Abstract
Simple Summary
The use of essential oils (EOs) in the food industry is a popular research topic, as they have antioxidant and antimicrobial activity and could be used as ingredients directly in food or as bioactive component in food coating and food packaging. Thus, the study of their antioxidant and antimicrobial activity is a crucial step to evaluate their use in food packaging/coating. In this work, we evaluate the antioxidant and antimicrobial activities of 13 EOs from herbs, spices, fruits, and vegetables. Briefly, the EOs from aromatic herbs and spices showed the highest antioxidant and antimicrobial activity. Fennel essential oil reported the lowest antioxidant activity, however it showed very good antimicrobial activity against Botrytis cinerea, one of the post-harvest pathogen microorganisms in fruits and vegetables.
Abstract
In the field of food preservation, encapsulated Essential Oils (EOs) could be the best non-toxic and eco-friendly tool for food preservative applications substituting the chemicals ones that have several disadvantages for the environment and health. Thirteen commercial EOs from plants, fruits, and vegetables were characterized by GC-MS. The antioxidant activity was measured by DPPH and ABTS techniques. Antimicrobial activity was assessed by agar well-diffusion method and the Minimum Inhibitory Concentration (MIC) by agar dilution method against six bacteria, Candida albicans, and Botrytis cinerea. All the EOs tested have demonstrated antioxidant activity in the range of IC50 0.01–105.32 mg/mL. Between them, cinnamon EOs were the best, followed by oregano and thyme EOs. Fennel EO showed the lowest radical scavenging. MIC values ranged from 0.14 to 9 mg/mL. C. cassia, thyme, and oregano EOs were the most effective against the bacterial species tested, and the yeast C. albicans. On the contrary, citric fruit EOs showed low or no inhibition against most bacterial strains. The percentages of inhibition of mycelia growth of B. cinerea ranged from 3.4 to 98.5%. Thyme, oregano, mint, and fennel EOs showed the highest inhibition.
Keywords: essential oils, DPPH, ABTS, food spoilage, antiradical activity
1. Introduction
Food security is supported in four main pillars: food access, food utilization, food stability, and food preservation. The latter mainly consists of the degradation and microbial contamination that can affect food. Food spoilage is one of the problems that should be avoided. A lot of chemical preservatives have been developed and proved to have a significant contribution in controlling this degradation. However, they have often raised negative concerns to the consumers as they are not from a green source. They need long term degradation cycles, they are environmental toxicology and have potential risks of carcinogenesis and teratogenesis in humans and animals. Due to this, essential oils (EOs) and their active components are under study for their potential use as preservatives due to their wide antibacterial, antifungal, antimycotoxigenic spectrum, and antioxidant properties [1]. Therefore, the use of EOs in food industry is growing, as they could be directly added to edible products or used for active packaging and edible coatings [2]. Thus, the use of essential oil in food industry has a double action due to their antioxidant and antimicrobial properties [3]. Moreover, the bioactive compounds contained in the EOs could also be used for pharmaceutical and cosmetic applications [4].
Overall, EOs are products that can be isolated from leaves, bark, seeds, fruit peels, roots, flowers, buds, and stems, namely, from agro-industrial by-products in the majority of cases. The antioxidant activity of the EOs is related to the complex mixture of terpenes, terpenoids, and phenylpropanoids that compose them. Among others, it was noticed that carvacrol, thymol, and eugenol are able to inhibit the oxidation processes [5].
The same happens with the antimicrobial activity of the EOs, but in this case the mechanism depends on the specific chemical components. The most common mechanism seems to be related with the alteration in the membranes, modifying their dynamicity and permeability, and in consequence releasing the cytoplasmic constituents. However, the effect is different for each microorganism depending on the variability of the membrane thickness, composition, and cellular metabolic activities [1].
The limitations that could carry the use of EOs as preservatives in food can be due to the intense aroma, high reactivity, hydrophobicity, reduced solubility, and possible negative interaction with the matrices, leading to changes in the intestinal absorbance and organoleptic properties. However, nowadays they are avoidable due to the new mechanisms studied and developed for encapsulating those EOs. These methods can improve the stability and solubility of the EOs, protecting them from the environmental interactions [6].
Therefore, for agriculture and food fields, encapsulated EOs could be the best non-toxic and eco-friendly option for preservative applications as active food packaging, enhancing food shelf life. In this context, one of the goals of the SHEALTHY project is to find essential oils that could be incorporated in food packaging in order to obtain active packaging/coating for fruit and vegetables. In this way, it is important to identify essential oils with antibacterial and anti-fungus properties that can act against foodborne pathogens, e.g., Botrytis cinerea, a necrotrophic fungus that affects several fruits, causing the production of high quantities of fruit wastes. Thus, the aim of this work is to evaluate and compare the antioxidant and antimicrobial activities of 13 commercial EOs from plants and fruit and vegetables. To achieve this, the EOs were characterized by GC-MS. The antioxidant activity was evaluated by DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) assays. The antimicrobial activity was tested against microorganisms that usually spoil food and are dangerous for human health.
2. Materials and Methods
2.1. Reagents and Samples
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solvents were from Honeywell (Wabash, Lafayette, IN, USA) except ethanol, which was from PanReac (Barcelona, Spain). Water was purified using a Milli-Q system (Millipore, Bedford, MA, USA).
Thirteen essential oils were purchased from a local supplier; seven of them hail from aromatic plants and spices, and others were from fruits and vegetables (Table 1). All samples were obtained by hydrodistillation (purity 100%). Selection of the essential oils took the literature data and the availability in the Spanish market into account.
Table 1.
List of the EOs used in this work.
| Common Name | Scientific Name | Part Used |
|---|---|---|
| Aromatic herbs and spices | ||
| True cinnamon | Cinnamomum zeylanicum J. Presl | Leaf |
| Cinnamon | Cinnamomum cassia J. Presl | Bark |
| Oregano | Origanum vulgare L. | Leaf |
| Thyme | Thymus vulgaris L. | Flower/leaf |
| Rosemary | Rosmarinus officinalis (L.) Schleid. | Leaf |
| Peppermint | Mentha piperita L. | Leaf |
| Sage | Salvia lavandulifolia Vahl | Leaf |
| Fruits and vegetables | ||
| Celery | Apium graveolens L. | Seed |
| Fennel | Foeniculum vulgare Mill. var. dulce | Not reported |
| Mandarin | Citrus reticulata Blanco | Peel |
| Sweet orange | Citrus sinensis Osbeck | Peel |
| Lemon | Citrus limon L. | Peel |
| Grapefruit | Citrus paradisi Macfad. | Peel |
2.2. DPPH Free Radical-Scavenging Capacity
DPPH radical scavenging activity was assayed with a method proposed by several authors [7,8]. Briefly, 2.9 mL of 100 µM DPPH (in methanol or n-hexane depending on the polarity of the essential oil) was mixed with 100 μL of each essential oil at different concentrations. They were incubated during 30 min at 25 °C and were measured at 517 nm. The antioxidant activity of the EOs was expressed as the concentration of extract that inhibited the DPPH radical formation by 50% (IC50). IC50 for each sample was calculated by elaborating a curve where is represented the concentration (mg/mL) and the percentage of inhibition calculated as in the following Equation (1):
| Inhibition (%) = (blank − sample)/blank × 100 | (1) |
where “blank” is the absorbance of DPPH with the sample replaced with methanol and “sample” is referred to the absorbance of the DPPH mixed with the essential oil or the control. Ascorbic acid was used as a reference of positive control from 0.0001 to 0.5 mg/mL.
2.3. ABTS Acid Cation Radical-Scavenging Capacity
This technique was developed by Re at al. [9] in which the monocation ABTS•+ is generated by oxidation of the ABTS with potassium persulfate in the dark at room temperature for 12–24 h. For the analyses, 2 mL of 7 mM ABTS solution was added to 20 µL of different concentrations of each sample, and they were measured at 734 nm after 30 min of incubation at 30 °C. The antioxidant activity of the EOs was expressed as the concentration of extract that inhibited the ABTS radical formation by 50% (IC50). The IC50 for each sample was calculated by elaborating a curve where is represented the concentration (mg/mL) and the percentage of inhibition calculated as in the Equation (1). Ascorbic acid was used as a reference of positive control from 0.0001 to 0.5 mg/mL.
2.4. Antimicrobial Activity
2.4.1. Test Microorganisms
The antimicrobial activity of essential oils was tested against Staphylococcus aureus (S. aureus), methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli (E. coli), Salmonella enterica serovar Typhimurium (S. Typhimurium), Listeria monocytogenes (L. monocytogenes), Candida albicans (C. albicans), and Botrytis cinerea (B. cinerea). All bacterial strains and C. albicans were isolated from various clinical samples in the Microbiology Service of the Virgen de las Nieves Hospital (Granada, Spain) and were stored as glycerol stocks and reactivated by incubation in tryptic soy agar at 37 °C for 24 h. B. cinerea was obtained from the Spanish Type Culture Collection (CECT 2100) and was maintained and grown at 25 °C in Sabouraud dextrose agar.
2.4.2. Agar-Well-Diffusion Method
For bacteria and C. albicans, antimicrobial activity was assessed following the method described by Hayes and Markovic [10] with modifications, as follows: 15 mL of molten Mueller Hinton agar were poured into sterile Petri dishes and allowed to set to form a base layer. An 8 mm diameter stainless steel cylinder was placed over the base layer, and 10 mL of molten Mueller Hinton agar containing the inoculum were poured over the surface of the base layer and left to set. For the preparation of inoculate, cultures of the strains were suspended in buffered saline solution until reaching a turbidity corresponding to 0.5 McFarland standard and were inoculated in molten Mueller Hinton agar to obtain a final concentration of approximately 1 × 106 CFU/ mL. After solidification of the upper layer, the cylinders were carefully removed and 25 μL of essential oils were added into the resulting holes. As a positive control for antimicrobial activity, ciprofloxacin was used at 2 mg/mL for MRSA, 0.1 mg/mL for L. monocytogenes, and 0.01 mg/mL for the remaining bacteria, and ketoconazole at 0.01 mg/mL for C. albicans. After incubation at 4 °C for 30 min to allow extracts to diffuse into the medium, the plates were incubated at 37 °C for 24 h. The inhibition zone diameters were measured (mm) and recorded as the mean ± standard deviation (SD). Three replicates were conducted.
For B. cinerea, Sabouraud dextrose plates were prepared following the previous procedure but testing one extract per plate. After 30 min incubation to allow the extract to diffuse, a 4-mm plug of mycelium was placed at a 3 cm distance from the essential oil. The plates were incubated for 7 days at 25 °C. The growth control consisted of Sabouraud dextrose plates inoculated with mycelium alone. Ketoconazole at 20 µg/mL was used as antimicrobial control. The percentage of mycelium inhibition for each essential oil was calculated by measuring the area of fungal growth and comparing it to the control using the ImageJ software [11]. Three replicates were conducted.
