Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive disease that is characterized by a state of persistent inflammation and oxidative stress. The presence of oxidative stress in COPD is the result of an imbalance between pro-oxidant and antioxidant mechanisms. The aim of this review was to investigate a possible association between glutathione peroxidase (GPx), a key component of antioxidant defense mechanisms, and COPD. A systematic search for relevant studies was conducted in the electronic databases PubMed, Web of Science, Scopus, and Google Scholar, from inception to June 2021. Standardized mean differences (SMDs) were used to express the differences in GPx concentrations between COPD patients and non-COPD subjects. Twenty-four studies were identified. In 15 studies assessing whole blood/erythrocytes (GPx isoform 1), the pooled results showed that GPx concentrations were significantly lower in patients with COPD (SMD = −1.91, 95% CI −2.55 to −1.28, p < 0.001; moderate certainty of evidence). By contrast, in 10 studies assessing serum/plasma (GPx isoform 3), the pooled results showed that GPx concentrations were not significantly different between the two groups (very low certainty of evidence). The concentration of GPx-1, but not GPx-3, is significantly lower in COPD patients, suggesting an impairment of antioxidant defense mechanisms in this group.
Keywords: chronic obstructive pulmonary disease, oxidative stress, antioxidant defense systems, glutathione peroxidase
1. Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease characterized by persistent airflow limitation due to airway obstruction and/or lung tissue damage [1]. With a global prevalence of 13.1%, COPD is the third leading cause of death worldwide [2,3]. Oxidative stress and inflammation are considered key drivers of the pathophysiology of COPD [4,5,6]. The lungs are particularly exposed to environmental insults, such as tobacco smoke and air pollutants, that represent important sources of reactive oxygen species (ROS). The latter directly promote lung damage, resulting from alterations of DNA, lipids, carbohydrates, and proteins, as well as activate local inflammatory responses which contribute to the development and progression of COPD [6]. ROS can also activate epithelial cells and macrophages as well as facilitate the recruitment of neutrophils, monocytes, and lymphocytes. Recruited inflammatory cells become activated and then generate further ROS, enhancing the pro-oxidant burden [7,8]. These events lead to a state of sustained inflammation and chronic oxidative stress. Moreover, it has been reported that traffic-related air pollution increases airway inflammation which induces the expression of inflammatory factors through the activation of the NF-κB signaling pathway [9]. Environmental exposure can also induce oxidative stress through a disruption in the expression of micro-RNA, short sequences of non-coding RNA molecules which are involved in the regulation of gene expression [10]. Increased oxidative stress in COPD patients, which has been convincingly demonstrated with various biomarkers, reflects both an increase in oxidant molecules and a decrease in antioxidant defense mechanisms [11,12]. Specifically, the antioxidant defense mechanisms are overwhelmed in the presence of excess ROS. These antioxidant defenses mainly consist of non-enzymatic molecules, such as vitamins, glutathione, and protein thiols, most notably albumin, and enzymatic molecules, such as superoxide dismutase, catalase and glutathione peroxidase. Among these enzymes, glutathione peroxidase (GPx) has received particular attention in COPD. GPx catalyzes the reduction of lipid hydroperoxides into their corresponding alcohols and the reduction of hydrogen peroxide into water, using glutathione as reducing substrate [13,14]. The GPx family includes eight isoforms with different expression and antioxidant properties in individual tissues [13]. Only the first four, all of which are selenoproteins, have been well characterized: GPx-1, the predominant isoform, is ubiquitously expressed in the cytosol and mitochondria; GPx-2 is localized in the gastrointestinal epithelium; GPx-3 is the only member of the GPx family that is present in the extracellular compartment; GPx-4 (phospholipids hydroperoxide GPx) has a different subcellular localization and protects the membrane against lipid peroxidation [13]. The assessment of GPx activity in COPD patients may be useful to detect an impaired antioxidant defense system in this group. Several studies have reported GPx activity in the blood of stable COPD patients and non-COPD subjects, however, the results were not always concordant or significant. Therefore, we sought to further investigate this issue by performing a comprehensive assessment of all published studies by means of systematic review and meta-analysis. We hypothesized that the presence of COPD would be associated with a significant reduction in GPx concentrations in the blood.
2. Materials and Methods
2.1. Search Strategy, Eligibility Criteria, and Study Selection
A systematic search was conducted in the electronic databases PubMed, Web of Science, Scopus, and Google Scholar, from inception to June 2021, using combinations of the following terms: “Glutathione Peroxidase” or “GPx” or “GSH-PX” and “Chronic Obstructive Pulmonary Disease” or “COPD”. Two investigators independently reviewed the full text of the articles once their abstracts were deemed relevant. Eligibility criteria were: (i) the assessment of GPx in blood, erythrocytes, plasma or serum; (ii) a comparison of adult human subjects with COPD and non-COPD (case–control design); (iii) a sample size of ≥ 10 patients with COPD; (iv) English language and (v) full-text available. The references of the retrieved articles were also searched to identify additional studies. To evaluate the risk of bias, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist was used, with scores ≥ 5, 4, and < 4 indicating low, moderate, and high risk, respectively [15]. We assessed the certainty of evidence following the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system. GRADE addresses the following domains: study design, the risk of bias, unexplained heterogeneity, indirectness of evidence, imprecision of the results, effect size, and the probability of publication bias [15].
2.2. Statistical Analysis
Since different units of measurement (U/L, U/gHb or U/mg protein) were used, standardized mean differences (SMDs) were calculated to build forest plots of continuous data and to express the differences in GPx concentrations in COPD patients vs. non-COPD subjects. A p-value < 0.05 was considered statistically significant, and 95% confidence intervals (CIs) were reported. If necessary, the mean and standard deviation values were extrapolated from median and interquartile ranges or medians and ranges, as reported by Wan et al. [16] and by Hozo et al. [17], respectively, or from graphs generated by using the Graph Data Extractor software (San Diego, CA, USA).
To test the heterogeneity of SMD across studies the Q-statistic (the significance level at p < 0.10) was used. We used fixed-effects and random-effects models for a pooled analysis with low heterogeneity (I2 statistic < 50% or p-value < 0.1) and high heterogeneity (I2 statistic > 50% or p-value ≤ 0.1), respectively [18,19]. A sensitivity analysis was also performed to evaluate the robustness of the pooled effect estimates by sequentially excluding each study and repeating the meta-analysis after each iteration [20].
