dysfunction. The mechanisms underlying these secondary diseases are poorly understood, but they can be categorized into several groups according to the origin of CoQ deficit: (1) disorders of the OXPHOS system; (2) pathogenic variants of nuclear and mitochondrial DNA-encoded proteins; (3) defects in other enzymes upstream of OXPHOS; (4) cholesterol metabolic impairment; (5) defects in protein carriers; and (6) defects in mitochondrial homeostasis.
Table 1.
Groups of disorders associated with secondary CoQ deficiency.
| Group of Disorders | Gene | Function | Diagnosis | Clinical Phenotype Described in Humans | Refs. |
|---|---|---|---|---|---|
| G1: Disorders due to impairment of oxidative phosphorylation (OXPHOS) system |
BSCL-1 | Defective BSCL-1 generates a catalytically and structurally inactive complex III | Leigh syndrome | Neurological symptoms and lactic acidosis | [123] |
| NDUFS4 | Defects on assembly of functional complex I | Leigh syndrome | Progressive loss of mental and psychomotor regression | [124,125] | |
| PARL | Instability of TTC19 expression, which is required for complex III activity | Leigh-like syndrome due to impairment of CIII activity | Necrotizing encephalomyelopathy | [67] | |
| G2: Defects on nuclear and mitochondrial DNA |
nDNA and mtDNA deletions | OXPHOS dysfunction | Mitochondrial DNA depletion syndrome (MDS) | Clinically heterogeneous mitochondrial phenotypes | [126] |
| POLG, MPV17, SUCLA2, FBXLA | mtDNA depletion | [127,128] | |||
| EARS2 | Defective mitochondrial aminoacyl-tRNA synthetase specific for glutamate | Combined OXPHOS deficiency | Leukoencephalopathy with high lactate | [129] | |
| MT-TL1 | Translational defects by punctual mutations in tRNA encoded by mtDNA | MELAS disease | Encephalomyopathy and lactic acidosis | [130] | |
| MT-TK | MERFF disease | Clinically heterogeneous mitochondrial phenotypes | [131] | ||
| G3: Defects on other proteins involved in enzymatic reactions upstream of OXPHOS |
ETFDH | Electron transfer defects from electron-transferring flavoprotein to ubiquinone | Multiple Acyl-CoA dehydrogenase deficiency (MADD) | Isolated myopathic phenotype | [132] |
| BRAF | Disruption of signals controlling cell growth | Cardiofaciocutaneous syndrome (CFS) | Psychomotor development delayed, muscular hypotonia, and ataxia | [133] | |
| ACADVL | Defects of first step of the mitochondrial fatty acid beta-oxidation pathway | Very long-chain Acyl-CoA dehydrogenase deficiency | Encephalopathy and rhabdomyolysis | [134] | |
| TBC1D24 | GTPase-activating protein for Rab family protein | Multifocal polymyoclorus | Multifocal polymyoclonus and neurodevelopmental delay | [135] | |
| G4: Cholesterol metabolism impairment |
PDZD8 | Impairment of mitochondrial Ca2+ uptake | Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis with associated frontotemporal dementia | Typical symptoms in neurodegenerative diseases | [136,137] |
| 3-Hydroxy-3-Methylglutaryl-CoA Rase | Defects on mevalonate pathway and CoQ synthesis | Niemann-Pick Type C1-deficiency, steatohepatitis, Alzheimer’s disease | [138,139,140,141] | ||
| StAR, MLN64, Aquaporin-8 | Cholesterol metabolism impairment | [142,143] | |||
| LDL-receptor and other proteins | Binding defects on LDL-cholesterol to plasma membrane receptor | Familial hypercholesterolemia | Early atherosclerosis and elevated serum cholesterol concentrations | [144] | |
| G5: Diseases caused by defects on protein carrier |
SLC25A11 | Disruption of transport of 2-oxoglutarate to mitochondria | Liver cancer | Hepatocellular carcinoma | [145] |
| ANO10 | Putative calcium-activated chloride channels defects | Autosomal recessive spinocerebellar ataxia-10 | Slowly progressive ataxia and dysarthria | [146] | |
| FXN | Defects in transport of iron to mitochondria | Friedreich’s ataxia | Ataxia, limn incoordination, dysarthria, dysphagia, eye movement defects, and muscle weakness | [122] | |
| G6: Mitochondrial homeostasis dysregulation |
LDL-receptor and other proteins | Autophagy flux impairment | Familial hypercholesterolemia | Early atherosclerosis and elevated serum cholesterol concentration | [144] |
| Point mutations in mtDNA genes encoding tRNAs | Defective protein synthesis | MELAS disease | Lactic acidosis | [130] | |
| MFN2 | Depletion of mitochondrial CoQ and respiratory chain dysfunction | n.d. | n.d. | [147] |
n.d.: not determined.
The first group of these diseases is related to the essential role of CoQ in the function of ETC. Therefore, the impairment of this system promotes mitochondrial bioenergetic disorders such as defects in proteins like BSC1L, necessary for assembling CIII [123], the CI subunit NDUFSA [124,125], and the mitochondrial protease PARL, necessary for the processing of TTC19 required for CIII activity [67]. In these cases, the reduction in CoQ levels could be the cause of a negative feedback of CoQ biosynthesis by decreasing the activity of respiratory complexes.
The maintenance of DNA copy number and its integrity is essential to preserving a proper mitochondrial function. The second diseases group is led by defective nuclear and mitochondrial-encoded OXPHOS proteins. Some of these alterations include nuclear DNA deletions [126] and mutations in POLG, MPV17, SUCLA2, or FBXLA genes [127,128], which cause mitochondrial DNA depletion syndrome (MDS) or Alper’s disease. Both mitochondrial and nuclear genome mutations can generate defects in the translation of mitochondrial proteins. Thus, mutations in EARS2, which encodes for mitochondrial aminoacyl-tRNA synthetase, cause mild-type leukoencephalopathy [129], and MT-TL1 and MT-TK mutations generate MELAS [130] and MERRF [131] diseases, respectively.
CoQ also participates as a cofactor in other enzymatic reactions upstream and downstream of the OXPHOS system. For example, as indicated above, CoQ receives electrons from many other dehydrogenases, such as electron-transferring flavoprotein dehydrogenase (ETFDH). Mutation in this gene causes multiple acyl-CoA dehydrogenation deficiency (MADD) characterized by a decrease in CoQ levels [132]. CoQ deficiency has been found in cardiofaciocutaneous syndrome where the BRAF gene, encoding a serine/threonine-protein kinase, is mutated [133]. Mutations in ACADVL, causing very long-chain Acyl-CoA dehydrogenase deficiency, and TBC1D24, which causes multifocal polymyoclonus and neurodevelopmental delay, have been associated with secondary CoQ deficiency [134,135].
CoQ is distributed throughout the cell membranes after synthesis inside mitochondria, and extracellular CoQ can be imported into mitochondria [15,148]. In yeast, the distribution of endogenously synthesized CoQ in mitochondria is affected by the Ypl109c and Ylr253w genes. Ypl109c (Cqd1) is required to maintain CoQ in mitochondria. Its deletion increases CoQ exit from mitochondria, increasing the resistance to oxidative stress. Ylr253w (Cqd2) promotes the opposite effects [149]. Interestingly, Adck2 (a mammal orthologue of Ypl109c) deletion causes mitochondrial deficiency in CoQ, leading to mitochondrial myopathy in skeletal muscle [150]. To reach mitochondria, exogenous CoQ requires a mechanism of import based on endocytosis and intracellular vesicular trafficking pathways, well conserved in all species. To date, six yeast transporter proteins (CDC10, RTS1, RVS161, RVS167, VPS1, and NAT3) represent the essential steps in the pathways responsible for the transport of exogenous CoQ to its functional sites in the cell [151]. Additionally, in yeast, the CoQ biosynthetic complex is organized in domains related to endoplasmic reticulum-mitochondria encounter structures called ER-mitochondria contact sites. Disorganization of these sites triggers secondary CoQ deficiency through a loss of mitochondrial membrane integrity compromising Q-synthome organization and the transfer of CoQ from and to mitochondria [21,152].
CoQ-synthome and mitochondrial machinery organization could also be affected by a disruption of intracellular cholesterol trafficking towards mitochondria. In fact, an elevated mitochondrial cholesterol level has been detected in several mitochondrial pathological conditions, including Niemann-Pick Type C1-deficiency (NPC) [141], steatohepatitis [140], and Alzheimer’s disease [139]. A study using in vitro and in vivo models of mitochondrial cholesterol enrichment displayed an impairment in oxidative phosphorylation and respiratory supercomplex assembly. This effect is due to the dysregulation of the enzyme in cholesterol synthesis 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMG-CoAR), which may down-regulate the mevalonate pathway and CoQ synthesis [139]. Furthermore, mitochondrial cholesterol trafficking is controlled by proteins such as STAR and MLN64, among others. An overexpression of MLN64 [142] and mitochondrial aquaporin-8 (mtAQP8) [143] induce an increase in both mitochondrial cholesterol and dysfunction. In fact, it has been hypothesized that statin-induced CoQ deficiency inhibits HMG-CoAR, a common enzyme of CoQ and the cholesterol biosynthesis pathways [138].
The fifth group of causes of secondary CoQ deficiency is represented by a deficit in the activity of transporter proteins. The cholesterol could perturb other membrane carriers such as SLC25A11, which catalyzes the transport of 2-oxoglutarate to mitochondria. A cholesterol-mediated impairment of this carrier promotes mitochondrial GSH (mGSH) depletion, leading to increased oxidative stress [145]. Furthermore, mutations in the ANO10 gene, which encodes for a carrier implicated in calcium signaling that is important for mitochondrial activity, has been associated with CoQ deficiency and ataxia [146]. Mutations in the FXN gene encoding for frataxin, a protein that regulates the transport of iron into mitochondria, is related to ferroptosis, a regulated cell death associated with lipid peroxidation. Some patients with Friedrich ataxia harbored the FXN mutation associated with low CoQ levels in skeletal muscle [122]. Recently, it has been observed that CoQ performs an important role in the DHODH-mediated defense mechanism against ferroptosis [153,154].