2.4.3. Determination of Minimum Inhibitory Concentration (MIC)
MIC values were assessed by agar-dilution method as follows: from 288 mg/mL solutions in 98% ethanol of each essential oil, serial dilutions in sterile water supplemented with 0.5% Tween 80 were prepared (144–0.56 mg/mL). A total of 5 mL of the essential oil’s dilutions were added in 15 mL of molten Mueller Hinton or Sabouraud agar, which was prepared previously with three-quarters of its volume of water, and plated onto sterile Petri dishes. The essential oils were tested in concentrations ranging from 72 to 0.14 mg/mL for bacteria and C. albicans, and from 72 to 0.07 mg/mL for B. cinerea. Bacterial suspensions corresponding to 0.5 McFarland’s standard were adjusted to obtain a concentration of approximately 1 × 106 CFU/mL, and a spot of 10 μL of each bacterial suspension was added onto the plates. B. cinerea was inoculated placing a 4-mm plug of mycelium at the center of each plate. A plate containing 5 mL of sterile water supplemented with 0.5% Tween 80 in 15 mL of molten Mueller Hinton or Sabouraud agar prepared and inoculated as before, was used as a growth control. All experiments were performed in duplicate. The MIC was defined as the lowest concentration of the essential oils that completely inhibited microbial growth after incubation at 37 °C for 24 h for bacteria and C. albicans, and 25 °C for 7 days for B. cinerea.
2.5. Determination of Essential Oil Compounds by GC-MS
Essential oils were diluted in trichloromethane and analysed by GC-MS according to Ben Lajnef et al. [12]. Separation was achieved using an Agilent 7890A GC coupled to a Waters QUATTRO microTM GC mass spectrometer. The compounds were separated on a capillary column DB-5MS (30 m × 0.25 mm; f.t. 0.25 μm) purchased from Agilent Technologies (J&W Scientific, Folsom, CA, USA). Oven temperature was set at 40 °C for 2 min, after that the temperature increased from 40 to 250 °C at 3 °C min−1, and remained at 250 °C for 10 min.
MS detector parameters were set at: scan range: 40–450 m/z; solvent delay time: 3.0 min; transfer line temperature 250 °C; ion source 230 °C; and ionisation energy 70 eV. Carrier gas was (He) at a flow of 1.0 mL min−1. Samples were injected in splitless mode. GS-MS chromatograms are showed in Supplementary Figure S1.
2.6. Statistical Analysis
Pearson’s correlations between antioxidant methods were evaluated using Statistica 6.0 (2001, StatSoft, Tulsa, OK, USA).
3. Results and Discussion
3.1. Composition of the Essential Oils
Essential oils were analyzed by GC-MS and a total of 56 compounds were detected. Table 2 reported the relative amounts (%) of each compound determined in aromatic plant EOs.
Table 2.
Composition (expressed as %) of aromatic plant EOs determined by GC-MS.
| Compound |
Cinnamomum
cassia |
Cinnamomum
zeylanicum |
Mentha
piperita |
Origanum
vulgare |
Rosmarinus
officinalis |
Salvia
lavandulifolia |
Thymus
vulgaris |
|
|---|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | n.d. | n.d. | n.d. | n.d. | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.08 ± 0.00 |
| 2 | Thujene | n.d. | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.3 ± 0.01 | 0.2 ± 0.00 | 0.2 ± 0.01 | 0.6 ± 0.03 |
| 3 | α-pinene | 0.05 ± 0.00 | 0.2 ± 0.03 | 0.7 ± 0.07 | 1.0 ± 0.10 | 11.9 ± 0.90 | 5.5 ± 0.30 | 0.7 ± 0.06 |
| 4 | Camphene | n.d. | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.2 ± 0.00 | 5.0 ± 0.30 | 7.7 ± 0.40 | 0.7 ± 0.01 |
| 5 | Sabinene | n.d. | n.d. | 0.2 ± 0.00 | n.d. | n.d. | 1.1 ± 0.30 | 0.004 ± 0.00 |
| 6 | β-pinene | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.9 ± 0.02 | 0.07 ± 0.01 | 2.5 ± 0.30 | 5.1 ± 0.20 | 0.1 ± 0.01 |
| 7 | β-myrcene | n.d. | 0.008 ± 0.00 | n.d. | 0.5 ± 0.30 | 30.7 ± 0.40 | 2.0 ± 0.08 | 0.7 ± 0.03 |
| 8 | α-phellandrene | n.d. | 0.2 ± 0.01 | 0.01 ± 0.00 | 0.07 ± 0.00 | 0.7 ± 0.02 | 0.04 ± 0.00 | 0.1 ± 0.00 |
| 9 | α-terpinene | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.1 ± 0.00 | 0.5 ± 0.02 | 0.6 ± 0.02 | 0.3 ± 0.01 | 1.2 ± 0.10 |
| 10 | p-cymene | 0.05 ± 0.00 | 0.1 ± 0.01 | 0.3 ± 0.03 | 5.3 ± 0.20 | 1.6 ± 0.10 | 0.3 ± 0.02 | 16.5 ± 0.30 |
| 11 | D-limonene | 1.5 ± 0.20 | 0.09 ± 0.00 | 2.1 ± 0.20 | 0.2 ± 0.01 | 2.9 ± 0.30 | 4.7 ± 0.40 | 0.3 ± 0.02 |
| 12 | 1,8-cineole (Eucalyptol) | n.d. | n.d. | 4.9 ± 0.30 | 0.07 ± 0.00 | 14.8 ± 0.50 | 38.6 ± 0.80 | n.d. |
| 13 | cis-ocimene | n.d. | n.d. | n.d. | n.d. | 0.1 ± 0.00 | 0.06 ± 0.00 | n.d. |
| 14 | γ-terpinene | n.d. | n.d. | 0.1 ± 0.03 | 2.4 ± 0.20 | 0.7 ± 0.02 | 0.4 ± 0.01 | 7.1 ± 0.30 |
| 15 | Terpinolene | n.d. | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.3 ± 0.03 | 0.2 ± 0.00 | 0.05 ± 0.00 |
| 16 | Linalool | 1.8 ± 0.10 | 0.4 ± 0.01 | n.d. | 0.7 ± 0.04 | 0.5 ± 0.06 | 0.7 ± 0.00 | 2.5 ± 0.08 |
| 17 | Pinone | n.d. | n.d. | 0.004 ± 0.00 | n.d. | n.d. | 0.3 ± 0.01 | n.d. |
| 18 | Camphor | n.d. | n.d. | 0.09 ± 0.00 | 0.07 ± 0.00 | 20.7 ± 0.30 | 23.6 ± 0.50 | 0.6 ± 0.02 |
| 19 | Menthone | n.d. | n.d. | 29.8 ± 0.50 | n.d. | n.d. | n.d. | n.d. |
| 20 | Menthofuran | 0.002 ± 0.00 | n.d. | 1.9 ± 0.30 | n.d. | 0.004 ± 0.00 | n.d. | n.d. |
| 21 | Isomenthone | 0.002 ± 0.00 | 0.002 ± 0.00 | 4.2 ± 0.20 | n.d. | 0.01 ± 0.00 | n.d. | n.d. |
| 22 | Isomenthol | n.d. | n.d. | 3.6 ± 0.10 | n.d. | n.d. | n.d. | n.d. |
| 23 | Borneol | n.d. | 0.004 ± 0.00 | n.d. | 0.3 ± 0.04 | 0.9 ± 0.10 | 2.0 ± 0.20 | 1.3 ± 0.10 |
| 24 | Menthol | n.d. | n.d. | 39.7 ± 0.30 | n.d. | n.d. | n.d. | n.d. |
| 25 | Terpinen-4-ol | n.d. | 0.02 ± 0.00 | n.d. | 0.3 ± 0.00 | 0.4 ± 0.01 | 0.4 ± 0.02 | 1.0 ± 0.1 |
| 26 | α-terpineol | n.d. | 0.04 ± 0.00 | 0.2 ± 0.00 | n.d. | 1.0 ± 0.06 | 0.4 ± 0.01 | 0.1 ± 0.01 |
| 27 | Estragole | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 28 | Verbenone | 0.006 ± 0.00 | n.d. | n.d. | n.d. | 0.3 ± 0.00 | n.d. | 0.01 ± 0.00 |
| 29 | Methyl thymyl ether | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 ± 0.02 |
| 30 | Pulegone | n.d. | n.d. | 0.8 ± 0.10 | n.d. | n.d. | n.d. | n.d. |
| 31 | Linalyl anthranilate | n.d. | n.d. | n.d. | n.d. | n.d. | 2.2 ± 0.30 | n.d. |
| 32 | Piperitone | n.d. | n.d. | 0.5 ± 0.03 | n.d. | n.d. | n.d. | n.d. |
| 33 | Neomenthol acetate | 0.003 ± 0.00 | n.d. | 0.3 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 34 | Cinnamaldehyde | 91.9 ± 1.70 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 35 | Bornyl acetate | n.d. | n.d. | n.d. | n.d. | 0.8 ± 0.02 | 0.8 ± 0.01 | n.d. |
| 36 | Myrtenyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. | 1.1 ± 0.02 | n.d. |
| 37 | Menthyl acetate | n.d. | n.d. | 6.1 ± 0.70 | n.d. | n.d. | n.d. | n.d. |
| 38 | β-isosafrole | n.d. | 0.1 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 39 | Thymol | n.d. | n.d. | n.d. | 1.6 ± 0.20 | n.d. | n.d. | 61.2 ± 1.10 |
| 40 | Carvacrol | n.d. | n.d. | n.d. | 84.5 ± 1.60 | n.d. | n.d. | 2.6 ± 0.5 |
| 41 | Terpinyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. | 0.6 ± 0.02 | 0.06 ± 0.00 |
| 42 | Eugenol | 4.2 ± 0.30 | 95.2 ± 1.40 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 43 | β-caryophyllene | 0.5 ± 0.02 | 1.6 ± 0.20 | 2.7 ± 0.10 | 1.6 ± 0.10 | 2.2 ± 0.20 | 0.7 ± 0.10 | 1.9 ± 0.20 |
| 44 | α-caryophyllene | 0.03 ± 0.00 | 0.3 ± 0.04 | 0.2 ± 0.00 | 0.1 ± 0.00 | 0.7 ± 0.08 | 0.4 ± 0.03 | 0.05 ± 0.00 |
| 45 | β-eudesmene | n.d. | n.d. | 0.08 ± 0.00 | n.d. | n.d. | n.d. | 0.07 ± 0.00 |
| 46 | Eremophilane | n.d. | n.d. | 0.03 ± 0.00 | n.d. | n.d. | 0.2 ± 0.01 | 0.01 ± 0.00 |
| 47 | Bisabolene | n.d. | n.d. | n.d. | 0.12 | n.d. | n.d. | 0.07 ± 0.00 |
| 48 | Isoeugenol | n.d. | 0.4 ± 0.10 | 0.2 ± 0.00 | n.d. | 0.3 ± 0.03 | 0.1 ± 0.00 | 0.07 ± 0.00 |
| 49 | 2-(2-propenyl)-furan | n.d. | 0.007 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 50 | Benzyl benzoate | n.d. | 1.0 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpenes | 3.4 | 1.3 | 39.9 | 11.4 | 58.2 | 28.9 | 30.8 | |
| Oxygenated monoterpenes | n.d. | n.d. | 5.0 | 0.1 | 35.5 | 62.2 | 0.6 | |
| Alcohols | n.d. | 0.1 | 43.6 | 0.5 | 2.3 | 2.8 | 2.4 | |
| Ethers | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 | |
| Esthers | n.d. | 1.0 | 6.4 | n.d. | 0.8 | 2.5 | 0.1 | |
| Sesquiterpene hydrocarbons | 0.5 | 1.8 | 3.1 | 1.8 | 2.9 | 1.3 | 2.1 | |
| Aldehydes | 91.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Phenols | 4.2 | 95.7 | 0.2 | 86.1 | 0.3 | 0.1 | 63.9 | |
| Others | n.d. | 0.2 | 1.9 | n.d. | n.d. | 2.2 | n.d. |
n.d. = not detected.