The Begg’s adjusted rank correlation test and the Egger’s regression asymmetry test, at the p < 0.05 level of significance, were also performed to evaluate the presence of publication bias [21,22]. The latter was further investigated using the Duval and Tweedie “trim-and-fill” method [23]. Univariate meta-regression analyses were conducted to investigate the presence of associations between the effect size and the following parameters: age, gender, FEV1 (forced expiratory volume in the 1st second), FEV1/FVC (forced expiratory volume in in the 1st second /forced vital capacity), and the guidelines used for diagnosis (GOLD vs. ATS guidelines). Information regarding missing data in the original articles was not queried upon to the authors. This study followed the guidelines for systematic reviews which are illustrated in the PRISMA Statement [24]. Statistical analyses were performed using Stata 14 (STATA Corp., College Station, TX, USA). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42021276524).
3. Results
3.1. Systematic Research
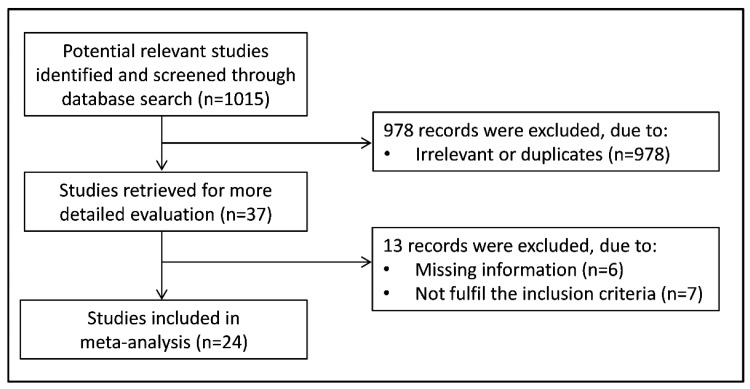
Figure 1 shows the flow chart depicting the screening process. We identified 1,015 articles from the database search. After screening the abstracts and titles of the studies, 37 were selected for full-text evaluation. Of these, 13 were further excluded, either because of missing information or they did not fulfil the inclusion criteria. Finally, 24 studies were included in the meta-analysis [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. A total of 2214 COPD patients (mean age 60 years, 74% male), and 1608 non-COPD subjects (mean age 55 years, 71% male) were evaluated. The characteristics of the retrieved studies, published between 1994 and 2019, are described in Table 1.
Figure 1.
Flow chart of study selection.
Table 1.
Summary of the studies on non-COPD subjects vs COPD patients included in the meta-analysis.
| Non-COPD | COPD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author Year, Country |
Matrix Type |
n | Age Mean |
Gender (M/F) |
GPx Mean ± SD |
n | Age Mean |
Gender (M/F) |
GPX Mean ± SD |
| WHOLE BLOOD/ERYTHROCYTES | |||||||||
| Santos MC et al. 2004, Portugal |
Er | 24 | NR | NR | 0.092 ± 0.027 U/g Hb | 21 | NR | NR | 0.068 ± 0.027 U/g Hb |
| Nadeem A et al. 2005, India |
Er | 21 | NR | NR | 65.96 ± 13.56 mU/g Hb | 67 | NR | NR | 54.17 ± 16.12 mU/g Hb |
| Joppa P et al. 2007, Slovakia |
Er | 21 | 48 | 9/12 | 51.30 ± 14.66 U/g Hb | 75 | 65 | 58/17 | 41.80 ± 18.05 U/g Hb |
| Biljak VR et al. 2010, Croatia |
Er | 51 | 52 | 21/30 | 7904 ± 1096 U/L | 109 | 71 | 82/27 | 6418 ± 1657 U/L |
| Lakhdar R et al. 2011, Tunisia |
Er | 182 | 56 | 173/9 | 85.70 ± 13.61 U/g Hb | 234 | 62 | 222/12 | 63.66 ± 4.95 U/g Hb |
| Tavilani H et al. 2012, Iran |
Er | 60 | 67 | NR | 64.7 ± 31.70 U/g Hb | 30 | 66 | NR | 80.74 ± 46.50 U/g Hb |
| Ahmad H et al. 2013, India |
Er | 75 | 42 | 53/22 | 48.32 ± 14.20 U/g Hb | 140 | 45 | 111/29 | 43.04 ± 9.93 U/g Hb |
| Arja C et al. 2013, India |
Er | 150 | 61 | NR | 43.63 ± 1.61 U/g Hb | 236 | 63 | NR | 36.36 ± 4.57 U/g Hb |
| Wozniak A et al. 2013, Poland |
Er | 35 | 45 | 19/16 | 13.8 ± 46 U/g Hb | 108 | 49 | 61/47 | 8.4 ± 3.1 U/g Hb |
| Montoya-Estrada A et al. 2013, Mexico |
Er | 11 | 61 | 1/10 | 13.90 ± 1.98 mU/mg protein | 43 | 69 | 30/13 | 12.89 ± 2.51 mU/mg protein |
| Bukowska B et al. 2015, Poland |
Er | 18 | NR | NR | 53.66 ± 9.56 mU/g Hb | 30 | NR | NR | 43.70 ± 11.20 mU/g Hb |
| Elmasry SA et al. 2015, Egypt |
