Abstract
Respiratory syncytial virus (RSV) infection is an important cause of hospitalization in infants and young children. Monthly administration of palivizumab during the RSV season is effective in preventing severe infections in children with comorbidities. However, determining the onset of the RSV season for starting palivizumab is often challenging. The present study aimed to evaluate the ideal timing to start palivizumab and its effect on hospitalization in the real world.
We performed a retrospective, observational study to identify the relationship between the timing of the first dose of palivizumab administration and RSV-related hospitalization. Medical records from 2015 to 2019 were reviewed. We included patients who had indications for palivizumab as of July 1 in each year. We counted the proportion of children receiving palivizumab and the number of RSV infection-related hospitalizations each month. We also evaluated the differences in background and underlying disease between children with and without hospitalization.
A total of 498 patients were included, and 105 (21.0%) completed the first dose in July when the RSV season usually begins in Japan. Twenty-three (4.6%) patients were hospitalized for RSV infection during the observation period, with 13 (56.5%) hospitalizations before their first dose of palivizumab. The remaining 10 patients were hospitalized after receiving 1 or more doses of palivizumab. Children living with siblings and children with cyanosis originating from congenital heart disease had a higher risk of RSV with odds ratios of 5.1 (95% confidence interval 1.48-17.6, P < .01) and 3.3 (95% confidence interval 1.33-7.94, P < .01), respectively.
Delays in administering palivizumab at the beginning of the season increases the rate of RSV infection-related hospitalization. To maximize prophylactic effectiveness, administering the first dose as early as possible in the RSV season is crucial, with priority for cyanotic children or those with siblings.
Keywords: palivizumab, prophylaxis, respiratory syncytial virus, season
1. Introduction
Respiratory syncytial virus (RSV) is the leading cause of respiratory tract infections in infants, with nearly all cases occurring before 2 years old.[1] Preterm birth, chronic lung disease, congenital heart disease (CHD), immunocompromised status, and Down syndrome are significant risks for severe RSV infection.[2–4] Children with these underlying medical conditions are more susceptible to severe lower respiratory tract infections, which are potentially fatal.[4–6] There is no specific treatment for RSV infection. Currently, prevention of severe disease can be achieved by administering palivizumab, which is an anti-RSV monoclonal antibody.[7]
Palivizumab has been approved by the Japanese healthcare insurance system to prevent severe RSV infections in preterm infants and in patients with bronchopulmonary dysplasia since 2002. Palivizumab has also been approved in patients with CHD since 2005 and in patients with immunodeficiency disease and Down syndrome since 2013. Guidelines recommend that the first dose of palivizumab should be administered at the beginning of the RSV season.[8,9] However, determining the onset of the RSV season is difficult because the timing varies from year to year and differs with geographical location. Gutfraind et al[9] investigated the optimal timing of palivizumab administration using a mathematical modeling study. They concluded that their modified palivizumab regimens improved protection for children at risk for severe outcomes of RSV infection.[9] However, their mathematical model regimens have not been proven to be effective in the real world, and their data suggest that there is potential for further refinement in the currently recommended regimens.
The present study primarily aimed to verify the hypothesis that a delay in palivizumab administration increases the incidence of RSV-related hospitalization. This study also aimed to investigate whether underlying medical conditions or the presence of siblings are risk factors for RSV-related hospitalization in a specific population living in a particular area with similar exposure to RSV infection. The results of this study will provide critical information for determining the optimal timing of initiation and duration of palivizumab administration.
2. Materials and methods
We performed a retrospective, observational study in a Children's Hospital in Fukuoka, Japan. Study protocols were approved by the Fukuoka Children's Hospital Institutional Review Board (#2019-64). The inclusion criteria were as follows: children who received palivizumab from 2015 to 2019 at Fukuoka Children's Hospital; and children who were already under outpatient management as of 1 July in each year. The indications for palivizumab administration under the Japanese healthcare insurance system were one of the following: infants (≤12 months) born before 29 weeks’ gestation; infants (≤6 months) born between 29 and 35 weeks’ gestation; children (≤2 years) who were treated for bronchopulmonary dysplasia within the past 6 months; children (≤2 years) with hemodynamically significant CHD; children (≤2 years) with immunodeficiency; and children (≤2 years) with Down syndrome.