According to the literature [13], cinnamon samples presented different composition according to the part of the plant that was used for the extraction. Briefly, eugenol was the main compound in C. zeylanicum that was extracted from leaves; on the other hand, cinnamaldehyde was the first compound in C. cassia sample that was extracted from bark.
Oregano EO showed carvacrol as the main compound (84.5%) followed by p-cymene, γ-terpinene and thymol. Similar composition has been reported by Diniz do Nascimento et al. [4]; as remarked by the same authors, the oregano essential oil composition is highly influenced by the part of the plant and the agronomic and technological processes.
Thymus EO composition is also affected from the different parts of plant that are used for extraction (among agronomical and processing factors). The samples that were analyzed in this work proceed from a mix of flowers and leaves; thymol was the first compound accounting for more than 60%, followed by p-cymene, γ-terpinene, carvacrol, and linalool, respectively, accounting for about the 90% of total compound. The same compounds were described by Diniz do Nascimento et al. [4] in thymus EOs.
β-myrcene was the first compound in Rosmarinus EO, the second one was camphor, followed by eucalyptol and α-pinene; the present composition is in the same order of magnitude than that reported by Diniz do Nascimento et al. [4].
Mentha EO reported the typical compounds previously found in this matrix [4] and are characteristic of this EO. Briefly, menthol and menthone accounted for 69.5% of the total compounds, followed by menthyl-acetate, eucalyptol, isomenthone, isomenthol, and mentho-furan. Other minor compounds that are usually found in this plant are piperitone and neomenthol acetate.
Finally, according to Porres-Martínez et al. [14], 1,8-cineole (eucalyptol) followed by camphor, camphene, and β- and α-pinene were the main compounds of sage EO.
Table 3 reports the composition of essential oil obtained from fruits and vegetables.
Table 3.
Composition (expressed as %) of fruit and vegetable EOs determined by GC-MS.
| Compound |
Apium
graveolens |
Citrus
limon |
Citrus
paradisi |
Citrus
reticulata |
Citrus
sinensis |
Foeniculum
vulgare |
|
|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | n.d. | n.d. | n.d. | 0.003 ± 0.00 | n.d. | 0.004 ± 0.00 |
| 2 | Thujene | 0.009 ± 0.00 | 0.1 ± 0.02 | n.d. | 0.01 ± 0.00 | n.d. | n.d. |
| 3 | α-pinene | 0.3 ± 0.04 | 0.8 ± 0.05 | 0.1 ± 0.00 | 0.2 ± 0.02 | 0.2 ± 0.03 | 1.2 ± 0.10 |
| 4 | Camphene | n.d. | 0.03 ± 0.00 | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 5 | Sabinene | 0.06 ± 0.00 | 0.8 ± 0.10 | 0.2 ± 0.01 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.03 ± 0.00 |
| 6 | β -pinene | 2.4 ± 0.20 | 6.0 ± 0.30 | 0.08 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 |
| 7 | β-myrcene | 0.4 ± 0.02 | 0.4 ± 0.10 | 0.3 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.01 ± 0.00 |
| 8 | α-terpinene | n.d. | 0.08 ± 0.00 | n.d. | n.d. | n.d. | 0.003 ± 0.00 |
| 9 | Cymene | 0.06 ± 0.00 | 0.1 ± 0.02 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| 10 | D-limonene | 71.4 ± 1.50 | 87.0 ± 1.20 | 99.0 ± 1.60 | 99.0 ± 1.10 | 99.2 ± 1.50 | 1.2 ± 0.40 |
| 11 | 1,8-cineole (Eucalyptol) | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 12 | γ-terpinene | n.d. | 3.8 ± 0.07 | 0.04 ± 0.00 | 0.06 ± 0.00 | n.d. | 0.01 ± 0.00 |
| 13 | Terpinolene | n.d. | 0.1 ± 0.02 | n.d. | n.d. | n.d. | 0.03 ± 0.00 |
| 14 | Fenchone | 0.003 ± 0.00 | n.d. | n.d. | 0.002 ± 0.00 | n.d. | 0.3 ± 0.01 |
| 15 | Linalool | n.d. | n.d. | n.d. | n.d. | n.d. | 0.001 ± 0.00 |
| 16 | Pinone | 0.01 ± 0.00 | 0.001 ± 0.00 | n.d. | 0.002 ± 0.00 | n.d. | 0.0005 ± 0.00 |
| 17 | Camphor | n.d. | n.d. | n.d. | n.d. | 0.005 ± 0.00 | 0.003 ± 0.00 |
| 18 | 5-undecen-3-yne, (E)- | 1.2 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 19 | Menthofuran | n.d. | n.d. | 0.001 ± 0.00 | 0.002 ± 0.00 | 0.002 ± 0.00 | 0.001 ± 0.00 |
| 20 | Isomenthone | 0.002 ± 0.00 | 0.008 ± 0.00 | 0.001 ± 0.00 | n.d. | n.d. | n.d. |
| 21 | Isomenthol | n.d. | n.d. | 0.01 ± 0.00 | n.d. | n.d. | 0.001 ± 0.00 |
| 22 | Borneol | n.d. | 0.004 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 23 | Menthol | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 24 | Terpinen-4-ol | n.d. | 0.04 ± 0.00 | n.d. | n.d. | 0.005 ± 0.00 | 0.001 ± 0.00 |
| 25 | α-terpineol | n.d. | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.01 ± 0.00 | n.d. | 0.002 ± 0.00 |
| 26 | Estragole | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. | 0.3 ± 0.02 |
| 27 | Verbenone | n.d. | n.d. | n.d. | n.d. | 0.003 ± 0.00 | 0.002 ± 0.00 |
| 28 | Anethol | n.d. | n.d. | n.d. | n.d. | n.d. | 96.8 ± 1.80 |
| 29 | β-caryophyllene | 0.4 ± 0.02 | 0.1 ± 0.00 | 0.1 ± 0.01 | n.d. | n.d. | n.d. |
| 30 | Bergamottin | n.d. | 0.2 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 31 | α-caryophyllene | 0.04 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 32 | β-eudesmene | 9.8 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 33 | Eremophilane | 1.2 ± 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 34 | Bisabolene | n.d. | 0.2 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 35 | Isoeugenol | 0.05 ± 0.00 | n.d. | 0.03 ± 0.00 | n.d. | n.d. | n.d. |
| 36 | 1-(2,4-Dimethylphenyl)propan-1-one | 2.5 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 37 | Allyl phenoxyacetate | 8.2 ± 0.40 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 38 | 2-(2-propenyl)-furan | 1.9 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpenes | 74.6 | 99.4 | 99.8 | 100 | 100 | 2.6 | |
| Oxygenated monoterpenes | n.d. | n.d. | n.d. | n.d. | n.d. | 0.4 | |
| Alcohols | n.d. | 0.1 | 0.1 | n.d. | n.d. | n.d. | |
| Phenylpropanoids | n.d. | n.d. | n.d. | n.d. | n.d. | 97.0 | |
| Esthers | 8.2 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Sesquiterpene hydrocarbons | 11.5 | 0.4 | 0.1 | n.d. | n.d. | n.d. | |
| Phenols | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Others | 5.6 | 0.2 | n.d. | n.d. | n.d. | n.d. |
n.d. = not detected.
Apium EO showed the limonene as main compound accounting more than 70% followed by β-eudesmene; similar composition was noticed by Zorga et al. [15]. Allyl phenoxyacetate was identified as a third compound; it was recently found in celery leaves EO by Stan et al. [16].
As expected, citrus samples reported limonene as the main compound. Its content ranged from 87 to 99%. The lowest content was detected in citrus lemon sample; all the other ones showed a content higher than 99%. β-pinene and γ-terpinene were the second and third compounds, respectively, in citrus lemon EO (6%). Similar results were reported by Singh et al. [17]. All Citrus samples, except orange essential oil, did not comply with the ISO standards; this could be justified with the provenience of the raw material (Turkey) as reported by Singh et al. [17].
F. vulgare EO reported high amount of anethol that was about 97% of total compounds. Other compounds were limonene and α-pinene; fenchone and estragole were also detected in small amounts. These results agreed with those reported by Ferioli and co-workers [18] in sweet fennel.
3.2. Antioxidant Activity of the Essential Oils
The antioxidant activity of the essential oils was evaluated by two different assays such as DPPH and ABTS. Table 4 shows the results of DPPH assay.
Table 4.
DPPH Free Radical-Scavenging Capacity of different essential oils from plants and fruits.
| Essential Oils | IC50 DPPH (mg/mL) |
|---|---|
| Aromatic plants EOs | |
| Cinnamomum cassia | 0.05 |
| Cinnamomum zeylanicum | 0.01 |
| Mentha piperita | 22.98 |
| Origanum vulgare | 0.29 |
| Rosmarinus officinalis | 11.19 |
| Salvia lavandulifolia | 41.82 |
| Thymus vulgaris | 0.31 |
| Fruit and vegetables EOs | |
| Apium graveolens | 25.89 |
| Citrus limon | 49.06 |
| Citrus paradisi | 87.67 |
| Citrus reticulata | 38.79 |
| Citrus sinensis | 47.30 |
| Foeniculum vulgare | 105.32 |
| Ascorbic acid | 0.003 |
Aromatic plants EOs. Cinnamon EOs have demonstrated to have the strongest antioxidant activity between the others and the minor differences with ascorbic acid. From them, C. zeylanicum leaf EO is slightly better with an IC50 five times lower than C. cassia bark EO.