Finally, mitochondrial homeostasis is the result of a balance between mitochondrial biogenesis and mitophagy. This last group of secondary CoQ deficiencies is represented by diseases in which mitochondrial dysfunction is the result of an unbalance in mitochondrial turnover. In Familiar Hypercholesterolemia (FH), pathology has been associated with the persistent activation of mitophagy in fibroblasts [144]. Similarly, an increase in the degradation of impaired mitochondria was observed in fibroblasts of patients with MELAS [130]. Furthermore, MFN1 and MFN2 are also proteins implicated in mitochondrial dynamics through the activation of fusion to increase the amount of active mitochondria [155]. Specifically, it has been described that MFN2 is essential for CoQ biosynthesis [147].
In summary, CoQ synthesis can be affected by many different processes located not only in mitochondria, but also in other organelles (Figure 2).
Figure 2.
Causes of secondary CoQ deficiency. Mechanisms proposed to trigger the secondary variant are grouped in several categories (G1 to G6) indicated in different colors. The synthesis of CoQ can be reduced by defects in proteins implicated directly or indirectly in the assembly and proper respiratory machinery functioning (G1 (blue) and G3 (red)). In orange (G2) are the essential proteins for the maintenance of intact mtDNA. Point mutations in mitochondrial aminoacyl-tRNA synthetases are also associated with low CoQ levels. The stability of Q-synthome depends on ER-mitochondrial contact sites (ERMES) and the cholesterol synthesis pathway (G4 (purple)). Defects in the proteins implicated in the transport of several metabolites, such as glucose or Fe2+, throughout to plasm or mitochondrial membrane cause channelopathies, marked in green (G5). The mechanisms to maintain mitochondrial mass by processes such as fusion-fission proteins, MFN1, and MFN2 (G6) are shown in yellow.
5. Role of CoQ in Aging and Age-Associated Metabolic Disorders
The accumulation of dysfunctional mitochondria is a shared feature of both aging and metabolic disorders [156,157,158,159]. Evidence indicating the essential role of mitochondrial physiology in the increasing dysfunction of tissues and organs during aging has been accumulating over the last few years [160,161]. This indicates that any therapy able to maintain a balanced mitochondrial turnover and dynamics can delay age-associated metabolic diseases and improve functionality during the progression of aging [1,4,39].
The essential role of CoQ in the maintenance of mitochondrial activities and in the prevention of oxidative damage in cells and plasma lipoproteins points to its importance in aging and in age-related diseases [1,162]. However, it is not clear if the decrease in CoQ levels associated with aging is the cause of mitochondrial dysfunction or a consequence of the deterioration of the turnover and dynamics of mitochondria found in metabolic diseases and aging [157,160,163].
We can consider that the secondary CoQ deficiency associated with aging may be a consequence of OXPHOS dysfunction [126]. Several CoQ biosynthesis genes were downregulated in mice showing impaired mtDNA gene expression [126]. Many components of the CoQ-synthome suffered a clear decrease in mitochondria: COQ3, COQ5, COQ6, COQ7, COQ8A/ADCK3, COQ9, and COQ10A. On the other hand, two of these enzymes (PDSS2 and COQ8B/ADCK4) increased, indicating a different response of the members of the synthome to mitochondrial dysfunction [126]. Additionally, COQ8A/ADCK3 and COQ8B/ADCK4 are regulated depending on the glycolytic or respiratory conditions, indicating a response to the metabolic conditions [164]. It has been recently reported that in glioma cells, the inhibition of CoQ biosynthesis is associated with the stabilization of HIF-1 and the switch toward glycolysis, introducing a mechanism in the regulation of the metabolism and the development of cancer [165].
The decrease in CoQ levels found in mutants and in aged animals could be a response to the equilibrium to maintain a balanced activity of the ETC. However, we cannot discard a side effect of OXPHOS dysfunction that affects activities in the inner mitochondrial membrane that regulate CoQ synthesis [67], and the transport of proteins into the mitochondrial matrix that can destabilize the CoQ-synthome [126].
Interestingly, the maintenance of balanced mitochondrial dynamics is essential to avoid mitochondrial dysfunction during aging and metabolic diseases [156]. The transport of polyprenyl pyrophosphate from endoplasmic reticulum to mitochondria is severely affected in mitofusin2 (MFN2) KO mice and causes CoQ deficiency [147]. MFN2 is directly involved in mitochondrial fusion [166], and also in the tethering of mitochondria to the ER [167]. Communication between the ER and mitochondria affects many physiological processes, and its alteration has been associated with aging [168] and many chronic pathologies associated with aging, such as neurodegenerative diseases, metabolic syndromes, and cancer [169].
On the other hand, a depletion in CoQ levels activates mitophagy [170] and increases the dysfunction of mitochondria associated with higher oxidative stress and apoptosis in cultured cells [171]. Further, a depletion in CoQ levels would not only add more disturbing factors for mitochondrial physiology, but also affects membrane antioxidant activities. This cycle would accelerate mitochondrial dysfunction while aggravating oxidative stress.
In support of the key role of CoQ in the delay of this vicious cycle, recent studies have proposed the therapeutic use of CoQ, or bioactive compounds able to increase its levels, to reduce the progression of age-related diseases and to improve healthy aging [39,172,173]. Treatments with ubiquinol can rescue statin-associated mitochondrial dysfunction and rhabdomyolysis, indicating that a depletion in CoQ levels in muscle causes mitochondrial dysfunction through the chronic use of statins [174]. Lower CoQ levels have been recently associated with the progress of chronic kidney diseases [175].
6. Supplementation with CoQ: Metabolic Recovery
The efficacy of CoQ as supplement for the treatment of diseases associated with mitochondrial dysfunction is not completely clear. Many studies and clinical trials have been performed in order to find the positive effects of dietary supplementation with CoQ in different metabolic and age-related diseases [176]. However, the design of the trials and the presence of an important percentage of the population showing a low incorporation of dietary CoQ has not allowed us to obtain clear therapeutic procedures for this compound [177,178].
The hydrophobicity of CoQ and its large molecular weight reduces the amount of nutritional CoQ that reaches blood plasma [179]. Fat-solubilized formulations are preferred to powder-based presentations as solubilization in the fat matrix permits superior bioavailability [178,179]. Recently, it has been found that CoQ transport in intestinal epithelium requires Niemann-Pick Like 1 protein, a transporter involved in the intestinal absorption of fat-soluble components such as cholesterol [180]. This is an interesting aspect as people showing low fat absorption from their diet may suffer from an insufficient incorporation of dietary CoQ. In any case, supplementation with CoQ produces null or very low toxicity and no serious side effects in doses up to 1.2 to 3 g/day [181]. This aspect is important, especially in those patients suffering CoQ deficiency syndrome, which needs supplementation with high levels of this compound.
CoQ supplementation has shown therapeutic benefits in aging-related disorders, principally in cardiovascular and metabolic diseases in the elderly [182,183]. Studies on the effect of CoQ supplementation in human aging need to be performed rigorously, but studies in rat life-long supplementation have demonstrated the attenuation of an age-related decrease in total antioxidant activity, and also a reduction in DNA-damage in lymphocytes [184].
The supplementation of a Mediterranean diet with CoQ improves the metabolism of advanced glycation end products in the post-prandial period in elderly people [185], the lipid and glycemic profile in dyslipidemia individuals [186], and the glucose metabolism and oxidative damage markers in the plasma of patients with diabetic nephropathy [187]. In people suffering metabolic syndrome, CoQ supplementation may reduce serum triglyceride levels and improve lipid profiles [188].
Metabolic syndrome courses with a chronic inflammatory profile and CoQ supplementation has demonstrated the capacity to reduce this profile, possibly by improving mitochondrial activity in immune cells. In general, supplementation with CoQ can reduce the levels of plasmatic inflammatory cytokines [189], although the number of studies are limited, and in some cases, the results are conflicting [190,191,192]. The effect of supplementation with CoQ in a decrease in inflammation and lipid peroxidation in metabolic syndrome patients has also been associated with an improvement in liver function [193].
Mitochondrial dysfunction is associated with aging-dependent myalgia, and supplementation with CoQ shows some therapeutic efficacy in reducing inflammation and restoring antioxidant and mitochondrial activities [194]. Interestingly, CoQ and carotene deficiencies, accompanied by metabolic disorders, are common in patients with oral cancer. This deficiency is associated with an increase in the risk of central obesity, hyperglyceridemia, and metabolic disease [195].
Interestingly, CoQ10 appears as an efficient reducer of inflammation in people showing chronic inflammation processes, such as ulcerative colitis [196]. In fact, its use has been proposed in the modulation of autoimmune disorders accompanied by inflammatory processes [197], such as antiphospholipid syndrome [198]. We have recently proposed that supplementation with CoQ could reverse the mitochondrial dysfunction affecting immune cells such as cytotoxic T-lymphocytes and proinflammatory macrophages that are associated with immune deficiency and the inflammatory responses associated with respiratory diseases [49].
A reduction in proinflammatory cytokines levels through the modulation of mitochondrial dysfunction in endothelial and immune cells can also be related with a protective effect on neurodegenerative diseases. In these diseases, oxidative stress is considered a key modulator of the progression of cell dysfunction and degeneration [43]. In fact, supplementation with CoQ reduces chronic inflammation and endothelial dysfunction in patients suffering mild cognitive impairment [199]. It seems clear that inflammation can be considered a therapeutic target of CoQ supplementation for neuronal and muscular degenerative diseases [98,173].
The most clear therapeutic effect of supplementation with CoQ is in cardiovascular diseases [200], and lowering lipid levels in patients with coronary artery disease [201]. In fact, CoQ supplementation is considered to be a therapeutic tool to prevent cardiomyopathies, coronary artery disease, and the preservation of the myocardium [200]. In recent years, the combination of selenium and CoQ has shown positive effects in reducing cardiovascular mortality and the levels of plasma markers of cardiovascular damage [182,183,202,203,204].