To reduce the bias due to RSV exposure status, we used the following exclusion criteria: children who were administered palivizumab during hospital admission owing to an underlying medical condition; children who did not receive their first dose of palivizumab in our hospital; children who lived outside of Fukuoka Prefecture; children who did not visit the regular outpatient clinic for palivizumab administration; children who were hospitalized for longer than 4 weeks for reasons other than RSV infection; and children who had undergone radical cardiac surgery and their hemodynamic abnormalities were resolved.
The timing of starting palivizumab, background information, and the clinical course of these patients up to March of the following year were reviewed in the electronic medical records. Patient information collected from the medical records included sex, underlying medical conditions, family history, group childcare use, cyanosis and home oxygen use, surgical history and planned surgery, gestational week at birth, and residence area. During the observation period, we reviewed the details of palivizumab administration, RSV infection-related hospitalization and treatment, hospitalization for other diseases, and surgery.
3. Analysis
We evaluated the proportion of children receiving palivizumab and the number of RSV-related hospitalizations each month. Results are presented as numbers and proportions for categorical data and as medians and interquartile ranges for continuous data. Data analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan, version 1.40), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.2).[10] Demographic data and risk factors for RSV infection were compared between patients with and without hospitalization for RSV using the independent sample t test, Mann–Whitney U test, and Pearson χ2 test. Seasonal hospitalization rates were compared by Fisher exact test (2-sided). Multivariable logistic regression analysis adjusted for having siblings, cyanosis originating from CHD, and preschool was also performed to identify the determinants for hospitalization due to RSV infection. A 2-tailed P value < .05 was considered statistically significant.
4. Results
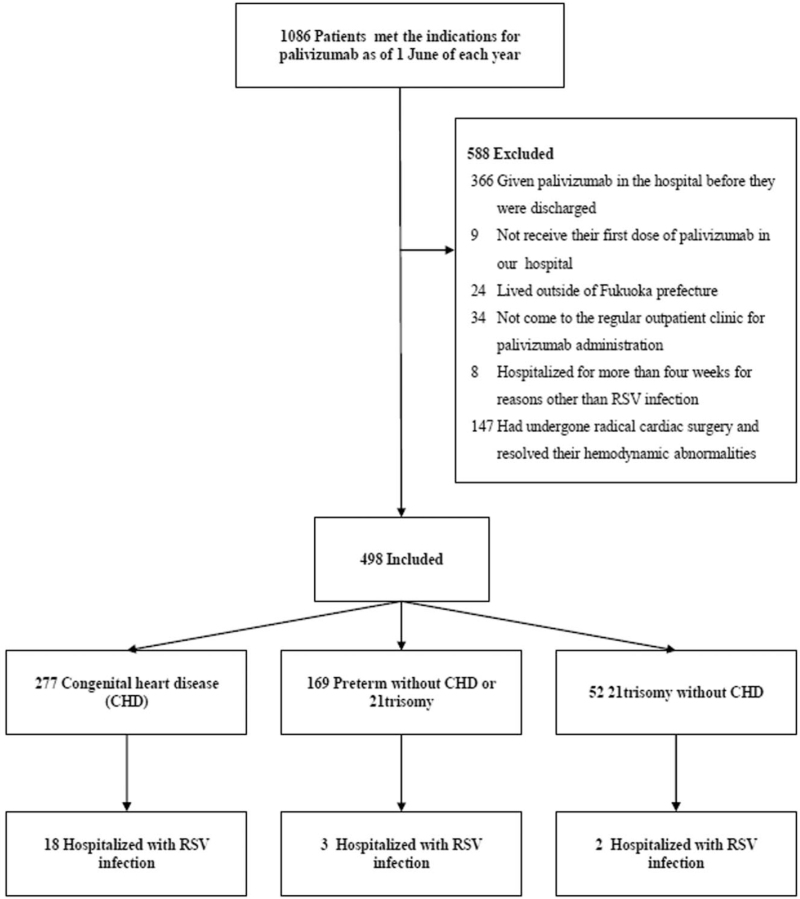
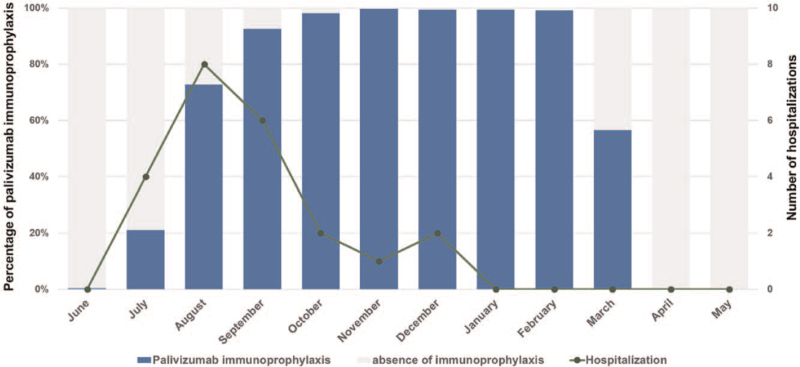