Comparing with other authors, this C. zeylanicum leaf EO has higher free radical-scavenging than the EOs from Cinnamomum leaves as C. tamala (IC50: 1.65 mg/mL) [19], C. griffithii (IC50: 0.082 mg/mL), C. macrocarpum (IC50: 0.099 mg/mL) [20], and C. malabathrum (IC50: 1.7 mg/mL) [21]. These differences are due to their content in eugenol, as they only reached EOs with a content between 38.5–52% [19,20], while ours had 95.2% (Table 4). The same happens with the EO reported by Srirmavaratharajan et al. [22] from C. wightii leaves that contain 72.6–85.9% of benzyl benzoate as major compound, causing them to have an IC50 of 3.49 mg/mL, much higher than the reported in this work. Moreover, it has 23 times higher antioxidant activity than other commercial EO from the same Cinnamomum specie leaves (IC50: 0.23 mg/mL) that had 48.8% of eugenol [23]. Therefore, the antioxidant activity of Cinamomum leaf EO can be attributed to the content in eugenol.
C. cassia bark EO has reported similar IC50 to other authors in other varieties of Cinnamomum bark EOs, such as C. altissium (IC50: 0.04 mg/mL) [24] and C. griffithii (IC50: 0.07 mg/mL) [20], and lower than others (IC50: 0.10 mg/mL) [20,23], although in all cases the major compounds are different. It is remarkable that they did not name the compound cinnamaldehyde, which is the major compound reported by several studies [25], and it is in concordance with this work in which it has been found in an amount of 91.9%.
Following them, O. vulgare flower/leaf and T. vulgaris EOs have also demonstrated high antioxidant activity compared with the others, occupying the third and fourth positions, respectively. The IC50 obtained for the O. vulgare EO with major compound carvacrol (84.5%) is in concordance with other authors in EOs obtained by hydrodistillation as Hamada et al. [26] with an IC50: 0.30 mg/mL (48.4% of carvacrol), and Sokmen et al. [27] who reported IC50: 0.31 mg/mL (64.3% of carvacrol). Boskovic et al. [28] also agreed with us, reporting an IC50 of 0.33 mg/mL for the O. vulgare EO obtained from a Serbian company.
T. vulgaris EO has demonstrated slightly lower antioxidant activity than those obtained by hydrodistillation (IC50: 0.159–0.243 mg/mL) [29,30] maybe as the differences in composition. They reported thymol as major compound at concentrations of 36.5–55.3% followed by carvacrol 28.7–29.8% and p-cymene 10–11.2%. In this case, we have found thymol 61.2%, p-cymene 16.7%, and carvacrol 2.6%, so the difference can be attributed to the reduced content in carvacrol compared with them. However, it is in the range of values reported by Boskovic et al. [28] (IC50: 0.48 mg/mL) and Aazza et al. [31] (IC50: 0.26 mg/mL) in commercial ones from Serbia and Morocco, respectively.
After those four, the EOs with higher antioxidant activity are from R. officinalis leaf and M. piperita leaf. R. officinalis leaf EO has demonstrated less effectivity than those obtained by other authors that have reported IC50 from 0.52 to 3.48 mg/mL [32,33,34,35,36]. However, in contrast, the scavenging activity is better than the reported by Risaliti et al. [37] who obtained 25% of inhibition with 4.23 mg/mL of R. officinalis EO from a Greek company (our EO would need 3.77 mg/mL for this 25% of inhibition). They found lower content in β-myrcene (0.9% in front of 30.75%), camphor (11.7% in front of 20.7%), and β-pinene (8.3% in front of 11.9%), despite the fact that its content in eucalyptol is higher (48.7% in front of 14.8%). This seems to indicate that the minor compounds also contribute to the reducing power.
The antioxidant activity shown by M. piperita EO is in concordance with that reported by Wu et al. [38] (IC50: 22.77 mg/mL) in an USA commercial sample, and higher than that reported by Fatemi et al. [39] (IC50: 25.80 mg/mL) in an EO obtained by hydro-distillation, and Stanojevic et al. [40] (IC50: 58.41 mg/mL) in a EO from a Serbian company. All of them had very similar composition being the major compounds menthol (38.4–52.4%), menthone (13.8–24.9%), menthyl acetate (3.9–6.5%), and eucalyptol (3.9–5.6%), which is in concordance with us (menthol 39.7%, menthone 29.8%, menthyl acetate 6.1%, and eucalyptol 4.9%).
Following them is S. lavandulifolia EO. It has demonstrated higher IC50 than other S. lavandulifolia leaves EO obtained by hydro-distillation (IC50: 0.97–8.31 mg/mL) [41,42]. This lower antioxidant activity can be attributed to the inversion in amount in the major compounds, namely camphor (20.3–33.6%) > eucalyptol (15.0–22.2%) > α-thujene (14.9–21.4%) in front of eucalyptol > camphor > camphene that are found in our S. lavandulifolia EO. Risaliti et al. [37] who used Salvia triloba leaves EO from a Greek company achieved a 25% of inhibition with 4.47 mg/mL and ours could achieve that with 14.5 mg/mL, although the compositions are very similar (S. triloba eucalyptol (46.68%) > camphor (10.5%) > α-pinene (8.5%) > camphene (6.7%) > β-pinene (6.7%), and S. lavandulifolia eucalyptol (38.6%) > camphor (23.6%) > camphene (7.7%) > α-pinene (5.5%) > β-pinene (5.1%)). However, it seems to have better antioxidant activity than other EOs from other varieties, such as Salvia kiangsiensis of which, according with Fang et al. [43], 10 mg/mL are needed to have 4.3% of inhibition, meanwhile with ours only 1.5 mg/mL would be necessary, and the compositions are totally different.
Fruit and vegetables EOs.A. graveolens seed EO is the vegetable EO that has shown higher antioxidant activity. However, its activity is lower than that reported by Hassanen et al. [44] who, with 0.9 mg/mL of A. graveolens seed EO hydro-distillated, achieved 74.3% of inhibition. With this concentration of our EO, we could reach only 35% of inhibition. Although the compositions are similar, they obtained an EO with higher amount of β-selinene (27.0% in front of 9.8%) which could be the responsible of this increment in the antioxidant activity.
Taking into account the citrus EOs, the order according with their antioxidant strength is C. reticulata > C. sinensis > C. paradisii. They have very similar composition with D-limonene as major compound counting for 99% in all cases. However, the difference in the reducing power between them could be due to its content in the second major compound β-myrcene that counts for 0.40, 0.36, and 0.31%, respectively. Comparing with Kamal et al. [45], they obtained 24.1, 18.5, and 14.0% of inhibition with 0.1 mg/mL of C. reticulata, C. sinensis, and C. paradisii EOs, respectively, which corroborate the order and differences between them found in this study. The values of IC50 obtained for C. sinensis and C. paradisii EOs are in the range of the values reported by Phi et al. [46] (28.5–63.43 and 45.7–86.3 mg/mL, respectively). If comparing our C. sinensis peel EO with C. sinensis leaves EO, the studies revealed that those that come from leaves have much higher antioxidant activities with IC50 between 0.75–1.49 mg/mL [47]. This is mainly attributed to the composition that consists of 16.9% β-pinene, 13.8% D-limonene, and 7.5% of β-ocimene as major compounds. In this group of essential oils, it can be appreciated that the antioxidant activity can clearly be attributed to the limonene content, but also to the content in other monoterpenoids. For the C. paradisii peel EO, although the composition reported by Kaanin-Boudraa et al. [48] and Ou et al. [49] are very similar to that reported here, they obtained an IC50 of 40 mg/mL, amounting to half of our total. Another Citrus EO, C. limon peel EO, has been evaluated apart from the rest due to its different composition found (Table 3). Compared with the others, it has lower D-limonene (87.0%), β-pinene (6.0%), and γ-terpineno. Ben Miri et al. [50] found that C. sinensis EO had the double antioxidant activity than C. limon, while in this study, both EOs have demonstrated similar IC50. However, the antioxidant activity found is in concordance with Guo et al. [51], who reported 32.8% of inhibition with 30 mg/mL. Moreover, it has been demonstrated that C. limon peel EO has lower antioxidant activity than C. limon leaves EO according with Fancello et al. [52] who reported an IC50 of 11.9 mg/mL.
The EO which has shown least antioxidant activity between all tested is F. vulgare var. dulce EO. The result obtained is very far from those reported by Kalleli et al. [53] from Tunisian (IC50: 0.20–0.49 mg/mL) and French (IC50: 0.59–0.63 mg/mL) F. vulgare seeds EOs. However, it is closer to the values reported by Ahmed et al. [54] for Chinese samples (IC50: 15.66 mg/mL) and totally in concordance with the Egyptian ones (IC50: 141.82 mg/mL). According to them, the Tunisian and Chinese ones agree with us about the major compound, anethole (54.3–78.3%) but they revealed higher amounts of estragole (17.1–20.2%), L-fenchone (7.4–12.1%), and D-limonene (2.4–4.7%). Otherwise, the French one had as major compound estragole (44.7–88.9%), with lower amounts of anethole (14.0–36.3%). These compositional differences could be responsible of the changes in the antioxidant activity.
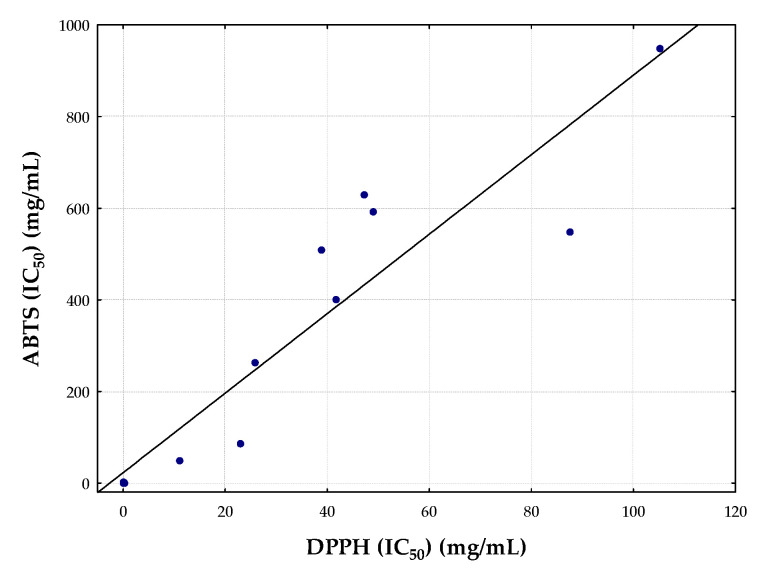
Correlation DPPH vs. ABTS. All the exposed data obtained by the DPPH technique is in concordance with the results obtained with the ABTS technique. As shown in Figure 1, there was significant correlation between them (r = 0.9291, r2 = 0.8681, and p < 0.001), which indicated that both techniques could be used to investigate the antioxidant activity of plants and fruit and vegetables EOs although they have not been found enough references to compare.