Apart from an improvement in cardiovascular function, the protection of endothelial function against oxidative damage in cardiovascular disease seems to be one of the most promising activities of supplementation with CoQ as it can rapidly reach these cells from plasma [205]. CoQ can act as an antioxidant both reducing oxidative damage and improving mitochondrial activity reducing oxidative stress [206], which would improve endothelial function [207]. The endothelial dysfunction induced by dyslipidemia can be improved by CoQ supplementation through increasing NO production and reducing LDL oxidation [208].
Mitochondrial activity and oxidative damage are also associated with exercise. The antioxidant capacity of CoQ can be essential in the protection against oxidative damage and inflammation during intense exercise [209], and can even improve aerobic capacity in athletes during heavy training sessions or competition [210,211]. Short-term moderate CoQ supplementation can alleviate tissue damage and fatigue after strenuous exercise in runners [212]. Supplementation with CoQ has been proposed to restore moderate CoQ deficiency in athletes in order to maintain antioxidant protection and exercise performance [213], to induce pro-angiogenic effects by improving hemoglobin levels and reducing inflammatory markers [209], and to produce an ergogenic effect [214].
7. Concluding Remarks
It is clear that CoQ is an essential factor involved in many metabolic and antioxidant processes controlling energy in cells and in molecular protection against oxidation and even cell survival against ferroptosis. As essential factor for many different metabolic activities in the cells mainly involving catabolic, anabolic, and antioxidant activities, CoQ plays a pivotal role in cell and organ physiology. Its deficiency can displace the metabolism to those activities in which CoQ interaction occurs at higher affinity, whereas those enzymes that interact with CoQ at lower affinity are seriously affected. This effect can be important in mitochondria, in which CoQ shares its redox activity with catabolic and anabolic processes.
For this reason, levels of CoQ in humans can be key in the promotion, initial phases, and development of many metabolic and degenerative diseases, and even in the proper aging process. Mitochondrial dysfunction and CoQ levels can be part of a vicious cycle in which dysfunctional mitochondria can reduce CoQ levels, enhancing mitochondrial destruction by mitophagy, and further increasing mitochondrial dysfunction. Supplementation with CoQ in many of the diseases involved in chronic metabolic diseases and aging can improve mitochondrial and metabolic activities and decrease oxidative damage, reinforcing the role of CoQ as a hinge in metabolism regulation in health and disease.
Author Contributions
J.D.H.-C., L.G.-C., D.J.M.F.-A., G.L.-L. and P.N., writing—original draft preparation; G.L.-L. and P.N., writing—review and editing; and J.D.H.-C. and P.N., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Junta de Andalucía grants P18-RT-4572, UPO-126247, UPO-1265673 and BIO-177, the Instituto de Salud Carlos III FIS grant FIS PI20/00541, the FEDER Funding Program from the European Union, and CIBERER (U729)-ISCIII, and Spanish Ministry of Science, Innovation and Universities grant RED2018-102576-T to P.N. This work was supported by the Spanish Ministry of Education, Culture and Sports through fellowship FPU16/03264 to J.D.H-C.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopez-Lluch G., Rodriguez-Aguilera J.C., Santos-Ocana C., Navas P. Is coenzyme Q a key factor in aging? Mech. Ageing Dev. 2010;131:225–235. doi: 10.1016/j.mad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Festenstein G.N., Heaton F.W., Lowe J.S., Morton R.A. A constituent of the unsaponifiable portion of animal tissue lipids (lambda max. 272 m mu) Biochem. J. 1955;59:558–566. doi: 10.1042/bj0590558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane F.L., Hatefi Y., Lester R.L., Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta Bioenerg. 1957;25:220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Camacho J.D., Bernier M., Lopez-Lluch G., Navas P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentinger M., Tekle M., Dallner G. Coenzyme Q—biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 6.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta Bioenergy. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Forsmark-Andree P., Dallner G., Ernster L. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Radic. Biol. Med. 1995;19:749–757. doi: 10.1016/0891-5849(95)00076-A. [DOI] [PubMed] [Google Scholar]
- 8.Godic A., Poljsak B., Adamic M., Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014;2014:860479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroz A., Anderson R.F., Smith R.A., Murphy M.P. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: Implications for in vivo antioxidant activity. Free Radic. Biol. Med. 2009;46:105–109. doi: 10.1016/j.freeradbiomed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Bentinger M., Brismar K., Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Kagan V.E., Arroyo A., Tyurin V.A., Tyurina Y.Y., Villalba J.M., Navas P. Plasma membrane NADH-coenzyme Q0 reductase generates semiquinone radicals and recycles vitamin E homologue in a superoxide-dependent reaction. FEBS Lett. 1998;428:43–46. doi: 10.1016/S0014-5793(98)00482-7. [DOI] [PubMed] [Google Scholar]
- 12.Navas P., Villalba J.M., Lenaz G. Coenzyme Q-dependent functions of plasma membrane in the aging process. AGE. 2005;27:139–146. doi: 10.1007/s11357-005-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villalba J.M., Navas P. Regulation of coenzyme Q biosynthesis pathway in eukaryotes. Free Radic. Biol. Med. 2021;165:312–323. doi: 10.1016/j.freeradbiomed.2021.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Navas P., Cascajo M.V., Alcazar-Fabra M., Hernandez-Camacho J.D., Sanchez-Cuesta A., Rodriguez A.B.C., Ballesteros-Simarro M., Arroyo-Luque A., Rodriguez-Aguilera J.C., Fernandez-Ayala D.J.M., et al. Secondary CoQ10 deficiency, bioenergetics unbalance in disease and aging. Biofactors. 2021;47:551–569. doi: 10.1002/biof.1733. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Ayala D.J., Brea-Calvo G., Lopez-Lluch G., Navas P. Coenzyme Q distribution in HL-60 human cells depends on the endomembrane system. Biochim. Biophys. Acta Biomembr. 2005;1713:129–137. doi: 10.1016/j.bbamem.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Kawamukai M. Biosynthesis of coenzyme Q in eukaryotes. Biosci. Biotechnol. Biochem. 2016;80:23–33. doi: 10.1080/09168451.2015.1065172. [DOI] [PubMed] [Google Scholar]
- 17.Stefely J.A., Pagliarini D.J. Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem. Sci. 2017;42:824–843. doi: 10.1016/j.tibs.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez M.J.A., Trevisson E., Canton M., Vazquez-Fonseca L., Morbidoni V., Baschiera E., Frasson C., Pelosi L., Rascalou B., Desbats M.A., et al. Vanillic Acid Restores Coenzyme Q Biosynthesis and ATP Production in Human Cells Lacking COQ6. Oxid. Med. Cell. Longev. 2019;2019:3904905. doi: 10.1155/2019/3904905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcazar-Fabra M., Rodriguez-Sanchez F., Trevisson E., Brea-Calvo G. Primary Coenzyme Q deficiencies: A literature review and online platform of clinical features to uncover genotype-phenotype correlations. Free Radic. Biol. Med. 2021;167:141–180. doi: 10.1016/j.freeradbiomed.2021.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg-Bord M., Tsui H.S., Antunes D., Fernandez-Del Rio L., Bradley M.C., Dunn C.D., Nguyen T.P.T., Rapaport D., Clarke C.F., Schuldiner M. The Endoplasmic Reticulum-Mitochondria Encounter Structure Complex Coordinates Coenzyme Q Biosynthesis. Contact. 2019;2:2515256418825409. doi: 10.1177/2515256418825409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian K., Jochem A., Le Vasseur M., Lewis S., Paulson B.R., Reddy T.R., Russell J.D., Coon J.J., Pagliarini D.J., Nunnari J. Coenzyme Q biosynthetic proteins assemble in a substrate-dependent manner into domains at ER-mitochondria contacts. J. Cell Biol. 2019;218:1353–1369. doi: 10.1083/jcb.201808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashby M.N., Edwards P.A. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J. Biol. Chem. 1990;265:13157–13164. doi: 10.1016/S0021-9258(19)38280-8. [DOI] [PubMed] [Google Scholar]
- 23.Payet L.A., Leroux M., Willison J.C., Kihara A., Pelosi L., Pierrel F. Mechanistic Details of Early Steps in Coenzyme Q Biosynthesis Pathway in Yeast. Cell Chem. Biol. 2016;23:1241–1250. doi: 10.1016/j.chembiol.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Stefely J.A., Kwiecien N.W., Freiberger E.C., Richards A.L., Jochem A., Rush M.J.P., Ulbrich A., Robinson K.P., Hutchins P.D., Veling M.T., et al. Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat. Biotechnol. 2016;34:1191–1197. doi: 10.1038/nbt.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson K.P., Jochem A., Johnson S.E., Reddy T.R., Russell J.D., Coon J.J., Pagliarini D.J. Defining intermediates and redundancies in coenzyme Q precursor biosynthesis. J. Biol. Chem. 2021;296:100643. doi: 10.1016/j.jbc.2021.100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awad A.M., Bradley M.C., Fernandez-Del Rio L., Nag A., Tsui H.S., Clarke C.F. Coenzyme Q10 deficiencies: Pathways in yeast and humans. Essays Biochem. 2018;62:361–376. doi: 10.1042/EBC20170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozeir M., Muhlenhoff U., Webert H., Lill R., Fontecave M., Pierrel F. Coenzyme Q biosynthesis: Coq6 is required for the C5-hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem. Biol. 2011;18:1134–1142. doi: 10.1016/j.chembiol.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Jonassen T., Clarke C.F. Isolation and functional expression of human COQ3, a gene encoding a methyltransferase required for ubiquinone biosynthesis. J. Biol. Chem. 2000;275:12381–12387. doi: 10.1074/jbc.275.17.12381. [DOI] [PubMed] [Google Scholar]