A total of 1086 patients met the indications for palivizumab as of June 1 in each year. Of these, 588 patients who met the exclusion criteria were excluded from the study (Fig. 1). The primary indications for palivizumab were 277 for CHD, 169 for preterm without CHD or 21trisomy, and 52 for 21 trisomy without CHD. No patients took palivizumab for immunodeficiency. The background characteristics of the patients are shown in Table 1. Palivizumab administration was started in June of each year. The accumulated administration rate gradually increased from 0.4% (2/498) in June to 21.1% (105/498) in July and to 72.7% (362/498) in August, with 95% (473/498) of children initiating administration of palivizumab by September. Palivizumab prophylaxis was stopped in the middle of March each year because we decided that the epidemic had ended. As a result, 53.4% (281/498) of children received palivizumab in March, and no children received palivizumab in April (Fig. 2). The number of patients per total number of doses was 2 for 4 times, 17 for 5 times, 47 for 6 times, 163 for 7 times, 265 for 8 times, and 4 for 9 times.
Figure 1.
Flow chart of the 23 patients who were hospitalized with RSV infection. RSV = respiratory syncytial virus.
Table 1.
Demographics and patients’ characteristics at baseline.
| Main indication for palivizumab | ||||
| Total | Congenital heart disease (CHD) | Preterm | 21trisomy | |
| N | 498 | 277 | 169 | 52 |
| Mean age (mo) [min-max] | 9.5 (0-24) | 11.6 (0-24) | 5.2 (1-23) | 12.5 (1-24) |
| Male | 267 (53.6%) | 130 (46.9%) | 100 (59.2%) | 37 (71.2%) |
| Female | 231 (46.4%) | 147 (53.1%) | 69 (40.8%) | 15 (28.8%) |
| Mean gestational age (wks) [min-max] | 35.6 (22-42) | 37.8 (24-42) | 31.1 (22-35) | 37.6 (36-40) |
| High flow humidified nasal cannula | 7 (1.4%) | 7 (2.5%) | 0 (0.0%) | 0 (0.0%) |
| Mechanical ventilation | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| Presence of siblings | 304 (61.0%) | 149 (53.8%) | 113 (66.9%) | 42 (80.8%) |
| Attending preschool | 78 (15.7%) | 43 (15.5%) | 4 (2.4%) | 31 (59.6%) |
| With cyanosis originating from CHD | 115 (23.1%) | 115 (41.5%) | 2 (1.2%) | 0 (0.0%) |
| History of cardiac surgery | 179 (35.9%) | 179 (64.6%) | 0 (0.0%) | 0 (0.0%) |
| Scheduled for radical or Fontan operation | 33 (6.6%) | 33 (11.9%) | – | – |
Figure 2.