WB | 40 | 54 | 31/9 | 12.2 ± 0.7 U/mL | 34 | 55 | 27/7 | 12 ± 0.9 U/mL |
| Mohammed A et al. 2017, India |
Er | 59 | 51 | 38/21 | 63.77 ± 3.38 U/mg protein | 127 | 60 | 98/29 | 59.43 ± 5.63 U/mg protein |
| Di Stefano A et al. 2018, Italy |
WB | 27 | NR | 12/15 | 293 ± 332 U/mL | 45 | NR | 39/6 | 143 ± 159 U/mL |
| Al-Azzawi MA et al. 2019, Egypt |
Er | 40 | 45 | 28/12 | 47.5 ± 1.82 U/mL | 30 | 65 | 21/9 | 15.9 ± 1.2 U/mL |
| SERUM/PLASMA | |||||||||
| Premanand R et al. 1994, India |
S | 100 | NR | 60/40 | 0.231 ± 0.040 U/mL | 75 | NR | 43/32 | 0.204 ± 0.040 U/mL |
| Nadeem A et al. 2005, India |
P | 21 | NR | NR | 129.9 ± 24.1 mU/g Hb | 51 | NR | NR | 156.4 ± 30.8 mU/g Hb |
| Vibhuti A et al. 2007, India |
P | 136 | 50 | 110/26 | 19.1 ± 3.5 U/mL | 202 | 59 | 160/42 | 17.9 ± 7.1 U/mL |
| Montaño M et al. 2010, Mexico |
P | 30 | 65 | 0/30 | 0.11 ± 0.03 U/mL | 60 | 73 | 0/60 | 0.23 ± 0.22 U/mL |
| Wassem SM et al. 2012, India |
S | 60 | 38 | 46/14 | 57.21 ± 0.39 mU/mg protein | 121 | 48 | 80/41 | 51.46 ± 2.77 mU/mg protein |
| Zeng M et al. 2013, China |
P | 28 | 69 | 23/5 | 214.2 ± 6.9 U | 35 | 71 | 31/4 | 183.0 ± 4.6 U |
| Ben Anes A et al. 2014, Tunisia |
P | 229 | 58 | NR | 33.6 ± 24.1 U/ml | 153 | 61 | NR | 121.3 ± 33.0 U/ml |
| Ambade VN et al. 2015, India |
P | 96 | 60 | 73/23 | 50.95 ± 15.30 U/L | 96 | 68 | 73/23 | 89.73 ± 27.84 U/L |
| Hartmann SE et al. 2015, Canada |
P | 14 | 68 | 6/8 | 41.41 ± 8.75 U/ml | 12 | 69 | 4/8 | 36.41 ± 9.84 U/ml |
| Al-Azzawi MA et al. 2017, Egypt |
P | 80 | 53 | 62/18 | 11.98 ± 1.01 mU/ml | 80 | 55 | 58/22 | 12.13 ± 0.92 mU/ml |
Er = Erythrocytes; Hb: Haemoglobin; NR = Not reported; P = Plasma; S = Serum; WB = Whole Blood
3.2. Meta-Analysis of Whole Blood/Erythrocyte GPx Concentrations
3.2.1. Study Characteristics
Fifteen studies on 1329 COPD patients (mean age 60 years, 79% male) and 814 non-COPD subjects (mean age 54 years, 71% male) were identified [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. COPD was diagnosed according to the Global Obstructive Lung Disease (GOLD) guidelines in 12 studies [25,26,28,29,31,32,33,34,35,36,37,38], and the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines in three [27,30,39]. Thirteen studies assessed erythrocytes [25,26,27,28,29,30,31,32,33,34,35,37,39] whereas the remaining two assessed whole blood [36,38].
3.2.2. Risk of Bias
The risk of bias was considered low in seven studies [26,27,28,29,30] and moderate in the remaining eight [25,31,33,34,35,36,38,39] (Table 2).
Table 2.
The Joanna Briggs Institute critical appraisal checklist for analytical cross-sectional studies.
| Study | Were the Criteria for Inclusion in the Sample Clearly Defined? | Were the Study Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Santos et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Nadeem et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Joppa et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Biljak et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Lakhdar et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tavilani et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ahmad et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Arja et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Wozniak et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Montoya et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Bukowska et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Elmasry et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Mohammed et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Di Stefano et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Al-Azzawi et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Premanand et al. | No | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Nadeem et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Vibhuti et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Montano et al. | No | No | Yes | No | No | No | Yes | No | High |