- 29.Poon W.W., Barkovich R.J., Hsu A.Y., Frankel A., Lee P.T., Shepherd J.N., Myles D.C., Clarke C.F. Yeast and rat Coq3 and Escherichia coli UbiG polypeptides catalyze both O-methyltransferase steps in coenzyme Q biosynthesis. J. Biol. Chem. 1999;274:21665–21672. doi: 10.1074/jbc.274.31.21665. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen T.P., Casarin A., Desbats M.A., Doimo M., Trevisson E., Santos-Ocana C., Navas P., Clarke C.F., Salviati L. Molecular characterization of the human COQ5 C-methyltransferase in coenzyme Q10 biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2014;1841:1628–1638. doi: 10.1016/j.bbalip.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marbois B.N., Clarke C.F. The COQ7 gene encodes a protein in Saccharomyces cerevisiae necessary for ubiquinone biosynthesis. J. Biol. Chem. 1996;271:2995–3004. doi: 10.1074/jbc.271.6.2995. [DOI] [PubMed] [Google Scholar]
- 32.Tauche A., Krause-Buchholz U., Rodel G. Ubiquinone biosynthesis in Saccharomyces cerevisiae: The molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 2008;8:1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 33.Xie L.X., Hsieh E.J., Watanabe S., Allan C.M., Chen J.Y., Tran U.C., Clarke C.F. Expression of the human atypical kinase ADCK3 rescues coenzyme Q biosynthesis and phosphorylation of Coq polypeptides in yeast coq8 mutants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2011;1811:348–360. doi: 10.1016/j.bbalip.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefely J.A., Reidenbach A.G., Ulbrich A., Oruganty K., Floyd B.J., Jochem A., Saunders J.M., Johnson I.E., Minogue C.E., Wrobel R.L., et al. Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme Q biosynthesis. Mol. Cell. 2015;57:83–94. doi: 10.1016/j.molcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marbois B., Gin P., Gulmezian M., Clarke C.F. The yeast Coq4 polypeptide organizes a mitochondrial protein complex essential for coenzyme Q biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2009;1791:69–75. doi: 10.1016/j.bbalip.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh E.J., Gin P., Gulmezian M., Tran U.C., Saiki R., Marbois B.N., Clarke C.F. Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch. Biochem. Biophys. 2007;463:19–26. doi: 10.1016/j.abb.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohman D.C., Forouhar F., Beebe E.T., Stefely M.S., Minogue C.E., Ulbrich A., Stefely J.A., Sukumar S., Luna-Sanchez M., Jochem A., et al. Mitochondrial COQ9 is a lipid-binding protein that associates with COQ7 to enable coenzyme Q biosynthesis. Proc. Natl. Acad. Sci. USA. 2014;111:E4697–E4705. doi: 10.1073/pnas.1413128111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui T.Z., Kawamukai M. Coq10, a mitochondrial coenzyme Q binding protein, is required for proper respiration in Schizosaccharomyces pombe. FEBS J. 2009;276:748–759. doi: 10.1111/j.1742-4658.2008.06821.x. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Lluch G. Coenzyme Q homeostasis in aging: Response to non-genetic interventions. Free Radic. Biol. Med. 2021;164:285–302. doi: 10.1016/j.freeradbiomed.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Diaz-Casado M.E., Quiles J.L., Barriocanal-Casado E., Gonzalez-Garcia P., Battino M., Lopez L.C., Varela-Lopez A. The Paradox of Coenzyme Q10 in Aging. Nutrients. 2019;11:2221. doi: 10.3390/nu11092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee R., Purhonen J., Kallijarvi J. The mitochondrial coenzyme Q junction and complex III: Biochemistry and pathophysiology. FEBS J. 2021 doi: 10.1111/febs.16164. [DOI] [PubMed] [Google Scholar]
- 42.Sanz A., Navas P. Editorial: Coenzyme Q Redox State and Cellular Homeostasis. Front. Physiol. 2018;9:912. doi: 10.3389/fphys.2018.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scialo F., Sanz A. Coenzyme Q redox signalling and longevity. Free Radic. Biol. Med. 2021;164:187–205. doi: 10.1016/j.freeradbiomed.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orr A.L., Quinlan C.L., Perevoshchikova I.V., Brand M.D. A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase. J. Biol. Chem. 2012;287:42921–42935. doi: 10.1074/jbc.M112.397828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushita F., Yoshikawa H. The effect of epidural anesthesia on the differential pulmonary blood flow when one lung was challenged with hypoxia. Masui. Jpn. J. Anesthesiol. 1988;37:1044–1051. [PubMed] [Google Scholar]
- 49.Fernandez-Ayala D.J.M., Navas P., Lopez-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020;142:111147. doi: 10.1016/j.exger.2020.111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alcazar-Fabra M., Trevisson E., Brea-Calvo G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018;62:377–398. doi: 10.1042/EBC20170107. [DOI] [PubMed] [Google Scholar]
- 51.Hidalgo-Gutierrez A., Gonzalez-Garcia P., Diaz-Casado M.E., Barriocanal-Casado E., Lopez-Herrador S., Quinzii C.M., Lopez L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants. 2021;10:520. doi: 10.3390/antiox10040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauchova H., Battino M., Fato R., Lenaz G., Drahota Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1992;24:235–241. doi: 10.1007/BF00762682. [DOI] [PubMed] [Google Scholar]
- 53.Missaglia S., Tavian D., Angelini C. ETF dehydrogenase advances in molecular genetics and impact on treatment. Crit. Rev. Biochem. Mol. Biol. 2021;56:360–372. doi: 10.1080/10409238.2021.1908952. [DOI] [PubMed] [Google Scholar]
- 54.Summitt C.B., Johnson L.C., Jonsson T.J., Parsonage D., Holmes R.P., Lowther W.T. Proline dehydrogenase 2 (PRODH2) is a hydroxyproline dehydrogenase (HYPDH) and molecular target for treating primary hyperoxaluria. Biochem. J. 2015;466:273–281. doi: 10.1042/BJ20141159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Garcia P., Hidalgo-Gutierrez A., Mascaraque C., Barriocanal-Casado E., Bakkali M., Ziosi M., Abdihankyzy U.B., Sanchez-Hernandez S., Escames G., Prokisch H., et al. Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism. Hum. Mol. Genet. 2020;29:3296–3311. doi: 10.1093/hmg/ddaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sahebekhtiari N., Fernandez-Guerra P., Nochi Z., Carlsen J., Bross P., Palmfeldt J. Deficiency of the mitochondrial sulfide regulator ETHE1 disturbs cell growth, glutathione level and causes proteome alterations outside mitochondria. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:126–135. doi: 10.1016/j.bbadis.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 57.Baschiera E., Sorrentino U., Calderan C., Desbats M.A., Salviati L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021;166:277–286. doi: 10.1016/j.freeradbiomed.2021.02.039. [DOI] [PubMed] [Google Scholar]
- 58.Drabikowska A.K., Szarkowska L. The reduction of ubiquinone in rat liver mitochondria associated with the oxidation of choline. Acta Biochim. Pol. 1965;12:387–394. [PubMed] [Google Scholar]
- 59.Loffler M., Carrey E.A., Knecht W. The pathway to pyrimidines: The essential focus on dihydroorotate dehydrogenase, the mitochondrial enzyme coupled to the respiratory chain. Nucleosides Nucleotides Nucleic Acids. 2020;39:1281–1305. doi: 10.1080/15257770.2020.1723625. [DOI] [PubMed] [Google Scholar]
- 60.Woyda-PLoSzczyca A.M., Jarmuszkiewicz W. The conserved regulation of mitochondrial uncoupling proteins: From unicellular eukaryotes to mammals. Biochim. Biophys. Acta Bioenerg. 2017;1858:21–33. doi: 10.1016/j.bbabio.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Reyes I., Cardona L.R., Kong H., Vasan K., McElroy G.S., Werner M., Kihshen H., Reczek C.R., Weinberg S.E., Gao P., et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 2020;585:288–292. doi: 10.1038/s41586-020-2475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tocilescu M.A., Zickermann V., Zwicker K., Brandt U. Quinone binding and reduction by respiratory complex I. Biochim. Biophys. Acta Bioenerg. 2010;1797:1883–1890. doi: 10.1016/j.bbabio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Bartoschek S., Johansson M., Geierstanger B.H., Okun J.G., Lancaster C.R., Humpfer E., Yu L., Yu C.A., Griesinger C., Brandt U. Three molecules of ubiquinone bind specifically to mitochondrial cytochrome bc1 complex. J. Biol. Chem. 2001;276:35231–35234. doi: 10.1074/jbc.C100365200. [DOI] [PubMed] [Google Scholar]