Cumulative percentage of children who received palivizumab and the number of RSV-related hospitalizations by month. The blue bars show the cumulative percentage of children who received palivizumab. The gray bars show the percentage of children who did not receive palivizumab. The line graph shows the number of RSV-related hospitalizations. RSV = respiratory syncytial virus.
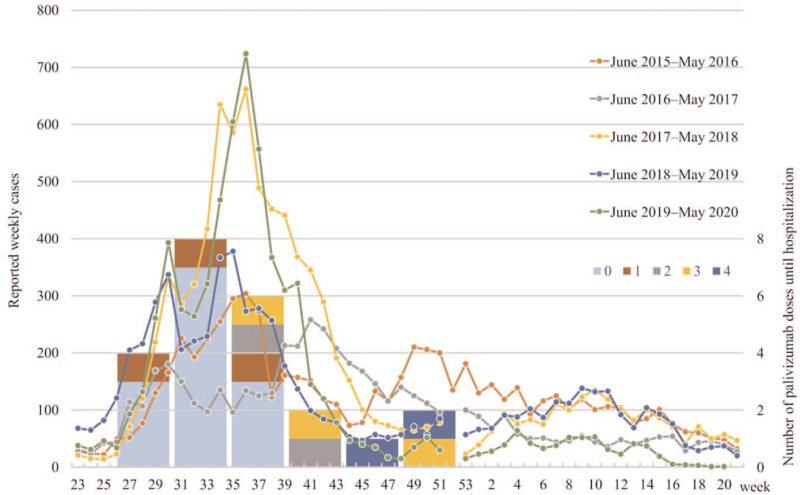
Twenty-three (4.6%, 23/498) patients were hospitalized with RSV infection during the follow-up period. They were all confirmed to diagnose RSV by an immunochromatographic rapid test kit on nasal swabs. There was no significant difference in the hospitalization rate between the 3 groups (Table 2). The numbers of hospitalizations in each season were 3/106 (2.8%), 3/107 (2.8%), 5/84 (6.0%), 7/106 (6.6%), and 5/95 (5.3%) in order from the 2015 season, with no significant difference among the seasons (P = .56). One child was diagnosed with RSV infection in December, but had a mild case and was not hospitalized. With regard to monthly RSV-related hospitalizations, there were 4 in July, 8 in August, 6 in September, 2 in October, 1 in November, and 2 in December. There were no RSV-related hospitalizations after January (Fig. 2). We referred to the Infectious Disease Weekly Report of the National Institute of Infectious Diseases (https://www.niid.go.jp/niid/en/survaillance-data-table-english.html) for comparison with prefectural epidemic data. The number of reported RSV infections in Fukuoka Prefecture from 2015 to 2019 followed a pattern that began to increase in July, peaked in September, and ended in January and February each year (Fig. 3).
Table 2.
Analysis of incidence of hospitalization for RSV.
| No. | Univariate | Multivariate | ||||
| Hospitalization (n = 23) | Non hospitalization (n = 475) | P value | Odds ratio | 95% CI | P value | |
| Demographic characteristics | ||||||
| Sex | .39 | |||||
| Female | 13 (5.6%) | 218 (94.4%) | ||||
| Mean age in months at entry (SD) | 9.8 (5.7) | 9.5 (6.5) | .81 | |||
| Mean gestational age in weeks (SD) | 36.5 (3.8) | 35.6 (4.1) | .27 | |||
| Main indications for palivizumab | .05 | |||||
| Congenital heart disease (CHD) | 18 (3.6%) | 259 (52.0%) | ||||
| Preterm | 3 (0.6%) | 166 (33.3%) | ||||
| 21 trisomy without CHD | 2 (0.4%) | 50 (10.0%) | ||||
| RSV risk factors | ||||||
| History of cardiac surgery | 8 (34.8%) | 171 (36.0%) | 1.00 | |||
| Scheduled cardiac surgery | 7 (30.4%) | 26 (5.5%) | <.01 | |||
| Siblings | 20 (87.0%) | 284 (59.8%) | <.01 | 5.10 | 1.48–17.6 | <.01 |
| Cyanosis originating from CHD | 10 (43.5%) | 108 (22.7%) | .04 | 3.25 | 1.33–7.94 | <.01 |
| Preschool | 4 (17.4%) | 74 (15.6%) | .77 | 1.38 | 0.44–4.39 | .58 |
RSV = respiratory syncytial virus.