| Waseem et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Zeng et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ben Anes et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Ambade et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Hartmann et al. | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Al-Azzawi et al. | No | Yes | No | No | No | No | Yes | No | High |
3.2.3. Results of Individual Studies and Syntheses
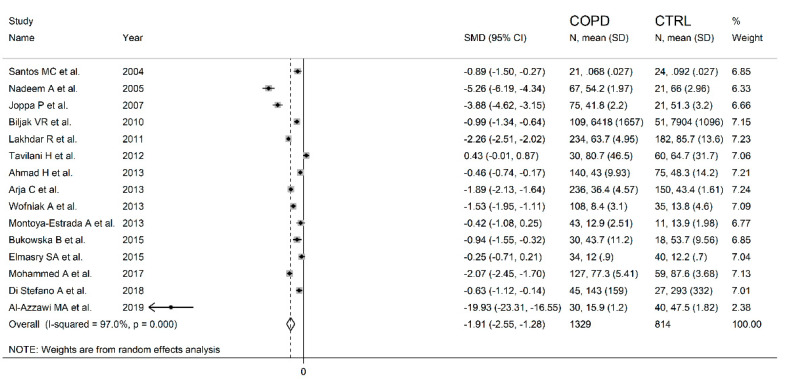
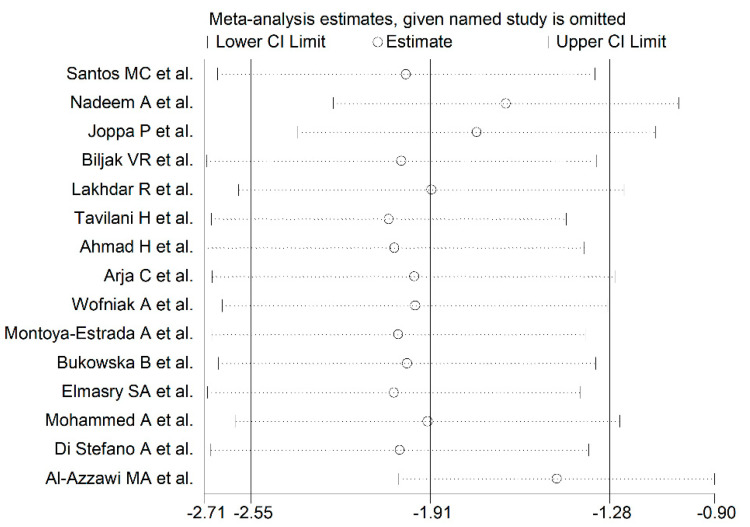
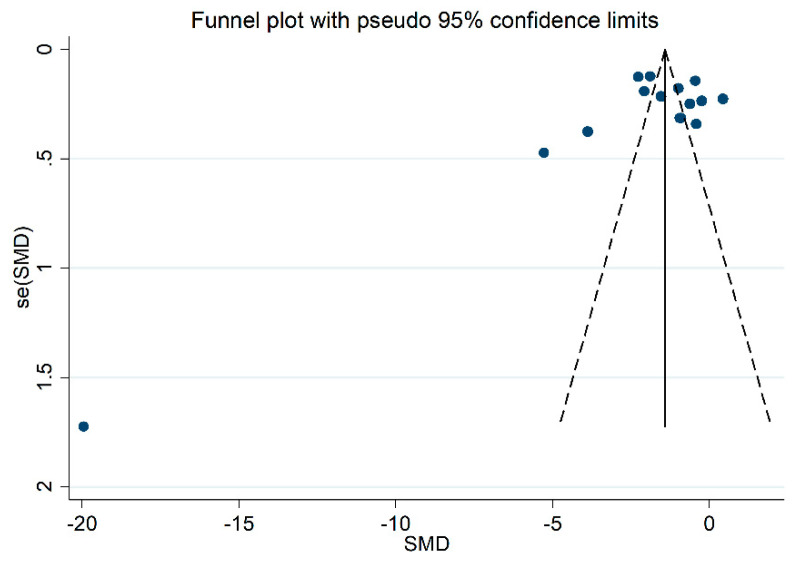
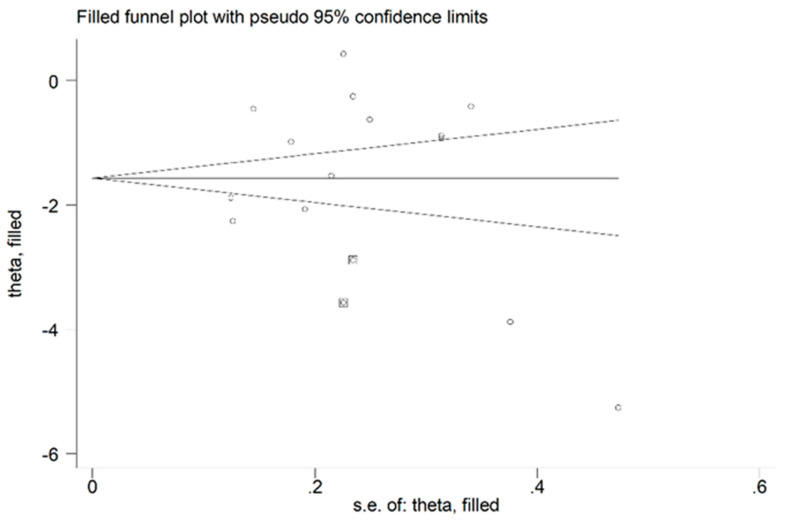
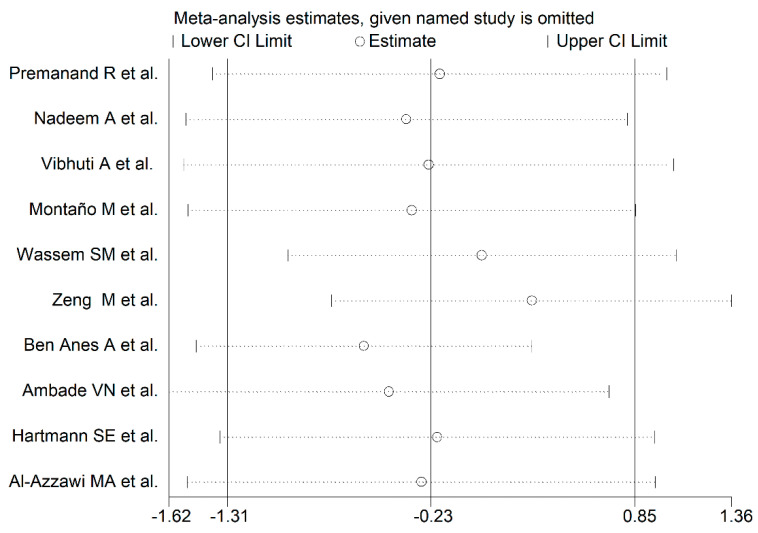
The forest plot for the blood GPx concentrations in COPD patients and non-COPD subjects is reported in Figure 2. In 14 studies [25,26,27,28,29,31,32,33,34,35,36,37,38,39], COPD patients had lower blood GPx concentrations when compared to non-COPD subjects (mean difference range, −0.42 to −19.93), however, the difference was statistically significant in only two studies [34,36]. Extreme heterogeneity between studies was observed (I2 = 97.0%, p < 0.001). Thus, random-effects models were used. Overall, the pooled results showed that blood GPx concentrations were significantly lower in COPD patients (SMD= −1.91, 95% CI −2.55 to −1.28; p < 0.001). Sensitivity analysis showed that the corresponding pooled SMD values were not altered when any single study was sequentially omitted (effect size range, between −2.04 and −1.46, Figure 3). However, funnel plot analysis showed that the study by Al-Azzawy et al. [39] influenced graph symmetry which had a possible effect on the magnitude of the results (Figure 4). After removing this study, the SMD was attenuated but remained significant (SMD = −1.46, 95% CI −2.02 to −0.90, p < 0.001) with persistent, extreme heterogeneity (I2 = 96.2%, p < 0.001).
Figure 2.
Forest plot of studies examining blood/erythrocytes GPx values of COPD and non-COPD.
Figure 3.
Sensitivity analysis of the association between blood/erythrocytes GPx and COPD disease. For each study, the displayed effect size (hollow circles) corresponds to an overall effect size computed from a meta-analysis excluding that study.
Figure 4.
Funnel plot of the 15 retrieved studies evaluating the association between blood/erythrocytes GPx concentration and COPD disease.
3.2.4. Publication Bias
There was no publication bias, after removing the study by Al-Azzawy et al. [39] (Begg’s test, p = 0.74; Egger’s test, p = 0.94). The “trim-and-fill” method identified two potential missing studies to be added to the left side of the funnel plot to ensure symmetry (Figure 5). The adjusted SMD was further increased as a result (SMD = −1.69, 95% CI −2.26 to −1.12, p < 0.001).
Figure 5.
Funnel plot of studies investigating the association between blood/erythrocytes GPx concentration and COPD disease after trimming and filling. Dummy studies and genuine studies are represented by enclosed circles and free circles, respectively.