- 64.Santos-Ocana C., Do T.Q., Padilla S., Navas P., Clarke C.F. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J. Biol. Chem. 2002;277:10973–10981. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 65.Guaras A., Perales-Clemente E., Calvo E., Acin-Perez R., Loureiro-Lopez M., Pujol C., Martinez-Carrascoso I., Nunez E., Garcia-Marques F., Rodriguez-Hernandez M.A., et al. The CoQH2/CoQ Ratio Serves as a Sensor of Respiratory Chain Efficiency. Cell Rep. 2016;15:197–209. doi: 10.1016/j.celrep.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Giachin G., Bouverot R., Acajjaoui S., Pantalone S., Soler-López M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front. Mol. Biosci. 2016;3:43. doi: 10.3389/fmolb.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spinazzi M., Radaelli E., Horre K., Arranz A.M., Gounko N.V., Agostinis P., Maia T.M., Impens F., Morais V.A., Lopez-Lluch G., et al. PARL deficiency in mouse causes Complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc. Natl. Acad. Sci. USA. 2019;116:277–286. doi: 10.1073/pnas.1811938116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acin-Perez R., Fernandez-Silva P., Peleato M.L., Perez-Martos A., Enriquez J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Letts J.A., Fiedorczuk K., Degliesposti G., Skehel M., Sazanov L.A. Structures of Respiratory Supercomplex I+III2 Reveal Functional and Conformational Crosstalk. Mol. Cell. 2019;75:1131–1146.e1136. doi: 10.1016/j.molcel.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapuente-Brun E., Moreno-Loshuertos R., Acin-Perez R., Latorre-Pellicer A., Colas C., Balsa E., Perales-Clemente E., Quiros P.M., Calvo E., Rodriguez-Hernandez M.A., et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi K., Ohsawa I., Shirasawa T., Takahashi M. Optic atrophy 1 mediates coenzyme Q-responsive regulation of respiratory complex IV activity in brain mitochondria. Exp. Gerontol. 2017;98:217–223. doi: 10.1016/j.exger.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Genova M.L., Bianchi C., Lenaz G. Supercomplex organization of the mitochondrial respiratory chain and the role of the Coenzyme Q pool: Pathophysiological implications. Biofactors. 2005;25:5–20. doi: 10.1002/biof.5520250103. [DOI] [PubMed] [Google Scholar]
- 73.Lenaz G., Genova M.L. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim. Biophys. Acta Bioenerg. 2009;1787:563–573. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 74.Calvo E., Cogliati S., Hernansanz-Agustin P., Loureiro-Lopez M., Guaras A., Casuso R.A., Garcia-Marques F., Acin-Perez R., Marti-Mateos Y., Silla-Castro J.C., et al. Functional role of respiratory supercomplexes in mice: SCAF1 relevance and segmentation of the Qpool. Sci. Adv. 2020;6:eaba7509. doi: 10.1126/sciadv.aba7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genova M.L., Baracca A., Biondi A., Casalena G., Faccioli M., Falasca A.I., Formiggini G., Sgarbi G., Solaini G., Lenaz G. Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim. Biophys. Acta Bioenerg. 2008;1777:740–746. doi: 10.1016/j.bbabio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Acin-Perez R., Enriquez J.A. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys. Acta Bioenergy. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Hernansanz-Agustín P., Enríquez J.A. Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radic. Biol. Med. 2021;167:232–242. doi: 10.1016/j.freeradbiomed.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Genova M.L., Lenaz G. New developments on the functions of coenzyme Q in mitochondria. Biofactors. 2011;37:330–354. doi: 10.1002/biof.168. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Landry A.P., Ding H. The mitochondrial outer membrane protein mitoNEET is a redox enzyme catalyzing electron transfer from FMNH(2) to oxygen or ubiquinone. J. Biol. Chem. 2017;292:10061–10067. doi: 10.1074/jbc.M117.789800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bjornstedt M., Nordman T., Olsson J.M. Extramitochondrial reduction of ubiquinone by flavoenzymes. Methods Enzym. 2004;378:131–138. doi: 10.1016/S0076-6879(04)78008-8. [DOI] [PubMed] [Google Scholar]
- 81.Arroyo A., Navarro F., Gomez-Diaz C., Crane F.L., Alcain F.J., Navas P., Villalba J.M. Interactions between ascorbyl free radical and coenzyme Q at the plasma membrane. J. Bioenerg. Biomembr. 2000;32:199–210. doi: 10.1023/A:1005568132027. [DOI] [PubMed] [Google Scholar]
- 82.Villalba J.M., Navas P. Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid. Redox Signal. 2000;2:213–230. doi: 10.1089/ars.2000.2.2-213. [DOI] [PubMed] [Google Scholar]
- 83.Francesco A.D., Choi Y., Bernier M., Zhang Y., Diaz-Ruiz A., Aon M.A., Kalafut K., Ehrlich M.R., Murt K., Ali A., et al. NQO1 protects obese mice through improvements in glucose and lipid metabolism. NPJ Aging Mech. Dis. 2020;6:13. doi: 10.1038/s41514-020-00051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diaz-Ruiz A., Lanasa M., Garcia J., Mora H., Fan F., Martin-Montalvo A., Di Francesco A., Calvo-Rubio M., Salvador-Pascual A., Aon M.A., et al. Overexpression of CYB5R3 and NQO1, two NAD(+)-producing enzymes, mimics aspects of caloric restriction. Aging Cell. 2018;17:e12767. doi: 10.1111/acel.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin-Montalvo A., Sun Y., Diaz-Ruiz A., Ali A., Gutierrez V., Palacios H.H., Curtis J., Siendones E., Ariza J., Abulwerdi G.A., et al. Cytochrome b5 reductase and the control of lipid metabolism and healthspan. NPJ Aging Mech. Dis. 2016;2:16006. doi: 10.1038/npjamd.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ross D., Siegel D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017;8:595. doi: 10.3389/fphys.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Grocin A.G., da Silva T.N.X., Panzilius E., Scheel C.H., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 89.Niki E., Noguchi N., Gotoh N. Inhibition of oxidative modification of low density lipoprotein by antioxidants. J. Nutr. Sci. Vitaminol. 1993;39:S1–S8. doi: 10.3177/jnsv.39.Supplement_S1. [DOI] [PubMed] [Google Scholar]
- 90.Thomas S.R., Neuzil J., Stocker R. Inhibition of LDL oxidation by ubiquinol-10. A protective mechanism for coenzyme Q in atherogenesis? Mol. Asp. Med. 1997;18:S85–S103. doi: 10.1016/S0098-2997(97)00031-9. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi T., Mine Y., Okamoto T. Extracellular coenzyme Q10 (CoQ10) is reduced to ubiquinol-10 by intact Hep G2 cells independent of intracellular CoQ10 reduction. Arch. Biochem. Biophys. 2019;672:108067. doi: 10.1016/j.abb.2019.108067. [DOI] [PubMed] [Google Scholar]
- 92.Ogasahara S., Engel A.G., Frens D., Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc. Natl. Acad. Sci. USA. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gueguen N., Baris O., Lenaers G., Reynier P., Spinazzi M. Secondary coenzyme Q deficiency in neurological disorders. Free Radic. Biol. Med. 2021;165:203–218. doi: 10.1016/j.freeradbiomed.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 94.Schijvens A.M., van de Kar N.C., Bootsma-Robroeks C.M., Cornelissen E.A., van den Heuvel L.P., Schreuder M.F. Mitochondrial Disease and the Kidney With a Special Focus on CoQ10 Deficiency. Kidney Int. Rep. 2020;5:2146–2159. doi: 10.1016/j.ekir.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sardet A., Grimfeld A. Oxygen therapy at home in children. Agressologie. 1988;29:529–531. [PubMed] [Google Scholar]
- 96.Christiansen L.B., Reimann M.J., Schou-Pedersen A.M.V., Larsen S., Lykkesfeldt J., Olsen L.H. Depleted Myocardial Coenzyme Q10 in Cavalier King Charles Spaniels with Congestive Heart Failure Due to Myxomatous Mitral Valve Disease. Antioxidants. 2021;10:161. doi: 10.3390/antiox10020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acosta M.J., Vazquez Fonseca L., Desbats M.A., Cerqua C., Zordan R., Trevisson E., Salviati L. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta Bioenerg. 2016;1857:1079–1085. doi: 10.1016/j.bbabio.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 98.Manzar H., Abdulhussein D., Yap T.E., Cordeiro M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020;21:9299. doi: 10.3390/ijms21239299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desbats M.A., Lunardi G., Doimo M., Trevisson E., Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ10) deficiency. J. Inherit. Metab. Dis. 2015;38:145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 100.Emmanuele V., Lopez L.C., Berardo A., Naini A., Tadesse S., Wen B., D’Agostino E., Solomon M., DiMauro S., Quinzii C., et al. Heterogeneity of coenzyme Q10 deficiency: Patient study and literature review. Arch. Neurol. 2012;69:978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boitier E., Degoul F., Desguerre I., Charpentier C., Francois D., Ponsot G., Diry M., Rustin P., Marsac C. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J. Neurol. Sci. 1998;156:41–46. doi: 10.1016/S0022-510X(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 102.Rahman S., Hargreaves I., Clayton P., Heales S. Neonatal presentation of coenzyme Q10 deficiency. J. Pediatr. 2001;139:456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- 103.Desbats M.A., Vetro A., Limongelli I., Lunardi G., Casarin A., Doimo M., Spinazzi M., Angelini C., Cenacchi G., Burlina A., et al. Primary coenzyme Q10 deficiency presenting as fatal neonatal multiorgan failure. Eur. J. Hum. Genet. 2015;23:1254–1258. doi: 10.1038/ejhg.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Musumeci O., Naini A., Slonim A.E., Skavin N., Hadjigeorgiou G.L., Krawiecki N., Weissman B.M., Tsao C.Y., Mendell J.R., Shanske S., et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/WNL.56.7.849. [DOI] [PubMed] [Google Scholar]