Figure 3.
The line graph shows the reported weekly cases of RSV infection from 2015 to 2019 in Fukuoka Prefecture, where the research facility is located (axis 1). The bar graph shows the number of patients with RSV who were admitted to the hospital during the month. The number of palivizumab doses (0-4) administered before admission is indicated by color coding (axis 2). RSV = respiratory syncytial virus.
The background information of the 23 patients with RSV-related hospitalizations is shown in Table 2. The number of doses of palivizumab administered before hospitalization was 0 in 13 (56.5%, 13/23) patients, 1 in 3 patients, 2 in 2 patients, 3 in 3 patients, and 4 in 2 patients. There were no significant differences in patient's outcomes (intensive care unit admission rate, inotropic use rate, mechanical and non-invasive ventilation use rate, duration of oxygen administration, and the number of hospital days) between patients who were hospitalized during the observation period without palivizumab and those who were hospitalized while already receiving the palivizumab. None of the patients included in the study had multiple RSV-related hospitalizations or deaths (Table S1, Supplemental Digital Content). Multivariable analysis showed that children with older siblings and cyanosis that originated from CHD had a higher risk of RSV, with an odds ratio of 5.1 (95% confidence interval [CI] 1.48-17.6, P < .01) and 3.25 (95% CI 1.33-7.94, P < .01), respectively (Table 2).
5. Discussion
In this study, we found that patients were hospitalized for RSV infection before administration or after only 1 dose of palivizumab in the early stages of the RSV season. Additionally, having older siblings and cyanosis originating from CHD were notable risk factors for RSV-related hospitalization in patients who had an indication for palivizumab prophylaxis.
Currently, antiviral drugs and vaccines for RSV infection are being developed, and palivizumab is the only tool available for clinical use. The efficacy of this monoclonal antibody in preventing severe RSV infection has been demonstrated in several studies[11–14] and passive immunization with palivizumab is recommended worldwide. However, the effectiveness of palivizumab in preventing hospitalization is only 74% (95% CI 56%-85%) in preterm infants (born at 29-35 weeks of gestational age) aged ≤6 months without comorbid conditions, including CHD and chronic lung disease, even in developed countries (e.g., United States of America and Canada).[15] Additionally, the reduction in the rate of intensive care unit admission from using palivizumab is 62% (95% CI 35%-78%).[15] This rate is not satisfactory when taking into consideration the high acquisition costs of palivizumab. The effectiveness of palivizumab needs to be maximized to reduce the cost of hospitalization, productivity loss, and most importantly, suffering of the children.
The majority of hospitalized patients in our cohort (13, 56.5%) had never received palivizumab before admission to hospital. Three children were also hospitalized for RSV infection after the first dose of palivizumab. They developed symptoms approximately 3 weeks after their first dose of palivizumab. In the IMpact-RSV Study, intramuscular administration of palivizumab (15 mg/kg) resulted in mean trough serum concentrations of 37 ± 21 mg/mL after the initial injection and they gradually increased with each dose.[12] The lowest blood levels were shown observed just before the second palivizumab administration in the previous reports.[12,16] Our 3 patients might not have had sufficient serum palivizumab levels. If these 16 patients had received palivizumab at an earlier stage of the season, RSV-related hospitalizations might have been avoided.