Figure 1.
Correlation between the IC50values obtained by DPPH and ABTS assays in aromatic plants and fruits and vegetables EOs.
3.3. Antimicrobial Activity against Bacteria and C. albicans
Of 13 essential oils tested, 11 showed inhibitory activity against one or more bacteria or C. albicans (Table 5). Those essential oils that showed diameters of the zones of inhibition higher than or equal to 28 mm (8 mm well-diameter included) were considered to have a strong inhibitory effect, between 16 and 28 mm as moderately active inhibitors, between 12 and 16 mm as mild inhibitors, and less than 12 mm as no or low inhibitors [55]. Thus, Eos obtained from C. cassia, T. vulgaris, and O. vulgare proved to be strong inhibitors against most of the bacterial species tested, both Gram-negative and Gram-positive, and the yeast C. albicans.
Table 5.
Measure of inhibition zone diameters (mm) for essential oils against bacterial strains.
| Essential Oils | S. aureus | MRSA | E. coli | S. Typhimurium | L. monocytogenes | C. albicans |
|---|---|---|---|---|---|---|
| Thymus vulgaris | 33.0 ± 1.0 | 33.0 ± 2.0 | 38.0 ± 3.6 | 41.0 ± 1.0 | 35.3 ± 5.0 | 58.0 ± 2.6 |
| Origanum vulgare | 28.7 ± 5.5 | 30.7 ± 3.8 | 33.3 ± 4.2 | 35.7 ± 1.2 | 31.7 ± 2.9 | 50.7 ± 1.2 |
| Rosmarinus officinalis | 10.7 ± 1.2 | 10.0 ± 0.0 | 11.0 ± 1.7 | 12.7 ± 1.2 | N | 18.7 ± 2.3 |
| Apium graveolens | 12.0 ± 2.6 | 11.3 ± 0.6 | N | 13.0 ± 1.0 | 11.7 ± 1.5 | 20.7 ± 1.2 |
| Salvia lavandulifolia | 10.3 ± 0.6 | 10.3 ± 0.6 | 10.7 ± 0.6 | 13.3 ± 0.6 | N | 25.0 ± 3.0 |
| Cinnamomum zeylanicum | 18.3 ± 2.3 | 18.3 ± 1.2 | 21.3 ± 1.2 | 21.7 ± 1.2 | 14.3 ± 0.6 | 34.7 ± 0.6 |
| Cinnamomum cassia | 36.0 ± 3.5 | 34.7 ± 2.3 | 29.0 ± 4.6 | 28.0 ± 1.7 | 28.0 ± 2.0 | 55.7 ± 3.5 |
| Citrus sinensis | N | N | N | N | N | N |
| Citrus reticulata | 10.3 ± 0.6 | 10.0 ± 0.0 | N | 14.0 ± 1.0 | 10.3 ± 0.6 | 22.0 ± 1.7 |
| Citrus limon | N | N | N | N | N | N |
| Citrus paradise | N | N | N | N | N | N |
| Foeniculum vulgare | N | N | N | 12.0 ± 2.0 | N | 12.7 ± 2.5 |
| Mentha piperita | 11.0 ± 0.0 | 11.0 ± 1.0 | 13.3 ± 1.5 | 22.3 ± 2.5 | N | 40.3 ± 4.0 |
| AA | 15.3 ± 0.6 | 17.3 ± 0.6 | 32.3 ± 4.0 | 30.7 ± 1.2 | 19.0 ± 1.0 | 25.7 ± 1.2 |
Results are presented by mean values from three experiments ± standard deviations. S. aureus: Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; E. coli: Escherichia coli; S. Typhimurium: Salmonella enterica serovar Typhimurium; L. monocytogenes: Listeria monocytogenes; C. albicans: Candida albicans; N: no inhibition zone; AA: antimicrobial agents (ciprofloxacin 2 mg/mL for MRSA, 0.1 mg/mL for L. monocytogenes, 0.01 mg/mL for the rest of bacteria, and ketoconazole 0.01 mg/mL for C. albicans).
Cassia bark essential oil was the most effective oil against all the strains tested showing zones of inhibition that ranged between 28.0 and 55.7 mm of diameters and higher than inhibition zones of the antimicrobial agents. Previous research is consistent with our results as essential oils of C. cassia have shown potent antimicrobial activity against L. monocytogenes, E.coli, S. Typhimurium [56], Candida glabrata, and C. albicans [57]. Firmino et al. [58] showed that essential oils extracted from cassia bark, as well as its main component, trans-cinnamaldehyde, in a concentration range of 0.25 to 0.50 mg/mL, inhibited the growth of the planktonic forms of S. aureus and E. coli, and reduced biomass in biofilms of both bacteria by more than 99.9%. Trans-Cinnamaldehyde is an unsaturated aldehyde that possesses an acrolein group (α,β- unsaturated carbonyl moiety) which is essential for antimicrobial activity [59]. Trans-Cinnamaldehyde has been shown to possess substantial antimicrobial activity against Gram-positive and Gram-negative bacteria, including L. monocytogenes, S. aureus, S. Typhimurium, E. coli, and Pseudomonas aeruginosa [56,60,61,62]. At sublethal concentrations, this compound is capable of inhibiting cell division by acting on the FtsZ protein, but at higher concentrations, it has a bactericidal action as it affects the integrity of bacterial membranes [63]. The antimicrobial activity of the essential oils of cassia observed in this work is attributable to trans-cinnamaldehyde, which represented 91.9% of the extract.
Of particular interest is the strong antibacterial activity of the essential oils of thyme and oregano EOs, herbs used frequently in gastronomy, against all the bacterial strains tested, as most of them have been implicated as causal agents of foodborne disease outbreaks and food quality degradation [64,65]. This strong antimicrobial activity is in concordance with other studies. Silva et al. [66] reported that thyme and oregano EOs showed significant antibacterial activity against L. monocytogenes, S. Typhimurium, S. aureus, and E. coli, and Bozin et al. [67] found a strong antibacterial activity of oregano and thyme EOs, even on multiresistant strains of E. coli, S. Typhimurium, and S. aureus. Our results showed inhibition diameters similar or higher than those of the ciprofloxacin against the tested bacterial strains, and higher than those of ketoconazole diameters against C. albicans.
The efficacy of these EOs can be attributed to the activity of phenolic compounds carvacrol and thymol, the major compounds of oregano and thyme essential oils, respectively. In this work, oregano EO was mainly composed of carvacrol (84.52%) and thymol (1.62%), whereas thyme oil contained 61.21% of thymol and 2.58% of carvacrol. Carvacrol has been reported to be active against C. albicans, L. monocytogenes, E. coli, S. Typhimurium, S. aureus, Shigella sonnei, and Shigella flexneri [56,68,69,70,71] and thymol has shown activity against E. coli, S. Typhimurium, S. aureus, L. monocytogenes, S. sonnei, S. flexneri, and Bacillus cereus [56,70,71,72]. Thymol is structurally analogous to carvacrol, but have a free hydroxyl group, the radical essential for antimicrobial activity [69], at a different location on the phenolic ring. Both compounds interact with the cell membrane, making it permeable due to the introduction of lipophilic group into the ordered structure of the lipid bilayer [63,73].
The essential oil obtained from C. zeylanicum leaf showed moderate inhibition against bacterial strains with inhibition zones between 14.3 and 21.7 mm, and strong inhibition for C. albicans. Regarding antimicrobial agents, this essential oil showed inhibition zones similar to ciprofloxacin against bacterial strains, but higher than those of ketoconazole against C. albicans. In agreement with our results, Ebani et al. [74], Prabuseenivasan et al. [75], and Brnawi et al. [76] reported antibacterial activity of cinnamon oil against several Gram-positive and Gram-negative bacteria. The antimicrobial activity of cinnamon oil can be ascribed to eugenol, a phenylpropene that was found in this work at 95.23%. This compound alters the membrane and the transport of ions and ATP and modifies the fatty acid profile [63]. Eugenol is active against foodborne pathogens such as E. coli, L. monocytogenes, S. aureus, S. Typhimurium, Bacillus subtilis, and B. cereus [69,77,78].
On the contrary to most of the EOs, essential oils obtained from citric fruits exhibited low or no inhibition against all tested bacteria, except the mandarin essential oil that mildly inhibited S. Typhimurium with inhibition zones about 14 mm and moderately to C. albicans with inhibition zones of 22 mm. D-limonene is one of the major compounds of the citrus essential oils, a monoterpene whose antimicrobial activity depends on the alkyl group [63]. In this work, it was found as a major compound in lemon, mandarin, sweet orange, and pink grapefruit EOs (87.0, 99.0, 99.2, and 99.0, respectively). In addition to limonene, β-pinene, and γ-terpinene, biological precursors of phenolic compounds, were found in lemon at 6.0 y 3.8%, respectively. Some terpenes do not possess high antimicrobial activity when they are used as a single compound. Such is the case of p-cymene, one of the most important components of thyme essential oil, which did not show antimicrobial activity against E. coli, S. sonnei, and S. flexneri using the agar well diffusion assay [71]. Similarly, 21 terpenoids such as limonene, α-pinene, β-pinene, γ-terpinene δ-3-carene, (+)-sabinene, and α-terpinene showed low inhibition of the bacterial growth, whereas all essential oils exhibited considerable inhibitory effects against 25 different genera of bacteria, including plant pathogens, food poisoning, and spoilage bacteria [79], suggesting that a combination of bioactive compounds in a suitable proportion, is needed to achieve a high and effective overall activity.
The minimal inhibitory concentration (MIC) of essential oils against bacteria and C. albicans was determined by the agar dilution method and was expressed in mg/mL (Table 6). MIC values obtained are consistent with previous diameters of inhibition zones. The essential oil obtained from C. cassia bark showed the lowest MIC values between 0.14 and 0.28 mg/mL for bacteria, and < 0.14 mg/mL for C. albicans. Similarly, cinnamon, thyme and oregano essential oils showed great MIC values for all tested strains showing MIC values between 0.28 and 2.25 mg/mL. A moderate effect was observed with the rosemary, celery, sage, and fennel EOs that showed MIC values between 1.125 and 4.5 mg/mL, and the citric fruits essential oils were the less effective EOs with MIC values from 18 to 72 mg/mL for bacteria, and between 4.5 and 18 mg/mL for C. albicans.