3.2.5. Meta-Regression and Sub-group Analysis
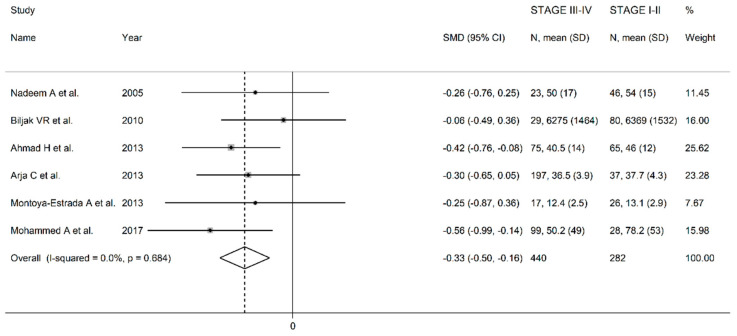
In univariate meta-regression, no significant associations were observed between the effect size and age (t = −1.89, p = 0.10), gender (t = 0.78, p = 0.46), FEV1 (t = 0.62, p = 0.55), FEV1/FVC (t = −0.50, p = 0.63), or specific guideline used (t = −0.20, p = 0.85). In the sub-group analysis, the pooled SMD value in studies which measured GPx in whole blood (SMD = −0.43, 95% CI −0.80 to −0.06, p = 0.023; I2 = 18.4%, p = 0.268) was non-significantly higher (t = 1.07, p = 0.31) than that observed in studies which assessed GPx in erythrocytes (SMD= −1.64, 95% CI −2.25 to −1.03, p < 0.001; I2 = 96.4%, p < 0.001). The search for more homogeneous study sub-groups, according to diagnostic guidelines, matrix type and continent, led to the identification of four studies conducted in Europe which used the GOLD guidelines and assessed erythrocytes [25,28,33,35]. The effect size was still significant (SMD= −1.12, 95% CI −1.43 to −0.81, p < 0.001) with a substantially lower heterogeneity (I2 = 41.4%, p = 0.16). In order to evaluate the relationship between the effect size and disease severity we performed a further meta-analysis in a sub-group of six studies which reported the erythrocyte GPx concentrations in groups with different disease severities (GOLD stage I-II vs III-IV) [26,28,31,32,34,37]. The forest plot for the GPx concentrations in mild/moderate vs severe/very severe COPD patients is reported in Figure 6. In all studies, the GPx concentrations were lower in patients with severe disease (mean difference range −0.56 to −0.06), with a significant difference in two studies [31,37]. The pooled results showed that GPx concentrations were significantly lower in patients with more severe disease (SMD = −0.33; 95% CI −0.50 to −0.16, p < 0.001; I2 = 0.0%, p = 0.68).
Figure 6.
Forest plot of studies examining erythrocyte GPx concentrations of COPD patients in stage I-II vs stage III-IV.
3.2.6. Certainty of Evidence
The initial level of certainty for the blood/erythrocyte GPx SMD values was considered low because of the observational nature of the selected studies (rating 2, ⊕⊕⊝⊝). After considering the presence of a moderate risk of bias in 8 out of 15 studies (a serious limitation, downgrade one level), a generally extreme heterogeneity that was partly explained by specific diagnostic guidelines, the matrix type, and continent (no rating change required), the lack of indirectness (no rating change required), the relatively low imprecision (relatively narrow confidence intervals without threshold crossing, no rating change required), the relatively large effect size (SMD −1.91, upgrade one level), and the absence of publication bias (upgrade one level), the overall level of certainty was considered moderate (rating 3, ⊕⊕⊕⊝).
3.3. Meta-analysis of Serum/Plasma GPx Concentrations
3.3.1. Study Characteristics
Ten studies in 885 COPD patients (mean age 60 years, 66% male) and 794 non-COPD subjects (mean age 55 years, 70% male) were identified [26,40,41,42,43,44,45,46,47,48]. A COPD diagnosis was made according to the Global Obstructive Lung Disease (GOLD) guidelines in 8 studies [26,42,43,44,45,46,47,48], and the American Thoracic Society/ European Respiratory Society (ATS/ERS) guidelines in two [40,41]. Plasma was analyzed in 8 studies [26,41,42,44,45,46,47,48], whereas serum was assessed in two [40,43].
3.3.2. Risk of Bias
The risk of bias was considered low in seven studies [26,41,43,44,45,46,47], moderate in one [40] and high in the remaining two [42,48] (Table 2).
3.3.3. Results of Individual Studies and Syntheses
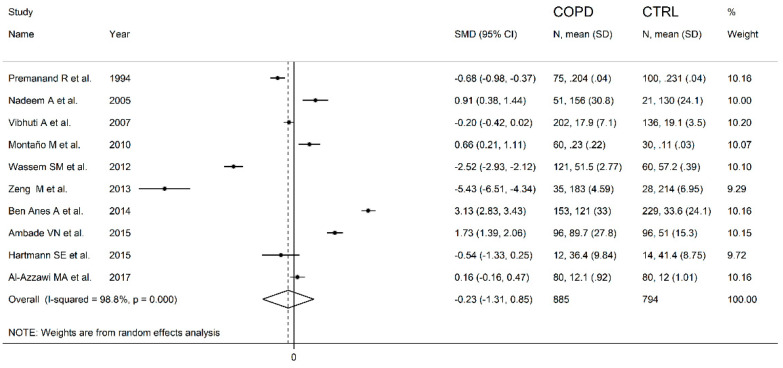
The forest plot for the serum/plasma GPx concentrations in COPD patients and non-COPD subjects is described in Figure 7. In five studies [40,41,43,44,47], COPD patients had lower serum GPx concentrations when compared to non-COPD subjects (mean difference range, −5.43 to −0.20), and this difference was statistically significant in three studies [40,43,44]. In the remaining five studies [26,42,45,46,48], COPD patients had higher serum GPx concentrations (mean difference range, 0.16 to 3.13), and this difference was statistically significant in four studies [26,42,45,46]. Extreme heterogeneity between studies was observed (I2 = 98.8%, p < 0.001). Thus, random-effects models were used. Overall, the pooled results showed that the serum/plasma GPx concentrations were not significantly different between the two groups (SMD= −0.23, 95% CI −1.31 to 0.85, p = 0.67). The effect size was not substantially altered (range between −0.59 and 0.30, Figure 8) after sequentially removing individual studies.