Approximately 2 months were required to complete the first dose of palivizumab in all eligible children after the beginning of the RSV season. Informing the parents that administration of palivizumab has started can take a while, and the number of children that can have administration in a day is limited. During this period, many children are exposed to RSV, and some of them are hospitalized with severe RSV infection. The finding of the low rate of RSV-related hospitalization after the administration rate reached 100% suggests that early administration of the first dose is essential. However, identifying the onset of the RSV season is challenging. We need to overcome the difficulty in accessing medical services, and human and non-human resource shortages in hospitals to administer palivizumab as early as possible.
Another major problem that prevents us from starting administration at the correct time is that the beginning of the RSV season varies from year to year. The RSV season usually starts in early July, but it can be late July or early August, which causes difficulty in determining the start of the RSV season. Our study showed that early July was crucial for preventing RSV related-hospitalization. Yamagami et al[17] found that 0.31 reports per week per sentinel medical institution was the estimated starting point of the RSV epidemic. This finding was based on backward-looking analysis of data on the number of infectious disease reports in Tokyo. However, when we attempted to apply the same model to other regions, there were 0.22 to 1.0 reports per week per sentinel medical institution, which is too wide to be clinically applicable. Further research is needed for the practical application of a tool to accurately predict the start of the RSV season.
A realistic approach is that children who have risk factors should start administration of palivizumab quickly and efficiently if there is any sign that the RSV season has started. In our study, having older siblings in the family and cyanosis originating from CHD were risk factors for RSV-related hospitalization. Of the 23 patients who were admitted, 20 (87.0%) had older siblings. Large birth cohort studies have shown that RSV infection can be transmitted from older siblings.[18] Eliminating the risk posed by older siblings could reduce the number of admissions for RSV by one third across the first 3 years of life.[18–20] If an RSV vaccine is developed, targeting older siblings would also indirectly protect younger children with underlying diseases. However, such a vaccine effect is not feasible. Vaccination will take a considerable amount of time because the historical RSV vaccine has failed.
Children with cyanotic CHD also had a higher risk of RSV-associated hospitalization than those with acyanotic CHD in our cohort. Children with cyanotic heart disease are discharged from the hospital after a palliative operation and continue their daily lives at home and in the community. At this stage, they have a low cardiac reserve with varying degrees. Hypoxia and elevated carbon dioxide from RSV infection can easily disrupt hemodynamics by affecting somatic and pulmonary vascular resistance. Therefore, children with cyanotic CHD are a priority target for administration of palivizumab.
This study has potential limitations. First, the criteria for admission were not uniform. In many cases, the decision to admit patients might have been made owing to the severity of the underlying disease, which might have affected the incidence of RSV infection-related hospitalizations. Second, the status of RSV epidemics in each child's area or residence would be different, which was not considered in the analysis. These issues could be addressed by following up the children separately according to underlying disease and by creating a cohort of large numbers of cases at multiple institutions in different regions. Third, blood levels of palivizumab were not measured. Therefore, we were not able to confirm that blood palivizumab levels were insufficient in those who were hospitalized after administration of palivizumab.
In conclusion, administering the first dose of palivizumab as early as possible after the start of the RSV season is important, especially in children with older siblings and cyanotic heart disease, to avoid RSV-related hospitalization. Our findings could be applied to maximize the cost-effectiveness of palivizumab.
Acknowledgments
We would like to thank Ami Saito for her assistance in data collection. We also thank Ellen Knapp, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
Akiko Kamori contributed to the data acquisition and paper writing. Yuya Morooka contributed to the data verification and the manuscript review. Kenichiro Yamamura contributed to the design of the work and the analysis of data for the work. Pin Fee Chong and Noriko Kuga contributed to drafting the work and revising critically for important intellectual content. Yasushi Takahata and Koichi Sagawa contributed to the design and data acquisition. Kenji Furuno contributed to the conception and design of the work, the analysis and interpretation of data for the work, and the manuscript review. All authors read and approved the final manuscript.