Table 6.
Minimum Inhibitory Concentration MIC (mg/mL) for essential oils against bacterial strains.
| Essential Oils | S. aureus | MRSA | E. coli | S. Typhimurium | L. monocytogenes | C. albicans |
|---|---|---|---|---|---|---|
| Thymus vulgaris | 2.25 | 1.125 | 1.125 | 1.125 | 1.125 | 0.56 |
| Origanum vulgare | 1.125 | 1.125 | 1.125 | 0.56 | 1.125 | 0.56 |
| Rosmarinus officinalis | 36 | 36 | 18 | 9 | 18 | 4.5 |
| Apium graveolens | 4.5 | 4.5 | 36 | 9 | 4.5 | 1.125 |
| Salvia lavandulifolia | 9 | 9 | 9 | 4.5 | 4.5 | 2.25 |
| Cinnamomum zeylanicum | 2.25 | 1.125 | 1.125 | 1.125 | 2.25 | 0.28 |
| Cinnamomum cassia | 0.28 | 0.28 | 0.28 | 0.28 | 0.14 | <0.14 |
| Citrus sinensis | 72 | 72 | 36 | 36 | 36 | 18 |
| Citrus reticulata | 36 | 36 | 36 | 36 | 18 | 4.5 |
| Citrus limon | 72 | 72 | 72 | 36 | 36 | 18 |
| Citrus paradise | 72 | 72 | 72 | 36 | 72 | 9 |
| Foeniculum vulgare | 36 | 72 | 36 | 4.5 | 36 | 2.25 |
| Mentha piperita | 4.5 | 2.25 | 2.25 | 1.125 | 4.5 | 1.125 |
S. aureus: Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; E. coli: Escherichia coli; S. Typhimurium: Salmonella enterica serovar Typhimurium; L. monocytogenes: Listeria monocytogenes; and C. albicans: Candida albicans.
3.4. Antifungal Activity of Essential Oils against B. cinerea
Antifungal properties of the thirteen essential oils were assessed against B. cinerea by agar well-diffusion and agar dilution methods. The percentage of inhibition of mycelial growth and the MIC values were determined after 7 days of incubation (Table 7). Among all the essential oils tested, oregano and thyme EOs showed the highest inhibition (98.5 and 98.2%, respectively), followed by fennel and mint EOs that inhibited 93.8 and 93.1% of the mycelia growth, respectively. Mild inhibition was observed by celery EO (48.8%) and low or no inhibition by EOs obtained from citric fruits (3.4 to 18.4%). Antimicrobial control with ketoconazole inhibited completely the mycelial growth. The minimal inhibitory concentration values ranged from 0.07 to 9 mg/mL. Although thyme and oregano EOs showed the highest mycelial reduction, cassia EO showed the lowest MIC value (0.14 mg/mL) followed by mint, oregano, fennel, thyme, cinnamon, and sage EOs that inhibited the fungus by concentrations of 0.56 to 2.25 mg/mL. Rosemary, celery, orange, lemon, mandarin, and pink grapefruit EOs showed MIC values between 4.5 and 9 mg/mL.
Table 7.
Percentage of mycelium inhibition of B. cinerea and minimal inhibitory concentration (MIC) of essential oils.
| Essential Oils | Mycelium Inhibition (%) | MIC (mg/mL) |
|---|---|---|
| Thymus vulgaris | 98.2 | 1.125 |
| Origanum vulgare | 98.5 | 0.56 |
| Rosmarinus officinalis | 79.0 | 9 |
| Apium graveolens | 48.8 | 9 |
| Salvia lavandulifolia | 79.8 | 2.25 |
| Cinnamomum zeylanicum | 70.4 | 1.125 |
| Cinnamomum cassia | 81.5 | 0.14 |
| Citrus sinensis | 3.4 | 9 |
| Citrus reticulata | 3.6 | 9 |
| Citrus limon | 9.7 | 4.5 |
| Citrus paradise | 18.4 | 9 |
| Foeniculum vulgare | 93.1 | 1.125 |
| Mentha piperita | 93.8 | 0.56 |
Previous research is in concordance with our results. The cassia and oregano EOs completely inhibited the mycelial growth of B. cinerea at 0.5 mg/mL, and concentrations of 250 mg/mL thymol and 300 mg/mL carvacrol inhibited completely its spore germination [80]. Additionally, the growth of B. cinerea was completely inhibited by cassia [81], oregano, and rosemary essential oils [82]. Other research reported the antifungal activity of carvacrol, the main compound of oregano EO, and eugenol, the main compound of cinnamon EO, against B. cinerea [69]. At 500 ppm, cinnamon EOs completely inhibited the growth of B. cinerea from 72 h of contact with the EOs, whereas thyme EO achieved the same from 120 h [83]. According to our results, Palfi et al. [84] reported inhibition of the mycelial growth of B. cinerea in the presence of thyme, fennel, peppermint, rosemary, and sage essential oils, whereas lemon oil lacked inhibitory activity.
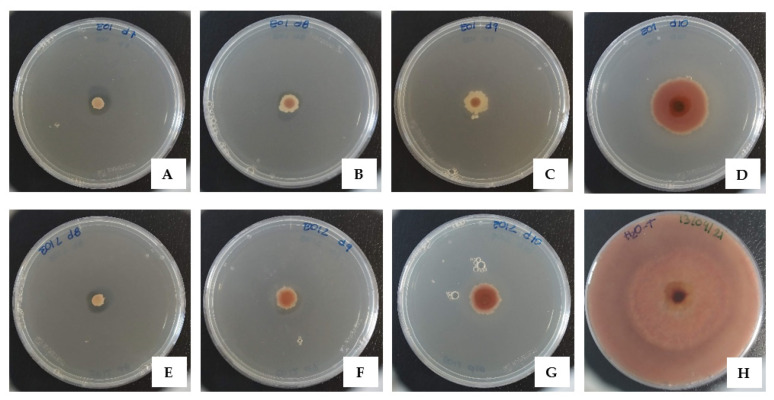
In addition to the dose-dependent effect observed in the MIC assay, some EOs inhibited the fungus by more than 90% at concentrations much lower than their minimum inhibitory concentration. Such is the case of the fennel EO that inhibited 95% of the fungus at a concentration as low as 0.07 mg/mL and thyme EO that inhibited the mycelia growth in 97.6% at 0.14 mg/mL, a concentration 8 times lower than its MIC value (Figure 2). Similarly, only 0.56 mg/mL of the celery EO inhibited 90.2% of mycelia growth, 16 times lower than its MIC value. This result is comparable with those of other researchers, such as the case of Grul’ová et al. [85] that reported complete inhibition of B. cinerea with 500 ppm oregano EO, and more than 80% with 100 ppm of oil incorporated into Potato Dextrose Agar.
Figure 2.
Inhibition of B. cinerea mycelium growth by thyme EO at 0.56 mg/mL (A), 0.28 mg/mL (B), 0.14 mg/mL (C), 0.07 mg/mL (D); and fennel EO at 0.28 mg/mL (E), 0.14 mg/mL (F), and 0.07 mg/mL (G). Control of mycelium growth (H) after 7 days of incubation at 25 °C.
B. cinerea is a phytopathogen that causes the grey mould disease in more than 200 crop species worldwide such as grapes, cucumbers, tomatoes, strawberries, and leading to vast economic losses due to the severe damage in pre-and post-harvest [86,87]. Control strategies are carried out including chemical control, resistance induction and biological control. Benzimidazoles, dicarboximides, phenylpyrroles, aromatic hydrocarbons, and phenylcarbamates are the main chemical fungicides used to control it [88]. In addition to the toxicological risk presented by their residues, B. cinerea has developed resistance to most of these substances [89,90,91]. Essential oils have a significant interest as an alternative to chemical treatments as they are bio-sourced products and therefore more ecological [92].
Essential oils can act on fungus via inhibition of sporulation or producing cell damage [93]. Hydrophobic character enables EOs to break through lipids of cell membranes and mitochondria increasing fungal membranes permeability [92]. The changes in the fluidity may leak electrolytes or cellular contents resulting in protein metabolism alteration and calcium ion concentration [93]. Moreover, permeabilization of out and inner mitochondrial membrane leads to cell death by apoptosis and necrosis [94].
Although most of the antimicrobial activities of essential oils have been attributed to their major components, the total antimicrobial effect is the result of the synergism of all their components [95]. Therefore, the antimicrobial activity is not related to a single mechanism of action, as the essential oils have different bioactive compounds, and each one of them has different structural groups in their composition [96].
4. Conclusions
EOs from herbs and spices showed the highest antioxidant activity. Essential oils obtained from aromatic plants showed higher antibacterial and antifungal activity than those obtained from citric fruits. The most effective essential oils were those of C. cassia, T. vulgaris, and O. vulgare. Although fennel essential oil reported the lowest antioxidant activity, it showed very good antimicrobial activity against B. cinerea, one of the post-harvest pathogen microorganisms in fruits and vegetables, thus it could be considered in active packaging production or food coating. The strong antimicrobial activity of essential oils and the broad spectrum they showed provide evidence that they may be used for prolonging the shelf life of food products, developing functional foods, and for protecting plants and crops.
Acknowledgments
Vito Verardo thanks the Spanish Ministry of Economy and Competitiveness (MINECO) for “Ramon y Cajal” contract (RYC-2015-18795).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10111091/s1, Figure S1: GS-MS chromatograms of fruit and vegetables, and aromatic herbs and spices essential oils.