Figure 7.
Forest plot of studies examining the serum/plasma GPx values of COPD and non-COPD.
Figure 8.
Sensitivity analysis of the association between serum/plasma GPx and COPD disease. For each study, the displayed effect size (hollow circles) corresponds to an overall effect size computed from a meta-analysis which excluded that study.
3.3.4. Publication Bias
There was no publication bias according to the Begg’s (p = 0.59) and Egger’s (p = 0.46) tests, or the “trim-and-fill method”.
3.3.5. Meta-regression and Sub-group Analysis
Sub-group analysis showed that the pooled SMD value for the studies measuring GPx in serum (SMD = −1.59, 95% CI −3.41 to −0.22, p = 0.084; I2 = 98.0%, p < 0.001) was non-significantly lower (t = 0.91, p = 0.39) than that observed in the studies assessing plasma (SMD = 0.12, 95% CI −1.00 to 1.25, p = 0.83; I2 = 98.6, p < 0.001). In addition, the pooled SMD value for the studies using the GOLD guidelines (SMD = −0.20, 95% CI −1.67 to 1.27, p = 0.79; I2 = 99.0%, p < 0.001) was similar (t = 0.12, p = 0.91) to that of the studies using the ATS guidelines (SMD = −0.43, 95% CI −0.89 to 0.04, p = 0.07, I2 = 83.4, p = 0.014).
3.3.6. Certainty of Evidence
The initial level of certainty for serum/plasma GPx SMD values was considered low because the selected studies were observational (rating 2, ⊕⊕⊝⊝). After considering the presence of a low risk of bias in 7 out of 10 studies (no rating change required), the generally extreme and unexplained heterogeneity (a serious limitation, downgrade one level), the lack of indirectness (no rating change required), the relatively high imprecision (relatively narrow confidence intervals with threshold crossing, downgrade one level), the relatively small effect size (SMD −0.23, downgrade one level), and the absence of publication bias (upgrade one level), the overall level of certainty was considered downgraded to very low (rating 0, ⊝⊝⊝⊝).
4. Discussion
This meta-analysis provides a critical appraisal of the association between blood GPx concentrations and the presence of COPD. Twenty-four case–control studies were included. and further analyzed according to whether the assessment was performed in whole blood/erythrocytes or serum/plasma.
The results showed that the GPx concentrations in whole blood or erythrocytes were significantly lower in COPD patients when compared to non-COPD subjects. The observed pooled SMD value (−1.91) indicated the presence of a large effect size [49], even after removing the study by Al-Azzawy [39] that appeared to influence the funnel plot symmetry (−1.46). Although a substantial heterogeneity between studies was observed, the sensitivity analysis showed that the pooled SMD value was not altered when individual studies were sequentially discarded. Furthermore, the Begg’s and Egger’s tests revealed the absence of a publication bias. The meta-regression analysis did not find associations between the effect size and age, gender or lung function parameters. The sub-group analysis identified four studies that were homogeneous regarding the diagnostic guidelines, matrix type, and continent. In this subgroup, the effect size confirmed that the GPx concentrations were significantly lower in COPD patients, but with a substantially lower heterogeneity between studies. This suggests that these factors can influence the observed heterogeneity. However, additional potential heterogeneity could also depend on other unreported factors, such as differences in sample handling and analytical procedure, or other inter-individual differences. Moreover, six studies allowed us to further evaluate the relationship between effect size and disease severity, which indicated that erythrocyte GPx concentrations were significantly lower in the patients with more severe disease.
In contrast to the assessment of whole blood and erythrocytes, the studies that assessed GPx in serum or plasma showed conflicting results. This could be due to differences in analytical approaches, age, gender, diet, or lifestyle, which might influence per se the concentration of antioxidant molecules. Therefore, the overall SMD value did not significantly differ between the two groups. There was a substantial heterogeneity between the studies, however the pooled SMD value was not altered when any single study was sequentially removed.
The observed differences in the pooled SMD between the two meta-analyses highlight the importance of the specific biological matrices GPx isoforms. In blood, two isoforms are mainly represented, the intracellular isoform GPx-1, which is ubiquitously expressed in the cytosol, and the extracellular GPx-3, which is actively released into the plasma where it is primarily present as a glycosylated protein [13,50]. Both isoforms are homo-tetramers containing a selenocysteine in their active site, which catalyzes the reduction of hydrogen peroxide or organic hydroperoxides to water or corresponding alcohols [51]. GPx-1, the first selenoprotein identified and characterized as an erythrocytic enzyme, protects hemoglobin from oxidative damage [52]. Red blood cells are normally exposed to high oxygen concentrations, which promote the production of ROS. Our meta-analysis has shown for the first time that GPx-1, but not GPx-3, is significantly lower in COPD patients when compared to non-COPD subjects, and in COPD patients with more severe disease when compared to those with milder forms, which further supports the pathophysiological role of oxidative stress in this disabling condition. The significant reduction of the erythrocytic isoform of GPx may be partly explained by a significant exposure of this type of cell to oxidative stress and an impaired antioxidant system. It has been shown that GPx-1 expression is diminished by selenium deficiency both in vitro and in vivo studies [14]. It is also known that patients affected by COPD often exhibit nutritional deficiencies, including selenium deficiency [25]. This could contribute to the reduced GPx-1 activity observed in this disease. Moreover, the diminished activity of this enzyme, which uses GSH as co-substrate, may also be the consequence of the reduced GSH concentrations that are reported in COPD [53]. Finally, a reduction in GPx-1 expression has been also described in the airway epithelial cells in COPD patients due to accelerated mRNA degradation [54]. Thus, a more thorough evaluation of this important component of the antioxidant defense system may provide useful insights into its role in COPD development and progression, and as a marker of therapeutic response.