- 105.Barca E., Kleiner G., Tang G., Ziosi M., Tadesse S., Masliah E., Louis E.D., Faust P., Kang U.J., Torres J., et al. Decreased Coenzyme Q10 Levels in Multiple System Atrophy Cerebellum. J. Neuropathol. Exp. Neurol. 2016;75:663–672. doi: 10.1093/jnen/nlw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lalani S.R., Vladutiu G.D., Plunkett K., Lotze T.E., Adesina A.M., Scaglia F. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch. Neurol. 2005;62:317–320. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- 107.Horvath R., Schneiderat P., Schoser B.G., Gempel K., Neuen-Jacob E., Ploger H., Muller-Hocker J., Pongratz D.E., Naini A., DiMauro S., et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- 108.Salviati L., Trevisson E., Doimo M., Navas P. Primary Coenzyme Q(10) Deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews(®) University of Washington; Seattle, WA, USA: 2017. [PubMed] [Google Scholar]
- 109.Yubero D., Montero R., Santos-Ocana C., Salviati L., Navas P., Artuch R. Molecular diagnosis of coenzyme Q10 deficiency: An update. Expert Rev. Mol. Diagn. 2018;18:491–498. doi: 10.1080/14737159.2018.1478290. [DOI] [PubMed] [Google Scholar]
- 110.Liu J.L., Yee C., Wang Y., Hekimi S. A single biochemical activity underlies the pleiotropy of the aging-related protein CLK-1. Sci. Rep. 2017;7:859. doi: 10.1038/s41598-017-00754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malicdan M.C.V., Vilboux T., Ben-Zeev B., Guo J., Eliyahu A., Pode-Shakked B., Dori A., Kakani S., Chandrasekharappa S.C., Ferreira C.R., et al. A novel inborn error of the coenzyme Q10 biosynthesis pathway: Cerebellar ataxia and static encephalomyopathy due to COQ5 C-methyltransferase deficiency. Hum. Mutat. 2018;39:69–79. doi: 10.1002/humu.23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwong A.K., Chiu A.T., Tsang M.H., Lun K.S., Rodenburg R.J.T., Smeitink J., Chung B.H., Fung C.W. A fatal case of COQ7-associated primary coenzyme Q10 deficiency. JIMD Rep. 2019;47:23–29. doi: 10.1002/jmd2.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bellman M., Byrne O., Sege R. Developmental assessment of children. BMJ. 2013;346:e8687. doi: 10.1136/bmj.e8687. [DOI] [PubMed] [Google Scholar]
- 114.Rotig A., Appelkvist E.L., Geromel V., Chretien D., Kadhom N., Edery P., Lebideau M., Dallner G., Munnich A., Ernster L., et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 115.Bezdicka M., Dluholucky M., Cinek O., Zieg J. Successful maintenance of partial remission in a child with COQ2 nephropathy by coenzyme Q10 treatment. Nephrology. 2020;25:187–188. doi: 10.1111/nep.13600. [DOI] [PubMed] [Google Scholar]
- 116.Doimo M., Trevisson E., Airik R., Bergdoll M., Santos-Ocana C., Hildebrandt F., Navas P., Pierrel F., Salviati L. Effect of vanillic acid on COQ6 mutants identified in patients with coenzyme Q10 deficiency. Biochim. Biophys. Acta Mol. Basis Dis. 2014;1842:1–6. doi: 10.1016/j.bbadis.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song X., Fang X., Tang X., Cao Q., Zhai Y., Chen J., Liu J., Zhang Z., Xiang T., Qian Y., et al. COQ8B nephropathy: Early detection and optimal treatment. Mol. Genet. Genom. Med. 2020;8:e1360. doi: 10.1002/mgg3.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu M.H., Tsang M.H., Lai S., Ho M.S., Tse D.M.L., Willis B., Kwong A.K., Chou Y.Y., Lin S.P., Quinzii C.M., et al. Primary coenzyme Q10 deficiency-7: Expanded phenotypic spectrum and a founder mutation in southern Chinese. NPJ Genom. Med. 2019;4:18. doi: 10.1038/s41525-019-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H., Wang F., Liu X., Zhong X., Yao Y., Xiao H. Steroid-resistant nephrotic syndrome caused by co-inheritance of mutations at NPHS1 and ADCK4 genes in two Chinese siblings. Intractable Rare Dis. Res. 2017;6:299–303. doi: 10.5582/irdr.2017.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Horvath R., Czermin B., Gulati S., Demuth S., Houge G., Pyle A., Dineiger C., Blakely E.L., Hassani A., Foley C., et al. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J. Neurol. Neurosurg. Psychiatry. 2012;83:174–178. doi: 10.1136/jnnp-2011-301258. [DOI] [PubMed] [Google Scholar]
- 121.Jakobs B.S., van den Heuvel L.P., Smeets R.J., de Vries M.C., Hien S., Schaible T., Smeitink J.A., Wevers R.A., Wortmann S.B., Rodenburg R.J. A novel mutation in COQ2 leading to fatal infantile multisystem disease. J. Neurol. Sci. 2013;326:24–28. doi: 10.1016/j.jns.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 122.Yubero D., Montero R., Martin M.A., Montoya J., Ribes A., Grazina M., Trevisson E., Rodriguez-Aguilera J.C., Hargreaves I.P., Salviati L., et al. Secondary coenzyme Q10 deficiencies in oxidative phosphorylation (OXPHOS) and non-OXPHOS disorders. Mitochondrion. 2016;30:51–58. doi: 10.1016/j.mito.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 123.Fernandez-Vizarra E., Bugiani M., Goffrini P., Carrara F., Farina L., Procopio E., Donati A., Uziel G., Ferrero I., Zeviani M. Impaired complex III assembly associated with BCS1L gene mutations in isolated mitochondrial encephalopathy. Hum. Mol. Genet. 2007;16:1241–1252. doi: 10.1093/hmg/ddm072. [DOI] [PubMed] [Google Scholar]
- 124.Bris C., Rouaud T., Desquiret-Dumas V., Gueguen N., Goudenege D., Barth M., Bonneau D., Amati-Bonneau P., Lenaers G., Reynier P., et al. Novel NDUFS4 gene mutation in an atypical late-onset mitochondrial form of multifocal dystonia. Neurol. Genet. 2017;3:e205. doi: 10.1212/NXG.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ortigoza-Escobar J.D., Oyarzabal A., Montero R., Artuch R., Jou C., Jimenez C., Gort L., Briones P., Muchart J., Lopez-Gallardo E., et al. Ndufs4 related Leigh syndrome: A case report and review of the literature. Mitochondrion. 2016;28:73–78. doi: 10.1016/j.mito.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 126.Kuhl I., Miranda M., Atanassov I., Kuznetsova I., Hinze Y., Mourier A., Filipovska A., Larsson N.G. Transcriptomic and proteomic landscape of mitochondrial dysfunction reveals secondary coenzyme Q deficiency in mammals. eLife. 2017;6:e30952. doi: 10.7554/eLife.30952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garone C., Gurgel-Giannetti J., Sanna-Cherchi S., Krishna S., Naini A., Quinzii C.M., Hirano M. A Novel SUCLA2 Mutation Presenting as a Complex Childhood Movement Disorder. J. Child Neurol. 2017;32:246–250. doi: 10.1177/0883073816666221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Montero R., Grazina M., Lopez-Gallardo E., Montoya J., Briones P., Navarro-Sastre A., Land J.M., Hargreaves I.P., Artuch R., Coenzyme Q10 Deficiency Study Group et al. Coenzyme Q10 deficiency in mitochondrial DNA depletion syndromes. Mitochondrion. 2013;13:337–341. doi: 10.1016/j.mito.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 129.Taskin B.D., Karalok Z.S., Gurkas E., Aydin K., Aydogmus U., Ceylaner S., Karaer K., Yilmaz C., Pearl P.L. Early-Onset Mild Type Leukoencephalopathy Caused by a Homozygous EARS2 Mutation. J. Child Neurol. 2016;31:938–941. doi: 10.1177/0883073816630087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cotan D., Cordero M.D., Garrido-Maraver J., Oropesa-Avila M., Rodriguez-Hernandez A., Gomez Izquierdo L., De la Mata M., De Miguel M., Lorite J.B., Infante E.R., et al. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. 2011;25:2669–2687. doi: 10.1096/fj.10-165340. [DOI] [PubMed] [Google Scholar]
- 131.Sacconi S., Trevisson E., Salviati L., Ayme S., Rigal O., Redondo A.G., Mancuso M., Siciliano G., Tonin P., Angelini C., et al. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscul. Disord. 2010;20:44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 132.Gempel K., Topaloglu H., Talim B., Schneiderat P., Schoser B.G., Hans V.H., Palmafy B., Kale G., Tokatli A., Quinzii C., et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aeby A., Sznajer Y., Cave H., Rebuffat E., Van Coster R., Rigal O., Van Bogaert P. Cardiofaciocutaneous (CFC) syndrome associated with muscular coenzyme Q10 deficiency. J. Inherit. Metab. Dis. 2007;30:827. doi: 10.1007/s10545-007-0612-0. [DOI] [PubMed] [Google Scholar]
- 134.Fragaki K., Chaussenot A., Benoist J.F., Ait-El-Mkadem S., Bannwarth S., Rouzier C., Cochaud C., Paquis-Flucklinger V. Coenzyme Q10 defects may be associated with a deficiency of Q10-independent mitochondrial respiratory chain complexes. Biol. Res. 2016;49:4. doi: 10.1186/s40659-015-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ngoh A., Bras J., Guerreiro R., McTague A., Ng J., Meyer E., Chong W.K., Boyd S., MacLellan L., Kirkpatrick M., et al. TBC1D24 Mutations in a Sibship with Multifocal Polymyoclonus. Tremor Other Hyperkinet. Mov. 2017;7:452. doi: 10.5334/tohm.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Area-Gomez E., Schon E.A. Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev. 2016;38:90–96. doi: 10.1016/j.gde.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H.W., Mueller S., Miller T., Miller C.C.J. There’s Something Wrong with my MAM; the ER—Mitochondria Axis and Neurodegenerative Diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cordes T., Metallo C.M. Statins Limit Coenzyme Q Synthesis and Metabolically Synergize with MEK Inhibition in Pancreatic Tumors. Cancer Res. 2020;80:151–152. doi: 10.1158/0008-5472.CAN-19-3415. [DOI] [PubMed] [Google Scholar]