Author Contributions
Conceptualization, V.V. and A.M.G.-C.; formal analysis, M.d.C.R.-D., S.D.-M.-P. and V.V.; investigation, M.d.C.R.-D., S.D.-M.-P. and V.V.; data curation, E.J.G.-H., M.J.-V. and A.R.-B.; writing—original draft preparation, M.d.C.R.-D., S.D.-M.-P. and V.V.; writing—review and editing, M.J.-V., A.R.-B., B.G.-V. and V.V.; supervision, V.V.; and funding acquisition, V.V. ands A.M.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the project SHEALTHY that has received funding from European Union’s Horizon 2020 research and innovation programme under grant agreement No 817936.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maurya A., Prasad J., Das S., Dwivedy A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021;5:133. doi: 10.3389/fsufs.2021.653420. [DOI] [Google Scholar]
- 2.Amorati R., Foti M.C., Valgimigli L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S., Barkauskaite S., Jaiswal A.K., Jaiswal S. Essential oils as additives in active food packaging. Food Chem. 2021;343:128403. doi: 10.1016/j.foodchem.2020.128403. [DOI] [PubMed] [Google Scholar]
- 4.Do Nascimento L.D., de Moraes A.A.B., da Costa K.S., Galúcio J.M.P., Taube P.S., Costa C.M.L., Cruz J.N., de Aguiar Andrade E.H., de Faria L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules. 2020;10:988. doi: 10.3390/biom10070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vianna T.C., Marinho C.O., Marangoni Júnior L., Ibrahim S.A., Vieira R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021;182:1803–1819. doi: 10.1016/j.ijbiomac.2021.05.170. [DOI] [PubMed] [Google Scholar]
- 6.del Carmen Razola-Díaz M., Guerra-Hernández E.J., García-Villanova B., Verardo V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021;354:129575. doi: 10.1016/j.foodchem.2021.129575. [DOI] [PubMed] [Google Scholar]
- 7.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free redical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 8.Parejo I., Codina C., Petrakis C., Kefalas P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH·(2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods. 2000;44:507–512. doi: 10.1016/S1056-8719(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 9.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 10.Hayes A.J., Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 2002;40:535–543. doi: 10.1016/S0278-6915(01)00103-X. [DOI] [PubMed] [Google Scholar]
- 11.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Lajnef H., Ferioli F., Pasini F., Politowicz J., Khaldi A., D’Antuono L.F., Caboni M.F., Nasri N. Chemical composition and antioxidant activity of the volatile fraction extracted from air-dried fruits of Tunisian Eryngium maritimum L. ecotypes. J. Sci. Food Agric. 2018;98:635–643. doi: 10.1002/jsfa.8508. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-Santos R., Andrade M., Madella D., Martinazzo A.P., de Aquino Garcia Moura L., de Melo N.R., Sanches-Silva A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017;62:154–169. doi: 10.1016/j.tifs.2017.02.011. [DOI] [Google Scholar]
- 14.Porres-Martínez M., González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. Influence of phenological stage on chemical composition and antioxidant activity of Salvia lavandulifolia Vahl. essential oils. Ind. Crops Prod. 2014;53:71–77. doi: 10.1016/j.indcrop.2013.12.024. [DOI] [Google Scholar]
- 15.Zorga J., Kunicka-Styczynska A., Gruska R., Smigielski K. Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profile. Molecules. 2020;25:5322. doi: 10.3390/molecules25225322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stan M., Soran M.L., Varodi C., Lung I., Copolovici L., Mǎruţoiu C. Extraction and GC determination of volatile aroma compounds from extracts of three plant species of the Apiaceae family. AIP Conf. Proc. 2013;1565:75–78. [Google Scholar]
- 17.Singh B., Singh J.P., Kaur A., Yadav M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021;143:110231. doi: 10.1016/j.foodres.2021.110231. [DOI] [PubMed] [Google Scholar]
- 18.Ferioli F., Giambanelli E., D’Antuono L.F. Fennel (Foeniculum vulgare Mill. subsp. piperitum) florets, a traditional culinary spice in Italy: Evaluation of phenolics and volatiles in local populations, and comparison with the composition of other plant parts. J. Sci. Food Agric. 2017;97:5369–5380. doi: 10.1002/jsfa.8426. [DOI] [PubMed] [Google Scholar]
- 19.Heer A., Guleria S., Razdan V.K. Chemical composition, antioxidant and antimicrobial activities and characterization of bioactive compounds from essential oil of Cinnamomum tamala grown in north-western Himalaya. J. Plant Biochem. Biotechnol. 2017;26:191–198. doi: 10.1007/s13562-016-0381-7. [DOI] [Google Scholar]
- 20.Salleh W.M.N.H.W., Ahmad F., Yen K.H. Antioxidant and anticholinesterase activities of essential oils of cinnamomum griffithii and C. macrocarpum. Nat. Prod. Commun. 2015;10:1465–1468. doi: 10.1177/1934578X1501000838. [DOI] [PubMed] [Google Scholar]
- 21.Kumar B.H., Shani B. Haseena Antioxidant potential and antimicrobial activity of Cinnamomum malabathrum (Batka) Orient. J. Chem. 2010;26:1449–1453. [Google Scholar]
- 22.Sriramavaratharajan V., Murugan R. Chemical profile of leaf essential oil of cinnamomum walaiwarense and comparison of its antioxidant and hypoglycemic activities with the major constituent benzyl benzoate. Nat. Prod. Commun. 2018;13:779–782. doi: 10.1177/1934578X1801300633. [DOI] [Google Scholar]
- 23.Gogoi R., Sarma N., Loying R., Pandey S.K., Begum T., Lal M. A Comparative Analysis of Bark and Leaf Essential Oil and their Chemical Composition, Antioxidant, Anti-inflammatory, Antimicrobial Activities and Genotoxicity of North East Indian Cinnamomum zeylanicum Blume. Nat. Prod. J. 2021;11:74–84. [Google Scholar]
- 24.Abdelwahab S.I., Mariod A.A., Taha M.M.E., Zaman F.Q., Abdelmageed A.H.A., Khamis S., Sivasothy Y., Awang K. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae) Arab. J. Chem. 2017;10:131–135. doi: 10.1016/j.arabjc.2014.02.001. [DOI] [Google Scholar]
- 25.Foudah A.I., Shakeel F., Alqarni M.H., Ross S.A., Salkini M.A., Alam P. Simultaneous Estimation of Cinnamaldehyde and Eugenol in Essential Oils and Traditional and Ultrasound-Assisted Extracts of Different Species of Cinnamon Using a Sustainable/Green HPTLC Technique. Molecules. 2021;26:2054. doi: 10.3390/molecules26072054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamada H., Al-Waili N., Aboulghazi A., Abdellaoui A., Al-Waili T., Lyoussi B. Chemical composition and antioxidant content of Thymus vulgaris honey and Origanum vulgare essential oil; their effect on carbon tetrachlorideinduced toxicity. Vet. World. 2021;14:292–301. doi: 10.14202/vetworld.2021.292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokmen A., Abdel-Baki A.A.S., Al-Malki E.S., Al-Quraishy S., Abdel-Haleem H.M. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud Univ.-Sci. 2020;32:2377–2382. doi: 10.1016/j.jksus.2020.03.018. [DOI] [Google Scholar]
- 28.Boskovic M., Glisic M., Djordjevic J., Starcevic M., Glamoclija N., Djordjevic V., Baltic M.Z. Antioxidative Activity of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare) Essential Oils and Their Effect on Oxidative Stability of Minced Pork Packaged Under Vacuum and Modified Atmosphere. J. Food Sci. 2019;84:2467–2474. doi: 10.1111/1750-3841.14788. [DOI] [PubMed] [Google Scholar]
- 29.Sokmen A., Gulluce M., Akpulat H.A., Daferera D., Tepe B., Polissiou M., Sokmen M., Sahin F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004;15:627–634. doi: 10.1016/j.foodcont.2003.10.005. [DOI] [Google Scholar]
- 30.Gedikoğlu A., Sökmen M., Çivit A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019;7:1704–1714. doi: 10.1002/fsn3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aazza S., Lyoussi B., Miguel M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules. 2011;16:7672–7690. doi: 10.3390/molecules16097672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouyahya A., Et-Touys A., Bakri Y., Talbaui A., Fellah H., Abrini J., Dakka N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017;111:41–49. doi: 10.1016/j.micpath.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Chraibi M., Farah A., Elamin O., Iraqui H., Fikri-Benbrahim K. Characterization, antioxidant, antimycobacterial, antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020;11:25–29. doi: 10.4103/japtr.JAPTR_74_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leporini M., Bonesi M., Loizzo M.R., Passalacqua N.G., Tundis R. The essential oil of salvia rosmarinus spenn. From Italy as a source of health-promoting compounds: Chemical profile and antioxidant and cholinesterase inhibitory activity. Plants. 2020;9:798. doi: 10.3390/plants9060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakehal S., Chaouia C., Benrebiha F.Z. Antibacterial and Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Essential Oil Growing in Djelfa (Algeria) In: Kallel A., Ksibi M., BenDhia H., Khelifi N., editors. Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, Vols I and II. Springer International Publishing AG; Cham, Switzerland: 2018. pp. 1253–1254. [Google Scholar]
- 36.Bajalan I., Rouzbahani R., Pirbalouti A.G., Maggi F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind. Crops Prod. 2017;107:305–311. doi: 10.1016/j.indcrop.2017.05.063. [DOI] [Google Scholar]
- 37.Risaliti L., Kehagia A., Daoultzi E., Lazari D., Bergonzi M.C., Vergkizi-Nikolakaki S., Hadjipavlou-Litina D., Bilia A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In Vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019;51:493–498. doi: 10.1016/j.jddst.2019.03.034. [DOI] [Google Scholar]
- 38.Wu Z., Tan B., Liu Y., Dunn J., Martorell Guerola P., Tortajada M., Cao Z., Ji P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatemi F., Dini S., Rezaei M.B., Dadkhah A., Dabbagh R., Naij S. The effect of γ-irradiation on the chemical composition and antioxidant activities of peppermint essential oil and extract. J. Essent. Oil Res. 2014;26:97–104. doi: 10.1080/10412905.2013.871670. [DOI] [Google Scholar]
- 40.Stanojevic L.P., Stanojevic J.S., Savic V.L., Cvetkovic D.J., Kolarevic A., Marjanovic-Balaban Z., Nikolic L.B. Peppermint and Basil Essential Oils: Chemical Composition, in vitro Antioxidant Activity and in vivo Estimation of Skin Irritation. J. Essent. Oil-Bearing Plants. 2019;22:979–993. doi: 10.1080/0972060X.2019.1661793. [DOI] [Google Scholar]
- 41.El Jery A., Hasan M., Rashid M.M., Al Mesfer M.K., Danish M., Ben Rebah F. Phytochemical characterization, and antioxidant and antimicrobial activities of essential oil from leaves of the common sage Salvia officinalis L. from Abha, Saudi Arabia. Asian Biomed. 2020;14:261–270. doi: 10.1515/abm-2020-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Euch S.K., Hassine D.B., Cazaux S., Bouzouita N., Bouajila J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019;120:253–260. doi: 10.1016/j.sajb.2018.07.010. [DOI] [Google Scholar]
- 43.Fang S.P., Xing X., Lai P.X., Huang J.J. Chemical Composition and Antioxidant Activity of the Essential Oil from Salvia kiangsiensis. Chem. Nat. Compd. 2018;54:591–592. doi: 10.1007/s10600-018-2418-8. [DOI] [Google Scholar]