5. Conclusions
This meta-analysis had some limitations, in particular the presence of high heterogeneity, and the lack of sub-studies on the relation between GPx expression and clinical parameters, such as smoking habits or other environmental exposure. Furthermore, the number of studies included was limited to those written in English. On the other hand, strengths of our study include the assessment of individual matrix types, hence isoforms, and a comprehensive evaluation of the certainty of evidence for the SMD values. Whilst the presence of extreme heterogeneity might curtail the generalizability of our findings, we also identified that the use of specific COPD diagnostic guidelines, matrix types, and geographical areas are important contributors to such heterogeneity. Our findings support the presence of an impaired antioxidant defense system in COPD. The identification of GPx-1 as a potential biomarker of oxidative stress in COPD warrants longitudinal studies to determine its prognostic role in terms of disease progression and mortality, and to investigate the effects of specific antioxidant therapies in these patients.
Author Contributions
Conceptualization, E.Z., A.Z and P.P.; methodology, E.Z., B.P., S.M. and M.C.P.; formal analysis, A.Z., S.M., A.A.M. and A.G.F.; investigation and data curation, A.Z., A.G.F., B.P., M.C.P. and C.C.; writing—original draft preparation, E.Z. and A.Z.; writing—review and editing, E.Z., A.Z., A.A.M., and P.P.; visualization, A.Z., A.A.M.; supervision, E.Z., and P.P.; funding acquisition, C.C., and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Sardinian Fondo di Sviluppo e Coesione (FSC) 2014–2020, Patto per lo Sviluppo della Regione Sardegna, L.R.7-2017 (RASSR82005) and Fondo di Ateneo per la Ricerca- annualità 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in review.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Chen R., Decramer M., Fabbri L.M., et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Blanco I., Diego I., Bueno P., Casas-Maldonado F., Miravitlles M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur. Respir J. 2019;54:1900610. doi: 10.1183/13993003.00610-2019. [DOI] [PubMed] [Google Scholar]
- 3.Quaderi S.A., Hurst J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018;3:e4. doi: 10.1017/gheg.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo L., Wijegunawardana D. Redox Role of ROS and Inflammation in Pulmonary Diseases. Adv. Exp. Med. Biol. 2021;1304:187–204. doi: 10.1007/978-3-030-68748-9_11. [DOI] [PubMed] [Google Scholar]
- 5.McGuinness A.J., Sapey E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017;6:21. doi: 10.3390/jcm6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 7.Rahman I. The role of oxidative stress in the pathogenesis of COPD: Implications for therapy. Treat. Respir Med. 2005;4:175–200. doi: 10.2165/00151829-200504030-00003. [DOI] [PubMed] [Google Scholar]
- 8.Barnes P.J., Burney P.G., Silverman E.K., Celli B.R., Vestbo J., Wedzicha J.A., Wouters E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J., Pu J., Hao B., Huang L., Chen J., Hong W., Zhou Y., Li B., Ran P. LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Sci. Rep. 2020;10:11587. doi: 10.1038/s41598-020-68327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finicelli M., Squillaro T., Galderisi U., Peluso G. Micro-RNAs: Crossroads between the Exposure to Environmental Particulate Pollution and the Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020;21:7221. doi: 10.3390/ijms21197221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinellu E., Zinellu A., Fois A.G., Fois S.S., Piras B., Carru C., Pirina P. Reliability and Usefulness of Different Biomarkers of Oxidative Stress in Chronic Obstructive Pulmonary Disease. Oxid Med. Cell Longev. 2020;2020:4982324. doi: 10.1155/2020/4982324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. doi: 10.1016/j.redox.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Sarıkaya E., Doğan S. Chapter: Glutathione Peroxidase in Health and Diseases in Glutathione System and Oxidative Stress in Health and Disease, Margarete Dulce Bagatini. IntechOpen; London, UK: 2020. [Google Scholar]
- 15.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K., et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E., Munn Z., editors. JBI Manual for Evidence Synthesis. JBI; Adelaide, Australia: 2020. [Google Scholar]
- 16.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999;47:15–17. [Google Scholar]
- 21.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 23.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos M.C., Oliveira A.L., Viegas-Crespo A.M., Vicente L., Barreiros A., Monteiro P., Pinheiro T., Bugalho De Almeida A. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers. 2004;9:461–469. doi: 10.1080/13547500400024768. [DOI] [PubMed] [Google Scholar]
- 26.Nadeem A., Raj H.G., Chhabra S.K. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation. 2005;29:23–32. doi: 10.1007/s10753-006-8965-3. [DOI] [PubMed] [Google Scholar]
- 27.Joppa P., Petrásová D., Stancák B., Dorková Z., Tkácová R. Oxidative stress in patients with COPD and pulmonary hypertension. Wien. Klin Wochenschr. 2007;119:428–434. doi: 10.1007/s00508-007-0819-y. [DOI] [PubMed] [Google Scholar]
- 28.Biljak V.R., Rumora L., Cepelak I., Pancirov D., Popović-Grle S., Sorić J., Grubisić T.Z. Glutathione cycle in stable chronic obstructive pulmonary disease. Cell Biochem. Funct. 2010;28:448–453. doi: 10.1002/cbf.1675. [DOI] [PubMed] [Google Scholar]
- 29.Lakhdar R., Denden S., Mouhamed M.H., Chalgoum A., Leban N., Knani J., Lefranc G., Miled A., Ben Chibani J., Khelil A.H. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp. Lung Res. 2011;37:195–204. doi: 10.3109/01902148.2010.535093. [DOI] [PubMed] [Google Scholar]
- 30.Tavilani H., Nadi E., Karimi J., Goodarzi M.T. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care. 2012;57:2090–2094. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad A., Shameem M., Husain Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2013;17:1104–1109. doi: 10.5588/ijtld.12.0512. [DOI] [PubMed] [Google Scholar]