- 139.Desai R., Campanella M. Exploring mitochondrial cholesterol signalling for therapeutic intervention in neurological conditions. Br. J. Pharmacol. 2019;176:4284–4292. doi: 10.1111/bph.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solsona-Vilarrasa E., Fucho R., Torres S., Nunez S., Nuno-Lambarri N., Enrich C., Garcia-Ruiz C., Fernandez-Checa J.C. Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes. Redox Biol. 2019;24:101214. doi: 10.1016/j.redox.2019.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Torres S., Garcia-Ruiz C.M., Fernandez-Checa J.C. Mitochondrial Cholesterol in Alzheimer’s Disease and Niemann—Pick Type C Disease. Front. Neurol. 2019;10:1168. doi: 10.3389/fneur.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balboa E., Castro J., Pinochet M.J., Cancino G.I., Matias N., Saez P.J., Martinez A., Alvarez A.R., Garcia-Ruiz C., Fernandez-Checa J.C., et al. MLN64 induces mitochondrial dysfunction associated with increased mitochondrial cholesterol content. Redox Biol. 2017;12:274–284. doi: 10.1016/j.redox.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Danielli M., Marrone J., Capiglioni A.M., Marinelli R.A. Mitochondrial aquaporin-8 is involved in SREBP-controlled hepatocyte cholesterol biosynthesis. Free Radic. Biol. Med. 2019;131:370–375. doi: 10.1016/j.freeradbiomed.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 144.Suarez-Rivero J.M., de la Mata M., Pavon A.D., Villanueva-Paz M., Povea-Cabello S., Cotan D., Alvarez-Cordoba M., Villalon-Garcia I., Ybot-Gonzalez P., Salas J.J., et al. Intracellular cholesterol accumulation and coenzyme Q10 deficiency in Familial Hypercholesterolemia. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3697–3713. doi: 10.1016/j.bbadis.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 145.Baulies A., Montero J., Matias N., Insausti N., Terrones O., Basanez G., Vallejo C., de La Rosa L.C., Martinez L., Robles D., et al. The 2-oxoglutarate carrier promotes liver cancer by sustaining mitochondrial GSH despite cholesterol loading. Redox Biol. 2018;14:164–177. doi: 10.1016/j.redox.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Balreira A., Boczonadi V., Barca E., Pyle A., Bansagi B., Appleton M., Graham C., Hargreaves I.P., Rasic V.M., Lochmuller H., et al. ANO10 mutations cause ataxia and coenzyme Q10 deficiency. J. Neurol. 2014;261:2192–2198. doi: 10.1007/s00415-014-7476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mourier A., Motori E., Brandt T., Lagouge M., Atanassov I., Galinier A., Rappl G., Brodesser S., Hultenby K., Dieterich C., et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 2015;208:429–442. doi: 10.1083/jcb.201411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fernandez-Ayala D.J., Lopez-Lluch G., Garcia-Valdes M., Arroyo A., Navas P. Specificity of coenzyme Q10 for a balanced function of respiratory chain and endogenous ubiquinone biosynthesis in human cells. Biochim. Biophys. Acta Bioenerg. 2005;1706:174–183. doi: 10.1016/j.bbabio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 149.Kemmerer Z.A., Robinson K.P., Schmitz J.M., Manicki M., Paulson B.R., Jochem A., Hutchins P.D., Coon J.J., Pagliarini D.J. UbiB proteins regulate cellular CoQ distribution in Saccharomyces cerevisiae. Nat. Commun. 2021;12:4769. doi: 10.1038/s41467-021-25084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vazquez-Fonseca L., Schaefer J., Navas-Enamorado I., Santos-Ocana C., Hernandez-Camacho J.D., Guerra I., Cascajo M.V., Sanchez-Cuesta A., Horvath Z., Siendones E., et al. ADCK2 Haploinsufficiency Reduces Mitochondrial Lipid Oxidation and Causes Myopathy Associated with CoQ Deficiency. J. Clin. Med. 2019;8:1374. doi: 10.3390/jcm8091374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fernandez-Del-Rio L., Kelly M.E., Contreras J., Bradley M.C., James A.M., Murphy M.P., Payne G.S., Clarke C.F. Genes and lipids that impact uptake and assimilation of exogenous coenzyme Q in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2020;154:105–118. doi: 10.1016/j.freeradbiomed.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y., Hekimi S. The Complexity of Making Ubiquinone. Trends Endocrinol. Metab. 2019;30:929–943. doi: 10.1016/j.tem.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 153.Mao C., Liu X., Zhang Y., Lei G., Yan Y., Lee H., Koppula P., Wu S., Zhuang L., Fang B., et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garcia-Bermudez J., Birsoy K. A mitochondrial gatekeeper that helps cells escape death by ferroptosis. Nature. 2021;593:514–515. doi: 10.1038/d41586-021-01203-8. [DOI] [PubMed] [Google Scholar]
- 155.Escobar-Henriques M., Joaquim M. Mitofusins: Disease Gatekeepers and Hubs in Mitochondrial Quality Control by E3 Ligases. Front. Physiol. 2019;10:517. doi: 10.3389/fphys.2019.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lopez-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing Dev. 2017;162:108–121. doi: 10.1016/j.mad.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 157.Lopez-Lluch G., Hernandez-Camacho J.D., Fernandez-Ayala D.J.M., Navas P. Mitochondrial dysfunction in metabolism and ageing: Shared mechanisms and outcomes? Biogerontology. 2018;19:461–480. doi: 10.1007/s10522-018-9768-2. [DOI] [PubMed] [Google Scholar]
- 158.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lopez-Otin C., Galluzzi L., Freije J.M.P., Madeo F., Kroemer G. Metabolic Control of Longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 160.Lopez-Lluch G., Santos-Ocana C., Sanchez-Alcazar J.A., Fernandez-Ayala D.J., Asencio-Salcedo C., Rodriguez-Aguilera J.C., Navas P. Mitochondrial responsibility in ageing process: Innocent, suspect or guilty. Biogerontology. 2015;16:599–620. doi: 10.1007/s10522-015-9585-9. [DOI] [PubMed] [Google Scholar]
- 161.Gonzalez-Freire M., de Cabo R., Bernier M., Sollott S.J., Fabbri E., Navas P., Ferrucci L. Reconsidering the Role of Mitochondria in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lopez-Lluch G. The Important Role of CoQ10 in Aging. Antioxidants. 2019;8:570. doi: 10.3390/antiox8120570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Lluch G., Irusta P.M., Navas P., de Cabo R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Floyd B.J., Wilkerson E.M., Veling M.T., Minogue C.E., Xia C., Beebe E.T., Wrobel R.L., Cho H., Kremer L.S., Alston C.L., et al. Mitochondrial Protein Interaction Mapping Identifies Regulators of Respiratory Chain Function. Mol. Cell. 2016;63:621–632. doi: 10.1016/j.molcel.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liparulo I., Bergamini C., Bortolus M., Calonghi N., Gasparre G., Kurelac I., Masin L., Rizzardi N., Rugolo M., Wang W., et al. Coenzyme Q biosynthesis inhibition induces HIF-1α stabilization and metabolic switch toward glycolysis. FEBS J. 2021;288:1956–1974. doi: 10.1111/febs.15561. [DOI] [PubMed] [Google Scholar]
- 166.Rojo M., Legros F., Chateau D., Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 167.de Brito O.M., Scorrano L. Mitofusin 2: A mitochondria-shaping protein with signaling roles beyond fusion. Antioxid. Redox Signal. 2008;10:621–633. doi: 10.1089/ars.2007.1934. [DOI] [PubMed] [Google Scholar]
- 168.Picca A., Calvani R., Coelho-Junior H.J., Landi F., Bernabei R., Marzetti E. Inter-Organelle Membrane Contact Sites and Mitochondrial Quality Control during Aging: A Geroscience View. Cells. 2020;9:598. doi: 10.3390/cells9030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Filadi R., Theurey P., Pizzo P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium. 2017;62:1–15. doi: 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Rodriguez-Hernandez A., Cordero M.D., Salviati L., Artuch R., Pineda M., Briones P., Izquierdo L.G., Cotan D., Navas P., Sanchez-Alcazar J.A. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 171.Marcheggiani F., Cirilli I., Orlando P., Silvestri S., Vogelsang A., Knott A., Blatt T., Weise J.M., Tiano L. Modulation of Coenzyme Q10 content and oxidative status in human dermal fibroblasts using HMG-CoA reductase inhibitor over a broad range of concentrations. From mitohormesis to mitochondrial dysfunction and accelerated aging. Aging. 2019;11:2565–2582. doi: 10.18632/aging.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pagano G., Pallardó F.V., Lyakhovich A., Tiano L., Fittipaldi M.R., Toscanesi M., Trifuoggi M. Aging-Related Disorders and Mitochondrial Dysfunction: A Critical Review for Prospect Mitoprotective Strategies Based on Mitochondrial Nutrient Mixtures. Int. J. Mol. Sci. 2020;21:7060. doi: 10.3390/ijms21197060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Testai L., Martelli A., Flori L., Cicero A.F.G., Colletti A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients. 2021;13:1697. doi: 10.3390/nu13051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vaughan R.A., Garcia-Smith R., Bisoffi M., Conn C.A., Trujillo K.A. Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: Implications for statin-induced rhabdomyolysis. Eur. J. Pharmacol. 2013;711:1–9. doi: 10.1016/j.ejphar.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 175.Gvozdjakova A., Kucharska J., Sumbalova Z., Rausova Z., Chladekova A., Komlosi M., Szamosova M., Mojto V. The importance of coenzyme Q10 and its ratio to cholesterol in the progress of chronic kidney diseases linked to non-communicable diseases. Bratisl. Lek. Listy. 2020;121:693–699. doi: 10.4149/BLL_2020_113. [DOI] [PubMed] [Google Scholar]
- 176.Hargreaves I.P. Coenzyme Q10 as a therapy for mitochondrial disease. Int. J. Biochem. Cell Biol. 2014;49:105–111. doi: 10.1016/j.biocel.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 177.Langsjoen P.H., Langsjoen A.M. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin. Pharmacol. Drug Dev. 2014;3:13–17. doi: 10.1002/cpdd.73. [DOI] [PubMed] [Google Scholar]