- 44.Hassanen N.H., Eissa A.M.F., Hafez S.A.M., Mosa E.A. Antioxidant and antimicrobial activity of celery (Apium graveolens) and coriander (Coriandrum sativum) herb and seed essential oils. Int. J. Curr. Microbiol. Appl. Sci. 2015;4:284–296. [Google Scholar]
- 45.Kamal G.M., Ashraf M.Y., Hussain A.I., Shahzadi A., Chughtai M.I. Antioxidant potential of peel essential oils of three Pakistani Citrus species: Citrus reticulata, Citrus sinensis, and Citrus paradisii. Pak. J. Bot. 2013;45:1449–1454. [Google Scholar]
- 46.Phi N.T.L., Van Hung P., Chi P.T.L., Dung N.H. Impact of Extraction Methods on Antioxidant and Antimicrobial Activities of Citrus Essential Oils. J. Essent. Oil Bear. Plants. 2015;18:806–817. doi: 10.1080/0972060X.2014.977565. [DOI] [Google Scholar]
- 47.Chi P.T.L., Van Hung P., Le Thanh H., Phi N.T.L. Valorization of Citrus Leaves: Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oils. Waste Biomass Valorization. 2020;11:4849–4857. doi: 10.1007/s12649-019-00815-6. [DOI] [Google Scholar]
- 48.Kaanin-Boudraa G., Brahmi F., Wrona M., Nerín C., Hadjal S., Madani K., Boulekbache-Makhlouf L. Citrus × paradisi essential oil as a promising agent for margarine storage stability: Composition and antioxidant capacity. J. Food Process. Preserv. 2021;45:e15374. doi: 10.1111/jfpp.15374. [DOI] [Google Scholar]
- 49.Ou M.C., Liu Y.H., Sun Y.W., Chan C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid.-Based Complement. Altern. Med. 2015;2015:804091. doi: 10.1155/2015/804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben Miri Y., Arino A., Djenane D. Study of Antifungal, Anti-aflatoxigenic, Antioxidant Activity and Phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia Essential oils. J. Essent. Oil-Bearing Plants. 2018;21:345–361. doi: 10.1080/0972060X.2018.1456363. [DOI] [Google Scholar]
- 51.Guo J.J., Gao Z.P., Xia J.L., Ritenour M.A., Li G.Y., Shan Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. Lwt. 2018;97:825–839. doi: 10.1016/j.lwt.2018.07.060. [DOI] [Google Scholar]
- 52.Fancello F., Petretto G.L., Zara S., Sanna M.L., Addis R., Maldini M., Foddai M., Rourke J.P., Chessa M., Pintore G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT-Food Sci. Technol. 2016;69:579–585. doi: 10.1016/j.lwt.2016.02.018. [DOI] [Google Scholar]
- 53.Kalleli F., Bettaieb Rebey I., Wannes W.A., Boughalleb F., Hammami M., Saidani Tounsi M., M’hamdi M. Chemical composition and antioxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J. Food Biochem. 2019;43:e12935. doi: 10.1111/jfbc.12935. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed A.F., Shi M., Liu C., Kang W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.)seeds from Egypt and China. Food Sci. Hum. Wellness. 2019;8:67–72. doi: 10.1016/j.fshw.2019.03.004. [DOI] [Google Scholar]
- 55.Semeniuc C.A., Pop C.R., Rotar A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017;25:403–408. doi: 10.1016/j.jfda.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mith H., Duré R., Delcenserie V., Zhiri A., Daube G., Clinquart A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014;2:403–416. doi: 10.1002/fsn3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gucwa K., Milewski S., Dymerski T., Szweda P. Investigation of the antifungal activity and mode of action of thymus vulgaris, citrus limonum, pelargonium graveolens, cinnamomum cassia, ocimum basilicum, and eugenia caryophyllus essential oils. Molecules. 2018;23:1116. doi: 10.3390/molecules23051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Firmino D.F., Cavalcante T.T.A., Gomes G.A., Firmino N.C.S., Rosa L.D., De Carvalho M.G., Catunda F.E.A. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018;2018:7405736. doi: 10.1155/2018/7405736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasconcelos N.G., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018;120:198–203. doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 60.Ye H., Shen S., Xu J., Lin S., Yuan Y., Jones G.S. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control. 2013;34:619–623. doi: 10.1016/j.foodcont.2013.05.032. [DOI] [Google Scholar]
- 61.Friedman M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017;65:10406–10423. doi: 10.1021/acs.jafc.7b04344. [DOI] [PubMed] [Google Scholar]
- 62.Utchariyakiat I., Surassmo S., Jaturanpinyo M., Khuntayaporn P., Chomnawang M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016;16:158. doi: 10.1186/s12906-016-1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCabe-Sellers B.J., Beattie S.E. Food safety: Emerging trends in foodborne illness surveillance and prevention. J. Am. Diet. Assoc. 2004;104:1708–1717. doi: 10.1016/j.jada.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 65.Sergelidis D., Angelidis A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017;64:409–418. doi: 10.1111/lam.12735. [DOI] [PubMed] [Google Scholar]
- 66.Silva N., Alves S., Gonçalves A., Amaral J.S., Poeta P. Antimicrobial activity of essential oils from mediterranean aromatic plants against several foodborne and spoilage bacteria. Food Sci. Technol. Int. 2013;19:503–510. doi: 10.1177/1082013212442198. [DOI] [PubMed] [Google Scholar]
- 67.Bozin B., Mimica-Dukic N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- 68.Niu C., Wang C., Yang Y., Chen R., Zhang J., Chen H., Zhuge Y., Li J., Cheng J., Xu K., et al. Carvacrol Induces Candida albicans Apoptosis Associated With Ca2+/Calcineurin Pathway. Front. Cell. Infect. Microbiol. 2020;10:1–12. doi: 10.3389/fcimb.2020.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben Arfa A., Combes S., Preziosi-Belloy L., Gontard N., Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 70.Lambert R.J.W., Skandamis P.N., Coote P.J., Nychas G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 71.Bagamboula C.F., Uyttendaele M., Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21:33–42. doi: 10.1016/S0740-0020(03)00046-7. [DOI] [Google Scholar]
- 72.Cosentino S., Tuberoso C.I.G., Pisano B., Satta M., Mascia V., Arzedi E., Palmas F. In-Vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999;29:130–135. doi: 10.1046/j.1472-765X.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 73.Cristani M., D’Arrigo M., Mandalari G., Castelli F., Sarpietro M.G., Micieli D., Venuti V., Bisignano G., Saija A., Trombetta D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007;55:6300–6308. doi: 10.1021/jf070094x. [DOI] [PubMed] [Google Scholar]
- 74.Ebani V.V., Nardoni S., Bertelloni F., Tosi G., Massi P., Pistelli L., Mancianti F. In Vitro antimicrobial activity of essential oils against salmonella enterica serotypes enteritidis and typhimurium strains isolated from poultry. Molecules. 2019;24:900. doi: 10.3390/molecules24050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In Vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brnawi W.I., Hettiarachchy N.S., Horax R., Kumar-Phillips G., Ricke S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella typhimurium in broth system and on celery. J. Food Process. Preserv. 2019;43:e13888. doi: 10.1111/jfpp.13888. [DOI] [Google Scholar]
- 77.Blaszyk M., Holley R.A. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int. J. Food Microbiol. 1998;39:175–183. doi: 10.1016/S0168-1605(97)00134-7. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L.L., Zhang L.F., Xu J.G., Hu Q.P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017;61:1353356. doi: 10.1080/16546628.2017.1353356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 80.Hou H., Zhang X., Zhao T., Zhou L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ. 2020;8:e9626. doi: 10.7717/peerj.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Mogy M.M., Alsanius B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control. 2012;28:157–162. doi: 10.1016/j.foodcont.2012.04.036. [DOI] [Google Scholar]
- 82.Soylu E.M., Kurt Ş., Soylu S. In Vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010;143:183–189. doi: 10.1016/j.ijfoodmicro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 83.De Clerck C., Dal Maso S., Parisi O., Dresen F., Zhiri A., Haissam Jijakli M. Screening of Antifungal and Antibacterial Activity of 90 Commercial Essential Oils against 10 Pathogens of Agronomical Importance. Foods. 2020;9:1418. doi: 10.3390/foods9101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palfi M., Konjevoda P., Vrandečić K., Ćosić J. Antifungal activity of essential oils on mycelial growth of Fusarium oxysporum and Bortytis cinerea. Emir. J. Food Agric. 2019;31:544–554. doi: 10.9755/ejfa.2019.v31.i7.1972. [DOI] [Google Scholar]
- 85.Gruľová D., Caputo L., Elshafie H.S., Baranová B., de Martino L., Sedlák V., Gogaľová Z., Poráčová J., Camele I., de Feo V. Thymol Chemotype Origanum vulgare L. Essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules. 2020;25:595. doi: 10.3390/molecules25030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williamson B., Tudzynski B., Tudzynski P., Van Kan J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 87.Ahmadu T., Ahmad K., Ismail S.I., Rashed O., Asib N., Omar D. Antifungal efficacy of moringa oleifera leaf and seed extracts against botrytis cinerea causing gray mold disease of tomato (Solanum lycopersicum L.) Braz. J. Biol. 2021;81:1007–1022. doi: 10.1590/1519-6984.233173. [DOI] [PubMed] [Google Scholar]
- 88.Leroux P. Chemical Control of Botrytis and its Resistance to Chemical Fungicides. In: Elad Y., Williamson B., Tudzynski P., Delen N., editors. Botrytis: Biology, Pathology and Control. Springer; Dordrecht, The Netherlands: 2007. pp. 195–222. [Google Scholar]
- 89.Leroux P., Fritz R., Debieu D., Albertini C., Lanen C., Bach J., Gredt M., Chapeland F. Mechanisms of resistance to fungicides in field strains of B. cinerea. Pest Manag. Sci. 2002;58:876–888. doi: 10.1002/ps.566. [DOI] [PubMed] [Google Scholar]
- 90.Leroux P., Gredt M., Leroch M., Walker A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010;76:6615–6630. doi: 10.1128/AEM.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bardas G.A., Veloukas T., Koutita O., Karaoglanidis G.S. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag. Sci. 2010;66:967–973. doi: 10.1002/ps.1968. [DOI] [PubMed] [Google Scholar]
- 92.Raveau R., Fontaine J., Lounès-Hadj Sahraoui A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods. 2020;9:365. doi: 10.3390/foods9030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mani-López E., Cortés-Zavaleta O., López-Malo A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021;3:44. doi: 10.1007/s42452-020-04102-1. [DOI] [Google Scholar]
- 94.Akthar M.S., Degaga B., Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Biol. Sci. Pharm. Res. 2014;2:001–007. [Google Scholar]
- 95.Valdivieso-Ugarte M., Gomez-Llorente C., Plaza-Díaz J., Gil Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients. 2019;11:2786. doi: 10.3390/nu11112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wink M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines. 2015;2:251–286. doi: 10.3390/medicines2030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.