- 32.Arja C., Surapaneni K.M., Raya P., Adimoolam C., Balisetty B., Kanala K.R. Oxidative stress and antioxidant enzyme activity in South Indian male smokers with chronic obstructive pulmonary disease. Respirology. 2013;18:1069–1075. doi: 10.1111/resp.12118. [DOI] [PubMed] [Google Scholar]
- 33.Woźniak A., Górecki D., Szpinda M., Mila-Kierzenkowska C., Woźniak B. Oxidant-antioxidant balance in the blood of patients with chronic obstructive pulmonary disease after smoking cessation. Oxid. Med. Cell Longev. 2013;2013:897075. doi: 10.1155/2013/897075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montoya-Estrada A., Torres-Ramos Y.D., Flores-Pliego A., Ramirez-Venegas A., Ceballos-Reyes G.M., Guzman-Grenfell A.M., Hicks J.J. Urban PM2.5 activates GAPDH and induces RBC damage in COPD patients. Front. Biosci. 2013;5:638–649. doi: 10.2741/S396. [DOI] [PubMed] [Google Scholar]
- 35.Bukowska B., Sicińska P., Pająk A., Koceva-Chyla A., Pietras T., Pszczółkowska A., Górski P., Koter-Michalak M. Oxidative stress and damage to erythrocytes in patients with chronic obstructive pulmonary disease--changes in ATPase and acetylcholinesterase activity. Biochem. Cell Biol. 2015;93:574–580. doi: 10.1139/bcb-2015-0066. [DOI] [PubMed] [Google Scholar]
- 36.Elmasry S.A., Al-Azzawi M.A., Ghoneim A.H., Nasr M.Y., AboZaid M.M.N. Role of oxidant–antioxidant imbalance in the pathogenesis of chronic obstructive pulmonary disease. Egypt J. Chest Dis. Tuberc. 2015;64:813–820. doi: 10.1016/j.ejcdt.2015.06.001. [DOI] [Google Scholar]
- 37.Mohammed A., Gutta V., Ansari M.S., Venkata R.S., Jamil K. Altered antioxidant enzyme activity with severity and comorbidities of chronic obstructive pulmonary disease (COPD) in South Indian population. COPD Res. Pract. 2017;3:4. doi: 10.1186/s40749-017-0023-z. [DOI] [Google Scholar]
- 38.Di Stefano A., Coccini T., Roda E., Signorini C., Balbi B., Brunetti G., Ceriana P. Blood MCP-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int. J. Chron. Obs. Pulmon. Dis. 2018;13:1691–1700. doi: 10.2147/COPD.S159915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Azzawi M.A., Al-Rubaeaee A.A., Ghoneim A.H., AboZaid M.M. The effect of cigarette smoking on the oxidant–antioxidant imbalance in patients with chronic obstructive pulmonary disease. Egypt J. Chest Dis. Tuberc. 2019;68:462–470. [Google Scholar]
- 40.Premanand R., Naidu K.V.S., Kumari K.S., Reddy K.K. Lipid peroxides, vitamin E levels and glutathione peroxidase activity in serum of respiratory disease patients. Indian J. Clin. Biochem. 1994;9:50–53. doi: 10.1007/BF02867857. [DOI] [Google Scholar]
- 41.Vibhuti A., Arif E., Deepak D., Singh B., Pasha M.Q. Correlation of oxidative status with BMI and lung function in COPD. Clin. Biochem. 2007;40:958–963. doi: 10.1016/j.clinbiochem.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Montaño M., Cisneros J., Ramírez-Venegas A., Pedraza-Chaverri J., Mercado D., Ramos C., Sansores R.H. Malondialdehyde and superoxide dismutase correlate with FEV(1) in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal. Toxicol. 2010;22:868–874. doi: 10.3109/08958378.2010.491840. [DOI] [PubMed] [Google Scholar]
- 43.Waseem S.M., Mobarak M.H., Islam N., Ahmad Z. Comparative study of pulmonary functions and oxidative stress in smokers and non-smokers. Indian J. Physiol. Pharmacol. 2012;56:345–352. [PubMed] [Google Scholar]
- 44.Zeng M., Li Y., Jiang Y., Lu G., Huang X., Guan K. Local and systemic oxidative stress status in chronic obstructive pulmonary disease patients. Can. Respir J. 2013;20:35–41. doi: 10.1155/2013/985382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ben Anes A., Fetoui H., Bchir S., ben Nasr H., Chahdoura H., Chabchoub E., Yacoub S., Garrouch A., Benzarti M., Tabka Z., et al. Increased oxidative stress and altered levels of nitric oxide and peroxynitrite in Tunisian patients with chronic obstructive pulmonary disease: Correlation with disease severity and airflow obstruction. Biol. Trace Elem. Res. 2014;161:20–31. doi: 10.1007/s12011-014-0087-4. [DOI] [PubMed] [Google Scholar]
- 46.Ambade V.N., Sontakke A.N., Barthwal M.S., Tyagi R., Basannar D.R. Diagnostic Utility of Biomarkers in COPD. Respir Care. 2015;60:1729–1742. doi: 10.4187/respcare.03753. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann S.E., Waltz X., Kissel C.K., Szabo L., Walker B.L., Leigh R., Anderson T.J., Poulin M.J. Cerebrovascular and ventilatory responses to acute isocapnic hypoxia in healthy aging and lung disease: Effect of vitamin C. J. Appl. Physiol. 2015;119:363–373. doi: 10.1152/japplphysiol.00389.2015. [DOI] [PubMed] [Google Scholar]
- 48.Al-Azzawi M.A., Ghoneim A.H., Elmadbouh I. Evaluation of Vitamin D, Vitamin D Binding Protein Gene Polymorphism with Oxidant—Antioxidant Profiles in Chronic Obstructive Pulmonary Disease. J. Med. Biochem. 2017;36:331–340. doi: 10.1515/jomb-2017-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum; Mahwah, NJ, USA: 1988. [Google Scholar]
- 50.Brigelius-Flohé R., Flohé L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020;33:498–516. doi: 10.1089/ars.2019.7905. [DOI] [PubMed] [Google Scholar]
- 51.Toppo S., Flohé L., Ursini F., Vanin S., Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sotgia S., Paliogiannis P., Sotgiu E., Mellino S., Zinellu E., Fois A.G., Pirina P., Carru C., Mangoni A.A., Zinellu A. Systematic Review and Meta-Analysis of the Blood Glutathione Redox State in Chronic Obstructive Pulmonary Disease. Antioxidants. 2020;9:1146. doi: 10.3390/antiox9111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dabo A.J., Ezegbunam W., Wyman A.E., Moon J., Railwah C., Lora A., Majka S.M., Geraghty P., Foronjy R.F. Targeting c-Src Reverses Accelerated GPX-1 mRNA Decay in Chronic Obstructive Pulmonary Disease Airway Epithelial Cells. Am. J. Respir Cell Mol. Biol. 2020;62:598–607. doi: 10.1165/rcmb.2019-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in review.