- 178.Lopez-Lluch G., Del Pozo-Cruz J., Sanchez-Cuesta A., Cortes-Rodriguez A.B., Navas P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133–140. doi: 10.1016/j.nut.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 179.Bhagavan H.N., Chopra R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7:S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 180.Nashimoto S., Takekawa Y., Takekuma Y., Sugawara M., Sato Y. Transport via Niemann-Pick C1 Like 1 contributes to the intestinal absorption of ubiquinone. Drug Metab. Pharmacokinet. 2020;35:527–533. doi: 10.1016/j.dmpk.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 181.Hidaka T., Fujii K., Funahashi I., Fukutomi N., Hosoe K. Safety assessment of coenzyme Q10 (CoQ10) Biofactors. 2008;32:199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- 182.Alehagen U., Aaseth J. Selenium and coenzyme Q10 interrelationship in cardiovascular diseases—A clinician’s point of view. J. Trace Elem. Med. Biol. 2015;31:157–162. doi: 10.1016/j.jtemb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 183.Alehagen U., Aaseth J., Johansson P. Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. PLoS ONE. 2015;10:e0141641. doi: 10.1371/journal.pone.0141641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Quiles J.L., Ochoa J.J., Battino M., Gutierrez-Rios P., Nepomuceno E.A., Frias M.L., Huertas J.R., Mataix J. Life-long supplementation with a low dosage of coenzyme Q10 in the rat: Effects on antioxidant status and DNA damage. Biofactors. 2005;25:73–86. doi: 10.1002/biof.5520250109. [DOI] [PubMed] [Google Scholar]
- 185.Lopez-Moreno J., Quintana-Navarro G.M., Delgado-Lista J., Garcia-Rios A., Alcala-Diaz J.F., Gomez-Delgado F., Camargo A., Perez-Martinez P., Tinahones F.J., Striker G.E., et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:340–346. doi: 10.1093/gerona/glw214. [DOI] [PubMed] [Google Scholar]
- 186.Zhang P., Yang C., Guo H., Wang J., Lin S., Li H., Yang Y., Ling W. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J. Clin. Lipidol. 2018;12:417–427.e415. doi: 10.1016/j.jacl.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 187.Gholnari T., Aghadavod E., Soleimani A., Hamidi G.A., Sharifi N., Asemi Z. The Effects of Coenzyme Q10 Supplementation on Glucose Metabolism, Lipid Profiles, Inflammation, and Oxidative Stress in Patients With Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2018;37:188–193. doi: 10.1080/07315724.2017.1386140. [DOI] [PubMed] [Google Scholar]
- 188.Sharifi N., Tabrizi R., Moosazadeh M., Mirhosseini N., Lankarani K.B., Akbari M., Chamani M., Kolahdooz F., Asemi Z. The Effects of Coenzyme Q10 Supplementation on Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018;24:2729–2742. doi: 10.2174/1381612824666180406104516. [DOI] [PubMed] [Google Scholar]
- 189.Fan L., Feng Y., Chen G.C., Qin L.Q., Fu C.L., Chen L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017;119:128–136. doi: 10.1016/j.phrs.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 190.Mazidi M., Kengne A.P., Banach M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018;128:130–136. doi: 10.1016/j.phrs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 191.Farsi F., Heshmati J., Keshtkar A., Irandoost P., Alamdari N.M., Akbari A., Janani L., Morshedzadeh N., Vafa M. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;148:104290. doi: 10.1016/j.phrs.2019.104290. [DOI] [PubMed] [Google Scholar]
- 192.Zhai J., Bo Y., Lu Y., Liu C., Zhang L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dludla P.V., Orlando P., Silvestri S., Marcheggiani F., Cirilli I., Nyambuya T.M., Mxinwa V., Mokgalaboni K., Nkambule B.B., Johnson R., et al. Coenzyme Q10 Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2020;21:3247. doi: 10.3390/ijms21093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hargreaves I.P., Mantle D. Targeted Treatment of Age-Related Fibromyalgia with Supplemental Coenzyme Q10. Adv. Exp. Med. Biol. 2021;1286:77–85. doi: 10.1007/978-3-030-55035-6_5. [DOI] [PubMed] [Google Scholar]
- 195.Chan M.Y., Lee B.J., Chang P.S., Hsiao H.Y., Hsu L.P., Chang C.H., Lin P.T. The risks of ubiquinone and β-carotene deficiency and metabolic disorders in patients with oral cancer. BMC Cancer. 2020;20:310. doi: 10.1186/s12885-020-06839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Farsi F., Ebrahimi-Daryani N., Golab F., Akbari A., Janani L., Karimi M.Y., Irandoost P., Alamdari N.M., Agah S., Vafa M. A randomized controlled trial on the coloprotective effect of coenzyme Q10 on immune-inflammatory cytokines, oxidative status, antimicrobial peptides, and microRNA-146a expression in patients with mild-to-moderate ulcerative colitis. Eur. J. Nutr. 2021;60:3397–3410. doi: 10.1007/s00394-021-02514-2. [DOI] [PubMed] [Google Scholar]
- 197.López-Pedrera C., Villalba J.M., Patiño-Trives A.M., Luque-Tévar M., Barbarroja N., Aguirre M., Escudero-Contreras A., Pérez-Sánchez C. Therapeutic Potential and Immunomodulatory Role of Coenzyme Q10 and Its Analogues in Systemic Autoimmune Diseases. Antioxidants. 2021;10:600. doi: 10.3390/antiox10040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pérez-Sánchez C., Aguirre M.Á., Ruiz-Limón P., Ábalos-Aguilera M.C., Jiménez-Gómez Y., Arias-de la Rosa I., Rodriguez-Ariza A., Fernández-Del Río L., González-Reyes J.A., Segui P., et al. Ubiquinol Effects on Antiphospholipid Syndrome Prothrombotic Profile: A Randomized, Placebo-Controlled Trial. Arter. Thromb. Vasc. Biol. 2017;37:1923–1932. doi: 10.1161/ATVBAHA.117.309225. [DOI] [PubMed] [Google Scholar]
- 199.García-Carpintero S., Domínguez-Bértalo J., Pedrero-Prieto C., Frontiñán-Rubio J., Amo-Salas M., Durán-Prado M., García-Pérez E., Vaamonde J., Alcain F.J. Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment. Antioxidants. 2021;10:143. doi: 10.3390/antiox10020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh R.B., Niaz M.A., Rastogi V., Rastogi S.S. Coenzyme Q in cardiovascular disease. J. Assoc. Physicians India. 1998;46:299–306. [PubMed] [Google Scholar]
- 201.Jorat M.V., Tabrizi R., Mirhosseini N., Lankarani K.B., Akbari M., Heydari S.T., Mottaghi R., Asemi Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018;17:230. doi: 10.1186/s12944-018-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alehagen U., Johansson P., Bjornstedt M., Rosen A., Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013;167:1860–1866. doi: 10.1016/j.ijcard.2012.04.156. [DOI] [PubMed] [Google Scholar]
- 203.Alehagen U., Aaseth J., Alexander J., Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE. 2018;13:e0193120. doi: 10.1371/journal.pone.0193120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alehagen U., Aaseth J., Alexander J., Svensson E., Johansson P., Larsson A. Less fibrosis in elderly subjects supplemented with selenium and coenzyme Q10-A mechanism behind reduced cardiovascular mortality? Biofactors. 2018;44:137–147. doi: 10.1002/biof.1404. [DOI] [PubMed] [Google Scholar]
- 205.Dai Y.L., Luk T.H., Yiu K.H., Wang M., Yip P.M., Lee S.W., Li S.W., Tam S., Fong B., Lau C.P., et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 206.Chew G.T., Watts G.F. Coenzyme Q10 and diabetic endotheliopathy: Oxidative stress and the recoupling hypothesis. QJM Int. J. Med. 2004;97:537–548. doi: 10.1093/qjmed/hch089. [DOI] [PubMed] [Google Scholar]
- 207.Kawashima C., Matsuzawa Y., Konishi M., Akiyama E., Suzuki H., Sato R., Nakahashi H., Kikuchi S., Kimura Y., Maejima N., et al. Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study. Am. J. Cardiovasc. Drugs. 2020;20:363–372. doi: 10.1007/s40256-019-00384-y. [DOI] [PubMed] [Google Scholar]
- 208.Sabbatinelli J., Orlando P., Galeazzi R., Silvestri S., Cirilli I., Marcheggiani F., Dludla P.V., Giuliani A., Bonfigli A.R., Mazzanti L., et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients. 2020;12:1098. doi: 10.3390/nu12041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Diaz-Castro J., Moreno-Fernandez J., Chirosa I., Chirosa L.J., Guisado R., Ochoa J.J. Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise. Nutrients. 2020;12:424. doi: 10.3390/nu12020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sanchez-Cuesta A., Cortes-Rodriguez A.B., Navas-Enamorado I., Lekue J.A., Viar T., Axpe M., Navas P., Lopez-Lluch G. High coenzyme Q10 plasma levels improve stress and damage markers in professional soccer players during competition. Int. J. Vitam. Nutr. Res. 2020:1–12. doi: 10.1024/0300-9831/a000659. [DOI] [PubMed] [Google Scholar]
- 211.Emami A. The Impact of Pre-Cooling and CoQ(10) Supplementation on Mediators of Inflammatory Cytokines in Elite Swimmers. Nutr. Cancer. 2020;72:41–51. doi: 10.1080/01635581.2019.1614200. [DOI] [PubMed] [Google Scholar]
- 212.Suzuki Y., Nagato S., Sakuraba K., Morio K., Sawaki K. Short-term ubiquinol-10 supplementation alleviates tissue damage in muscle and fatigue caused by strenuous exercise in male distance runners. Int. J. Vitam. Nutr. Res. 2021;91:261–270. doi: 10.1024/0300-9831/a000627. [DOI] [PubMed] [Google Scholar]
- 213.Ho C.C., Tseng C.Y., Chen H.W., Chiu Y.W., Tsai M.C., Chang P.S., Lin P.T. Coenzyme Q10 status, glucose parameters, and antioxidative capacity in college athletes. J. Int. Soc. Sports Nutr. 2020;17:5. doi: 10.1186/s12970-020-0334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Diaz-Castro J., Mira-Rufino P.J., Moreno-Fernandez J., Chirosa I., Chirosa J.L., Guisado R., Ochoa J.J. Ubiquinol supplementation modulates energy metabolism and bone turnover during high intensity exercise. Food Funct. 2020;11:7523–7531. doi: 10.1039/D0FO01147A. [DOI] [PubMed] [Google Scholar]