Conceptualization: Kenji Furuno.
Data curation: Akiko Kamori, Yuya Morooka, Yasushi Takahata, Koichi Sagawa.
Formal analysis: Kenichiro Yamamura, Kenji Furuno.
Investigation: Kenji Furuno.
Methodology: Kenichiro Yamamura, Yasushi Takahata, Koichi Sagawa, Kenji Furuno.
Project administration: Kenji Furuno.
Validation: Yuya Morooka, Kenji Furuno.
Writing – original draft: Akiko Kamori, Pin Fee Chong, Noriko Kuga.
Writing – review & editing: Yuya Morooka, Pin Fee Chong, Noriko Kuga, Kenji Furuno.
Supplementary Material
Footnotes
Abbreviations: CHD = congenital heart disease, RSV = respiratory syncytial virus.
How to cite this article: Kamori A, Morooka Y, Yamamura K, Chong PF, Kuga N, Takahata Y, Sagawa K, Furuno K. Effect of delayed palivizumab administration on respiratory syncytial virus infection-related hospitalisation: a retrospective, observational study. Medicine. 2021;100:47(e27952).
The authors have no funding to disclose.
Kenji Furuno received speaker honoraria from AbbVie GK. The other authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–6. [DOI] [PubMed] [Google Scholar]
- [2].Simoes EA. Respiratory syncytial virus infection. Lancet 1999;354:847–52. [DOI] [PubMed] [Google Scholar]
- [3].Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995;126:212–9. [DOI] [PubMed] [Google Scholar]
- [4].Beckhaus AA, Castro-Rodriguez JA. Down syndrome and the risk of severe RSV infection: a meta-analysis. Pediatrics 2018;142:e20180225. [DOI] [PubMed] [Google Scholar]
- [5].Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J 2011;5:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol 2017;52:556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997;176:1215–24. [DOI] [PubMed] [Google Scholar]
- [8].American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134:415–20. Erratum in: Pediatrics. 2014;134:1221. [DOI] [PubMed] [Google Scholar]
- [9].Gutfraind A, Galvani AP, Meyers LA. Efficacy and optimization of palivizumab injection regimens against respiratory syncytial virus infection. JAMA Pediatr 2015;169:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother 2017;13:2138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- [13].Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532–40. [DOI] [PubMed] [Google Scholar]
- [14].Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013;4:CD006602. [DOI] [PubMed] [Google Scholar]
- [15].Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EAF. Effectiveness of palivizumab in high-risk infants and children. Pediatr Infect Dis J 2017;36:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].La Via WV, Notario GF, Yu XQ, Sharma S, Noertersheuser PA, Robbie GJ. Three monthly doses of palivizumab are not adequate for 5-month protection: a population pharmacokinetic analysis. Pulm Pharmacol Ther 2013;26:666–71. [DOI] [PubMed] [Google Scholar]
- [17].Yamagami H, Kimura H, Hashimoto T, Kusakawa I, Kusuda S. Detection of the onset of the epidemic period of respiratory syncytial virus infection in Japan. Front Public Health 2019;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jacoby P, Glass K, Moore HC. Characterizing the risk of respiratory syncytial virus in infants with older siblings: a population-based birth cohort study. Epidemiol Infect 2017;145:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Willmott M, Nicholson A, Busse H, Macarthur GJ, Brookes S, Campbell R. Effectiveness of hand hygiene interventions in reducing illness absence among children in educational settings: a systematic review and meta-analysis. Arch Dis Child 2016;101:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hardelid P, Verfuerden M, McMenamin J, Smyth RL, Gilbert R. The contribution of child, family and health service factors to respiratory syncytial virus (RSV) hospital admissions in the first 3 years of life: birth cohort study in Scotland, 2009 to 2015. Eurosurveill 2019;24:1800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.